Abstract
Decades of work have established the brain as a source of steroid hormones, termed ‘neurosteroids’. The neurosteroid neuroestradiol is produced in discrete brain areas and influences cognition, sensory processing, reproduction, neurotransmission, and disease. A prevailing research focus on neuroestradiol has essentially ignored whether its immediate synthesis precursor - the androgen testosterone - is also dynamically regulated within the brain. Testosterone itself can rapidly influence neurophysiology and behavior, and there is indirect evidence that the female brain may synthesize significant quantities of testosterone to regulate cognition, reproduction, and behavior. In songbirds, acoustic communication is regulated by neuroestrogens. Neuroestrogens are rapidly synthetized in the caudomedial nidopallium (NCM) of the auditory cortex of zebra finches in response to song and can influence auditory processing and song discrimination. Here, we examined the in vivo dynamics of NCM levels of the neuroestrogen synthesis precursor, testosterone. Unlike estradiol, testosterone did not appear to fluctuate in the female NCM during song exposure. However, a substantial song-induced elevation of testosterone was revealed in the left hemisphere NCM of females when local aromatization (i.e., conversion to estrogens) was locally blocked. This elevation was eliminated when local androgen synthesis was concomitantly blocked. Further, no parallel elevation was observed in the circulation in response to song playback, consistent with a local, neural origin of testosterone synthesis. To our knowledge, this study provides the first direct demonstration that testosterone fluctuates rapidly in the brain in response to socially-relevant environmental stimuli. Our findings suggest therefore that locally-derived ‘neuroandrogens’ can dynamically influence brain function and behavior.
Keywords: songbirds, aromatase, microdialysis, communication, non-genomic, neurosteroids
Introduction
Studies on rapid regulation of neurosteroids have focused almost exclusively on the potent estrogen 17β-estradiol (E2). E2 is produced via the local aromatization of testosterone within many brain regions, and influences major functions and behaviors including social communication (Ball and Balthazart, 2010; Balthazart et al., 2009; Garcia-Segura, 2008; Hull and Dominguez, 2015; Yoder and Vicario, 2012). The local dynamics and acute actions of E2 on social communication are most evident in songbirds.
One region of songbird secondary auditory cortex (caudomedial nidopallium; NCM) is enriched with estrogen receptors and aromatase (Metzdorf et al., 1999; Yoder and Vicario, 2012). Both male and female zebra finches exhibit a rapid elevation in NCM E2 in social and auditory contexts (Remage-Healey et al., 2012, 2008). Increases in local E2 also directly enhance the auditory-evoked activity of NCM neurons (Remage-Healey et al., 2010; Remage-Healey and Joshi, 2012; Tremere et al., 2009; Tremere and Pinaud, 2011), while infusion of fadrozole, a potent and selective aromatase inhibitor, causes acute decreases in the auditory-evoked activity of NCM neurons and the behavioral preference for familiar songs (Remage-Healey et al., 2010). Therefore, local E2 acts as a neuromodulator to directly influence both local neuronal response properties and downstream sensorimotor integration (Remage-Healey et al., 2013; Vahaba and Remage-Healey, 2018). The rapid fluctuations in local E2 levels in the songbird brain predict that upstream precursors like testosterone also exhibit dynamic regulation.
Theoretically, song-induced elevations in NCM E2 can depend on three related mechanisms, including (a) a rapid increase of aromatase activity, (b) a rise of the precursor testosterone in the plasma and diffusion into the brain and/or (c) a direct local testosterone synthesis independent from the periphery. Rapid changes in aromatase activity have been well demonstrated in the avian brain (Balthazart et al., 2006; Cornil and de Bournonville, 2017) including the telencephalon of zebra finches (Remage-Healey et al., 2009). Instead, this study focused specifically on the substrate fluctuation/synthesis hypotheses. This current work was conducted using female zebra finches for three reasons. First, females have lower peripheral testosterone levels than do males (Kabelik et al., 2011; Prior et al., 2014). Second, the key enzymes responsible for testosterone synthesis, 3β-hydroxysteroid-dehydrogenase (3β-HSD) and 17β-hydroxysteroid-dehydrogenase (17β-HSD), are expressed and active in the female NCM, exceeding that in males (London et al., 2006, 2003; Soma et al., 2004; Tomaszycki and Dzubur, 2013). Third, this high androgenic enzymatic activity is rapidly regulated during stress in females but not males (Soma et al., 2004). Therefore, testosterone synthesis in the female NCM could have a functional behavioral role in addition to providing a substrate for fast conversion to estrogens.
Methods
Animals
Females zebra finches (adults > 105 dph) were used for this study. Protocols were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts. All animals were raised from our colony or came from a commercial supplier.
General procedures
Three main experiments were performed using in vivo microdialysis or blood measurements for steroids according to previously published protocols (Remage-Healey et al., 2012, 2008). All animals were housed in acoustically isolated chambers one day before experiments began. For experiments involving acoustic playback, a subset of females was recorded in order to evaluate whether female behaviors were associated with the steroid fluctuations observed in NCM (see the behavioral analysis section).
Cannula implantation and microdialysis
For in vivo microdialysis experiments, a cannula (CMA Microdialysis) was implanted either in the left or in the right hemisphere NCM. On the day of the surgery, animals were anesthetized with an intramuscular injection of Equithesin (3.2 ml/Kg), and lidocaine (10–15 μl) was applied under the scalp. The skull was opened and a CMA-7 microdialysis guide cannula was lowered using stereotaxic coordinates that have been previously validated to target the most medial and caudal part of NCM (1.1 mm rostral and 0.7 mm lateral relative to the bifurcation of the midsagittal sinus, 1.4 mm from the surface of the brain). Dental cement and cyanoacrylate were used to fix the cannula to the skull. Meloxicam (1 μl/g weight; 0.1 mg/ml) was orally administered as an analgesic. Birds were then placed in sound attenuation chambers (Eckel Industries) with a female companion for at least 3 days until the experiment began. All animals were housed in acoustically isolated chambers one day before experiments began. The microdialysis probe (CMA-7; OD 0.24 mm; shaft length 7.0 mm; membrane length 1.0 mm) was inserted in the cannula the day before the first sample collection and was perfused overnight at a flow rate of 2 ul/min with artificial cerebrospinal fluid (aCSF; 199 mM NaCl, 26.2 mM NaHCO3, 2.5 mM KCl, 1 mM NaH2PO4, 1.3 mM MgSO4, 2.5 mM CaCl, 11 mM glucose, and 1% BSA, pH = 7.4) previously filtered through 0.2 μmHT syringe filters (Acrodisc 25-mm filters; Pall Life Sciences). This overnight period of acclimation before sample collection was carried out to avoid implantation-induced neurochemical contamination. The probe was connected to FEP tubing (CMA Microdialysis) and the outflow terminated in a microcentrifuge tube for sample collection. Perfusion fluid (aCSF as above) was infused at a rate of 2 ul/min for all experiments and samples collected every 30 minutes were immediately frozen at −20 °C.
Specific protocols
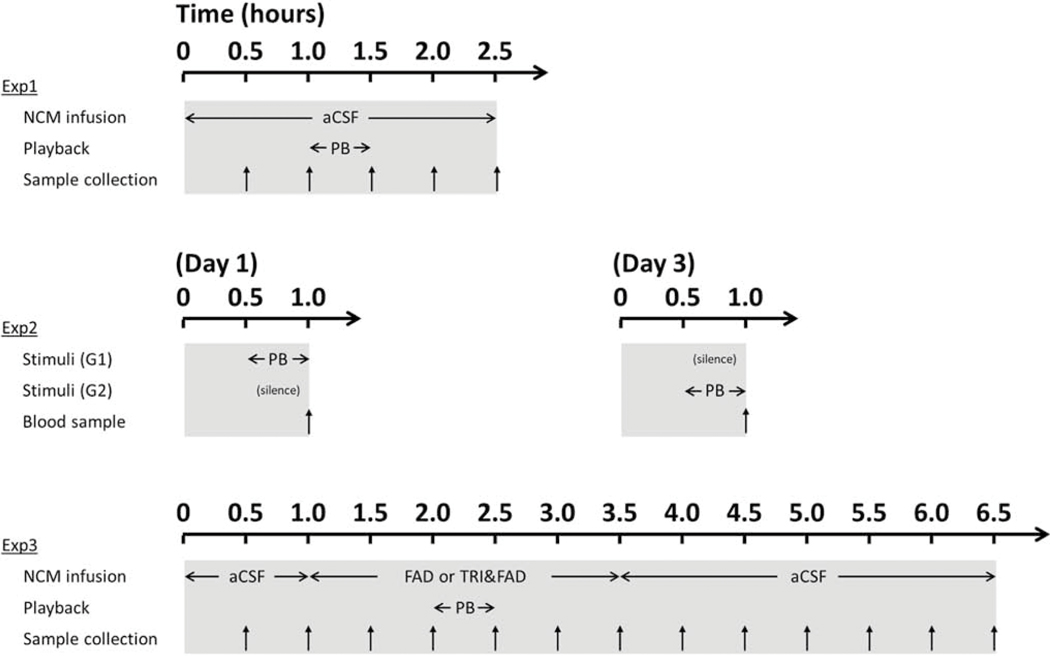
Specific protocols are described in Figure 1. The first experiment was set up to measure both testosterone and E2 fluctuations in NCM during acoustic playback of auditory stimuli, according to a previously published result for E2 alone (Remage-Healey et al., 2008). Dialysate samples were collected every 30 minutes during three experimental periods of time (see Figure 1): a baseline period (BL; 2 × 30 min) where females where not exposed to any acoustic stimulus, a playback period where females were exposed to songs (PB; 1 × 30 min) and a post-treatment silent period after the stimulus was over (POST; 2 × 30 min). The playback stimuli were identical in amplitude, duration, and repetition rate to that used in previously published studies (Remage-Healey et al., 2012, 2008). For a subset of animals (n = 13), both E2 and testosterone measurements were performed on the same dialysate samples using a serial assay design, for other subjects (n = 6) only testosterone was measured. Dialysates from some animals in the first subset were used for troubleshooting of the testosterone measurements resulting in the following sample sizes: 13 females were used to measure NCM E2 levels during playback while 12 females were used to measure NCM testosterone levels during playback.
Figure 1:
Specific protocols of experiments using steroid measurements in NCM dialysates (exp.1 and 3) or in blood samples (exp.2). For each experiment, the auditory stimulus is noted as “PB” and the time of sample collection is represented by vertical arrows. Abbreviations: aCSF, artificial cerebrospinal fluid; FAD, fadrozole; TRI, trilostane.
The second experiment aimed to determine whether the rise of E2 observed in NCM in response to song could be mediated by a rise of testosterone derived from the periphery. For 30 minutes, females were exposed to either acoustic playback (n = 10) or silence (n = 10). At the end of the stimulus, they were rapidly removed from the isolation chamber and a blood sample was collected from the brachial vein. Blood samples were centrifuged for 10 minutes at 10.000g to obtain serum, which was stored at −20 °C until testosterone assay. Two or three days later, the same experimental protocol was performed on the same subjects with the stimulus that they were not exposed to on the first trial (silence or song playback) and again, a blood sample was collected immediately after the end of the stimulus presentation and was processed for testosterone assay. Serum was extracted using a C18 SPE column (see steroids measurements below) before further ELISA analysis.
In a third experiment we aimed to assess the pattern of testosterone fluctuation during playback coupled with the blockade of selected neurosteroidogenic enzymes (see Figure 1). Specifically, fadrozole (100uM diluted in aCSF, stock solution 20mM in dH2O) was used in a first set of animals to inhibit E2 synthesis (Wade et al., 1994) while trilostane (15nM diluted in aCSF, stock solution 1.5mM in DMSO) was used to inhibit testosterone synthesis (Potts et al., 1978). Fadrozole only was used in 13 animals while a combination of trilostane and fadrozole was used in 7 animals. As reported in mammalian systems, the IC50 of fadrozole is 4.5 nM for aromatase and of trilostane is 4.1 μM for 3β-HSD (Browne et al., 1991; Coirini et al., 2003). Samples were collected every 30 minutes during the following experimental periods: After a baseline period (BL; 2 × 30 min) of aCSF perfusion, drugs were retrodialyzed under silent conditions (Fadrozole (FAD) or Trilostane and fadrozole (TRIFAD); 2 × 30 min), then during the playback (FAD+PB or TRIFAD+PB; 1 × 30 min), then again after the end of the stimulus (FAD or TRIFAD; 2 × 30 min). Thus, drug retrodialysis started an hour before the song stimulus was played and lasted another hour after the end of it. Perfusion fluid was finally switched back to aCSF for six more samples (POST). Since fadrozole and trilostane showed modest cross-reactivity with the testosterone ELISA kit, in vitro experiments were performed to evaluate the time-course interference of these drugs in order to correct the in vivo data (Figure 2 D–E). An extra validation was also performed to test whether natural testosterone fluctuations were still detectable and linearly displaced along the standard curve despite drug interferences (Figure 2 C). E2 as well as testosterone were measured in the same samples for 9 subjects in experiment 3 while only testosterone was assayed in the remaining subjects (n = 4).
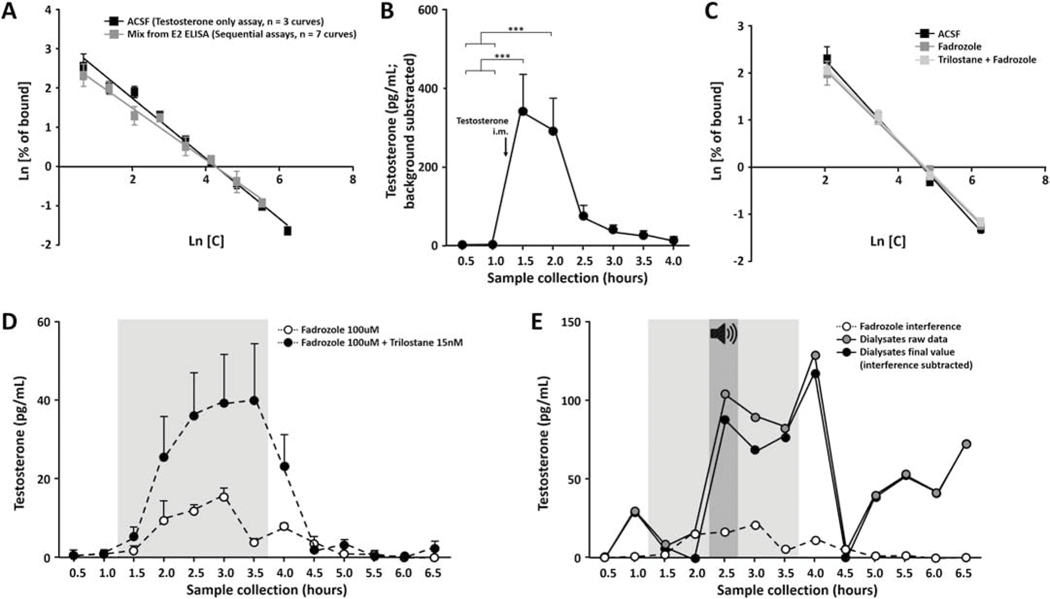
Figure 2:
Validations of the testosterone assay and control for drugs interferences. (A) Standard curves prepared with control aCSF or with a mix collected from the previous E2 assay. Both curves are parallel meaning that both techniques (testosterone-only, black squares; or sequential assays, gray squares) are thus able to quantify the same kind of fluctuations. X axis, logarithm of increasing concentration of testosterone; Y axis, logarithm of sample absorbance divided by total absorbance. (B) Testosterone injection causes a substantial and detectable increase in testosterone levels within NCM. Samples were collected every 30 minutes before (1.0 and −0.5) and immediately after (0.5 to 3.0 hours) the intramuscular injection (arrow). *** p < 0.001. (C) Standard curves prepared with increasing amount of testosterone in aCSF, in fadrozole (100uM) or in both fadrozole and trilostane (100uM and 15nM respectively). The three curves are parallel meaning that it is possible to quantify testosterone fluctuations in an accurate way despite the presence of these interfering drugs. X axis, logarithm of increasing concentration of testosterone; Y axis, logarithm of sample absorbance divided by total absorbance. (D) Over-time quantification of the fadrozole and trilostane interferences with the ELISA. ACSF alone (non-colored area; 0.5 to 1.0 and 4.0 to 6.5 hours) or ACSF with drugs (gray area; 1.5 to 3.5 hours) is perfused through probes dipped in an ACSF bath and samples are collected every 30 minutes and assayed by ELISA. The interference is higher when fadrozole is delivered in combination with trilostane (black dots, max interference 40.0 pg/mL) compared to fadrozole alone (white dots, max interference 15.4 pg/mL). (E) Example of individual data corrected for the drug interference. Dialysates for this animal were assayed for testosterone (raw data; gray dots) then corrected with the over-time quantification of the drug interference (white dots). All the results are finally expressed as the subtraction of the interference from the raw data (final value; black dots).
Behavioral analysis
For a subset of females in each experiment, video recordings were taken during the playback exposure. The camera was placed outside of the sound-attenuation chamber, facing the animal through a one-way glass partition window. The video duration was 30 minutes and included 15 minutes immediately prior to song exposure (baseline) and the first 15 minutes of song exposure (playback). Ten minutes of each period (baseline and playback) were then analyzed by a blind experimenter using JWatcher software (1.0) to quantify the frequency of the following behaviors: Head tilts, head orientation towards the speaker, head orientation away from the speaker, movement in the direction of the speaker, movement in the direction away from the speaker, eating, and drinking. The first three were combined in a composite measure of total head tilt frequency while the first five behaviors were combined in a composite measure of total activity in order to assess locomotor activity of females. Finally, eating, drinking and flying behaviors were combined and expressed as negative behaviors since they were used previously as a measure of inattention to the tutor in juvenile zebra finches (Chen et al., 2016). Other typical measures of female approaches (e.g. using perches close to or far away from the speaker; Howell and Derryberry, 2019) were not possible due to the microdialysis set up.
Brain histology
Females from in vivo microdialysis experiments (experiment 1 and 3) were killed by rapid decapitation and the brains were collected to determine the probe placement. Brains were dissected, immersed in a 20% sucrose in 10% formalin solution for 2–3 days until freezing and sectioning. Parasagittal sections of 45um, performed on a cryostat, were thaw-mounted onto gelatin-subbed slides (Fisher superfrost) and were stored at 4 °C. Sections were dehydrated, stained with thionin and cover-slipped before viewing under a light microscope (Zeiss) to identify probe placement. Brain histology revealed that most probes were located inside NCM (Figure 3A). More specifically, a few animals were found with a probe located outside of NCM in experiment 1 (E2 measurement during playback) while all further experiments had probes confirmed within NCM. Thus for experiment 1 with E2 measures only, we divided the set of data so that animals with a probe located outside of NCM were analyzed as an independent group.
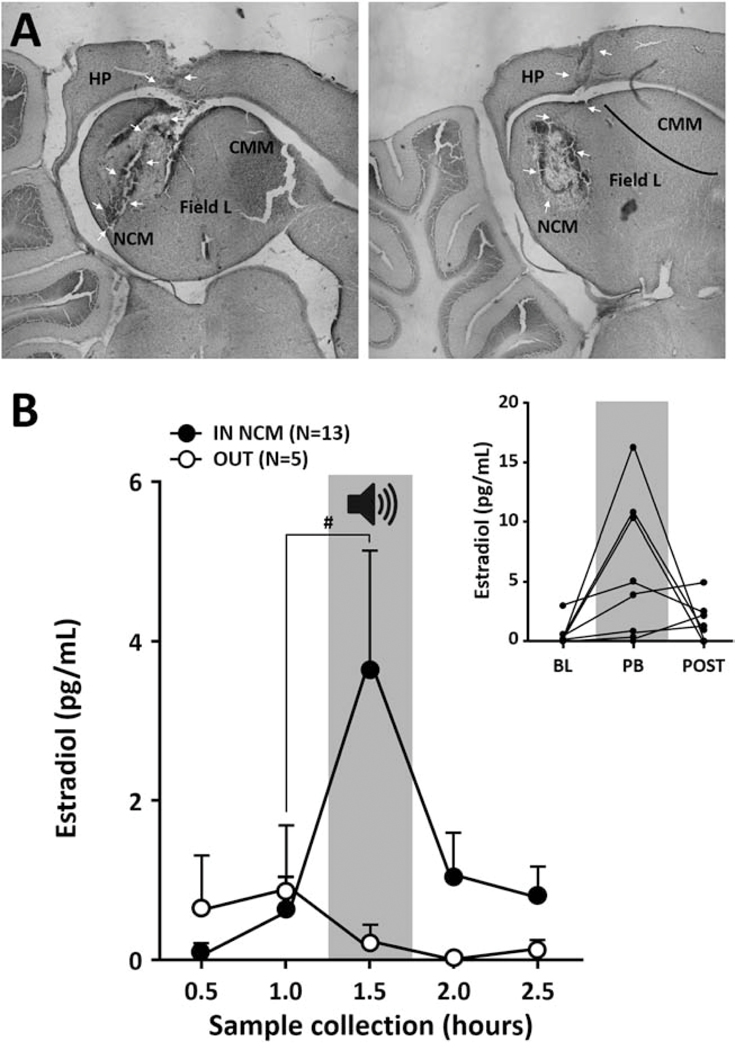
Figure 3:
Forebrain NCM E2 fluctuations during song exposure. (A) Photomicrographs showing microdialysis probes located inside NCM (medial NCM for the left picture, lateral NCM for the right picture), with the tip of the probe targeting the caudal part of NCM enriched with aromatase. (B) Acoustic playback (PB; gray area; 30 minutes duration centered on 1.5 hour) induces a significant increase in E2 levels within NCM. Average E2 level measured in samples collected every 30 minutes in females with a probe inside NCM (IN NCM) or outside of the region (OUT) under silent conditions (no color) or during playback (gray area) (Mean ± SEM). (Inset) Individual data of E2 level for animals with a probe positioned inside NCM during baseline (BL), playback (PB) and after the end of the stimulus (POST). # p = 0.055.
Steroid measurements
E2 levels were measured in dialysates as previously validated (Remage-Healey et al., 2012, 2008) using commercially available enzyme immunoassays kits (Cayman Chemical; Antibodies, mouse monoclonal anti-rabbit IgG and specific antiserum to Estradiol; Sensitivity 2.6 pg/ml). Briefly, 50uL of dialysate was directly pipetted on the 96-well plate and antiserum and E2 tracer were added to the wells. After one-hour of incubation, wells were washed, and the amount of linked conjugated E2 was measured via the colorimetric reaction induced by Ellman’s Reagent provided by the kit. Absorbance of dialysate E2 concentration were compared to the standard curve made with aCSF spiked with known increasing concentrations of E2. Dialysate concentrations were largely distributed throughout the linear portion of the ELISA standard curve. When samples showed undetectable E2 level, their value was fixed to zero. A background value from unmanipulated aCSF was automatically subtracted from sample’s values since the standard curve was prepared in aCSF.
At first, the goal of this study was to measure both E2 and testosterone in every sample using a serial assay (see next paragraph). However, when looking at individual data in experiment 1 (Figure 3B inset), we realized that only 3 animals out of the 13 with a probe correctly located, showed the expected increase (> 5 pg/ml) during playback. Others showed a minor increase or had low/undetectable E2 levels compared to the work previously published in our lab (Remage-Healey et al., 2012). These observations together with other troubleshooting experiments led to the conclusion that either the kit used for these measurements has changed (new antibody, etc) or the probes and the technique to collect the dialysates became inefficient to detect estrogens. Given the fact that another steroid, testosterone, was detected in higher amount in the same samples (see figure 4), the latter hypothesis is unlikely. For the remainder of the study, therefore we concentrated on testosterone only since it was reliably detectable using these methods.
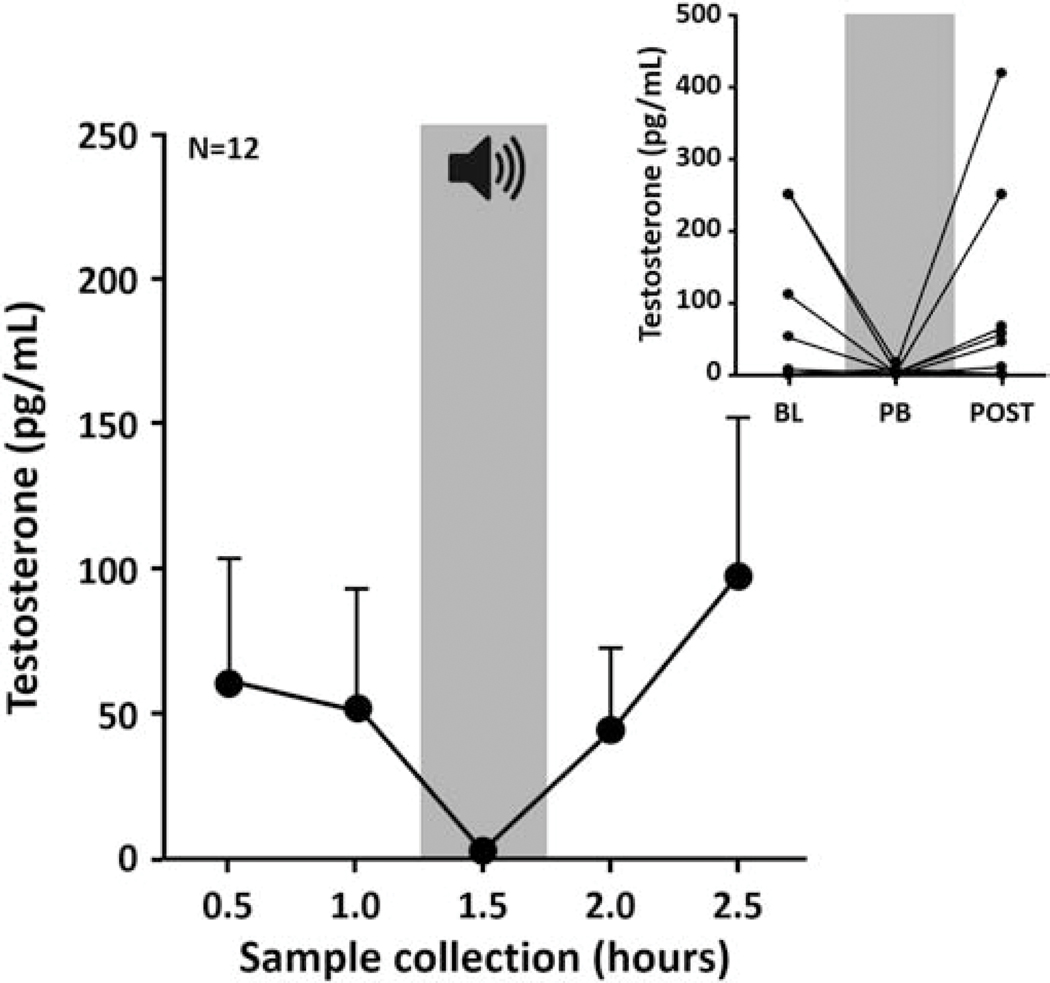
Figure 4:
NCM testosterone fluctuations during song exposure. Acoustic playback (PB; gray area; 30 minutes duration centered on 1.5 hour) induces no significant changes in testosterone levels within NCM. Average testosterone level measured in samples collected every 30 minutes in females NCM under silent conditions (no color) or during playback (gray area) (Mean ± SEM). (Inset) Individual data of testosterone levels in NCM during baseline (BL), playback (PB) and after the end of the stimulus (POST).
A serial assay design providing quantification of both E2 and testosterone from the same samples was processed on a subset of samples (see the specific protocols section). In this case, the mix containing dialysates, antiserum and E2 tracer was collected from the wells before the washing step in the ELISA for E2 and were frozen at −20 °C until testosterone assay.
Testosterone was measured in sera and brain dialysates as previously described (Remage-Healey et al., 2008). Blood sera were extracted for steroids (Newman et al., 2008) using a solidphase extraction. Diluted samples were eluted through C18 columns under vacuum pressure and washed with ddH2O to elute hydrophilic polar compounds. Hydrophobic compounds, including steroids, were then eluted using 100% methanol washes followed by evaporation under air in a 50 °C water bath. Dried samples were suspended in EIA buffer and testosterone content was assayed using a commercial ELISA kit (Cayman Chemical; Antibodies, mouse monoclonal anti-rabbit IgG and specific antiserum to Testosterone; Sensitivity 1.95 pg/ml). Dialysates were also measured for testosterone using the same kits. In samples where only testosterone was assayed, 50uL of dialysate was directly pipetted on the 96-well plate and antiserum and testosterone tracer were added to the wells. After a two-hour incubation, wells were washed, and the amount of linked conjugated testosterone was measured via the colorimetric reaction induced by Ellman’s Reagent provided by the kit. Dialysate testosterone concentrations were compared to the standard curve made with aCSF spiked with known increasing amount of testosterone. Each plate of testosterone ELISA contained a sample with fadrozole and/or fadrozole plus trilostane perfusion fluid in order to assess the total interference of the drug. The drug interference was removed from sample’s raw data as explained in figure 2D–E. In samples where both E2 and testosterone were assayed, 50 uL of the mix containing dialysate coming from the E2 plate was directly pipetted on the 96-well plate. For these samples, testosterone concentrations were extrapolated from a specific standard curve that was made by adding known increasing amount of testosterone in the mix containing aCSF from the E2 assay. Again, a background value from unmanipulated aCSF was automatically subtracted from sample’s values since the standard curve was prepared in aCSF or in mix containing aCSF from the E2 assay. For each plate, the intra-assay coefficient of variation was always less than 5%.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.01. Standard curves were analyzed using a linear regression model assessing whether slopes were statistically different. Non-parametrical statistics were used to analyze microdialysis data since they were not normally distributed. Friedman tests were used to determine the fluctuation of E2 and testosterone over time. Independent Friedman tests were used for every separate group of subjects (i.e. left hemisphere, right hemisphere or inside NCM, outside NCM). When statistically significant changes over time were detected, the playback effect was assessed using the Dunn’s multiple comparisons test or the Wilcoxon Matched-pairs Signed-ranks test to directly compare the value of the sample collected during playback with the previous one. Plasma testosterone content was analyzed using a two-way ANOVA with the type of stimulus (playback or silence) as an independent factor and the sequence (playback first or silence first) as a repeated measure factor. Finally, behavioral data were analyzed using non-parametrical statistics given the low number of animals. Mann-Whitney U tests were used to compare behavior frequency between two experiments while Kruskal-Wallis tests were used when the comparison involved more than two groups. Independent Wilcoxon Matched-pairs Signed-ranks tests with Bonferroni correction were used to identify possible changes in behavior. When significant differences were observed, effect sizes were reported as eta squared for Wilcoxon tests.
Results
Validations of the testosterone assay on NCM dialysates
Testosterone was measured in NCM dialysates per previously published protocols (Remage-Healey et al., 2008). Here we used sequential assays of both steroids in the same samples and drugs that can interfere with these measurements, five validation steps were thus necessary.
First, the fidelity of both the testosterone-only and the sequential assays were compared. Increasing concentrations of testosterone were added to control aCSF or to the mix that came from the previous E2 assay. This mix contained aCSF, as well as tracer and antiserum that was used for E2 measurement. These increasing testosterone concentrations were then assayed via a testosterone ELISA. Results showed that the different concentrations of testosterone were accurately detected regardless of the solution in which they were prepared. Indeed, following linear regression model, standard curves from aCSF or from the mix were statistically indistinguishable (Figure 2A; F(1,45) = 1.79, p = 0.187), confirming that both techniques (testosterone-only or sequential assays) were sufficient to measure testosterone.
Second, we assessed whether our assay was sensitive enough to detect testosterone fluctuations in the brain. NCM dialysates (aCSF perfusion through the probe) were collected before and after an intramuscular injection of testosterone (100ul of a 2.75mM solution made in sesame oil; n = 4 females). Results showed significant changes in local NCM testosterone over time (Figure 2B; Friedman X2n=4 = 26.82, p = 0.0004). More specifically, a substantial increase in NCM testosterone levels occurred immediately after the injection compared to baseline (p = 0.001, Dunn’s multiple comparison test). Testosterone levels were still high one hour after the injection (p = 0.011) before gradually returning to basal levels (p > 0.099).
Third, we quantified assay interference induced by retrodialyzed drugs and tested whether natural testosterone fluctuations were still detectable despite the assay interference of steroidogenic enzyme inhibitors. Increasing concentrations of testosterone were thus added to either control aCSF (n = 4 replicates), fadrozole (100uM, n = 4 replicates) or to both fadrozole and trilostane (100uM and 15nM respectively, n = 4 replicates), and each concentration was then assayed. Results from the ELISA showed that the different concentrations of testosterone were clearly detectable regardless of the presence of the drugs. Indeed, a linear regression model confirmed that the three standard curves were statistically indistinguishable (Figure 2C; F(2,40) = 1.77, p = 0.184).
Fourth, in order to accurately quantified the drug interference, probes immersed in aCSF bath were perfused sequentially with regular aCSF for 60 minutes, with drugs (fadrozole 100uM, n = 3 probes or a combination of fadrozole 100uM and trilostane 15nM, n = 9 probes) for 150 minutes, then with regular aCSF again for 180 minutes. Samples were collected every 30 minutes and testosterone content was measured using ELISA. Specific assay interference for fadrozole-only or the combination of trilostane and fadrozole are shown in figure 2D. Once quantified, the interference of both drugs was converted into a time-dependent percentage of total interference. This percentage was then used to account for assay interference for every subject. Specifically, before perfusion, an aliquot of the drug used for each animal was saved and stored in the freezer with other samples. On the day of the ELISA, the total assay interference of the drugs was quantified for every subject. Data from each animal were then corrected using its specific total interference and the percentage of the established interference for that drug. Final data for each animal were expressed as the subtraction of the over-time interference from the raw testosterone data (Figure 2E). Together, these experiments validated the detection and quantification of testosterone in dialysates.
Finally, the effect of fadrozole infusion on NCM testosterone levels was assessed. Nine animals were placed in the microdialysis set up and samples were collected during a baseline period (BL; 1 × 30 min), during fadrozole infusion (FAD; 1 × 30 min) and during a post-treatment period (POST1 to 6; 6 × 30 min). Importantly, these animals did not receive any song stimulation during the whole collection period. Testosterone was assayed in these dialysates and revealed that fadrozole infusion alone within NCM did not induce fluctuations in local testosterone levels (Friedman X2n=9 = 2.81, p = 0.90; MEAN ± SEM, BL 30.0 pg/ml ± 15.5, FAD 33.8 pg/ml ± 26.9, POST1 31.0 pg/ml ± 13.5, POST2 31.3 pg/ml ± 12.0, POST3 4.1 pg/ml ± 1.3, POST4 20.1 pg/ml ± 10.0, POST5 8.1 pg/ml ± 3.8, POST6 47.2 pg/ml ± 24.1).
Experiment 1 – NCM E2 and testosterone levels fluctuate during acoustic playback
We first replicated a prior result that local E2 levels are rapidly elevated in the NCM of females in response to a potent sensory stimulus, male song (Remage-Healey et al., 2012). This was observed in animals with a probe within NCM (Figure 3; IN Friedman X2n=13 = 11.23, p = 0.024; E2 levels comparing baseline to playback p = 0.055 Wilcoxon, n2 = 5.07) but not in animals with a probe positioned outside NCM (Figure 3 OUT; Friedman X2n=5 = 2.09, p = 0.718). However, once split, the time course analyses for each hemisphere became non-significant (Right, Friedman X2n=6 = 5.32, p = 0.256; Left, Friedman X2n=7 = 6.62, p = 0.158), probably due to low statistical power and detectability issues (see methods).
A parallel experiment revealed that testosterone levels in NCM did not change in response to song playback, unlike E2. Despite a qualitative decrease in almost every subject during playback compared to baseline (Figure 4 inset), we did not detect statistically significant changes over time (Figure 4; Friedman X2n=12 = 4.56, p = 0.336). These findings were consistent across both hemispheres (Right, Friedman X2n=4 = 1.63, p = 0.85; Left, Friedman X2n=8 = 4.9, p = 0.298). Interestingly, all animals implanted in the right NCM showed low basal levels of testosterone (average level of 1.85 pg/ml) as compared to animals with a probe in the left NCM (average level of 83.95 pg/ml) although this difference did not reach statistical significance (MannWhitney Un=4,n=8 = 6.5; p = 0.121, n2 = 0.22).
Experiment 2 – Playback and peripheral testosterone levels
The rapid elevations in local E2 levels in NCM could in principle originate from a rise the precursor testosterone from peripheral endocrine glands such as the gonads and adrenals (Soma et al., 2015) and be locally converted by NCM aromatase. In experiment 3, blood testosterone levels were assessed in a separate set of females exposed to either song or silence for 30 minutes. Results showed no significant effect of the stimulus on plasma T levels (Table 1; paired t10 = 0.46, p = 0.657).
Table 1:
Testosterone concentrations in plasma collected from the brachial vein (Mean±SEM)
| Testosterone (pg/ml) | ||
|---|---|---|
| Silence | Playback exposed-females | |
| Subgroup 1 (silence first) | 19.25 ± 7.06 (6) | 17.89 ± 7.17 (6) |
| Subgroup 2 (playback first) | 18.27 ± 6.89 (5) | 15.22 ± 6.59 (5) |
| AVERAGE | 18.80 ± 4.68 (11) | 16.68 ± 4.72 (11) |
Note: Sample sizes are in parentheses. No significant differences.
Because we counterbalanced the stimulus presentation order by day, we examined the effect of the sequence of stimuli, but did not observe an effect on testosterone levels (Sequence F(1,9) = 0.04, p = 0.838; Stimulus F(1,9) = 0.2, p = 0.664; Interaction F(1,9) = 0.29, p = 0.867). Levels of testosterone found in female plasma were low in accordance with previously published data (Kabelik et al., 2011). Therefore, these findings are consistent with a neural origin of testosterone detected in NCM in our microdialysis experiments.
Experiment 3 – Playback induces NCM testosterone changes during neurosteroid synthesis blockade
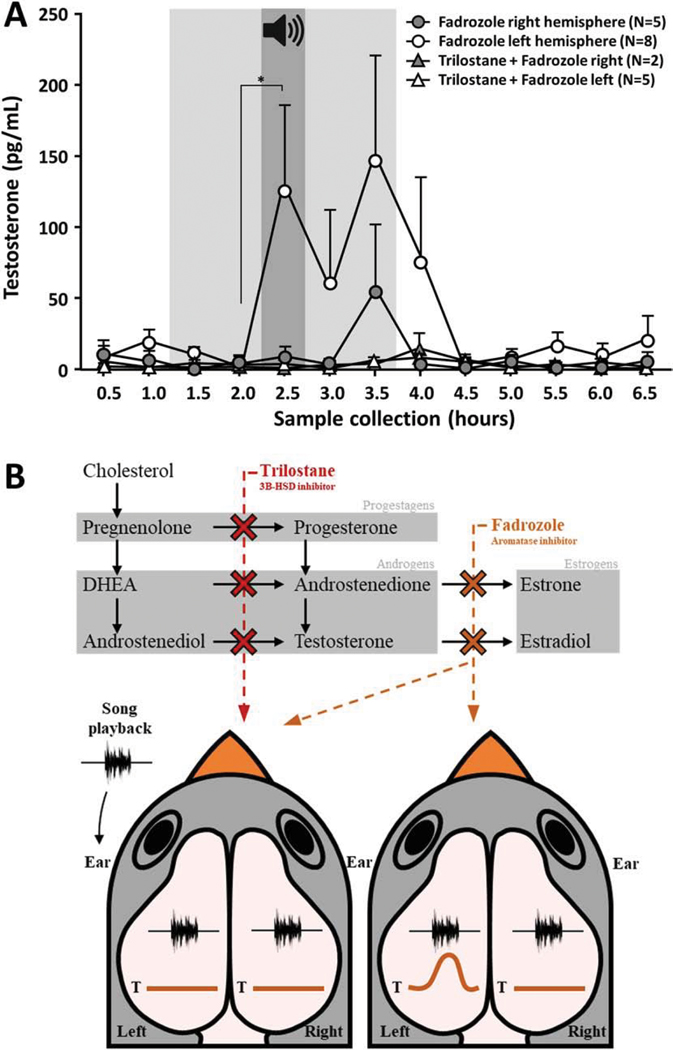
Since a rapid elevation of testosterone in response to song did not occur nor in NCM nor in the periphery, we reasoned that rapid increases in local E2 in NCM were either due to rapid changes in aromatase kinetics or the acute local synthesis of testosterone. This latter mechanism was directly tested in experiment 3 in a new set of animals exposed to song playback as in experiment 1. In this case, either neuro-E2 or neuro-testosterone synthesis was blocked pharmacologically by retrodialysis of the selective synthesis inhibitors fadrozole and trilostane, respectively (Potts et al., 1978; Soma et al., 2004; Wade et al., 1994). When E2 synthesis was locally inhibited, we observed rapid increases in local testosterone levels that were markedly different in the left vs. right NCM. Specifically, testosterone levels substantially increased in the left NCM (Friedman X2n=8 = 10.86, p = 0.028) while there was no significant change in the right NCM (Figure 5A; Friedman X2n=5 = 3.81, p = 0.432). This substantial (~ 770%) increase in local testosterone occurred specifically at the moment of the playback in the left NCM but not the right NCM (p = 0.031 Wilcoxon, n2 = 3.23). Testosterone levels also tended to be higher than baseline 60 minutes after the end of the stimulus, also in the left hemisphere (p = 0.078 Wilcoxon, n2 = 4.72).
Figure 5:
NCM song-evoked testosterone fluctuations during steroidogenesis blockade. (A) When E2 and/or testosterone syntheses are pharmacologically inhibited (light gray area, 2.5 hours duration, from 1.5 to 3.5 hours), acoustic playback (dark gray area; 30 minutes duration centered on 2.5 hours) induces differential changes in testosterone levels within NCM. Testosterone (Mean ± SEM) rises during playback only in the left NCM and only when E2 synthesis alone is blocked, whereas no such change occurs in the right hemisphere nor when the upstream testosterone synthesis is blocked in either hemisphere. * p < 0.05. (B) Schematic view of trilostane and fadrozole actions on the steroidogenesis pathway and their effects on left vs. right NCM testosterone levels during song playback.
If testosterone is locally synthesized in NCM, but only detectable when rapid conversion to estrogens via aromatase is blocked, we reasoned that combined blockade of local androgen and estrogen synthesis within NCM would directly test our hypothesis. Consistent with this hypothesis, in a separate set of animals, when local synthesis of both neuro-E2 and testosterone were blocked specifically in the left NCM, no significant changes in local testosterone levels were observed in response to playback (Figure 5A; Friedman X2n=5 = 7.6, p = 0.47). The same experimental design was also run in two animals implanted in the right hemisphere as a negative control since no rise was observed in T in this hemisphere during FAD infusion and song playback. A schematic representation of these findings is presented in Figure 5B.
Behavioral data
We analyzed several behaviors displayed by females in order to ensure that the microdialysis set up (tether, attached tubing, etc.) did not interfere with the expression of common speciestypical behaviors in the laboratory. Behaviors displayed under silent conditions (baseline period) was compared to behaviors in animals receiving aCSF infusion in the microdialysis chamber (tethered females from experiment 1) and animals put in the same test chamber without being tethered (untethered females from experiment 2). The same behavioral analysis was also performed in animals placed in the microdialysis set up and exposed to either aCSF (control females, from experiment 1) fadrozole or both fadrozole and trilostane (females from experiment 3) in order to test whether steroidogenic enzyme inhibitors alone caused overt behavioral changes. Locomotor activity as measure by the total activity of the female (see methods) were not affected by the use of a tether (Untethered females vs. tethered females Mann-Whitney UN1=10, N2=5 = 18.00, p = 0.422) or by drug treatment (Kruskal-Wallis H = 1.85, p = 0.413).
Negative behaviors recorded during playback (considered representative of attention Chen et al., 2016) did not differ between tethered females compared to untethered females (Mann-Whitney UN1=10, N2=5 = 18.00, p = 0.401) or between treatment groups (Kruskal-Wallis H = 0.54, p = 0.775). Therefore, the use of a tether did not interfere with the display of typical female behavior and the infusion of steroidogenic enzyme inhibitors did not alter overt behaviors like locomotor activity.
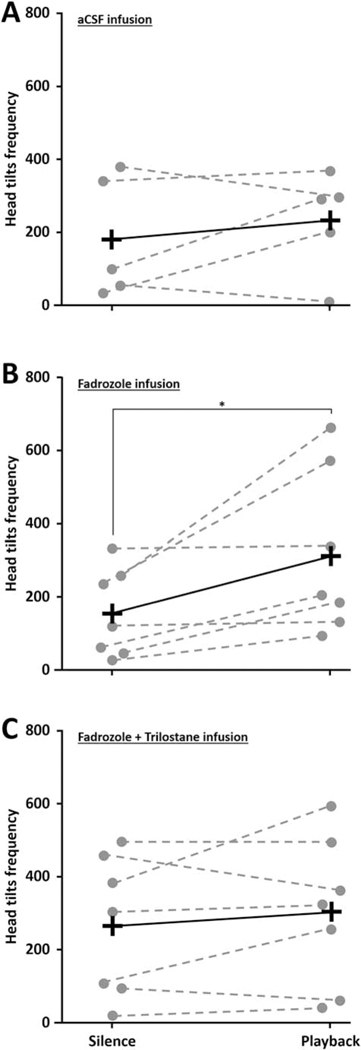
Interestingly, among all the behaviors recorded, head tilts (an indication of stimulus attending) increased during playback only when estrogen synthesis was blocked (WN=7 = 28, p = 0.016, n2 = 3.20; Figure 6). This behavior was not increased during playback when testosterone synthesis was inhibited (WN=7 = 8, p = 0.563) or when animals were infused with control aCSF (WN=5 = 28, p = 0.625). Thus, head tilts increased during playback concurrent with the increased production of testosterone observed in left NCM. A tendency in the same direction was observed for total activity, with an increased activity in response to song during fadrozole infusion (WN=7 = 22, p = 0.078) while no song-induced changes were observed during aCSF (WN=5 = 3, p = 0.813) or both fadrozole and trilostane infusions (WN=7 = 4, p = 0.813). Negative behaviors did not change in response to song playback during any treatment (WN=5 = 4, p = 0.625 for aCSF; WN=7 = 1, p = 0.999 for fadrozole; WN=6 = 0, p = 0.999 for both trilostane and fadrozole retrodialyses). Together these findings suggest that testosterone in NCM could rapidly affect song-induced female behaviors, although this possibility remains to be directly tested.
Figure 6:
Female behavior during song exposure and steroidogenesis blockade. The frequency of head tilts increased during playback compared to silence conditions in all animals that received fadrozole infusion (B) while it was not the case for animals that received trilostane in combination with fadrozole (C) or control aCSF (A). Each dot represents individual data. Crosses represent group averages. * p < 0.05.
Discussion
To our knowledge, this study provides the first evidence that testosterone is locally synthesized in the brain, in vivo. Our findings also clarify rapid neuroestrogen fluctuations in the forebrain. Neuroestrogens can fluctuate in several species, including within the hippocampus and hypothalamus (Kenealy et al., 2013; Sato and Woolley, 2016). Our findings replicated a song induced-elevation in local neuroestrogen levels that was previously observed in the NCM of male (Remage-Healey et al., 2008) and female (Remage-Healey et al., 2012) zebra finches. We further demonstrate that this rise in local E2 occurs specifically in NCM and that probes positioned outside the nucleus did not report such changes (experiment 1). Next, we find that acute song-driven neuroestrogen increases are mediated by an increase in the androgenic substrate, testosterone, in the left hemisphere NCM. Implications about the origin, function, timing, and lateralization of testosterone fluctuations within the brain itself are discussed below.
Synthesis of testosterone in the NCM of females during song
Unlike E2, local testosterone levels did not increase within NCM of females when they were hearing male song. Instead, NCM testosterone was elevated during playback during local E2 synthesis blockade. Interestingly, peripheral testosterone did not fluctuate in response to playback, indicating that acute changes occur within the brain itself. Furthermore, testosterone elevations were completely abolished when the neural enzymatic production of testosterone was also blocked. Androgen synthesis in the brain has been suggested in other systems, especially in the avian brain where all the enzymes necessary are present and active (Tsutsui, 2011). Local androgen production has been evident in brain explant studies (Mensah-Nyagan et al., 1996; Mukai et al., 2006; Pradhan et al., 2010; Soma et al., 2004; Tam and Schlinger, 2007). We build on these findings to directly show that testosterone synthesis occurs rapidly in the female brain in response to socially-relevant stimuli.
Our evidence for local brain testosterone production raises the question of its functional significance. Brain aromatization is required for the acute regulation of many responses and behaviors, including male sexual behavior, nociception, neuroprotection, memory and cognition (Balthazart et al., 2018; Garcia-Segura, 2008). Studies in birds provided important discoveries in the rapid regulation of aromatase kinetics related to such responses (Cornil and de Bournonville, 2017; Saldanha et al., 2011). Importantly, rapid changes in aromatase activity could mediate the song-induced elevation of estrogens observed in males, as it has been shown that the enzymatic activity is elevated in the NCM of singers compared to non-singers (Remage-Healey et al., 2009). The current study highlights the key-role of local aromatization in auditory processing in females since the data show increased testosterone levels only when the local aromatase is blocked.
Besides being the substrate to neuroestrogens, this study also raises the possibility of direct effects of androgens in the female brain. This question is somewhat unexpected, since testosterone has been traditionally viewed as a male-typical hormone. Neuromodulators, including neuroestrogens, are synthesized within the brain to rapidly control local neural circuits and behavior (Remage-Healey, 2014; Saldanha et al., 2011). Testosterone has been suggested to control important functions such as female sexual desire in humans (Alexander et al., 2006; Davison and Davis, 2011, 2003; Pluchino et al., 2013) and women often report an increased sexual desire when they are treated with testosterone (Arlt et al., 1999; Tuiten et al., 2000; Van Goozen et al., 1997) despite concurrent aromatase inhibition (Dahir and Travers-Gustafson, 2014). Cognitive performance has also been linked to androgens in women (Davison and Davis, 2003; Pluchino et al., 2015), and testosterone can affect attention, emotion processing and memory within a few hours of treatment (Aleman et al., 2004; Bos et al., 2016; Postma et al., 2000), consistent with a direct neural site-of-action. In nonhuman animals testosterone can regulate neural circuits and behaviors within short time-scales, including anxiety-like behavior (Aikey et al., 2002), social vocalizations (Fernández-Vargas, 2017; Remage-Healey and Bass, 2004), lordosis behavior (Gladue et al., 1978) and aggression (Ardia et al., 2010). In this study, the changes in head movements in the group of females experiencing a significant peak of testosterone in NCM suggests similarly rapid effects of neuroandrogens. However, the lack of difference between the control aCSF and the fadrozole infused animals coupled to the low number of animals in which these behavioral measures were recorded significantly constrain these conclusions. Still, these findings provide a basis for further in-depth investigations.
The hypothesis that neuroandrogens could act on behavior directly, independent of aromatization, is bolstered by dense expression of androgen receptors in songbird NCM (Fusani et al., 2000; Metzdorf et al., 1999; Tomaszycki and Dzubur, 2013). However, the direct and local action of testosterone within NCM on song processing and related behaviors still need to be tested. Testosterone could rise in NCM then act somewhere else in the brain or even in the periphery to affect behavior. This study presents evidence for brain testosterone synthesis but its local action (either by androgen receptor activation or by its conversion and eventual estrogen receptor activation) still need to be determined by local infusion of androgen/estrogen receptor antagonists and/or by measures of blood testosterone levels under trilostane and/or fadrozole retrodialyses.
There is considerable evidence supporting rapid, non-genomic actions mediated by membraneassociated androgen receptors in many species (Bennett et al., 2010; Foradori et al., 2008; Li et al., 2018; Revelli et al., 1998; Shihan et al., 2015, 2014; Vicencio et al., 2011; Walker, 2010). Whether androgenic effects take place through direct action of testosterone on androgen receptors or via its active metabolites such as 5α-dihydrotestosterone is also of great interest. In any case, the current evidence that testosterone can fluctuate in a local and dynamic manner is consistent with the emerging view that androgens are neuromodulators, similar to recent considerations of neuroestrogens.
The current findings suggest that neuro-testosterone locally synthesized within NCM could regulate song processing and sensorimotor integration, perhaps influencing downstream vocal production (Remage-Healey and Joshi, 2012). Rapid actions of androgens in sensory areas have received little attention to date but a few studies seem to support it. Indeed, the androgen receptor antagonist flutamide showed antinociceptive effects within 30 min in rats (Nayebi and Rezazadeh, 2004). Another study in rats showed that inhibiting androgen synthesis influences the acoustic startle reflex within minutes after drugs infusions, although these effects seem to occur independently of AR activation (Frau et al., 2014). In human and birds, testosterone appears to be able to modify acoustic parameters of voice/songs such as lowering fundamental frequency (Abitbol et al., 1999; Beani et al., 1995; Cynx et al., 2005), and rapid actions of androgens have been identified on important features of vocalizations in several vertebrates (Fernández-Vargas, 2017; Pultorak et al., 2015; Remage-Healey and Bass, 2004; Yazaki et al., 1998). These effects likely take place in motor regions controlling vocalizations but could also be a consequence of steroid actions on sensory integration regions, although this last hypothesis has never been studied directly. Thus, androgens and/or their estrogen metabolites rapidly modulate several key aspects of social communication. Now that neuroandrogen fluctuations have been established, the dynamics of concurrent fluctuating neuro-androgens and -estrogens in the context of social communication becomes of increasing interest.
Time course of neurotestosterone fluctuations
Our findings reveal that one mechanism governing acute increases of neuroestrogen in response to song is via an increase in the substrate, testosterone. This acute elevation likely provides substrate for rapid conversion to estrogens by the enzyme aromatase. In our experiment, the magnitude of T changes is larger than the magnitude of E2 changes. This discrepancy could be due to sensitivity differences in the two assays employed. Additionally, although 17-beta-estradiol has been shown to regulate auditory processing in zebra finch NCM (Remage-Healey et al., 2010; Remage-Healey and Joshi, 2012; Tremere et al., 2009; Tremere and Pinaud, 2011), other estrogens (estrone, estriol) could also contribute to rapid regulation of auditory functions and could themselves be dependent on the increased song-induced testosterone rise we observe here in NCM. The activity of the enzyme aromatase has been shown to be rapidly regulated within timeframes of 2 to 30 minutes in behavioral contexts such as sexual interactions (Cornil et al., 2005; de Bournonville et al., 2017, 2013a) stress (Dickens et al., 2014, 2011), and aggressive interactions (Schlinger and Callard, 1989). Interestingly, forebrain synaptic aromatase activity is elevated in singing male zebra finches compared to non-singers (Remage-Healey et al., 2009). Thus it is likely that, within the 30 minutes of playback, a rapid change in aromatase activity occurs in parallel with a change in the androgenic substrate, leading to acute neuroestrogen elevations in female NCM.
Interestingly, when E2 synthesis was blocked, testosterone levels tended to remain elevated for the next 60 minutes after the playback (experiment 3) while E2 returned to baseline (experiment 1 and (Remage-Healey et al., 2012)). The extended elevation of testosterone compared to E2 suggests that neuroandrogens could be involved in neural and behavioral outcomes aside from the conversion into E2, including a direct role for testosterone on auditory processing and encoding. However, it could also be explained by a direct effect of fadrozole on local testosterone levels. Indeed, in male zebra finches, 30 minutes of fadrozole infusion was enough to induce a rise in NCM testosterone. On the contrary, our experiment included two time points with fadrozole infusion preceding song playback and these values suggest that fadrozole infusion alone does not directly affect local testosterone levels. However, a long-term effect of fadrozole or its accumulation provide an alternative explanation of this later peak in testosterone and thus cannot be excluded.
It is also likely that several steps along the steroidogenesis pathway are involved in local testosterone elevations observed here. The central nervous system of vertebrates has the capacity to synthesize neurosteroids de novo from cholesterol (Tsutsui, 2011) and stress studies have identified extremely rapid changes in testosterone and upstream precursors in the periphery (Dawson and Howe, 1983; Romero and Reed, 2005; Wingfield and Smith, 1982) and brain (Minni et al., 2012). Sensory stimuli could cause rapid synthesis of multiple precursors upstream of testosterone (pregnenolone, DHEA) for direct action and/or conversion (Soma et al., 2015). Similarly, the drug treatments used here are likely to influence local levels of these other neurosteroid precursors. Direct measurement and manipulation of these precursor molecules alongside quantification of the behavioral consequences are now necessary in order to fully understand how the neurosteroidogenic pathway is activated in response to social cues.
Lateralization of NCM testosterone synthesis
We observed song-induced testosterone synthesis only in the left hemisphere NCM. This finding adds further evidence for lateralization of the auditory forebrain of songbirds and other vocal learning species. The left hemisphere Wernicke’s area of humans is dominant for language processing (Doupe and Kuhl, 1999; Price, 2012) and is considered a functional analog of the songbird NCM (Bolhuis et al., 2010; Jarvis, 2004). The temporal cortex of humans is also enriched for local neurosteroid capacity, including abundant aromatase expression (Azcoitia et al., 2011; Yague et al., 2006). In the songbird brain, evidence for lateralization of auditory function has accumulated from studies of immediate early gene expression (Avey et al., 2005; Chirathivat et al., 2015; Lampen et al., 2017; Moorman et al., 2015, 2012; Olson et al., 2016), electrophysiological recordings (Bell et al., 2015), epigenetic markers (Phan et al., 2017), retrodialysis and behavior (Remage-Healey et al., 2010), and neurogenesis (Tsoi et al., 2014). Concurrent with our findings, a recent FMRI study showed that auditory-evoked activation was blunted in the NCM of male European starlings when E2 synthesis was blocked, and only in the left hemisphere (De Groof et al., 2017). The modulation of auditory processing by steroids is therefore lateralized and the production of testosterone within the left NCM of females in particular could be important for auditory coding and sensorimotor integration.
Several mechanisms could mediate this lateralized steroid production in NCM, including lateralization of enzymes for steroid synthesis. However, somal aromatase is expressed and active at comparable levels in both hemispheres (De Groof et al., 2017; Ikeda et al., 2017; Saldanha et al., 2000). But since aromatase contained in the synaptic terminals rather than in the soma seem to be involved in song processing at least in male zebra finches (Remage-Healey et al., 2009), a study of the lateral distribution of synaptic aromatase would be of great interest. To our knowledge, hemispheric differences in the expression of testosteronesynthesizing enzymes have not been reported (London et al., 2006, 2003). Rapid and contextspecific changes in enzymatic activity have been reported for both aromatase (Cornil et al., 2005; de Bournonville et al., 2017, 2013a, 2013b, Dickens et al., 2014, 2011) and 3β-HSD (Pradhan et al., 2010; Soma et al., 2004), and further clarification about the lateralization of these phenomena is warranted. Other neurotransmitter systems are also known to interact with steroid production and action. Interestingly, the left NCM contains more fibers for tyrosine hydroxylase and dopamine beta hydroxylase fibers (Matragrano et al., 2011). Although norepinephrine does not appear to regulate song-induced neuroestrogen fluctuations (Ikeda et al., 2015), catecholaminergic regulation of androgen synthesis remains to be studied.
Conclusions
Our findings demonstrate that testosterone is actively synthetized in the female brain and suggest that neuroandrogens might thus regulate brain function and behavior. These data focus attention on reproduction and cognition for neuroandrogen actions (Davison and Davis, 2003; Pluchino et al., 2015). It is also now important to evaluate the translational implications of androgen synthesis in the female brain, since steroidal drugs are used by women for sexual desire disorder and anti-cancer treatment, and future neurotherapeutics could incorporate this ‘neuroandrogen’ perspective to help mitigate side effects such as loss of libido or cognitive dysfunction (Ramdhan et al., 2018; Sabatini et al., 2011; Warren et al., 2014).
Highlights (85 characters each including space).
Estradiol (E2) acutely increases in the female songbird auditory cortex during song
Testosterone (T) rises in response to song when its conversion to E2 is blocked
This rise in T only happens in the left hemisphere and does not happen in the blood
This rise is abolished when the production of T is locally blocked
The female brain thus produces T in response to socially-relevant stimuli
Significance statement.
This study demonstrates that androgen synthesis occurs rapidly in vivo in the brain in response to social cues, in a lateralized manner. Specifically, testosterone synthesis occurs within the left secondary auditory cortex when female zebra finches hear male song. Therefore, testosterone could act as a neuromodulator to rapidly shape sensory processing. Androgens have been linked to functions such as the control of female libido, and many steroidal drugs used for contraception, anti-cancer treatments, and sexual dysfunction likely influence the brain synthesis and action of testosterone. The current findings therefore establish a clear role for androgen synthesis in the female brain with implications for understanding neural circuit function and behavior in animals, including humans.
Acknowledgements
This work was supported by National Institutes of Health - Grant R01NS082179 (to LRH). CdB was supported by a Belgian American Education Foundation (BAEF) fellowship.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abitbol J, Abitbol P, Abitbol B, 1999. Sex hormones and the female voice. J. Voice 13, 424–446. [DOI] [PubMed] [Google Scholar]
- Aikey JL, Nyby JG, Anmuth DM, James PJ, 2002. Testosterone Rapidly Reduces Anxiety in Male House Mice (Mus musculus). Horm. Behav 42, 448–460. doi: 10.1006/hbeh.2002.1838 [DOI] [PubMed] [Google Scholar]
- Aleman A, Bronk E, Kessels RPC, Koppeschaar HPF, Van Honk J, 2004. A single administration of testosterone improves visuospatial ability in young women. Psychoneuroendocrinology 29, 612–617. doi: 10.1016/S0306-4530(03)00089-1 [DOI] [PubMed] [Google Scholar]
- Alexander JL, Dennerstein L, Burger H, Graziottin A, A JL,, D L, B H, G A, 2006. Testosterone and libido in surgically and naturally menopausal women. Women’s Heal. 2, 459–477. doi: 10.2217/17455057.2.3.459 [DOI] [PubMed] [Google Scholar]
- Ardia DR, Broughton DR, Gleicher MJ, 2010. Short-term exposure to testosterone propionate leads to rapid bill color and dominance changes in zebra finches. Horm. Behav 58, 526–532. doi: 10.1016/j.yhbeh.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Arl W., Callie F., van Vlijme J., Koehle I., Reinck M., Bidlingmaie M., Hueble D., Oette M., Erns M., Schult H., Alloli B., 1999. Dehydroepiandrosterone Replacement in Women With Adrenal Insufficiency. N. Engl. J. Med 341, 1013––1020.. [DOI] [PubMed] [Google Scholar]
- Avey MT, Phillmore LS, MacDougall-Shackleton SA, 2005. Immediate early gene expression following exposure to acoustic and visual components of courtship in zebra finches. Behav. Brain Res. 165, 247–253. doi: 10.1016/j.bbr.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, Garcia-Segura LM, 2011. Estradiol synthesis within the human brain. Neuroscience 191, 139–147. doi: 10.1016/j.neuroscience.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J, 2010. Neuroendocrine regulation of reproductive behavior in birds, in: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin (Eds.), Hormones, Brain and Behavior. San Diego, pp. 855–897. doi: 10.1016/B978-008088783-8.00025-5 [DOI] [Google Scholar]
- Balthazart J, Baillien M, Ball GF, 2006. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology 147, 359–366. doi: 10.1210/en.2005-0845 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Choleris E, Remage-Healey L, 2018. Steroids and the brain: 50 years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm. Behav 99, 1–8. doi: 10.1016/j.yhbeh.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Cornil CA, Charlier TD, Taziaux M, Ball GF, 2009. Estradiol, a key endocrine signal in the sexual differentiation and activation of reproductive behavior in Quail. J. Exp. Zool. Part A Ecol. Genet. Physiol 311, 323–345. doi: 10.1002/jez.464 [DOI] [PubMed] [Google Scholar]
- Beani L, Panzica GC, Briganti F, Persichella P, Dessi-Fulgheri F, 1995. Testosteroneinduced changes of the call structure and vocal apparatus in partridges. Physiol. Behav 58, 1149–1157. [DOI] [PubMed] [Google Scholar]
- Bell BA, Phan ML, Vicario DS, 2015. Neural responses in songbird forebrain reflect learning rates, acquired salience, and stimulus novelty after auditory discrimination training. J. Neurophysiol 113, 1480–1492. doi: 10.1152/jn.00611.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet NC., Gardine RA., Hoope JD., Johnso DW., Gob GC., 2010. Molecular cell biology of androgen receptor signalling. Int. J. Biochem. Cell Biol 42, 813––827.. doi: 10.1016/j.biocel.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C, 2010. Twitter evolution: Converging mechanisms in birdsong and human speech. Nat. Rev. Neurosci 11, 747–759. doi: 10.1038/nrn2931 [DOI] [PubMed] [Google Scholar]
- Bos PA, Hofman D, Hermans EJ, Montoya ER, Baron-Cohen S, van Honk J, 2016. Testosterone reduces functional connectivity during the “Reading the Mind in the Eyes” Test. Psychoneuroendocrinology 68, 194–201. doi: 10.1016/j.psyneuen.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne LJ, Gude C, Rodriguez H, Steele RE, Bhatnager A, 1991. Fadrozole hydrochloride: a potent, selective, nonsteroidal inhibitor of aromatase for the treatment of estrogen-dependent disease. J Med Chem 34, 725–736. [DOI] [PubMed] [Google Scholar]
- Chen Y, Matheson LE, Sakata JT, 2016. Mechanisms underlying the social enhancement of vocal learning in songbirds. Proc. Natl. Acad. Sci 113, 6641–6646. 10.107/pnas.1522306113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirathivat N, Raja SC, Gobes SMH, 2015. Hemispheric dominance underlying the neural substrate for learned vocalizations develops with experience. Sci. Rep 5, 11359. doi: 10.1038/srep11359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coirini H, Goue M, Delespierre B, Liere P, Pianos A, Eychenne B, Schumacher M, Guennoun R, 2003. Characterization and regulation of the 3 b -hydroxysteroid dehydrogenase isomerase enzyme in the rat sciatic nerve 119–126. [DOI] [PubMed] [Google Scholar]
- Corni CA., Dall C., Papadopoulou-Daifot Z., Baillie M., Dejac C., Bal GF., Balthazar J., 2005. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology 146, 3809––3820.. doi: 10.1210/en.2005-0441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, de Bournonville C, 2017. Dual action of neuro-estrogens in the regulation of male sexual behavior. Gen. Comp. Endocrinol doi: 10.1016/j.ygcen.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynx J, Bean NJ, Rossman I, 2005. Testosterone implants alter the frequency range of zebra finch songs. Horm. Behav 47, 446–451. doi: 10.1016/j.yhbeh.2004.11.018 [DOI] [PubMed] [Google Scholar]
- Dahir M, Travers-Gustafson D, 2014. Breast cancer, aromatase inhibitor therapy, and sexual functioning: A pilot study of the effects of vaginal testosterone therapy. Sex. Med 2, 8–15. doi: 10.1002/sm2.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison SL, Davis SR, 2011. Androgenic hormones and aging - The link with female sexual function. Horm. Behav 59, 745–753. doi: 10.1016/j.yhbeh.2010.12.013 [DOI] [PubMed] [Google Scholar]
- Davison SL, Davis SR, 2003. Androgens in women. J. Steroid Biochem. Mol. Biol 85, 363–366. doi: 10.1016/S0960-0760(03)00204-8 [DOI] [PubMed] [Google Scholar]
- Dawson A, Howe PD, 1983. Plasma corticosterone in wild starlings (Sturnus vulgaris) immediately following capture and in relation to body weight during the annual cycle. Gen. Comp. Endocrinol 51, 303–308. doi: 10.1016/0016-6480(83)90085-0 [DOI] [PubMed] [Google Scholar]
- de Bournonville C, Ball GF, Balthazart J, Cornil CA, 2017. Rapid changes in brain aromatase activity in the female quail brain following expression of sexual behaviour. J. Neuroendocrinol 29, e12542. doi: 10.1002/mrd.22357 [DOI] [PubMed] [Google Scholar]
- de Bournonville C, Dickens MJ, Ball GF, Balthazart J, Cornil CA, 2013a. Dynamic changes in brain aromatase activity following sexual interactions in males: Where, when and why? Psychoneuroendocrinology 38, 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bournonville C, Dickens MJ, Ball GF, Balthazart J, Cornil CA, 2013b. Dynamic changes in brain aromatase activity following sexual interactions in males: Where, when and why? Psychoneuroendocrinology 38, 789–799. doi: 10.1016/j.psyneuen.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groof G, Balthazart J, Cornil CA, Van der Linden A, 2017. Topography and Lateralized Effect of Acute Aromatase Inhibition on Auditory Processing in a Seasonal Songbird. J. Neurosci 37, 4243–4254. doi: 10.1523/JNEUROSCI.1961-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, Cornil CA, Balthazart J, 2011. Acute stress differentially affects aromatase activity in specific brain nuclei of adult male and female quail. Endocrinology 152, 4242–4251. doi: 10.1210/en.2011-1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, de Bournonville C, Balthazart J, Cornil CA, 2014. Relationships between rapid changes in local aromatase activity and estradiol concentrations in male and female quail brain. Horm. Behav 65, 154–164. doi: 10.1016/j.yhbeh.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK, 1999. BIRDSONG AND HUMAN SPEECH: Common Themes and Mechanisms. Annu. Rev. Neurosci 22, 567–631. doi: 10.1146/annurev.neuro.22.1.567 [DOI] [PubMed] [Google Scholar]
- Fernández-Vargas M, 2017. Rapid effects of estrogens and androgens on temporal and spectral features in ultrasonic vocalizations. Horm. Behav 94, 69–83. doi: 10.1016/j.yhbeh.2017.06.010 [DOI] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ, 2008. Non-genomic actions of androgens. Front. Neuroendocrinol 29, 169–181. doi: 10.1016/j.yfrne.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra R., Bin V., Pe R., Pilloll G., Sab P., Devot P., Bortolat M., 2014. Inhibition of 17α-hydroxylase/C17,20 lyase reduces gating deficits consequent to dopaminergic activation. Psychoneuroendocrinology 39, 204––213.. doi: 10.1016/j.psyneuen.2013.09.014.Inhibition [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusani L, Van’t Hof T, Hutchison J, Gahr M, 2000. Seasonal Expression of Androgen Receptors, Estrogen Receptors, and Aromatase in the Canary Brain in Relation to Circulating Androgens and Estrogens. J Neurobiol 43, 254–258. doi:Doi [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, 2008. Aromatase in the brain: Not just for reproduction anymore. J. Neuroendocrinol 20, 705–712. doi: 10.1111/j.1365-2826.2008.01713.x [DOI] [PubMed] [Google Scholar]
- Gladue BA, Dohanich GP, Clemens LG, 1978. Hormonally Mediated Lordosis in Female Rats : Actions of Flutamide and an Aromatization Inhibitor. Pharmacol. Biochem. Behav 9, 827–832. [DOI] [PubMed] [Google Scholar]
- Howell C, Derryberry RAEP, 2019. Female cognitive performance and mass are correlated with different aspects of mate choice in the zebra finch ( Taeniopygia guttata ). Anim. Cogn doi: 10.1007/s10071-019-01299-6 [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM, 2015. Male Sexual Behavior, in: Knobil and Neill’s Physiology of Reproduction. pp. 2211–2285. doi: 10.1016/B978-0-12-397175-3.00049-1 [DOI] [Google Scholar]
- Ikeda MZ, Jeon SD, Cowell RA, Remage-Healey L, 2015. Norepinephrine Modulates Coding of Complex Vocalizations in the Songbird Auditory Cortex Independent of Local Neuroestrogen Synthesis. J. Neurosci 35, 9356–68. doi: 10.1523/JNEUROSCI.4445-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iked MZ., Krentze AA., Olive TJ., Scarp GB., Remage-Heale L., 2017. Clustered organization and region-specific identities of estrogen-producing neurons in the forebrain of Zebra Finches ( Taeniopygia guttata ). J. Comp. Neurol 525, 3636––3652.. doi: 10.1002/cne.24292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, 2004. Learned Birdsong and the Neurobiology of Human Language. Ann N Y Acad Sci 1016, 749–777. doi: 10.1196/annals.1298.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik D, Schrock SE, Ayres LC, Goodson JL, 2011. Estrogenic regulation of dopaminergic neurons in the opportunistically breeding zebra finch. Gen. Comp. Endocrinol 173, 96–104. doi: 10.1016/j.ygcen.2011.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Kapoor A, Guerriero K. a, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E, 2013. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J. Neurosci. 33, 19051–9. doi: 10.1523/JNEUROSCI.3878-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen J, McAuley J, Chang S, Wade J, 2017. ZENK induction in the zebra finch brain by song: Relationship to hemisphere, rhythm, oestradiol and sex. J Neuroendocr. 29, e12543. doi: 10.1111/ijlh.12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Fu X, Cao S, Li J, Xing S, Li D, Dong Y, Cardin D, Park H, Mauvais-Jarvis F, Zhang H, 2018. Membrane-associated androgen receptor (AR) potentiates its transcriptional activities by activating heat shock protein 27 (HSP27). J Biol Chem 293, 12719–12729. doi: 10.1074/jbc.R115.662759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Boulter J, Schlinger BA, 2003. Cloning of the Zebra Finch Androgen Synthetic Enzyme CYP17: A Study of Its Neural Expression throughout Posthatch Development. J. Comp. Neurol 467, 496–508. doi: 10.1002/cne.10936 [DOI] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA, 2006. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology 147, 5975–5987. doi: 10.1210/en.2006-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matragran LL., Sanfor SE., Salvant KG., Sockma KW., Mane DL., 2011. Estradioldependent catecholaminergic innervation of auditory areas in a seasonally breeding songbird 34, 416––425.. doi: 10.1111/j.1460-9568.2011.07751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah-Nyagan A, Do-Rego J, Feuilloley M, Marcual A, Lange C, Pelletier G, Vaudry H, 1996. In vivo and in vitro evidence for the biosynthesis of testosterone in the telencephalon of the female frog. J. Neurochem 67, 413–422. [DOI] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L, 1999. Distribution of aromatase, estrogen receptor, and androgen receptor mrna in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol 407, 115–129. doi: [DOI] [PubMed] [Google Scholar]
- Minni AM, Dorey R, Piérard C, Dominguez G, Helbling JC, Foury A, Berácochéa D, Moisan MP, 2012. Critical role of plasma corticosteroid-binding-globulin during stress to promote glucocorticoid delivery to the brain: Impact on memory retrieval. Endocrinology 153, 4766–4774. doi: 10.1210/en.2012-1485 [DOI] [PubMed] [Google Scholar]
- Moorman S, Gobes SMH, Kuijpers M, Kerkhofs A, Zandbergen MA, Bolhuis JJ, 2012. Human-like brain hemispheric dominance in birdsong learning. Proc. Natl. Acad. Sci 109, 12782–12787. doi: 10.1073/pnas.1207207109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman S, Gobes SMH, Van De Kamp FC, Zandbergen MA, Bolhuis JJ, 2015. Learning-related brain hemispheric dominance in sleeping songbirds. Sci. Rep 5, 1–7. doi: 10.1038/srep09041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muka H., Takat N., Ishi HT., Tanab N., Hoj Y., Furukaw A., Kimot T., Kawat S., 2006. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: Synaptocrinology. Neuroscience 138, 757––764.. doi: 10.1016/j.neuroscience.2005.09.010 [DOI] [PubMed] [Google Scholar]
- Nayebi RAM, Rezazadeh H, 2004. Involvement of serotoninergic mechanism in analgesia by castration and flutamide, a testosterone antagonist, in the rat formalin test 77, 9–14. doi: 10.1016/j.pbb.2003.09.009 [DOI] [PubMed] [Google Scholar]
- Newman AEM, Chin EH, Schmidt KL, Bond L, Wynne-Edwards KE, Soma KK, 2008. Analysis of steroids in songbird plasma and brain by coupling solid phase extraction to radioimmunoassay. Gen. Comp. Endocrinol. 155, 503–510. doi: 10.1016/j.ygcen.2007.08.007 [DOI] [PubMed] [Google Scholar]
- Olson EM, Maeda RK, Gobes SMH, 2016. Mirrored patterns of lateralized neuronal activation reflect old and new memories in the avian auditory cortex. Neuroscience 330, 395–402. doi: 10.1016/j.neuroscience.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Gergues MM, Mahidadia S, Jimenez-Castillo J, Vicario DS, Bieszczad KM, 2017. HDAC3 Inhibitor RGFP966 Modulates Neuronal Memory for Vocal Communication Signals in a Songbird Model. Front. Syst. Neurosci 11, 1–12. doi: 10.3389/fnsys.2017.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino N, Carmignani A, Cubeddu A, Santoro A, Cela V, Alcalà TE, 2013. Androgen therapy in women: For whom and when. Arch. Gynecol. Obstet 288, 731–737. doi: 10.1007/s00404-013-2969-7 [DOI] [PubMed] [Google Scholar]
- Pluchino N, Drakopoulos P, Bianchi-Demicheli F, Wenger JM, Petignat P, Genazzani AR, 2015. Neurobiology of DHEA and effects on sexuality, mood and cognition. J. Steroid Biochem. Mol. Biol 145, 273–280. doi: 10.1016/j.jsbmb.2014.04.012 [DOI] [PubMed] [Google Scholar]
- Postma A, Meyer G, Tuiten A, Honk J van, 2000. Effects of testosterone administration on selective aspects of object-location memory in healthy young women. Psychoneuroendocrinology 25, 563–575. [DOI] [PubMed] [Google Scholar]
- Potts G, Creange J, Harding H, 1978. Trilostane, an orally active inhibitor of steroid biosynthesis. Steroids 32, 257–267. [DOI] [PubMed] [Google Scholar]
- Pradha DS., Newma AEM., Wacke DW., Wingfiel JC., Schlinge BA., Som KK., 2010. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm. Behav 57, 381––389.. doi: 10.1016/j.yhbeh.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, 2012. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62, 816–847. doi: 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior NH, Yap KN, Soma KK, 2014. Acute and chronic effects of an aromatase inhibitor on pair-maintenance behavior of water-restricted zebra finch pairs. Gen. Comp. Endocrinol 196, 62–71. doi: 10.1016/j.ygcen.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Pultorak JD, Fuxjager MJ, Kalcounis-Rueppell MC, Marler CA, 2015. Male fidelity expressed through rapid testosterone suppression of ultrasonic vocalizations to novel females in the monogamous California mouse. Horm. Behav 70, 47–56. doi: 10.1016/j.yhbeh.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Ramdhan RC, Simonds E, Wilson C, Loukas M, Oskouian RJ, Tubbs RS, 2018. Complications of Subcutaneous Contraception: A Review. Cureus 10, 1–10. doi: 10.7759/cureus.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, 2014. Frank Beach Award Winner: Steroids as neuromodulators of brain circuits and behavior. Horm. Behav 66, 552–560. doi: 10.1016/j.yhbeh.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH, 2004. Rapid, Hierarchical Modulation of Vocal Patterning by Steroid Hormones. J. Neurosci 24, 5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger B. a, 2010. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc. Natl. Acad. Sci. U. S. A 107, 3852–3857. doi: 10.1073/pnas.0906572107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Heale L., Don SM., Cha A., Schlinge BA., 2012. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J. Neurophysiol 107, 1621––1631.. doi: 10.1152/jn.00749.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Jeon SD, Joshi NR, 2013. Recent evidence for rapid synthesis and action of oestrogens during auditory processing in a songbird. J. Neuroendocrinol 25, 1024–1031. doi: 10.1111/jne.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR, 2012. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci 32, 8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA, 2008. Forebrain steroid levels fluctuate rapidly during social interactions. Nat. Neurosci 11, 1327–34. doi: 10.1038/nn.2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Oyama RK, Schlinger BA, 2009. Elevated aromatase activity in forebrain synaptic terminals during song. J. Neuroendocrinol 21, 191–199. doi: 10.1111/j.1365-2826.2009.01820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelli A, Massobrio M, Tesarik J, 1998. Nongenomic actions of steroid hormones in reproductive tissues. Endocr. Rev 19, 3–17. doi: 10.1210/edrv.19.1.0322 [DOI] [PubMed] [Google Scholar]
- Romero LM, Reed JM, 2005. Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comp. Biochem. Physiol. - A Mol. Integr. Physiol 140, 73–79. doi: 10.1016/j.cbpb.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Sabatini R, Cagiano R, Rabe T, 2011. Adverse Effects of Hormonal Contraception. Reprod. Med. Endocrinol 8, 130–156. doi: 10.1783/147118910791749425 [DOI] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA, 2011. Synaptocrine signaling: Steroid synthesis and action at the synapse. Endocr. Rev 32, 532–549. doi: 10.1210/er.2011-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanh CJ., Tuer MJ., Ki YH., Fernande AO., Arnol AP., Schlinge BA., 2000. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J. Comp. Neurol 423, 619––630.. doi: [DOI] [PubMed] [Google Scholar]
- Sato SM, Woolley CS, 2016. Acute inhibition of neurosteroid estrogen synthesis suppresses status epilepticus in an animal model. Elife 5, e12917. doi: 10.7554/eLife.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV, 1989. Aromatase activity in quail brain: Correlation with aggressiveness. Endocrinology 124, 437–443. doi: 10.1210/endo-124-1-437 [DOI] [PubMed] [Google Scholar]
- Shihan M, Bulldan A, Scheiner-Bobis G, 2014. Non-classical testosterone signaling is mediated by a G-protein-coupled receptor interacting with Gnα11. Biochim. Biophys. Acta - Mol. Cell Res 1843, 1172–1181. doi: 10.1016/j.bbamcr.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Shihan M, Chan KH, Konrad L, Scheiner-Bobis G, 2015. Non-classical testosterone signaling in spermatogenic GC-2 cells is mediated through ZIP9 interacting with Gnα11. Cell. Signal 27, 2077–2086. doi: 10.1016/j.cellsig.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Hau M, Schlinger BA, 2004. Dehydroepiandrosterone metabolism by 3B-hydroxysteroid dehydrogenase/D5-D4 isomerase in adult zebra finch brain: Sex difference and rapid effect of stress. Endocrinology 145, 1668–1677. doi: 10.1210/en.2003-0883 [DOI] [PubMed] [Google Scholar]
- Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE, 2015. DHEA effects on brain and behavior : Insights from comparative studies of aggression. J. Steroid Biochem. Mol. Biol 145, 261–272. doi: 10.1016/j.jsbmb.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Tam H, Schlinger BA, 2007. Activities of 3β-HSD and aromatase in slices of developing and adult zebra finch brain. Gen. Comp. Endocrinol 150, 26–33. doi: 10.1016/j.ygcen.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszyck ML., Dzubu E., 2013. 17β-Hydroxysteroid dehydrogenase Type IV, a Z-linked gene, is higher in females than in males in visual and auditory regions of developing zebra finches. Brain Res. 1520, 95––106.. doi: 10.1016/j.brainres.2013.05.017 [DOI] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R, 2009. Estradiol Shapes Auditory Processing in the Adult Brain by Regulating Inhibitory Transmission and Plasticity-Associated Gene Expression. J. Neurosci 29, 5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R, 2011. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci 31, 3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi SC, Aiya UV, Wasner KD, Phan ML, Pytte CL, Vicario DS, 2014. Hemispheric asymmetry in new neurons in adulthood is associated with vocal learning and auditory memory. PLoS One 9, e108929. doi: 10.1371/journal.pone.0108929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K, 2011. Neurosteroid biosynthesis and function in the brain of domestic birds. Front. Endocrinol 2, 1–14. doi: 10.3389/fendo.2011.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuiten A, Van Honk J, Koppeschaar H, Bernaards C, Thijssen J, Verbaten R, 2000. Time Course of Effects of testosterone Administration on Sexual Arousal in Women. Arch Gen Psychiatry 57, 149–153. [DOI] [PubMed] [Google Scholar]
- Vahaba DM, Remage-Healey L, 2018. Neuroestrogens rapidly shape auditory circuits to support communication learning and perception: Evidence from songbirds. Horm. Behav 104, 77–87. doi: 10.1016/j.yhbeh.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goozen SHM, Wiegant VM, Endert E, Helmond FA, Van De Poll NE, 1997. Psychoendocrinological assessment of the menstrual cycle: The relationship between hormones, sexuality, and mood. Arch. Sex. Behav 26, 359–382. doi: 10.1023/A:1024587217927 [DOI] [PubMed] [Google Scholar]
- Vicenci JM., Estrad M., Galvi D., Brav R., Contrera AE., Rotte D., Szabadka G., Hil JA., Rotherme BA., Jaimovic E., Lavander S., 2011. Anabolic androgenic steroids and intracellular calcium signaling: A mini review on mechanisms and physiological implications. Mini Rev Med Chem 11, 390––398.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Schlinger BA, Hodges L, Arnold AP, 1994. Fadrozole: A Potent and Specific Inhibitor of Aromatase in the Zebra Finch Brain. Gen Comp Endocrinol 94, 53–61. [DOI] [PubMed] [Google Scholar]
- Walker WH, 2010. Non-classical actions of testosterone and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci 365, 1557–1569. doi: 10.1098/rstb.2009.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren AM, Gurvich C, Worsley R, Kulkarni J, 2014. A systematic review of the impact of oral contraceptives on cognition. Contraception 90, 111–116. doi: 10.1016/j.contraception.2014.03.015 [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Smith JANP, 1982. Endocrine Responses of White-Crowned Sparrows To Environmental Stress 84, 399–409. [Google Scholar]
- Yague JG, Muñoz A, De Monasterio-Schrader P, DeFelipe J, Garcia-Segura LM, Azcoitia I, 2006. Aromatase expression in the human temporal cortex. Neuroscience 138, 389–401. doi: 10.1016/j.neuroscience.2005.11.054 [DOI] [PubMed] [Google Scholar]
- Yazaki Y, Yamamoto K, Matsushima T, Aoki K, 1998. Non-genomic action of testosterone mediates avian vocal behavior. Proc. Japan Acad 74, 132–135. [Google Scholar]
- Yoder KM, Vicario DS, 2012. To Modulate and Be Modulated: Estrogenic Influences on Auditory Processing of Communication Signals within a Socio- Neuro-Endocrine Framework. Behav. Neurosci 126, 17–28. doi: 10.1002/ar.20849.3D [DOI] [PMC free article] [PubMed] [Google Scholar]