Abstract
Neurofilaments are proteins selectively expressed in the cytoskeleton of neurons, and increased levels are a marker of damage. Elevated neurofilament levels can serve as a marker of ongoing disease activity as well as a tool to measure response to therapeutic intervention. The potential utility of neurofilaments has drastically increased as recent advances have made it possible to measure levels in both the cerebrospinal fluid and blood. There is mounting evidence that neurofilament light chain (NfL) and phosphorylated neurofilament heavy chain (NfH) are abnormal in a host of neurodegenerative diseases. In this review we examine how both of these proteins behave across diseases and what we know about how these biomarkers relate to in vivo white matter pathology and each other.
Keywords: neurodegeneration, NFL, NFH, NFM, white matter
Introduction
Neurodegenerative disease such as Alzheimer disease (AD), stroke, traumatic brain injury, and multiple sclerosis (MS) are common causes of disability and mortality. These diseases have a social and financial cost to the affected individuals and society more broadly. Biomarkers aid diagnosis and impact treatment, in turn improving the quality of life for patients and caregivers. No one biomarker fully characterizes a disorder, and new measures are constantly introduced to improve our understanding of disease.
Neurofilaments are type IV intermediate filaments sharing structural elements with nestin, peripherin, and α-internexin [1-3]. Neurofilaments fall into three categories, separated by their molecular weights of 68, 150, and 190-210 kilodaltons into light (NfL), medium (NfM), and heavy (NfH) chains [1,2,4]. Neurofilaments are heteropolymers; NfL forms the core of the structure and dimerizes with NfH or NfM to form tetramers, protofilaments, and finally ~10 nm fibers [1,2,4-6]. Neurofilaments are specifically expressed in neurons and are a major cytoskeleton protein[1,7]. Their gene expression [8] and phosphorylation levels [9] directly affect axonal diameter, myelination, and conduction velocity [9-13]. When neurons are damaged neurofilaments are released into the interstitial fluid then diffuse into the cerebrospinal fluid (CSF) and then the blood [2,4]. Antibodies have been successfully developed to measure NfL and phosphorylated NfH. These proteins may be good markers of acute disease activity, monitors of therapeutic intervention, and predictors of future disease trajectories.
Measuring Neurofilaments
CSF NfL was first measured using enzyme-linked immunosorbent assays (ELISAs) [14-16]. These anti-bodies have been adapted for electrochemiluminescence (ECL) assays [17], and subsequently for the ultra-sensitive single molecule array (SIMOA) platform. The SIMOA system is more sensitive than either ELISAs and ECL at measuring the low concentrations of neurofilaments present in the blood [18,19]. Work directly comparing CSF and either serum or plasma levels of NfL have generally found a high concordance (Pearson r or Spearman ρ =0.7-0.9, [20-28]) although other analyses have found only moderate (~ 0.5-0.69) associations [29-33]. There has been no obvious evidence that either plasma or serum provide better measurements, and NfL levels in the two are highly corelated [34]. Phosphorylated levels of NfH in both CSF and blood are commonly measured using ELISAs [35,36]or ECL [37,38] approaches, although translation of antibodies to SIMOA is possible [19]. Correlations between blood and CSF levels of NfH are lower than that observed for NfL [39,40], and this lower correlation may be due to sensitivity limits of ELISA and ECL [19]. There is some evidence that blood based levels of neurofilaments may partially be influenced by body size and blood volume in the brain [41], which may introduce discrepancies between CSF and blood levels.
Neurofilaments in Neurodegenerative Disease
As neurofilament levels reflect nonspecific damage to axons, they have been examined across many neurological disorders. NfL levels are elevated in most conditions relative to healthy controls, but the degree of elevation varies considerably between disorders [14,16,23,34,42-50]. Prion diseases have some of the highest levels [43,46,51], with frontotemporal dementia (FTD) [20,43-45,48,49,51,52] and amyotrophic lateral sclerosis (ALS) [44,53,54] also typically high relative to other diseases. Vascular dementia (VAD), multiple systems atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD) are moderately elevated [23,34,43,45,47,49,55]. Alzheimer disease (AD), mild cognitive impairment (MCI), Parkinson disease (PD), and dementia with Lewy bodies (DLB) are only modestly elevated over controls [34,43-45,47-49,54] (See meta-analysis of CSF NfL by Bridel et al., 2019). Cross-disease comparisons of NfH are limited, but show that NfH levels are generally increases across neurodegenerative conditions [37,50,56-58].
Multiple Sclerosis (MS)
MS is chronic neurodegenerative disease where an immune-mediated process causes inflammation that damages myelin. White matter lesions can be seen as hyperintensities on fluid-attenuated inversion recovery (FLAIR) scans while gadolinium-enhancing lesions can identify areas of inflammation. CSF and blood-based measures of NfL are consistently elevated in MS patients [16,59-65], tied to relapses [29,60,64], and elevated in clinically isolated syndrome (CIS) patients that later convert to MS [64,66,67]. Higher levels of NfL in both CSF and blood have consistently been related to T2-weighted hyperintensity and gadolinium-enhancing lesions [61,62,66-71]. Perhaps the most intriguing finding is that patients on disease modifying treatments show significant reductions in NfL levels relative to placebo [33,61,63,70,72-74]. Work examining NfH is consistent with those of NfL, levels are elevated in MS patients [37,38,64,68,72,75] and increases in those that relapse [38,64]. In CIS NfH has been found to be both elevated [64,76] and no different from controls [66]. There have been mixed results relating NfH to gadolinium enhancing lesions, with both significant [69] and no [64] associations observed.
Amyotrophic Lateral Sclerosis (ALS)
ALS is a rapidly progressing disorder that affects motor neurons. NfL levels in both the CSF and blood [14,16,22,24,44,50,54,77-82] are highly elevated in ALS patients relative to controls, particularly for upper motor dominant ALS. NfL values discriminate ALS from mimics [22,77,82,83], indicating a utility identifying the 7% of diagnoses that turn out to be other diseases [50]. NfL levels are strongly tied to symptomology, and elevated values predict shorter survival times and greater progression rates [22,24,80]. There is also evidence that elevated NfL is associated with altered white matter integrity in descending motor tracts [78,81]. Whereas MS researchers have been a driving force exploring NfL, much of what we know about NfH comes from ALS research. As with NfL, both CSF and blood levels of NfH are dramatically increases in ALS patients relative to controls [37,39,50,56,82-89], levels discriminate ALS patients from mimics [50,77,82,83,88,89], values increase near the onset of clinical symptoms [88], and higher levels predict faster decline [56,82-84,88,90] and shorter survival times [39,84,87,89].
Frontotemporal Dementia (FTD)
FTD is an umbrella term for heterogeneous disorders that affect the frontal and temporal lobes, commonly occur in individuals in their 50’s and 60’s, and prominently manifest in changes in behavior, language, or movement. FTD can be grouped into disorders that involve tau or TDP43 protein and there are genetic links between FTD and ALS [91]. NfL is consistently elevated in FTD, with patients having some of the highest levels seen across disorders [20,42-46,48,49,52,53,92-98]. In genetic forms of FTD, levels of NfL are not elevated in asymptomatic individuals but increase with the onset of impairment [98,99]. There is some evidence that NfH levels are elevated in FTD [93,100], but such effects are modest and have not always been found when excluding FTD patients with motor involvement [39,84].
Alzheimer Disease (AD)
AD is the leading cause of dementia and its hallmark pathologies are extracellular beta-amyloid plaques (Aβ) and intracellular tau tangles. There is an extensive literature examining CSF, neuroimaging, and blood-based biomarkers of these pathologies. Still, there is a desire to develop new biomarkers to better understand neurodegeneration occurring in AD. In autosomal dominant cohorts NfL levels are elevated in mutation carriers relative to controls [28,101,102] and show highly significant relationships with levels of Aβ [28,103], cognition [28,101,103], and atrophy [28,101]. In sporadic, late onset AD, the predictive value of NfL is less clear. Levels are elevated in impaired individuals [31,34,44,46,49,51,92,96,104-110] relative to controls, although this is not always found [100,111], and AD is one of the least elevated neurodegenerative disorders [34,43,45,47-49]. NfL has been significantly tied to abnormal Aβ levels [28,32,107,112], although no association is also commonly found [31,34,106,112,113]. NfH is typically not elevated in AD [37,56,93,111] when age is considered, although positive results exist [57]
The most consistent finding is that higher levels of NfL are associated with worse cross-sectional and longitudinal cognition [28,31,44,101,106,110,112-114]. However the influence of NfL on cognition is often found to be similar between those with or without abnormal Aβ levels [114] or is independent of Aβ [106,113], and the relationship with cognition may be driven by the high correlations of neurofilaments with age [28,38,45,104,110,115,116]. Neurofilaments in AD appear highly similar to MRI volumetries; they can be abnormal due to disease pathophysiology, levels are associated with cognition, but other comorbidities such as cerebrovascular disease and diabetes could be contributing factors. Still, as clinical trials shift to include anti-tau therapies, neurofilaments are a cost-effective marker of neurodegeneration not directly engaged by the drugs. Neurofilaments could also serve as a compliment to blood measures of Aβ [117] and tau [118] to screen participants to increase the efficiency of clinical trials, or serve as biomarker endpoints themselves.
Parkinson Disease (PD)
PD is common degenerative disease characterized by a loss of dopaminergic neurons leading to motor difficulties as well as cognitive symptoms. CSF NfL levels are generally low in PD, often not different than controls [34,47,55,92]. The low levels in PD distinguish it from the high levels seen in multiple system atrophy (MSA) [34,47,55,119,120] and progressive supranuclear palsy (PSP) [120] which have overlapping symptomology. NfL levels are increased in PD patients additionally suffering from dementia (PDD) [44,49,55,121]. NfH mirrors NfL, being elevated in MSA and PSP relative to Parkinson’s disease [58,119] and controls [58].
Creutzfeldt–Jakob disease (CJD)
CJD is a rapidly progressive neurodegenerative condition caused by prions. NfL levels are elevated in those with CJD [27,43,46,122,123], with higher levels of being associated with more aggressive diseases courses and shorter survival time [27,46]. CSF NfH levels are also elevated in both sporadic and genetic forms of CJD, with levels increasing before the onset of symptoms [123,124]. The utility of neurofilaments in CJD is unclear, as CSF total tau has been shown to be a better predictor of disease severity [27].
Traumatic Brain Injury (TBI)
TBI represents a “silent epidemic” with as many as sixty-nine million individuals affected annually [125]. Blood levels of NfL are consistently increased in individuals with TBI [26,118,126,127]. Competitive athletes provide unique cohorts to study TBI. Blood-based levels of NfL are elevated in active but not retired boxers [128], increase after a bout [26], and levels negatively correlate with brain volumes measured with MRI [128]. In hockey players, serum NfL levels increase after a concussion, and levels were related to how long it took to return to play [118]. In football players serum NfL levels are higher in starters relative to non-starters, and the difference between groups gradually increases over the course of the season [129]. A similar pattern emerges for NfH; NfH is increased in animal models of TBI [130]. CSF levels increase after a bout in boxers [131], and serum levels are elevated in TBI and higher levels predict poorer outcomes [132]. Given that they can be measured in blood and correlate with meaningful outcomes, neurofilaments have a high utility in assessing and monitoring TBI.
Cardiovascular Disease
Impaired vascular systems can manifest as both chronic and acute neurological insults. CSF NfL and NfH are elevated in vascular dementia (VAD) [16,43,45,49,57], and these elevations may contribute to the mixed findings in the AD literature. Levels of NfL and NfH are increased after stroke [37,40,133-138], and increased levels of NfL are related to greater infarct size [133,135,138], as well as white matter changes [133], although this is not always the case [134]. With cardiac arrest blood NfL and NfH are elevated, and levels predict long-term neurological outcomes [139-141]. These results suggest that neurofilaments could aid neurological prognosis.
Other Neurodegenerative Conditions
The number and topical breadth of publications examining neurofilaments has drastically increased over the last few years. This trend will only increase given the relative low cost of neurofilament assays, and the ubiquity proved by blood-based measurements. Neurofilaments are examined in diverse diseases, representing both chronic and acute conditions. NfL levels are elevated in PSP [23,34,47,55,142,143], DLB [34,44,46,47], CBD [34,47], primary progressive aphasia (PPA) [144], Huntington disease [25,145], drug naïve subjects with HIV [146,147], psychiatric disorders such as bipolar disorder, depression, schizophrenia, and anorexia [94,148,149], spinal cord injuries [150], and postoperative delirium [151]. NfH has been shown to be a sensitive marker in DLB [100], PPA [144], PSP [58], VAD [57] spinal muscular atrophy [152], seizures [153], optic neuritis [154], and Guillain-Barré syndrome [37]. While promising, these results generally represent small samples and only a handful of publications.
Linking neurofilaments to pathology
Neurofilaments levels are interpreted as a marker of axonal damage across diverse diseases with demyelination, neuropathy, dendritic loss, frank cell death, and chronic inflammation. The most common link with white matter in vivo has been relating NfL levels to white matter hyperintensity (WMH) lesions in MS patients [61,62,66-71]. Diffusion tensor imaging (DTI) is a MRI technique that is sensitive to the movement of water in the brain and is considered a measure of fiber integrity [155]. NfL has been related to altered DTI metrics in stroke patients [133], TBI [126], and ALS [78,81]. However, cross-sectional and longitudinal studies in older adult cohorts are an inconsistent, with a mixture of significant and null findings [32,156-158]. There is a dearth of research relating neurofilament levels to white matter health, particularly for NfH. While neurofilament levels are clearly meaningful, a greater understanding of what damage they represent is needed.
The relationships between NfL and NfH
Due to their interrelated nature, it is unclear if light, medium, and heavy chains would be expected to have divergent diagnostic utility. Still, the proteins are not identical, NfL forms the backbone of neurofilaments, with NfM and NfH forming the side arms [1,2,4-6], and different phosphorylation rates and molecular weights could impact solubility, diffusion, and proteolysis. NfL and NfH generally have similar overarching patterns; both are increased in neurodegenerative conditions, and the more rapidly a disease progresses the higher the neurofilament levels. When examined concurrently in the CSF, NfL and NfH have been reasonably correlated (~0.4-0.6) [40,64,66,68,77] but less so in serum [69]. The results relating NfL to outcomes in disease are more robust, but NfL is more commonly examined as NfH has primarily been embraced by researchers studying motor neuron disease. The exact cause of this differential adoption is unclear but may be driven by differential availability and promotion of commercial assays, greater heterogeneity of NfH antibody utilized in assays, as well as the fact that there has been minimal adoption of NfH antibodies to the SIMOA system.
Conclusions
Neurofilaments have been widely implemented in research, and it is a matter of time before they are routinely adopted in clinical settings. While neurofilament levels are significantly elevated in patient populations, measures overlap between diseases and with healthy control populations. Neurofilaments are unlikely to have diagnostic utility except when values drastically differ between conditions with similar clinical presentations (e.g. ALS and mimics). Instead, levels are best interpreted as an individual difference marker of neuronal damage that can aid prognosis and therapeutic monitoring in combination with clinical judgment. Longitudinal clinical, cognitive, and biomarker data collected in parallel with neurofilaments are crucial to this endeavor and something that has been done in only a handful of published studies. The gaps in our understanding of how neurofilament levels relate to in vivo white matter, as well as the relationships between NfL, NfM, and NfH, are further questions that also need to be addressed before neurofilaments become biomarkers included by default to study neurodegenerative diseases. Finally, although excellent preclinical animal work has been done [34], research is overwhelmingly in human populations. This is a missed opportunity, as animal work provides an unsurpassed ability to directly manipulate biological properties to ask questions that simply cannot be asked in human studies. Neurofilaments are a promising biomarker of neuronal damage that may greatly aid disease prognosis and therapeutic intervention.
Table 1:
Key Findings in Neurodegenerative Disorders
| Disease | NfL Key Publications | NfH Key Publications | Finding Overview |
|---|---|---|---|
| Multiple Sclerosis (MS) | Lycke et al., 1998 [59] Malmeström et al. 2003 [60] Gunnarsson et al., 2011 [73] Kuhle et al. 2015 [74] Disanto et al., 2017 [29] |
Teunissen et al. 2009 [64] Kuhle et al. 2010 [37] Kuhle et al. 2011 [38] Kuhle et al. 2013 [72] Kuhle et al. 2017 [69] |
|
| Amyotrophic Lateral Sclerosis (ALS) |
Rosengren et al. 1996 [14] Lu et al. 2015 [24] Menke et al. 2015 [81] Steinacker et al. 2016 [50]* Poesen et al. 2017 [82]* |
Brettschneider et al. 2006 [56] Kuhle et al. 2010 [37] Ganesalingam et al. 2011 [39] Boylan et al. 2013 [90] Gendron et al. 2017 [84] |
|
| Frontotemporal Dementia (FTD) | Sjögren et al. 2000 [ 52] Landqvist et al. 2013 [42] Scherling et al. 2015 [48] Rohrer et al. 2016 [95] |
De Jong et al. 2007 [100] Pijnenburg et al. 2007 [93]* Ganesalingam et al. 2011 [39] |
|
| Alzheimer Disease (AD) |
Bacioglu et al 2016 [34] Zetterberg et al. 2016 [106] Mattsson et al. 2016 [107] Mattsson et al. 2017 [31] Preische et al. 2019 [28] |
Brettschneider et al. 2006 [56] Brettschneider et al. 2006 [57] Pijnenburg et al. 2007 [93] Kuhle et al. 2010 [37] |
|
| Parkinson Disease (PD) | Holmberg et al. 1998 [121] Abdo et al. 2007 [120] Hall et al. 2012 [47] Hall et al. 2018 [55] |
Brettschneider et al. 2006 [58] Abdo et al. 2007 [120] |
|
| Creutzfeldt-Jakob Disease (CJD) | Staffaroni et al. 2019 [27] Abu-Rumeileh et al. 2018 [46] |
Van Eijk et al. 2010 [125]* Steinacker et al. 2016 [124]* |
|
| Traumatic Brain Injury (TBI) | Oliver et al. 2016 [130] Shahim et al. 2017 [26] Shahim et al. 2018 [119] |
Anderson et al. 2008 [131] Neselius et al. 2013 [132] Shibahaski et al. 2016 [133] |
|
| Stroke and Ischemia | Tiedt et al. 2018 [134] Pujol-Calderon et al. 2019 [40] Moseby-Knappe et al. 2018 [141] |
Singh et al. 2011 [137] Rundgren et al. 2012 [142] |
|
examines both NfL and pNfH
Highlights.
Neurofilament levels are elevated in most neurodegenerative conditions
Levels predict cross-sectional and longitudinal cognitive and clinical outcomes
There is minimal work relating neurofilaments to in vivo white matter damage
It is unclear how neurofilament light and heavy chains relate to one another
Neurofilaments are promising biomarkers, but more work needs to be done
Acknowledgements
Support was provided by NIH grants K01AG053474, U19AG032438, R01AG05255002, P30NS09857704, and U19AG03243808. Illustration in Figure 1 was provided by Dylan Lawrence and Amanda Dicks in association with InPrint at Washington University in St. Louis.
Figure 1:
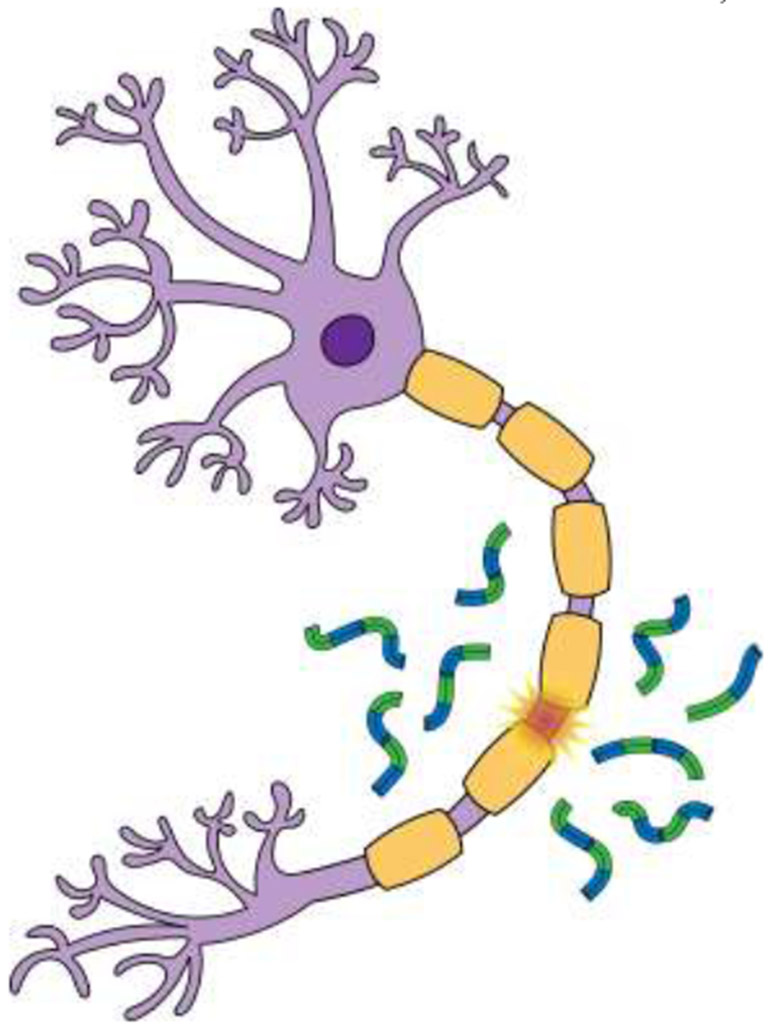
When neurons are damaged neurofilaments are released into the interstitial fluid, before moving into the cerebrospinal fluid and blood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest statement
Nothing declared.
References
· of special interest
·· of outstanding interest
- 1.Liu Q, Xie F, Siedlak SL, Nunomura A, Honda K, Moreira PI, Zhua X, Smith MA, Perry G: Neurofilament proteins in neurodegenerative diseases. Cell Mol Life Sci 2004, 61:3057–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, et al. : Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 2018, 14:577–589. [DOI] [PubMed] [Google Scholar]
- 3.Yuan A, Rao MV, Veeranna, Nixon RA: Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb Perspect Biol 2017, 9:a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petzold A: Neurofilament phosphoforms: Surrogate markers for axonal injury, degeneration and loss. J Neurol Sci 2005, 233:183–198. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs E, Weber K: Intermediate Filaments: Structure, Dynamics, Function and Disease. Annu Rev Biochem 1994, 63:345–382. [DOI] [PubMed] [Google Scholar]
- 6.Lee MK, Xu Z, Wong PC, Cleveland DW: Neurofilaments are obligate heteropolymers in vivo. J Cell Biol 1993, 122:1337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris JR, Lasek RJ: Monomer-polymer equilibria in the axon: direct measurement of tubulin and actin as polymer and monomer in axoplasm. J Cell Biol 1984, 98:2064–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL: Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A 1987, 84:3472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs E, Cleveland DW: A structural scaffolding of intermediate filaments in health and disease. Science (80-) 1998, 279:514–9. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Q, Couillard-Després S, Julien J-P: Delayed Maturation of Regenerating Myelinated Axons in Mice Lacking Neurofilaments. Exp Neurol 1997, 148:299–316. [DOI] [PubMed] [Google Scholar]
- 11.Jacomy H, Zhu Q, Couillard-Després S, Beaulieu J-M, Julien J-P: Disruption of Type IV Intermediate Filament Network in Mice Lacking the Neurofilament Medium and Heavy Subunits. J Neurochem 2001, 73:972–984. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki H, Itakura C, Mizutani M: Hereditary hypotrophic axonopathy with neurofilament deficiency in a mutant strain of the Japanese quail. Acta Neuropathol 1991, 82:427–434. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi T, Okada M, Kitamura T, Kawasaki K: Reduced diameter and conduction velocity of myelinated fibers in the sciatic nerve of a neurofilament-deficient mutant quail. Neurosci Lett 1993, 153:65–68. [DOI] [PubMed] [Google Scholar]
- 14.Rosengren LE, Karlsson J-E, Karlsson J-O, Persson LI, Wikkelsø C: Patients with Amyotrophic Lateral Sclerosis and Other Neurodegenerative Diseases Have Increased Levels of Neurofilament Protein in CSF. J Neurochem 1996, 67:2013–2018. [DOI] [PubMed] [Google Scholar]
- 15.Norgren N, Karlsson J-E, Rosengren L, Stigbrand T: Monoclonal Antibodies Selective for Low Molecular Weight Neurofilaments. Hybrid Hybridomics 2002, 21:53–59. [DOI] [PubMed] [Google Scholar]
- 16.Norgren N, Rosengren L, Stigbrand T: Elevated neurofilament levels in neurological diseases. Brain Res 2003, 987:25–31. [DOI] [PubMed] [Google Scholar]
- 17.Limberg M, Disanto G, Barro C, Kuhle J: Neurofilament light chain determination from peripheral blood samples In Methods in Molecular Biology. . Humana Press, New York, NY; 2015:93–98. [DOI] [PubMed] [Google Scholar]
- 18.Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius Å, Liman V, Norgren N, Blennow K, Zetterberg H: Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016, 54:1655–1661. [DOI] [PubMed] [Google Scholar]
- 19.Wilke C, Pujol-Calderón F, Barro C, Stransky E, Blennow K, Michalak Z, Deuschle C, Jeromin A, Zetterberg H, Schüle R, et al. : Correlations between serum and CSF pNfH levels in ALS, FTD and controls: a comparison of three analytical approaches. Clin Chem Lab Med 2019, 57:1556–1564.The authors compared serum and CSF levels of phosphorylated NfH in controls and patients with ALS or FTD, using ELISA and SIMOA. The results demonstrated a high concordance between platforms when analyzing CSF samples, but only serum values measured with the SIMOA strongly correlated with levels measured in CSF.
- 20.Steinacker P, Anderl-Straub S, Diehl-Schmid J, Semler E, Uttner I, Arnim CAF von, Barthel H, Danek A, Fassbender K, Fliessbach K, et al. : Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology 2018, 91:e1390–e1401. [DOI] [PubMed] [Google Scholar]
- 21.Weydt P, Oeckl P, Huss A, Müller K, Volk AE, Kuhle J, Knehr A, Andersen PM, Prudlo J, Steinacker P, et al. : Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol 2016, 79:152–158. [DOI] [PubMed] [Google Scholar]
- 22.Gille B, De Schaepdryver M, Goossens J, Dedeene L, De Vocht J, Oldoni E, Goris A, Van Den Bosch L, Depreitere B, Claeys KG, et al. : Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with Amyotrophic Lateral Sclerosis. Neuropathol Appl Neurobiol 2019, 45:291–304.Serum levels were elevated in 149 ALS patients relative to 19 ALS mimics and 82 controls, and Nfl values were related to rates of progress, survival time, as well as motor neuron degeneration. Effects were driven by upper motor neuron degeneration.
- 23.Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, et al. : Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 2017, 88:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C-H, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, Giovannoni G, Fratta P, Sidle K, Fish M, et al. : Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015, 84:2247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrne LM, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RAC, Scahill RI, Tabrizi SJ, Zetterberg H, Langbehn D, et al. : Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. Lancet Neurol 2017, 16:601–609.In retrospective analyses plasma NfL levels were elevated in a cohort of 201 carriers with CAG repeat expansions in the HTT gene over 97 controls and baseline values in carriers predicted subsequent cognitive decline, brain atrophy, and risk of clinical onset.
- 26.Shahim P, Zetterberg H, Tegner Y, Blennow K: Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017, 88:1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staffaroni AM, Kramer AO, Casey M, Kang H, Rojas JC, Orrú CD, Caughey B, Allen IE, Kramer JH, Rosen HJ, et al. : Association of Blood and Cerebrospinal Fluid Tau Level and Other Biomarkers With Survival Time in Sporadic Creutzfeldt-Jakob Disease. JAMA Neurol 2019.CSF Aβ42, ptau181, total tau, and NfL along with plasma total tau, NFL and glial fibrillary acidic protein were measured in 193 individuals with sporadic Creutzfeldt-Jakob Disease. CSF Total tau levels were the best predictor of survival time, although CSF and plasma NfL were also robust predictors.
- 28.Preische O, Schultz SA, Apel A, Kuhle J, Kaeser SA, Barro C, Gräber S, Kuder-Buletta E, LaFougere C, Laske C, et al. : Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med 2019, 25:277–283.Longitudinal serum levels were examined in large cohort of autosomal dominant AD mutation carriers and familial controls. Rates of change in NfL were highest in those individuals that converted from cognitive normality to impairment, and at the group level rates of change become abnormal in mutation carriers more than a decade before the predicted onset of dementia. In mutation carriers NfL levels were significant related to change in AD biomarker values as well as cognition.
- 29.Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, Zecca C, Blennow K, Zetterberg H, Leppert D, et al. : Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017, 81:857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhle J, Barro C, Disanto G, Mathias A, Soneson C, Bonnier G, Yaldizli Ö, Regeniter A, Derfuss T, Canales M, et al. : Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler J 2016, 22:1550–1559. [DOI] [PubMed] [Google Scholar]
- 31.Mattsson N, Andreasson U, Zetterberg H, Blennow K: Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 2017, 74:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I, Machulda MM, Kremers WK, Knopman DS, Jack C, et al. : Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 2019, 93:e252–e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piehl F, Kockum I, Khademi M, Blennow K, Lycke J, Zetterberg H, Olsson T: Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler J 2018, 24:1046–1054. [DOI] [PubMed] [Google Scholar]
- 34.Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, et al. : Neurofilament Light Chain in Blood and CSF as Marker of Disease Progression in Mouse Models and in Neurodegenerative Diseases. Neuron 2016, 91:56–66. [DOI] [PubMed] [Google Scholar]
- 35.Petzold A, Keir G, Green AJ., Giovannoni G, Thompson E.: A specific ELISA for measuring neurofilament heavy chain phosphoforms. J Immunol Methods 2003, 278:179–190. [DOI] [PubMed] [Google Scholar]
- 36.Shaw G, Yang C, Ellis R, Anderson K, Parker Mickle J, Scheff S, Pike B, Anderson DK, Howland DR: Hyperphosphorylated neurofilament NF-H is a serum biomarker of axonal injury. Biochem Biophys Res Commun 2005, 336:1268–1277. [DOI] [PubMed] [Google Scholar]
- 37.Kuhle J, Regeniter A, Leppert D, Mehling M, Kappos L, Lindberg RLP, Petzold A: A highly sensitive electrochemiluminescence immunoassay for the neurofilament heavy chain protein. J Neuroimmunol 2010, 220:114–119. [DOI] [PubMed] [Google Scholar]
- 38.Kuhle J, Leppert D, Petzold A, Regeniter A, Schindler C, Mehling M, Anthony DC, Kappos L, Lindberg RLP: Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology 2011, 76:1206–13. [DOI] [PubMed] [Google Scholar]
- 39.Ganesalingam J, An J, Shaw CE, Shaw G, Lacomis D, Bowser R: Combination of neurofilament heavy chain and complement C3 as CSF biomarkers for ALS. J Neurochem 2011, 117:528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pujol-Calderón F, Portelius E, Zetterberg H, Blennow K, Rosengren LE, Höglund K: Neurofilament changes in serum and cerebrospinal fluid after acute ischemic stroke. Neurosci Lett 2019, 698:58–63. [DOI] [PubMed] [Google Scholar]
- 41.Manouchehrinia A, Piehl F, Hillert J, Kuhle J, Alfredsson L, Olsson T, Kockum I: Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann Clin Transl Neurol 2020, 7:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landqvist Waldö M, Frizell Santillo A, Passant U, Zetterberg H, Rosengren L, Nilsson C, Englund E: Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC Neurol 2013, 13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zerr I, Schmitz M, Karch A, Villar-Piqué A, Kanata E, Golanska E, Díaz-Lucena D, Karsanidou A, Hermann P, Knipper T, et al. : Cerebrospinal fluid neurofilament light levels in neurodegenerative dementia: Evaluation of diagnostic accuracy in the differential diagnosis of prion diseases. Alzheimer’s Dement 2018, 14:751–763. [DOI] [PubMed] [Google Scholar]
- 44.Olsson B, Portelius E, Cullen NC, Sandelius Å, Zetterberg H, Andreasson U, Höglund K, Irwin DJ, Grossman M, Weintraub D, et al. : Association of Cerebrospinal Fluid Neurofilament Light Protein Levels with Cognition in Patients with Dementia, Motor Neuron Disease, and Movement Disorders. JAMA Neurol 2019, 76:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosengren LE, Karlsson JE, Sjögren M, Blennow K, Wallin A: Neurofilament protein levels in CSF are increased in dementia. Neurology 1999, 52:1090–1093. [DOI] [PubMed] [Google Scholar]
- 46.Abu-Rumeileh S, Capellari S, Stanzani-Maserati M, Polischi B, Martinelli P, Caroppo P, Ladogana A, Parchi P: The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alzheimers Res Ther 2018, 10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, Nilsson C, Widner H, Decraemer H, Nägga K, et al. : Accuracy of a Panel of 5 Cerebrospinal Fluid Biomarkers in the Differential Diagnosis of Patients With Dementia and/or Parkinsonian Disorders. Arch Neurol 2012, 69:1445. [DOI] [PubMed] [Google Scholar]
- 48.Scherling CS, Hall T, Berisha F, Klepac K, Karydas A, Coppola G, Kramer JH, Rabinovici G, Ahlijanian M, Miller BL, et al. : Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol 2014, 75:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skillbäck T, Farahmand B, Bartlett JW, Rosén C, Mattsson N, Nägga K, Kilander L, Religa D, Wimo A, Winblad B, et al. : CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 2014, 83:1945–53. [DOI] [PubMed] [Google Scholar]
- 50.Steinacker P, Feneberg E, Weishaupt J, Brettschneider J, Tumani H, Andersen PM, Arnim CAFV, Böhm S, Kassubek J, Kubisch C, et al. : Neurofilaments in the diagnosis of motoneuron diseases: A prospective study on 455 patients. J Neurol Neurosurg Psychiatry 2016, 87:12–20. [DOI] [PubMed] [Google Scholar]
- 51.Abu-Rumeileh S, Steinacker P, Polischi B, Mammana A, Bartoletti-Stella A, Oeckl P, Baiardi S, Zenesini C, Huss A, Cortelli P, et al. : CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimer’s Res Ther 2019, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sjögren M, Rosengren L, Minthon L, Davidsson P, Blennow K, Wallin A: Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology 2000, 54:1960–4. [DOI] [PubMed] [Google Scholar]
- 53.Skillbäck T, Mattsson N, Blennow K, Zetterberg H: Cerebrospinal fluid neurofilament light concentration in motor neuron disease and frontotemporal dementia predicts survival. Amyotroph Lateral Scler Front Degener 2017, 18:397–403. [DOI] [PubMed] [Google Scholar]
- 54.Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, Bestwick JP, Monsch AU, Regeniter A, Lindberg RL, et al. : Increased Neurofilament Light Chain Blood Levels in Neurodegenerative Neurological Diseases. PLoS One 2013, 8:e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall S, Janelidze S, Surova Y, Widner H, Zetterberg H, Hansson O: Cerebrospinal fluid concentrations of inflammatory markers in Parkinson’s disease and atypical parkinsonian disorders. Sci Rep 2018, 8:13276.CSF Aβ42, ptau181, total tau, α-synuclein, NfL and well as a host of CSF inflammatory markers were examined in 50 controls, 131 PD, 27 PDD, 24 MSA, and 14 PSP patients. NfL was elevated in PDD, MSA, and PSP but not PD. Across the entire cohort levels of CSF were correlated with total tau, α-synuclein and multiple inflammatory biomarkers.
- 56.Brettschneider J, Petzold A, Süßmuth SD, Ludolph AC, Tumani H: Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 2006, 66:852–856. [DOI] [PubMed] [Google Scholar]
- 57.Brettschneider J, Petzold A, Schöttle D, Claus A, Riepe M, Tumani H: The neurofilament heavy chain (NfHSMI35) in the cerebrospinal fluid diagnosis of Alzheimer’s disease. Dement Geriatr Cogn Disord 2006, 21:291–295. [DOI] [PubMed] [Google Scholar]
- 58.Brettschneider J, Petzold A, Süßmuth SD, Landwehrmeyer GB, Ludolph AC, Kassubek J, Tumani H: Neurofilament heavy-chain NfHSMI35 in cerebrospinal fluid supports the differential diagnosis of Parkinsonian syndromes. Mov Disord 2006, 21:2224–2227. [DOI] [PubMed] [Google Scholar]
- 59.Lycke JN, Karlsson JE, Andersen O, Rosengren LE: Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 1998, 64:402–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malmeström C, Haghighi S, Rosengren L, Andersen O, Lycke J: Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 2003, 61:1720–5. [DOI] [PubMed] [Google Scholar]
- 61.Novakova L, Zetterberg H, Sundström P, Axelsson M, Khademi M, Gunnarsson M, Malmeström C, Svenningsson A, Olsson T, Piehl F, et al. : Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017, 89:2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhle J, Kropshofer H, Haering DA, Kundu U, Meinert R, Barro C, Dahlke F, Tomic D, Leppert D, Kappos L: Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92:E1007–E1015.Plasma NfL values were measured using the SIMOA platform in 589 patients with relapsing remitting MS on fingolimod and placebo and 35 healthy controls. NfL levels were higher in patients than controls, and levels correlated with T2 and gadolinium-enhancing lesions. Treatment with fingolimod reduced levels of NfL by six months, and this reduction was maintained until the trial cessation.
- 63.Thebault S, R. Tessier D, Lee H, Bowman M, Bar-Or A, Arnold DL, L. Atkins H, Tabard-Cossa V, Freedman MS: High serum neurofilament light chain normalizes after hematopoietic stem cell transplantation for MS. Neurol - Neuroimmunol Neuroinflammation 2019, 6:e598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teunissen CE, Iacobaeus E, Khademi M, Brundin L, Norgren N, Koel-Simmelink MJA, Schepens M, Bouwman F, Twaalfhoven HAM, Blom HJ, et al. : Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 2009, 72:1322–9. [DOI] [PubMed] [Google Scholar]
- 65.Bjornevik K, Munger KL, Cortese M, Barro C, Healy BC, Niebuhr DW, Scher AI, Kuhle J, Ascherio A: Serum Neurofilament Light Chain Levels in Patients with Presymptomatic Multiple Sclerosis. JAMA Neurol 2019, doi: 10.1001/jamaneurol.2019.3238.The authors examined serum NfL levels in a case-control study of military personnel. The study found that serum NfL was elevated in those that subsequently developed multiple sclerosis compared to matched controls suggestion a prodromal phase.
- 66.Arrambide G, Espejo C, Eixarch H, Villar LM, Alvarez-Cermeño JC, Picón C, Kuhle J, Disanto G, Kappos L, Sastre-Garriga J, et al. : Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 2016, 87:1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dalla Costa G, Martinelli V, Sangalli F, Moiola L, Colombo B, Radaelli M, Leocani L, Furlan R, Comi G: Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology 2019, 92:E733–E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Håkansson I, Tisell A, Cassel P, Blennow K, Zetterberg H, Lundberg P, Dahle C, Vrethem M, Ernerudh J: Neurofilament light chain in cerebrospinal fluid and prediction of disease activity in clinically isolated syndrome and relapsing-remitting multiple sclerosis. Eur J Neurol 2017, 24:703–712. [DOI] [PubMed] [Google Scholar]
- 69.Kuhle J, Nourbakhsh B, Grant D, Morant S, Barro C, Yaldizli Ö, Pelletier D, Giovannoni G, Waubant E, Gnanapavan S: Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 2017, 88:826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siller N, Kuhle J, Muthuraman M, Barro C, Uphaus T, Groppa S, Kappos L, Zipp F, Bittner S: Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Mult Scler J 2019, 25:678–686. [DOI] [PubMed] [Google Scholar]
- 71.Barro C, Benkert P, Disanto G, Tsagkas C, Amann M, Naegelin Y, Leppert D, Gobbi C, Granziera C, Yaldizli Ö, et al. : Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018, 141:2382–2391. [DOI] [PubMed] [Google Scholar]
- 72.Kuhle J, Malmeström C, Axelsson M, Plattner K, Yaldizli Ö, Derfuss T, Giovannoni G, Kappos L, Lycke J: Neurofilament light and heavy subunits compared as therapeutic biomarkers in multiple sclerosis. Acta Neurol Scand 2013, 128:e33–e36. [DOI] [PubMed] [Google Scholar]
- 73.Gunnarsson M, Malmeström C, Axelsson M, Sundström P, Dahle C, Vrethem M, Olsson T, Piehl F, Norgren N, Rosengren L, et al. : Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol 2011, 69:83–89. [DOI] [PubMed] [Google Scholar]
- 74.Kuhle J, Disanto G, Lorscheider J, Stites T, Chen Y, Dahlke F, Francis G, Shrinivasan A, Radue E-W, Giovannoni G, et al. : Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology 2015, 84:1639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herrera MI, Kölliker-Frers RA, Otero-Losada M, Perez Lloret S, Filippo M, Tau J, Capani F, Villa AM: A Pilot Cross-Sectional Study to Investigate the Biomarker Potential of Phosphorylated Neurofilament-H and Immune Mediators of Disability in Patients With 5 Year Relapsing-Remitting Multiple Sclerosis. Front Neurol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khalil M, Enzinger C, Langkammer C, Ropele S, Mader A, Trentini A, Vane M, Wallner-Blazek M, Bachmaier G, Archelos J-J, et al. : CSF neurofilament and N-acetylaspartate related brain changes in clinically isolated syndrome. Mult Scler J 2013, 19:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reijn TS, Abdo WF, Schelhaas HJ, Verbeek MM: CSF neurofilament protein analysis in the differential diagnosis of ALS. J Neurol 2009, 256:615–619. [DOI] [PubMed] [Google Scholar]
- 78.Schreiber S, Spotorno N, Schreiber F, Acosta-Cabronero J, Kaufmann J, Machts J, Debska-Vielhaber G, Garz C, Bittner D, Hensiek N, et al. : Significance of CSF NfL and tau in ALS. J Neurol 2018, 265:2633–2645.In this work the authors examined 84 asymptomatic individuals carrying ALS-causing gene mutations, 17 ALS patients, 10 phenoconverters, and 34 controls. They found that serum and CSF NfL levels were not elevated in the stable asymptomatic individuals, but in the phenoconverters levels increased as early as a year before clinical symptoms.
- 79.Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A: Neurofilament light: A candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol 2018, 84:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaiani A, Martinelli I, Bello L, Querin G, Puthenparampil M, Ruggero S, Toffanin E, Cagnin A, Briani C, Pegoraro E, et al. : Diagnostic and Prognostic Biomarkers in Amyotrophic Lateral Sclerosis. JAMA Neurol 2017, 74:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menke RAL, Gray E, Lu C-H, Kuhle J, Talbot K, Malaspina A, Turner MR: CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol 2015, 2:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poesen K, De Schaepdryver M, Stubendorff B, Gille B, Muckova P, Wendler S, Prell T, Ringer TM, Rhode H, Stevens O, et al. : Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 2017, 88:2302–2309. [DOI] [PubMed] [Google Scholar]
- 83.Rossi D, Volanti P, Brambilla L, Colletti T, Spataro R, La Bella V: CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. J Neurol 2018, 265:510–521. [DOI] [PubMed] [Google Scholar]
- 84.Gendron TF, C9ORF72 Neurofilament Study Group LM, Daughrity LM, Heckman MG, Diehl NN, Wuu J, Miller TM, Pastor P, Trojanowski JQ, Grossman M, et al. : Phosphorylated neurofilament heavy chain: A biomarker of survival for C9ORF72-associated amyotrophic lateral sclerosis. Ann Neurol 2017, 82:139–146.CSF phosphorylated NfH was examined in 135 C9ORF72 mutation carriers and 107 noncarriers that were either healthy or were diagnosed with ALS. pNfH levels were low in asymptomatic carriers but abnormal in symptomatic carriers. For carriers and noncarriers, those with symptoms of both ALS and FTD had greater pNfH levels than those with FTD alone and higher pNfH levels predicted shorter survival times.
- 85.Lehnert S, Costa J, de Carvalho M, Kirby J, Kuzma-Kozakiewicz M, Morelli C, Robberecht W, Shaw P, Silani V, Steinacker P, et al. : Multicentre quality control evaluation of different biomarker candidates for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Front Degener 2014, 15:344–3 50. [DOI] [PubMed] [Google Scholar]
- 86.Boylan K, Yang C, Crook J, Overstreet K, Heckman M, Wang Y, Borchelt D, Shaw G: Immunoreactivity of the phosphorylated axonal neurofilament H subunit (pNF-H) in blood of ALS model rodents and ALS patients: evaluation of blood pNF-H as a potential ALS biomarker. J Neurochem 2009, 111:1182–1191. [DOI] [PubMed] [Google Scholar]
- 87.De Schaepdryver M, Goossens J, De Meyer S, Jeromin A, Masrori P, Brix B, Claeys KG, Schaeverbeke J, Adamczuk K, Vandenberghe R, et al. : Serum neurofilament heavy chains as early marker of motor neuron degeneration. Ann Clin Transl Neurol 2019, doi: 10.1002/acn3.50890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Schaepdryver M, Jeromin A, Gille B, Claeys KG, Herbst V, Brix B, Van Damme P, Poesen K: Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2018, 89:367–373.Phosphorylated NfH in serum was measured using ELISA in 95 ALS patients, 35 individuals with mild cognitive impairment, and 85 controls. In the ALS patients serum levels were elevated up to 18 months before the onset of clinical symptoms and prediagnostic values were a predictor of survival time.
- 89.Ganesalingam J, An J, Bowser R, Andersen PM, Shaw CE: pNfH is a promising biomarker for ALS. Amyotroph Lateral Scler Front Degener 2013, 14:146–149. [DOI] [PubMed] [Google Scholar]
- 90.Boylan KB, Glass JD, Crook JE, Yang C, Thomas CS, Desaro P, Johnston A, Overstreet K, Kelly C, Polak M, et al. : Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2013, 84:467–72. [DOI] [PubMed] [Google Scholar]
- 91.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. : Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron 2011, 72:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bridel C, Van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, Alvarez-Cermeño JC, Andreasson U, Axelsson M, Bäckström DC, Bartos A, et al. : Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol 2019, doi: 10.1001/jamaneurol.2019.1534.The authors performed a metanalysis of papers published between 2006 and 2016 reporting on CSF NfL levels. This paper shows that NfL levels are elevated in most neurodegenerative conditions relative to healthy controls, but the degree of abnormality varies greatly between conditions.
- 93.Pijnenburg YAL, Janssen JC, Schoonenboom NSM, Petzold A, Mulder C, Stigbrand T, Norgren N, Heijst H, Hack CE, Scheltens P, et al. : CSF neurofilaments in frontotemporal dementia compared with early onset Alzheimer’s disease and controls. Dement Geriatr Cogn Disord 2007, 23:225–30. [DOI] [PubMed] [Google Scholar]
- 94.Al Shweiki MR, Steinacker P, Oeckl P, Hengerer B, Danek A, Fassbender K, Diehl-Schmid J, Jahn H, Anderl-Straub S, Ludolph AC, et al. : Neurofilament light chain as a blood biomarker to differentiate psychiatric disorders from behavioural variant frontotemporal dementia. J Psychiatr Res 2019, 113:137–140. [DOI] [PubMed] [Google Scholar]
- 95.Rohrer JD, Woollacott IOC, Dick KM, Brotherhood E, Gordon E, Fellows A, Toombs J, Druyeh R, Cardoso MJ, Ourselin S, et al. : Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 2016, 87:1329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alcolea D, Vilaplana E, Suárez-Calvet M, Illán-Gala I, Blesa R, Clarimón J, Lladó A, Sánchez-Valle R, Molinuevo JL, García-Ribas G, et al. : CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology 2017, 89:178–188. [DOI] [PubMed] [Google Scholar]
- 97.Verde F, Steinacker P, Weishaupt JH, Kassubek J, Oeckl P, Halbgebauer S, Tumani H, Arnim CAF von, Dorst J, Feneberg E, et al. : Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2019, 90:157–164. [DOI] [PubMed] [Google Scholar]
- 98.van der Ende EL, Meeter LH, Poos JM, Panman JL, Jiskoot LC, Dopper EGP, Papma JM, de Jong FJ, Verberk IMW, Teunissen C, et al. : Serum neurofilament light chain in genetic frontotemporal dementia: a longitudinal, multicentre cohort study. Lancet Neurol 2019, 18:1103–1111.In participants from the Genetic Frontotemporal Dementia Initiative (GENFI), researchers found that serum NfL is elevated in symptomatic individuals and increases in those individuals that become symptomatic. The longitudinal rate of change in NfL was related to changes in cognition and grey matter atrophy.
- 99.Meeter LH, Dopper EG, Jiskoot LC, Sanchez-Valle R, Graff C, Benussi L, Ghidoni R, Pijnenburg YA, Borroni B, Galimberti D, et al. : Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol 2016, 3:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Jong D, Jansen RWMM, Pijnenburg YAL, van Geel WJA, Borm GF, Kremer HPH, Verbeek MM: CSF neurofilament proteins in the differential diagnosis of dementia. J Neurol Neurosurg Psychiatry 2007, 78:936–8.Aβ42, Aβ40, ptau181, total tau, and NfL were measured in the CSF and plasma in a cohort of 282 participants with Down syndrome and 67 controls. CSF markers robustly discriminated between Down syndrome participants with and without evidence of AD pathology. Plasma NfL, but not the other blood markers discriminated between the Down syndrome subgroups.
- 101.Weston PSJ, Poole T, Ryan NS, Nair A, Liang Y, Macpherson K, Druyeh R, Malone IB, Ahsan RL, Pemberton H, et al. : Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology 2017, 89:2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weston PSJ, Poole T, O’Connor A, Heslegrave A, Ryan NS, Liang Y, Druyeh R, Mead S, Blennow K, Schott JM, et al. : Longitudinal measurement of serum neurofilament light in presymptomatic familial Alzheimer’s disease. Alzheimers Res Ther 2019, 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sánchez-Valle R, Heslegrave A, Foiani MS, Bosch B, Antonell A, Balasa M, Lladó A, Zetterberg H, Fox NC: Serum neurofilament light levels correlate with severity measures and neurodegeneration markers in autosomal dominant Alzheimer’s disease. Alzheimer’s Res Ther 2018, 10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lleó A, Alcolea D, Martínez-Lage P, Scheltens P, Parnetti L, Poirier J, Simonsen AH, Verbeek MM, Rosa-Neto P, Slot RER, et al. : Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimer’s Dement 2019, 15:742–753. [DOI] [PubMed] [Google Scholar]
- 105.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K: Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol 2019, doi: 10.1001/jamaneurol.2019.0765.Longitudinal plasma NfL was measured from 1583 cognitively normal and impaired individuals from the ADNI study. NfL levels were elevated in those with mild cognitive impairment and AD, and greater rates of change in NfL were associated with faster rates of change in CSF total tau, grey matter, and metabolism.
- 106.Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, Weiner M, Blennow K, Hansson O, Alzheimer’s Disease Neuroimaging Initiative the ADN: Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol Med 2016, 8:1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zetterberg H, Skillbäck T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K: Association of Cerebrospinal Fluid Neurofilament Light Concentration With Alzheimer Disease Progression. JAMA Neurol 2016, 73:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fortea J, Carmona-Iragui M, Benejam B, Fernández S, Videla L, Barroeta I, Alcolea D, Pegueroles J, Muñoz L, Belbin O, et al. : Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol 2018, 17:860–869. [DOI] [PubMed] [Google Scholar]
- 109.Hampel H, Toschi N, Baldacci F, Zetterberg H, Blennow K, Kilimann I, Teipel SJ, Cavedo E, Melo dos Santos A, Epelbaum S, et al. : Alzheimer’s disease biomarker-guided diagnostic workflow using the added value of six combined cerebrospinal fluid candidates: Aβ1–42, total-tau, phosphorylated-tau, NFL, neurogranin, and YKL-40. Alzheimer’s Dement 2018, 14:492–501. [DOI] [PubMed] [Google Scholar]
- 110.Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, Maler JM, Kornhuber J, Blennow K, Zetterberg H: Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res Ther 2018, 10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kester MI, Scheffer PG, Koel-Simmelink MJ, Twaalfhoven H, Verwey NA, Veerhuis R, Twisk JW, Bouwman FH, Blankenstein MA, Scheltens P, et al. : Serial CSF sampling in Alzheimer’s disease: specific versus non-specific markers. Neurobiol Aging 2012, 33:1591–1598. [DOI] [PubMed] [Google Scholar]
- 112.Bos I, Vos S, Verhey F, Scheltens P, Teunissen C, Engelborghs S, Sleegers K, Frisoni G, Blin O, Richardson JC, et al. : Cerebrospinal fluid biomarkers of neurodegeneration, synaptic integrity, and astroglial activation across the clinical Alzheimer’s disease spectrum. Alzheimer’s Dement 2019, 15:644–654. [DOI] [PubMed] [Google Scholar]
- 113.Kern S, Syrjanen JA, Blennow K, Zetterberg H, Skoog I, Waern M, Hagen CE, Van Harten AC, Knopman DS, Jack CR, et al. : Association of Cerebrospinal Fluid Neurofilament Light Protein with Risk of Mild Cognitive Impairment among Individuals Without Cognitive Impairment. JAMA Neurol 2019, 76:187–193.CSF levels of NfL and neurogranin were measured in 648 individuals without cognitive impairment. Elevated NfL levels were associated with a greater risk of developing mild cognitive impairment, but this effect was independent of Aβ42 levels.
- 114.Chatterjee P, Goozee K, Sohrabi HR, Shen K, Shah T, Asih PR, Dave P, Manyan C, Taddei K, Chung R, et al. : Association of Plasma Neurofilament Light Chain with Neocortical Amyloid-β Load and Cognitive Performance in Cognitively Normal Elderly Participants. J Alzheimer’s Dis 2018, 63:479–487. [DOI] [PubMed] [Google Scholar]
- 115.Sandelius å, Zetterberg H, Blennow K, Adiutori R, Malaspina A, Laura M, Reilly MM, Rossor AM: Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 2018, 90:e518–e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iverson GL, Reddi PJ, Posti JP, Kotilainen A-K, Tenovuo O, Öhman J, Zetterberg H, Blennow K, Luoto TM: Serum Neurofilament Light Is Elevated Differentially in Older Adults with Uncomplicated Mild Traumatic Brain Injuries. J Neurotrauma 2019, doi: 10.1089/neu.2018.6341. [DOI] [PubMed] [Google Scholar]
- 117.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, Sullivan M, Paumier K, Holtzman DM, Morris JC, et al. : Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer’s Dement 2017, 13:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shahim P, Tegner Y, Marklund N, Blennow K, Zetterberg H: Neurofilament light and tau as blood biomarkers for sports-related concussion. Neurology 2018, 90:e1780–e1788.Serum levels of NfL were examined in 87 professional hockey players with sampling starting at 1 hour and extending to 144 hours after sports-related concussions. Serum NfL levels predicted time to return to play and outperformed tau, S100B, and neuron-specific enolase.
- 119.Abdo WF, Bloem BR, Van Geel WJ, Esselink RAJ, Verbeek MM: CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiol Aging 2007, 28:742–747. [DOI] [PubMed] [Google Scholar]
- 120.Holmberg B, Rosengren L, Karlsson J-E, Johnels B: Increased cerebrospinal fluid levels of neurofilament protein in progressive supranuclear palsy and multiple-system atrophy compared with Parkinson’s disease. Mov Disord 1998, 13:70–77. [DOI] [PubMed] [Google Scholar]
- 121.Báckström DC, Eriksson Domellöf M, Linder J, Olsson B, Öhrfelt A, Trupp M, Zetterberg H, Blennow K, Forsgren L: Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol 2015, 72:1175. [DOI] [PubMed] [Google Scholar]
- 122.Kanata E, Golanska E, Villar-Piqué A, Karsanidou A, Dafou D, Xanthopoulos K, Schmitz M, Ferrer I, Karch A, Sikorska B, et al. : Cerebrospinal fluid neurofilament light in suspected sporadic Creutzfeldt-Jakob disease. J Clin Neurosci 2019, 60:124–127. [DOI] [PubMed] [Google Scholar]
- 123.Steinacker P, Blennow K, Halbgebauer S, Shi S, Ruf V, Oeckl P, Giese A, Kuhle J, Slivarichova D, Zetterberg H, et al. : Neurofilaments in blood and CSF for diagnosis and prediction of onset in Creutzfeldt-Jakob disease. Sci Rep 2016, 6:38737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Van Eijk JJJ, Van Everbroeck B, Abdo WF, Kremer BPH, Verbeek MM: CSF neurofilament proteins levels are elevated in sporadic Creutzfeldt-Jakob disease. J Alzheimer’s Dis 2010, 21:569–576. [DOI] [PubMed] [Google Scholar]
- 125.Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung Y-C, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, et al. : Estimating the global incidence of traumatic brain injury. J Neurosurg 2019, 130:1080–1097. [DOI] [PubMed] [Google Scholar]
- 126.Ljungqvist J, Zetterberg H, Mitsis M, Blennow K, Skoglund T: Serum Neurofilament Light Protein as a Marker for Diffuse Axonal Injury: Results from a Case Series Study. J Neurotrauma 2017, 34:1124–1127. [DOI] [PubMed] [Google Scholar]
- 127.Shahim P, Gren M, Liman V, Andreasson U, Norgren N, Tegner Y, Mattsson N, Andreasen N, Öst M, Zetterberg H, et al. : Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep 2016, 6:36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bernick C, Zetterberg H, Shan G, Banks S, Blennow K: Longitudinal Performance of Plasma Neurofilament Light and Tau in Professional Fighters: The Professional Fighters Brain Health Study. J Neurotrauma 2018, 35:2351–2356. [DOI] [PubMed] [Google Scholar]
- 129.Oliver JM, Jones MT, Kirk KM, Gable DA, Repshas JT, Johnson TA, Andréasson U, Norgren N, Blennow K, Zetterberg H: Serum Neurofilament Light in American Football Athletes over the Course of a Season. J Neurotrauma 2016, 33:1784–1789. [DOI] [PubMed] [Google Scholar]
- 130.Anderson KJ, Scheff SW, Miller KM, Roberts KN, Gilmer LK, Yang C, Shaw G: The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. J Neurotrauma 2008, 25:1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Neselius S, Zetterberg H, Blennow K, Marcusson J, Brisby H: Increased CSF Levels of Phosphorylated Neurofilament Heavy Protein following Bout in Amateur Boxers. PLoS One 2013, 8:e81249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shibahashi K, Doi T, Tanaka S, Hoda H, Chikuda H, Sawada Y, Takasu Y, Chiba K, Nozaki T, Hamabe Y, et al. : The Serum Phosphorylated Neurofilament Heavy Subunit as a Predictive Marker for Outcome in Adult Patients after Traumatic Brain Injury. J Neurotrauma 2016, 33:1826–1833. [DOI] [PubMed] [Google Scholar]
- 133.Tiedt S, Duering M, Barro C, Kaya AG, Boeck J, Bode FJ, Klein M, Dorn F, Gesierich B, Kellert L, et al. : Serum neurofilament light: A biomarker of neuroaxonal injury after ischemic stroke. Neurology 2018, 91:e1338–e1347..Serum NfL in two cohorts with stroke as well as controls was examined. NFL levels were elevated in patients until 6 months after the stroke, levels tracked new lesions, and NfL values predicted altered diffusion tensor imaging metrics.
- 134.De Marchis GM, Katan M, Barro C, Fladt J, Traenka C, Seiffge DJ, Hert L, Gensicke H, Disanto G, Sutter R, et al. : Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol 2018, 25:562–568. [DOI] [PubMed] [Google Scholar]
- 135.Onatsu J, Vanninen R, Jäkälä P, Mustonen P, Pulkki K, Korhonen M, Hedman M, Zetterberg H, Blennow K, Höglund K, et al. : Serum Neurofilament Light Chain Concentration Correlates with Infarct Volume but Not Prognosis in Acute Ischemic Stroke. J Stroke Cerebrovasc Dis 2019, 28:2242–2249. [DOI] [PubMed] [Google Scholar]
- 136.Singh P, Yan J, Hull R, Read S, O’Sullivan J, Henderson RD, Rose S, Greer JM, McCombe PA: Levels of phosphorylated axonal neurofilament subunit H (pNfH) are increased in acute ischemic stroke. J Neurol Sci 2011, 304:117–121. [DOI] [PubMed] [Google Scholar]
- 137.Sellner J, Patel A, Dassan P, Brown MM, Petzold A: Hyperacute detection of neurofilament heavy chain in serum following stroke: A transient sign. Neurochem Res 2011, 36:2287–2291. [DOI] [PubMed] [Google Scholar]
- 138.Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, Pichler A, Barro C, Gröbke S, Voortman M, et al. : Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 2017, 89:2108–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rana OR, Schröder JW, Baukloh JK, Saygili E, Mischke K, Schiefer J, Weis J, Marx N, Rassaf T, Kelm M, et al. : Neurofilament light chain as an early and sensitive predictor of long-term neurological outcome in patients after cardiac arrest. Int J Cardiol 2013, 168:1322–1327. [DOI] [PubMed] [Google Scholar]
- 140.Moseby-Knappe M, Mattsson N, Nielsen N, Zetterberg H, Blennow K, Dankiewicz J, Dragancea I, Friberg H, Lilja G, Insel PS, et al. : Serum Neurofilament Light Chain for Prognosis of Outcome After Cardiac Arrest. JAMA Neurol 2018, doi: 10.1001/jamaneurol.2018.3223.Serum NfL was measured 24, 48, and 72 hours after cardiac arrest in 717 patients. Serum levels were increased in patients with poor neurological outcomes at 24, 48, and 72 hours and performed comparably or better prognostically than electroencephalogram, CT, somatosensory-evoked potentials, and pupillary and corneal reflexes.
- 141.Rundgren M, Friberg H, Cronberg T, Romner B, Petzold A: Serial soluble neurofilament heavy chain in plasma as a marker of brain injury after cardiac arrest. Crit Care 2012, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rojas JC, Bang J, Lobach IV, Tsai RM, Rabinovici GD, Miller BL, Boxer AL, AL-108-231 Investigators: CSF neurofilament light chain and phosphorylated tau 181 predict disease progression in PSP. Neurology 2018, 90:e273–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rojas JC, Karydas A, Bang J, Tsai RM, Blennow K, Liman V, Kramer JH, Rosen H, Miller BL, Zetterberg H, et al. : Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol 2016, 3:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Steinacker P, Semler E, Anderl-Straub S, Diehl-Schmid J, Schroeter ML, Uttner I, Foerstl H, Landwehrmeyer B, von Arnim CAF, Kassubek J, et al. : Neurofilament as a blood marker for diagnosis and monitoring of primary progressive aphasias. Neurology 2017, 88:961–969. [DOI] [PubMed] [Google Scholar]
- 145.Johnson EB, Byrne LM, Gregory S, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RA, Zetterberg H, Tabrizi SJ, et al. : Neurofilament light protein in blood predicts regional atrophy in Huntington disease. Neurology 2018, 90:e717–e723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gisslén M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, Blennow K, Zetterberg H: Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 2016, 3:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anderson AM, Easley KA, Kasher N, Franklin D, Heaton RK, Zetterberg H, Blennow K, Gisslen M, Letendre SL: Neurofilament light chain in blood is negatively associated with neuropsychological performance in HIV-infected adults and declines with initiation of antiretroviral therapy. J Neurovirol 2018, 24:695–701.The authors examined plasma levels of NfL in 37 HIV+ and 54 HIV− adults. Longitudinal measurements were available in 11 HIV+ individuals before and after 24 weeks on combination antiretroviral therapy (cART). After being on cART plasma NfL levels dropped.
- 148.Jakobsson J, Bjerke M, Ekman CJ, Sellgren C, Johansson AG, Zetterberg H, Blennow K, Landén M: Elevated Concentrations of Neurofilament Light Chain in the Cerebrospinal Fluid of Bipolar Disorder Patients. Neuropsychopharmacology 2014, 39:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nilsson IAK, Millischer V, Karrenbauer VD, Juréus A, Salehi AM, Norring C, von Hausswolff-Juhlin Y, Schalling M, Blennow K, Bulik CM, et al. : Plasma neurofilament light chain concentration is increased in anorexia nervosa. Transl Psychiatry 2019, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuhle J, Gaiottino J, Leppert D, Petzold A, Bestwick JP, Malaspina A, Lu C-H, Dobson R, Disanto G, Norgren N, et al. : Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J Neurol Neurosurg Psychiatry 2015, 86:273–9. [DOI] [PubMed] [Google Scholar]
- 151.Casey CP, Lindroth H, Mohanty R, Farahbakhsh Z, Ballweg T, Twadell S, Miller S, Krause B, Prabhakaran V, Blennow K, et al. : Postoperative delirium is associated with increased plasma neurofilament light. Brain 2020, 143:47–54.Plasma levels of NfL were measured before and after surgery in a cohort of 114 individuals. Plasma levels increased after surgery, and greater increases were associated with a greater risk of postoperative delirium.
- 152.Darras BT, Crawford TO, Finkel RS, Mercuri E, De Vivo DC, Oskoui M, Tizzano EF, Ryan MM, Muntoni F, Zhao G, et al. : Neurofilament as a potential biomarker for spinal muscular atrophy. Ann Clin Transl Neurol 2019, 6:932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matsushige T, Inoue H, Fukunaga S, Hasegawa S, Okuda M, Ichiyama T: Serum neurofilament concentrations in children with prolonged febrile seizures. J Neurol Sci 2012, 321:39–42. [DOI] [PubMed] [Google Scholar]
- 154.Petzold A, Rejdak K, Plant GT: Axonal degeneration and inflammation in acute optic neuritis. J Neurol Neurosurg Psychiatry 2004, 75:1178–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Le Bihan D, Mangin J-F, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H: Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging 2001, 13:534–546. [DOI] [PubMed] [Google Scholar]
- 156.Moore EE, Hohman TJ, Badami FS, Pechman KR, Osborn KE, Acosta LMY, Bell SP, Babicz MA, Gifford KA, Anderson AW, et al. : Neurofilament relates to white matter microstructure in older adults. Neurobiol Aging 2018, 70:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Racine AM, Merluzzi AP, Adluru N, Norton D, Koscik RL, Clark LR, Berman SE, Nicholas CR, Asthana S, Alexander AL, et al. : Association of longitudinal white matter degeneration and cerebrospinal fluid biomarkers of neurodegeneration, inflammation and Alzheimer’s disease in late-middle-aged adults. Brain Imaging Behav 2019, 13:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim WH, Racine AM, Adluru N, Hwang SJ, Blennow K, Zetterberg H, Carlsson CM, Asthana S, Koscik RL, Johnson SC, et al. : Cerebrospinal fluid biomarkers of neurofibrillary tangles and synaptic dysfunction are associated with longitudinal decline in white matter connectivity: A multi-resolution graph analysis. NeuroImage Clin 2019, 21:101586. [DOI] [PMC free article] [PubMed] [Google Scholar]


