Abstract
Behavioral responses to a perceptual stimulus are typically faster with repeated exposure to the stimulus (behavioral priming). This implicit learning mechanism is critical for survival but impaired in a variety of neurological disorders, including Alzheimer’s disease. Many studies of the neural bases for behavioral priming have encountered an interesting paradox: in spite of faster behavioral responses, repeated stimuli usually elicit weaker neural responses (repetition suppression). Several neurophysiological models have been proposed to resolve this paradox, but noninvasive techniques for human studies have had insufficient spatial-temporal precision for testing their predictions. Here, we used the unparalleled precision of electrocorticography (ECoG) to analyze the timing and magnitude of task-related changes in neural activation and propagation while patients named novel vs repeated visual objects. Stimulus repetition was associated with faster verbal responses and decreased neural activation (repetition suppression) in ventral occipito-temporal cortex (VOTC) and left prefrontal cortex (LPFC). Interestingly, we also observed increased neural activation (repetition enhancement) in LPFC and other recording sites. Moreover, with analysis of high gamma propagation we observed increased top-down propagation from LPFC into VOTC, preceding repetition suppression. The latter results indicate that repetition suppression and behavioral priming are associated with strengthening of top-down network influences on perceptual processing, consistent with predictive coding models of repetition suppression, and they support a central role for changes in large-scale cortical dynamics in achieving more efficient and rapid behavioral responses.
Keywords: language, expectation, intracranial EEG, effective connectivity, event related causality (ERC), large scale brain network
Introduction
Repetition suppression, a decrease in cortical activation upon repeated stimulus exposure, has been widely observed under a variety of experimental conditions (Grill-Spector et al., 2006; Henson et al., 2014). Repeated stimulus exposure is also associated with faster and more accurate behavioral responses, referred to as “behavioral priming”. The joint observation of repetition suppression and behavioral priming mandates some form of improved neural processing efficiency, which is of fundamental importance for understanding cortical mechanisms of learning and implicit memory (Squire, 1992; McClelland et al., 1995; Schacter and Buckner, 1998; Wiggs and Martin, 1998; Henson, 2003; Gotts, 2016). Furthermore, the occurrence of repetition suppression and priming across a range of cognitive tasks and domains suggests that these phenomena have important utility in uncovering the mechanisms that relate brain and behavior across many distinct systems. A number of theoretical models have been proposed, yet the neural mechanisms remain unresolved (Gotts et al., 2012). The importance of behavioral priming is highlighted by studies of lexical access (Scarborough et al., 1977), which is impaired in Alzheimer’s disease even at its early stages (Fleischman, 2007). This brain disorder irremediably alters the proficiency of word search and retrieval processes.
Competing models of the neurophysiological mechanisms of repetition suppression have been framed as different repetition-related changes in the activity and/or collective dynamics of neural networks (Gotts et al., 2012, 2015). Some of these models, such as the perceptual “sharpening” model (Desimone, 1996; Wiggs and Martin, 1998), make direct predictions about alterations in the tuning preferences of individual cells but are less clear about predictions regarding neural dynamics. Others, in contrast, make predictions specifically about dynamics. For example, the “synchrony” model (Gotts, 2003; Ghuman et al., 2008; Gilbert et al., 2010) holds that neurons fire more synchronously after repetition while at overall reduced rates, improving the reliability of activity propagation. The “facilitation” model (James et al., 2000; Henson, 2003) proposes that neural activity is advanced in time with repetition and terminates more rapidly. Given that perception relies on the integration of sensory information and prior expectations, the “predictive coding” model claims that repetition leads to optimized neural representations, with top-down activity serving as model-based predictions about the current stimulus and bottom-up activity representing the prediction error related to various stimuli and their constituent elements. With repetition, top-down causal influences are enhanced as the predictions become more accurate and bottom-up activity is accordingly reduced. Repetition suppression on this view reflects a relative reduction in perceptual prediction error (Henson, 2003; Friston, 2005; Cope et al., 2017).
The majority of studies of repetition suppression and priming in humans have been conducted using methods with relatively poor temporal resolution (e.g. BOLD fMRI), preventing the evaluation of the dynamical predictions of these models. In the current study, we evaluate changes in neural activity and large-scale dynamics that result from stimulus repetition using electrocorticography (ECoG), which records local field potentials from the surface of the brain with excellent temporal resolution. Compared to scalp EEG, ECoG has far higher spatial resolution and superior signal quality, especially for high gamma responses, which reflect overall firing rates in neural populations (Crone et al., 2011; Lachaux et al., 2012). Indeed, a recent study showed that reductions in ECoG high gamma responses to repeated stimuli support the time course of MEG and localization of fMRI (McDonald et al., 2010). Although these responses cannot be used to test models that posit changes in the tuning preferences of individual neurons (e.g. sharpening), they can be used to measure neural dynamics at time scales relevant to the evaluation of other models. Here, we used ECoG recordings to study the fine temporal dynamics of neural activity during behavioral priming, and we evaluated changes in the propagation of high gamma activity using a recently developed technique – event related causality (ERC), based on the concept of Granger causality, to measure the timing, magnitude and directionality of task-related changes in neural propagation across cortical networks activated during visual object naming (Korzeniewska et al., 2008, 2011; Flinker et al., 2015; Nishida et al., 2017). This analysis strategy was designed to provide information about neural dynamics relevant to multiple theoretical models of repetition suppression and behavioral priming as comprehensively as possible. We used the ERC method for this because it does not require any a priori model of the investigated network.
Materials and methods
Patients
Ten patients provided informed consent to participate in experimental testing while electrocorticogram (ECoG) signals were recorded for epilepsy surgery under a Johns Hopkins University School of Medicine Institutional Review Board approved protocol. The cortical areas covered by subdural ECoG electrodes were solely determined by clinical needs, with significant variation among patients. Localization of implanted electrodes was performed using the BioImage Suite (Duncan et al., 2004) with pre-implantation MRI and post-implantation CT. For details on the MNI electrode registration, see Cervenka et al., 2013. Freesurfer (Fischl, 2012) was used for final presentation of results. Because of the importance of ventral occipito-temporal cortex (VOTC) and left prefrontal cortex (LPFC) in previous studies of visual object naming (e.g. van Turennout et al., 2003; Gilmore et al., 2019), as well as studies of behavioral priming (e.g. Dobbins et al., 2004; Ghuman et al., 2008; Horner and Henson, 2008; Race et al., 2009; Wig et al., 2005), patients were included in the study if they had electrodes implanted over both of these cortical areas in the left hemisphere, dominant for language in all patients. None were excluded based on their task performance.
Experimental Paradigm
For visual object naming, we used E-Prime (Psychology Software Tools, Sharpsburg, PA, USA) to present color images of objects on a white background on a computer monitor 1 meter from the patient. Each stimulus was displayed for 1 s and then replaced by a fixation cross. Patients were asked to name each picture as quickly and as accurately as possible. The inter-trial interval was jittered randomly in four 250-msec increments around 4 seconds. Two hundred fifty images were displayed in pseudo-randomized order, including 50 stimuli that were repeated identically after a short delay (100 trials total), either consecutively (no intervening stimulus) or with one different stimulus intervening between them. Object names were matched for word frequency, and lists of repeated versus non-repeated (novel) objects were counterbalanced across patients. Pairs of novel/repeated trials with artifacts in the ECoG signals and trials in which the patient did not promptly and correctly name the picture were excluded from analysis, resulting in 26–45 novel-repeat pairs, i.e. 26–45 trials with a novel stimulus, and their 26–45 counterparts with the repeated stimulus (mean=43 trials, median=44), included for each patient. The number of trials was sufficient given the high signal quality of ECoG and the needs of the ERC analysis (Korzeniewska et al., 2008).
Signal Acquisition and Preprocessing
ECoG signals were recorded subdurally with 2.3 mm diameter platinum-iridium electrodes (Ad-Tech Medical Instrument Corporation, Racine, WI, USA) spaced 10 mm apart center-to-center. A Stellate (Stellate Systems Inc., Montreal, Canada), Nihon Kohden (Nihon Kohden Corporation, Tokyo, Japan), or NeuroPort (Blackrock Microsystems, Salt Lake City, UT, USA) amplifier was used to record neural activity, which was referenced to a relatively inactive intracranial electrode, usually on a strip facing the dura. Signals were digitized at 2000 Hz (Nihon Kohden) or 1000 Hz (Stellate, NeuroPort); the former were downsampled to 1000 Hz after being preprocessed with a digital antialiasing filter.
Pathological ECoG signals, e.g. signals with spikes, as well as signals with artifacts or excessive noise, were manually excluded from analysis. The remaining ECoG signals were re-referenced using a common average reference (CAR) to remove any spatial bias from the choice of reference. To eliminate bias from epileptogenic activity and thereby focus our analysis on task-related changes in neural activation and propagation, we statistically compared, or normalized, all post-stimulus time points to baseline intervals immediately preceding the onset of each stimulus (see below).
Identification of Event-Related Activation and Repetition Effects
Spectral power was extracted from ECoG signals, band-pass filtered in the 16–200 Hz range, in 128 ms long sliding windows with a 16 ms overlap, using a multi-taper algorithm from the Chronux toolbox (Bokil et al., 2010). Signal power was extracted from each window using 5 orthogonal tapers with a time-bandwidth product of 3. In each signal, the power after stimulus onset was normalized to the baseline power from the 1 s prior to the stimulus onset, in each frequency bin. This normalization adjusted for the 1/f power decay of the signal. An estimate of high gamma power at each time point in each trial was obtained by averaging the power in each bin between 70 and 120 Hz.
For each ECoG recording site in each patient, event-related changes in high gamma power relative to the pre-stimulus baseline were statistically tested separately for novel and repeated trials using a nonparametric sign test at every post-stimulus time point. A signal exhibited event-related activation if the normalized power was significantly greater than one, representing an increase over baseline (p < 0.05, one-sided test for each signal with FDR correction across time points) (Benjamini and Hochberg, 1995).
Event-related repetition effects were tested with a paired sign test for each signal in each patient at every time point, i.e. the same time window measured from the stimulus onset. The effect of repetition was marked as significant if activation of either novel or repeated trials was both: 1) significantly greater than baseline (p < 0.05, one-sided test for each signal with FDR correction across time points), and 2) significantly greater than that in either repeated or novel trials, respectively (p < 0.05, two-sided test for each region of interest with FDR correction across time points).
In addition to testing repetition effects on neural activation in individual signals, these effects were also tested in group analyses, over all patients. We used the same signal inclusion criteria as for event-related causality described below. Pooled variance over trials with novel or repeated stimuli, for each patient, was used for further two-sided paired t-tests (p < 0.05, FDR corrected for multiple comparisons at every time point), for all patients combined. The effect of repetition was marked as significant if the average power for repeated trials was significantly different than for novel trials (either increased or decreased), as indicated by the paired t-test.
Event-Related Causality and Repetition Effects
Event-related causality (ERC, implemented in custom analysis interface (ANIN) software (Franaszczuk and Jouny, 2004), finds statistically significant event-related effective connectivity between ECoG recording sites using multivariate autoregressive (MVAR) models of ECoG activity in a subset of recorded signals. A detailed description of the method may be found in (Korzeniewska et al., 2008). A particular strength of this method for the current study is that it does not require any a priori model of network interactions and thus is unbiased with respect to competing models of priming. Because an MVAR model encompassing all recorded signals would include signals unrelated to the investigated process, i.e. noise that would prevent a good fit with the investigated process, a subset of the recorded sites, limited to sites involved in the investigated process, must be used. Ideally, this subset provides a balanced representation of the system of sites potentially responsible for task performance. However, unavoidable variations in anatomical coverage across individuals can potentially lead to under- or over-representation of this system’s constituents. For example, too many sites recording from one cortical area can introduce redundant information and bias the system. Thus, ERC requires careful selection of sites for inclusion in the MVAR model.
As in previous studies using this method (Korzeniewska et al., 2011; Flinker et al., 2015; Nishida et al., 2017), we used a small set of inclusion/exclusion criteria based on the principles described above to select ECoG sites for analysis. First, we included all sites with statistically significant high gamma responses under either novel or repeated stimulus conditions. This included sites with or without a statistically significant difference between novel and repeat conditions. Second, we included all sites where electrocortical stimulation mapping (ESM) interfered with speech, even if they had no statistically significant high gamma response during the task, reasoning that high gamma propagation can occur without an overall increase in high gamma power relative to baseline. In a previous study of visual object naming using ERC, high gamma propagation was often observed without statistically significant high gamma responses at the source and/or the destination of propagation but was usually observed at nodes with multiple outgoing propagations (Korzeniewska et al., 2011). Finally, we excluded sites if more than three were adjacent to each other, based on the aforementioned rationale that signals recorded from the same cortical area are more likely to contribute redundant information. Excluding these electrodes does not remove information from the overall system’s dynamics and avoids over-representation of one cortical area relative to others (Korzeniewska et al., 2011). Indeed, in previous studies we have observed that a cluster of more than three adjacent electrodes can emphasize propagation within the cluster at the expense of other interactions in the system. Therefore, we excluded sites with the weakest event-related high gamma responses from these clusters, and we included the three sites with the strongest responses. Using the aforementioned criteria, we selected 12–21 electrodes for inclusion in the MVAR model for each patient. Our selection criteria ensured there was sufficient data to fit MVAR model parameters in each patient.
It is important to note that implantation of electrodes was performed solely according to clinical needs. Therefore, the numbers of sites revealing repetition suppression, repetition enhancement, or temporal shift were limited by the electrode locations unique to each patient. We mitigated this limitation by using group statistical analyses subsequent to statistical analyses in single patients.
The selected ECoG signals were re-referenced using a common average reference (CAR). This referencing helped ensure that signal components related to the investigated physiological system were included in the MVAR model, while components not originating in the system (e.g. from volume conduction) were subtracted from the model. Signals were then digitally band-pass filtered backward and forward to avoid phase shifts using a 490 order FIR filter in the 70–120 Hz frequency band. The filter characteristics were designed to capture high gamma responses while avoiding line noise from 60 Hz and its harmonics.
The following multivariate autoregressive (MVAR) model was fitted to the ECoG signals in the selected electrodes:
| (1) |
Here x(t) represents a vector of ECoG signals of zero mean and variance equal to 1 from the selected signals at time t and consecutive time shifts j = 1, 2, …, p, and e(t) is zero mean uncorrelated residual noise. The model order p was determined using the Akaike Information Criterion (AIC) (Burnham and Anderson, 2003). The coefficients in each Aj were calculated by solving the Yule-Walker equations (Yule, 1927; Walker, 1931). To follow the temporal course of brief changes in signal propagation between different brain regions, an algorithm introduced by (Ding et al., 2000), using the property that multiple repetitions of the same task can be treated as multiple realizations of a stochastic process with locally stationary segments, was applied for MVAR coefficient estimation.
In the frequency domain, the MVAR model is given by:
| (2) |
| (3) |
Here f is frequency, Δt is the sampling interval, X(f) is the transformed ECoG signals, and E(f) is the transformed residual noise. To map only direct connections, we multiplied the elements of the transfer matrix H with elements of the partial coherence matrix:
| (4) |
where c is an element of the inverse of the spectral matrix S, found with:
| (5) |
The asterisk indicates transposition and complex conjugation, and V is the residual noise covariance matrix, which can be estimated by summing the products of the estimated MVAR coefficients and the estimate of the covariance matrix.
The short-time direct directed function (SdDTF) is given by:
| (6) |
This equation describes the SdDTF from the k-th channel to the l-th channel, where hkl(f) is an element of the transfer function H and χkl(f) is partial coherence.
We calculated the SdDTF with a 140 ms sliding window to investigate the dynamics of repetition related effects. Consecutive windows had a 96% overlap to smooth the connectivity estimates. ERC values were then obtained using statistical testing (penalized thin-plate spline) (Ruppert et al., 2003) to compare the estimates of connectivity during post-stimulus intervals to baseline connectivity estimates. One second preceding the stimulus presentation in each trial was used as a baseline. A 95% joint confidence interval was constructed for the difference between the estimates of connectivity during trials and the baseline estimate of connectivity. Only positive values of ERC were considered for this analysis because decreases in connectivity, as compared to baseline, are unlikely to be related to task performance, let alone enhance it (Korzeniewska et al., 2008).
Given previous results in MEG of early (~ 200 ms) top-down phase locking between LPFC and VOTC in priming (Ghuman et al., 2008) and a priori predictions of the predictive coding model with respect to increased top-down inputs for repeated stimuli, we tested the effect of repetition on the increase in strength of neural activity propagation in repeated trials (as compared to novel) with one-tailed paired t-tests, corrected for multiple comparisons. These tests were conducted at the group level at every time-point (sliding window of 140 ms). The effect of repetition was marked as significant if average propagation between regions of interests for repeated trials was significantly greater than for novel trials (p<0.05, FDR corrected).
Results
Behavioral priming
Behavioral priming effects were highly significant in a group analysis of all patients (paired t-test with Bonferroni correction for multiple comparisons: t(9) = 6.015, p < 0.001, with each patient showing a numerical decrease in response time (RT) for repeated relative to novel trials (mean = 153 ms, and median = 128 ms). Behavioral priming effects were also significant for all patients except Patient E. Mean response times and their standard deviations, as well as median response times and median absolute deviations (MAD) for each patient for successful novel and repeated trials are shown in Table 2.
Table 2.
Priming Effect, i.e. the difference between reaction time for novel and repeated stimuli, by patient
| Patient | Mean RT ± STD RT (s) | Median RT ± MAD RT (s) | Priming effect | Significance by paired t-test |
|||
|---|---|---|---|---|---|---|---|
| Novel | Repeated | Novel | Repeated | Mean | Median | ||
| A | 1.17 ± 0.41 | 1.02 ± 0.28 | 1.09 ± 0.26 | 1.01 ± 0.17 | 0.15 | 0.08 | p<0.05 |
| B | 0.94 ± 0.31 | 0.81 ± 0.20 | 0.89 ± 0.10 | 0.72 ± 0.05 | 0.13 | 0.17 | p<0.05 |
| C | 1.27 ± 0.47 | 1.05 ± 0.40 | 1.17 ± 0.27 | 0.94 ± 0.24 | 0.22 | 0.23 | p<0.01 |
| D | 0.96 ± 0.23 | 0.71 ± 0.17 | 0.90 ± 0.13 | 0.67 ± 0.10 | 0.25 | 0.23 | p<0.01 |
| E | 0.93 ± 0.22 | 0.86 ± 0.22 | 0.87 ± 0.10 | 0.81 ± 0.08 | 0.07 | 0.06 | p>0.05 |
| F | 0.71 ± 0.15 | 0.62 ± 0.14 | 0.69 ± 0.07 | 0.60 ± 0.10 | 0.09 | 0.09 | p<0.01 |
| G | 0.87 ± 0.15 | 0.78 ± 0.09 | 0.82 ± 0.07 | 0.79 ± 0.07 | 0.09 | 0.03 | p<0.01 |
| H | 2.08 ± 0.32 | 1.95 ± 0.30 | 2.01 ± 0.17 | 1.88 ± 0.09 | 0.13 | 0.13 | p<0.01 |
| I | 1.04 ± 0.21 | 0.92 ± 0.25 | 1.00 ± 0.11 | 0.88 ± 0.07 | 0.12 | 0.12 | p<0.01 |
| J | 2.33 ± 0.53 | 2.05 ± 0.26 | 2.17 ± 0.19 | 2.03 ± 0.13 | 0.28 | 0.14 | p<0.01 |
Repetition Suppression, Repetition Enhancement, and Temporal Shift
We observed three types of neural responses to repeated stimuli. First, at least two recording sites (maximum of 15, median of 5) in each patient demonstrated repetition suppression (RS, purple sites in Fig. 1), i.e. less activation for repeated vs. novel stimuli (p < 0.05, sign test with FDR correction). Second, at least one site (maximum of 8, median of 2.5) in each patient demonstrated repetition enhancement (RE, green sites in Fig. 1), i.e. greater activation with repeated stimuli, except for Patient H, for whom repetition enhancement was not observed. Although this may seem unexpected given the emphasis on repetition suppression in the literature, repetition enhancement has also been reported by others in both humans and monkeys (e.g. de Gardelle et al., 2013; Henson et al., 2000; Merzagora et al., 2014; Miller et al., 1993; Segaert et al., 2013), and multiple potential mechanisms have been offered for them (Segaert et al., 2013). Finally, a small number of cortical sites (10 in five patients) showed no difference in the magnitude of activation for repeated vs. novel stimuli, but did demonstrate a temporal shift (TS, blue sites in Fig. 1) in the peak of activation relative to stimulus onset. This shift, however, was no longer apparent when cortical signals were aligned to response onset, suggesting that activation at these sites was linked to response processing and that the temporal shift was due to shorter latencies to respond to repeated stimuli. Indeed, all but one of these sites were located in ventral sensorimotor cortex or posterior superior temporal gyrus, commonly activated during verbal responses.
Fig. 1.
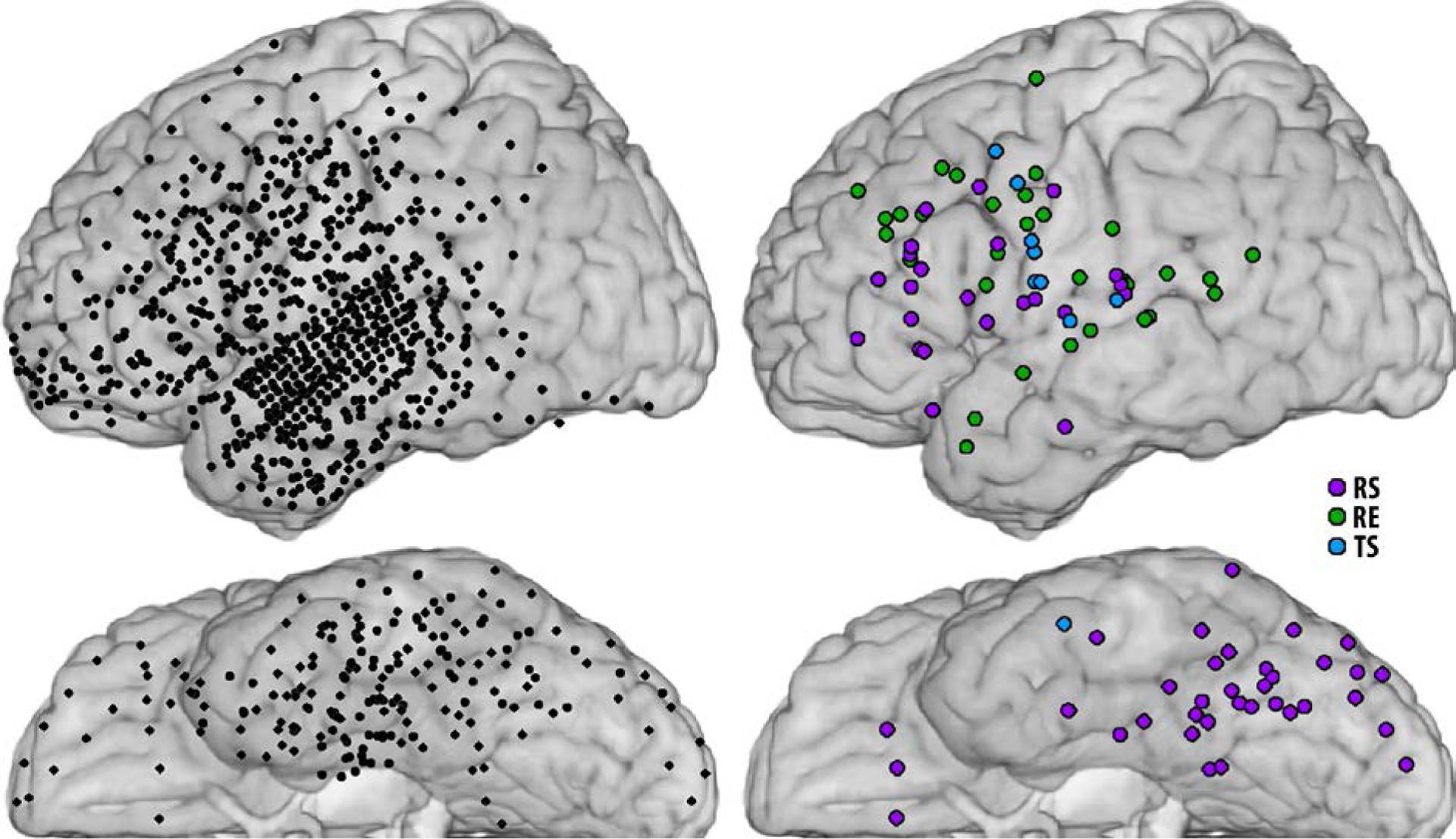
Sites with responses to repeated stimuli. Left panel - all sites recorded and analyzed in 10 patients, projected onto an MNI brain atlas. Note that one patient had a high-density grid over left superior and middle temporal gyri. Right panel - sites for all patients with repetition suppression (purple, RS), repetition enhancement (green, RE), or a temporal shift in peak activation i.e. earlier responses (blue, TS).
The anatomical distribution of these repetition effects was sampled with a total of 962 sites recorded across all 10 patients (Fig. 1 left). Repetition suppression (RS) was observed at 58 sites. The majority of these sites were located in ventral occipital-temporal cortex (VOTC, observed in all patients) and in left prefrontal cortex (LPFC, 7 patients). Repetition enhancement (RE) was observed in 30 sites located in lateral frontal, parietal, and temporal cortices, but not in VOTC. Temporal shift (TS) was observed in 10 sites, as noted above.
Because behavioral priming can have perceptual and conceptual components, with task-related activation in ventral stream and left prefrontal areas (Schacter and Buckner, 1998; Wig et al., 2005), we compared task-related activation for initial and repeated stimuli in these two regions of interests (LPFC and VOTC), and in all other activated sites (OAS) with task related activation. In analyses at the level of individual patients, aggregating sites included in subsequent ERC analyses, we observed significant repetition suppression (RS) in VOTC in 7 patients; repetition suppression (RS) and/or significant repetition enhancement (RE) in LPFC in 7 patients (in one patient - Patient C – we did not observe activation change in LPFC), and temporal shifts (TS) of activation in other activated sites (OAS) in 4 patients. These effects were also significant in group analyses across all patients (Fig. 2).
Fig. 2.
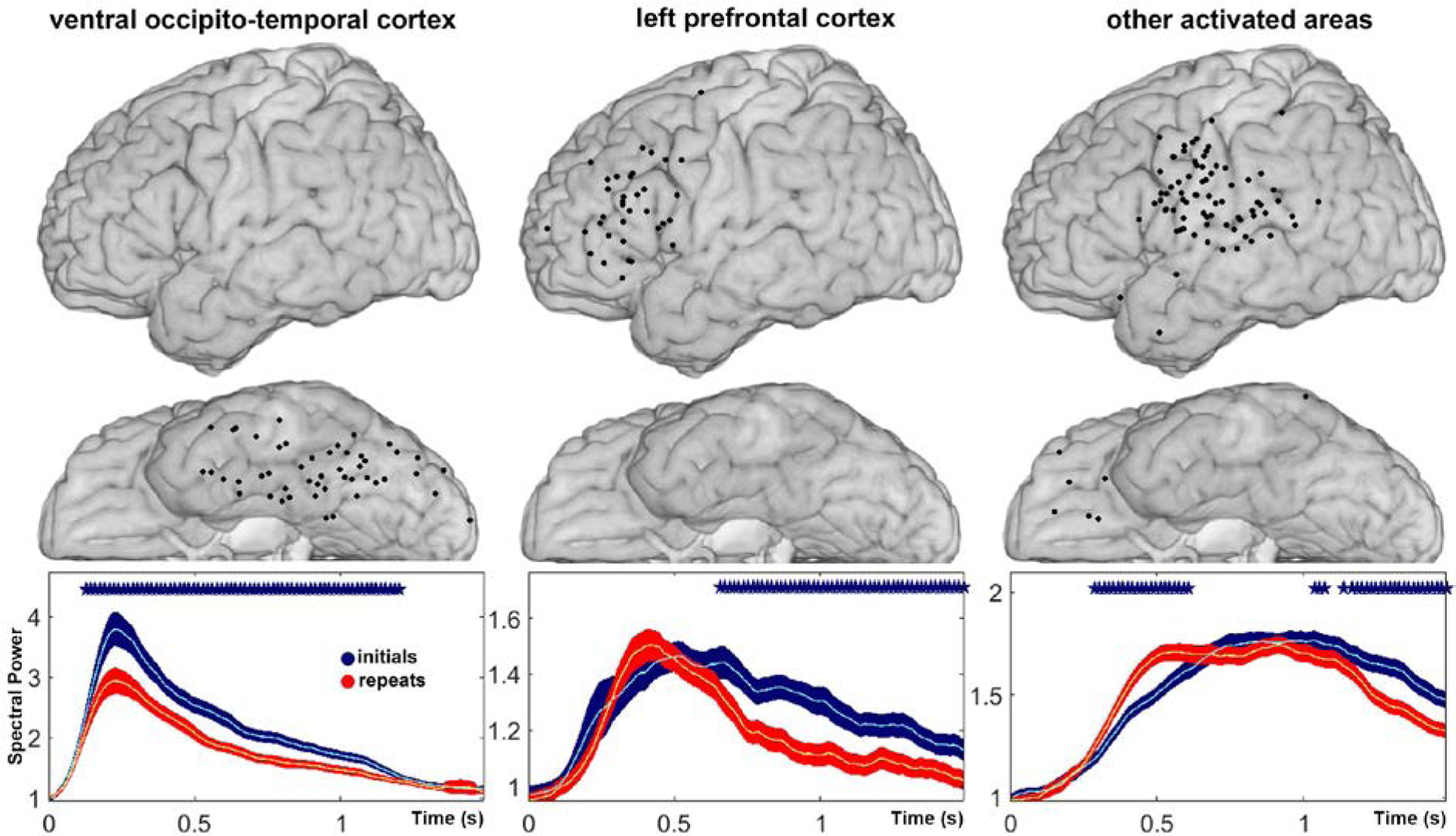
Mean change in high gamma power relative to baseline power, with confidence intervals, aligned to stimulus onset, for novel trials (blue) and repeated trials (red), averaged over sites selected for ERC analysis in ventral occipito-temporal cortex (VOTC), left prefrontal cortex (LPFC), and other activated sites (OAS). Individual recording sites included in the group analyses are depicted above each plot. X-axis represents time in seconds, which indicates the beginning of a sliding window (window length - 128 ms). Y-axis represents mean power. Asterisks indicate time points in which power changes in novel and repeated trials were significantly different (p < 0.05, two-sided t-test with FDR correction). The locations of electrodes were determined for each patient, based on their individual brain reconstructions, and then projected onto the MNI brain (Montreal Neurological Institute standard brain).
We observed significant repetition suppression (RS) in VOTC starting shortly after stimulus onset (~125 ms), and lasting about 1 second (Fig 2. left) in responses averaged across patients within this region of interest. We observed a temporal shift (TS) in peak high gamma responses averaged over other activated sites (OAS, Fig 2. right). In our group analysis of left prefrontal cortex (LPFC, Fig 2. center) we observed a more complex pattern of changes, reflecting repetition suppression (RS) at some sites, and repetition enhancement (RE) in others, the latter often followed by repetition suppression (~600 ms). Interestingly, a shift from initial RE to RS at a later time has been observed using MEG (Marinkovic et al., 2003). To better appreciate the relative timing of RE and RS in LPFC, as well as RS in VOTC, we performed separate statistical analyses over all sites exhibiting statistically significant RE or RS at the level of single recording sites (as shown in Fig. 1).
Group analyses aggregating recording sites with statistically significant repetition enhancement (RE) did not reach statistical significance for VOTC, LPFC, or OAS. Previous studies have attributed RE to multiple potential mechanisms, including attention, explicit memory, and expectation (Segaert et al., 2013), and it is possible that variations in the timing and magnitude of RE, given our sparse sampling of LPFC and other activated sites, did not allow us to observe a significant effect of stimulus repetition. However, group statistics aggregating recording sites with statistically significant repetition suppression (RS) revealed statistically significant and sustained RS in VOTC, LPFC, and other activated sites (Fig. 3).
Fig. 3.
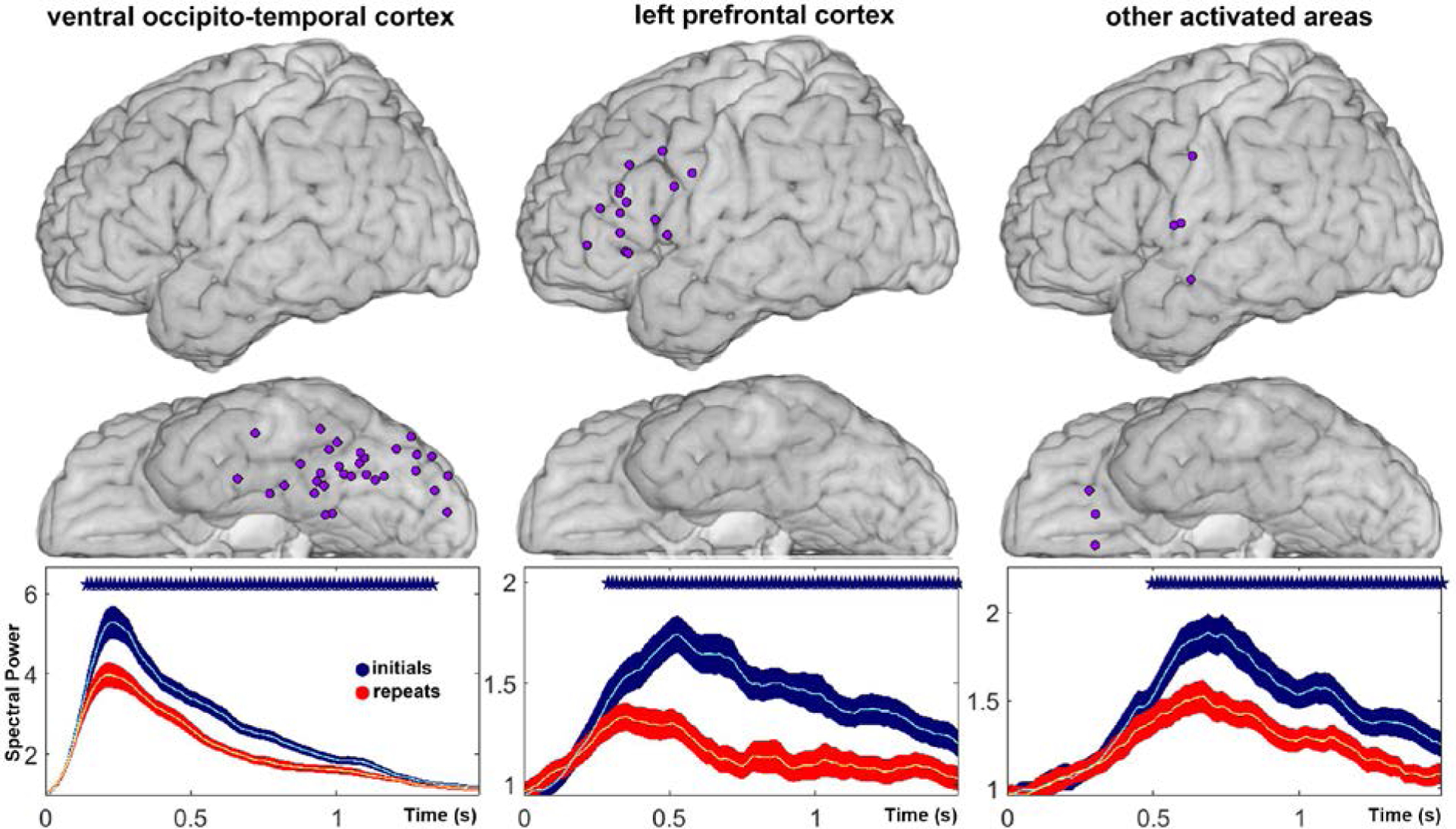
Mean change in high gamma power relative to baseline power, aligned to stimulus onset, for novel trials (blue) and repeated trials (red), averaged over all sites with statistically significant repetition suppression (RS) in ventral occipito-temporal cortex (VOTC), left prefrontal cortex (LPFC), and other activated sites (OAS). Recording sites included in the analysis are depicted above each plot. X-axis represents time in seconds. Y-axis represents mean power. Asterisks indicate time points at which power changes during novel and repeated trials were significantly different (p < 0.05, two-sided t-test with FDR correction).
Interestingly, in group analyses of repetition suppression (RS), it became statistically significant in LPFC later (~ 280 ms, Fig. 3 center) than in VOTC (~ 140 ms, Fig. 3 left), with a slight time-shift in the peak activation in LPFC to earlier time points in the repeat condition (Fig. 3 center). The average peaks in activation at LPFC sites occurred after the average peaks in activation at VOTC sites (Fig. 3 left), consistent with previous reports of the timing of activation in this region, based on phase-locked responses, during visual object perception (Allison et al., 1994). Importantly, we did not observe a consistent temporal shift in the temporal envelope of VOTC activation to earlier latencies, as predicted by the “facilitation” model. Instead, we observed a reduction in the peak of activation and/or an early return to baseline activity. Note that we also observed significant RS within individual patients. In analyses at the level of individual patients, aggregating only sites with significant RS within regions of interest, we observed significant RS in VOTC in 8 patients, in LPFC in 7 patients (in Patient C we did not observe activation over LPFC), and in other activated sites (OAS) in 4 patients (in 6 patients we did not observe RS at individual sites in OAS).
Repetition-related Changes in Effective Connectivity
In ERC analyses within individual patients we observed that for trials with novel stimuli, the most prominent neural propagation (ERC flow) was from VOTC to LPFC, with far less propagation in the opposite direction. In contrast, for trials with repeated stimuli, the most prominent neural propagation was from LPFC to VOTC, with only weak flows in the opposite direction. Examples of these results for two representative patients are shown in Fig. 4.
Fig. 4.
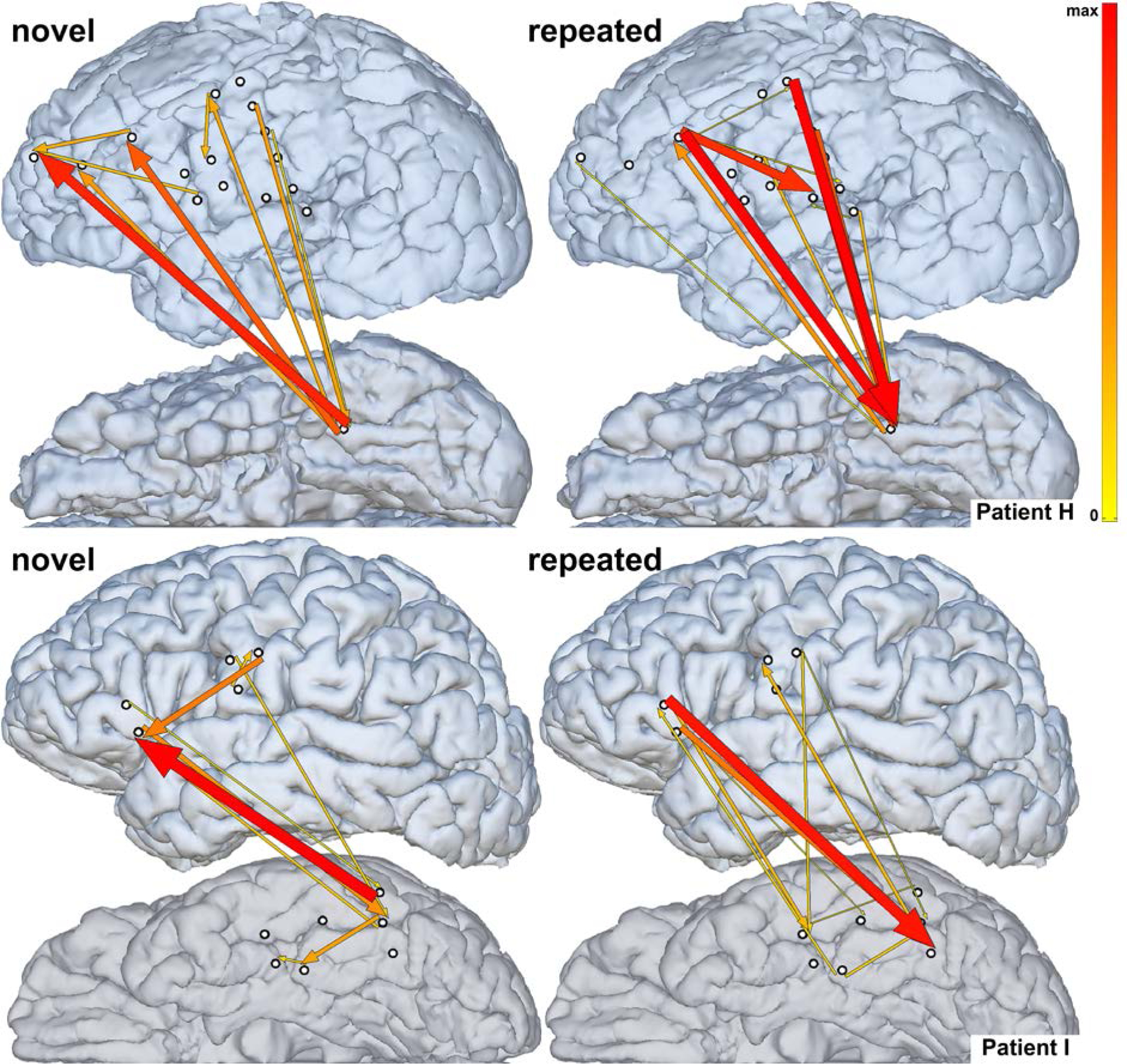
Neural propagation during novel (left) vs. repeated (right) task conditions in two representative patients (top - Patient H, bottom - Patient I) early after stimulus onset (H - 85–450 msec, I - 0–300 msec, taking into account the sliding window length of 140 ms). Arrow width and color both correspond to the strength of estimated ERC flows. Only sites used for estimating ERC flows are shown. Top-down propagation into VOTC is stronger during repeated than during novel task conditions.
Our findings within individual patients suggested that behavioral priming was accompanied by an increase in top-down propagation from LPFC to VOTC. Since this is a key prediction of the predictive coding model of priming and repetition suppression, we performed a group analysis of all observed ERC flows between LPFC and VOTC, as well as other activated sites (OAS) across all patients. Specifically, we tested for increases in responses to repeated stimuli as compared to novel stimuli, using a one-sided t-test with FDR correction. This analysis revealed a statistically significant (p < 0.05, FDR-corrected to q<.05) increase in propagation from LPFC to VOTC for repeated stimuli relative to novel stimuli, which started around 100 ms after stimulus onset (Fig. 5). In addition, we observed a small but significant early increase in propagation among other activated sites (OAS) starting around 50 ms (Fig. 5).
Fig. 5.
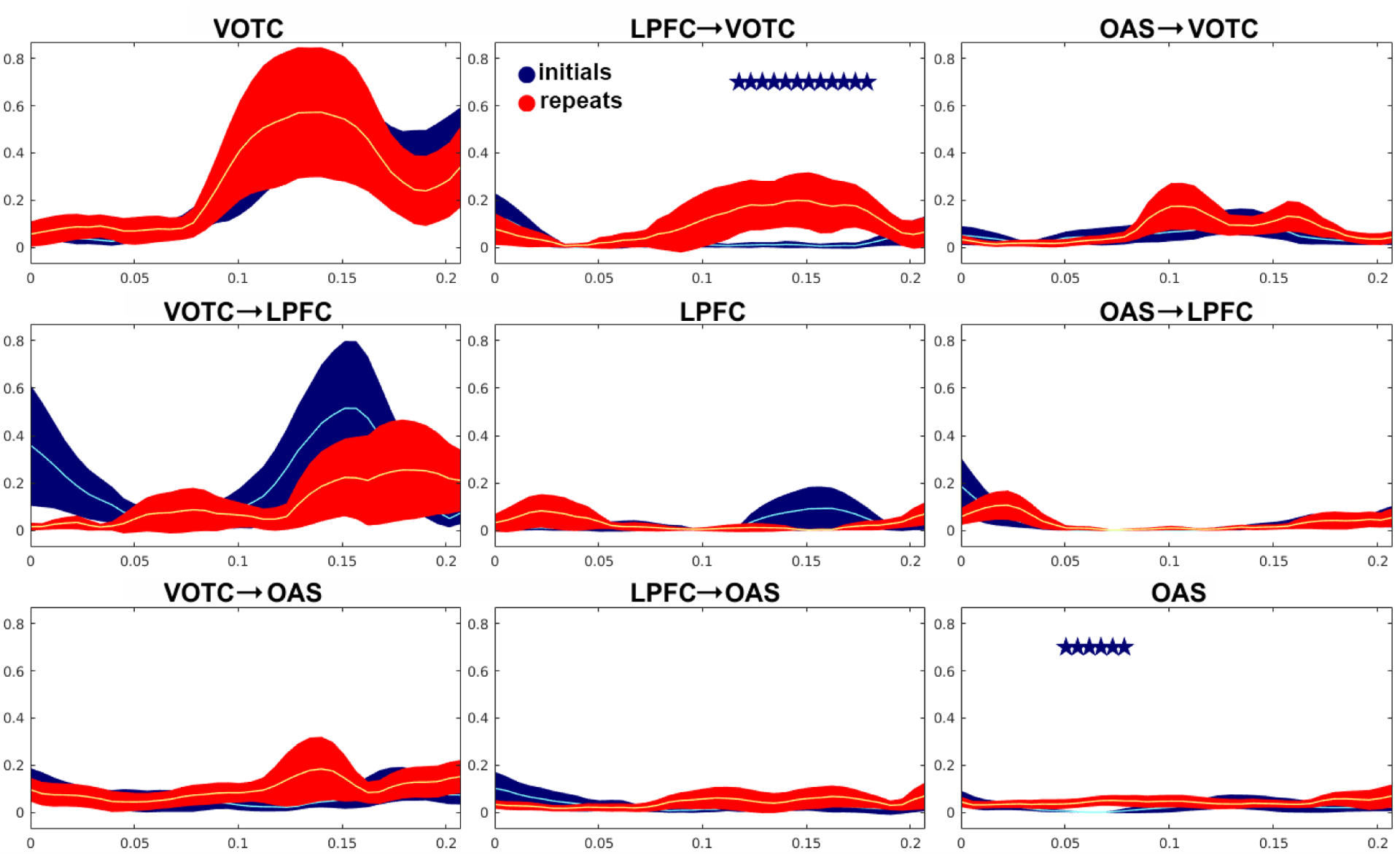
Dynamics of effective connectivity for novel (blue), and repeated (red) stimuli, as revealed by ERC analysis, averaged over 9 patients (no repetition effects in frontal region, or activation there, were observed for Patient C, therefore the patient was not included). Each plot represents a propagation of neural activity from one brain region to another, with 95% confidence intervals for the mean. Recording sites included in the analysis as in Fig. 2. The X-axis represents time in seconds, which indicates the beginning of sliding windows (window length - 140 ms). Y-axis represents the intensity of the propagation. Asterisks represent intervals with significant differences by one-sided t-test (p < 0.05, q<.05).
The effect of stimulus repetition on top-down propagation from LPFC to VOTC, and its temporal relationship with repetition suppression in VOTC, is illustrated in Fig. 6. Note that the repetition-related increase in top-down propagation from LPFC to VOTC became significant numerically earlier than repetition suppression in VOTC did.
Fig. 6.
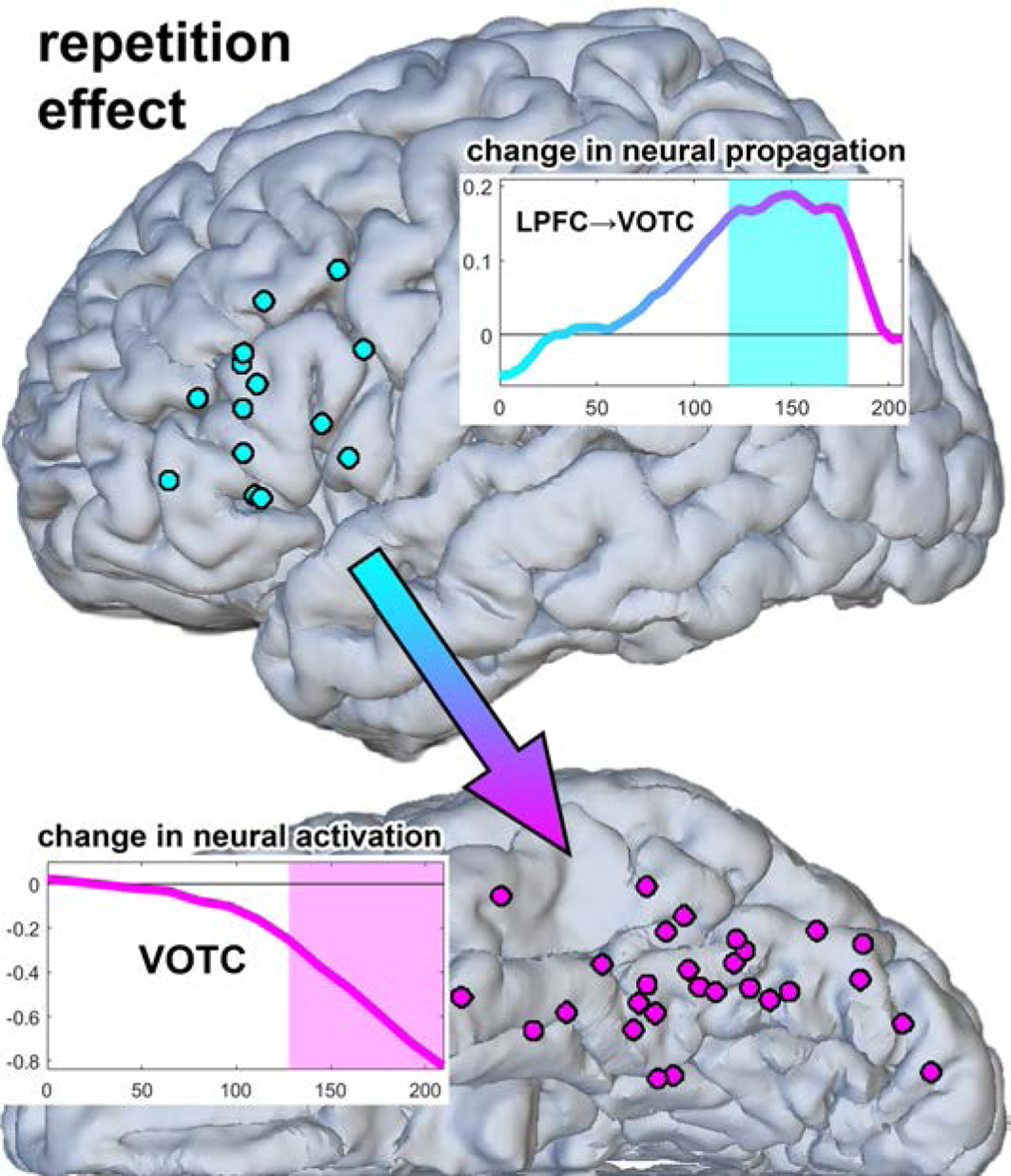
Timing of repetition-related increase in top-down propagation from LPFC to VOTC relative to the timing of repetition suppression. Rectangular inserts show time plots of changes (differences between repeated and initial conditions) in neural activity propagation from LPFC to VOTC (top right), and in neural activation in VOTC (bottom left). The X-axis represents time in seconds, which indicates the beginning of sliding windows. Y-axis represents the magnitude of the change. The shaded areas in the insertions mark time intervals of significant change.
Discussion
Repetition-related Changes in Neural Processing
We used electrocorticography (ECoG) during a visual object naming task to examine changes in neural activity levels (high gamma) and inter-regional network dynamics as a function of short-term stimulus repetition. Using high gamma power changes as an index of changes in population firing rates (Ray et al., 2008), we observed repetition suppression in ventral occipito-temporal cortex (VOTC), left prefrontal cortex (LPFC), and other activated sites, repetition enhancement in a smaller number of sites in LPFC and superior temporal gyrus, and a temporal shift to earlier responses in ventral sensorimotor cortices and superior temporal gyrus, consistent with cortical processing of verbal responses at shorter latencies (behavioral priming). These results were in agreement with other studies of behavioral priming reporting repetition suppression, repetition enhancement, and behavioral facilitation (for reviews please see Gotts et al.,2012; Segaert et al., 2013). Although most studies of the neural basis of behavioral priming have emphasized repetition suppression, especially in cortical areas mediating perceptual processing of stimuli, our findings contribute to a growing literature recognizing repetition enhancement (RE) effects in a variety of behavioral priming paradigms (Segaert et al., 2013). Although we observed statistically significant RE at 30 recording sites across 10 patients, our group analyses did not reach statistical significance for any of the regions of interest, potentially due to variations in the timing and magnitude of RE across patients, as well as inter-individual variations in spatial sampling of our regions of interest. Nevertheless, our observations of RE suggest that further investigation of its role in behavioral priming, at a comparable spatial-temporal resolution, is warranted.
We observed the earliest neural responses in VOTC, with peak activity around 200 ms (Fig. 2 and 3), for both novel and repeated stimuli. Neural responses in LPFC had a later peak (around 500 ms for novel stimuli, and a little earlier than 500 ms for repeated stimuli), with the latest responses observed in sensorimotor cortex just prior to the onset of verbal responses. These findings were consistent with the expected evolution of functional anatomical activation during visual object naming (Indefrey and Levelt, 2004).
In VOTC the only statistically significant effect of stimulus repetition was repetition suppression (RS). Group analyses across ten patients confirmed consistent, statistically significant RS in VOTC, starting shortly after stimulus onset, and lasting for almost the entire duration of task performance. In LPFC, we observed both suppression (RS) and enhancement (RE). This diversity occurred early, and sustained suppression followed, consistent with previous findings with MEG that repetition effects can shift from initial RE to RS (Marinkovic et al., 2003). We also observed RS (starting around 500 ms) in other activated sites for which repetition suppression was significant in individual sites.
Effective connectivity analysis at the group level showed a repetition related increase in top-down propagation from LPFC to VOTC early in task performance (beginning around 100 milliseconds after stimulus onset). This became significant slightly before the onset of statistically significant repetition suppression in VOTC and other activated sites. These findings are suggestive of a role for repetition-related top-down propagation from LPFC in driving effects on repetition suppression that are observed in VOTC and elsewhere. These effects may also underlie earlier responses in brain regions responsible for articulation (ventral sensorimotor cortex), thereby producing faster responses (behavioral priming).
Our finding of early increased top-down propagation with repetition complements a previous study by Ghuman et al. (2008) using MEG, which reported enhanced phase-locking between LPFC and temporal cortex in low-beta frequencies (12–14 Hz) for repeated objects during object identification, beginning ~200 ms after stimulus onset. That study also indicated that the phase of LPFC activity was predictive of the phase of subsequent temporal cortex activity, suggesting information transfer from LPFC to temporal cortex. MEG recordings may be biased by field spread across large distances between neural sources and MEG sensors. Our multivariate autoregressive analysis of ECoG signals provides more direct evidence for neural activity in LPFC having a causal influence on neural activity in VOTC, propagated in high gamma frequencies that are tightly correlated with overall neural population firing rates.
Implications for Models of Repetition Suppression and Priming
As discussed earlier, not all models of repetition suppression and behavioral priming have clear predictions about changes in neural population dynamics. For example, the perceptual “sharpening” model (Desimone, 1996; Wiggs and Martin, 1998) holds that neural tuning becomes more stimulus-selective following repetition, with most of the effects of repetition suppression being driven by the dropping out of poorly selective and responsive cells. This can then lead to reduced support for competing responses downstream. However, the predictions for dynamics are unclear, since responses of cells that prefer the repeated stimulus may be advanced in time and responses of cells that do not prefer the repeated stimulus may be delayed in time (due to reduced synaptic input from the cells that have been sharpened or “pruned” away). It is also unclear if the effects of sharpening should be exclusive to occipito-temporal regions or could occur more generally throughout the brain (Grill-Spector et al., 2006; Gotts et al., 2015).
The “synchrony” model (Gotts, 2003; Ghuman et al., 2008; Gilbert et al., 2010; Gotts et al., 2012) makes certain predictions about dynamics--namely that neural activity should be more synchronized with repetition (Hansen and Dragoi, 2011; Wang et al., 2011; Brunet et al., 2014). However, it is much less clear what the synchrony model predicts with regard to changes in top-down and bottom-up causal influences, and the effective connectivity analyses employed here remove the shared temporal variance across sites about which this model makes direct claims. Appropriate tests of both the sharpening and the synchrony model will require further studies with somewhat different methods (Gotts et al., 2015).
Two of the models that have been proposed previously do make strong predictions in the context of the current study and analysis methods: the “facilitation” model and the “predictive coding” model. The “facilitation” model (James et al., 2000; Henson, 2003, 2012; James and Gauthier, 2006) holds that neural responses should start and finish earlier in repeated than in novel trials, perhaps with an overall reduction in the total amount of neural activity. The facilitation model is partly supported by our current results in the observation of earlier responses at frontal and sensorimotor sites showing accordingly repetition enhancement and a temporal shift of neural activity to earlier time points. However, this model, which was originally proposed to explain repetition suppression in occipito-temporal and prefrontal cortex, does not readily explain the pattern of neural responses we observed in VOTC (i.e. no discernable shift in the peak of repeated trials toward earlier time-points). Nor does it obviously explain our observation of an increase in top-down propagation into VOTC with repetition. Nevertheless, the core idea of the facilitation model, that responses are shifted earlier, appears to be congruent with a number of the findings of this study.
The entire pattern of results is perhaps in best accordance with the “predictive coding” model of repetition suppression and priming (Henson, 2003; Friston, 2005). In this model, the cortex is considered a form of hierarchical generative Bayesian statistical model. Perceptual inference occurs as a progressive interaction between bottom-up sensory input (“evidence”) and top-down expectations (“prediction”) throughout the cortical hierarchy. A critical aspect of this view is that top-down predictions serve to inhibit or suppress the bottom-up sensory evidence, with residual activity in the lower levels of the cortical hierarchy serving as “prediction error” that is, in turn, relayed back toward the higher levels. As the model improves its predictions with stimulus repetition and experience, the top-down input grows stronger and activity in cells that encode prediction error decreases, yielding repetition suppression. While many previous tests of predictive coding have focused on modulating repetition likelihood to induce a high-level strategic expectation (or “prediction”) of stimulus repetition (Larsson and Smith, 2012; Summerfield et al., 2008, 2011; Todorovic et al., 2011; Kaliukhovich and Vogels, 2011), predictions are argued to occur within much of the cortical hierarchy automatically and in a manner insensitive to higher-level context (Ewbank and Henson, 2012; Gotts et al., 2012). On the predictive coding view, top-down “predictions” should serve as feedback from each point in the cortical hierarchy even when all stimuli in a paradigm are novel.
The observed dynamics of increased neural propagation from LPFC into VOTC, followed by sustained repetition suppression, are consistent with a key feature of the predictive coding model, namely an increase in top-down signaling with repetition. Moreover, the effects we observed occurred at latencies that are consistent with the neural model of picture naming and other word production tasks proposed by Indefrey and Levelt (Indefrey and Levelt, 2004), in which the object recognition component of the task lasts up to ~200 ms, and thus add to evidence from previous fMRI studies using effective connectivity methods (Ewbank et al., 2011). Our findings of repetition enhancement (RE) at many LPFC sites may also be relevant in this context, namely because of the possibility that top-down signaling is accompanied by increased activation at the source of this signaling, for example generating predictions based on the expectation of randomly repeated stimuli. Although our group analyses did not demonstrate statistically significant RE, possibly due to variable timing and sampling within LPFC, we observed early RE at a number of individual LPFC sites in our patients. Given that similar observations have been reported with MEG (Marinkovic et al., 2003), future studies of this phenomenon may be warranted.
It will be important to examine repetition effects on neural dynamics over much longer repetition delays than examined here (a few seconds), as both behavioral priming and repetition suppression have been observed over delays of hours and days, and these longer delays are of paramount importance to models positing long-lasting changes to cortical representations (e.g. perceptual sharpening and predictive coding models).
Conclusions
In the current study, we have shown that behavioral priming following short-term repetitions in humans is accompanied by suppression of neural activity in VOTC, LPFC, and other activated sites, as well as temporal shifts of activity to earlier time points in sensorimotor regions mediating verbal responses. Aside from the novel observations of repetition enhancement and temporal shifts of activity in an object naming task, we also observed enhanced top-down feedback from LPFC into VOTC consistent with the claims of the predictive coding model. Regardless of the status of any of the existing models of repetition suppression and priming, our results highlight the important role of altered large-scale dynamics in these phenomena and the utility of ECoG studies in humans for elucidating these dynamics.
Table 1.
Specification of number of sites (electrodes) included in MVAR model, by patient
| Patient | Sites with repetition suppression | Sites with repetition enhancement | Adjacent sites excluded from MVAR model | ESM+ sites | Sites included in MVAR model | |||
|---|---|---|---|---|---|---|---|---|
| VOTC | LPFC | VOTC | LPFC | VOTC | LPFC | |||
| A | 4 | 2 | 0 | 2 | 0 | 0 | 7 | 18 |
| B | 7 | 1 | 0 | 2 | 2 | 0 | 1 | 19 |
| C | 3 | 0 | 0 | 0 | 0 | 0 | 6 | 15 |
| D | 1 | 1 | 0 | 3 | 0 | 0 | 5 | 20 |
| E | 1 | 4 | 0 | 3 | 0 | 0 | 10 | 21 |
| F | 2 | 3 | 0 | 0 | 0 | 0 | 5 | 16 |
| G | 2 | 2 | 0 | 0 | 0 | 0 | 6 | 15 |
| H | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 16 |
| I | 3 | 0 | 0 | 0 | 0 | 0 | 11 | 12 |
| J | 2 | 0 | 0 | 3 | 0 | 0 | 2 | 13 |
Highlights.
Repetition suppression occurred in ventral temporal and left prefrontal cortex
Early top-down neural propagation preceded repetition suppression
These results provide support for predictive coding in behavioral priming
Acknowledgement
We thank our colleagues Christopher Coogan, Piyush Routray, and Adeshola Lawal for their contribution in reconstruction of electrodes sites in patient brain, and projecting onto an MNI brain atlas, that greatly improved the manuscript.
Funding
This study was supported by NINDS R01 NS 40596 and NINDS R01 NS 091139
Abbreviations:
- ECoG
electrocorticogram
- ERC
event related causality
- ESM
electrostimulation mapping
- LPFC
left prefrontal cortex
- MVAR
multivariate autoregressive model
- RE
repetition enhancement
- RS
repetition suppression
- SdDTF
short-time direct directed function
- TS
time shift
- VOTC
ventral occipito-temporal cortex
References
- Allison T, McCarthy G, Nobre A, Puce A, Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cereb Cortex 1994; 4: 544–54. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol 1995; 57: 289–300. [Google Scholar]
- Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: A platform for analyzing neural signals. J. Neurosci. Methods 2010; 192: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet NM, Bosman CA, Vinck M, Roberts M, Oostenveld R, Desimone R, et al. Stimulus repetition modulates gamma-band synchronization in primate visual cortex. Proc. Natl. Acad. Sci 2014; 111: 3626–3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer Science & Business Media; 2003. [Google Scholar]
- Cervenka MC, Corines J, Boatman-Reich DF, Eloyan A, Sheng X, Franaszczuk PJ, et al. Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. Neuroimage 2013; 69: 267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TE, Sohoglu E, Sedley W, Patterson K, Jones PS, Wiggins J, et al. Evidence for causal top-down frontal contributions to predictive processes in speech perception [Internet]. Nat. Commun 2017; 8[cited 2018 Apr 10] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5735133/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical gamma responses: searching high and low. Int. J. Psychophysiol 2011; 79: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gardelle V, Waszczuk M, Egner T, Summerfield C. Concurrent repetition enhancement and suppression responses in extrastriate visual cortex. Cereb Cortex 2013; 23: 2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U A 1996; 93: 13494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Bressler SL, Yang W, Liang H. Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: data preprocessing, model validation, and variability assessment. Biol Cybern 2000; 83: 35–45. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, and Schacter DL. Cortical activity reductions during repetition priming can result from rapid response learning. Nature 2004; 428: 316–319. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH. Geometric strategies for neuroanatomic analysis from MRI. NeuroImage 2004; 23 Suppl 1: S34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank MP, Henson RN. Explaining away repetition effects via predictive coding. Cogn. Neurosci 2012; 3: 239–240. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Lawson RP, Henson RN, Rowe JB, Passamonti L, Calder AJ. Changes in “Top-Down” Connectivity Underlie Repetition Suppression in the Ventral Visual Pathway. J. Neurosci 2011; 31: 5635–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B FreeSurfer. Neuroimage 2012; 62: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA. Repetition priming in aging and Alzheimer’s disease: an integrative review and future directions. Cortex 2007; 43: 889–97. [DOI] [PubMed] [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, et al. Redefining the role of Broca’s area in speech. Proc. Natl. Acad. Sci. U. S. A 2015; 112: 2871–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franaszczuk PJ, Jouny CC. Software system for data management and distributed processing of multichannel biomedical signals. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf 2004; 2: 983–985. [DOI] [PubMed] [Google Scholar]
- Friston K A theory of cortical responses. Philos Trans R Soc Lond B Biol Sci 2005; 360: 815–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuman AS, Bar M, Dobbins IG, Schnyer DM. The effects of priming on frontal-temporal communication. Proc Natl Acad Sci U A 2008; 105: 8405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JR, Gotts SJ, Carver FW, Martin A. Object repetition leads to local increases in the temporal coordination of neural responses [Internet] . Front. Hum. Neurosci 2010; 4[cited 2017 Oct 27] Available from: https://www.frontiersin.org/articles/10.3389/fnhum.2010.00030/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AW, Kalinowski SE, Milleville SC, Gotts SJ, and Martin A. Identifying task-general effects of stimulus familiarity in the parietal memory network. Neuropsychologia 2019; 124: 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ. Mechanisms underlying enhanced processing efficiency in neural systems. 2003
- Gotts SJ. Incremental learning of perceptual and conceptual representations and the puzzle of neural repetition suppression. Psychon. Bull. Rev 2016; 23: 1055–1071. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Chow CC, Martin A. Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization. Cogn. Neurosci 2012; 3: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Milleville SC, Martin A. Object identification leads to a conceptual broadening of object representations in lateral prefrontal cortex. Neuropsychologia 2015; 76: 62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci 2006; 10: 14–23. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol Amst 2001; 107: 293–321. [DOI] [PubMed] [Google Scholar]
- Hansen BJ, Dragoi V. Adaptation-induced synchronization in laminar cortical circuits. Proc. Natl. Acad. Sci 2011; 108: 10720–10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science 2000; 287: 1269–72. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Prog Neurobiol 2003; 70: 53–81. [DOI] [PubMed] [Google Scholar]
- Henson RN. Repetition accelerates neural dynamics: In defense of facilitation models. Cogn. Neurosci 2012; 3: 240–241. [DOI] [PubMed] [Google Scholar]
- Henson RN, Eckstein D, Waszak F, Frings C, Horner AJ. Stimulus–response bindings in priming. Trends Cogn. Sci 2014; 18: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AJ and Henson RN. Priming, response learning and repetition suppression. Neuropsychologia 2008; 46(7): 1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition 2004; 92: 101–44. [DOI] [PubMed] [Google Scholar]
- James TW, Gauthier I. Repetition-induced changes in BOLD response reflect accumulation of neural activity. Hum Brain Mapp 2006; 27: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Menon RS, Goodale MA. The effects of visual object priming on brain activation before and after recognition. Curr. Biol 2000; 10: 1017–1024. [DOI] [PubMed] [Google Scholar]
- Kaliukhovich DA, Vogels R. Stimulus repetition probability does not affect repetition suppression in macaque inferior temporal cortex. Cereb. Cortex N. Y. N 1991 2011; 21: 1547–1558. [DOI] [PubMed] [Google Scholar]
- Korzeniewska A, Crainiceanu CM, Kus R, Franaszczuk PJ, Crone NE. Dynamics of event-related causality in brain electrical activity. Hum. Brain Mapp 2008; 29: 1170–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewska A, Franaszczuk PJ, Crainiceanu CM, Kus R, Crone NE. Dynamics of large-scale cortical interactions at high gamma frequencies during word production: event related causality (ERC) analysis of human electrocorticography (ECoG). Neuroimage 2011; 56: 2218–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Prog. Neurobiol 2012; 98: 279–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Smith AT. fMRI repetition suppression: neuronal adaptation or stimulus expectation? Cereb. Cortex N. Y. N 1991 2012; 22: 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophysiol 1993; 69: 1918–29. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol 1997; 77: 24–42. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron 2003; 38(3): 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 1995; 102: 419–57. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Carlson C, Blumberg M, Girard HM, Trongnetrpunya A, et al. Multimodal imaging of repetition priming: Using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. NeuroImage 2010; 53: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKone E The decay of short-term implicit memory: unpacking lag. Mem. Cognit 1998; 26: 1173–1186. [DOI] [PubMed] [Google Scholar]
- McMahon DB, Olson CR. Repetition suppression in monkey inferotemporal cortex: relation to behavioral priming. J Neurophysiol 2007; 97: 3532–43. [DOI] [PubMed] [Google Scholar]
- Merzagora AR, Coffey TJ, Sperling M, Sharan A, Litt B, Baltuch G, Jacobs J. Repeated stimuli elicit diminished high-gamma electrocorticographic responses. NeuroImage 2014; 85 Pt 2: 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, and Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. Journal of Neuroscience 1993; 13: 1460–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science 1999; 285: 1405–1408. [DOI] [PubMed] [Google Scholar]
- Nishida M, Korzeniewska A, Crone NE, Toyoda G, Nakai Y, Ofen N, et al. Brain network dynamics in the human articulatory loop. Clin. Neurophysiol 2017; 128: 1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J-P, Midgley K, Holcomb P, Grainger J. On the time course of letter perception: A masked priming ERP investigation. Psychonomic Bulletin& Review 2006; 13(4): 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race EA, Shanker S, and Wagner AD. Neural priming in human frontal cortex: Multiple forms of learning reduce demands on the prefrontal executive system. Journal of Cognitive Neuroscience 2009; 21: 1766–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Miller EK. Effects of visual experience on the representation of objects in the prefrontal cortex. Neuron 2000; 27: 179–89. [DOI] [PubMed] [Google Scholar]
- Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci 2008; 28: 11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert D, Wand MP, Carroll RJ. Semiparametric regression. New York: Cambridge University Press; 2003. [Google Scholar]
- Segaert K, Weber K, deLange FP, Petersson KM, Hagoort P. The suppression of repetition enhancement: A review of fMRI studies. Neuropsychologia 2013; 51: 59–66. [DOI] [PubMed] [Google Scholar]
- Scarborough DL, Cortese C, Scarborough HS. Frequency and repetition effects in lexical memory. J. Exp. Psychol. Hum. Percept. Perform 1977; 3: 1–17. [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron 1998; 20: 185–95. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 1992; 99: 195–231. [DOI] [PubMed] [Google Scholar]
- Summerfield C, Trittschuh EH, Monti JM, Mesulam MM, Egner T. Neural repetition suppression reflects fulfilled perceptual expectations. Nat. Neurosci 2008; 11: 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Wyart V, Johnen VM, de Gardelle V. Human Scalp Electroencephalography Reveals that Repetition Suppression Varies with Expectation. Front. Hum. Neurosci 2011; 5: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Schall JD. Antecedents and correlates of visual detection and awareness in macaque prefrontal cortex. Vision Res. 2000; 40: 1523–1538. [DOI] [PubMed] [Google Scholar]
- Todorovic A, van Ede F, Maris E, de Lange FP. Prior expectation mediates neural adaptation to repeated sounds in the auditory cortex: an MEG study. J. Neurosci. Off. J. Soc. Neurosci 2011; 31: 9118–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Turennout M, Bielamowicz L, Martin A. Modulation of neural activity during object naming: effects of time and practice. Cereb Cortex 2003; 13: 381–91. [DOI] [PubMed] [Google Scholar]
- Walker G On Periodicity in Series of Related Terms. Proc. R. Soc. Lond. Ser. Contain. Pap. Math. Phys. Character 1931; 131: 518–532. [Google Scholar]
- Wang Y, Iliescu BF, Ma J, Josić K, Dragoi V. Adaptive changes in neuronal synchronization in macaque V4. J. Neurosci. Off. J. Soc. Neurosci 2011; 31: 13204–13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat Neurosci 2005; 8: 1228–33. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol 1998; 8: 227–33. [DOI] [PubMed] [Google Scholar]
- Yule GU. On a Method of Investigating Periodicities in Disturbed Series, with Special Reference to Wolfer’s Sunspot Numbers. Philos. Trans. R. Soc. Lond. Ser. Contain. Pap. Math. Phys. Character 1927; 226: 267–298. [Google Scholar]


