Abstract
Objective
Assess outcomes after intraoperative plasma transfusion in patients undergoing cardiac surgery.
Design
Retrospective study of adult cardiac surgical between 2011– 2015. Relationships between plasma transfusion volume, coagulation test values, and a primary outcome of early postoperative red blood cell (RBC) transfusion were assessed via multivariable regression analyses. Secondary outcomes included hospital mortality, intensive care unit (ICU) and hospital free days, intraoperative RBCs, estimated blood loss, and reoperation for bleeding.
Setting
Academic tertiary referral center.
Participants
1794 patients received intraoperative plasma transfusions during the study period.
Interventions
None.
Measurements and Main Results
Higher plasma transfusion volumes were associated with worse clinical outcomes, with each 1 unit increase being associated with greater odds for postoperative RBCs [OR 1.12 (1.04, 1.20); P=0.002], intraoperative [OR 1.85 (1.69, 2.03); P<0.001], and fewer hospital free days [mean −0.20 (−0.39, −0.01); P=0.04]. Each 0.1 increase in pre-transfusion International Normalized Ratio (INR) was associated with increased odds of postoperative and intraoperative RBCs, reoperation for bleeding, and fewer ICU and hospital free days. For given plasma volumes, patients achieving greater reduction in elevated pre-transfusion INR values experienced more favorable outcomes.
Conclusions
In patients undergoing cardiac surgery who received intraoperative plasma transfusion, higher plasma transfusion volumes were associated with inferior clinical outcomes. Higher pre-transfusion INR values were also associated with worse outcomes; however, those achieving a greater degree of INR correction after plasma transfusion demonstrated more favorable outcomes. Prospective studies related to plasma transfusion are needed to address this important topic.
Keywords: Plasma, INR, cardiac surgery, coagulation, bleeding, transfusion
Introduction
Perioperative bleeding necessitating allogenic transfusion is common in cardiac surgery with a prior study citing overall transfusion rates in excess of 50%.1 Intraoperative transfusion rates for plasma in patients undergoing cardiac surgery exceeds 20% in some studies1, 2 The coagulopathy associated with cardiac surgery utilizing cardiopulmonary bypass (CPB) is complex and multifactorial. There can be a quantitative reduction of coagulation factors resulting from both hemodilution and consumption. Fibrinolysis and platelet abnormalities (quantitative/qualitative) can further contribute to microvascular bleeding in the immediate post CPB period.3 Additionally, the presence of residual anticoagulants (e.g. heparin, warfarin, etc.) and ongoing surgical site bleeding can contribute to further derangements in the coagulation cascade. The historical mainstay of therapy to combat intraoperative bleeding due to coagulation factor deficiencies not related to congenital causes (i.e. hemophilia) has been allogenic plasma transfusion.4 The American Society of Anesthesiologists (ASA) Practice Guidelines for Perioperative Blood Management endorses the use of plasma for microvascular bleeding when the International Normalized Ratio (INR) is > 2.0.5 Additionally, more liberal plasma transfusion strategies (e.g. triggered by INR >1.6) have been suggested in cardiac surgical patients with microvascular bleeding.6 While the INR has long been recognized for its inadequacies in predicting bleeding risk, it remains a commonly utilized laboratory modality for assessing perioperative coagulation status as well as patient-specific responses to plasma transfusion.
Despite the guidelines mentioned above, there is an overall paucity of data to guide clinicians regarding intraoperative plasma transfusion in the cardiac surgical population. A prior study assessed outcomes related to intraoperative plasma administration amongst all surgical patients at our institution and determined that higher intraoperative plasma transfusion volumes were associated inferior clinical outcomes.7 However, the impact of intraoperative plasma transfusion in cardiac surgical patients remain incompletely defined.
The purpose of this study was to assess the relationships between intraoperative plasma transfusion volume, changes in coagulation test results, and the associations with clinical outcomes in patients undergoing cardiac surgery. We hypothesized that patients receiving higher intraoperative plasma volumes would have inferior clinical outcomes.
Materials & Methods
This is a retrospective, single-center, cohort study conducted with approval from the Mayo Clinic (Rochester, Minnesota) Institutional Review Board with waived written informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used in the design and conduct of this study, as well as in the reporting of results.8
Inclusion criteria were: 1) age ≥ 18 years; 2) cardiac surgery; 3) presence of an intraoperative INR value obtained prior to any intraoperative plasma transfusion(s); 4) presence of intraoperative plasma transfusion (occurring after the qualifying pre-transfusion INR value and before surgical closure) between January 1st 2011 and December 31st 2015, and; 5) presence of a post-transfusion INR value, defined as the first INR value measured after the last unit of intraoperative plasma (within 24 hours). Only INR values and plasma transfusion episodes occurring after the cessation of cardiopulmonary bypass (CPB) were included.
Exclusion criteria included: 1) lack of research authorization; 2) plasma utilized as part of therapeutic plasma exchange or apheresis; 3) a normal pre-transfusion INR (i.e. ≤ 1.1); 4) prior inclusion in the study; 5) non-cardiac surgery; 6) congenital cardiac operations, ventricular assist device placement or exchange, and extracorporeal membrane oxygenator initiation; and 7) cardiac operations without use of CPB. For patients receiving intraoperative plasma transfusion during multiple surgical encounters during the study period, only the first intraoperative encounter was included.
Screening for potential study participants was performed using an institutional data warehouse called the OR DataMart, which captures transfusion data for all patients at the study institution.9 In addition, this resource contains clinical and procedural data for all patients admitted to an acute care environment. Transfusion details were extracted from a related data warehouse called the Transfusion DataMart, which provides detailed information regarding all transfused blood products (e.g. product type, volumes), exact transfusion timings (i.e. order time, issue time, administration times), and related laboratory variables (e.g. pre-transfusion and post-transfusion hemoglobin, INR, platelet counts, and fibrinogen values). Additional pertinent baseline characteristics were obtained from a second validated database, the Advanced Cohort Explorer.10 Both databases have undergone extensive validation with accuracy superior to manual data collection alone.11 Data pertinent to cardiac surgical operations were obtained from The Society of Thoracic Surgeons (STS) database.
The primary exposure variables of interest were the volume of plasma transfused (aim 1) and the change in INR and R-time values (aim 2). Additional potentially confounding variables of interest included demographic features, surgical characteristics (e.g. cardiac operation type, redo-sternotomy, surgery length, perfusion time and aortic cross clamp time, emergency surgery, estimated blood loss), clinical features [e.g. left ventricular ejection fraction, STS score, Charlson comorbidity scores, Sequential Organ Failure Assessment (SOFA) scores at the time of surgical incision, ASA physical status, comorbid medical conditions], perioperative transfusions, and perioperative laboratory tests. Cardiac operations were categorized as isolated coronary artery bypass surgery (CAB) or valve surgery (Valve), combined CAB+Valve, combined CAB+Valve+Other, or Other. The “Other” operations included for example, aorta surgery, cardiac mass removal, myectomy, patent foramen ovale closure, left atrial appendage ligation, surgical MAZE procedure, or combinations of the above not classified elsewhere. Thromboelastography derived R-time values were included if the pre-transfusion TEG was obtained within the hour preceding the first intraoperative plasma transfusion and the post-transfusion TEG was obtained within 6 hours following the last unit of intraoperative plasma. R-time values obtained after the first intraoperative unit but preceding additional units of intraoperative plasma were not included. Similarly, R-time values obtained after the last intraoperative plasma unit but without corresponding pre-transfusion values were not included.
The primary outcome of interest was the need for early postoperative RBC transfusion (defined as RBC transfusion in the first 24 postoperative hours), with secondary outcomes of reoperation for bleeding, hospital mortality, estimated blood loss (EBL) ICU free days, and hospital free days. Free days were defined by subtracting the ICU or hospital length of stay in days from 28, with patients dying during the ICU or hospital stay receiving a score of zero. Patients with ICU or hospital lengths of stay greater than 28 days also received a score of zero. For example, if a patient was discharged alive after a length of stay of three days, their hospital free days was 28–3=25. If, however, they died after 4 days, their hospital free days were 0. This outcome was chosen instead of simple length of stay to account for death, and avoid early death (hence short length of stay) being viewed as a favorable outcome statistically. Of note, intraoperative RBC transfusions and EBL were utilized as covariates to account for the severity of the surgical insult rather than outcome variables in primary analyses. However, recognizing that these variables may represent clinically important outcomes for intraoperative plasma transfusion, sensitivity analyses were performed utilizing 1) intraoperative RBC transfusions occurring after the first plasma unit, and 2) EBL as outcome variables. In these instances, only pre-transfusion characteristics were utilized as covariates with explicit exclusion of intraoperative features (i.e. surgery length, intraoperative transfusions of platelets and cell-salvaged blood, intraoperative RBC transfusions given prior to plasma, intraoperative factor concentrate use).
Throughout the study period a well-established transfusion algorithm was used to guide plasma transfusion (and other hemostatic interventions).6 The indication for algorithm based plasma transfusion is presence of microvascular bleeding as determined by anesthesiologist and surgeon consensus and PT > 16.6 sec / INR >1.6, and/or aPTT >57 sec via ACL-IL Top 500 ® analyzer (Werfen; Bedford, MA). Indication for platelet transfusion is a platelet count <102 × 109/L and/or TEG MA <48mm, and for cryoprecipitate when the fibrinogen is < 144 mg/dl (all assuming return of ACT to within 10% baseline). Of note, at the study institution, antithrombin concentrates are used for treatment of antithrombin deficiency with inadequate heparinization, and hence plasma is not utilized for this purpose. Red blood cell transfusion triggers were not standardized in cardiac surgical patients during the study period, however, institutionally-endorsed guidelines for RBC transfusion formulated by a multidisciplinary team of anesthesiologists, hematologists, surgeons, pathologists, and transfusion medicine specialists were readily available to all providers (accessed through the internal web server), which included the following indications for RBC transfusion: 1) active bleeding with cardiovascular instability at any hemoglobin; 2) at hemoglobin ≤ 8 g/dL in the setting of coronary artery disease, signs of end-organ ischemia, acute brain injury, or symptoms related to anemia (e.g. unexplained hypotension, tachycardia, chest pain, heart failure); 3) at hemoglobin from 8 to 10 g/dL in the setting of acute coronary syndromes; and 4) at hemoglobin ≤ 7 g/dL in hemodynamically stable, non-bleeding patients. In cardiac surgery, it is common practice to target a hemoglobin concentration of ≥ 8.0 g/dL at surgical completion and in the early postoperative period (e.g. first 24 hours). Institutional guidelines for RBC transfusion, as described above, were similarly applicable in the postoperative care of these patients.
Statistical Analysis
We followed methods similar to those employed in a study of plasma transfusion in patients across all surgical subspecialties.7 This current work focuses solely on patients undergoing cardiac surgery, with expanded surgical details extracted from the surgical record and the STS database. Briefly, descriptive statistics, frequencies and percentages for categorical variables and medians and interquartile ranges for continuous variables, were used to summarize baseline demographics and intraoperative characteristics. Differences in the distribution of baseline characteristics across categorized plasma dose per ml/kg (<10 vs. 10+) were compared using chi-square/fisher exact tests for categorical variables (where appropriate) and Wilcoxon rank-sum tests for continuous variables. The outcomes of postoperative RBCs within 24 hours, in hospital mortality, ICU and hospital free days, RBC transfusion intraoperatively after the first plasma unit transfused, intraoperative EBL, and reoperation for bleeding were also descriptively summarized by plasma dose per mL/kg.
Associations between intraoperative plasma dose (per 1000 ml) with postoperative INR and R were analyzed using multivariable linear regression. Associations between plasma dose (per 1 unit, defined by a typical unit volume of 300 ml), pre-transfusion INR, pre – post transfusion INR, pre-transfusion R, and pre – post transfusion R with postoperative and intraoperative outcomes were analyzed using multivariable regression models. Postoperative RBC use (yes/no?), hospital mortality, intraoperative RBC use after initial plasma transfusion (yes/no?), and reoperation for bleeding were analyzed using logistic regression, while ICU free days, hospital free days, and estimated blood loss were analyzed using linear regression. Each model was adjusted for potentially important confounding variables including demographic features (age, sex, BMI), preoperative laboratory values (hemoglobin, creatinine, platelet count), intraoperative resuscitation features (platelet transfusion volume, total crystalloid volume, colloid volume, allogeneic RBC volume, cell-salvage volume), severity of comorbid illness (Charlson score, SOFA score, left ventricular ejection fraction), and surgical features (perfusion time, aortic cross-clamp time, operation type, and redo-sternotomy). When analyzing INR and plasma volumes, and for all outcomes but hospital mortality, we had the power to additionally adjust for the effects of preoperative medications (aspirin, clopidogrel, warfarin, NSAIDs, heparin or low-molecular-weight heparin, vasopressors, inotropes within 24 hours of the procedure), intraoperative factor concentrate administration (including prothrombin complex concentrates and single factor replacements), and additional surgical features (surgery length, emergency surgery, and EBL). Only the preoperative adjustment terms were included for the intraoperative outcomes, since inclusion of intraoperative terms in those models would be taking into account future information. Finally, only adjusted models were used (as detailed above) when analyzing relationships between outcomes and pre-transfusion R-times and changes in R-times, since TEG data were only available for roughly 20% of the sample.
We used a multiple imputation approach with 10 independent imputed data sets to fill in missing values (sex 0.1%, surgery time 1.0%, hemoglobin and ASA physical status 1.6%, creatinine and plasma volume 3.6%, anesthesia type 4.2%, body mass index 5.0%, and EBL 6.2%).12, 13
Multiple sensitivity analyses were planned a priori, including additionally adjusting for STS prediction score (only applicable for 40% of patients), and repeating the analysis based upon 3 unique categories of pre-transfusion INR (≤ 1.5, between 1.5 and 2, and ≥ 2) and high or low intraoperative allogeneic RBC requirements (≥3 units for high, <3 units for low). Additionally, multivariable regression analyses to examine the association between plasma dose (ml/kg) and the primary and secondary outcomes were performed. All statistical analyses were performed using computer software (SAS Version 9.4, SAS Institute Inc., Cary, NC). A two-sided P value < 0.05 was considered significant.
Results
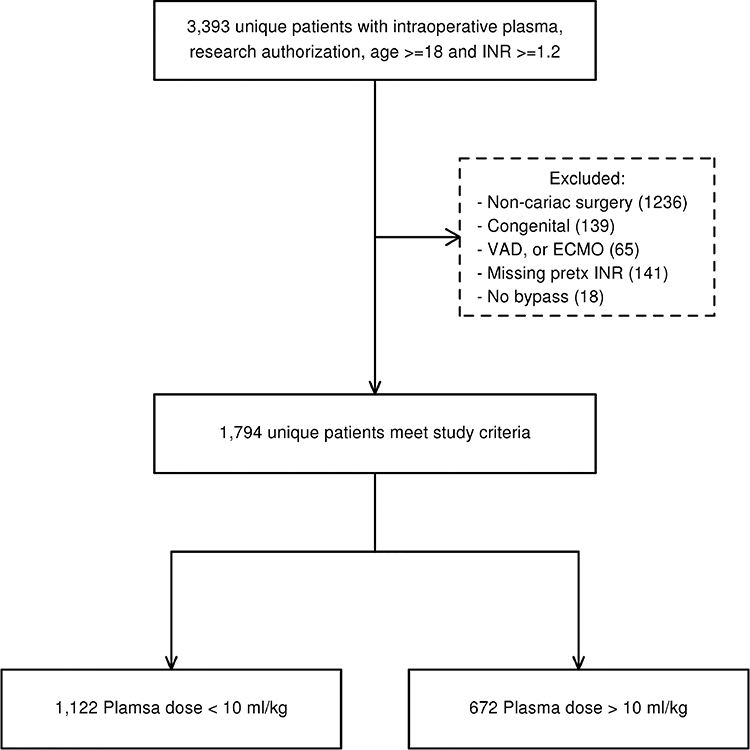
A total of 1794 unique patients were included (Figure 1). Table 1 displays the baseline demographic, clinical, and laboratory features for the cohort, categorized into plasma volumes at a threshold of 10 ml/kg. The median (IQR) pre-transfusion and post-transfusion INR values for the cohort were 1.7 (1.6–1.9) and 1.3 (1.2–1.4), respectively. The median time from pre-transfusion INR measurement to plasma transfusion was 0.9 (0.7– 1.9) hours, and the median time from last intraoperative plasma transfusion end to post-transfusion INR measurement was 1.2 (0.8– 1.6) hours. The median (IQR) pre-transfusion and post-transfusion R values were 7.3 (6.1, 9.9) and 6.8 (5.7, 8.3) respectively. The median (IQR) number of plasma units transfused was 2 (2– 4). Patients with a pre-transfusion INR ≥2 tended to receive more plasma units, as 51% of those with INR ≥2.0 received 4+ plasma units, compared to 22% and 38% for those with INR between 1.5–1.9 and < 1.5, respectively (p < 0.001). Thirty-six percent of patients underwent isolated CAB or Valve surgery, followed by 13.4% CAB+Valve, 8.8% CAB+Valve+Other, and 41.5% were “Other”. The median CPB perfusion and cross-clamp times were 115 minutes and 80 minutes respectively. Approximately 39.9% of patients received intraoperative RBCs (median, 1 unit) after the qualifying plasma transfusion, 64% received platelets (median volume 326 mL), and 12% received cryoprecipitate (median volume 208 mL). Unadjusted postoperative outcomes are displayed in Table 2. With regard to postoperative event rates, 250 (18.0%) patients received a postoperative RBC transfusion within 24 hours, 88 (4.9 %) died during the hospitalization, and 127 (7.1%) required reoperation for bleeding (Table 2).
Figure 1.
Study population flow diagram.
Table 1.
Demographic and clinical characteristics of patients receiving intraoperative plasma transfusion by categorized plasma ml per kg.
| Characteristic | <10 N=1122 | 10+ N=672 | Total N=1794 | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 73 (64, 80) | 72 (63, 80) | 73 (63, 80) | 0.44 † |
| Male sex | 794 (71.8%) | 418 (62.2%) | 1212 (68.2%) | <0.001 ‡ |
| BMI (kg/m2) | 29.1 (25.9, 33.4) | 26.9 (23.5, 30.8) | 28.3 (24.9, 32.5) | <0.001 † |
| Patient comorbidities | ||||
| Preoperative Charlson score | 5 (4, 7) | 5 (4, 7) | 5 (4, 7) | 0.83 † |
| Preoperative SOFA score | 4 (2, 5) | 5 (4, 6) | 4 (3, 6) | <0.001 † |
| STS Predicted Mortality Applicable | 560 (49.9%) | 185 (27.5%) | 745 (41.5%) | <0.001 ‡ |
| STS Predicted Mortality (%) | 2.2 (1.1, 4.4) | 3.8 (1.6, 7.1) | 2.4 (1.2, 5.1) | <0.001 † |
| Laboratory values | ||||
| Preoperative INR | 1.7 (1.6, 1.8) | 1.8 (1.6, 2.1) | 1.7 (1.6, 1.9) | <0.001 † |
| Preoperative INR | <0.001 ‡ | |||
| <1.5 | 75 (6.7%) | 55 (8.2%) | 130 (7.2%) | |
| 1.5–1.9 | 882 (78.6%) | 383 (57.0%) | 1265 (70.5%) | |
| ≥2 | 165 (14.7%) | 234 (34.8%) | 399 (22.2%) | |
| Postoperative INR* | 1.3 (1.2, 1.4) | 1.3 (1.2, 1.4) | 1.3 (1.2, 1.4) | 0.13 † |
| INR Decrease (Pre-Post) | 0.4 (0.3, 0.5) | 0.5 (0.3, 0.7) | 0.4 (0.3, 0.6) | <0.001 † |
| N with TEG R time values | 204 (18.2%) | 170 (25.3%) | 374 (20.8%) | <0.001 ‡ |
| Preoperative R time | 7.3 (5.9, 9.2) | 7.6 (6.3, 10.9) | 7.3 (6.1, 9.9) | 0.08 † |
| Postoperative R time | 6.5 (5.7, 8.1) | 7.2 (5.7, 8.7) | 6.8 (5.7, 8.3) | 0.03 † |
| R time Decrease (Pre-Post) | 0.6 (−0.9, 2.5) | 0.7 (−1.5, 3.4) | 0.6 (−1.0, 2.9) | 0.70 † |
| Preoperative hemoglobin | 13.2 (11.6, 14.4) | 12.3 (10.7, 13.7) | 12.9 (11.1, 14.2) | <0.001 † |
| Preoperative creatinine | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.3) | 0.50 † |
| Preoperative platelet count | 191 (160, 232) | 185.0 (142.5, 234.5) | 188 (156, 232) | 0.01 † |
| Preoperative medications | ||||
| Clopidogrel within 14 days | 184 (16.6%) | 82 (12.2%) | 266 (14.9%) | 0.01 ‡ |
| Aspirin within 7 days | 761 (68.7%) | 446 (66.4%) | 1207 (67.8%) | 0.31 ‡ |
| NSAIDs within 7 days | 94 (8.5%) | 45 (6.7%) | 139 (7.8%) | 0.17 ‡ |
| Warfarin within 5 days | 331 (29.9%) | 257 (38.2%) | 588 (33.0%) | <0.001 ‡ |
| Factor Xa inhibitor within 5 days | 23 (2.1%) | 8 (1.2%) | 31 (1.7%) | 0.17 ‡ |
| Direct thrombin inhibitor within 5 days | 22 (2.0%) | 13 (1.9%) | 35 (2.0%) | 0.94 ‡ |
| Therapeutic heparin infusion within 1 day | 8 (0.7%) | 4 (0.6%) | 12 (0.7%) | 1.00 § |
| Therapeutic LMW heparin within 1 day | 25 (2.3%) | 22 (3.3%) | 47 (2.6%) | 0.19 ‡ |
| Vitamin K within 1 day | 11 (1.0%) | 12 (1.8%) | 23 (1.3%) | 0.15 ‡ |
| Vasopressors or inotropes within 24 hours | 33 (3.0%) | 50 (7.4%) | 83 (4.7%) | <0.001 ‡ |
| Surgical characteristics | ||||
| Operation Category | <0.001 ‡ | |||
| CAB or Valve only | 467 (41.6%) | 185 (27.5%) | 652 (36.3%) | |
| CAB+Valve | 164 (14.6%) | 77 (11.5%) | 241 (13.4%) | |
| CAB+Valve+Other | 100 (8.9%) | 57 (8.5%) | 157 (8.8%) | |
| Other | 391 (34.8%) | 353 (52.5%) | 744 (41.5%) | |
| REDO-sternotomy | 186 (16.6%) | 187 (27.8%) | 373 (20.8%) | <0.001 ‡ |
| Ejection fraction | 60 (52, 65) | 58 (45, 65) | 60 (50, 65) | <0.001 † |
| Perfusion time | 104 (73, 145) | 137 (96, 202) | 115 (79, 166) | <0.001 † |
| Cross clamp time | 75.5 (51.5, 105.0) | 90 (56, 131) | 80.0 (53.5, 112.0) | <0.001 † |
| ASA PS | 3 (3, 3) | 3 (3, 4) | 3 (3, 4) | <0.001 † |
| Emergency Procedure | 22 (2.0%) | 41 (6.1%) | 63 (3.5%) | <0.001 ‡ |
| Intraoperative transfusion characteristics | ||||
| Intraoperative EBL (ml) | 1313 (904, 1758) | 1654 (1131, 2769) | 1423 (972, 1994) | <0.001 † |
| Number of plasma units given | 2 (2, 2) | 4 (2, 4) | 2 (2, 3) | <0.001 † |
| Number of plasma units given | <0.001 ‡ | |||
| Missing | 4 | 0 | 4 | |
| 1 | 111 (9.9%) | 0 (0.0%) | 111 (6.2%) | |
| 2 | 819 (73.3%) | 57 (8.5%) | 876 (48.9%) | |
| 3 | 151 (13.5%) | 117 (17.4%) | 268 (15.0%) | |
| 4+ | 37 (3.3%) | 498 (74.1%) | 535 (29.9%) | |
| Plasma volume (ml) | 560 (537, 595) | 1142.5 (932.5, 1885.5) | 599 (551, 1095) | <0.001 † |
| Plasma dose (ml/kg) | 6.7 (5.5, 8.0) | 15.2 (12.1, 22.9) | 8.3 (6.2, 13.0) | <0.001 † |
| RBCs Transfused Intraop After Primary FFP | 280 (25.0%) | 435 (64.7%) | 715 (39.9%) | <0.001 ‡ |
| RBC units after FFP (if any) | 1 (1, 2) | 2 (1, 3) | 1 (1, 2) | <0.001 † |
| Platelets | 602 (53.7%) | 554 (82.4%) | 1156 (64.4%) | <0.001 ‡ |
| Platelet volume (ml) | 290 (252, 393) | 534 (293, 810) | 326 (278, 580) | <0.001 † |
| Cryoprecipitate | 46 (4.1%) | 166 (24.7%) | 212 (11.8%) | <0.001 ‡ |
| Cryoprecipitate volume (ml) | 203 (189, 214) | 210 (196, 387) | 208.0 (194.0, 267.5) | 0.002 † |
| Cell saver volume (ml) | 661.5 (457.0, 882.0) | 831 (571, 1388) | 713 (492, 998) | <0.001 † |
| Total IV Crystalloid volume (ml) | 1678 (1100, 2300) | 1800 (1234, 2640) | 1730 (1171, 2430) | <0.001 † |
| Intraop CPB Crystalloid (ml) | 2923 (2222, 3753) | 3140 (2350, 4348) | 3000 (2264, 3945) | <0.001 † |
| Colloid volume (ml) | 2279.9 (1633.7, 3121.3) | 4079.3 (2817.7, 6264.0) | 2785.0 (1910.7, 3966.6) | <0.001 † |
| Prothrombin complex concentrates | 4 (0.4%) | 25 (3.7%) | 29 (1.6%) | <0.001 § |
Numbers indicate N (%) and median (Q1, Q3).
Wilcoxon
Chi-square
Fisher exact
Total plasma units was unavailable for 4 patients (all in the < 10 ml/kg group), though total plasma volume was available
Abbreviations: ASA PS – American Society of Anesthesiologists Physical Status classification score; CAB – coronary artery bypass; CPB – cardiopulmonary bypass; EBL – estimated blood loss; FFP – fresh frozen plasma; INR – international normalized ration; Intraop – intraoperative; IV – intravenous; NSAIDs – non-steroidal anti-inflammatory drugs; R time – thromboelastography R time; RBC – red blood cells; SOFA – sequential organ failure assessment; STS – Society of Thoracic Surgeons; TEG – thromboelastography
Table 2.
Outcomes of patients receiving intraoperative plasma transfusion by plasma volume (ml/kg).
| Characteristic | <10 N=1122 | 10+ N=672 | Total N=1794 |
|---|---|---|---|
| Early postoperative RBCs | 150 (19.4%) | 100 (16.2%) | 250 (18.0%) |
| In-hospital Mortality | 32 (2.9%) | 56 (8.3%) | 88 (4.9%) |
| ICU Free Days | 26.9 (25.4, 27.1) | 25.2 (20.3, 27.0) | 26.3 (24.1, 27.1) |
| Hospital Free Days | 20.7 (16.6, 22.6) | 16.7 (1.3, 20.7) | 19.7 (13.3, 21.8) |
| Intraoperative RBCs after plasma | 280 (25.0%) | 435 (64.7%) | 715 (39.9%) |
| Intraoperative RBC volume (units) | 0 (0, 0) | 1 (0, 3) | 0 (0, 1) |
| Intraoperative EBL (ml) | 1313 (904, 1758) | 1654 (1131, 2769) | 1423 (972, 1994) |
| Reoperation for bleeding | 42 (3.8%) | 85 (12.7%) | 127 (7.1%) |
Numbers indicate N (%) and median (Q1, Q3).
Abbreviations: EBL – estimated blood loss; ICU – intensive care unit; RBC – red blood cells
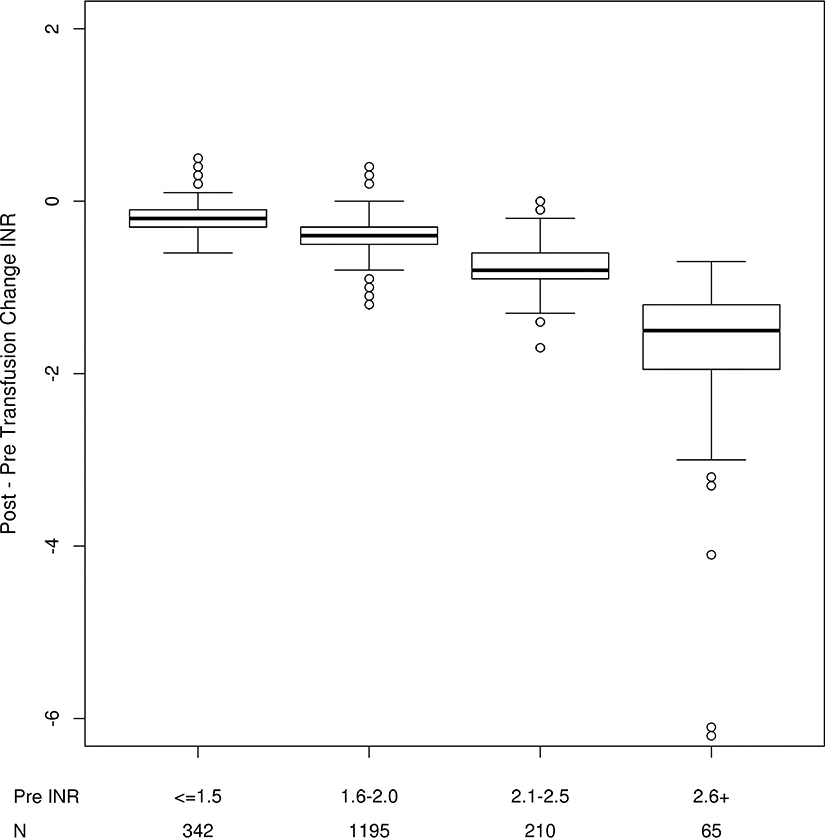
The magnitude of INR change (pre - post transfusion) was significantly different between groups with differing pre-transfusion INR values (Figure 2). Patients with higher pre-transfusion INR values tended to have larger decreases from pre –post INR. There was no significant association between increased plasma volume (per 1000 ml) and change in post-transfusion R times [mean decrease = 0.51 seconds; 95% CI (−1.70, 2.71); P=0.65].
Figure 2.
Change in INR by severity of pre-transfusion INR.
Plasma Transfusion Volume & Clinical Outcomes (aim 1)
Intraoperative plasma dose (per unit) was significantly associated with multiple outcomes (Table 3), even after multivariable adjustment. Each additional plasma unit was associated with increased odds of RBC transfusion in the 24 hours following surgery [OR 1.12 (1.04, 1.20); P=0.002], increased odds of intraoperative RBCs after the 1st unit of plasma [OR 1.85 (1.69, 2.03); P<0.001], and decreased hospital free days [mean −0.20 (−0.39, −0.01); P=0.04]. When analyzed by increases in plasma dose per ml/kg, the findings assimilated the primary analysis with the addition of less ICU free days (data not shown).
Table 3.
Multivariable Regression Models Examining Associations With Outcomes.
| Postoperative RBCs † | Hospital Mortality †¶ | ICU Free Days ‡ | Hospital Free Days ‡ | Intraop RBCs †§ | EBL (per 100 ml) ‡§ | Reoperation for Bleed † | |
|---|---|---|---|---|---|---|---|
| Outcome | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Mean Estimate (95% CI) | Mean Estimate (95% CI) | Odds Ratio (95% CI) | Mean Estimate (95% CI) | Odds Ratio (95% CI) |
| Plasma dose (per unit) †† | 1.12 (1.04, 1.20) ** | 0.94 (0.84, 1.05) | −0.01 (−0.19, 0.18) | −0.20 (−0.39, −0.01) * | 1.85 (1.69, 2.03) *** | 28.55 (−144.09, 201.20) | 0.97 (0.87, 1.09) |
| Pre-transfusion INR (per 0.1) †† | 1.25 (1.17, 1.34) *** | 1.12 (1.00, 1.26) | −0.33 (−0.50, −0.16) *** | −0.25 (−0.43, −0.07) ** | 1.12 (1.04, 1.21) ** | 7.73 (−41.91, 57.38) | 1.15 (1.03, 1.27) * |
| Pre - post INR (per 0.1) †† | 0.78 (0.73, 0.85) *** | 0.90 (0.79, 1.02) | 0.36 (0.17, 0.55) *** | 0.19 (−0.00, 0.39) | 0.88 (0.81, 0.96) ** | −9.63 (−71.87, 52.60) | 0.88 (0.78, 0.99) * |
| Pre-transfusion R time (per 0.1) §§ | 1.00 (1.00, 1.01) | 1.00 (0.97, 1.02) | −0.01 (−0.02, 0.01) | −0.02 (−0.03, 0.00) | 1.00 (0.99, 1.00) | −0.17 (−1.56, 1.22) | 1.00 (0.99, 1.01) |
| Pre - post R time (per 0.1) §§ | 1.00 (0.99, 1.00) | 1.00 (0.98, 1.03) | −0.01 (−0.02, 0.01) | 0.00 (−0.01, 0.02) | 1.00 (1.00, 1.01) | 0.17 (−1.10, 1.45) | 1.00 (0.99, 1.01) |
Analyzed using multivariable logistic regression
Analyzed using multivariable linear regression
Only preoperative characteristics were included as adjutment terms when analyzing the outcomes of RBC units and estimated blood loss
Regression models included plasma units (per 300 mL), pre-transfusion INR, and decrease in INR plus preoperative demographics (age, sex, body mass index); preoperative laboratory values (creatinine, hemoglobin, platelet count); preoperative medications (aspirin, clopidogrel, warfarin, NSAIDs, heparin or low-molecular-weight heparin, vasopressors, inotropes within 24 hours of the procedure); preoperative PLT, plasma, and RBC transfusions; intraoperative transfusion volumes (plasma, PLTs, allogeneic RBCs, cell-salvaged blood); total intraoperative crystalloid and colloid volumes; intraoperative factor concentrate administration (including prothrombin complex concentrates and single factor replacements); surgical features (surgery type, surgery length, REDO-sternotomy, ejection fraction, perfusion time, cross clamp time, emergency surgery, and estimated blood loss); and patient comorbidities (preoperative Charlson score, preoperative SOFA scores).
Regression models included plasma dose (per mL/kg), pretransfusion R, and decrease in R plus age, sex, BMI, preoperative hemoglobin, creatinine, platelet count, crystalloid, colloid, RBC, Charlson, SOFA, ejection fraction, perfusion time, cross clamp time, and operation type.
Abbreviations: EBL – estimated blood loss; ICU – intensive care unit; INR – international normalized ratio; Intraop – intraoperative; R time – thromboelastography R time; RBC – red blood cells
p<0.05
p<0.01
p<0.001
Coagulation Test Values & Clinical Outcomes (aim 2)
After adjustment for potential confounders, pre-transfusion INR values and the magnitude of change in INR after transfusion were associated with multiple outcomes (Table 3). Higher pre-transfusion INR values (per 0.1 increase) were associated with increased odds of postoperative RBCs [1.25 (1.17, 1.34); P <0.001] and intraoperative RBCs [1.12 (1.04, 1.21); P=0.002], fewer mean ICU [−0.33 (−0.50, −0.16); P<0.001] and hospital free days [−0.25 (−0.43, −0.07; P=0.006], and increased odds of re-operation for bleeding [1.15 (1.03, 1.27); P=0.01]. Each 0.1 decrease from pre to post transfusion INR was significantly associated with decreased odds of postoperative RBCs [0.78 (0.73, 0.85); P<0.001], intraoperative RBCs [0.88 (0.81, 0.96); P=0.003], more ICU free days [0.36 (0.17, 0.55); P<0.001] and decreased odds of reoperation for bleeding [0.88 (0.78, 0.99); P=0.01]. For given plasma volumes, patients achieving greater reduction in elevated pre-transfusion INR values experienced more favorable outcomes. Pre-transfusion R times and changes in R times after plasma transfusion were not significantly associated with any of the outcomes.
Sensitivity analyses
Only 41% of cardiac surgeries had an applicable STS risk score. When we subset our analyses to this group and additionally adjusted for STS score, we observed similar findings as in the primary analysis, with the exception that the association between plasma dose (per unit) and postoperative RBCs was no longer significant, and pre-transfusion INR was associated with increased hospital mortality [1.45 (1.08, 1.96); P=0.02] while greater decreases in pre-post transfusion INR was associated with decreased hospital mortality [0.67 (0.49, 0.93); P =0.02] (Supplemental Table 1). Analyses by pre-transfusion INR categories are displayed in Supplemental Table 2. In the INR ≥ 2 group, plasma dose was not significantly associated with need for postoperative RBCs. However, plasma dose was associated with a higher rate of intraoperative RBC transfusion and a lower rate of reoperation for bleeding. In those with INR values from 1.5 to 2.0, the same trends in the primary analysis between plasma dose and outcomes were observed with the exception of hospital free days. In those with INR < 1.5, plasma dose was associated with increased odds for intraoperative RBCs. Analyses subset by high or low intraoperative RBC transfusion volumes are displayed in Supplemental Table 3, with 66.0% of the cohort receiving < 3 RBC units. The outcomes were generally consistent across groups.
Discussion
The primary aim of this study was to evaluate the effect of plasma transfusion volumes on clinical outcomes in nearly 2000 patients undergoing cardiac surgery. To that end, we found that increasing plasma transfusion volumes were associated with inferior clinical outcomes. Specifically, after adjustment for potentially confounding variables, increasing plasma transfusion volumes were associated with increased odds for postoperative RBC transfusion, intraoperative RBC transfusion (analyses limited to RBCs administered after 1st plasma unit), and fewer hospital free days. However, higher pre-transfusion INR values were strongly associated with increased risk for postoperative and intraoperative RBC transfusion, fewer ICU and hospital free days, and increased re-operation for bleeding. Moreover, patients who experienced a greater degree of INR correction after plasma transfusion had more favorable clinical outcomes, including lower odds of postoperative/intraoperative RBC transfusion, and more ICU free days. The optimal endpoint for plasma-mediated INR correction remains unknown. Intuitively, the cessation of microvascular bleeding may serve as a suitable endpoint, and it is possible that additional plasma administration beyond this may contribute to worse outcomes. However, the precise time for which cessation of microvascular bleeding is achieved can be difficult to reliably ascertain in real-time as this is a clinical diagnosis that requires assessment of the surgical field from both surgeons and anesthesiologists.
Perioperative coagulopathy and bleeding in patients undergoing cardiac surgery is common with some studies reporting overall transfusion rates in excess of 50%.1 Transfusion rates for non-RBC hemostatic products (e.g. plasma, platelets, cryoprecipitate) have been reported from 11% to greater than 20% in this population.1, 2, 6 It is well accepted that perioperative bleeding increases risk for major morbidity and mortality in patients undergoing cardiac surgery.14 Bleeding in this cohort is multifactorial, and partially due to a complex coagulopathy induced by blood contact with the cardiopulmonary bypass circuit. Specifically, a coagulation factor mediated coagulopathy is common in this population for which historically, allogenic plasma transfusion has been the therapy of choice. Unfortunately, little is known regarding the optimal plasma transfusion practice in this surgical setting.
Algorithm driven transfusion practices have become increasingly common in most cardiac surgical practices in recent years, and the use of such pathways has been shown to reduce transfusion rates.6 Transfusion algorithms commonly contain laboratory based cutoffs for which transfusions are suggested in the presence of ongoing microvascular bleeding. There is certainly a debate as to the best perioperative tests of coagulation, much of which is far beyond the scope of this manuscript. Despite a national trend toward use of viscoelastic assays (e.g. TEG, ROTEM) in the perioperative period, many centers still utilize standard coagulation testing (e.g INR, activated partial thromboplastin time, platelet count, and fibrinogen) to guide perioperative transfusion. The INR test has several shortcomings when used as a predictor for bleeding in the acute operative setting.15–17 Furthermore, the INR threshold for plasma transfusion is debatable. While the ASA supports plasma transfusion when the INR is >2.0 in the presence of microvascular bleeding,5 other cardiac surgery specific algorithms use an INR >1.6 in the presence microvascular bleeding as the cutoff for plasma transfusion.6 As mentioned above, the coagulopathy induced by utilization of cardiopulmonary bypass is complex and causes alterations in nearly every aspect of the coagulation cascade. Thus, in instances of ongoing microvascular bleeding, plasma transfusion in patients whose INR falls in an intermediate zone (INR 1.6–2.0) is common, and often occurs simultaneously with the correction of other hemostatic laboratory abnormalities. While comprehensive discussion of various coagulation testing modalities and management approaches for coagulopathy in cardiac surgery is beyond the scope of this investigation, it is important to note that there is likely substantial heterogeneity in laboratory test utilization and provider-specific practice patterns. With regard to INR values and outcomes in this study, overall, elevation in INR was associated with worse clinical outcomes including higher rates of intraoperative and postoperative RBC transfusion, fewer ICU and hospital free days, and higher rates of re-operation for bleeding. On the other hand, TEG R-times were not significantly associated with any of these outcomes, though values were only available for 20% of the study cohort and therefore may have been underpowered to detect outcome differences.
When considering the use of plasma transfusion to combat intraoperative bleeding and coagulation factor derangements, providers are faced with the dilemma regarding optimal plasma transfusion volumes. Prior studies have shown a greater improvement in coagulation test values with larger plasma transfusion volumes; however, this does not necessarily translate to improvements in clinical outcomes. Furthermore, there is little known about clinical outcomes associated with plasma transfusion volumes in patients undergoing cardiac surgery.18, 19 The goal of plasma transfusion in these circumstances is to increase coagulation factor activity levels to an acceptable range to assist hemostasis. The ideal coagulation factor activity level in bleeding cardiac surgical patients is unknown, however, an arbitrary individual coagulation factor level of 30 IU/dL has been thought to be sufficient for hemostasis.18 Prior studies have shown a direct correlation between INR and factor activity levels.20 This theoretical 30% factor activity target correlates roughly with an INR value near 2.0.21 Larger plasma transfusion volumes (30 mL/kg vs 10–15 mL/kg) have been demonstrated to improve coagulation factor concentration levels to a greater extent, yet how this correlates with bleeding and other more important clinical outcomes is poorly defined.18 The American Society of Anesthesiologist’s Practice Guidelines for Blood Component Therapy recommended a plasma transfusion dose of 10–15 mL/kg which aligns with common practice in most centers, though the median plasma dose in this investigation was slightly lower at 8.3 ml/kg.22 In a study by Mazzeffi et al., patients undergoing cardiac surgery who required massive transfusion (>8 units RBCs), and received plasma transfusion ratios >1:1 (plasma:RBC) had improved survival rates, lower re-operation rates, and less AKI, yet had prolonged mechanical ventilation and higher rates of atrial fibrillation.23 In the current study only 39.9% received intraoperative RBC transfusions (median 1 unit), with plasma transfusion volumes across the entire cohort exceeding RBC transfusion volumes, hence also a plasma:RBC ratio of > 1:1.
Patients receiving plasma transfusion in this study had inferior outcomes with increasing transfusion volumes despite controlling for potentially confounding variables. Approximately 75% of patients had a pre-transfusion INR value < 2, with the majority falling between 1.5 and 1.9. While the exact context for each transfusion episode is not known, perhaps this intermediate group (INR 1.5–1.9) with some preservation of coagulation factor activity is not receiving the intended hemostatic benefit but rather is simply exposed to the risks of transfusion. It is well appreciated that transfusion of allogenic blood products is associated with various complications that may impact patient outcomes, including excessively positive postoperative fluid balances, transfusion-related acute lung injury (TRALI), transfusion associated circulatory overload (TACO), febrile and allergic reactions, infection, and multi-organ failure.21, 24–27 Additionally, risk for these complications escalates with increasing plasma transfusion volumes.27 Interestingly, in patients with pre-transfusion INR values ≥ 2, higher plasma doses were no longer associated with some of the unfavorable outcomes seen in the intermediate INR group (i.e. postoperative RBCs), and was actually protective with regard to re-operation for bleeding. Hence, this may support an INR threshold ≥ 2 as a potential cutoff for less restricted plasma transfusion (i.e. 10–15 mL/kg), as this group may be most likely to benefit from such therapy. Conversely, plasma transfusion for patients with more modest elevations in INR (i.e. INR < 2), when deemed clinically necessary, should perhaps be limited to low volumes with incremental re-assessment of bleeding and coagulation testing. There is no evidence to support routine plasma transfusion for INR values <1.5, which represented only a fraction of the study cohort (7%).
In addition to analyses by pre-transfusion INR, we analyzed outcomes by the magnitude of intraoperative allogeneic RBC transfusion, which may serve as a surrogate for the severity of the surgical bleeding insult. Outcomes related to plasma transfusion volume were similar regardless of intraoperative RBC transfusion volume, with increased plasma volumes associated with more postoperative RBCs, fewer ICU and hospital free days, and increased reoperation for bleeding.
Another potential explanation for the observed association between higher plasma transfusion volumes and inferior clinical outcomes in the overall study cohort might relate to transfusion-mediated hypervolemia. In the absence of ongoing large-volume hemorrhage, it is possible that increasing transfusion volumes may cause an appreciable rise in circulating blood volume, which may result in increased intravascular pressures and downstream disruption of newly formed hemostatic plugs, leading to further microvascular blood loss. This theoretical mechanism has been proposed when considering superior outcomes observed with more restrictive transfusion strategies for those with acute upper gastrointestinal bleeding.28 Similar benefits have been described with hypotensive resuscitation strategies for patients with acute hemorrhage, though research is limited almost exclusively to trauma. In that setting, this strategy has shown promise with regard to reductions in bleeding, mortality, acute respiratory dysfunction, and organ dysfunction.29 Furthermore, low central venous pressure strategies have shown hemostatic benefit in those undergoing liver resection.30 Current adoption of similar practices to cardiac surgery is premature; however, an appreciation for the potential relationship between hypervolemia, elevated vascular pressures, and bleeding may have important implications for cardiac surgical patients. Moreover, the potential consequences on important downstream clinical outcomes such as acute kidney injury are unknown.
Beyond the observed relationships between plasma transfusion volumes and clinical outcomes, it is important to note that elevated pre-transfusion INR values were associated with unfavorable outcomes and that a greater degree of INR correction after plasma transfusion was associated with more favorable outcomes. Paradoxically, increasing plasma transfusion volumes, for any degree of INR correction, were associated with inferior outcomes. Given that a majority of patients in this study had pre-transfusion INR values < 2, the margin for INR correction was quite small given that the intrinsic INR of the plasma unit itself may be as high as 1.5.21 Thus, many of these patients may not have experienced hemostatic or INR-correction benefits from increasing doses of plasma, but would have been subjected to potential unfavorable transfusion associated outcomes, as described previously. Certainly, there may also be patients who received plasma and experienced no correction or even worsening of INR values in the setting of ongoing surgical bleeding. While this latter subset is likely small, it is well established that ongoing surgical bleeding is associated with unfavorable outcomes.
Limitations
The limitations of this study include those inherent to all retrospective analyses including charting omissions and inaccuracies. The exact circumstances associated with each plasma transfusion event are unknown (e.g. severity of microvascular bleeding, decision to administer a given transfusion volume), though the timings for all transfusions are precise. Additionally, the primary endpoint of postoperative RBC transfusion was used as a surrogate for ongoing blood loss, though decisions to transfuse may have deviated from standardized institutional guidelines. While the common practice is to target a hemoglobin ≥ 8.0 g/dL in the immediate post cardiac surgical period, the exact circumstances surrounding each RBC transfusion event are unknown. This study included only patients receiving plasma transfusion, so comparing outcomes to similar patients not receiving plasma transfusion did not occur. Additionally, data related to appropriate transfusion algorithm adherence for other hemostatic products (i.e. platelets and cryoprecipitate) was not explored, hence uncorrected abnormalities related to such may have contributed to bleeding, transfusion, and outcomes. While we have attempted to carefully control for confounding, the potential exists that those receiving higher plasma transfusion volumes represent a more chronically complex cohort or more complicated operative subset beyond what could be captured with adjustment. This study also represents a single center experience and the results may not be generalizable to all practice settings.
Conclusion
Overall, higher pre-transfusion INR values and higher plasma transfusion volumes were associated with unfavorable clinical outcomes. Those with pre-transfusion INR values ≥ 2 may benefit from higher plasma transfusion volumes than other patient groups; however, optimal transfusion volumes are not known. Ultimately, large prospective studies are needed to define optimal plasma transfusion triggers, targets, and volumes in patients undergoing cardiac surgery. Moreover, future studies should be designed to compare clinical outcomes between plasma-based versus factor-based coagulopathy correction (e.g. prothrombin complex concentrates) in cardiac surgery.
Supplementary Material
Acknowledgments
Financial Support
This study was made possible by funding from the Mayo Clinic Department of Anesthesiology and Perioperative Medicine and the Critical Care Integrated Multidisciplinary Practice, Rochester, Minnesota. In addition, this study was supported by an NIH R01 grant (HL121232) to Dr. Kor and by CTSA Grant Number KL2 TR002379 to Dr. Warner from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Declarations of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geissler RG, Rotering H, Buddendick H, et al. Utilisation of blood components in cardiac surgery: a single-centre retrospective analysis with regard to diagnosis-related procedures. Transfus Med Hemother 2015;42:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank SM, Savage WJ, Rothschild JA, et al. Variability in blood and blood component utilization as assessed by an anesthesia information management system. Anesthesiology 2012;117:99–106. [DOI] [PubMed] [Google Scholar]
- 3.Thiele RH, Raphael J. A 2014 Update on Coagulation Management for Cardiopulmonary Bypass. Semin Cardiothorac Vasc Anesth 2014;18:177–189. [DOI] [PubMed] [Google Scholar]
- 4.Kor DJ, Gajic O. Blood product transfusion in the critical care setting. Current opinion in critical care 2010;16:309–316. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Anesthesiologists Task Force on Perioperative Blood M. Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology 2015;122:241–275. [DOI] [PubMed] [Google Scholar]
- 6.Nuttall GA, Oliver WC, Santrach PJ, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology 2001;94:773–781; discussion 775A-776A. [DOI] [PubMed] [Google Scholar]
- 7.Warner MA, Frank RD, Weister TJ, et al. Higher intraoperative plasma transfusion volumes are associated with inferior perioperative outcomes. Transfusion 2019;59:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 9.Herasevich V, Kor DJ, Li M, et al. ICU data mart: a non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform 2011;28:42, 44–45. [PubMed] [Google Scholar]
- 10.Chute CG, Beck SA, Fisk TB, et al. The Enterprise Data Trust at Mayo Clinic: a semantically integrated warehouse of biomedical data. J Am Med Inform Assoc 2010;17:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc 2012;87:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585–598. [DOI] [PubMed] [Google Scholar]
- 13.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 14.Ruel M, Chan V, Boodhwani M, et al. How detrimental is reexploration for bleeding after cardiac surgery? J Thorac Cardiovasc Surg 2017;154:927–935. [DOI] [PubMed] [Google Scholar]
- 15.Darcy MD, Kanterman RY, Kleinhoffer MA, et al. Evaluation of coagulation tests as predictors of angiographic bleeding complications. Radiology 1996;198:741–744. [DOI] [PubMed] [Google Scholar]
- 16.Dzik WH. Predicting hemorrhage using preoperative coagulation screening assays. Current hematology reports 2004;3:324–330. [PubMed] [Google Scholar]
- 17.Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 2005;45:1413–1425. [DOI] [PubMed] [Google Scholar]
- 18.Chowdary P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. British journal of haematology 2004;125:69–73. [DOI] [PubMed] [Google Scholar]
- 19.Dara SI, Rana R, Afessa B, et al. Fresh frozen plasma transfusion in critically ill medical patients with coagulopathy. Critical care medicine 2005;33:2667–2671. [DOI] [PubMed] [Google Scholar]
- 20.Gulati G, Hevelow M, George M, et al. International normalized ratio versus plasma levels of coagulation factors in patients on vitamin K antagonist therapy. Arch Pathol Lab Med 2011;135:490–494. [DOI] [PubMed] [Google Scholar]
- 21.Kor DJ, Stubbs JR, Gajic O. Perioperative coagulation management--fresh frozen plasma. Best Pract Res Clin Anaesthesiol 2010;24:51–64. [DOI] [PubMed] [Google Scholar]
- 22.Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology 1996;84:732–747. [PubMed] [Google Scholar]
- 23.Mazzeffi MA, Chriss E, Davis K, et al. Optimal Plasma Transfusion in Patients Undergoing Cardiac Operations With Massive Transfusion. Ann Thorac Surg 2017;104:153–160. [DOI] [PubMed] [Google Scholar]
- 24.Clifford L, Jia Q, Subramanian A, et al. Characterizing the epidemiology of postoperative transfusion-related acute lung injury. Anesthesiology 2015;122:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clifford L, Jia Q, Yadav H, et al. Characterizing the epidemiology of perioperative transfusion-associated circulatory overload. Anesthesiology 2015;122:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest 2007;131:1308–1314. [DOI] [PubMed] [Google Scholar]
- 27.Inaba K, Branco BC, Rhee P, et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J Am Coll Surg 2010;210:957–965. [DOI] [PubMed] [Google Scholar]
- 28.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21. [DOI] [PubMed] [Google Scholar]
- 29.Owattanapanich N, Chittawatanarat K, Benyakorn T, et al. Risks and benefits of hypotensive resuscitation in patients with traumatic hemorrhagic shock: a meta-analysis. Scand J Trauma Resusc Emerg Med 2018;26:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes MJ, Ventham NT, Harrison EM, et al. Central venous pressure and liver resection: a systematic review and meta-analysis. HPB (Oxford) 2015;17:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.