Abstract
Background & Aims:
Celiac disease could be treated, and potentially cured, by restoring T-cell tolerance to gliadin. We investigated the safety and efficacy of negatively charged, 500 nm, poly(lactide-co-glycolide) nanoparticles encapsulating gliadin protein (TIMP-GLIA) in 3 mouse models of celiac disease. Uptake of these nanoparticles by antigen-presenting cells was shown to induce immune tolerance in other animal models of autoimmune disease.
Methods:
We performed studies with C57BL/6, RAG1−/− (C57BL/6), and HLA-DQ8, huCD4 transgenic Ab0 NOD mice. Mice were given 1 or 2 tail-vein injections of TIMP-GLIA or control nanoparticles. Some mice were given intradermal injections of gliadin in complete Freund’s adjuvant (immunization), or of soluble gliadin or ovalbumin (ear challenge). RAG−/− mice were given intraperitoneal injections of CD4+CD62L–CD44hi T cells from gliadin-immunized C57BL/6 mice, and were fed with AIN-76A-based diet containing wheat gluten (oral challenge), or without gluten. Spleen or lymph node cells were analyzed in proliferation and cytokine secretion assays, or by flow cytometry, RNA sequencing or real-time quantitative PCR. Serum samples were analyzed by gliadin antibody ELISA, and intestinal tissues were analyzed by histology. Human PBMC, or immature dendritic cells derived from human PBMC, were cultured in medium containing TIMP-GLIA, anti-CD3 antibody, or LPS (controls) and analyzed in proliferation and cytokine secretion assays or by flow cytometry. Whole blood or plasma from healthy volunteers was incubated with TIMP-GLIA, and hemolysis, platelet activation and aggregation, and complement activation or coagulation were analyzed.
Results:
TIMP-GLIA did not increase markers of maturation on cultured human dendritic cells or induce activation of T cells from patients with active or treated celiac disease. In the delayed-type hypersensitivity (model 1), the HLA-DQ8 transgenic (model 2), and the gliadin memory T cell enteropathy (model 3) models of celiac disease, intravenous injections of TIMP-GLIA significantly decreased gliadin-specific T cell proliferation (in models 1 and 2), inflammatory cytokine secretion (in models 1, 2, and 3), circulating gliadin-specific IgG/IgG2c (in models 1 and 2), ear swelling (in model 1), gluten-dependent enteropathy (in model 3), and body weight loss (in model 3). In model 1, the effects were shown to be dose dependent. Splenocytes from HLA-DQ8 transgenic mice given TIMP-GLIA nanoparticles, but not control nanoparticles, had increased levels of FOXP3, and gene expression signatures associated with tolerance induction.
Conclusions:
In mice with gliadin sensitivity, injection of TIMP-GLIA nanoparticles induced unresponsiveness to gliadin, and reduced markers of inflammation and enteropathy. This strategy might be developed for treatment of celiac disease.
Keywords: Gluten sensitivity, tolerogenic vaccine, immunotherapy, immunomodulation
Introduction
Celiac disease is a gluten-sensitive enteropathy with a prevalence of 0.3–2.4% in most populations. Celiac disease results from the failed immune regulation of gluten-specific CD4+ T cells in individuals carrying human leukocyte antigen (HLA)-DQ2 or HLA-DQ8 risk alleles.1 In celiac disease patients, exposure to gluten leads to the activation of gluten-specific-T cells, culminating in immune-mediated intestinal damage.2 Therapeutic approaches that render T cells tolerant to gluten have the potential to cure celiac disease. The induction of sustained unresponsiveness to gluten could eliminate the life-time burden of dietary restriction, clinical symptoms associated with accidental gluten exposure, and the risk of severe long-term complications, such as malignancies, secondary autoimmune diseases or bone loss.3 However, until now no attempt to induce tolerance in autoimmune disease patients has shown clinical efficacy.
Recently, we demonstrated the tolerogenic potential of antigen loaded, negatively charged nanoparticles (referred to as Tolerogenic Immune Modifying Nanoparticles, TIMP) in murine models of multiple sclerosis (MS), transplantation, airway allergy, and type 1 diabetes (T1D).4,5,6,7 Previous studies have shown that TIMP, when injected intravenously, are mainly directed to the spleen and the liver.8,9,10 In these organs, tissue-resident antigen presenting cells (APC), including splenic marginal zone macrophages expressing macrophage receptor with collagenous structure (MARCO), take up TIMP.8,9,10,11 A recent mechanistic study demonstrated uptake of TIMP by both macrophages and dendritic cells, TIMP localization to endolysosomes, altered APC transcriptional activity following TIMP uptake, upregulation of surface MHC class II presentation of specific peptide, and induced downregulation of co-stimulatory molecules CD80 and CD86 on the surface of APC.12 Studies using nanoparticles or apoptotic cells have shown that treatment resulted in the upregulation of inhibitory ligands on macrophages, such as PD-L1, and the production of regulatory cytokines, such as interleukin 10 (IL10) and transforming growth factor beta (TGFB), in the spleen.7,13,14 Additionally, these antigen-specific therapies induced CD4+ T cell anergy, and the activation of CD4+ T-regulatory (Treg) cells.4,7,13,14 The overall result was a decrease in pro-inflammatory CD4+ and CD8+ T cell activity, reduction in leukocyte accumulation in tissues, and decreased clinical signs of disease.
Based on these observations, we hypothesized that intravenous administration of gliadin protein encapsulated in TIMP (referred to as TIMP-GLIA), harboring immunodominant and subdominant gliadin epitopes15,16, may restore peripheral tolerance to gluten. Here, we report results of the generation and subsequent preclinical evaluation of TIMP-GLIA, produced to good-manufacturing practices, and currently being tested in human phase 1/2 clinical trials. Administration of TIMP-GLIA proved highly effective at inducing gliadin immune tolerance and reduction of gliadin-specific inflammatory responses, including gluten enteropathy, in three rodent models of celiac disease. Previously identified gene targets of TIMP were confirmed, and several novel targets identified. Additional studies showed that TIMP-GLIA were biocompatible in human blood.
Materials and Methods
Particle synthesis
PLGA particles encapsulating gliadin from wheat (Sigma-Aldrich, St. Louis, MO) were fabricated using a double emulsion technique, by first dissolving 400 mg of PLGA (Lactel Absorbable Polymers, Birmingham, AL) in 2 mL of ethyl acetate. Subsequently, 10 mg of gliadin was dissolved at 25 mg/ml in 0.05 M acetic acid, and added to the PLGA solution. The solution was emulsified by sonication for 30s at 100% amplitude using a Cole-Parmer CPX130 Ultrasonic Processor, equipped with a Cole-Parmer CV 18 ultrasonic probe adapter and a Cole-Parmer 3 mm probe with stepped tip. Immediately after sonication, 10 ml of 1% w/v poly(ethylene-alt-maleic anhydride) (PEMA) or 2% poly(vinyl alcohol) (PVA; both from Polysciences, Warrington, PA) was poured into the first emulsion, and sonicated for an additional 30s at 100% amplitude. Following the second sonication, the emulsion was poured into 200 ml 0.5% PEMA or 0.5% PVA, and was stirred overnight to remove residual organic solvents. The nanoparticles were then washed three times in 0.1 M sodium carbonate-sodium bicarbonate buffer, pH 9.6. The nanoparticles were then resuspended in 20 ml 3% w/v aqueous D-mannitol and 4% w/v aqueous sucrose, frozen in liquid nitrogen, lyophilized and stored for future use. The amount of protein encapsulated were determined from nanoparticles dissolved in DMSO, using the CBQCA Protein assay (Molecular Probes, Waltham, MA). PLGA nanoparticles encapsulating Cy5.5 dye, ovalbumin or lysozyme were prepared as previously described.4,6
Animals
C57BL/6, RAG1−/− (C57BL/6) and HLA-DQ8, huCD4 transgenic Ab0 NOD mice17 were purchased from The Jackson Laboratory, Bar Harbor, ME. All mice were housed under specific pathogen-free conditions in the Northwestern University Center for Comparative Medicine, or the University of Helsinki Laboratory Animal Centre, and were raised on normal chow, or gluten-free, standardized diet (AIN-76A; Research Diets, New Brunswick, NJ), as indicated. Some groups of mice were challenged with AIN-76A-based diet containing 2.5 g wheat gluten/kg (Sigma Aldrich; prepared by Research Diets). To compare the effects of different treatments, some groups of mice were injected one or two times into the tail vein with TIMP-GLIA, control nanoparticles containing lysozyme (TIMP-LYS), ovalbumin (TIMP-OVA) or unloaded (TIMP; nanoparticle dose range 0.025–2.5mg, time points as indicated; each in 200μl of PBS), or 25–40μg soluble gliadin in 200μl of PBS.
Gliadin delayed-type hypersensitivity (DTH) model
Female C57BL/6 mice (6–7 weeks old) were immunized subcutaneously with 100μl of an emulsion containing 200μg of M. tuberculosis H37Ra (BD Biosciences, San Jose, CA) and 100μg of gliadin, distributed over three sites on the flank. Gliadin (SAFC, Madison, WI) was reconstituted from lyophilized powder in 50mM acetic acid (5 mg/ml). This gliadin solution was then further diluted for use in PBS. Mice were treated intravenously with different nanoparticle preparations, on days −7, 0 or 7, as indicated. On day 14 after priming, mice were bled, and tested for delayed type hypersensitivity (DTH). Mice were anaesthetized by inhalation of isoflurane, and baseline pinna thickness was measured for both ears using calipers (Mitutoyo Thickness Gage; Global Industrial, Port Washington, NY). Immediately following pinna thickness assessments, using a Hamilton syringe with a 30G1/2 needle, gliadin or negative control OVA protein (10μl at 1mg/ml) in PBS was intradermally injected into the left and right ear, respectively. The increase in ear thickness was determined after 24h, and the change in pinna thickness (Δ T) was calculated using the following equation: (ΔT = (pinna thickness at 24hrs following elicitation) – (pinna thickness prior to elicitation)). Mice were then sacrificed, and cell suspensions from draining lymph nodes or spleens were prepared.
HLA-DQ8 transgenic mouse model
2 groups of female HLA-DQ8 mice were treated intravenously on days -11 and -3 with TIMP-GLIA, or TIMP-OVA, while 2 other groups remained without treatment (2.5mg/dose; n=11–19, 10–12 weeks of age, raised on gluten-free diet AIN-76A). On day 0, mice from both the TIMP-GLIA, the TIMP-OVA and 1 control group of mice (IMMU ONLY) were injected at the base of the tail with 100 μg gliadin in complete Freund’s adjuvant, followed by 50 μg gliadin in incomplete Freund’s adjuvant on day 14 (Sigma-Aldrich). The second control group (NO TREATMENT) remained untreated. On days 28–30, mice were anesthetized with ketamine/xylazine. Blood was collected retro-orbitally, and serum stored at −20°C. Spleens were harvested for analyses.
Gliadin Memory T cell Enteropathy Model.
Male C57BL/6 donor mice, raised on gluten-free diet AIN-76A, were injected at the base of the tail with 100 μg gliadin in complete Freund’s adjuvant, followed by 50 μg gliadin in incomplete Freund’s adjuvant after 2 weeks (Sigma-Aldrich). CD3+ T cells were isolated after 4–5 weeks from spleen cell suspensions, using antibody-coated columns. Memory CD4+ T cells were isolated from CD3+ T cells using CD4+CD62L-CD44hi T cell columns, as reported (both columns from R&D Systems, Minneapolis, MN).18,19 Four groups of male Rag1−/− mice (n=16, 6–10 weeks of age, matched for body weight) were injected intraperitoneally on day 0 with 3 × 105 splenic CD4+CD62L-CD44hi T cells. Recipient mice were challenged until the end of the experiment with AIN-76A-based diets containing 2.5 g wheat gluten/kg, or no gluten added. Mice from 2 groups were either injected into the tail vein with TIMP-GLIA (2.5mg dose, in 200μl of PBS), or with 2.5mg of TIMP-LYS (nanoparticle treatment control), both on day 10 and 24. Mice were monitored and body weights were recorded for 8 weeks after adoptive transfers, and then mice were sacrificed for organ harvest.
Statistics
RNA sequencing data were analyzed with edgeR.20 Other data were analyzed with Prism 8 software (GraphPad, La Jolla, CA). Statistical comparisons were performed using paired or unpaired Student’s t-tests, t-tests corrected for multiple testing using the Holm-Sidak method (alpha=0.05), one-way ANOVA and Tukey’s tests for comparisons between multiple groups, or Kruskal-Wallis and Dunn’s tests for nonparametric data, as indicated. Means, medians, SD, SEM and interquartile ranges were calculated.
Results
Development of Tolerogenic Immune-Modifying Nanoparticles encapsulating gliadin extracted from wheat (TIMP-GLIA)
Previous studies have demonstrated the importance of negative charge in nanoparticle tolerance induction.21 Therefore, we tested the potential for poly(lactide-coglycolide) (PLGA) nanoparticles with different zeta potentials to be taken up by macrophages, and for inducing immune tolerance in a gliadin-specific delayed-type hypersensitivity (DTH) model. Different formulations were prepared, using a double emulsion-solvent evaporation, and either poly(ethylene-alt-maleic acid) (PEMA) as the stabilizing anionic surfactant, or poly(vinyl acetate) (PVA) as the neutral stabilizing surfactant. Particles encapsulated either the fluorescent dye Cy5.5, gliadin isolated from wheat, ovalbumin or no dye/protein (Fig 1A). PLGA-PEMA-Cy5.5 nanoparticles with high negative zeta potential (approx. −40 mV) interacted strongly with bone marrow-derived macrophages, whereas PLGA-PVA-Cy5.5 nanoparticles with a more neutral zeta potential (approx. −20 mV) did not reach the same levels (Fig 1B). Correspondingly, in a gliadin DTH mouse model, intravenous treatment on days 0 and 7 with two injections of 2.5 mg/mouse PLGA-PEMA-GLIA following gliadin/CFA priming was associated with significant decreases in ear swelling when compared to control animals receiving either PLGA-PVA-GLIA, or PLGA-PEMA-OVA (Fig 1C). This result was similar to our previous findings8,21, demonstrating that nanoparticles with higher negative charge showed increased efficacy in reducing ear swelling in DTH models. Furthermore, the importance of nanoparticle encapsulation of protein was also confirmed, as the intravenous injection of 25 μg soluble (free) gliadin alone did not show any efficacy in reducing ear swelling in the gliadin DTH model (Fig 1D).
Figure 1: Development of Tolerogenic Immune-Modifying Nanoparticles encapsulating gliadin (TIMP-GLIA) (I).
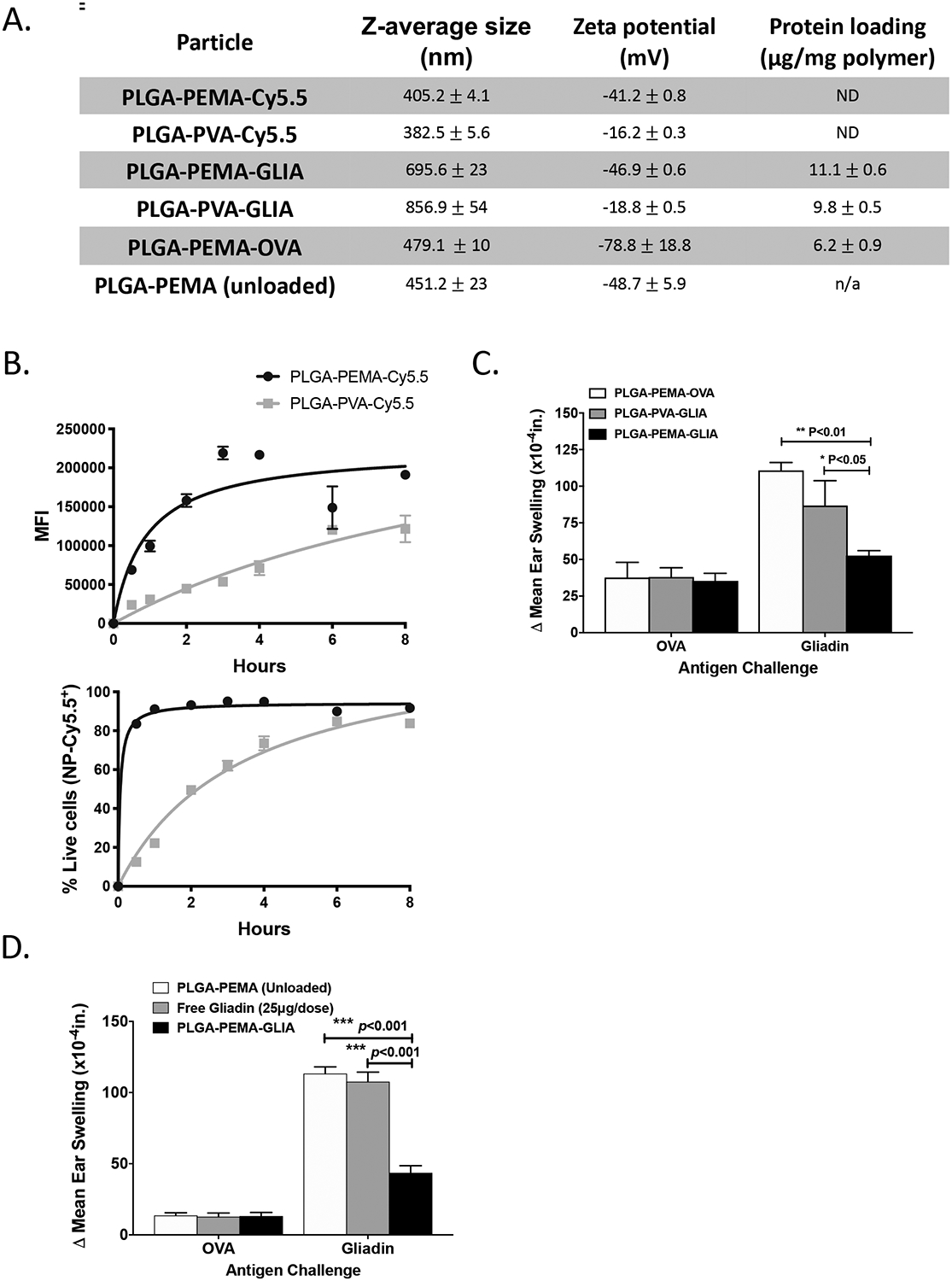
A) Six different formulations of nanoparticles were prepared for testing, using either PEMA or PVA as stabilizing surfactant, and encapsulating either Cy5.5 dye, gliadin or ovalbumin (or remaining unloaded). Size, charge and protein loading were measured (mean +/− SD). B) PLGA-PEMA-Cy5.5 or PLGA-PVA-Cy5.5 particles were added to bone marrow-derived macrophage cultures. Cells were analyzed by flow cytometry for mean fluorescence intensity, or for percentage of Cy5.5+/DAPI- live cells (triplicates; mean +/− SD). C) Intravenous treatment effect of PLGA-PEMA-GLIA, PLGA-PVA-GLIA or PLGA-PEMA-OVA in the gliadin DTH mouse model. Ear thickness was measured 24h after injection of either gliadin or ovalbumin (n=5; Δ mean ear thickness +/− SEM; x10−4in). D) Treatment effect of PLGA-PEMA-GLIA, soluble gliadin or PLGA-PEMA (unloaded) in the gliadin DTH mouse model (n=5; Δ mean ear thickness +/− SEM; x10−4in). Statistical analyses were performed using one-way ANOVA and Tukey’s multiple comparison test (C, D; *p≤0.05, **p≤0.01, ***p≤0.001)
Based on this experience, PLGA-PEMA nanoparticles encapsulating gliadin were prepared (now referred to as TIMP-GLIA; structure shown schematically in Fig 2A). Nanoparticles containing no protein, ovalbumin (TIMP-OVA), or lysozyme (TIMP-LYS) were synthesized as control particles (Fig 2B).6,8 The loading of TIMP with gliadin was controlled by adjusting the concentration of gliadin during formulation, and was aimed at 10 ± 5 μg protein per mg of PLGA. To determine whether TIMP-GLIA or TIMP-OVA displayed antigen on the particle surface, surface protein binding of antigen-specific antibodies was measured by FACS. Less than 1% of TIMP were found to be positive for surface protein. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western Blot confirmed the presence of gliadin α/β, γ and ω proteins in TIMP-GLIA (Fig 2C; Supplementary Fig 1). Scanning electron microscopy of TIMP-GLIA suspensions confirmed the size and a spherical morphology with a smooth surface (Fig 2D). Additionally, in use stability testing showed that reconstituted TIMP-GLIA remained stable for up to at least 8 hours after rehydration, with burst release, average size and zeta potential remaining unchanged during this time (Fig 2E–G).
Figure 2: Development of Tolerogenic Immune-Modifying Nanoparticles encapsulating gliadin (TIMP-GLIA) (II).
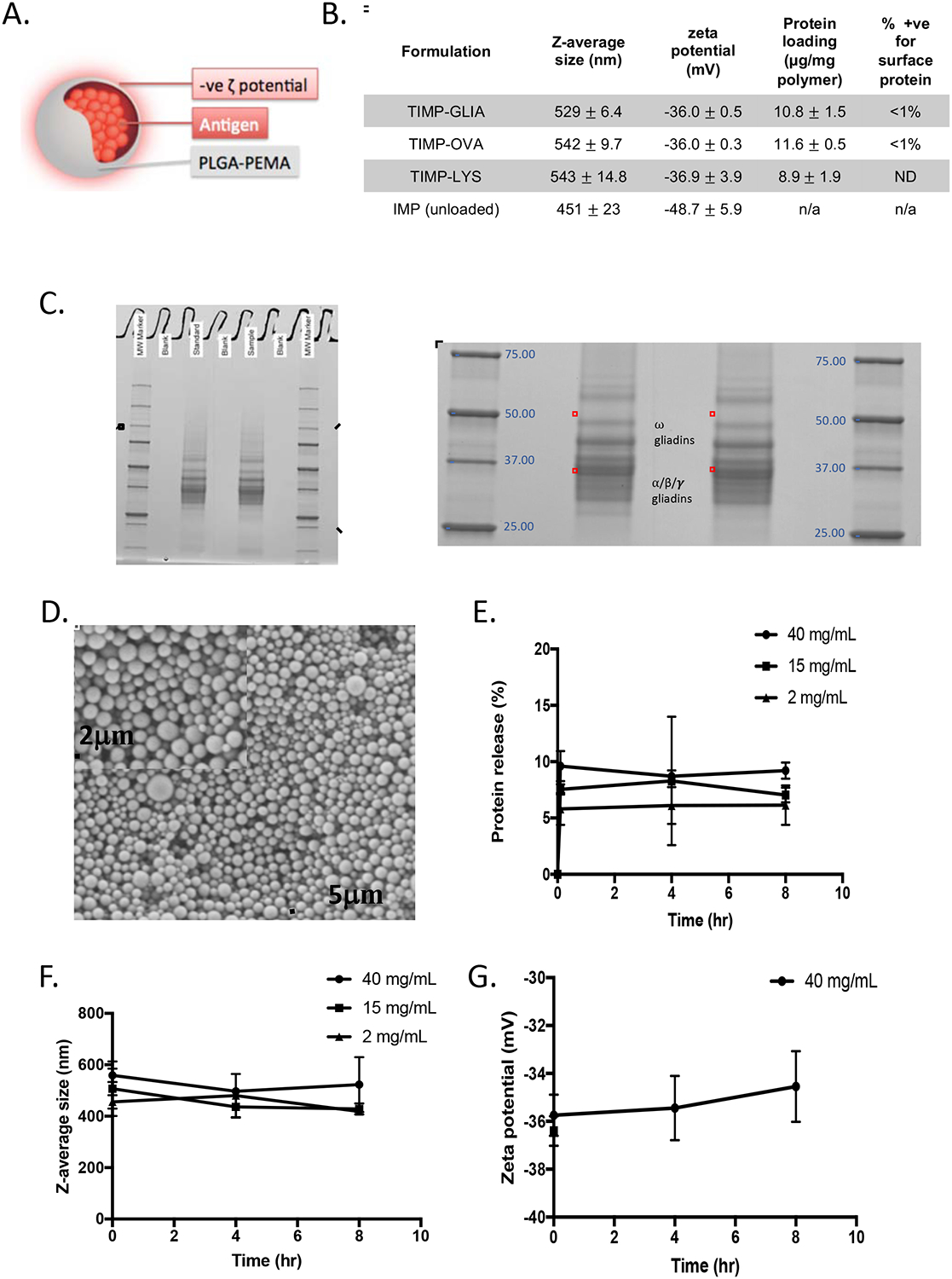
. A) Schematic representation of TIMP. B) Four different formulations of PLGA-PEMA nanoparticles were prepared for testing, encapsulating either gliadin (TIMP-GLIA), ovalbumin (TIMP-OVA) or lysozyme (TIMP-LYS), or remaining unloaded (IMP). Size, charge, protein loading (mean +/− SD) and percentage of particles positive for surface protein (FACS) were analyzed. C) SDS-PAGE of gliadin preparation used for production of TIMP-GLIA (duplicates, central rows). D) Scanning electron microscopy of a representative TIMP-GLIA suspension. E-G) Analysis of TIMP-GLIA stability in water over 8h, measuring protein release (E), size (F) and charge (G; triplicates, mean +/− SD).
TIMP-GLIA tolerance induction in mice with delayed-type hypersensitivity to gliadin is dose-dependent, and effective when given before or after gliadin immunization.
To analyze further the efficacy and specificity of TIMP-GLIA tolerance induction, we pre-treated mice on days −7 and day 0 in the DTH model. Intravenous pre-treatment with two injections of 0.025 mg - 2.5 mg/mouse (1.25 – 125 mg/kg) TIMP-GLIA prior to gliadin/CFA priming was not associated with any clinical symptoms or adverse events in mice. While pre-treatment with 0.025 mg/mouse (1.25 mg/kg) failed to show efficacy, doses of 0.25–2.5 mg/mouse (12.5–125 mg/kg) TIMP-GLIA were associated with significant decreases (p<0.0001) in ear swelling when compared to control animals receiving unloaded nanoparticles (IMP; Fig 3A). Immediately after measuring ear swelling, the spleens were collected and the CD4+ T cell populations present within the spleen examined by flow cytometry. The spleens of animals that had received two infusions of TIMP-GLIA had significantly reduced numbers of proliferating, interferon gamma (IFNG)-producing CD4+ effector T cells in the spleen, as determined by intracellular cytokine staining (Fig 3B; gating strategy in Supplementary Fig 2). The decrease in effector T cells observed in TIMP-GLIA treated animals correlated with a dose-dependent reduction in T cell proliferation (Fig 3C) in splenocyte cultures when restimulated with gliadin. Therefore, pre-treatment of mice with TIMP-GLIA prior to gliadin/CFA immunization induced a tolerogenic phenotype in gliadin-specific CD4+ T cells. Furthermore, this phenotype appeared to result in reduced T cell help, required for anti-gliadin antibody production, as treatment with 2.5mg/mouse TIMP-GLIA resulted in significant reduction in circulating levels of gliadin-specific IgG (Fig 3D).
Figure 3: TIMP-GLIA tolerance induction in mice with delayed-type hypersensitivity to gliadin.
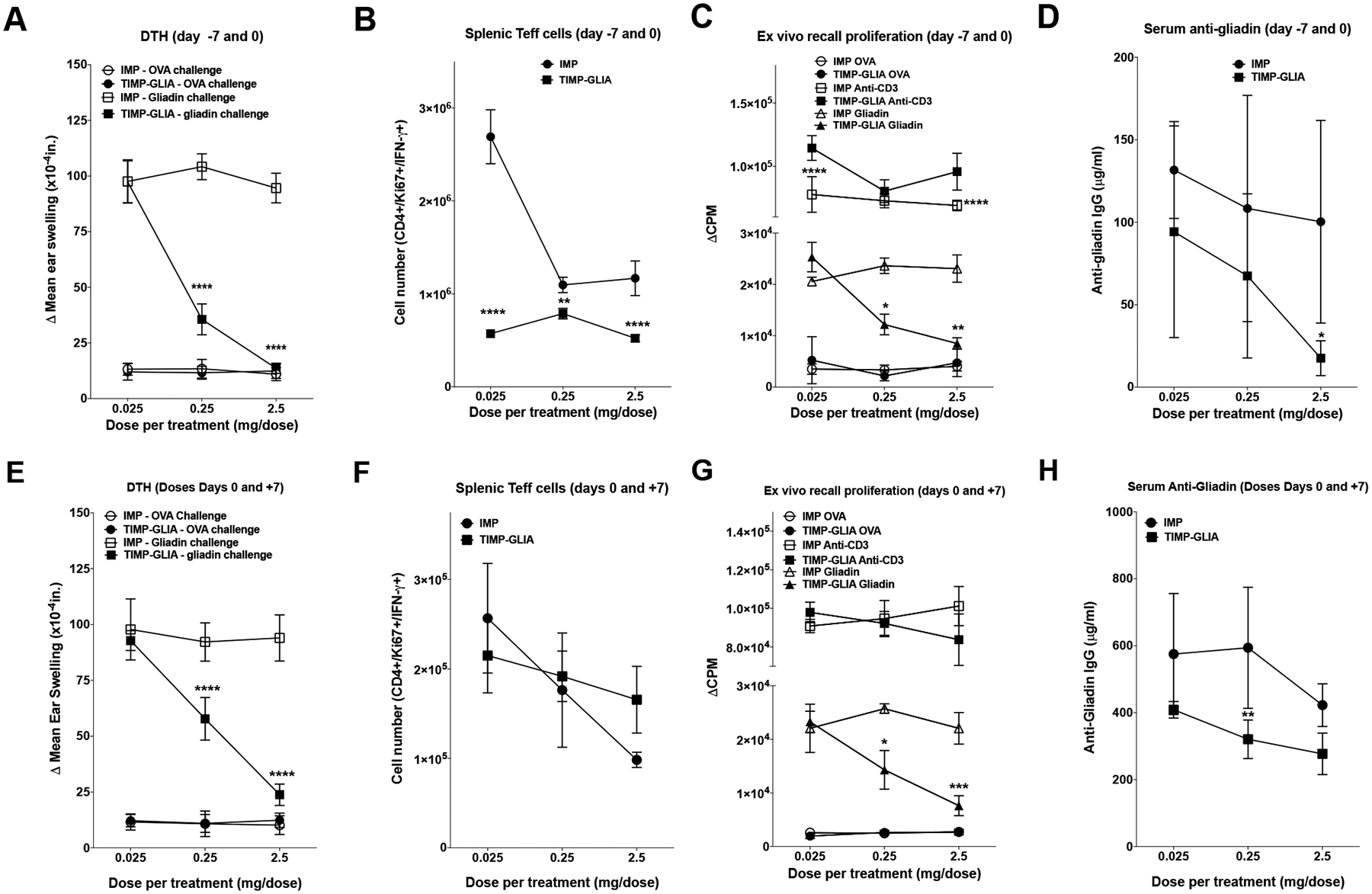
A-H) C57BL/6 female mice (n=5) were treated with TIMP-GLIA, or unloaded control particles (IMP), either on days −7 and 0 (A-D), or days 0 and 7 (E-H). Mice were primed with gliadin in CFA on day 0. A, E) On day 14 post priming, mice were injected with gliadin or ovalbumin (OVA) into the ear pinna for DTH analysis (Δ mean ear thickness after 24h +/− SEM; x10−4in). B, F) The numbers of live, CD3+/CD4+/Ki67+/IFNG+ splenic effector T cells were determined (Teffs; mean +/− SEM; flow cytometry). C, G) To assess proliferation, spleen cells were stimulated with anti-CD3, OVA, or gliadin (mean counts per minute +/− SEM; 3H-TdR incorporation). D, H) Serum anti-gliadin IgG antibody levels were analyzed, testing serial dilutions (mean concentration +/− SEM; ELISA). Statistical analyses were performed using one-way ANOVA and Tukey’s multiple comparison test (A-H; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001).
Subsequently, TIMP-GLIA tolerization was also tested in mice following immunization. TIMP-GLIA was administered several hours after priming (day 0), and again on day 7 after immunization. While treatment with 0.025 mg/mouse (1.25 mg/kg) failed to show efficacy, doses of 0.25–2.5 mg/mouse (12.5–125 mg/kg) were associated with significant decreases in ear swelling when compared to controls (Fig 3E). The most robust decrease in DTH was observed in animals receiving 2.5 mg/mouse (a human equivalent of 10.16 mg/kg using body surface area). Animals that had received 0.25–2.5 mg/mouse TIMP-GLIA had reduced numbers of proliferating, IFNG-producing CD4+ effector T cells in the spleen, compared to the lowest dose (Fig 3F). Gliadin recall experiments further demonstrated a dose-dependent effect. While spleen cells from animals receiving 0.025mg/mouse TIMP-GLIA showed unchanged T cell proliferation, two infusions of 0.25–2.5 mg/mouse/dose resulted in a significant inhibition of T cell proliferation (Fig 3G). Furthermore, TIMP-GLIA administration after immunization was associated with a reduction in circulating gliadin specific IgG (Fig 3H).
In an additional experiment, we tested the effects of 2.5mg TIMP-GLIA vs. IMP, administered intravenously to mice both on days 0 and 7 after gliadin immunization, on cytokine secretion of ear draining lymph node cells or spleen cells restimulated with gliadin on day 15. The results demonstrated significant reductions in inflammatory cytokines IFNG and interleukin 17 (IL17), but not in regulatory cytokine IL10, in mice treated with TIMP-GLIA (Supplementary Fig 3A–F). Taken together, the data obtained in the DTH model showed that TIMP-GLIA induced gliadin-specific tolerance in a dose-dependent fashion even in the context of a robustly activated immune response, without causing any apparent adverse effects.
TIMP-GLIA tolerance induction in transgenic mice expressing celiac disease-associated HLA-DQ8
To address the potential of TIMP-GLIA to induce tolerance in the context of the human leukocyte antigen DQ8 risk allele (HLA-DQ8) associated with celiac disease, Ab0 NOD mice (mouse MHC II deficient) expressing HLA-DQ8 and human CD4 transgenes were used.15 These mice, raised and maintained on a gluten free diet and subsequently immunized with gliadin/CFA, develop gliadin-specific B cell and CD4+ T cell responses. In this study, treatment of HLA-DQ8 mice with 2.5 mg/mouse (125 mg/kg) TIMP-GLIA or TIMP-OVA control on days −11 and −3 prior to immunization with gliadin/CFA (Fig 4A), did not appear to alter the levels of circulating anti-gliadin IgG1 (Supplementary Fig 4), but reduced the amount of T-helper 1 (Th1) cell-associated, complement-fixing anti-gliadin IgG2c, compared to TIMP-OVA control-treated mice (Fig 4B). In addition, TIMP-GLIA treatment resulted in significant reductions of T cell proliferation (Fig 4C) and inflammatory cytokine secretion in response to gliadin restimulation of splenocytes (IFNG, IL17; Fig 4D, E). While only non-significant reductions were observed for interleukin 2 (IL2) and the regulatory cytokine IL10 (Fig 4F, G), TIMP-GLIA treatment increased regulatory T cell specific forkhead box P3 (Foxp3) mRNA expression by gliadin-restimulated splenocytes in RT-qPCR (resulting in significant reductions of ΔCT values vs. TIMP-OVA, Fig 4H). Together, these results demonstrate gliadin-specific tolerance induction by TIMP-GLIA in HLA-DQ8 mice, and the involvement of FOXP3+ Tregs in the modulation of the gliadin recall response.
Figure 4. TIMP-GLIA tolerance induction in transgenic mice expressing celiac disease-associated HLA-DQ8.
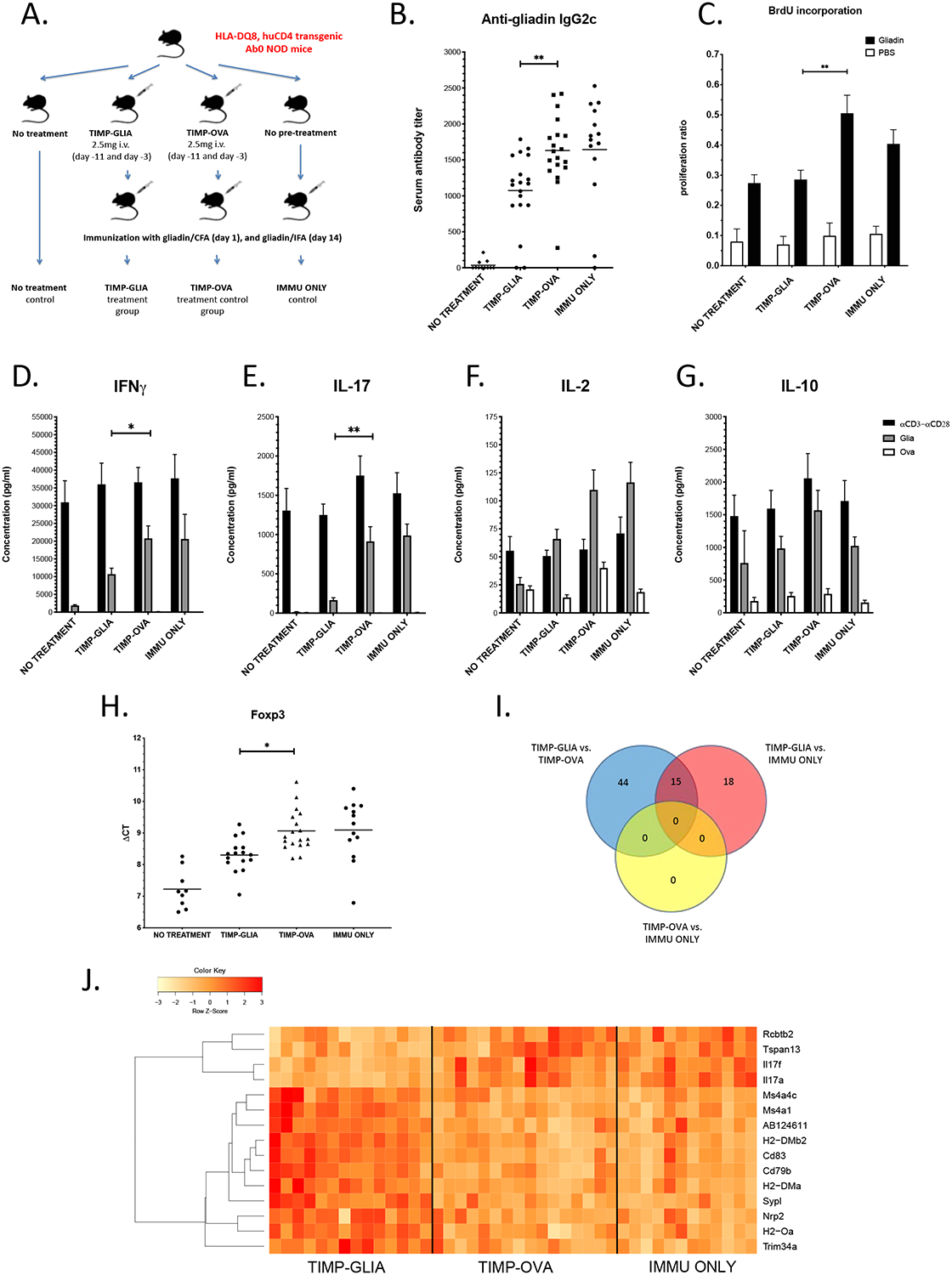
A) Experimental design. B) Serum anti-gliadin IgG2c antibody titers in groups of HLA-DQ8 mice, treated according to A (n=11–19; ELISA). C) Proliferation of spleen cells (n=8–9; BrdU ELISA). Data expressed as proliferation ratios, relating to anti-CD3/anti-CD28 positive control. D-G) IFNG, IL17, IL2 and IL10 cytokine concentrations in supernatants of spleen cells stimulated with gliadin, ovalbumin (negative control) or anti-CD3/anti-CD28 (positive control; n=11–19; ELISA). H) Foxp3 mRNA expression by spleen cells in response to gliadin restimulation (n=9–18; RT-qPCR). Results expressed in ΔCT values (reductions of ΔCT reflect increases in Foxp3 mRNA expression). I) Venn diagram depicting the numbers of genes differentially expressed in spleen cells restimulated with gliadin, in 3 separate comparisons between 3 groups of HLA-DQ8 mice (n=13–16; RNAseq). J) Heat map depicting the up- (red) or down-regulation (yellow) of 15 genes differentially expressed after treatment with TIMP-GLIA (n=13–16, adjusted p-value p≤0.05; RNAseq). Statistical analyses were performed using one-way ANOVA and Tukey’s multiple comparison test (B, H), t-tests corrected for multiple testing using the Holm-Sidak method (C-G) or edgeR (I, J). In plots B-H, significant results are indicated for comparisons between TIMP-GLIA and TIMP-OVA groups only (*p≤0.05, **p≤0.01).
To further characterize mechanistic pathways involved in TIMP-GLIA tolerance induction, we performed RNA sequencing of gliadin-restimulated splenocytes from three groups of treated HLA-DQ8 mice (TIMP-GLIA, TIMP-OVA, IMMU ONLY; Fig 4A), more than four weeks after the last dose of TIMP-GLIA had been administered. We found 77 genes differentially expressed, either between TIMP-GLIA vs. TIMP-OVA treated mice, or TIMP-GLIA vs. IMMU ONLY (Supplementary Fig 5). Of these, 15 genes showed significant differences in both comparisons, thus reconfirming results. A comparison between TIMP-OVA and IMMU ONLY did not reveal any differentially expressed genes, supporting an antigen-specific effect on gene regulation by TIMP-GLIA (Venn diagram, Fig 4I). The identities of these 15 genes, and their expression levels between samples from different groups, revealed long-term changes induced by TIMP-GLIA treatment in pathways of APC function (Tspan13, Cd83, Nrp2), B cell activation and differentiation (Cd79a, Ms4a1/Cd20, Ms4a4c), MHC II peptide loading (H2-DM, H2-O) and T cell cytokine secretion (Il17a, Il17f; gene expression heatmap, Fig 4J). The RNA sequence data has been deposited to NCBI Gene Expression Omnibus (Accession No.: GSE140736).
TIMP-GLIA tolerance induction reverses gliadin memory T cell enteropathy in mice
Based on the robust treatment responses observed above, we determined the efficacy of TIMP-GLIA in an adoptive gliadin memory T cell transfer model of celiac disease. In contrast to the animal models above, this model mimics the gluten-dependent enteropathy characteristic of celiac disease.18,19 Splenic CD4+CD62L-CD44hi memory T cells isolated from gliadin immunized C57BL/6 donor mice were transferred into four groups of male Rag1−/− mice (n=16; matched for body weight). On the same day, three of the groups were introduced to a gluten-containing diet, while one group remained on gluten-free diet (GFD negative control). Mice from two groups on gluten-containing diet were subsequently treated on days 10 and 24 with 2.5 mg/mouse (125 mg/kg) of TIMP-GLIA i.v., or TIMP-LYS (nanoparticle treatment control), while the third group on gluten-containing diet did not receive injections with nanoparticles (GLUTEN diet positive control; Fig 5A). For statistical analysis, we combined four matched experiments, all showing similar results. As is typically seen in this model, the GLUTEN positive control group gained less body weight until week 4, and subsequently lost weight more rapidly, when compared to the GFD control. However, mice in the TIMP-GLIA group were protected from weight loss over the entire study period, similar to gluten free diet control animals. In comparison, the TIMP-LYS treatment control group lost weight, and did not differ significantly from GLUTEN diet control (Fig 5B). In agreement with this result, treatment with TIMP-GLIA significantly reduced the severity of histological duodenitis compared to GLUTEN positive control, while TIMP-LYS treatment did not, indicating that the effect of TIMP-GLIA on small bowel pathology was antigen-specific (Fig 5C). Duodenal sections that exemplify histological duodenitis severity scores of normal, mild, moderate or severe duodenitis are shown (Fig 5D–G; hematoxylin/eosin staining).
Figure 5. TIMP-GLIA tolerance induction reverses gliadin memory T cell enteropathy in mice.
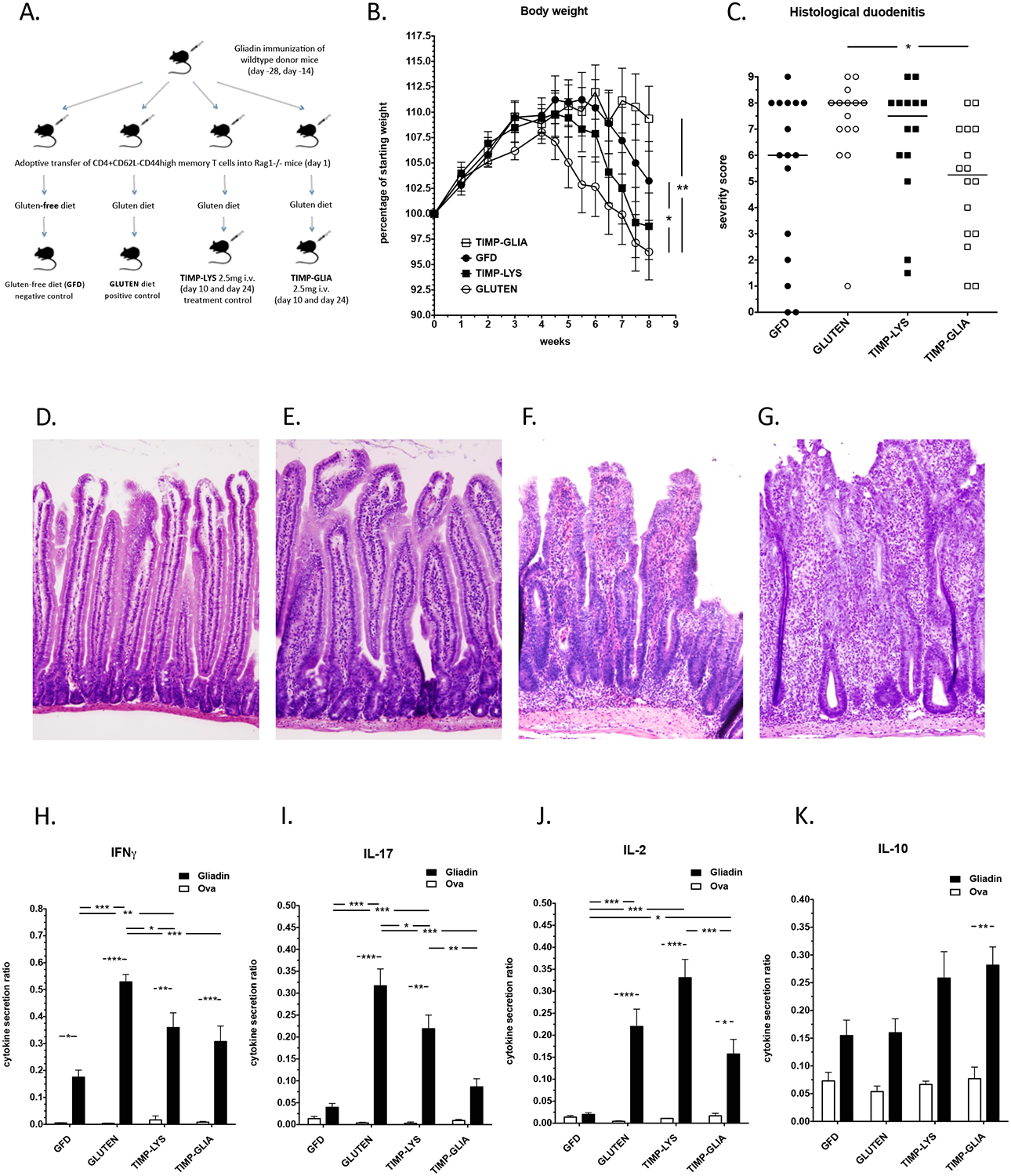
A) Experimental design. B) Total body weight development in four groups of Rag1−/− mice, treated according to A (n=14–16; data expressed as percentage of starting weight). C) Histological duodenitis severity scores (n=14–16; max. score 9). D-G) Hematoxylin/eosin staining of duodenal sections. Examples represent histological scores of D) 0 (normal), E) 3 (mild), F) 6 (moderate), or G) 9 (severe) duodenitis. Note increased villus and basal mononuclear cell infiltration, reduced villus-to-crypt ratios, and development of crypt abscesses with increasing scores. H-K). IFNG, IL17, IL2 and IL10 cytokine secretion in response to gliadin, or ovalbumin (Ova, negative control), in relation to anti-CD3/anti-CD28 antibody (positive control; n=9–12; ELISA; data expressed as cytokine secretion ratios). Statistical analyses were performed using one-way ANOVA and Tukey’s multiple comparison test (*p≤0.05, **p≤0.01, ***p≤0.001).
Consistent with results in HLA-DQ8 mice, treatment with TIMP-GLIA reduced secretion of pro-inflammatory IFNG, IL17, and IL2 cytokines by spleen cells restimulated with gliadin, compared to GLUTEN positive control or TIMP-LYS treatment control (Fig 5H–J), but not of regulatory cytokine IL10 (Fig 5K). Thus, TIMP-GLIA treatment inhibited gliadin-specific Th1/T-helper 17 (Th17) cell responses, while not altering the IL10-mediated regulatory response. In summary, treatment with TIMP-GLIA reversed the effects of dietary gluten exposure also in this intestinal celiac disease model, similar to the effect of GFD (negative control), while control treatment with TIMP-LYS showed minor effects only. Therefore, the findings confirmed that TIMP-GLIA treatment induced a tolerogenic phenotype within the gliadin-specific T cell population.
TIMP-GLIA clearance and biodistribution
To determine TIMP-GLIA circulating time, C57BL/6 mice were injected intravenously with 2.5mg TIMP-GLIA vs. 40μg soluble gliadin. Gliadin in plasma was quantified over 24h. The gliadin plasma concentration after injection of TIMP-GLIA peaked at 1h, rapidly declined until 4h, and had returned to basline at 24h after injection, as determined by ELISA (Fig 6A).
Figure 6. TIMP-GLIA clearance, and interaction with human peripheral blood mononuclear cells (PBMC).
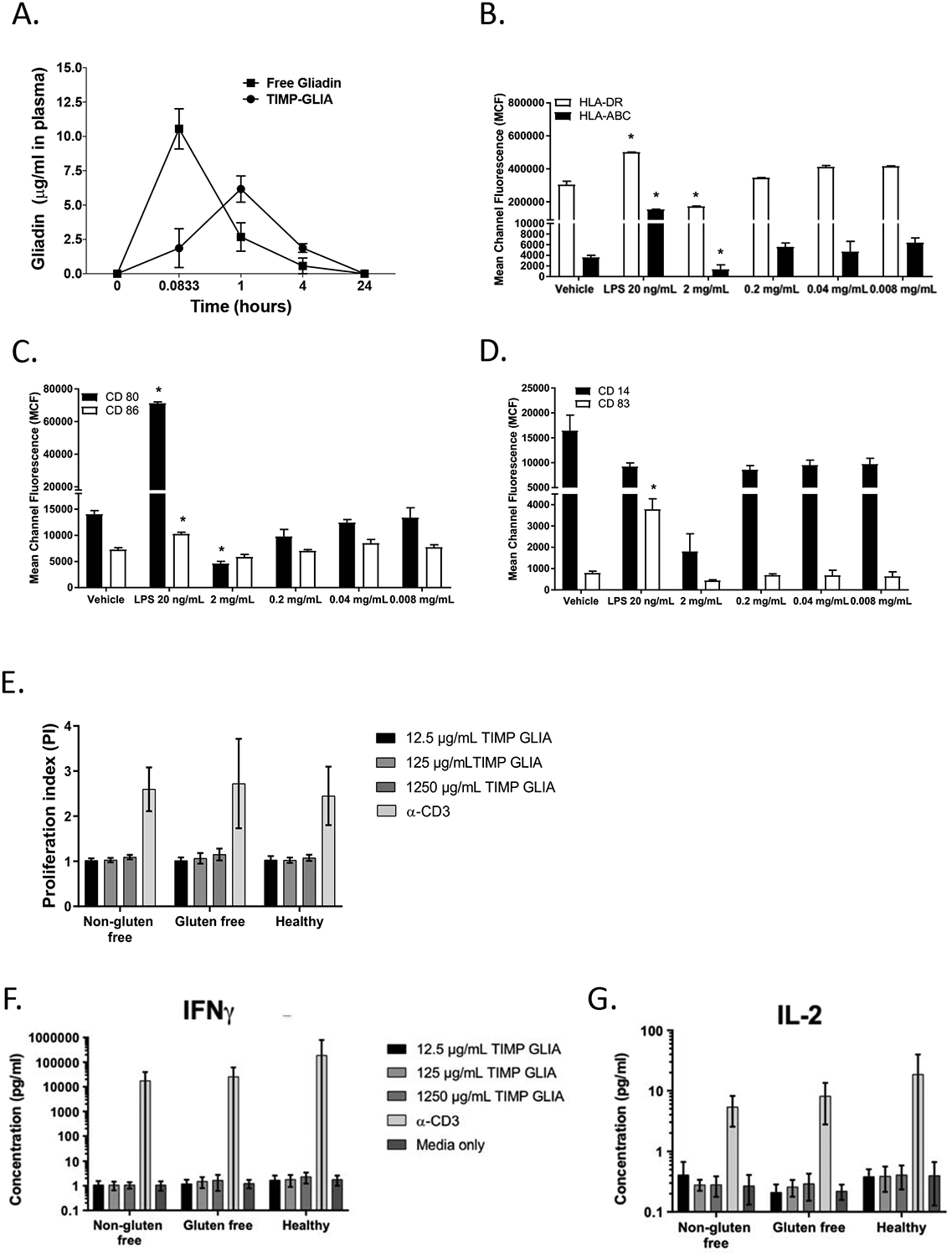
A) Naïve C57BL/6 mice (n=3 per time point) were injected intravenously with either 2.5mg of TIMP-GLIA, or 40ug of free gliadin (corresponding amount). Mice were bled at 5min, 1h, 4h, and 24h. Collected plasma samples were assessed for the level of free gliadin (ELISA; mean concentration +/− SEM). B-D) Immature dendritic cells derived from human PBMC (n=6–9) were treated with vehicle (PBS), LPS 20 ng/mL (positive control) or TIMP-GLIA at increasing concentrations for 48 hours. Surface expression of HLA-ABC and HLA-DR (B), CD80 and CD86 (C) and CD14 and CD83 (D) were determined by flow cytometry (mean channel fluorescence; mean ± SD). E-G) Human PBMC from celiac disease patients on normal or gluten free diet, or healthy controls, were stimulated with anti-CD3 antibody (positive control) or TIMP-GLIA at increasing concentrations (triplicates; n=9–11). Proliferation (E), and IFNG (F) or IL2 (G) cytokine secretion were measured after 72h. Data is expressed as proliferation index (relating to unstimulated cells; luminescent cell viability assay), or cytokine concentrations (V-Plex assay). Statistical analyses were performed using paired t-test, compared to vehicle group (B-D; *p≤0.05).
Human peripheral blood monocyte (PBMC)-derived dendritic cells maintain an immature phenotype when treated with TIMP-GLIA, consistent with the induction of tolerance
TIMP induced antigen-specific tolerance has been shown in rodent models to harnesses physiological systems of apoptotic cellular debris clearance and homeostasis to induce T cell non-responsiveness. To address whether TIMP-GLIA is being perceived as apoptotic by APCs, such as dendritic cells (DC), in humans, we next examined the potential for TIMP-GLIA to induce PBMC derived DC maturation, following a reference protocol. Primary cultures of human monocytes were treated with interleukin 4 (IL4) and granulocyte-macrophage colony-stimulating factor (GM-CSF) for 7 days to generate immature DC. Maturation of DC was assessed by flow cytometry, following culture of the resultant immature DCs in the presence of TIMP-GLIA at increasing doses for 48hrs (0.008 mg/ml – 2 mg/ml). No increases in the surface expression of HLA-ABC, HLA-DR, CD14, CD83, CD80 or CD86 were observed in comparison to negative control, or immature DC that were cultured in the presence of LPS (Fig 6 B–D). Together the data show that TIMP-GLIA did not result in activation of human monocyte-derived DC, but in maintenance of low levels of co-stimulatory molecules and HLA, suggesting a tolerogenic DC phenotype.
TIMP-GLIA shows promising human in vitro biocompatibility, and is non-activating when incubated with PBMC from CD patients and controls
The induction of tolerance using the TIMP platform is contingent upon intravenous infusion. Following reference protocols, we determined the biocompatibility of TIMP-GLIA, by adding into human blood increasing doses of TIMP-GLIA, spanning the theoretical plasma concentration, calculated from the TIMP-GLIA mouse optimum/human equivalent dose (0.127mg/ml; Supplementary Fig 6A). TIMP-GLIA did not trigger hemolysis (Supplementary Fig 6B), platelet activation (Supplementary Fig 6C), platelet aggregation (Supplementary Fig 6D), or complement activation (as determined by levels of Bb plus, C4d and iC3b; Supplementary Fig 6E–G). Coagulation studies showed no abnormalities in prothrombin time (extrinsic pathway) at any concentration, whereas prolonged partial thromboplastin time (intrinsic pathway) and thrombin time (common pathway) were observed only at concentrations exceeding the theoretical plasma concentration (Supplementary Figure 5H–J). Taken together, the findings suggest compatibility of TIMP-GLIA for intravenous infusion.
Finally, the potential of TIMP-GLIA to non-specifically activate CD3+ T cells of celiac disease patients or healthy controls was analyzed (clinical data shown in Supplementary Table 2). No proliferation of PBMC was detected with increasing concentrations of TIMP-GLIA, exceeding the theoretical plasma concentration, confirming that no direct activation of blood T cells by TIMP-GLIA occurred (Fig. 6E). This was further supported by a lack of IFNG or IL2 T cell cytokine secretion (Fig. 6F, G).
Discussion
Immune tolerance is a state of unresponsiveness of the immune system to foreign or self-antigens. The natural development of unresponsiveness to harmless stimuli from food, including gluten, is called oral tolerance. Oral tolerance to gluten is broken in celiac disease, for reasons unknown, and the re-establishment of oral tolerance to gluten is the goal of antigen-specific immunotherapy of celiac disease with TIMP-GLIA. While sustained unresponsiveness to food antigen may be achieved as a result of an intervention, as exemplified by oral immunotherapy of food allergy22, such therapies are not available for the treatment of celiac disease.
Gliadin-specific CD4+T cells of celiac disease patients secrete cytokines IFNG and IL2 in response to dietary gluten, provide T cell help to gliadin- or TG2-specific B cells for antibody production, and orchestrate the immune response in the celiac mucosa, leading to villous atrophy, crypt hyperplasia and mononuclear cell infiltration.1, 2 In addition, several reports demonstrate that the CD4+ T cell cytokine IL17 is overexpressed in active celiac disease23,24, and is detected early in serum in response to gluten challenge.25 There is no single experimental model that fully reflects human celiac disease.26 Therefore, testing of TIMP-GLIA was performed using three different mouse models, each featuring major disease characteristics associated with human celiac disease. The presented results demonstrate preclinical efficacy and safety supporting the clinical translation of TIMP-GLIA for the treatment of celiac disease. TIMP-GLIA inhibited the proliferation and cytokine IL2, IFNG and IL17 secretion of gliadin-stimulated T cells, while secretion of the regulatory cytokine IL10 remained unchanged. In addition, TIMP-GLIA increased Foxp3 expression by Treg cells stimulated with gliadin, decreased the anti-gliadin antibody production by B cells, and reduced gliadin-specific DTH, histological gluten-dependent enteropathy and body weight loss. Unloaded or control antigen-loaded TIMP used as treatment control did not show comparable effects. In summary, TIMP-GLIA induced gluten unresponsiveness in mice in an antigen-specific and dose-dependent fashion. If similar effects were induced by treatment of celiac disease patients with TIMP-GLIA, this might lead to unresponsiveness to oral gluten, and potentially, cure of celiac disease.
TIMP-GLIA was designed with antigenic gliadin protein that encompasses an array of gliadin-specific T cell epitopes. Importantly, not a single one of the many identified gliadin T cell peptides is recognized by more than two thirds of celiac disease patients.27 Therefore, treatments aimed at restoring unresponsiveness to gliadin may need to modulate a variety of T cell clones, specific for a broad set of gliadin peptides.15,16,28 Gliadin was successfully solubilized and encapsulated into PLGA nanoparticles with a zeta potential of approximately −40 mV, under current good manufacturing practices. The TIMP-GLIA treatment approach is based on harnessing the physiological propensity of ‘tolerogenic’ APCs to process and present peptide epitopes from intact proteins, thus avoiding gliadin peptide selection bias, and allowing APCs to present the full spectrum of T cell epitopes from gliadin protein.
Since gliadin T cell epitopes frequently contain deamidated glutamine (glutamic acid) residues15,16,28, we note that as a result of the protein solubilization procedure in acetic acid, gliadin encapsulated in TIMP-GLIA is already partially deamidated.29 Processing of TIMP-GLIA by APC, equipped with enzymes that can further deamidate glutamine residues of gliadin peptides, most importantly tissue transglutaminase (TG2)30,31, may lead to the presentation of both native and deamidated gliadin peptides. Irrespective, gliadin-specific T cells isolated from the intestine of celiac disease patients have frequently been shown to respond to both deamidated and native gliadin protein.
In this study, TIMP-GLIA was not associated with any drug-related toxicity in mice. In addition, a good laboratory practice repeat dose toxicology study was conducted in rats. This study found that TIMP-GLIA had a no observed adverse effect level of 75 mg/kg (human equivalent dose of ~12 mg/kg/day; HED), a level exceeding the HED calculated from the mouse optimum dose in our study (10.16 mg/kg/day). Further, a toxicokinetic (pK) analysis was performed in rats with increasing doses of TIMP-GLIA. This study determined that the maximum concentration and the area under the curve increased in proportion to the dose of TIMP-GLIA administered. The maximum plasma fraction was reached at 0.083 hours after dosing. The half-life of TIMP-GLIA ranged from 5.94 to 6.94 hours. In a completed, FDA-monitored phase 1 safety study of TIMP-GLIA (NCT03486990), and in a completed randomized, double-blind, placebo-controlled phase 2 trial of TIMP-GLIA (NCT03738475), no toxicity was noted in celiac disease patients (unpublished results). Our human studies in vitro showed that TIMP-GLIA does not cause complement activation, hemolysis, platelet activation or aggregation, or activation/interference with coagulation at the estimated plasma concentration. Similar findings have been described for other PLGA nanoparticle formulations.32 Surface characteristics, charge, and size have been identified as key factors important in limiting adverse interactions of nanoparticles with blood components and the innate immune system. Although TIMP-GLIA does not possess direct T cell targeting moieties, in this study the response by PBMCs isolated from celiac disease patients to TIMP-GLIA was also assessed. The results demonstrate that TIMP-GLIA does not lead to direct, broad T cell activation or T cell inflammatory cytokine secretion. Together these observations indicate safety of TIMP-GLIA, and its compatibility with intravenous administration.
Until now, none of the attempts to induce tolerance in human autoimmune diseases through oral, subcutaneous, or intramuscular antigen administration have led to the development of an approved drug. This has been proposed to be a result of antigen delivery to pro-inflammatory APC populations in the periphery, expressing high levels of MHC II and positive co-stimulatory molecules, which are associated with robust Th1 cell immune responses.33 Induction of tolerance with intradermal injection of a solution of three immunodominant gliadin peptides has recently been attempted (NexVax2; ImmusanT, Inc.).34, 35 Administration of gliadin peptides via this route triggered cytokine release, nausea and vomiting in celiac patients. Interestingly, plasma cytokine levels and treatment-emergent adverse events were diminished upon repeated intradermal administration of peptides. No evidence for unresponsiveness to dietary gluten was demonstrated in these clinical studies. Here, we show that the targeted delivery of gliadin to apoptotic pathways in the spleen by intravenous infusion of TIMP-GLIA in mice leads to the suppression of inflammatory T and B cell responses upon gliadin recall, and the reduction of gliadin-/gluten-dependent organ pathology. Clinical efficacy, safety and durability of gliadin-specific immunotherapies remains to be demonstrated in celiac disease patients.
TIMP-GLIA size, charge, and route of administration are designed to direct gliadin to APCs within the spleen and liver. Similar PLGA particles have been shown to be taken up in a tolerogenic fashion by APCs.4,5,6,7,8,12 Here we show that TIMP-GLIA at concentrations derived from mouse optimum/human equivalent doses does not trigger human monocyte-derived dendritic cell maturation, or proinflammatory pathways. In addition to these direct effects on APC, we characterized the long-term immunomodulatory signature of TIMP-GLIA. Changes in the gliadin recall response of spleen cells from tolerized HLA-DQ8 transgenic mice were identified more than four weeks after the last dose had been administered (RNAseq; RT-qPCR; cytokine ELISA). Genes modulating APC function (Tspan13, Cd83, Nrp2), MHC class II peptide presentation (H2-DM, H2-O), B cell activation/differentiation (Cd79b, Ms4a1/Cd20, Ms4a4c) and T cell differentiation/cytokine secretion (Foxp3, Ifng, Il17, Il2) were identified as directly or indirectly regulated targets, confirming earlier but more limited results using antigens coupled to apoptotic cells 13,14 or contained in PLGA nanoparticles.7 Collectively, these results indicate that TIMP-GLIA modulates the response of gliadin memory B and T cells, consistent with the treatment outcomes in three celiac mouse models in our study.
Interestingly, the modulation of HLA class II gliadin presentation by HLA-DM and HLA-DO has already been identified as a therapeutic opportunity in celiac disease. The celiac disease-associated risk alleles HLA-DQ2.5 and HLA-DQ8 appear to interact poorly with HLA-DM, resulting in prolonged retention of gliadin peptides, and increased effector T cell stimulation.36 Increased expression of HLA-DM and/or HLA-DO in APCs, as a long-term effect of TIMP-GLIA treatment, may improve the efficiency of gliadin peptide exchange, thus inhibiting HLA-DQ2.5- and HLA-DQ8-restricted gliadin presentation.
Successful reintroduction of durable immune tolerance to gluten would represent a cure for celiac disease patients. The evidence provided here supports the ability for TIMP-GLIA to establish sustained unresponsiveness to gluten in mice, which in the context of human disease may not only alleviate the need for a gluten-free diet, but might also reduce disease complications such as secondary autoimmune diseases, bone loss or lymphoma. The therapeutic potential of TIMP-GLIA is currently under investigation in phase 1/2 clinical trials (NCT03486990 and NCT03738475).
Supplementary Material
Acknowledgements
This study was performed with funding by Cour Pharmaceuticals Development, Inc., National Health and Medical Research Council grant 1030897 (to D.R.G and N.J.C.K), National Institutes of Health Grant EB-013198 (to S.D.M), Helsinki University Central Hospital (TYH2014307) and Academy of Finland (292393) grants (both to S.K.M).
We thank Mari Rissanen (Centre for Drug Research, University of Helsinki) for technical assistance with mouse intravenous injections, and Ulla Kiiski and Leena Saikko (both at the Dept. of Pathology, University of Helsinki) for technical assistance with histology and immunohistochemistry, respectively.
Abbreviations
- Ag
Antigen
- APC
antigen-presenting cells
- CFA
complete Freund’s adjuvant
- DTH
delayed-type hypersensitivity
- DC
dendritic cells
- GFD
gluten-free diet
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HED
human equivalent dose
- HLA
human leukocyte antigen
- IFNG
interferon gamma
- IL2
interleukin 2
- IL4
interleukin 4
- IL10
interleukin 10
- IL17
interleukin 17
- MARCO
macrophage receptor with collagenous structure
- MS
multiple sclerosis
- PBMC
peripheral blood mononuclear cells
- PEMA
poly(ethylene-alt-maleic acid)
- PLGA
poly(lactide-co-glycolide)
- PVA
poly(vinyl acetate)
- (Th1) cell
T-helper 1
- (Th17) cell
T-helper 17
- TGFB
transforming growth factor beta
- Treg cells
T-regulatory cells
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SD
standard deviation
- SEM
standard error of the mean
- TIMP
tolerogenic immune-modifying nanoparticles
- TIMP-GLIA
tolerogenic immune-modifying nanoparticles containing gliadin
- T1D
type 1 diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
N.J.C.K, S.D.M, L.D.S and D.R.G are shareholders of Cour Pharmaceutical Development Company. N.J.C.K, S.D.M, L.D.S and D.R.G are inventors on patent applications describing TIMP-GLIA. Based on an agreement between the University of Helsinki and Cour Pharmaceuticals Development Company, T.L.F. received funding to conduct experiments. The other authors have no financial arrangements to disclose.
Study approval. Human blood cell and plasma samples were provided by Precision for Medicine (Frederick, MD, USA) and BTS Research (San Diego, CA, USA). Precision for Medicine and BTS Research obtained approvals from their respective institutional review boards for all the studies reported. Informed consent was obtained from all participants. All animal procedures were approved by the Northwestern University Institutional Animal Care and Use Committee (A3283–01), or the Southern Finnish State Administrative Agency (ESAVI/1064/04.10.03/2012 and ESAVI/1286/04.10.07/2016).
References
- 1.Lebwohl B, Sanders DS, Green PHR Coeliac disease. Lancet 391, 70–81 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Nilsen EM, Jahnsen FL, Lundin KE et al. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology 115, 551–63 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Anderson RP, Jabri B Vaccine against autoimmune disease: antigen-specific immunotherapy. Curr. Opin. Immunol 25, 410–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson RM, Casey LM, Hughes KR et al. Controlled delivery of single or multiple antigens in tolerogenic nanoparticles using peptide-polymer bioconjugates. Mol. Ther 25, 1655–1664 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hlavaty KA, McCarthy DP, Saito E et al. Tolerance induction using nanoparticles bearing HY peptides in bone marrow transplantation. Biomaterials 76, 1–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smarr CB, Yap WT, Neef TP et al. Biodegradable antigen-associated PLG nanoparticles tolerize Th2-mediated allergic airway inflammation pre- and postsensitization. Proc. Natl. Acad. Sci. USA 113, 5059–64 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad S, Neef T, Xu D, Podojil JR et al. Tolerogenic Ag-PLG nanoparticles induce tregs to suppress activated diabetogenic CD4 and CD8 T cells. J. Autoimmunity 89,112–124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Getts DR, Terry RL, Getts MT, et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med 6, 219ra7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy DP, Yap JW, Harp CT, et al. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomedicine 13, 191–200 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant J, Hlavaty KA, Zhang X, et al. Nanoparticle delivery of donor antigens for transplant tolerance in allogenic islet transplantation. Biomaterials 35, 8887–8894 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Getts DR, Martin AJ, Mc Carthy DP et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol 30, 1217–1224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo R, Saito E, Miller SD, Shea LD Peptide-conjugated nanoparticles reduce positive co-stimulatory expression and T cell activity to induce tolerance. Mol. Ther 25, 1676–1685 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Getts DR, Turley DM, Smith CE et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J. Immunol 187, 2405–17 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo X, Pothoven KL, McCarthy D et al. ECDI-fixed allogenic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc. Natl. Acad. Sci. USA 105, 14527–14532 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sollid LM, Qiao S-W, Anderson RP et al. Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics 64, 455–460 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tye-Din JA, Stewart JA, Dromey JA et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med 2, 41ra51 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Hayward SL, Bautista-Lopez N, Suzuki K et al. CD4 T cells play major effector role and CD8 T cells initiating role in spontaneous autoimmune myocarditis of HLA-DQ8 transgenic IAb knockout nonobese diabetic mice. J. Immunol 176, 7715–7725 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Freitag TL, Rietdijk S, Junker Y et al. Gliadin-primed CD4+CD45RBlowCD25- T cells drive gluten-dependent small intestinal damage after adoptive transfer into lymphopenic mice. Gut 58, 1597–1605 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freitag TL, Loponen J, Messing M et al. Testing safety of germinated rye sourdough in a celiac disease model based on the adoptive transfer of prolamin-primed memory T cells into lymphopenic mice. Am. J. Physiol. Gastrointest. Liver Physiol 306, G526–G534 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Macosko EZ, Basu A, Satija R et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 161, 1202–1214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter Z, McCarthy DP, Yap WT et al. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune diseases. ACS Nano 8, 2148–2160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anagnostou K, Clark C What do we mean by oral tolerance? Clin. Exp. Allergy 46, 782–784 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Monteleone I, Sarra M, Del Vecchio Blanco M et al. Characterization of IL-17A-producing cells in celiac disease mucosa. J. Immunol 184, 2211–2218 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Lahdenperä AI, Hölttä V, Ruohtula T et al. Up-regulation of small intestinal interleukin-17 immunity in untreated coeliac disease but not in potential coeliac disease or in type 1 diabetes. Clin. Exp. Immunol 167, 226–234 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goel G, Daveson AJM, Hooi CE et al. Serum cytokines elevated during gluten-mediated cytokine release in coeliac disease. Clin. Exp. Immunol 199, 68–78 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korneychuk N, Meresse B, Cerf-Bensussan N Lessons from rodent models in celiac disease. Mucosal Immunol. 8, 18–28 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Hardy MY, Girardin A, Pizzey C et al. Consistency in polyclonal T-cell responses to gluten between children and adults with celiac disease. Gastroenterology 149, 1541–1552.e2 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Vader W, Kooy Y, Van Veelen P et al. The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 122, 1729–1737 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Liao L, Zhao M, Ren J et al. Effect of acetic acid deamidation induced modification on functional and nutritional properties and confirmation of wheat gluten. J. Sci. Food Agric 90, 409–417 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Hodrea J, Demeny MA, Majai G et al. Transglutaminase 2 is expressed and active on the surface of human monocyte-derived dendritic cells and macrophages. Immunol. Lett 130, 74–81 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Toth B, Garabuczi E, Sarang Z et al. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J. Immunol 182, 2084–2092 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Guedj AS, Kell AJ, Barnes M et al. Preparation, characterization, and safety evaluation of poly(lactide-co-glycolide) nanoparticles for protein delivery into macrophages. Int. J. Nanomedicine 10, 5965–5979 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tozuka M, Oka T, Jounai N et al. Efficient antigen delivery to the draining lymph node is key component in the immunogenic pathway of the intradermal vaccine. J. Dermatol. Sci 82, 38–45 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Goel G, King T, Daveson AJ et al. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: two randomized, double-blind, placebo-controlled phase 1 studies. Lancet Gastroenterol. Hepatol 2, 479–493 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goel G, Tye-Din JA, Qiao SW et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci. Adv 5, eaaw7756 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou T, Macmillan H, Chen Z et al. An insertion mutant in DQA1*0501 restores susceptibility to HLA-DM: implications for disease associations. J. Immunol 187, 2442–2452 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


