Abstract
Background
Glycosaminoglycans (GAGs) are negatively charged long linear (highly sulfated) polysaccharides consisting of repeating disaccharide units that are expressed on the surfaces of all nucleated cells. The expression of GAGs is required for embryogenesis, regulation of cell growth and proliferation, maintenance of tissue hydration, and interactions of the cells via receptors. Mucopolysaccharidoses (MPS) are caused by deficiency of specific lysosomal enzymes that result in the accumulation of GAGs in multiple tissues leading to organ dysfunction. Therefore, GAGs are important biomarkers for MPS. Without any treatment, patients with severe forms of MPS die within the first two decades of life.
Scope of review
Accurate measurement of GAGs is important to understand the diagnosis and pathogenesis of MPS and to monitor therapeutic efficacy before, during, and after treatment of the disease. This review covers various qualitative and quantitative methods for measurement of GAGs, including dye specific, thin layer chromatography (TLC), capillary electrophoresis, high-performance liquid chromatography (HPLC), liquid chromatography-tandem mass spectrometry (LC-MS/MS), gas chromatography, ELISA, and automated high-throughput mass spectrometry.
Major conclusion
There are several methods for GAG detection however, specific GAG detection in the various biological systems requires rapid, sensitive, specific, and cost-effective methods such as LC-MS/MS.
General significance
This review will describe different methods for GAG detection and analysis, including their advantages and limitation.
Keywords: glycosaminoglycans, chondroitin sulfate, dermatan sulfate, heparan sulfate, keratan sulfate, tandem mass spectrometry (MS/MS)
2. Introduction
Glycosaminoglycans (GAGs) are linear, negatively charged polysaccharides classified into five families, based on their structure: chondroitin sulfate (CS) (glucuronic acid and N-acetylgalactosamine), dermatan sulfate (DS) (iduronic acid or glucuronic acid and N-acetylgalactosamine), heparan sulfate (HS) (iduronic acid or glucuronic acid and N-acetylglucosamine), keratan sulfate (KS) (galactose and N-acetylglucosamine), and hyaluronan (HA) (glucuronic acid and N-acetylglucosamine) [1, 2].
GAGs are important components of the extracellular matrix (ECM) and are found in multiple tissues [3]. Polymeric GAGs are covalently bound through a linkage region to core proteins to produce proteoglycans (PGs) or remain as free polysaccharides [4–7]. Studies on GAGs and PGs have shown their importance in biological roles, including cancer progression, angiogenesis, development, growth, microbial pathogenesis, cellular signaling (growth factors, cell surface receptors, cytokines, chemokines, enzymes, complement proteins), and anticoagulation [8–21]. GAGs are important biomarkers for inherited metabolic disorders particularly mucopolysaccharidoses (MPS). MPS are caused by the defeciency of specific lysosomal enzymes that lead to the accumulation of GAGs in multiple tissues and successive multiple organ dysfunctions. Without any treatment, patients with severe forms of MPS die within the first two decades of life [22]. Thus, it is important to establish GAG measurements, which will facilitate diagnosis, predict clinical severity and prognosis, enable therapeutic monitoring (biomarker), and provide disease screening for MPS [23].
This review manuscript focuses on the history of GAG assay development with qualitative and quantitative methods with a more detailed discussion on current applications of mass spectrometry for GAG analysis.
3. Methods for GAG analysis
There are several methods for measurement of GAG that have been reported in the literature, which are summarized below.
3.1. Dye-specific method
Dye specific methods include various stainings; toluidine blue, alcian blue, and dimethylmethylene blue, which detect intact polymer GAGs.
Toluidine blue (TB) is a simple staining technique, discovered by William Henry Perkin in 1856 [24]. Several applications of this method have been reported for the detection of GAGs in urine or other biological tissue or fluid [25–29]. TB is not suitable for quantitative analysis of GAG because of nonspecific binding with negatively charged molecules in the biological matrix.
Alcian blue (AB) has also been used for the detection of GAGs in biological fluid and urine [30, 31]. Although AB dye interacts with negatively charged sulfated GAGs, it cannot distinguish between specific GAG. There are several reports where GAG detection was performed in urine by AB in combination with electrophoresis [25, 29, 32–34]. AB staining is not applicable to measure GAGs in blood and tissue due to a lack of sensitivity and specificity.
Dimethylmethylene blue (DMMB) has also been used to detect GAGs in biological fluids. Taylor and Jeffree first described 1,9 dimethylmethylene blue as a sensitive histochemical stain for the induction of metachromasia [35]. The DMMB dye was later developed as a colorimetric assay [36], modified by Farndale et al. [37], and adapted by Panin et al. for urinary GAGs [30]. The method was modified by Whitley et al. and utilized as a direct assay for urinary GAGs [38, 39]. DMMB was used by Stone to measure 600 urine samples with one false negative sample (Morquio A) [40]. Later reports, however, indicate that the false negative data using this method is almost 15% for all MPS patients and an even higher percentage for MPS IV patients [41–43]. The purity of dye also impacts on GAG detection and may contribute to false negative data [44].
3.2. Paper and thin layer chromatography
Paper and thin-layer chromatography (TLC) uses the same principle using either paper (cellulose) or glass coated with a thin layer of silica, which is used as a stationary phase, and various solvents are used as mobile phase. Caster and Dorstewitz identified known mammalian mucopolysaccharides, including hyaluronic acid, heparin, heparin monosulfate, and chondroitin sulfates A, B, and C with some certainty but KS with less confidence [45]. The method is useful only as a tentative examination of intact GAGs with a limited type of specimen mixture. Stao and Gyorkey used paper chromatography to purify GAGs from tissue extracts using zinc acetate as a solvent system [46]. Lippiello and Mankin [47] used TLC with a different acidic solution of calcium acetate and ethanol and were able to separate CS, DS, and KS. Teller and Ziemann developed the TLC method and separated urinary GAGs from different MPS [48]. There are several reports [47–50] regarding the use of TLC for GAG analysis, mainly in the urine. The main limitation of TLC is that the retention factors of different GAGs overlap, thus leading to wrong identification of MPS, especially MPS IV. These methods are inadequate for the determination of GAGs from blood and tissue extracts without prior treatments. Lack of sensitivity and specificity also limit these methods for the identification of GAGs.
3.3. Fast atom bombardment mass spectrometry
Fast atom bombardment mass spectrometry (FAB-MS) is also known as liquid secondary ion mass spectrometry (LSI-MS). FAB-MS uses a beam of neutral atoms such as argon or xenon bearing high kinetic energy for the ionization of in volatile ionic or nonionic compounds present either in the solid state or as a solution in a glycerol matrix.
Linhardt et al. reported an analysis of larger, heparin-derived oligosaccharides and a variety of disaccharides obtained from different GAGs by FAB-MS, demonstrating the versatility of this technique [51]. Negative ion FAB-MS has been used in the analysis of monosulfated disaccharides. Lamb et al. analyzed the isomers of CS, DS, and HS with differences in sulfate position and linkage position [52]. The full-scan mass spectra are useful in differentiating isomers when the sulfate group resides on different saccharide units. Li et al. also used negative ion FAB-MS to characterize sulfated unsaturated disaccharides from heparin and heparan sulfate [53]. Analysis of GAGs using FAB-MS has been reported by several other investigators [54–60]. The major limitation of this method is that it is not readily compatible with HPLC; thus, isomers cannot be resolved.
3.4. Matrix-assisted laser desorption/ionization (MALDI)
Matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) method was first developed for protein analysis [61]. It was subsequently applied to the detection of complex substances such as peptides [62], oligonucleotides [63], and glycolipids [64], as well as larger molecules such as synthetic polymers [65], glycoproteins, and polysaccharides [66] without prior derivatization. Other investigators also have been successfully analyzed underivatized oligosaccharides by MALDI-MS with detection limits down to the low pmo1 range [67–70]. Tissotet et al. used strategies to analyze highly sulfated heparins by MALDI-TOF massspectrometry [71]. However, losses of sulfate group equivalents were reported by this method [72]. Bultel et al. minimized the extent of sulfate losses by adding sodium to the matrix solution [73]. Infrared MALDI is considered softer than UV-MALDI and has been used in the analysis of HS and CS disaccharides [74]. The results showed that disaccharides were detected as sodium adducts in negative mode with minimal losses of sulfate equivalents. Sisu et al. developed a method for CS and DS using MALDI-MS [75]. Since CS and DS constitute a subgroup of sulfated GAGs for which the degree of sulfation varies between species and tissues, Kisolova et al. [6] developed a new strategy applying MALDI-TOF MS in positive ion mode for semi-qualitative and quantitative analysis of CS/DS-derived disaccharide units. Hsieh et al. also developed nanodiamond-based affinity purification and detection of sulfated GAGs using MALDI-TOF claiming specific, sensitive, and potentially useful for clinical diagnosis of MPS [76]. This method is, however, incompatible with chromatographic systems and, therefore, unable to resolve isomers, which limit its usefulness in the clinical setting.
3.5. Gas chromatography
Gas chromatography (GC) is a simple, multifaceted, highly sensitive, and rapidly applied technique for the separation of molecules. Nakamura and Tamura used this technique to analyze mono and disaccharides in human blood and urine after oral administration [77]. Gordon and Haust reported urinary GAGs in 23 MPS patients [78]. Urinary GAGs analysis has been reported in normal [79, 80] and MPS patients by several researched using gas chromatography [81–83]. GC, coupled with mass spectrometry, has been used for the analysis of iduronic and glucuronic acid [84]. Traditional GC/MS uses “hard” ionization technique such as electron ionization (EI), which fragments the molecules in an extensive way. When GC is coupled with API/MS, it preserves molecular ion from fragmentation [85].
3.6. High-performance liquid chromatography (HPLC)
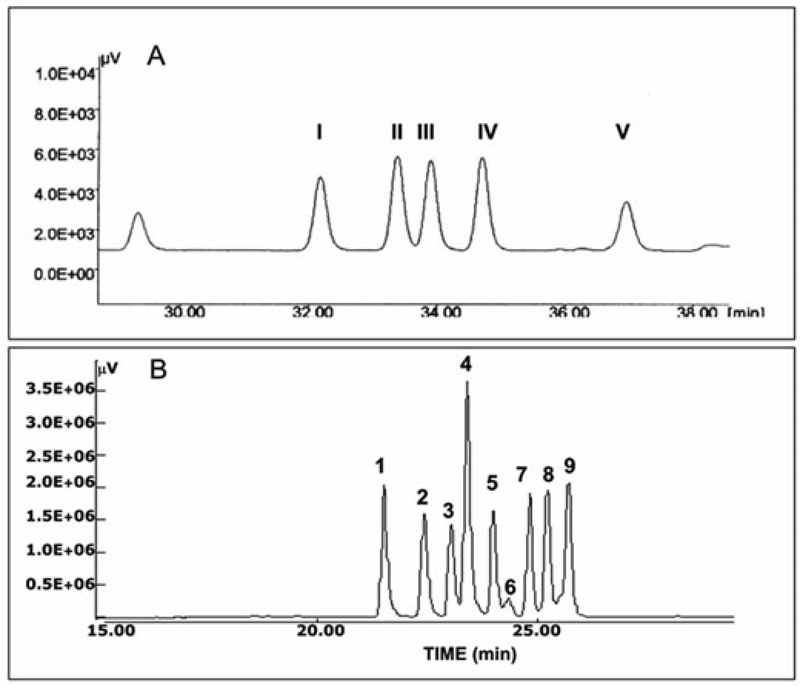
Toyoda et al. developed fluorometric detection of heparin and heparan sulfate using simple and reverse-phase ion-pair chromatography [86, 87]. Studelska et al. reported quantification of glucosamine- and galactosamine containing GAGs after the reversed-phase separation of their fluorescent isoindole derivatives, including heparin, HS, KS, CS, DS, and hyaluronan [88]. A similar method has been used by several investigators to identify oligosaccharides [88–91]. This method is sensitive and very specific and, therefore, has been used to identify various MPS patients by analyzing intact urinary GAGs [92]. An example of HPLC analysis of HA, CS/DS GAG-disaccharides using HPLC is presented in Figure1.
Figure 1.
Adapted from Karousou et al. [93]. HPLC analysis of HA, CS/DS GAG-disaccharides. The standard mixture of HA and CS/DS-disaccharide was derivatized with AMAC and analyzed with HPLC using a reversed-phase column (C-18, 4.6 × 150 mm). Products were detected with a fluorophore detector (ex = 442 nm and em = 520 nm). Panel A: separation of a mixture of commercial standard mono-sulfated-disaccharide from CS/DS and HA; I: di-mono4S; II: dimono6S; III: di-0 HA; IV: 0 CS; V: GalNAc. Panel B: separation of a mixture of commercial standard-disaccharide from HA and CS/DS GAGs. Peaks corresponds to: 1 di-tri(2,4,6)S; 2 di-di(2,4)S, 3 di-di(4,6)S, 4 di-di(2,6)S, 5 di-mono4S, 6 di-mono2S, 7 di-mono6S, 8 di-0S HA, 9 di-0S CS.
3.7. HPLC with ion-exchange chromatography
Ion exchange chromatography is also applied for intact GAG detection. Radhakrishnamurthy et al. have quantified uronic acid in chondroitin sulfates and dermatan sulfate mixtures by Dowex 1 Cl− column fractionation of GAG from aortas of different animal species [94]. Heparin, HS, CS, DS, and KS were analyzed using reverse-phase ion-pairing HPLC and ion-exchange HPLC with suppressed conductivity detection. The results were compared with those obtained by strong anion-exchange HPLC using UV detection [95].
Campo et al. used weak anion-exchange Ecteola-cellulose to detect GAGs in plasma and serum using HPLC [96]. This method is not frequently used since it is tedious and time-consuming.
3.8. Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent (ELISA) assay is a commonly used analytical biochemistry assay. Tomatsu et al. developed ELISA to measure KS and HS in blood and urine samples of MPS and ML patients [23, 41, 97, 98]. Several other investigators used ELISA to measure KS and HS in MPS patients [99–102]. Other GAGs, including CS, DS, and hyaluronan, were also identified by this method [103–107]. Although the ELISA method is a qualitative and quantitative method for the measurement of intact specific GAGs, it cannot be used to identify multiple GAGs simultaneously.
3.9. Capillary electrophoresis
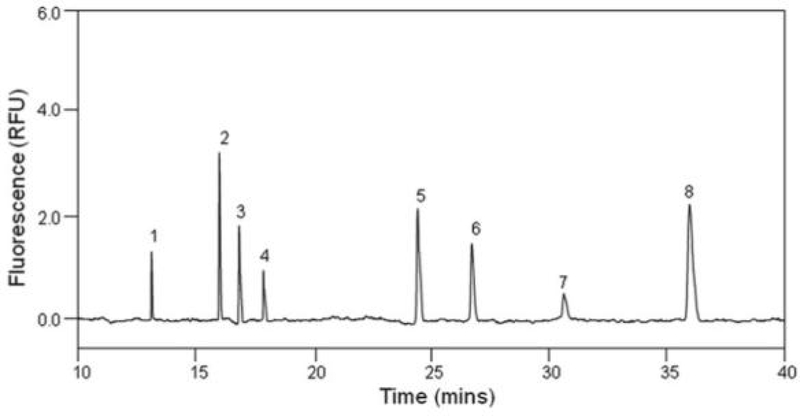
Capillary electrophoresis (CE) is a highly sensitive chromatographic method for intact GAGs and GAG-derived disaccharide analysis. This method has high resolving power, separation efficiency, and short analysis time. Chang et al. demonstrated 2-aminoacridone derivatized GAG disaccharides by CE-laser-induced fluorescence and stated that this technique is 100 times more sensitive than traditional UV detection at 232 nm [108]. Hopwood and Harrison have used CE to detect urinary GAGs from control and MPS patients [109]. Cappelletti et al. separated all animal GAGs using CE [110]. Several reports describe CE combined with mass spectrometry for simultaneous separation and determination of GAGs [111–113]. An example of capillary electrophoresis analysis of heparin (HP) and HS, GAG-disaccharides, is presented in Figure 2.
Figure 2.
Adapted from Chang et al. [108]. Electrophoregram of eight HP/HS Δ-disaccharides. The analysis was performed at 25°C, pressure injection of 50 mbar × 10 s, using 50 mM phosphate buffer, pH 3.5, under 30 kV with reversed polarity.
3.10. HPLC with ESI mass spectrometry
Electrospray ionization (ESI) is a soft ionization method that is generally used in combination with liquid chromatography and is most suitable for mass spectrometric (MS) analysis of glycosaminoglycans [114]. The spray conditions can be modified to suppress the loss of sulfo groups and enhance the abundance of certain types of ions, such as, for example, sodium cationized or protonated species and ions with specific charge states [115]. Oguma et al. developed methods to analyze GAGs (HS, KS, and DS) from serum/plasma using ESI mass spectrometry [116–120]. The method uses the lyase enzymes of chondroitinase B, keratinase II, and heparitinase, which digest polysaccharides to DS, KS, and HS disaccharides. The produced disaccharides were measured by LC/ESI/MS/MS. A similar enzymatic digestion method was used by Osago et al. [121], which was able to measure not only all classes of GAGs (CS/DS, KS, HS, and hyaluronic acid) but also isomeric disaccharide compounds. Numerous reports have described the measurement of GAGs in blood/plasma and urine samples from normal control and MPS patients by using enzyme-mediated digestion [3, 23, 122–128]. Detection of non-reducing end of GAGs was developed by Lawrence et al. [129, 130]. In this method, the lack of lysosomal enzyme results in the accumulation of characteristic non-reducing terminal carbohydrate structures. GAGs are enzymatically depolymerized, releasing unique mono-, di-, or trisaccharides from the non-reducing ends of the chains, which are subsequently labeled by reductive amination with heavy isotope-labeled aniline. The modified sugars are quantified by LC/MS/MS. This method is broadly applicable to all MPS disorders utilizing tissue, blood, and urine. However, this method cannot detect MPS IV.
Identification of GAGs (CS, DS, HS, and KS) from the urine of MPS patients by a chemical degradation by methanolysis followed by LC/MS/MS of the liberated disaccharides was developed by Auray-Blais et al. [131–133]. The method has been adapted for the identification and quantification of GAGs from urine [134, 135], cerebrospinal fluid (CSF), serum [136, 137], and animal tissues [138]. Recently, Trim et al. applied butanolysis followed by LC/MS/MS to quantify heparan sulfate in urine from MPS patients. (MPS I, II, III and VI) [139]. The sensitivity of this method is very high when optimized for HS.. The sensitivity of the new technique is illustrated by the quantification of HS in 5 μL urine samples from MPS patients and healthy controls. HS was quantifiable in all samples, including controls. Disaccharide reaction products were further characterized using exact mass MS/MS. However, this method is unable to identify subclasses of GAGs. Most recently, Forni et al. reported simultaneously quantification of urinary HS and DS from MPS (I, II, III, VI, and VII) patients using butanolysis derivatization followed by LC/MS/MS [140].
3.11. HPLC with atmospheric-pressure chemical ionization mass spectrometry
Atmospheric pressure chemical ionization (APCI) is a soft ionization method similar to chemical ionization used for polar and relatively less polar thermally stable compounds with molecular weight less than 1500 Da. McEwen et al. have described for the analysis of solids, liquids, and biological tissues by APCI LC/MS [141]. The method can be used in combination with either gas chromatography or liquid chromatography and is qualitative and quantitative analysis of mono and disaccharides. Ricochon et al. have reported the quantification of a large pool of mono and disaccharides in complex matrices by APCI mass spectrometry (APCI-MS) [142]. Zhou et al. have reported the determination of glucosamine in human plasma using HPLC-APCI-MS/MS [143].
3.12. Automated flow-injection mass spectrometry
Automated high-throughput mass spectrometry (HT-MS/MS) using flow-injection is a very rapid method for GAG analysis compared with standard LC-MS/MS. The HT-MS/MS system excludes chromatographic separation; thereby allowing sample-to-sample cycle times to be processed faster than LC-MS/MS. This method was originally developed for high-throughput newborn screening for inborn errors of metabolism by analysis of acylcarnitines and amino acids in DBS [144]. The complete automation of the sample introduction has been allowed the analysis of up to 200 samples in one injection sequence, at a rate of one sample every 3 min, with excellent separation.
Moreover, the innovative HT-MS/MS system (RapidFire; Agilent Technologies) enables the sample to be aspirated to a matrix for concentration and desalting, followed by direct injection into MS/MS without chromatographic separation. Each sample is processed within 10 seconds. In 2014, Shimada et al. have reported that this HT-MS/MS system is applied to assay HS in blood samples from control and patients with MPS II, III, or IV and in DBS from newborn controls and patients with MPS I, II, or III [145]. The study concluded that the innovative HT-MS/MS system provides 10–100 fold higher throughput than LC-MS/MS-based methods with similar sensitivity and specificity in an HS assay and suitable for diagnosis, monitoring, and newborn screening of MPS. Tomatsu et al. reported HS and KS analysis by HT-MS/MS, compared with LC/MS/MS and found a moderate correlation in serum HS and KS measurements of control subjects between LC-MS/MS and HT-MS/MS indicating that each assay is comparable [3].
The HT-MS/MS (RapidFire) system has been validated as suitable for many drug discoveries [146–150] and Absorption, Distribution, Metabolism, and Excretion (ADME) based applications [151–154]. The method could be valuable in newborn screening of MPS, whereby over one million of samples might be screened for GAG on an annual basis. The main drawback of this method is that disaccharides with identical molecular weights cannot be distinguished.
4. Advantages and limitations of methods for GAG analysis
Dye specific methods are simple and have been widely used for quantitative analysis of urinary GAGs but are unable to quantify specific GAG in blood and DBS, resulting in false negative data. Similarly, TLC is a very simple method used to identify specific GAGs in urine but not in blood and tissue extracts without prior treatment. Overlapping various GAGs affected by the retention factors results in misidentification. In contrast, HPLC is a very sensitive, highly reproducible, and efficient method for separation and detection of GAGs and other compounds within 10–30 min. and therefore produces quicker, and sometimes more accurate, results.
Co-elution of two compounds with similar structure and polarities is a limitation of this method. Capillary electrophoresis (CE) is also a highly sensitive method for GAGs and GAG-derived disaccharide analysis. Due to high resolving power, separation efficiency, and short analysis time, it is used by several investigators, as mentioned above. Another method for GAG analysis is ELISA, which is a sensitive and specific method; however, due to the high cost and inability to identify multiple GAGs simultaneously, this method is not widely used. LC/MS/MS is a highly specific and sensitive technique, which measures analytes in complex mixtures either in positive ion mode and/or negative ion mode. There are several protocols that have been developed in the past decades as described above. A variety of analyte separation techniques like capillary electrophoresis, gas chromatography, and HPLC are united with mass spectroscopy for simultaneous separation and determination of analytes called CE-MS, GC–MS, and HPLC-MS, respectively [111, 112, 133, 155, 156] which makes its application even wider. Although mass spectrometry has many advantages and its wider application makes it the best tool in the modern period however, it is very expensive, and not every lab can afford it.
Various LC-MS/MS-based GAG analysis methods, which are in clinical practice, their advantages and limitations have been summarized in table 1.
Table 1.
LC-MS/MS-based GAG analysis methods and their advantages and limitations.
| LC/MS/MS Methods | GAGs | Sample | Advantages | Disadvantage | Reference |
|---|---|---|---|---|---|
| Enzymatic degradation and LC/ESI/MS/MS | CS, DS, HS, KS | Plasma, serum, urine, DBS, tissue extracts, and CSF | Simultaneous identification of all GAGs Identification of subclasses of CS, HS, and KS Quantification of sulfation level | Variation in the ionization efficiency of different disaccharides. | [116–119, 123, 126, 157, 158] |
| Methanolysis and LC/MS/MS | CS, DS, HS, and KS | Urine, blood, CSF, and tissues | Simultaneous identification of all GAGs. Simple steps for preparing samples and reagents that are easily available. No use of an expensive enzyme. | Not applicable for blood KS. No detection of subclass of CS, HS, and KS | [131, 132, 136–138, 159] |
| Butanolysis and LC/MS/MS | DS, HS | Urine | Simultaneous identification of DS, HS. Simple steps for preparing samples and reagents that are easily available. No use of an expensive enzyme. | Not applicable in blood. No detection of subclass of CS, HS, and KS | [139, 140] |
| Atmospheric pressure chemical ionization mass spectrometry | Glucosamine Purified disaccharides from CS | Plasma | The main usage of APCI is for polar and relatively less polar, thermally stable compounds with molecular weight less than 1500 Da. Useful in drug discovery. | Not wide application in biological fluid for GAG detection. | [58, 143, 160] |
| MALDI-TOF massspectrometry | Heparin, CS, DS | Tissue | Creates singly charged species, greatly simplifies the interpretation of the data. Applicability to high-throughput experiments, robustness, relatively high tolerance to salts and other contaminants. | The low extent of desorption induced by the laser for samples containing a large number of acidic groups, together with the facile loss of sulfate moieties in the MALDI source has been major limitations of this technology for GAG analysis. | [6, 71, 75] |
| Fast atom bombardment mass spectrometry | Heparin, CS, DS, HS, hyaluronic acid, and KS | Commercially obtained disaccharides | The procedure is simple and rapid. Useful for high mass and thermally labile compounds. Relatively tolerant of variation in sampling. Good for a large variety of compounds | Analyte must be soluble in the matrix. Bad for multiple charged (>2 charges) compounds. Low sensitivity. Not compatible with HPLC. Cannot distinguish between isomers. | [51, 52] |
| Isotope Labeling mass spectrometry | CS, HS, and KS | CHO cells, and mice tissue | The technique is adaptable to all types of GAGs | Several different isotopes used, including reductive amination, biotin with single deuterium13C incorporation, etc. Procedures are tedious, time-consuming and have limited applications. | [130, 161–163] |
| Non-reducing end of GAG and mass spectrometry | HS, DS | Blood, urine, tissue | All MPS can be distinguished based on specific non-reducing end GAGs. | This method is not applicable to identify MPS IV based on non-reducing end KS. | [129] |
| Automated flow injection mass spectrometry (high throughput MS/MS; RapidFire) | HS, KS | Blood, DBS | Within 10 seconds per sample compared with LC/MS/MS. Much higher throughput than LC-MS/MS-based methods with similar sensitivity. | Isomeric disaccharides cannot be distinguished. | [3, 145] |
5. Conclusion
Although several procedures have been established to measure GAGs, dye specific and TLC methods are not used these days due to their limitation to detect all GAGs. Mass spectrometry is now widely used for measuring all species of GAGs in a biological systems including blood, urine, DBS, and tissue for prognosis, diagnosis, monitoring, and screening purposes. Due to itswide range of applications, LC-MS/MS is considered as the best analytical tool for basic or clinical research in the biomedical field and the method of choice for GAG analysis.
6. Acknowledgments
This work was supported by grants from Center for Biomedical Research Excellence (COBRE), The Carol Ann Foundation, Angelo R. Cali & Mary V. Cali Family Foundation, Inc., The Vain and Harry Fish Foundation, Inc., The Bennett Foundation, Jacob Randall Foundation, Austrian and Japanese MPS societies, and Nemours Funds. This work was supported by the project for baby and infant in research of health and development to Adolescent and young adult from Japan Agency for Medical Research and development, AMED, under grant number JP18gk0110017.R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of National Institutes of Health (NIH) under grant number P30GM114736. The content of the article has not been influenced by the sponsors.
7. References
- [1].Gama CI, Hsieh-Wilson LC, Chemical approaches to deciphering the glycosaminoglycan code Current opinion in chemical biology 9 (2005) 609–619. [DOI] [PubMed] [Google Scholar]
- [2].Jackson RL, Busch SJ, Cardin AD, Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes Physiological reviews 71 (1991) 481–539. [DOI] [PubMed] [Google Scholar]
- [3].Tomatsu S, Kubaski F, Sawamoto K, Mason RW, Yasuda E, Shimada T, Montano AM, Yamaguchi S, Suzuki Y, Orii T, Newborn screening and diagnosis of mucopolysaccharidoses: application of tandem mass spectrometry Nihon Masu Sukuriningu Gakkai Shi 24 (2014) 19–37. [PMC free article] [PubMed] [Google Scholar]
- [4].Couchman JR, Pataki CA, An introduction to proteoglycans and their localization The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 60 (2012) 885–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Esko JD, Kimata K, Lindahl U, Proteoglycans and Sulfated Glycosaminoglycans, in: nd, Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME (Eds.), Essentials of Glycobiology, Cold Spring Harbor; (NY), 2009. [PubMed] [Google Scholar]
- [6].Kiselova N, Dierker T, Spillmann D, Ramstrom M, An automated mass spectrometry-based screening method for analysis of sulfated glycosaminoglycans Biochemical and biophysical research communications 450 (2014) 598–603. [DOI] [PubMed] [Google Scholar]
- [7].Zhang L, Glycosaminoglycan (GAG) biosynthesis and GAG-binding proteins Progress in molecular biology and translational science 93 (2010) 1–17. [DOI] [PubMed] [Google Scholar]
- [8].Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB, Chondroitinase ABC promotes functional recovery after spinal cord injury Nature 416 (2002) 636–640. [DOI] [PubMed] [Google Scholar]
- [9].Carulli D, Laabs T, Geller HM, Fawcett JW, Chondroitin sulfate proteoglycans in neural development and regeneration Current opinion in neurobiology 15 (2005) 116–120. [DOI] [PubMed] [Google Scholar]
- [10].Casu B, Guerrini M, Torri G, Structural and conformational aspects of the anticoagulant and anti-thrombotic activity of heparin and dermatan sulfate Current pharmaceutical design 10 (2004) 939–949. [DOI] [PubMed] [Google Scholar]
- [11].Cavalcante LA, Garcia-Abreu J, Moura Neto V, Silva LC, Weissmuller G, Modulators of axonal growth and guidance at the brain midline with special reference to glial heparan sulfate proteoglycans Anais da Academia Brasileira de Ciencias 74 (2002) 691–716. [DOI] [PubMed] [Google Scholar]
- [12].Eswarakumar VP, Lax I, Schlessinger J, Cellular signaling by fibroblast growth factor receptors Cytokine & growth factor reviews 16 (2005) 139–149. [DOI] [PubMed] [Google Scholar]
- [13].Fareed J, Hoppensteadt DA, Bick RL, An update on heparins at the beginning of the new millennium Seminars in thrombosis and hemostasis 26 Suppl 1 (2000) 5–21. [DOI] [PubMed] [Google Scholar]
- [14].Fry EE, Lea SM, Jackson T, Newman JW, Ellard FM, Blakemore WE, Abu-Ghazaleh R, Samuel A, King AM, Stuart DI, The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex The EMBO journal 18 (1999) 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iozzo RV, Basement membrane proteoglycans: from cellar to ceiling Nature reviews. Molecular cell biology 6 (2005) 646–656. [DOI] [PubMed] [Google Scholar]
- [16].Mardberg K, Trybala E, Tufaro F, Bergstrom T, Herpes simplex virus type 1 glycoprotein C is necessary for efficient infection of chondroitin sulfate-expressing gro2C cells The Journal of general virology 83 (2002) 291–300. [DOI] [PubMed] [Google Scholar]
- [17].Perrimon N, Bernfield M, Cellular functions of proteoglycans--an overview Seminars in cell & developmental biology 12 (2001) 65–67. [DOI] [PubMed] [Google Scholar]
- [18].Raman R, Sasisekharan V, Sasisekharan R, Structural insights into biological roles of protein-glycosaminoglycan interactions Chemistry & biology 12 (2005) 267–277. [DOI] [PubMed] [Google Scholar]
- [19].Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A, Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies Journal of cellular biochemistry 96 (2005) 897–905. [DOI] [PubMed] [Google Scholar]
- [20].Sugahara K, Mikami T, Uyama T, Mizuguchi S, Nomura K, Kitagawa H, Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate Current opinion in structural biology 13 (2003) 612–620. [DOI] [PubMed] [Google Scholar]
- [21].Vlodavsky I, Goldshmidt O, Properties and function of heparanase in cancer metastasis and angiogenesis Haemostasis 31 Suppl 1 (2001) 60–63. [PubMed] [Google Scholar]
- [22].Neufeld E MJ, The Metabolic and Molecular Bases of Inherited Disease, in: Scriver CBASWVD (Ed.), The mucopolysaccharidoses, McGraw-Hill, New York, 2001, pp. 3421–3452. [Google Scholar]
- [23].Tomatsu S, Shimada T, Mason RW, Montano AM, Kelly J, LaMarr WA, Kubaski F, Giugliani R, Guha A, Yasuda E, Mackenzie W, Yamaguchi S, Suzuki Y, Orii T, Establishment of glycosaminoglycan assays for mucopolysaccharidoses Metabolites 4 (2014) 655–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perkin W, On mauveine and allied colouring matters J Chem Soc Trans 35 (1879) 717–732. [Google Scholar]
- [25].Kodama C, Kodama T, Yosizawa Z, Methods for analysis of urinary glycosaminoglycans Journal of chromatography 429 (1988) 293–313. [DOI] [PubMed] [Google Scholar]
- [26].Takemoto S, [A histochemical study of acid glycosaminoglycans on normal and pathological cartilage, ligaments and several other connective tissues] Nihon Seikeigeka Gakkai zasshi 61 (1987) 729–741. [PubMed] [Google Scholar]
- [27].Lamberg SI, Stimulatory effect of exogenous hyaluronic acid distinguishes cultured fibroblasts of Marfan’s disease from controls The Journal of investigative dermatology 71 (1978) 391–395. [DOI] [PubMed] [Google Scholar]
- [28].Lullmann-Rauch R, Experimental mucopolysaccharidosis: preservation and ultrastructural visualization of intralysosomal glycosaminoglycans by use of the cationic dyes cuprolinic blue and toluidine blue Histochemistry 93 (1989) 149–154. [DOI] [PubMed] [Google Scholar]
- [29].Terry DE, Chopra RK, Ovenden J, Anastassiades TP, Differential use of Alcian blue and toluidine blue dyes for the quantification and isolation of anionic glycoconjugates from cell cultures: application to proteoglycans and a high-molecular-weight glycoprotein synthesized by articular chondrocytes Analytical biochemistry 285 (2000) 211–219. [DOI] [PubMed] [Google Scholar]
- [30].Panin G, Naia S, Dall’Amico R, Chiandetti L, Zachello F, Catassi C, Felici L, Coppa GV, Simple spectrophotometric quantification of urinary excretion of glycosaminoglycan sulfates Clinical chemistry 32 (1986) 2073–2076. [PubMed] [Google Scholar]
- [31].Bjornsson S, Quantitation of proteoglycans as glycosaminoglycans in biological fluids using an alcian blue dot blot analysis Analytical biochemistry 256 (1998) 229–237. [DOI] [PubMed] [Google Scholar]
- [32].De Muro P, Faedda R, Formato M, Re F, Satta A, Cherchi GM, Carcassi A, Urinary glycosaminoglycans in patients with systemic lupus erythematosus Clinical and experimental rheumatology 19 (2001) 125–130. [PubMed] [Google Scholar]
- [33].De Muro P, Fresu P, Formato M, Tonolo G, Mameli M, Maioli M, Sanna GM, Cherchi GM, Urinary glycosaminoglycan and proteoglycan excretion in normoalbuminuric patients with type 1 diabetes mellitus Journal of nephrology 15 (2002) 290–296. [PubMed] [Google Scholar]
- [34].Maccari F, Volpi N, Glycosaminoglycan blotting on nitrocellulose membranes treated with cetylpyridinium chloride after agarose-gel electrophoretic separation Electrophoresis 23 (2002) 3270–3277. [DOI] [PubMed] [Google Scholar]
- [35].Taylor KB, Jeffree GM, A new basic metachromatic dye, I:9-dimethyl methylene blue The Histochemical journal 1 (1969) 199–204. [DOI] [PubMed] [Google Scholar]
- [36].Humbel R ES, A colorimetric method for the determination of sulphated glycosaminoglycans Rev Roum Biochim 11 (1974) 21–24. [Google Scholar]
- [37].Farndale RW, Sayers CA, Barrett AJ, A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures Connective tissue research 9 (1982) 247–248. [DOI] [PubMed] [Google Scholar]
- [38].Whitley CB, Ridnour MD, Draper KA, Dutton CM, Neglia JP, Diagnostic test for mucopolysaccharidosis. I. Direct method for quantifying excessive urinary glycosaminoglycan excretion Clinical chemistry 35 (1989) 374–379. [PubMed] [Google Scholar]
- [39].de Jong JG, Wevers RA, Laarakkers C, Poorthuis BJ, Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses Clinical chemistry 35 (1989) 1472–1477. [PubMed] [Google Scholar]
- [40].Stone JE, Urine analysis in the diagnosis of mucopolysaccharide disorders Annals of clinical biochemistry 35 ( Pt 2) (1998) 207–225. [DOI] [PubMed] [Google Scholar]
- [41].Tomatsu S, Okamura K, Taketani T, Orii KO, Nishioka T, Gutierrez MA, Velez-Castrillon S, Fachel AA, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Noguchi A, Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA Pediatric research 55 (2004) 592–597. [DOI] [PubMed] [Google Scholar]
- [42].Gray G, Claridge P, Jenkinson L, Green A, Quantitation of urinary glycosaminoglycans using dimethylene blue as a screening technique for the diagnosis of mucopolysaccharidoses: an evaluation Annals of clinical biochemistry 44 (2007) 360–363. [DOI] [PubMed] [Google Scholar]
- [43].Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA, Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix Clinical chemistry 35 (1989) 2074–2081. [PubMed] [Google Scholar]
- [44].Stone JE, Akhtar N, Botchway S, Pennock CA, Interaction of 1,9-dimethylmethylene blue with glycosaminoglycans Annals of clinical biochemistry 31 ( Pt 2) (1994) 147–152. [DOI] [PubMed] [Google Scholar]
- [45].Castor CW, Dorstewitz EL, Identification of Acid Mucopolysaccharides by Paper Chromatography Journal of chromatography 13 (1964) 157–165. [DOI] [PubMed] [Google Scholar]
- [46].Sato CS, Gyorkey F, Purification of protease-extracted glycosaminoglycans by short-distance paper chromatography with a zinc acetate solvent Analytical biochemistry 87 (1978) 540–544. [DOI] [PubMed] [Google Scholar]
- [47].Lippiello L, Mankin HJ, Thin-layer chromatographic separation of the isomeric chondroitin sulfates, dermatan sulfate, and keratan sulfate Analytical biochemistry 39 (1971) 54–58. [DOI] [PubMed] [Google Scholar]
- [48].Teller WM, Ziemann A, Thin layer chromatography of urinary acid glycosaminoglycans as screening procedure for mucopolysaccharidoses Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 1 (1969) 32–35. [DOI] [PubMed] [Google Scholar]
- [49].Humbel R, Chamoles NA, Sequential thin layer chromatography of urinary acidic glycosaminglycans Clinica chimica acta; international journal of clinical chemistry 40 (1972) 290–293. [DOI] [PubMed] [Google Scholar]
- [50].Zhang Z, Xie J, Zhang F, Linhardt RJ, Thin-layer chromatography for the analysis of glycosaminoglycan oligosaccharides Analytical biochemistry 371 (2007) 118–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Linhardt RJ, Wang HM, Loganathan D, Lamb DJ, Mallis LM, Analysis of glycosaminoglycan-derived oligosaccharides using fast-atom-bombardment mass-spectrometry Carbohydrate research 225 (1992) 137–145. [DOI] [PubMed] [Google Scholar]
- [52].Lamb DJ, Wang HM, Mallis LM, Linhardt RJ, Negative ion fast-atom bombardment tandem mass spectrometry to determine sulfate and linkage position in glycosaminoglycan-derived disaccharides Journal of the American Society for Mass Spectrometry 3 (1992) 797–803. [DOI] [PubMed] [Google Scholar]
- [53].Ii T, Kubota M, Okuda S, Hirano T, Ohashi M, Negative-ion fast atom bombardment tandem mass spectrometry for characterization of sulfated unsaturated disaccharides from heparin and heparan sulfate Glycoconjugate journal 12 (1995) 162–172. [DOI] [PubMed] [Google Scholar]
- [54].Reinhold VN, Carr SA, Green BN, Petitou M, Choay J, Sinay P, Structural characterization of sulfated glycosaminoglycans by fast atom bombardment mass spectrometry: application to heparin fragments prepared by chemical synthesis Carbohydrate research 161 (1987) 305–313. [DOI] [PubMed] [Google Scholar]
- [55].Takagaki K, Nakamura T, Kon A, Tamura S, Endo M, Characterization of beta-D-xyloside-induced glycosaminoglycans and oligosaccharides in cultured human skin fibroblasts Journal of biochemistry 109 (1991) 514–519. [DOI] [PubMed] [Google Scholar]
- [56].Mallis LM, Wang HM, Loganathan D, Linhardt RJ, Sequence analysis of highly sulfated, heparin-derived oligosaccharides using fast atom bombardment mass spectrometry Analytical chemistry 61 (1989) 1453–1458. [DOI] [PubMed] [Google Scholar]
- [57].Barker CG, Blakemore WF, Dell A, Palmer AC, Tiller PR, Winchester BG, GM1 gangliosidosis (type 1) in a cat The Biochemical journal 235 (1986) 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Scudder P, Tang PW, Hounsell EF, Lawson AM, Mehmet H, Feizi T, Isolation and characterization of sulphated oligosaccharides released from bovine corneal keratan sulphate by the action of endo-beta-galactosidase European journal of biochemistry 157 (1986) 365–373. [DOI] [PubMed] [Google Scholar]
- [59].Ii T, Kubota M, Hirano T, Ohashi M, Yoshida K, Suzuki S, FAB CID-MS/MS characterization of tetrasaccharide tri- and tetrasulfate derived from the antigenic determinant recognized by the anti-chondroitin sulfate monoclonal antibody MO-225 Glycoconjugate journal 12 (1995) 282–289. [DOI] [PubMed] [Google Scholar]
- [60].Khoo KH, Morris HR, McDowell RA, Dell A, Maccarana M, Lindahl U, FABMS/derivatisation strategies for the analysis of heparin-derived oligosaccharides Carbohydrate research 244 (1993) 205–223. [DOI] [PubMed] [Google Scholar]
- [61].Karas M, Hillenkamp F, Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons Analytical chemistry 60 (1988) 2299–2301. [DOI] [PubMed] [Google Scholar]
- [62].Cohen SL, Chait BT, Influence of matrix solution conditions on the MALDI-MS analysis of peptides and proteins Analytical chemistry 68 (1996) 31–37. [DOI] [PubMed] [Google Scholar]
- [63].Juhasz P, Roskey MT, Smirnov IP, Haff LA, Vestal ML, Martin SA, Applications of delayed extraction matrix-assisted laser desorption ionization time-of-flight mass spectrometry to oligonucleotide analysis Analytical chemistry 68 (1996) 941–946. [DOI] [PubMed] [Google Scholar]
- [64].Meri S, Lehto T, Sutton CW, Tyynela J, Baumann M, Structural composition and functional characterization of soluble CD59: heterogeneity of the oligosaccharide and glycophosphoinositol (GPI) anchor revealed by laser-desorption mass spectrometric analysis The Biochemical journal 316 ( Pt 3) (1996) 923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wu KJ, Odom RW, Peer reviewed: characterizing synthetic polymers by MALDI MS Analytical chemistry 70 (1998) 456A–461A. [DOI] [PubMed] [Google Scholar]
- [66].Harvey DJ, Matrix-assisted laser desorption/ionisation mass spectrometry of oligosaccharides and glycoconjugates Journal of chromatography. A 720 (1996) 429–446. [DOI] [PubMed] [Google Scholar]
- [67].Wu KJ, Steding A, Becker CH, Matrix-assisted laser desorption time-of-flight mass spectrometry of oligonucleotides using 3-hydroxypicolinic acid as an ultraviolet-sensitive matrix Rapid communications in mass spectrometry : RCM 7 (1993) 142–146. [DOI] [PubMed] [Google Scholar]
- [68].F H, M K, A I, B S, Biological mass Spectrometry, Flsevier, Amsterdam, 1990, pp. 49–60. [Google Scholar]
- [69].H E., J P-K., M K., B S, Pure Appl. Chem 63 (1991) 491. [Google Scholar]
- [70].B S., S T., J Z., M K., F H, M S, G S, Anal. Biochem 223 (1994) 218.7887467 [Google Scholar]
- [71].Tissot B, Gasiunas N, Powell AK, Ahmed Y, Zhi Z.-l., Haslam SM, Morris HR, Turnbull JE, Gallagher JT, Dell A, Towards GAG glycomics: Analysis of highly sulfated heparins by MALDI-TOF mass spectrometry Glycobiology 17 (2007) 972–982. [DOI] [PubMed] [Google Scholar]
- [72].Juhasz P, Biemann K, Mass spectrometric molecular-weight determination of highly acidic compounds of biological significance via their complexes with basic polypeptides Proceedings of the National Academy of Sciences of the United States of America 91 (1994) 4333–4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bultel L, Landoni M, Grand E, Couto AS, Kovensky J, UV-MALDI-TOF mass spectrometry analysis of heparin oligosaccharides obtained by nitrous acid controlled degradation and high performance anion exchange chromatography J Am Soc Mass Spectrom 21 (2010) 178–190. [DOI] [PubMed] [Google Scholar]
- [74].Takano T, Yamanouchi Y, Assignment of human beta-galactosidase-A gene to 3p21.33 by fluorescence in situ hybridization Human genetics 92 (1993) 403–404. [DOI] [PubMed] [Google Scholar]
- [75].Sisu E, Flangea C, Serb A, Zamfir AD, Modern developments in mass spectrometry of chondroitin and dermatan sulfate glycosaminoglycans Amino acids 41 (2011) 235–256. [DOI] [PubMed] [Google Scholar]
- [76].Hsieh CC, Guo JY, Hung SU, Chen R, Nie Z, Chang HC, Wu CC, Quantitative analysis of oligosaccharides derived from sulfated glycosaminoglycans by nanodiamond-based affinity purification and matrix-assisted laser desorption/ionization mass spectrometry Analytical chemistry 85 (2013) 4342–4349. [DOI] [PubMed] [Google Scholar]
- [77].Nakamura H, Tamura Z, Gas chromatographic analysis of mono- and disaccharides in human blood and urine after oral administration of disaccharides Clinica chimica acta; international journal of clinical chemistry 39 (1972) 367–381. [DOI] [PubMed] [Google Scholar]
- [78].Gordon BA, Haust MD, The mucopolysaccharidoses types I, II, and 3: urinary findings in 23 cases Clinical biochemistry 3 (1970) 203–215. [PubMed] [Google Scholar]
- [79].Pennock CA, Charles RG, Stansbie D, Glycosaminoglycan fractions in normal human urine Annals of clinical biochemistry 12 (1975) 207–211. [DOI] [PubMed] [Google Scholar]
- [80].Taniguchi N, Age differences in the pattern of urinary glycosaminoglycan excretion in normal individuals Clinica chimica acta; international journal of clinical chemistry 37 (1972) 225–233. [DOI] [PubMed] [Google Scholar]
- [81].Murphy D, Pennock CA, London KJ, Gas-liquid chromatographic measurement of glucosamine and galactosamine content of urinary glycosaminoglycans Clinica chimica acta; international journal of clinical chemistry 53 (1974) 145–152. [DOI] [PubMed] [Google Scholar]
- [82].O’Brien JF, Gerritsen T, Helmuth AC, Identification of acid mucopolysaccharides by gas chromatographic analysis of component sugars after chemical depolymerization Analytical biochemistry 56 (1973) 465–479. [DOI] [PubMed] [Google Scholar]
- [83].Bach G, Friedman R, Weissmann B, Neufeld EF, The defect in the Hurler and Scheie syndromes: deficiency of -L-iduronidase Proceedings of the National Academy of Sciences of the United States of America 69 (1972) 2048–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].TOIDA T, QIU G, MATSUNAGA T, SAGEHASHI Y, IMANARI T, Gas chromatography-mass spectrometric determinations of iduronic and glucuronic acids in glycosaminoglycans after reduction of carboxylic group using sodium borodeuteride Analytical sciences 8 (1992) 799–804. [Google Scholar]
- [85].Li DX, Gan L, Bronja A, Schmitz OJ, Gas chromatography coupled to atmospheric pressure ionization mass spectrometry (GC-API-MS): review Anal Chim Acta 891 (2015) 43–61. [DOI] [PubMed] [Google Scholar]
- [86].Toyoda H, Yamamoto H, Ogino N, Toida T, and Imanari T, Rapid and sensitive analysis of disaccharide composition in heparin and heparan sulfate by reversed-phase ion-pair chromatography on a 2 μm porous silica gel column. Journal of Chromatography A 830 (1999) 197–201. [Google Scholar]
- [87].Toyoda H, Nagashima T, Hirata R, Toida T, Imanari T, Sensitive high-performance liquid chromatographic method with fluorometric detection for the determination of heparin and heparan sulfate in biological samples: application to human urinary heparan sulfate Journal of chromatography. B, Biomedical sciences and applications 704 (1997) 19–24. [DOI] [PubMed] [Google Scholar]
- [88].Studelska DR, Giljum K, McDowell LM, Zhang L, Quantification of glycosaminoglycans by reversed-phase HPLC separation of fluorescent isoindole derivatives Glycobiology 16 (2006) 65–72. [DOI] [PubMed] [Google Scholar]
- [89].Cheetham N, Sirimanne P, High-performance liquid chromatographic separation of carbohydrate oligomers Journal of Chromatography A 207 (1981) 439–444. [Google Scholar]
- [90].Cheetham N.W.H.a.T., G., Some applications of reversed-phase high-performance liquid chromatography to oligosaccharide separations Journal of Chromatography B Biomedical Sciences and Applications (J Chrom B Biomed Sci Appl) 336 (1984) 161–172. [Google Scholar]
- [91].Cheng PW, High-performance liquid chromatographic analysis of galactosamine, glucosamine, glucosaminitol, and galactosaminitol Analytical biochemistry 167 (1987) 265–269. [DOI] [PubMed] [Google Scholar]
- [92].Kodama C, Ototani N, Isemura M, Aikawa J, Yosizawa Z, Liquid-chromatographic determination of urinary glycosaminoglycans for differential diagnosis of genetic mucopolysaccharidoses Clinical chemistry 32 (1986) 30–34. [PubMed] [Google Scholar]
- [93].Karousou EG, Viola M, Vigetti D, Genasetti A, Rizzi M, Clerici M, Bartolini B, Luca GD, Passi A, Analysis of glycosaminoglycans by electrophoretic approach Current Pharmaceutical Analysis 4 (2008) 78–89. [Google Scholar]
- [94].Radhakrishnamurthy B, Dalferes ER Jr, Berenson GS, Determination of hexuronic acids in glycosaminoglycans by automated ion-exchange chromatography Analytical biochemistry 75 (1976) 160–167. [DOI] [PubMed] [Google Scholar]
- [95].Linhardt R, Gu K, Loganathan D, Carter S, Analysis of glycosaminoglycan-derived oligosaccharides using reversed-phase ion-pairing and ion-exchange chromatography with suppressed conductivity detection Analytical biochemistry 181 (1989) 288–296. [DOI] [PubMed] [Google Scholar]
- [96].Campo GM, Campo S, Ferlazzo AM, Vinci R, Calatroni A, Improved high-performance liquid chromatographic method to estimate aminosugars and its application to glycosaminoglycan determination in plasma and serum Journal of Chromatography B: Biomedical Sciences and Applications 765 (2001) 151–160. [DOI] [PubMed] [Google Scholar]
- [97].Tomatsu S, Okamura K, Maeda H, Taketani T, Castrillon SV, Gutierrez MA, Nishioka T, Fachel AA, Orii KO, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Laybauer LS, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Haskins M, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Okuyama T, Tanaka A, Noguchi A, Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses Journal of inherited metabolic disease 28 (2005) 187–202. [DOI] [PubMed] [Google Scholar]
- [98].Tomatsu S, Gutierrez MA, Ishimaru T, Pena OM, Montano AM, Maeda H, Velez-Castrillon S, Nishioka T, Fachel AA, Cooper A, Thornley M, Wraith E, Barrera LA, Laybauer LS, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ullrich K, Isogai K, Suzuki Y, Orii T, Noguchi A, Heparan sulfate levels in mucopolysaccharidoses and mucolipidoses Journal of inherited metabolic disease 28 (2005) 743–757. [DOI] [PubMed] [Google Scholar]
- [99].Hintze JP, Tomatsu S, Fujii T, Montano AM, Yamaguchi S, Suzuki Y, Fukushi M, Ishimaru T, Orii T, Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine Biomarker insights 6 (2011) 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Dung VC, Tomatsu S, Montano AM, Gottesman G, Bober MB, Mackenzie W, Maeda M, Mitchell GA, Suzuki Y, Orii T, Mucopolysaccharidosis IVA: correlation between genotype, phenotype and keratan sulfate levels Molecular genetics and metabolism 110 (2013) 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Perkins KJ, Muller V, Weber B, Hopwood JJ, Prediction of Sanfilippo phenotype severity from immunoquantification of heparan-N-sulfamidase in cultured fibroblasts from mucopolysaccharidosis type IIIA patients Molecular genetics and metabolism 73 (2001) 306–312. [DOI] [PubMed] [Google Scholar]
- [102].Ginsberg SD, Galvin JE, Lee VM, Rorke LB, Dickson DW, Wolfe JH, Jones MZ, Trojanowski JQ, Accumulation of intracellular amyloid-beta peptide (A beta 1–40) in mucopolysaccharidosis brains Journal of neuropathology and experimental neurology 58 (1999) 815–824. [DOI] [PubMed] [Google Scholar]
- [103].Shibutani T, Nishino W, Shiraki M, Iwayama Y, ELISA detection of glycosaminoglycan (GAG)-linked proteoglycans in gingival crevicular fluid Journal of periodontal research 28 (1993) 17–20. [DOI] [PubMed] [Google Scholar]
- [104].Yang JA, Kim ES, Kwon JH, Kim H, Shin JH, Yun SH, Choi KY, Hahn SK, Transdermal delivery of hyaluronic acid -- human growth hormone conjugate Biomaterials 33 (2012) 5947–5954. [DOI] [PubMed] [Google Scholar]
- [105].Langford-Smith KJ, Mercer J, Petty J, Tylee K, Church H, Roberts J, Moss G, Jones S, Wynn R, Wraith JE, Bigger BW, Heparin cofactor II-thrombin complex and dermatan sulphate:chondroitin sulphate ratio are biomarkers of short- and long-term treatment effects in mucopolysaccharide diseases Journal of inherited metabolic disease 34 (2011) 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pal AR, Langereis EJ, Saif MA, Mercer J, Church HJ, Tylee KL, Wynn RF, Wijburg FA, Jones SA, Bruce IA, Bigger BW, Sleep disordered breathing in mucopolysaccharidosis I: a multivariate analysis of patient, therapeutic and metabolic correlators modifying long term clinical outcome Orphanet journal of rare diseases 10 (2015) 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Langford-Smith K, Arasaradnam M, Wraith JE, Wynn R, Bigger BW, Evaluation of heparin cofactor II-thrombin complex as a biomarker on blood spots from mucopolysaccharidosis I, IIIA and IIIB mice Molecular genetics and metabolism 99 (2010) 269–274. [DOI] [PubMed] [Google Scholar]
- [108].Chang Y, Yang B, Weyers A, Linhardt RJ, Capillary electrophoresis for the analysis of glycosaminoglycan-derived disaccharides Methods Mol Biol 984 (2013) 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hopwood JJ, Harrison JR, High-resolution electrophoresis of urinary glycosaminoglycans: an improved screening test for the mucopolysaccharidoses Analytical biochemistry 119 (1982) 120–127. [DOI] [PubMed] [Google Scholar]
- [110].Cappelletti R, Del Rosso M, Chiarugi VP, A new electrophoretic method for the complete separation of all known animal glycosaminoglycans in a monodimensional run Analytical biochemistry 99 (1979) 311–315. [DOI] [PubMed] [Google Scholar]
- [111].Duteil S, Gareil P, Girault S, Mallet A, Feve C, Siret L, Identification of heparin oligosaccharides by direct coupling of capillary electrophoresis/Ionspray- Mass spectrometry Rapid communications in mass spectrometry 13 (1999) 1889–1898. [DOI] [PubMed] [Google Scholar]
- [112].Lamoree M, Reinhoud N, Tjaden U, Niessen W, Van der Greef J, On- capillary isotachophoresis for loadability enhancement in capillary zone electrophoresis/mass spectrometry of β- agonists Biological mass spectrometry 23 (1994) 339–345. [DOI] [PubMed] [Google Scholar]
- [113].Lazar IM, Xin B, Lee ML, Lee ED, Rockwood AL, Fabbi JC, Lee HG, Design of a time-of-flight mass spectrometer as a detector for capillary electrophoresis Analytical Chemistry 69 (1997) 3205–3211. [Google Scholar]
- [114].Bielik AM, Zaia J, Historical overview of glycoanalysis Methods Mol Biol 600 (2010) 9–30. [DOI] [PubMed] [Google Scholar]
- [115].Zaia J, Principles of mass spectrometry of glycosaminoglycans J Biomacromol Mass Spectrom 1 (2005) 3–36. [Google Scholar]
- [116].Oguma T, Toyoda H, Toida T, Imanari T, Analytical method of heparan sulfates using high-performance liquid chromatography turbo-ionspray ionization tandem mass spectrometry Journal of chromatography. B, Biomedical sciences and applications 754 (2001) 153–159. [DOI] [PubMed] [Google Scholar]
- [117].Oguma T, Toyoda H, Toida T, Imanari T, Analytical method for keratan sulfates by high-performance liquid chromatography/turbo-ionspray tandem mass spectrometry Analytical biochemistry 290 (2001) 68–73. [DOI] [PubMed] [Google Scholar]
- [118].Oguma T, Toyoda H, Toida T, Imanari T, Analytical method of chondroitin/dermatan sulfates using high performance liquid chromatography/turbo ionspray ionization mass spectrometry: application to analyses of the tumor tissue sections on glass slides Biomedical chromatography : BMC 15 (2001) 356–362. [DOI] [PubMed] [Google Scholar]
- [119].Oguma T, Tomatsu S, Montano AM, Okazaki O, Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry Analytical biochemistry 368 (2007) 79–86. [DOI] [PubMed] [Google Scholar]
- [120].Oguma T, Tomatsu S, Okazaki O, Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry Biomedical chromatography : BMC 21 (2007) 356–362. [DOI] [PubMed] [Google Scholar]
- [121].Osago H, Shibata T, Hara N, Kuwata S, Kono M, Uchio Y, Tsuchiya M, Quantitative analysis of glycosaminoglycans, chondroitin/dermatan sulfate, hyaluronic acid, heparan sulfate, and keratan sulfate by liquid chromatography-electrospray ionization-tandem mass spectrometry Analytical biochemistry 467 (2014) 62–74. [DOI] [PubMed] [Google Scholar]
- [122].Kubaski F, Brusius-Facchin AC, Mason RW, Patel P, Burin MG, Michelin-Tirelli K, Kessler RG, Bender F, Leistner-Segal S, Moreno CA, Cavalcanti DP, Giugliani R, Tomatsu S, Elevation of glycosaminoglycans in the amniotic fluid of a fetus with mucopolysaccharidosis VII Prenatal diagnosis 37 (2017) 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kubaski F, Mason RW, Nakatomi A, Shintaku H, Xie L, van Vlies NN, Church H, Giugliani R, Kobayashi H, Yamaguchi S, Suzuki Y, Orii T, Fukao T, Montano AM, Tomatsu S, Newborn screening for mucopolysaccharidoses: a pilot study of measurement of glycosaminoglycans by tandem mass spectrometry Journal of inherited metabolic disease 40 (2017) 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kubaski F, Suzuki Y, Orii K, Giugliani R, Church HJ, Mason RW, Dung VC, Ngoc CT, Yamaguchi S, Kobayashi H, Girisha KM, Fukao T, Orii T, Tomatsu S, Glycosaminoglycan levels in dried blood spots of patients with mucopolysaccharidoses and mucolipidoses Molecular genetics and metabolism 120 (2017) 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Kida K, Kubota M, Barrera L, Orii T, Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry Journal of inherited metabolic disease 33 Suppl 3 (2010) S35–42. [DOI] [PubMed] [Google Scholar]
- [126].Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Sakura N, Barrera L, Kida K, Kubota M, Orii T, Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis I Journal of inherited metabolic disease 33 (2010) 141–150. [DOI] [PubMed] [Google Scholar]
- [127].Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Barrera LA, Kida K, Kubota M, Orii T, Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry Molecular genetics and metabolism 99 (2010) 124–131. [DOI] [PubMed] [Google Scholar]
- [128].Tomatsu S, Shimada T, Mason RW, Kelly J, LaMarr WA, Yasuda E, Shibata Y, Futatsumori H, Montano AM, Yamaguchi S, Suzuki Y, Orii T, Assay for Glycosaminoglycans by Tandem Mass Spectrometry and its Applications Journal of analytical & bioanalytical techniques 2014 (2014) 006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons GJ, Esko JD, Crawford BE, Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses Nature chemical biology 8 (2012) 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Lawrence R, Olson SK, Steele RE, Wang L, Warrior R, Cummings RD, Esko JD, Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling The Journal of biological chemistry 283 (2008) 33674–33684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Auray-Blais C, Bherer P, Gagnon R, Young SP, Zhang HH, An Y, Clarke JT, Millington DS, Efficient analysis of urinary glycosaminoglycans by LC-MS/MS in mucopolysaccharidoses type I, II and VI Molecular genetics and metabolism 102 (2011) 49–56. [DOI] [PubMed] [Google Scholar]
- [132].Auray-Blais C, Lavoie P, Zhang H, Gagnon R, Clarke JT, Maranda B, Young SP, An Y, Millington DS, An improved method for glycosaminoglycan analysis by LC-MS/MS of urine samples collected on filter paper Clinica chimica acta; international journal of clinical chemistry 413 (2012) 771–778. [DOI] [PubMed] [Google Scholar]
- [133].Auray-Blais C, Lavoie P, Tomatsu S, Valayannopoulos V, Mitchell JJ, Raiman J, Beaudoin M, Maranda B, Clarke JT, UPLC-MS/MS detection of disaccharides derived from glycosaminoglycans as biomarkers of mucopolysaccharidoses Analytica chimica acta 936 (2016) 139–148. [DOI] [PubMed] [Google Scholar]
- [134].Zhang H, Wood T, Young SP, Millington DS, A straightforward, quantitative ultra-performance liquid chromatography-tandem mass spectrometric method for heparan sulfate, dermatan sulfate and chondroitin sulfate in urine: an improved clinical screening test for the mucopolysaccharidoses Molecular genetics and metabolism 114 (2015) 123–128. [DOI] [PubMed] [Google Scholar]
- [135].Zhang H, Young SP, Millington DS, Quantification of glycosaminoglycans in urine by isotope- dilution liquid chromatography- electrospray ionization tandem mass spectrometry Current protocols in human genetics 76 (2013) 17.12. 11–17.12. 14. [DOI] [PubMed] [Google Scholar]
- [136].Zhang H, Young SP, Auray-Blais C, Orchard PJ, Tolar J, Millington DS, Analysis of glycosaminoglycans in cerebrospinal fluid from patients with mucopolysaccharidoses by isotope-dilution ultra-performance liquid chromatography-tandem mass spectrometry Clinical chemistry 57 (2011) 1005–1012. [DOI] [PubMed] [Google Scholar]
- [137].Tanaka N, Kida S, Kinoshita M, Morimoto H, Shibasaki T, Tachibana K, Yamamoto R, Evaluation of cerebrospinal fluid heparan sulfate as a biomarker of neuropathology in a murine model of mucopolysaccharidosis type II using high-sensitivity LC/MS/MS Molecular genetics and metabolism 125 (2018) 53–58. [DOI] [PubMed] [Google Scholar]
- [138].Trim PJ, Lau AA, Hopwood JJ, Snel MF, A simple method for early age phenotype confirmation using toe tissue from a mouse model of MPS IIIA Rapid communications in mass spectrometry : RCM 28 (2014) 933–938. [DOI] [PubMed] [Google Scholar]
- [139].Trim PJ, Hopwood JJ, Snel MF, Butanolysis derivatization: improved sensitivity in LC-MS/MS quantitation of heparan sulfate in urine from mucopolysaccharidosis patients Analytical chemistry 87 (2015) 9243–9250. [DOI] [PubMed] [Google Scholar]
- [140].Forni G, Malvagia S, Funghini S, Scolamiero E, Mura M, Della Bona M, Villanelli F, Damiano R, la Marca G, LC-MS/MS method for simultaneous quantification of heparan sulfate and dermatan sulfate in urine by butanolysis derivatization Clinica chimica acta; international journal of clinical chemistry 488 (2019) 98–103. [DOI] [PubMed] [Google Scholar]
- [141].McEwen CN, McKay RG, Larsen BS, Analysis of solids, liquids, and biological tissues using solids probe introduction at atmospheric pressure on commercial LC/MS instruments Analytical Chemistry 77 (2005) 7826–7831. [DOI] [PubMed] [Google Scholar]
- [142].Ricochon G, Paris C, Girardin M, Muniglia L, Highly sensitive, quick and simple quantification method for mono and disaccharides in aqueous media using liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry (LC-APCI-MS) Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 879 (2011) 1529–1536. [DOI] [PubMed] [Google Scholar]
- [143].Zhou X, Cheng Z, Liling RL, Guo X, Liu Z, Yu P, Determination of Glucosamine in Human Plasma by High-Performance Liquid Chromatography-Atmospheric Pressure Chemical Ionization Source-TandemMass Spectrometry Chromatography Research International 2011 (2011) 1–7. [Google Scholar]
- [144].Rashed MS, Ozand PT, Bucknall MP, Little D, Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry Pediatric research 38 (1995) 324–331. [DOI] [PubMed] [Google Scholar]
- [145].Shimada T, Kelly J, LaMarr WA, van Vlies N, Yasuda E, Mason RW, Mackenzie W, Kubaski F, Giugliani R, Chinen Y, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T, Tomatsu S, Novel heparan sulfate assay by using automated high-throughput mass spectrometry: Application to monitoring and screening for mucopolysaccharidoses Molecular genetics and metabolism 113 (2014) 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kyranos JN, Cai H, Wei D, Goetzinger WK, High-throughput high-performance liquid chromatography/mass spectrometry for modern drug discovery Current opinion in biotechnology 12 (2001) 105–111. [DOI] [PubMed] [Google Scholar]
- [147].Cai Z, Han C, Harrelson S, Fung E, Sinhababu AK, High-throughput analysis in drug discovery: application of liquid chromatography/ion-trap mass spectrometry for simultaneous cassette analysis of alpha-1a antagonists and their metabolites in mouse plasma Rapid communications in mass spectrometry : RCM 15 (2001) 546–550. [DOI] [PubMed] [Google Scholar]
- [148].Gao L, Cheng X, Zhang J, Burns DJ, A generic fast solid-phase extraction high-performance liquid chromatography/mass spectrometry method for high-throughput drug discovery Rapid communications in mass spectrometry : RCM 21 (2007) 3497–3504. [DOI] [PubMed] [Google Scholar]
- [149].Ding X, Ghobarah H, Zhang X, Jaochico A, Liu X, Deshmukh G, Liederer BM, Hop CE, Dean B, High-throughput liquid chromatography/mass spectrometry method for the quantitation of small molecules using accurate mass technologies in supporting discovery drug screening Rapid communications in mass spectrometry : RCM 27 (2013) 401–408. [DOI] [PubMed] [Google Scholar]
- [150].Rohman M, Wingfield J, High-Throughput Screening Using Mass Spectrometry within Drug Discovery Methods in molecular biology 1439 (2016) 47–63. [DOI] [PubMed] [Google Scholar]
- [151].Wu X, Wang J, Tan L, Bui J, Gjerstad E, McMillan K, Zhang W, In vitro ADME profiling using high-throughput rapidfire mass spectrometry: cytochrome p450 inhibition and metabolic stability assays Journal of biomolecular screening 17 (2012) 761–772. [DOI] [PubMed] [Google Scholar]
- [152].Paiva A, Shou WZ, Recent developments in software tools for high-throughput in vitro ADME support with high-resolution MS Bioanalysis 8 (2016) 1723–1733. [DOI] [PubMed] [Google Scholar]
- [153].Luippold AH, Arnhold T, Jorg W, Kruger B, Sussmuth RD, Application of a rapid and integrated analysis system (RIAS) as a high-throughput processing tool for in vitro ADME samples by liquid chromatography/tandem mass spectrometry Journal of biomolecular screening 16 (2011) 370–377. [DOI] [PubMed] [Google Scholar]
- [154].Zhang J, Vath M, Ferraro C, Li Y, Murphy K, Zvyaga T, Weller H, Shou W, A high-speed liquid chromatography/tandem mass spectrometry platform using multiplexed multiple-injection chromatography controlled by single software and its application in discovery ADME screening Rapid communications in mass spectrometry : RCM 27 (2013) 731–737. [DOI] [PubMed] [Google Scholar]
- [155].US B, S. A, S. D, S. M, Application of Mass Spectroscopy in Pharmaceutical and Biomedical Analysis, in: Sharmin E.a.Z., F (Ed.), Spectroscopic Analyses - Developments and Applications, IntechOpen; London, UK, 2017. [Google Scholar]
- [156].Yang B, Chang Y, Weyers AM, Sterner E, Linhardt RJ, Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography-mass spectrometry Journal of chromatography. A 1225 (2012) 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Khan SA, Mason RW, Giugliani R, Orii K, Fukao T, Suzuki Y, Yamaguchi S, Kobayashi H, Orii T, Tomatsu S, Glycosaminoglycans analysis in blood and urine of patients with mucopolysaccharidosis Molecular genetics and metabolism 125 (2018) 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Yu Y, Zhang F, Colon W, Linhardt RJ, Xia K, Glycosaminoglycans in human cerebrospinal fluid determined by LC-MS/MS MRM Analytical biochemistry 567 (2019) 82–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Auray-Blais C, Lavoie P, Maranda B, Boutin M, Evaluation of urinary keratan sulfate disaccharides in MPS IVA patients using UPLC-MS/MS Bioanalysis 8 (2016) 179–191. [DOI] [PubMed] [Google Scholar]
- [160].Da Col R, Silvestro L, Naggi A, Torri G, Baiocchi C, Moltrasio D, Cedro A, Viano I, Characterization of the chemical structure of sulphated glycosaminoglycans after enzymatic digestion. Application of liquid chromatography-mass spectrometry with an atmospheric pressure interface Journal of chromatography 647 (1993) 289–300. [DOI] [PubMed] [Google Scholar]
- [161].Bowman MJ, Zaia J, Tags for the stable isotopic labeling of carbohydrates and quantitative analysis by mass spectrometry Analytical chemistry 79 (2007) 5777–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hitchcock AM, Costello CE, Zaia J, Glycoform quantification of chondroitin/dermatan sulfate using a liquid chromatography-tandem mass spectrometry platform Biochemistry 45 (2006) 2350–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yuan J, Hashii N, Kawasaki N, Itoh S, Kawanishi T, Hayakawa T, Isotope tag method for quantitative analysis of carbohydrates by liquid chromatography-mass spectrometry Journal of chromatography. A 1067 (2005) 145–152. [DOI] [PubMed] [Google Scholar]