Abstract
Background.
IL-9 and IL-33 can profoundly influence immune responses. As a necessary first step toward defining their impact in the rhesus macaque model we confirmed their endogenous expression and sequence identity and generated expression vectors for the recombinant expression of rhesus IL-9 and IL-33.
Methods.
RT-PCR and Sanger sequencing was used to define the expression and sequences for rhesus IL-9 and IL-33. The resulting recombinant cytokines were tested by ELISA and proliferation assays.
Results.
Full length rhesus IL-9 and the mature form of rhesus IL-33 share 78% and 73% nucleotide similarity respectively with humans. Both cytokines are expressed in lymphocytes, with IL-9 expression also evident in CD4+ T cells. Recombinantly expressed rhesus IL-9 and IL-33 were each biologically active in vitro, including enhancing the proliferation of a rhesus B cell line.
Conclusions.
The recombinant rhesus IL-9 and IL-33 constructs produce biologically active cytokines that can act upon rhesus B cells.
Keywords: rhesus, IL-9, IL-33, B cell, proliferation
Introduction
The cytokines IL-9 and IL-33 can substantially influence immune responses including regulating T cells and B cells, yet the extent of their functions remains unclear. To enable translational research with these cytokines in nonhuman primates we defined their expression and sequence in rhesus macaque lymphocytes and generated plasmids for their functional expression.
Both IL-9 and IL-33 can influence B cell and antibody responses. IL-9 is an IL-2Rγc-chain cytokine that was first purified and characterized as a T cell and mast cell growth factor, termed P40, based on molecular weight and mast cell growth-enhancing activity, respectively [1, 2]. T cells are a major source of IL-9 and IL-9 production was first associated with the CD4+ T helper 2 (Th2) phenotype, and many of the primary functions of IL-9 were confirmed in models of Th2-associated immunity [3]. However, in the last decade Th9 cells, which appear in many ways to be distinct from Th2, have been described and increasingly better resolved [4–7]. TGF-β, IL-4, and IL-2 combined promote the differentiation of Th9 cells [8], additionally, IL-4 together with IL-1β in the absence of TGF-β has also been reported to promote Th9 differentiation [9]. Indeed, the ability of TGF-β to promote IL-9 expression is likely responsible for the ability of Th17 and T regulatory (Treg) subpopulations to also secrete IL-9 [7, 10, 11]. It has been shown that IL-9 can enhance B cell and antibody responses [12–15], including by a T follicular helper (Tfh) cell subset that produces IL-9 and promotes the germinal center response for memory B cell development [16, 17]. An IL-9 producing CD8+ T cell subset has also been reported, that may have tumoricidal activity [18]. In addition to T cells, IL-9 is produced by other cell types including type II innate lymphocyte cells (ILC2) and mast cells, that all crosstalk with Th9 cells in processes that still remain poorly resolved, yet likely have substantial implications.
There are two IL-9R subunits: the α-chain (IL-9Rα) and the shared γ-chain receptor that is present in other cytokines including IL-2, IL-4 and IL-7 [19, 20]. The human IL-9R gene has 11 exons which encodes a 522 aa protein, whereas the mouse IL-9R gene encodes a 468 aa protein [21]. Signaling through IL-9 results in the activation of STAT1, STAT3, STAT5, MAPK and insulin receptor substrate–PI3K pathway [22–25]. IL-9R is expressed on various T cell subsets including effector-T cells, Th2, and Th17 cells, but not on naïve T cells [25–28]. IL-9R is also expressed by B cells, particularly GC B cells [15–17]. IL-9R is expressed on mast cells, polymorphonuclear cells in the lungs, non-hematopoietic cells, including airway epithelial cells which increase mucus production in response to IL-9, and by smooth muscle cells which produce IL-8, IL-13, and eotaxin in response to IL-9 [29–31], underlining its role in regulating mucosal immunity.
IL-33, a member of the IL-1 family is considered as an ‘alarmin’, increased as the result of tissue damage and stress. The human IL-33 gene is located on chromosome 9p24.1, while its mouse counterpart can be found on the syntenic chromosome 19qC1 region [32]. This cytokine is expressed by endothelial cells, fibroblasts, smooth muscle cells, osteoblast, adipocytes and several immune cells including macrophages and dendritic cells. IL-33 is both an intracellular nuclear factor and an extracellular cytokine. Intracellularly, IL-33 is found to possess transcriptional repressor properties and nuclear IL-33 reduces nuclear factor kappaB (NF-κB)-induced gene expression by sequestering nuclear NF-κB and thus dampening pro-inflammatory signaling. On the contrary, the p65 subunit of the NF-κB complex in the nucleus is upregulated by IL-33 and promotes inflammation in endothelial cells. Extracellularly, IL-33 acts as an inflammatory cytokine and exerts its biological effects via binding to a heterodimer receptor complex consisting of ST2 and the IL-1 receptor accessory protein (IL-1RAcP).
The IL-33 receptor (ST2) belongs to the IL-1 receptor superfamily and is located on chromosome 2q12.1 in humans [33]. ST2 is present on cardiomyocytes and a variety of immune cells including T cells, especially Th2 CD4+ T cells, Tregs, innate lymphoid cell type 2 (ILC2), M2 macrophages, granulocytes, mast cells, iNKT cells and natural killer (NK) cells [34–41]. IL-33 can enhance B cell and antibody responses indirectly through acting on ILC2 cells and stimulating their production of the Th2 cytokines IL-5 and IL-33, and also act directly particularly on B1 B cells which constitutively express ST2 [42, 43]. IL-33 promotes the development of IgM memory and mucosal IgA plasma cells [42, 44–46], and has been suggested to reduce tolerance, and promote the development of auto-reactive antibodies through the induction of BAFF [47–49]. Additionally, IL-33 treatment upregulates FoxP3 expression and the development of Tregs [35, 50], further highlighting the unique role of IL-33 in shaping T cell and B cell immunity.
Although many human cytokines are highly homologous with their rhesus macaque counterparts, human IL-9 and IL-33 may be functionally active in the rhesus macaque model but has not been thoroughly defined. Research on IL-9 and IL-33 has thus far been confined primarily to mice and humans, hence not yet taking utilizing the many advantages of rhesus macaques to better understand their influence on immunity. As we are interested in utilizing the rhesus macaque model for the development of effective vaccine strategies, we sought to define the IL-9 and IL-33 genes in rhesus and create vectors for their functional testing.
Materials & Methods
Ethics statement.
This study was carried out in strict accordance with the recommendations described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, the Office of Animal Welfare, and the U.S. Department of Agriculture [51]. All animal work was approved by the Oregon Health & Science University (OHSU) West Campus Institutional Animal Care and Use Committee. The facilities at the Oregon National Primate Research Center (ONPRC) are accredited by the American Association for Accreditation of Laboratory Animal Care International (AAALACi). All efforts were made to minimize animal suffering, and all procedures involving potential pain were performed with the appropriate anesthetic or analgesic. The minimal number of animals was used in this study to obtain scientifically sound outcomes.
Animals.
Adult rhesus macaques (Macaca mulatta) were housed at the ONPRC in Beaverton, OR. All animals were free of cercopithecine herpesvirus 1, D-type simian retrovirus, simian T lymphotrophic virus type 1, and SIV infection. All procedures were performed according to standard operating procedures and protocols approved by the Institutional Animal Care and Use Committee at OHSU, and all records are kept in the PRIMe database, the electronic medical record and billing system at the ONPRC.
Endogenous expression of IL-9 and IL-33 and sequencing.
Total RNA was extracted from flow cytometry sorted peripheral blood mononuclear cells (PBMC) using RNeasy Mini kit (Qiagen) according to the manufacturer’s specifications. cDNA synthesis for sorted cells were performed using qScript cDNA Synthesis Kits (QuantaBio). cDNA was used as template for PCR with primers specific for rhesus macaque IL-9, and IL-33 open reading frames (ORF). Primer sequences used for amplification are IL-9 forward primer 5′-GAATTCATGCTGCTGGCCAC-3′, IL-9 reverse primer- 5′-TTGCTAGCTCAGATCTTGCCGC-3′, IL-33 forward primer- 5′-TTGAAGAATTCTTCGGAATCTCCGGCGTGC-3′, and IL-33 reverse primer- 5′-TGGGCTAGCTCAGATCTCGGACAGCTTGAA-3′. Thermocycling conditions were as follows: an initial denaturation at 94 °C for 30 s, followed by 35 cycles of 94 °C for 15 s, 55 °C (IL-9) or 61 °C (IL-33) annealing for 30 s and 68 °C extension for 1 min and an infinite hold at 10 °C. The nucleotide and amino acid sequence alignments were done by clustal omega [52].
Expression of recombinant rhesus IL-9 and rhesus IL-33.
The rhesus IL-33 (mature IL-3395-270), and rhesus IL-9 (full length) inserts were commercially codon optimized and synthesized (Geneart). The resulting rhesus IL-9 and rhesus IL-33 inserts were digested by EcoRI and NheI at 37 °C for 1 hour, ran on an agarose-gel, followed by gel extraction. The digested fragments were ligated into pUNO1-ss vector (Invivogen), followed by transformation into E.coli DH5a. Resulting transformed colonies were selected and colony PCR performed with vector specific primers- pUNO-1 forward 5′-CCGGCGCCTACCTGAGATCA-3′ and pUNO-1 reverse 5′-CACTGCATTCTAGTTGTGGTTTGTC -3′. Inserts were confirmed by sequencing.
Cell culture and bioassays.
HEK293T cells were cultured and maintained in DMEM medium containing 10% FCS, 1X antibiotic-antimycotic (Thermo fisher scientific) and 1X L-glutamine (Thermo fisher scientific). One day before transfection HEK293T cells were seeded in 10 cm tissue culture plate containing 10 ml DMEM (5% FCSII) and incubated at 37 °C for 24 hours. 25 μl jet PRIME transfection reagent was added to 500 μl of jet PRIME buffer (Polyplus). 10 μg of plasmid rhesus IL-9 or rhesus IL-33 were added to the transfection mix and incubated for 20 min at room temperature. Fresh DMEM media containing 5% FCSII were added to the seeded HEK293T cells, the plasmid-DNA transfection mix were added to the seeded HEK293T cells, incubated at 37 °C. Every two days, cell supernatant was harvested and concentrated for recombinant protein formation. The concentrated rhesus IL-9 and rhesus IL-33 supernatant was used in various bioassays. MO7e cells were incubated with rhesus IL-9 supernatant (culture supernatant of 1:10 dilution) and 30 μg/ml of IL-9 neutralizing antibody (cat # AB-209-NA, R&D systems) whereas D10.G4.T1 were treated with rhesus IL-33 supernatant (culture supernatant with no dilution) and 5 μg/ml of IL-33 neutralizing antibody (cat # MAB36254,R&D systems), incubated for 3 days at 37°C and 5% CO2 and cell proliferation was measured by cell count using acridine orange and propidium iodide staining and a LUNA-FL automated cell counter (Logos Biosystems). 20 ng/ml of human recombinant IL-9 (cat # 209-ILB/CF, R&D systems) and 20 ng/ml of IL-33 (cat #200-33, Peprotech) were used as positive controls and pUNO-1 empty vector transfected cell supernatant (no dilution) was used as a negative control to treat the MO7e and D10.G4.T1. Similarly, rhesus BLCL-C162 cells (NHP Reagent Resource) were treated with rhesus IL-9 (no dilution) and IL-33 (no dilution) supernatant, incubated for 3 days at 37 °C 5% CO2 and cell proliferation was measured by cell count as mentioned previously.
ELISA.
To measure the expression of rhesus IL-9 and IL-33, human IL-9 (cat #88795888, Invitrogen) and human IL-33 (cat # DY3625B-05, R&D systems) ELISA kits were purchased and ELISA was performed according to manufacturer’s protocol.
Flow Cytometry and Intracellular staining.
To measure IL-9 and IL-33 expressing T cells, Rhesus PBMCs were stimulated with 50 ng/ml of PMA (cat # 1201/1, Fisher Scientific) and 2.5 μM of Ionomycin in DMEM medium and incubated for 4 h in the presence of GolgiStop (BD-Biosciences) at 37°C. Cells were washed at 350 g for 5 min with MACS buffer (MACS buffer+1% BSA). Cells were stained with live dead stain (cat # L34961, Molecular Probes) and incubated for 20 mins at room temperature. Cells were washed and stained with cell surface markers (CD4-BB790 (BD-custom), CD19-AF700(Beckman Coulter- cat # A78837), CD20-APC-Cy7(BD Biosciences cat # 335794), IgM-BV605 (BD-Biosciences- cat # 562977, CD3-BV650 (BD Biosciences cat # 563916), CD8-BUV496-cat # 564805), incubated for 1 hr. at 4°C. Cells were washed twice with MACS buffer and fixed/permeabilized according to manufacturer’s protocol (BD-Cytofix/Cytoperm Fixation/Permeabilization kit- Cat # 554714). After fixation/permeabilization cells were stained with IL-9-AF647 (BD Biosciences cat # 560813), incubated for 30 min at 4°C, washed twice in BD Perm/Wash buffer and analyzed by flow cytometry.
Statistical analysis.
The data are expressed as mean ± SEM. Statistical significance of differences was analyzed using Prism software (GraphPad Prism (version 7.0e, GraphPad Software Inc, La Jolla, CA) by unpaired, two-tailed Student’s t test. Values of p < 0.05 were considered statistically significant.
Results
Expression of IL-9 and IL-33 in rhesus macaque.
To examine the endogenous expression of IL-9 and IL-33, total peripheral blood mononuclear cells (PBMC) and CD4+ T cells were isolated from adult rhesus macaques, and expression of IL-9 and IL-33 mRNA were measured by RT-PCR. IL-9 transcript was expressed by both PBMCs and CD4+ T cells, whereas IL-33 expression was observed only in PBMCs (Figure 1A). Intracellular cytokine staining with anti-human IL-9 antibody demonstrated increased IL-9 producing CD4+ T cells following stimulation, representing ~0.5% of total CD4+ T cells (Figure 1B). Sequencing of IL-9 and IL-33 RT-PCR products confirmed their identity. Rhesus IL-9 identity to human was 78% nucleotide (Figure 1B) and 86% amino acid (Figure 2A) similarity, respectively. Rhesus IL-9 was 82% (Figure 1B) and 99% (Figure 2A) similar with the predicted nucleotide and amino acid reference sequences for rhesus (XM_001110897), (XP_001110897.2) respectively, with the exception of the conservation of N36 we observe with the human. The mature form of rhesus IL-33 had 73% nucleotide (Figure 1C) and 94% amino acid (Figure 2B) similarity to human, respectively. Rhesus IL-33 was 74% (Figure 1C) similar to the predicted rhesus nucleotide reference sequence (XM_028835178.1) and 100% identical to the predicted rhesus amino acid reference sequence (XP_028691011.1) (Figure 2B).
Figure 1: Expression of IL-9 and IL-33 in rhesus macaque.
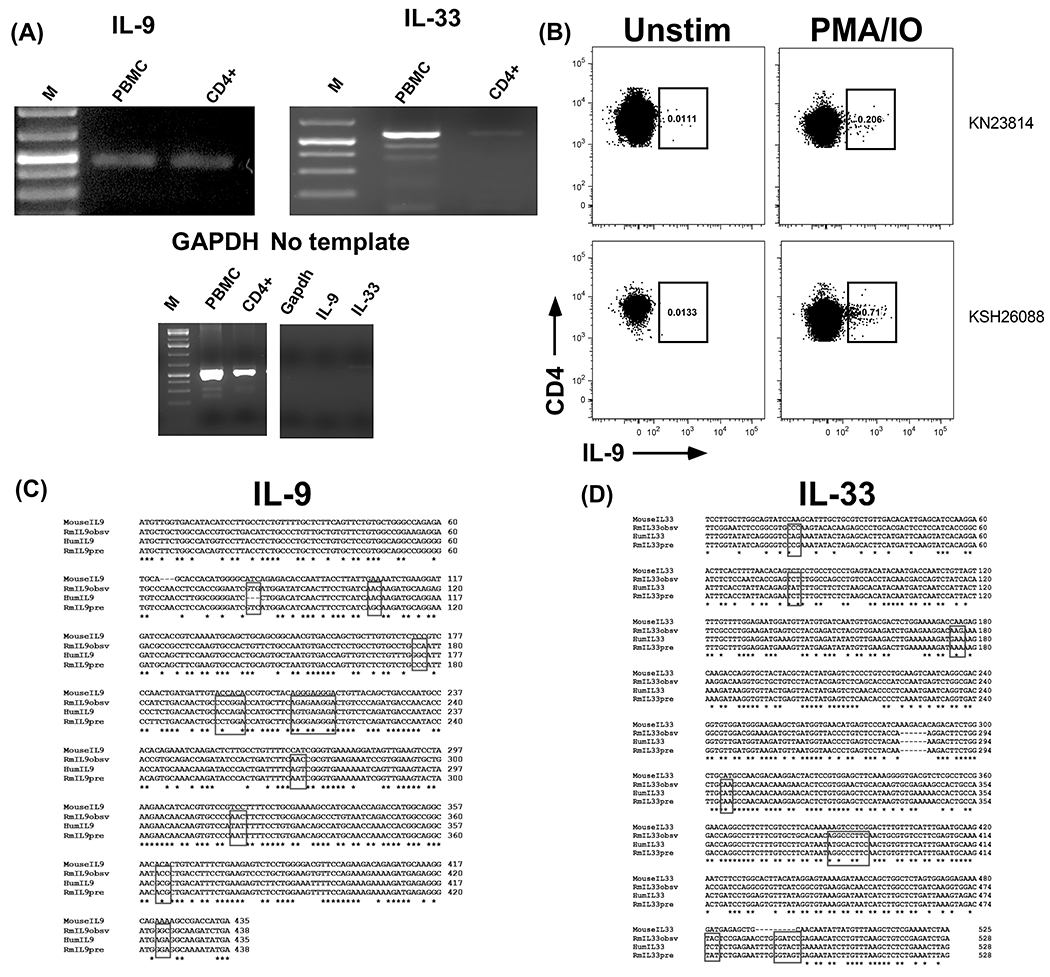
(A) RT-PCR amplification of IL-9 and IL-33 from rhesus PBMC and CD4+ T cells. (B) Expression of IL-9 by CD4+ T cells. PBMC from two rhesus macaques were stimulated with PMA and ionomycin for four hours, permeabilized, stained with anti-human IL-9 antibody, and analyzed by flow cytometry. Plots are gated on live, CD19-CD20-CD8-CD3+CD4+ T cells. Nucleotide alignment of rhesus observed (Rmobsv), predicted (Rmpre), mouse, and human (Hum) IL-9 (C) and IL-33 (D). The boxes indicate codon differences between the rhesus observed and human.
Figure 2: Amino acid sequences of rhesus macaque IL-9 and IL-33.
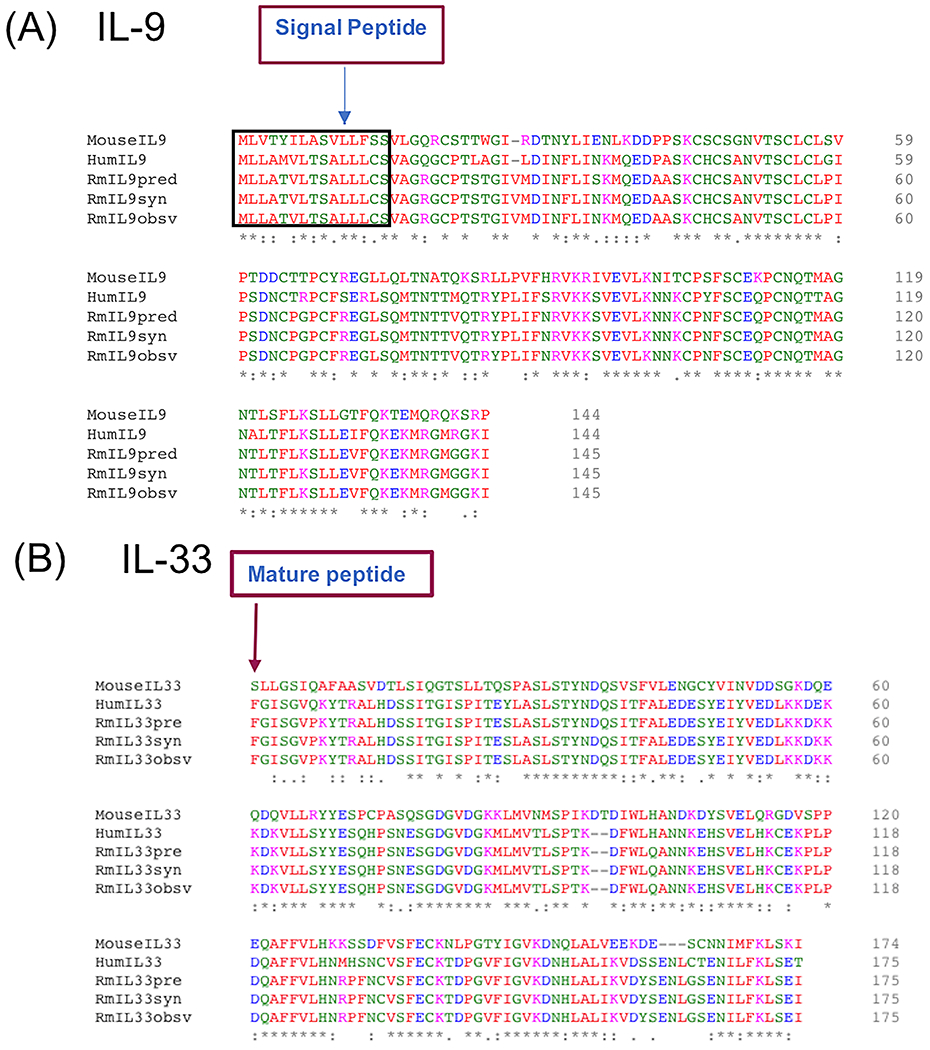
Amino acid alignments of mouse, human, predicted, and synthesized (syn) IL-9 (A) and IL-33 (B) proteins.
Cloning and expression of rhesus IL-9 and IL-33 proteins.
To enable future studies to discern the impact of IL-9 and IL-33 in rhesus we cloned rhesus IL-9 and IL-33 into the pUNO1 vector which drives gene expression through a hEF1/HTLV promoter. The resulting synthesized rhesus IL-9 (RmIL9syn) insert (Figure 2A) included the native rhesus IL-9 signal peptide, which differs from human IL-9 signal peptide by M5T. The pro-peptide of IL-33 was excluded from the insert (RmIL33syn), to enable efficient production and secretion of mature IL-33 (Figure 2B). HEK293T cells were transfected with the resulting rhesus IL-9 and IL-33 expression vectors to verify protein production. Testing of supernatant from transfected cells by ELISA demonstrated substantial recombinant rhesus IL-9 and IL-33 was produced (Figure 3).
Figure 3: Determination of recombinant IL-9 and IL-33 protein expression.
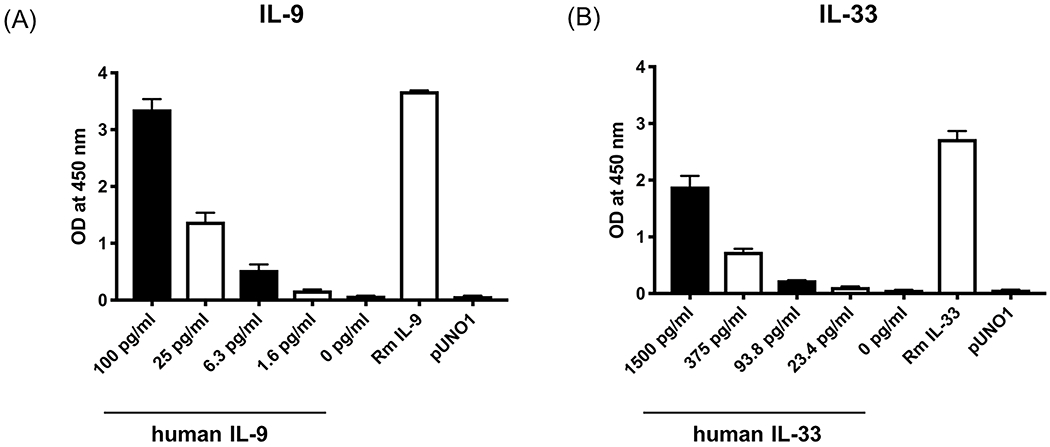
HEK293T cells were transfected with cloned rhesus IL-9 and IL-33 and supernatant was collected to measure the production of IL-9 (A) and IL-33 (B) protein by ELISA. Decreasing titrations of recombinant human cytokine included as positive control, supernatant from empty vector control transfected cells (pUNO1) included as negative control. Bars represent mean ± SEM of triplicate samples.
Rhesus IL-9 and IL-33 induce proliferation of MO7e cells and D10.G4.T cells
To confirm that the recombinant rhesus IL-9 and IL-33 were biologically active, the proliferation of cell lines with known responsiveness to human IL-9 or IL-33 was evaluated. Treatment of MO7e cells, a human megakaryocytic leukemia cell line responsive to IL-9 [53, 54], with supernatant from rhesus IL-9 transfected HEK293T cells induced significant proliferation (p<0.05) compared to empty vector control, that was abrogated in the presence of anti-IL-9 antibody (Figure 4A). Similarly, treatment of D10.G4.T1 cells, a murine Th2 CD4+ T cell line responsive to IL-33 [55], with rhesus IL-33 resulted in significant proliferation (p<0.05) as compared to empty vector control, that was abrogated by the presence of anti-IL-33 antibody (Figure 4B). The cell count-based assay was corroborated with MTT assay where similar results were observed (not shown).
Figure 4: Cell proliferation induced by recombinant IL-9 and IL-33.
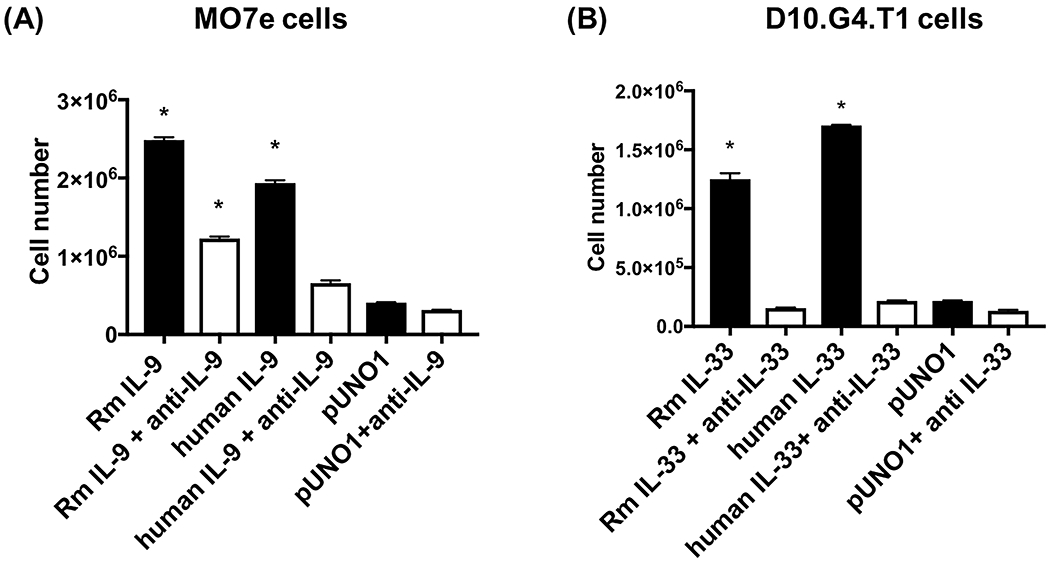
(A) Human MO7e cells were cultured with rhesus macaque IL-9 (RmIL-9), in the absence or presence of IL-9 neutralizing antibody for 3 days. (B) Mouse D10.G4.T1 cells were cultured with rhesus macaque IL-33 (RmIL-33) in the absence or presence of IL-33 neutralizing antibody for 3 days. Recombinant human IL-9 and IL-33 were used as positive control and empty vector pUNO1 as negative control. Bars represent mean ± SEM of triplicate samples. * indicates p<0.05 compared to pUNO1.
Rhesus IL-9 and IL-33 induce proliferation of rhesus BLCL cells.
To determine if the recombinant rhesus IL-9 and IL-33 have activity on rhesus lymphocytes, the BLCL-C162 rhesus B lymphoblastoid cell line was treated with increasing doses of rhesus IL-9 and IL-33. Both rhesus IL-9 and IL-33 significantly increased proliferation of BLCL cells (p<0.05), which was completely abrogated in the presence of corresponding neutralizing antibody (Figure 5), demonstrating that recombinant rhesus IL-9 and IL-33 produced in mammalian cells are each biologically active on rhesus lymphocytes.
Figure 5: BLCL-C162 cell proliferation induced by recombinant IL-9 and IL-33.
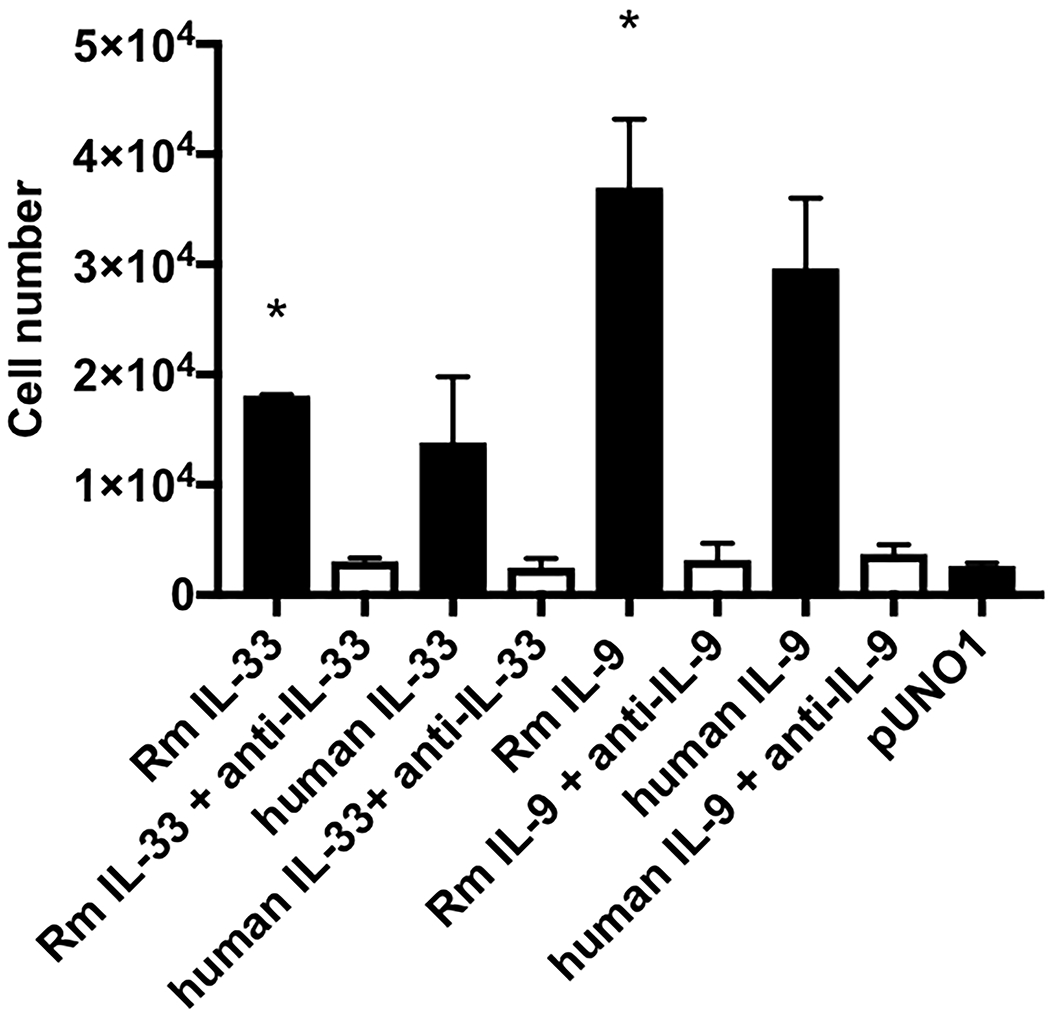
Rhesus BLCL-C162 cells were cultured for 3 days with rhesus macaque IL-9 or IL-33 in the presence or absence of neutralizing antibody. * indicates p<0.05 compared to pUNO1.
Discussion
To enable translational studies of IL-9 and IL-33 in the rhesus macaque model, we sought to determine their expression profiles, endogenous sequences, and generate subsequent vectors for their recombinant expression. IL-9 and IL-33 were expressed in rhesus PBMC, and IL-9, but not IL-33 expression was detected in rhesus CD4+ T cells. This is consistent with previous reports in mice and humans of the Th9 CD4+ helper T cell subset, which secretes IL-9. Isolation and functionally defining rhesus Th9, would likely be advantageous to basic immunology and vaccinology pursuits, as Th9 has been demonstrated to promote germinal center B cell responses, regulating the antibody somatic hypermutation and affinity maturation process. Among lymphocytes, IL-33 in mice and humans is expressed by Th2, ILC2, and B1 B cells, and further resolution of these cell populations in rhesus would likely confirm a similar expression profile in rhesus.
Both IL-9 and IL-33 are highly conserved between humans and rhesus, as evident by our sequencing results, detection with anti-human antibodies in ELISA, inhibition of rhesus IL-9 and IL-33 by anti-human antibodies, and the ability of the rhesus cytokines to act comparable to their human counterparts on the reporter cells. However, the range of impact of the differences in the cytokines between species is not defined in this study, and should be further defined, both by directed analysis of the differing residues in the described assays, but also by fully defining any potential structural and expression profile differences in the IL-9 and IL-33 receptors. This may be particularly relevant for IL-33 expression where different forms of IL-33 occur as a result of post translational modification, and have different biological activity with the mature form of IL-33 exhibiting 30 fold higher activity than the full length IL-33 [56, 57].
The recombinant rhesus IL-9 and IL-33 constructs we describe produce biologically active cytokines that can act upon rhesus B cells. With this basis, along with the known B cell enhancing activities of these cytokines from human and mice studies, we will advance these constructs in vivo in rhesus macaques to evaluate their impact on vaccine responses.
Acknowledgements
We are thankful for the expert primate veterinary oversight and care, provided by the staff of the Division of Comparative Medicine for husbandry and pathology services, and the staff of the Infectious Disease Nonhuman Primate Resource at the Oregon National Primate Research Center for sample collection.
This work was supported by funds from the U.S. Department of Health and Human Services, National Institutes of Health, R01DE027245 (JJK), and to the Oregon National Primate Research Center (P51OD011092 and U42OD010426).
References
- 1.Hultner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rude E, Dormer P, Van Snick J: Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9). Eur J Immunol 1990; 20:1413–1416. [DOI] [PubMed] [Google Scholar]
- 2.Uyttenhove C, Simpson RJ, Van Snick J: Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci U S A 1988; 85:6934–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gessner A, Blum H, Rollinghoff M: Differential regulation of IL-9-expression after infection with Leishmania major in susceptible and resistant mice. Immunobiology 1993; 189:419–435. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Chen S, Xiao X, Zhao Y, Ding W, Li XC: IL-9 and Th9 cells in health and diseases-From tolerance to immunopathology. Cytokine Growth Factor Rev 2017; 37:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK: IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol 2008; 9:1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B: Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 2008; 9:1341–1346. [DOI] [PubMed] [Google Scholar]
- 7.Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, Orban T, Elyaman W, Khoury SJ, Kuchroo VK, Baecher-Allan C, Hafler DA: TGF-beta induces IL-9 production from human Th17 cells. J Immunol 2010; 185:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kuhn R, Muller W, Palm N, Rude E: IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol 1994; 153:3989–3996. [PubMed] [Google Scholar]
- 9.Xue G, Jin G, Fang J, Lu Y: IL-4 together with IL-1beta induces antitumor Th9 cell differentiation in the absence of TGF-beta signaling. Nat Commun 2019; 10:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, Kuchroo VK, Khoury SJ: IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A 2009; 106:12885–12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, Maurer M, Rosenkranz AR, Wolf AM: IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol 2011; 186:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vink A, Warnier G, Brombacher F, Renauld JC: Interleukin 9-induced in vivo expansion of the B-1 lymphocyte population. J Exp Med 1999; 189:1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Li Q, Yang X, Li M: Interleukin-9 Is Associated with Elevated Anti-Double-Stranded DNA Antibodies in Lupus-Prone Mice. Mol Med 2015; 21:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leech MD, Grencis RK: Induction of enhanced immunity to intestinal nematodes using IL-9-producing dendritic cells. J Immunol 2006; 176:2505–2511. [DOI] [PubMed] [Google Scholar]
- 15.Fawaz LM, Sharif-Askari E, Hajoui O, Soussi-Gounni A, Hamid Q, Mazer BD: Expression of IL-9 receptor alpha chain on human germinal center B cells modulates IgE secretion. J Allergy Clin Immunol 2007; 120:1208–1215. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Shi J, Yan J, Xiao Z, Hou X, Lu P, Hou S, Mao T, Liu W, Ma Y, Zhang L, Yang X, Qi H: Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat Immunol 2017; 18:921–930. [DOI] [PubMed] [Google Scholar]
- 17.Takatsuka S, Yamada H, Haniuda K, Saruwatari H, Ichihashi M, Renauld JC, Kitamura D: IL-9 receptor signaling in memory B cells regulates humoral recall responses. Nat Immunol 2018; 19:1025–1034. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Hong B, Li H, Zheng Y, Zhang M, Wang S, Qian J, Yi Q: Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc Natl Acad Sci U S A 2014; 111:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renauld JC, Druez C, Kermouni A, Houssiau F, Uyttenhove C, Van Roost E, Van Snick J: Expression cloning of the murine and human interleukin 9 receptor cDNAs. Proc Natl Acad Sci U S A 1992; 89:5690–5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, et al. : Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science 1994; 266:1042–1045. [DOI] [PubMed] [Google Scholar]
- 21.Goswami R, Kaplan MH: A brief history of IL-9. J Immunol 2011; 186:3283–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer JH, Liu KD, You Y, Lai SY, Goldsmith MA: Heteromerization of the gammac chain with the interleukin-9 receptor alpha subunit leads to STAT activation and prevention of apoptosis. J Biol Chem 1998; 273:9255–9260. [DOI] [PubMed] [Google Scholar]
- 23.Demoulin JB, Louahed J, Dumoutier L, Stevens M, Renauld JC: MAP kinase activation by interleukin-9 in lymphoid and mast cell lines. Oncogene 2003; 22:1763–1770. [DOI] [PubMed] [Google Scholar]
- 24.Demoulin JB, Uyttenhove C, Van Roost E, DeLestre B, Donckers D, Van Snick J, Renauld JC: A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol Cell Biol 1996; 16:4710–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin T, Keller SR, Quelle FW, Witthuhn BA, Tsang ML, Lienhard GE, Ihle JN, Yang YC: Interleukin-9 induces tyrosine phosphorylation of insulin receptor substrate-1 via JAK tyrosine kinases. J Biol Chem 1995; 270:20497–20502. [DOI] [PubMed] [Google Scholar]
- 26.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ: IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med 2009; 206:1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosmi L, Liotta F, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Lasagni L, Vanini V, Romagnani P, Maggi E, Annunziato F, Romagnani S: Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood 2004; 103:3117–3121. [DOI] [PubMed] [Google Scholar]
- 28.Druez C, Coulie P, Uyttenhove C, Van Snick J: Functional and biochemical characterization of mouse P40/IL-9 receptors. J Immunol 1990; 145:2494–2499. [PubMed] [Google Scholar]
- 29.Neurath MF, Finotto S: IL-9 signaling as key driver of chronic inflammation in mucosal immunity. Cytokine Growth Factor Rev 2016; 29:93–99. [DOI] [PubMed] [Google Scholar]
- 30.Abdelilah S, Latifa K, Esra N, Cameron L, Bouchaib L, Nicolaides N, Levitt R, Hamid Q: Functional expression of IL-9 receptor by human neutrophils from asthmatic donors: role in IL-8 release. J Immunol 2001; 166:2768–2774. [DOI] [PubMed] [Google Scholar]
- 31.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, Kiener PA, Kolbeck R, Lloyd CM, Coyle AJ, Humbles AA: IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med 2011; 183:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA: IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005; 23:479–490. [DOI] [PubMed] [Google Scholar]
- 33.Griesenauer B, Paczesny S: The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front Immunol 2017; 8:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT: Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002; 106:2961–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Lohning M, Belkaid Y, Fallon PG, Powrie F: The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 2014; 513:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN: Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010; 464:1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, Shepherd M, McSharry C, McInnes IB, Xu D, Liew FY: IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol 2009; 183:6469–6477. [DOI] [PubMed] [Google Scholar]
- 38.Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R: The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J Immunol 1998; 161:4866–4874. [PubMed] [Google Scholar]
- 39.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H: A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol 2008; 121:1484–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE: IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol 2008; 20:1019–1030. [DOI] [PubMed] [Google Scholar]
- 41.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A, Kamradt T: T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A 1998; 95:6930–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komai-Koma M, Gilchrist DS, McKenzie AN, Goodyear CS, Xu D, Liew FY: IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol 2011; 186:2584–2591. [DOI] [PubMed] [Google Scholar]
- 43.Lund S, Walford HH, Doherty TA: Type 2 Innate Lymphoid Cells in Allergic Disease. Curr Immunol Rev 2013; 9:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kayamuro H, Yoshioka Y, Abe Y, Arita S, Katayama K, Nomura T, Yoshikawa T, Kubota-Koketsu R, Ikuta K, Okamoto S, Mori Y, Kunisawa J, Kiyono H, Itoh N, Nagano K, Kamada H, Tsutsumi Y, Tsunoda S: Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J Virol 2010; 84:12703–12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sattler S, Ling GS, Xu D, Hussaarts L, Romaine A, Zhao H, Fossati-Jimack L, Malik T, Cook HT, Botto M, Lau YL, Smits HH, Liew FY, Huang FP: IL-10-producing regulatory B cells induced by IL-33 (Breg(IL-33)) effectively attenuate mucosal inflammatory responses in the gut. J Autoimmun 2014; 50:107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarkar S, Piepenbrink MS, Basu M, Thakar J, Keefer MC, Hessell AJ, Haigwood NL, Kobie JJ: IL-33 enhances the kinetics and quality of the antibody response to a DNA and protein-based HIV-1 Env vaccine. Vaccine 2019; 37:2322–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose WA 2nd, Okragly AJ, Hu NN, Daniels MR, Martin AP, Koh YT, Kikly K, Benschop RJ: Interleukin-33 Contributes Toward Loss of Tolerance by Promoting B-Cell-Activating Factor of the Tumor-Necrosis-Factor Family (BAFF)-Dependent Autoantibody Production. Front Immunol 2018; 9:2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mu R, Huang HQ, Li YH, Li C, Ye H, Li ZG: Elevated serum interleukin 33 is associated with autoantibody production in patients with rheumatoid arthritis. J Rheumatol 2010; 37:2006–2013. [DOI] [PubMed] [Google Scholar]
- 49.Riviere E, Sellam J, Pascaud J, Ravaud P, Gottenberg JE, Mariette X: Serum IL-33 level is associated with auto-antibodies but not with clinical response to biologic agents in rheumatoid arthritis. Arthritis Res Ther 2018; 20:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao W, Guo S, Chen L, Luo Y: The role of Interleukin-33 in the modulation of splenic T-cell immune responses after experimental ischemic stroke. J Neuroimmunol 2019; 333:576970. [DOI] [PubMed] [Google Scholar]
- 51.Council NR: Guide for the care and use of laboratory animals, 8th ed Washington, DC: National Academies Press,, 2011. [PubMed] [Google Scholar]
- 52.Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, Lopez R: The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 2019; 47:W636–W641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang YC, Ricciardi S, Ciarletta A, Calvetti J, Kelleher K, Clark SC: Expression cloning of cDNA encoding a novel human hematopoietic growth factor: human homologue of murine T-cell growth factor P40. Blood 1989; 74:1880–1884. [PubMed] [Google Scholar]
- 54.Kang LY, Yang YC: Activation of junB and c-myc primary response genes by interleukin 9 in a human factor-dependent cell line. J Cell Physiol 1995; 163:623–630. [DOI] [PubMed] [Google Scholar]
- 55.Salmond RJ, Mirchandani AS, Besnard AG, Bain CC, Thomson NC, Liew FY: IL-33 induces innate lymphoid cell-mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol 2012; 130:1159–1166 e1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C: IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A 2012; 109:1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP: Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A 2014; 111:15502–15507. [DOI] [PMC free article] [PubMed] [Google Scholar]


