Abstract
Umbilical Cord Blood (UCB) transplant (UCBT) is a curative procedure for patients with hematologic malignancies and genetic disorders and expands access for non-Caucasian patients unable to find a fully matched unrelated donor. In 2011, the Food and Drug Administration (FDA) required that unrelated UCBT use either licensed UCB or unlicensed UCB via an Investigational New Drug (IND). The National Marrow Donor Program® (NMDP) manages an IND under which 2456 patients (1499 adults and 957 children (564 malignant disease and 393 non-malignant disease) received single or double UCBT between October 2011 and December, 2016. Median age was 31 years (<1 to 81); 50% of children and 36% of adults were non-Caucasian. Median days to neutrophil engraftment (absolute neutrophil count ≥ 500/mm3) were 22, 20 and 19 days and the incidence of engraftment at 42 days was 89%, 88%, and 90% for adult, pediatric malignant, and pediatric non-malignant, respectively. Acute GVHD Grades II-IV was 35%, 32%, and 24%, chronic GVHD was 24%, 26%, and 24% and one year overall survival (OS) was 57%, 71%, and 79% for adults, pediatric malignant, and pediatric non-malignant.. In multivariate analysis, younger age, lower HCT-CI, early stage chemotherapy sensitive disease, and higher performance score predicted improved OS for adults. In a subset analysis of children with malignancies receiving single UCBT, use of either licensed (n=48) or unlicensed UCB (n=382) was associated with similar engraftment and survival. Use of unlicensed UCB units is safe, effective and provides an important graft source for a diverse population.
Keywords: Cord Blood Transplantation, Leukemia, Non-malignant disease, licensure
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HCT) is a curative procedure for patients with some hematologic malignancies, bone marrow failure syndromes, and genetic diseases.1 The optimal donor is often an HLA-matched related donor (MRD), however only 30% of patients have a match in their family. There are over 20 million adult volunteer donors enrolled in the Be The Match® (BTM) international unrelated donor registry, but it remains difficult for Black, Hispanic, and Caucasian patients of non-Western European ancestry to identify a matched unrelated donor (MUD) in the registry. For example, only 16–19% of Black patients are able to find a full match on the BTM Registry.2 Unrelated cord blood donors, stored in public cord blood banks are a readily available alternative graft source for patients lacking a matched related or unrelated donor.3,4,5 A perfect HLA match to the recipient is not required; as such, BTM data indicates that UCB donors were utilized for 34% of unrelated transplants for Black patients. (Figure 1a)2,6,7 Over 40,000 UCBT has been performed worldwide to date, and multiple retrospective analyses have indicated similar survivals among UCB and other graft sources including MUD and haploidentical (haplo) HCT.8,9.10 UCB may be the only available graft source for some patients of diverse race/ethnicity11.
Figure 1. Distribution of Patient Race/Ethnicity.
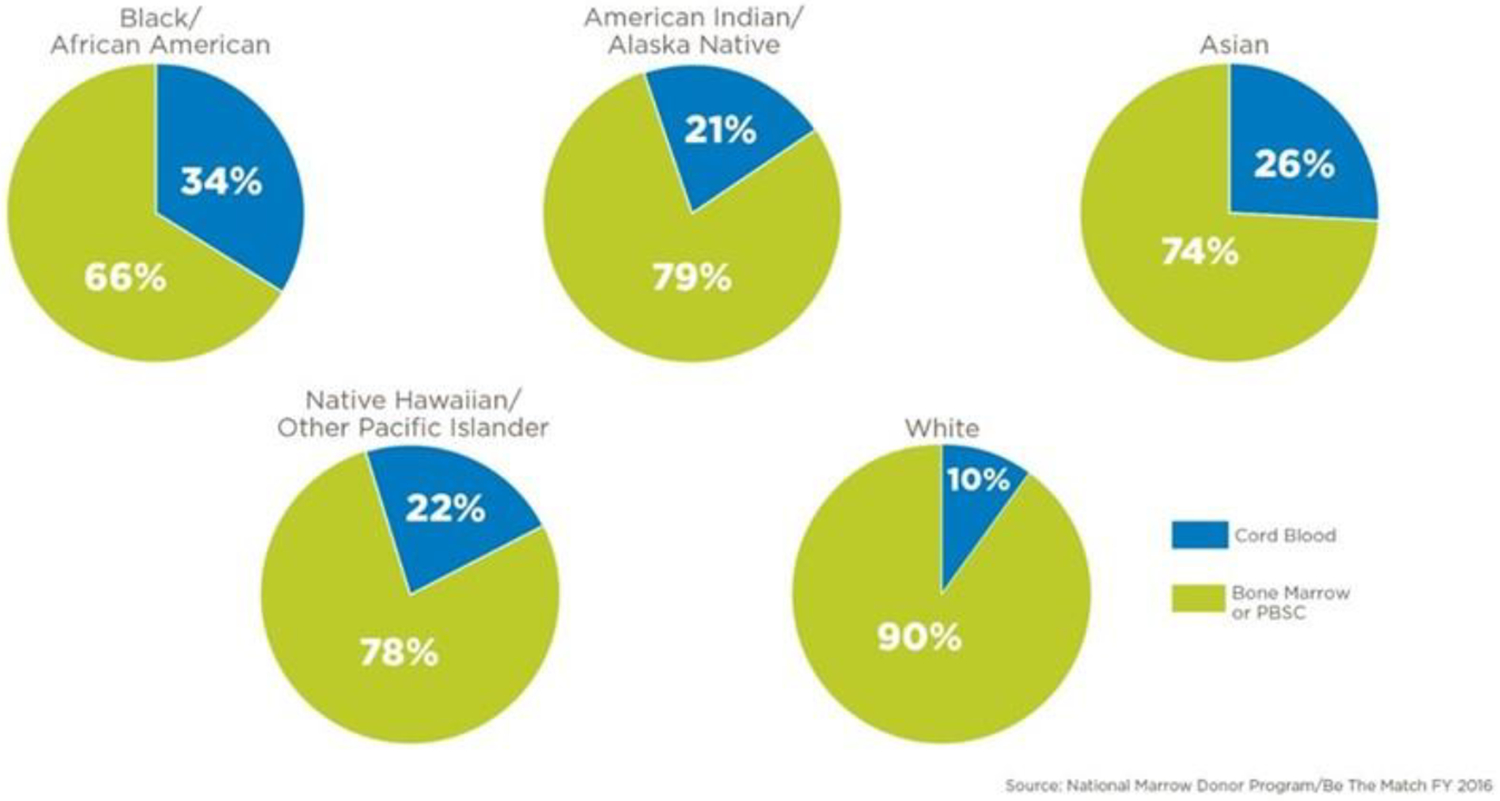
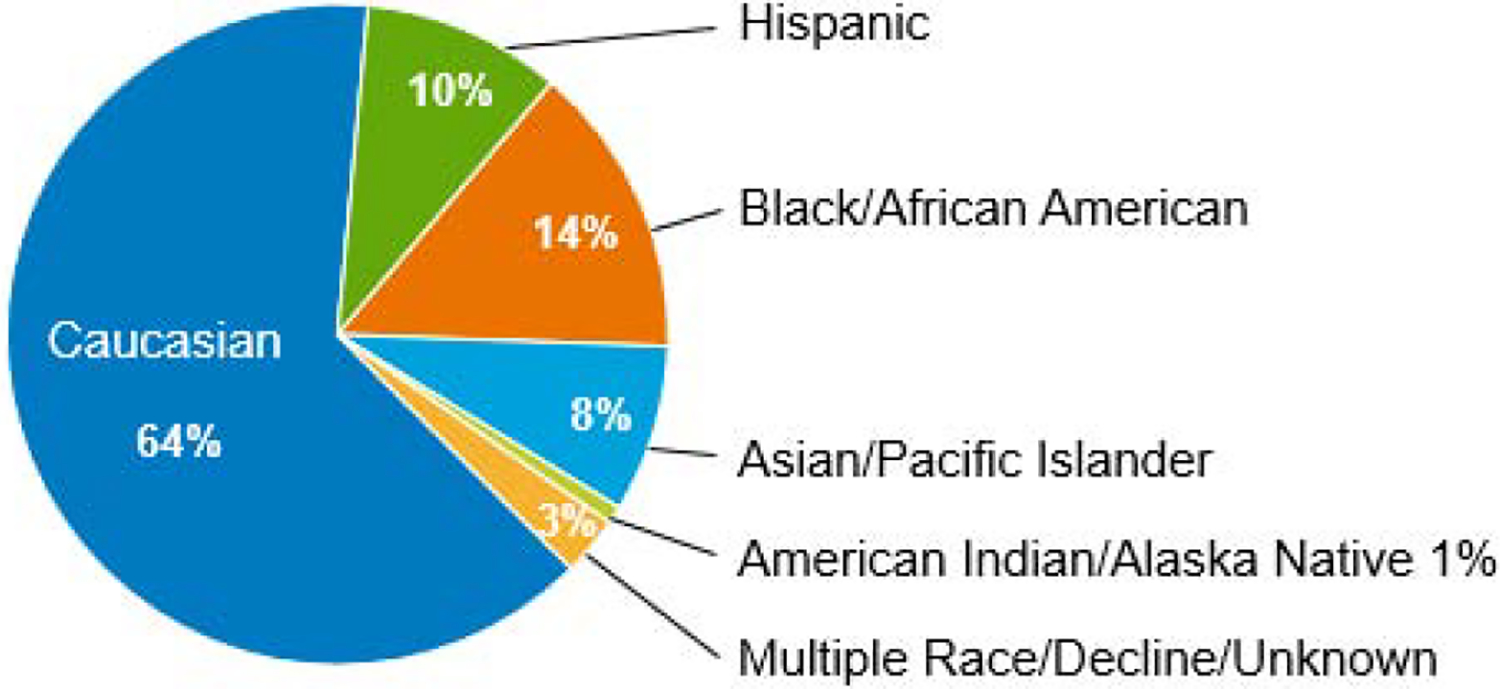
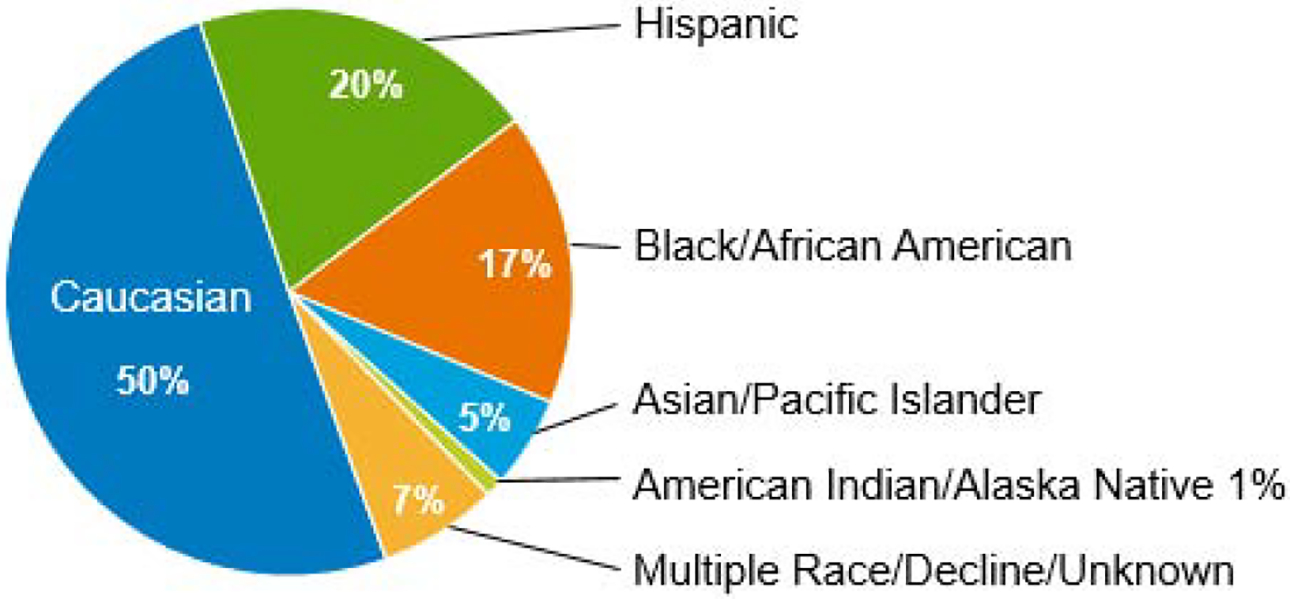
Figure 1a.Graft Source by Race/Ethnicity: Cord Blood vs. Adult Donor Bone Marrow or Peripheral Blood Stem Cells (distribution by the National Marrow Donor Program/Be The Match in 2016)
Figure 1b. Adult Patients with Malignant Diseases (N=1499)
Figure 1c. Pediatric Patients (N=957)
In 2011, the FDA required UCB units be licensed as a biologic drug, requiring public unrelated donor cord blood banks (CBB) to submit a Biologic License Application (BLA) and obtain a BLA approval from the FDA. Any unlicensed UCB units, including hundreds of thousands of CBUs banked in the prior 2 decades, were required to be distributed under IND.12,13 In order to provide access to UCB units that would not be licensed, the NMDP submitted a protocol under their existing UCB IND, entitled “A Multicenter Access and Distribution Protocol for Unlicensed Cryopreserved Cord Blood Units for Transplantation in Pediatric and Adult Patients with Hematologic Malignancies and Other Indications”.
The primary objective of the protocol was to examine neutrophil recovery after UCBT using unlicensed UCB units, and to provide access to and distribution of unlicensed UCB units to U.S. transplant centers. This report is an interim analysis of the ongoing IND study. 114 U.S. transplant centers, 22 U.S. CBBs, and 68 international CBBs participated. This report, one of the largest of its kind, examines outcomes for 2456 patients of diverse race/ethnicity (50% children and 36% of adults non-Caucasian) receiving UCBT using these unlicensed UCB units.
PATIENTS AND METHODS
Data source
Data were obtained from the Center for International Blood and Marrow Transplant Research® (CIBMTR), a research collaboration between the NMDP/BTM and the Medical College of Wisconsin. The data collection forms for the patients enrolled on this study include both the standard and study-specific CIBMTR data collection forms. Patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality and completeness. This study was performed in compliance with all applicable federal regulations pertaining to the protection of human research participants and under guidance of the NMDP Institutional Review Board (IRB). UCBT data are collected pre-HCT, 100 days and six months post-HCT, annually until 6 years post-HCT, and biannually thereafter until death.
STUDY POPULATION
In this prospective study, U.S. pediatric and adult patients who received a first allogeneic UCBT using an unlicensed UCB unit under the NMDP IND protocol between October 2011 and December 2016 were eligible. Pediatric and adult patients of any age with disorders treated by HCT were eligible. Patients who received a UCBT under a separate IND (for example, on a trial that studied ex vivo expansion or where UCB were more than minimally manipulated) were excluded, as were patients who received combined haplo/UCBT, and adults with non-malignant diseases (n=192) due to low numbers. In double UCBT, one of the two UCB units could be a licensed unit or accessed and distributed under another IND. U.S. transplant centers completed a site activation process to participate in the protocol. International and U.S. CBBs were qualified as suppliers of the UCB units.14 Criteria for UCB unit selection were determined by the transplant center, but cell dose > 2.5 × 107 total nucleated cell (TNC) dose/kg and ≥ 4/6 HLA match were recommended. Over 99% of the UCB units used in this study were from a CBB accredited by the American Association of Blood Banks (AABB) and/or Foundation for Accreditation of Cellular Therapy (FACT). Conditioning regimens, immune suppression, and supportive care were performed per institutional standards. In September 2013, the protocol was amended and approved by the local IRBs to require washing prior to infusion of all non-red blood cell (RBC) reduced UCB. The IRBs of the participating institutions provided approval for this protocol amendment. The NMDP facilitated 534 licensed UCBT from 2013 to October 2017. Data from this cohort was collected through the CIBMTR and made available for comparison to the 10-CBA cohort for the purposes of this analysis.
ENDPOINTS
The primary endpoint was incidence of neutrophil recovery. The secondary endpoints were platelet engraftment, acute GVHD, chronic GVHD, relapse, transplant-related mortality (TRM), overall survival (OS), and disease-free survival (DFS). Neutrophil engraftment was defined as an ANC ≥ 500 neutrophils/mm3 sustained for three consecutive days. Platelet engraftment was defined as a platelet count ≥ 20 × 109/L sustained for three consecutive days with no platelet transfusions in the previous seven days. Relapse was defined as occurrence of progressive disease or recurrence of disease post-HCT. Disease progression was not assessed in non-malignant disease. TRM was defined as death in continuous complete remission. Death from any cause was considered an event for OS. Death or relapse/progression was an event for DFS.
STATISTICAL CONSIDERATIONS
Patient-, disease-, transplant-, and UCB-related characteristics, such as cell dose, were examined. Univariate probabilities of neutrophil and platelet recovery, chronic GVHD, relapse, and TRM were calculated using the cumulative incidence function estimator with a subsequent HCT as a censoring event.15,16 For neutrophil and platelet engraftment and chronic GVHD, death without an event is the competing risk. For TRM, relapse was the competing risk; for relapse, TRM was the competing risk. The analyses of neutrophil and platelet engraftment were restricted to patients with conditioning regimens considered myeloablative (MAC) per CIBMTR guidelines.17 All other regimens were considered non-myeloablative or reduced intensity (RIC). Due to limited availability of GVHD onset date data, probabilities of acute GVHD were calculated using the binary outcome of whether acute GVHD was reported at 100 days. Univariate probabilities of OS and DFS used the Kaplan-Meier estimator; the log-rank test was used for comparisons of survival curves; the chi-square test was used for pointwise comparisons.18
Assessment of potential risk factors for day 42 neutrophil engraftment and day 100 acute GVHD was evaluated using logistic regression. Multivariate analyses of OS and chronic GVHD used Cox proportional hazards regression. The following risk factors were considered in the model building process: recipient sex, age, race/ethnicity, blood type, Karnofsky/Lansky Performance Score, HCT-specific comorbidity index (HCT-CI), cytomegalovirus (CMV) serology, prior autologous HCT, disease, disease risk, chemotherapy sensitivity for lymphomas, total body irradiation (TBI), conditioning intensity, antithymocyte globulin (ATG), GVHD prophylaxis, number of UCB units, Total Nucleated Count (TNC) dose, CD34 dose, and HLA, sex, race, and ABO matching between patient and UCB unit(s)19,20.
A stepwise selection technique with a significance level of 0.05 was used in all regression analyses. First-order interactions among significant prognostic factors were assessed. Analyses were performed using SAS software (SAS Institute, Cary, NC). Disease risk for acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), myelodysplastic syndrome (MDS), and myeloproliferative neoplasms (MPN) was classified into early, intermediate, or advanced as previously reported.21 The race/ethnicity of the UCB unit was self-reported by the mother at the time of donation. A sex or race/ethnicity mismatch was defined as either one or two UCB units collected from a donor that was the opposite sex or race/ethnicity of the recipient. Any cases with an unknown race for the patient of cord blood units were considered unknown for race match. HLA match for single cord transplants was based on the overall match between the patient and the unit at high resolution for HLA-A, B, C and DRB1 (8/8 match level). HLA match for double cord transplants was classified at the 8/8 level based on the worst matched cord.
RESULTS
Patients
Patient characteristics are outlined in Table 1. There were 1499 adults treated for malignant disease, 564 children with malignant disease and 393 children with nonmalignant disease. Figure 1 outlines the racial/ethnic distribution of the adult (Figure 1b) and pediatric (Figure 1c) recipients; UCBT were performed for a diverse group of patients. Thirty-six percent of adults and 50% of children were non-Caucasian. The most common diseases for both adults and children were AML and ALL. Eighty-nine percent of adults received double UCBT with the majority (82%) receiving 2 units supplied under the NMDP IND. As expected, most pediatric patients received a MAC regimen; 50% of adults were treated with a MAC regimen.
Table 1.
Characteristics of UCB Allogeneic Transplant Recipients
| Variable | Adult malignant N (%) | Pediatric Malignant N (%) | Pediatric Nonmalignant N (%) |
|---|---|---|---|
| Number of recipients | 1499 | 564 | 393 |
| Recipient sex | |||
| Male | 789 (53) | 328 (58) | 243 (62) |
| Female | 710 (47) | 236 (42) | 150 (38) |
| Recipient age at transplant | |||
| Median (range) | 50 (18–81) | 7 (0–17) | 1 (0–17) |
| Recipient race / ethnicity | |||
| Caucasian | 959 (64) | 283 (50) | 199 (51) |
| Hispanic | 146 (10) | 120 (21) | 69 (18) |
| Black / African American | 215 (14) | 93 (16) | 66 (17) |
| Asian / Pacific Islander | 123 (8) | 29 (5) | 21 (5) |
| American Indian / Alaska | 13 (1) | 5 (1) | 5 (1) |
| Native | |||
| Multiple race / decline / unknown | 46 (3) | 34 (6) | 33 (8) |
| Broad disease | |||
| AML | 703 (47) | 255 (40) | 0 |
| ALL | 257 (17) | 247 (44) | 0 |
| CML | 50 (3) | 8 (1) | 0 |
| CLL and PLL | 33 (2) | 0 | 0 |
| MDS | 162 (11) | 38 (7) | 0 |
| MPN | 22 (1) | 22 (4) | 0 |
| NHL | 191 (13) | 8 (1) | 0 |
| HL | 44 (3) | 1 (<1) | 0 |
| Other malignancies | 37 (3) | 15 (3) | 0 |
| Inherited erythrocyte abnormalities | 0 | 0 | 59 (15) |
| Inherited immune system disorders | 0 | 0 | 144 (37) |
| Inherited metabolism disorders | 0 | 0 | 136 (35) |
| Histiocytic disorders | 0 | 0 | 23 (6) |
| Other nonmalignant diseases | 0 | 0 | 31 (7) |
| HCT-specific comorbidity index (HCT-CI) | |||
| 0 | 358 (24) | 362 (64) | 277 (70) |
| 1–2 | 445 (30) | 123 (22) | 65 (17) |
| 3 or higher | 696 (46) | 79 (14) | 50 (13) |
| Unknown | 3 (<1) | 0 | 1 (<1) |
| Disease risk (AML, ALL, CML, MDS, MPN) | |||
| Early | 662 (56) | 253 (47) | |
| Intermediate | 261 (22) | 194 (36) | |
| Advanced | 267 (22) | 92 (17) | |
| Unknown | 4 (<1) | 1 (<1) | |
| Chemotherapy sensitivity (NHL, HL) | |||
| Sensitive | 197 (84) | 9 (100) | |
| Resistant | 35 (15) | 0 | |
| Untreated / unknown | 3 (1) | 0 | |
| Preparative regimen intensity | |||
| MAC | 751 (50) | 546 (97) | 327 (83) |
| RIC / Non-myeloablative | 748 (50) | 18 (3) | 66 (17) |
| GvHD prophylaxis | |||
| CSA+MMF | 739 (49) | 311 (55) | 196 (50) |
| CSA+MTX | 5 (<1) | 13 (2) | 8 (2) |
| CSA+Others | 4 (<1) | 49 (9) | 50 (13) |
| TAC+MMF | 554 (37) | 134 (24) | 102 (26) |
| TAC+MTX | 27 (2) | 35 (6) | 12 (3) |
| TAC+Others | 144 (10) | 15 (3) | 16 (4) |
| Others | 13 (1) | 5 (1) | 7 (2) |
| Unknown | 13 (1) | 2 (<1) | 2 (1) |
| Number of umbilical cord blood units | |||
| Single | 163 (11) | 456 (81) | 375 (95) |
| Double | 1336 (89) | 108 (19) | 18 (5) |
| Number of 10-CBA units in double UCBT | |||
| One | 234 (18) | 28 (26) | 6 (33) |
| Two | 1102 (82) | 80 (74) | 12 (67) |
| Single UCBT infused TNC dose, x107/kg | |||
| N Eval | 151 | 416 | 343 |
| Median (range) | 3.1 (1.2–7.9) | 6.9 (0.9–31.9) | 11.3 (0.8–72.6) |
| Double UCBT infused TNC dose, x107/kg | |||
| N Eval | 1228 | 89 | 17 |
| Median (range) | 4.8 (2.0–13.1) | 5.3 (2.0–16.3) | 6.8 (2.6–20.0) |
| Single UCBT infused CD34 dose, x106/kg | |||
| N Eval | 84 | 270 | 228 |
| Median (range) | 0.2 (0.0–1.7) | 0.2 (0.0–1.9) | 0.4 (0.0–2.4) |
| Double UCBT infused CD34 dose, x106/kg | |||
| N Eval | 792 | 71 | 7 |
| Median (range) | 0.2 (0.0–1.8) | 0.2 (0.0–0.7) | 0.2 (0.1–0.3) |
| HLA match grade (allele level typing at -A, -B, -C, -DRB1) | |||
| Better than 6/8 | 116 (8) | 137 (24) | 135 (34) |
| 6/8 | 152 (10) | 141 (25) | 106 (27) |
| Worse than 6/8 | 1155 (77) | 253 (45) | 131 (33) |
| Unknown | 76 (5) | 33 (6) | 21 (5) |
| UCB-recipient sex match | |||
| Matched | 398 (27) | 213 (38) | 178 (45) |
| Mismatched to female | 484 (32) | 129 (23) | 75 (19) |
| Mismatched to male | 553 (37) | 185 (33) | 122 (31) |
| Unknown | 64 (4) | 37 (7) | 18 (5) |
| UCB-recipient race match | |||
| Matched | 500 (33) | 257 (46) | 192 (49) |
| Mismatched | 717 (48) | 233 (41) | 147 (37) |
| Unknown | 282 (19) | 74 (13) | 54 (14) |
| UCB-recipient ABO match | |||
| Matched | 286 (19) | 212 (38) | 140 (36) |
| Bi-directional mismatch | 180 (12) | 58 (10) | 25 (6) |
| Major mismatch | 474 (32) | 128 (23) | 95 (24) |
| Minor mismatch | 401 (27) | 119 (21) | 100 (25) |
| Unknown | 158 (11) | 47 (8) | 33 (8) |
Abbreviations: AML is acute myeloid leukemia; ALL is acute lymphoblastic leukemia; CML is chronic myeloid leukemia; CLL is chronic lymphocytic leukemia; PLL is prolymphocytic leukemia; MDS is myelodysplastic syndrome; MPN is myeloproliferative neoplasms; NHL is non-Hodgkin lymphoma; HL is Hodgkin lymphoma; UCBT is umbilical cord blood transplant; CSA is cyclosporine A; MMF is mycophenylate mofetil; MTX is methotrexate; TAC is tacrolimus.
Engraftment
Engraftment is presented for patients receiving myeloablative UCBT (Table 2). For adults, the median days to ANC ≥ 500/mm3 were 22 days. At 42 days, 89% of adult patients had achieved neutrophil engraftment. Platelet engraftment, defined as the median days to platelet count ≥ 20 × 109/L, were 44 days for adults, and by day 100, 73% of adults had engrafted platelets. As expected, children had faster engraftment than adults. Children with malignant disease had a median days to neutrophil engraftment of 20 days and 88% had engrafted neutrophils by Day + 42. Median daysto platelet engraftment for this cohort were 48 days, with 75% engrafting platelets by Day 100. Median days to neutrophil and platelet engraftment in children with nonmalignant disease were 19 and 45 days respectively. 90% engrafted neutrophils by Day 42 and 79% engrafted platelets by Day 100.
Table 2.
Univariate Probability of Outcomes after Umbilical Cord Blood Transplantation
| Adult malignant | Pediatric Malignant | Pediatric Nonmalignant | ||||
|---|---|---|---|---|---|---|
| Outcome | N | Prob (95% CI) | N | Prob (95% CI) | N | Prob (95% CI) |
| Neutrophil engraftmenta | 737 | 543 | 326 | |||
| @ 42 days | 89 (87–91) | 88 (85–91) | 90 (87–93) | |||
| Median days to engraftment | 22 | 20 | 19 | |||
| Platelet 20K engraftmenta | 731 | 537 | 324 | |||
| @ 100 days | 73 (70–76) | 75 (72–79) | 79 (75–84) | |||
| Median days to engraftment | 44 | 48 | 45 | |||
| Overall survival | 1499 | 564 | 393 | |||
| @ 100 days | 81 (79–83) | 87 (84–90) | 90 (87–93) | |||
| @ 1 year | 57 (54–59) | 71 (67–75) | 79 (75–83) | |||
| @ 2 years | 46 (43–49) | 62 (58–66) | 77 (72–81) | |||
| Disease-free survivalb | 1407 | 533 | ||||
| @ 100 days | 76 (74–78) | 83 (79–86) | ||||
| @ 1 year | 50 (47–53) | 62 (58–66) | ||||
| @ 2 years | 40 (38–43) | 54 (50–59) | ||||
| Transplant-related mortalityb | 1407 | 533 | ||||
| @ 100 days | 14 (12–16) | 9 (7–12) | ||||
| @ 1 year | 27 (25–30) | 14 (12–18) | ||||
| @ 2 years | 31 (29–34) | 16 (13–19) | ||||
| Relapseb | 1407 | 533 | ||||
| @ 100 days | 10 (8–11) | 8 (6–11) | ||||
| @ 1 year | 23 (20–25) | 23 (20–27) | ||||
| @ 2 years | 28 (26–31) | 30 (26–31) | ||||
| aGVHD II-IV | 1451 | 552 | 390 | |||
| @ 100 days | 35 (33–38) | 32 (28–36) | 24 (19–28) | |||
| aGVHD III-IV | 1465 | 554 | 392 | |||
| @ 100 days | 16 (14–18) | 17 (14–20) | 9 (6–12) | |||
| cGVHD | 1458 | 550 | 384 | |||
| @ 1 year | 24 (22–27) | 26 (22–30) | 24 (20–28) | |||
Myeloablative conditioning only
Leukemia, myelodysplasia, myeloproliferative neoplasms, and lymphoma only
Graft vs Host Disease
The incidence of acute GVHD Grades II-IV was 35% (95% confidence interval [CI]: 33–38%), 32% (95% CI: 28–36%), and 24% (95% CI:19–28%) for adults, pediatric malignant and pediatric non-malignant cohorts, respectively. Acute GVHD grades III-IV was low at 16% (95%CI: 15–20%), 17% (95%CI: 14–18%), and 9% (95%CI: 6–12%) for adults, pediatric malignant and pediatric non-malignant, respectively. Chronic GVHD (limited and extensive) at one year was 24% (95% CI: 22–27%), 26% (95% CI: 22–30%), and 24% (95% CI: 20–28%) for adults, pediatric malignant, and pediatric non-malignant respectively. Of those that developed Chronic GVHD, 61% were classified as extensive.
Relapse and Transplant Related Mortality
The incidence of TRM and relapse are displayed in Table 2. TRM (death without relapse) was 14% and 27% at 100 days and one year respectively for adult patients. As expected, TRM was less frequent for children with malignancy, 9% at 100 days and 14% at one year. Relapse rate was evaluated for patients with hematologic malignancies. Relapse rate at 100 days was 10% for adults and 8% for children. Relapse rate at one year was 23% for both adults and children.
Adverse Events To Infusion
Serious adverse events (SAE) to the cord blood infusion were defined per protocol as follows:
Recipient seroconversion to any of the FDA-listed relevant communicable diseases within six months of UCB infusion which, upon investigation, is determined to be caused or potentially caused by the UCB unit
Recipient bacteremia related to a contaminated UCB unit
Recipient develops any of the FDA-listed relevant communicable diseases within six months of UCB infusion which, upon investigation, is determined to be caused or potentially caused by the UCB unit
Serious infusion reaction within first 24 hours after infusion
All SAE were monitored and reported as requested by the FDA. There were three cases of Takotsubo cardiomyopathy (stunned heart syndrome) related to infusion. The first patient received two RBC reduced UCB units on the same day and experienced chest pain approximately 16 hours after the infusion. The patient died 2 months later due to renal failure. The second patient received one RBC reduced UCB unit and one RBC replete UCB unit on the same day. The patient experienced acute respiratory distress syndrome upon completion of the second infusion, was transferred to the intensive care unit and subsequently recovered. The third patient received a single RBC reduced UCB unit and experienced respiratory distress shortly after the infusion. The patient developed multiorgan failure and died two weeks later. There was one additional death related to hemolysis and hyperkalemia, approximately 3 hours after receiving two RBC replete UCB units. These events were reported by the investigators at the individual transplant center and reviewed by the NMDP Medical Monitor. After the first reports of infusion related events with unwashed RBC replete UCB units, the protocol was amended in 2013 to mandate washing of RBC replete UCB units.
Survival and Disease-Free Survival
OS and DFS are outlined in Table 2 and Figures 2 and 3. For adults, OS was 81% (95% CI: 79–83) at 100 days, 57% (95% CI: 54–59%) at one year, and 46% (95% CI: 43–49) at two years. Children, as expected, had improved survival compared to adults. Overall survival for the pediatric malignant cohort was 87% (95% CI: 84–90) at 100 days, 71% (95% CI: 67–75) at one year, and 62% (95% CI: 58–66) at two years. Similarly, OS for the pediatric non-malignant cohort was 90% (95% CI: 87–93), 79% (95% CI: 75–83), and 77% (95% CI: 72–81) at 100 days, one year, and two years, respectively. Disease-free survival for adults was 76% (95% CI: 74–78%) at 100 days, 50% (95% CI: 47–53%) at 1 year, and 40% (95% CI: 38–43%) at 2 years. For pediatric patients, DFS was 83% (95% CI: 79–86%), 62% (95% CI: 58–66%), and 54% (95% CI: 50–59%) at 100 days, 1 year, and 2 years respectively.
Figure 2.
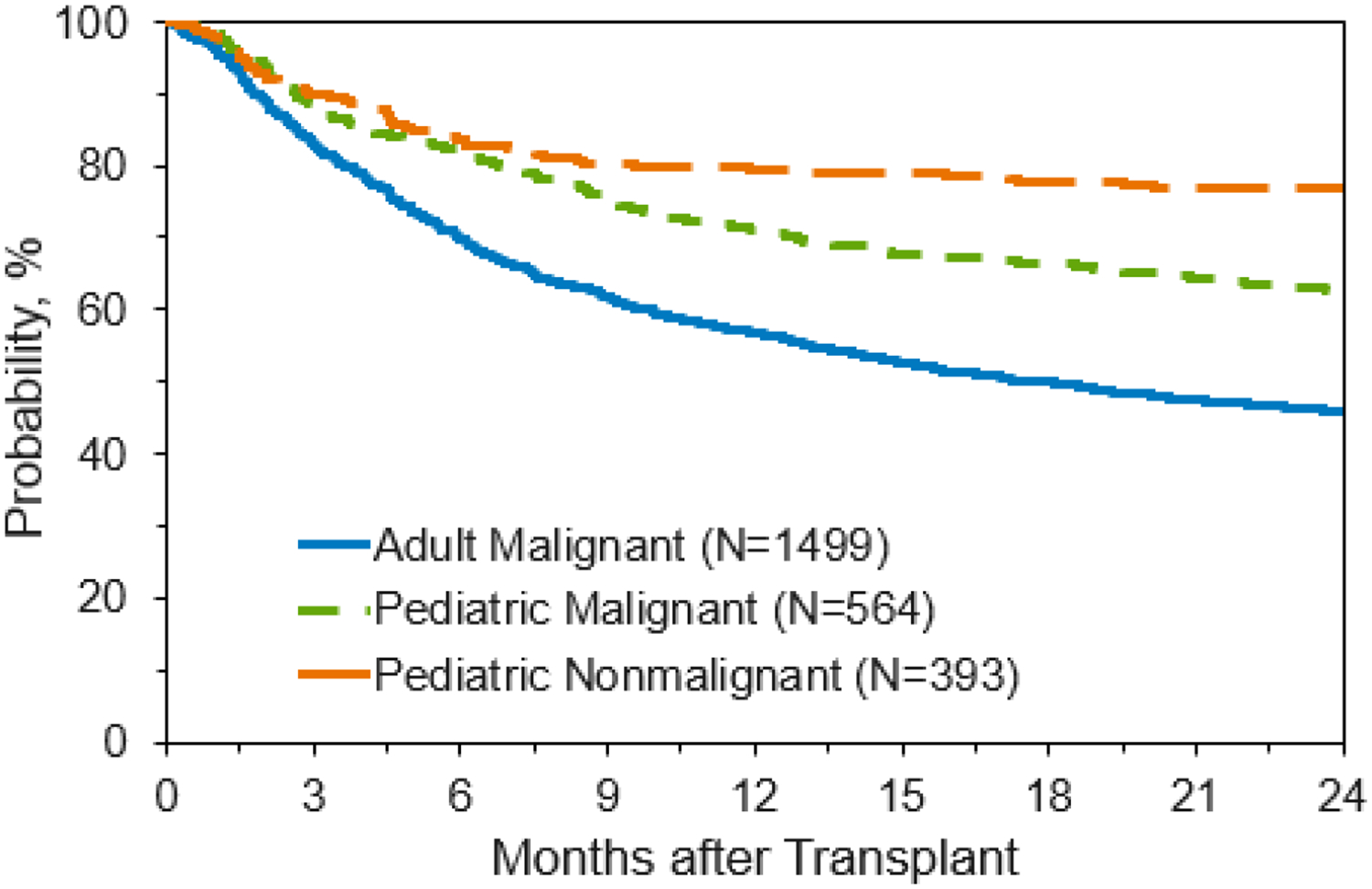
Overall Survival for Patients Receiving Unlicensed Umbilical Cord Blood Transplant
Figure 3.
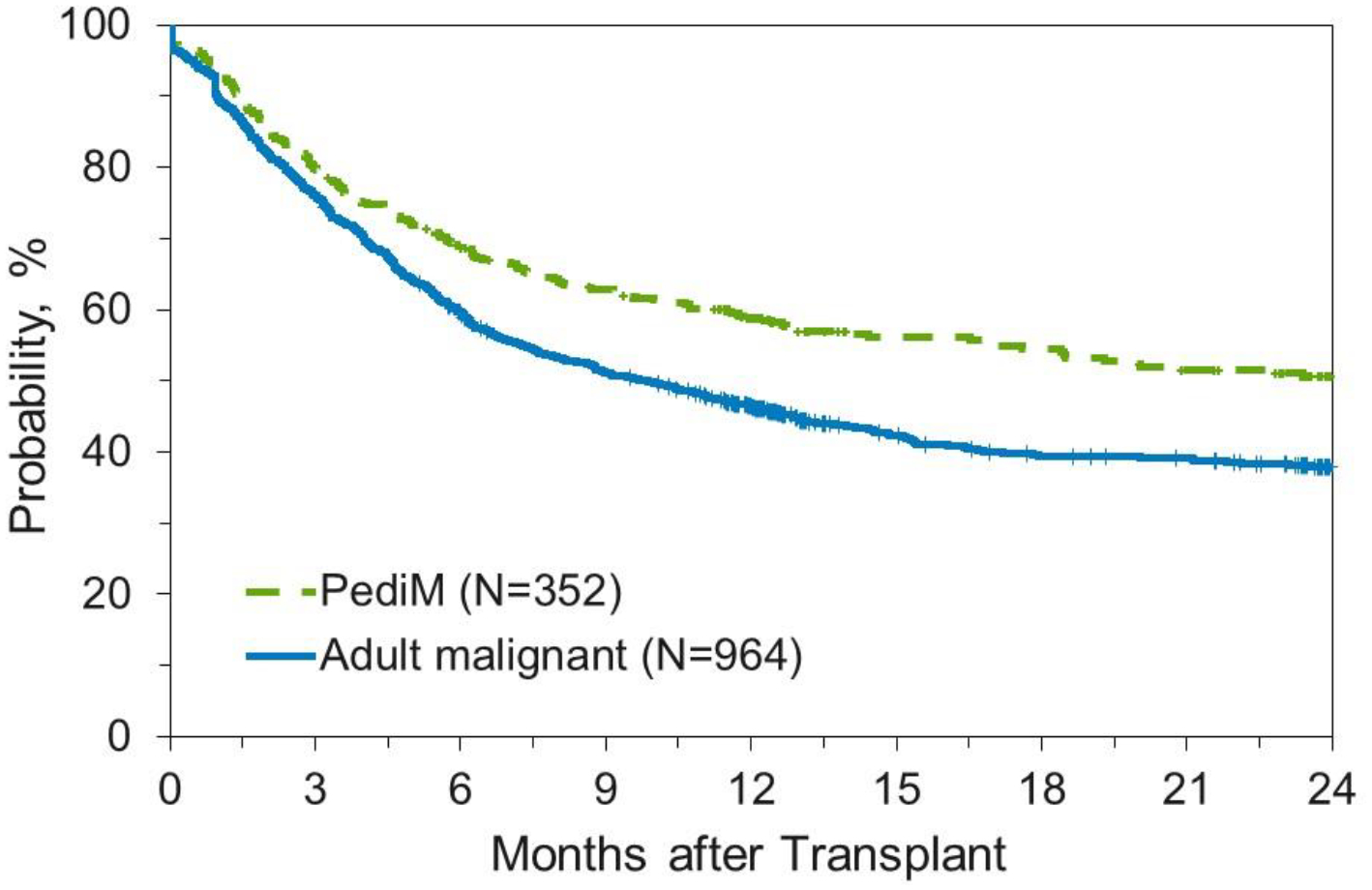
Disease-Free Survival for Patients Receiving Unlicensed Umbilical Cord Blood Transplant
As expected, patients with more advanced disease had lower DFS. DFS at 2 years for early vs advanced leukemia and MDS/MPN were 44% (95% CI: 40–48%) vs 28% (95% CI: 23–34%) for adults, and 60% (95% CI: 53–66%) vs 38% (95% CI: 28–48%) for children.
Single vs Double UCBT
The transplant center elected whether to use a single or double UCBT graft. For adult patients, there was no difference in OS or DFS between single and double UCBT in multivariate analysis. Double UCBT with one 10 CBA unit and one non 10 CBA unit was not analyzed separately due to low numbers.
Multivariable Analysis
Tables 3a–c outline the results of the multivariable analysis, using variables listed in Materials and Methods.
Table 3a:
Multivariate Models for Outcomes after Umbilical Cord Blood Transplantation: Adult Recipients with Malignant Diseases
| Neutrophil engraftment by day 42 | ||||
| (MAC only) | ||||
| Disease | 0.035 | |||
| AML | 346 | 1.00 | ||
| ALL | 180 | 1.66 | (0.81–3.38) | 0.165 |
| CML | 33 | 0.39 | (0.15–0.98) | 0.045 |
| MDS/MPN | 81 | 0.46 | (0.22–0.94) | 0.033 |
| NHL | 58 | 0.59 | (0.22–1.56) | 0.287 |
| HL | 10 | 0.59 | (0.07–4.75) | 0.625 |
| Other | 27 | 0.45 | (0.14–1.45) | 0.18 |
| Disease risk (AML, ALL, CML, MDS, MPN) | 0.008 | |||
| Early | 462 | 1.00 | ||
| Intermediate | 147 | 0.44 | (0.23–0.83) | 0.012 |
| Advanced | 126 | 0.43 | (0.23–0.83) | 0.011 |
| Karnofsky Performance Score | 0.05 | |||
| 90–100 | 476 | 1.00 | ||
| 10–80 | 247 | 0.54 | (0.33–0.89) | 0.015 |
| Unknown | 12 | 0.57 | (0.11–2.88) | 0.50 |
| Overall survivalb | ||||
| Recipient age at transplant | <0.001 | |||
| 18 to 29 | 248 | 1.00 | ||
| 30 to 39 | 252 | 1.15 | (0.88–1.50) | 0.319 |
| 40 to 49 | 233 | 1.43 | (1.10–1.87) | 0.007 |
| 50 to 59 | 359 | 1.60 | (1.25–2.05) | <0.001 |
| 60 to 64 | 195 | 1.85 | (1.41–2.43) | <0.001 |
| 65 or older | 208 | 1.79 | (1.35–2.37) | <0.001 |
| HCT-specific comorbidity index (HCT-CI) | <0.001 | |||
| 0 | 358 | 1.00 | ||
| 1–2 | 445 | 1.28 | <0.001 | |
| 3 or higher | 692 | 1.44 | 0.014 | |
| Disease risk (AML, ALL, CML, MDS, MPN) | 0.002 | |||
| Early | 1018 | 1.00 | ||
| Intermediate | 259 | 1.02 | (0.84–1.25) | 0.823 |
| Advanced | 218 | 1.45 | (1.17–1.78) | <0.001 |
| Chemotherapy sensitivity (NHL, HL) | ||||
| Sensitive | 199 | 1.00 | ||
| Resistant | 34 | 1.73 | (1.12–2.68) | 0.014 |
| Karnofsky Performance Score | 0.003 | |||
| 90–100 | 946 | 1.00 | ||
| 10–80 | 513 | 1.27 | (1.10–1.47) | 0.001 |
| Unknown | 36 | 1.28 | (0.83–1.99) | 0.268 |
| Conditioning regimen/intensity/ATG use(first 3 month post-HCT) | <0.001 | |||
| RIC, TBI based, No ATG | 515 | 1.00 | ||
| MAC, TBI based, No ATG | 590 | 1.42 | (1.02–1.96) | 0.035 |
| RIC, No TBI, ATG | 132 | 1.77 | (1.15–2.73) | 0.009 |
| Other RIC | 99 | 1.60 | (0.97–2.63) | 0.067 |
| Other MAC | 159 | 2.04 | (1.35–3.08) | <0.001 |
| Conditioning regimen/intensity/ATG use (>3 month post-HCT) | <0.001 | |||
| RIC, TBI based, No ATG | 444 | 1.00 | ||
| MAC, TBI based, No ATG | 498 | 0.76 | (0.61–0.94) | 0.010 |
| RIC, No TBI, ATG | 101 | 1.65 | (1.25–2.17) | <0.001 |
| Other RIC | 79 | 1.21 | (0.87–1.66) | 0.251 |
| Other MAC | 123 | 1.49 | (1.13–1.97) | 0.005 |
| Acute GVHD II-IV | ||||
| GVHD Prophylaxis | 0.008 | |||
| CSA+MMF+/− other | 714 | 1.00 | ||
| TAC+MMF+/− other | 540 | 0.78 | (0.59–1.03) | 0.08 |
| Other | 201 | 0.53 | (0.35–0.81) | 0.003 |
| ATG Use | ||||
| No ATG | 1138 | 1.00 | ||
| ATG | 317 | 0.51 | (0.36–0.73) | <0.001 |
| Age | <0.001 | |||
| 18 to 29 | 245 | 1 | ||
| 30 to 39 | 243 | 0.66 | (0.45–0.99) | 0.044 |
| 40 to 49 | 228 | 0.56 | (0.37–0.85) | 0.006 |
| 50 to 59 | 346 | 0.74 | (0.51–1.06) | 0.101 |
| 60 to 64 | 187 | 0.41 | (0.26–0.66) | <0.001 |
| 65+ | 206 | 0.41 | (0.26–0.66) | <0.001 |
| ABO Matching | 0.009 | |||
| Matched | 282 | 1 | ||
| Bidirectional mismatch | 177 | 1.12 | (0.73–1.71) | 0.092 |
| Minor Mismatch | 390 | 0.73 | (0.51–1.05) | 0.03 |
| Major Mismatch | 462 | 0.68 | (0.48–0.96) | 0.339 |
| Unknown | 144 | 1.24 | (0.79–1.95) | 0.608 |
| Chronic GVHD | ||||
| Patient Race/Ethnicity | 0.018 | |||
| Caucasian | 933 | 1 | ||
| Hispanic | 141 | 1.65 | (1.22–2.22) | 0.001 |
| Black or African American | 204 | 1.06 | (0.79–1.44) | 0.68 |
| Asian/Pacific Islander | 121 | 1.15 | (0.82–1.62) | 0.418 |
| Other/Unknown | 55 | 0.81 | (0.45–1.46) | 0.487 |
| GVHD Prophylaxis | 0.014 | |||
| CSA+MMF+/− other | 717 | 1 | ||
| FK+MMF+/− other | 534 | 1.35 | (1.06–1.72) | 0.015 |
| Other | 203 | 1.49 | (1.09–2.04) | 0.013 |
| Year of Transplant | 0.033 | |||
| 2011–2012 | 354 | 1 | ||
| 2013–2014 | 599 | 0.86 | (0.67–1.1) | 0.226 |
| 2015–2016 | 501 | 0.7 | (0.53–0.92) | 0.01 |
| Conditioning regimen/intensity/ATG use | <0.001 | |||
| RIC, TBI based, No ATG | 501 | 1 | ||
| MAC, TBI based, No ATG | 574 | 1.51 | (1.19–1.93) | <0.001 |
| RIC, No TBI, ATG | 128 | 0.85 | (0.56–1.3) | 0.463 |
| Other RIC | 94 | 1.19 | (0.78–1.8) | 0.421 |
| Other MAC | 157 | 0.67 | (0.44–1.01) | 0.053 |
| Sex Matching | 0.031 | |||
| Matched | 389 | 1 | ||
| Unknown | 60 | 0.95 | (0.59–1.53) | 0.825 |
| Mismatch to F | 466 | 0.77 | (0.6–0.99) | 0.044 |
| Mismatch to M | 539 | 0.69 | (0.54–0.89) | 0.005 |
| Recipient ABO type | 0.048 | |||
| A | 482 | 1 | ||
| AB | 39 | 2.17 | (1.27–3.7) | 0.005 |
| B | 185 | 1.08 | (0.77–1.5) | 0.654 |
| O | 610 | 0.95 | (0.75–1.19) | 0.661 |
| Unknown | 138 | 1.05 | (0.73–1.49) | 0.805 |
| Number of UCB | ||||
| 1 | 159 | 1 | ||
| 2 | 1295 | 0.64 | (0.47–0.88) | 0.005 |
OR for neutrophil engraftment and acute GVHD; HR for OS and chronic GVHD
Model stratified on disease due to nonproportional hazards
Abbreviations: OR is odds ratio; HR is hazard ratio; CI is confidence interval; HCT is hematopoietic cell transplantation; AML is acute myeloid leukemia; ALL is acute lymphoblastic leukemia; CML is chronic myeloid leukemia; MDS is myelodysplasia; MPN is myeloproliferative neoplasms; NHL is non-Hodgkin lymphoma; HL is Hodgkin lymphoma; RIC is reduced intensity conditioning; MAC is myeloablative conditioning; TBI is total body irradiation; ATG is anti-thymocyte globulin; CSA is cyclosporine A; TAC is tacrolimus; MMF is mycophenylate mofetil.
Table 3c.
Pediatric Recipients with Non-malignant Diseases
| Neutrophil engraftment by day 42 | ||||
| (MAC only) | ||||
| Recipient sex | ||||
| Female | 119 | 1.00 | ||
| Male | 207 | 3.27 | (1.51–7.10) | 0.003 |
| Recipient age at transplant | ||||
| 0 to 4 | 257 | 1.00 | ||
| 5 or older | 69 | 0.44 | (0.20–1.00) | 0.049 |
| Recipient race/ethnicity | 0.014 | |||
| Caucasian | 176 | 1.00 | ||
| Hispanic | 53 | 1.65 | (0.45–5.99) | 0.447 |
| Black or African American | 49 | 0.3 | (0.12–0.74) | 0.009 |
| Other/Unknown | 48 | 1.85 | (0.50–6.81) | 0.357 |
| Overall survival | ||||
| Disease | 0.018 | |||
| IEA | 59 | 1.00 | ||
| IIS | 144 | 2.93 | (0.16–0.89) | 0.006 |
| IMD | 136 | 1.75 | (1.36–6.30) | 0.167 |
| Other NMD | 54 | 1.7 | (0.79–3.90) | 0.248 |
| HLA match grade | 0.008 | |||
| Better than 6/8 | 135 | 1.00 | ||
| 6/8 | 106 | 2.07 | (1.19–3.57) | 0.009 |
| Less than 6/8 | 131 | 1.95 | (1.14–3.33) | 0.014 |
| Unknown | 21 | 0.41 | (0.10–1.77) | 0.233 |
| Karnofsky / Lansky Performance Score | 0.002 | |||
| 90–100 | 304 | 1.00 | ||
| 10–80 | 68 | 2.34 | (1.43–3.80) | <0.001 |
| Unknown | 21 | 0.98 | (0.39–2.46) | 0.967 |
| Acute GVHD II-IV | ||||
| HLA matching | 0.032 | |||
| Better than 6/8 | 135 | 1 | ||
| 6/8 | 105 | 1.94 | (0.9–4.17) | 0.089 |
| Less than 6/8 | 129 | 2.6 | (1.28–5.28) | 0.008 |
| Unknown | 21 | 0.47 | (0.06–3.79) | 0.478 |
| Chronic GVHD | ||||
| Conditioning intensity | ||||
| RIC | 63 | 1 | ||
| MAC | 321 | 2.26 | (1.1–4.67) | 0.027 |
| TNC dose | 0.015 | |||
| <=10 | 161 | 1 | ||
| >10 | 190 | 0.57 | (0.37–0.87) | 0.009 |
| Unknown | 33 | 1.17 | (0.61–2.26) | 0.63 |
OR for neutrophil engraftment and acute GVHD; HR for OS and chronic GVHD
Abbreviations: OR is odds ratio; CI is confidence interval; IEA is inherited erythrocyte abnormalities; IIS is inherited immune system disorders; IMD is inherited metabolism disorders; NMD is nonmalignant disease; RIC is reduced intensity conditioning; MAC is myeloablative conditioning.
Adult Malignant Cohort
For adult malignant patients with AML and ALL (p=0.035), early stage disease (p=0.008), and Karnofsky performance status (KPS) ≥ 90% (p=0.05) had faster neutrophil engraftment. Patients younger than 30 years (p <0.001), with lower comorbidity score (p<0.001), lower disease risk (p=0.002), chemotherapy sensitive disease (p=0.014), and KPS ≥ 90% (p=0.003), were associated with improved OS. A MAC/TBI based conditioning regimen with no ATG usage, compared to a RIC regimen, improved one-year OS (p <0.001). Factors associated with acute and chronic GVHD are described in table 3a. Patients greater than 30 years old (p <0.001), use of ATG (p<0.001), and use of non-cyclosporine/mycophenolate containing GVHD prophylaxis regimens (p=0.008) were associated with decreased Grades II-IV acute GVHD. Non-Hispanic race/ethnicity (p= 0.018), use of Tacrolimus-based GVHD prophylaxis (p=0.014), transplant after 2014 (p=0.033), sex mismatched UCB units (p=0.031), recipient blood group other than AB (p=0.048) and use of double UCBT (p=0.005) were associated with a lower risk of chronic GVHD.
Pediatric Malignant Cohort
In the pediatric malignant cohort, ALL (p=0.002) and cyclosporine based GVHD prophylaxis (p=0.005) were associated with faster neutrophil engraftment. Patients with MDS/MPN (p=0.005), lower disease risk (p=0.031), Lansky performance status of ≥90% (p<0.001), female patients (p=0.027), cyclosporine based GVHD prophylaxis (p=0.009), more recent transplants (p=0.024), and Caucasian race/ethnicity (p<0.001) had improved survival. Early stage disease (p=0.041), use of ATG (p=0.028), and closer HLA match (p=0.005) were associated with a lower incidence of acute GVHD grades II-IV. Use of ATG (p=0.002) and cyclosporine based GVHD prophylaxis (p=0.023) were associated with a lower risk of chronic GVHD.
Pediatric Non-malignant Cohort
In the pediatric nonmalignant cohort, patient age younger than 5 years (p=0.049), male (p=0.003), and race/ethnicity other than Black/African American (p=0.014) had faster neutrophil engraftment. Patients with inherited erythrocyte abnormalities (p=0.018), HLA match better than 6/8 (p=0.008), and Lansky performance status ≥90% (p=0.002) had improved survival. A closer HLA match was associated with decreased risk of Acute GVHD Grades II-IV (p=0.032). RIC transplant (p=0.027) and a TNC dose > 10 × 107/kg(p=0.015) were associated with decreased incidence of chronic GVHD.
Race/Ethnicity
One of the goals of the UCB program was to provide a stem cell source for a diverse population (Figure 1). Non-Caucasian patients represented 36% of adults, and 50% of children. Race/ethnicity of the patients was provided by the transplant center. For adult patients, 64% were Caucasian, 10% Hispanic, 14% Black/African American, 8% Asian, and 1% Native American. Pediatric patients were 50% Caucasian, 20% Hispanic, 17% Black/African American, 5% Asian, and 1% Native American. Other patients either had multiple race or chose not to identify a specific race/ethnicity. For adult patients, Hispanic patients had increased chronic GVHD (p=0.001) but there was no effect of race/ethnicity on survival or engraftment. OS at one year was 56% for Caucasians, 61% for Hispanics, and 52% for Black/African Americans.
For pediatric patients with malignant disease, Caucasian race/ethnicity was associatedwith improved survival (p <0.001) but had no effect on GVHD or neutrophil engraftment. For pediatric patients with non-malignant disease, Black/African American patients hadslower neutrophil engraftment (p=0.014), but there was no effect on survival or GVHD. Matching the patient and the UCB by race/ethnicity did not impact any outcomes. Caucasian children received more closely matched UCB units than Black children; 58%of Caucasian children with malignant disease received an HLA 6/8 match or better, and 41% of non-Caucasian children received an HLA 6/8 match or better (p<0.001).
LICENSED UNITS,
There were 534 licensed UCBT facilitated by the NMDP from 2013 to October 2017.42% of these were single UCBT and 58% were double UCBT. The majority (n=257) of double UCBT patients received 1 licensed and 1 unlicensed unit. During the study period, there were 145 licensed single UCBT. 33% of these UCB units were distributed for international patients. Therefore, a cohort of US pediatric malignant patients receiving either single licensed (n=48) or single unlicensed (n=382) UCB units were compared. There was no difference in 1-year OS (72% for both) and engraftment at 42 days was similar in these two cohorts (90% for licensed vs 88% for unlicensed).
DISCUSSION
UCB is a readily available stem cell source for patients without MRDs or MUDs. In this study, we explore outcomes for over 2456 diverse UCBT patients treated at multiple U.S. centers using unlicensed UCB units facilitated by the NMDP. The study was designed to track outcomes for patients receiving UCB units distributed under an IND maintained by the NMDP. The protocol activated in 2011 and currently continues accruing patients. The results reported here represent an interim analysis after five years. Patients, conditioning regimens, and GVHD prophylaxis regimens were selected by the transplant centers.
Our results suggest improving outcomes following UCBT with unlicensed units. The incidence of neutrophil engraftment at Day 42 was over 88% for all patients. Engraftment was similar between adults and children, suggesting improvement in adult outcomes over time.22,23 OS at one year was 57% for adults and over 70% for children. As expected, the incidence of severe acute GVHD was low. These results are comparable or superior to multiple other smaller studies in the literature.24,25
Adverse events to infusion, which were part of the required FDA reporting, were rare after UCBT. This protocol did not initially mandate any specific thawing procedure. However, after the report of severe infusion reactions, the protocol was amended in 2013 to mandate washing for RBC replete UCB units. Several modifications of the original Rubinstein washing procedure are now in clinical practice.26 Other centers have used a no-wash dilution strategy with good results.27 Adverse events after UCB unit infusion continue to be closely monitored.
In multivariate analysis, as expected, early stage disease and better performance status were associated with better outcomes. Interestingly, HLA match and cell dose were not associated with survival, in the adult and pediatric malignant cohorts, suggesting the importance of patient related factors, rather than UCB unit related, in outcomes after UCBT. Use of ATG containing regimens was associated with lower OS in adults, but did not affect OS in children, likely because children transplanted for non-malignant disease received ATG, and may be immunologically healthy pre transplant. The use of ATG in UCBT is controversial.28,29 Pascal et al reported decreased acute GVHD, higher NRM, and decreased OS in UCBT patients receiving rabbit ATG and a RIC regimen.30 Results with rabbit vs horse ATG were not compared in this study.
Race/Ethnicity
One of the goals of the NMDP UCB registry is to provide an adequate stem cell source for patients of diverse racial/ethnic backgrounds who are less likely to find fully matched adult donors. Thirty-six percent of adults and 50% of children were non-Caucasian. The NMDP continues to see higher utilization of cord blood in non-Caucasian populations with 34% of Black/African American patients receiving cord blood compared to just 10% for Caucasian patients in 2016 (Figure 1). There was no benefit to receiving a UCB unit matched by race/ethnicity. For adults, race/ethnicity had no effect on OS or DFS.
Black/African American children with malignant disease had lower OS and DFS than Caucasian patients and received less well matched UCB units. Black/African American children with non-malignant disease had poorer engraftment than Caucasian children. A prior CIBMTR study indicated that OS was lower for Blacks than Caucasians receiving single UCBT.31 However, Black/African American and Caucasian patients had similar survival when receiving UCBT of adequate cell dose. OS at one year for Black patients (median age 8 years) in the former study was 42%. Our larger study in a more recent era with more availability of larger units for Black/African American patients suggests that outcomes for UCBT are comparable for adults among different racial/ethnic groups, and that outcomes for Black patients have improved over time. However, for children (and cell dose should be less of a concern) we did not see similar outcomes among the racial/ethnic groups in the current study, suggesting that there may be other demographic and socioeconomic factors affecting outcomes. In addition, our series reflects a very diverse population compared to several single or multi-institution reports in the literature.10,24
The study was not designed to compare outcomes among UCBT and other graft sources including MUD, mismatched unrelated donor (MMUD), or haploidentical related (haplo) HCT. An ongoing randomized study via the Bone Marrow Transplant Clinical Trials Network (BMT CTN) compares haplo HCT to double UCBT using a RIC regimen in adults. Multiple retrospective studies have shown comparable survival among the different graft sources.32,33,34,10,35,36,37 Recently, Milano et al showed a decreased relapse rate in UCBT compared to MUD or MMUD for patients with AML and minimal residual disease (MRD).38 The relapse rate was low in our study, at 23% for adults and MRD was not able to be assessed. Late HCT complications and chronic GVHD may also be less following UCBT.39
Our study is limited by the heterogeneous nature of patients, conditioning regimens, and GVHD prophylaxis regimens in this cohort from multiple centers. Robust chimerism data was not available. We determined that good outcomes could be maintained in a diverse group of patients receiving unlicensed UCB units. This finding is important as licensure is expensive and less than 10% of the available UCB units are currently licensed. Currently, 8.7% of the available NMDP UCB units have been licensed by the FDA. There are currently 7 (5 during the study period) licensed CBBs in the U.S. Median start-up expenses to obtain licensure were $1.8 million and median incremental annual expenses are $365,000 per bank (Personal Communication, Cord Blood Advisory Committee of the NMDP). Therefore, the licensure cost (start-up and annual expenses) for the 5 CBBs who obtained licensure during this 3-year study period was approximately $14.5 million dollars. The policy of allowing unlicensed UCB units to be available to a diverse population under an IND currently serves the majority of patients who need a UCBT, and these UCB units are safe and effective. These findings may have implications for licensure of other cellular products, as licensure should not limit access.
Future studies will investigate long-term outcomes in this large cohort of UCB patients. Despite advances in treatment, novel strategies to decrease relapse and TRM are needed. UCBT continues to provide access to HCT for a racially/ethnically diverse population.
Supplementary Material
Figure 4. Overall Survival by Race/Ethnicity.
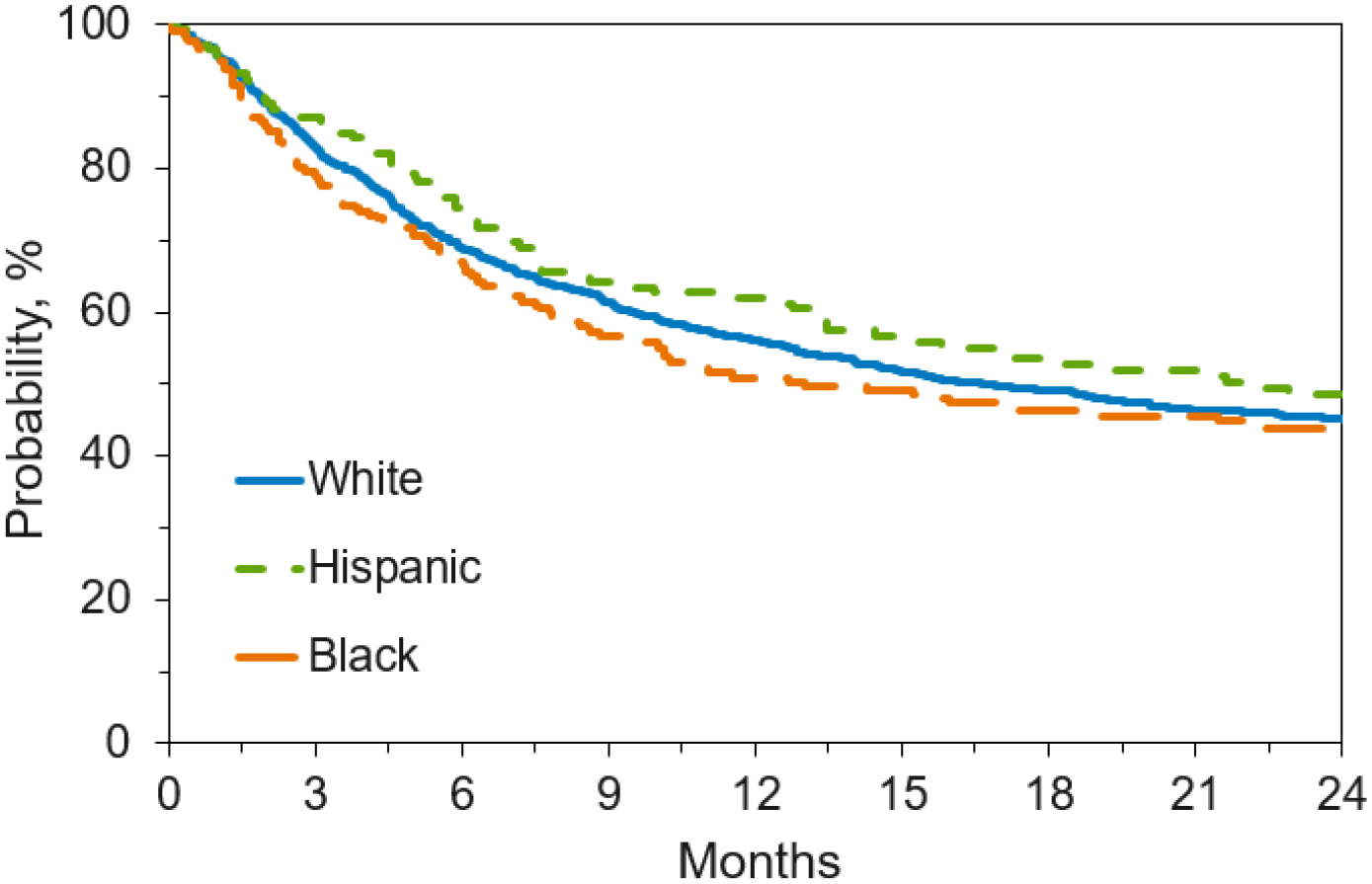

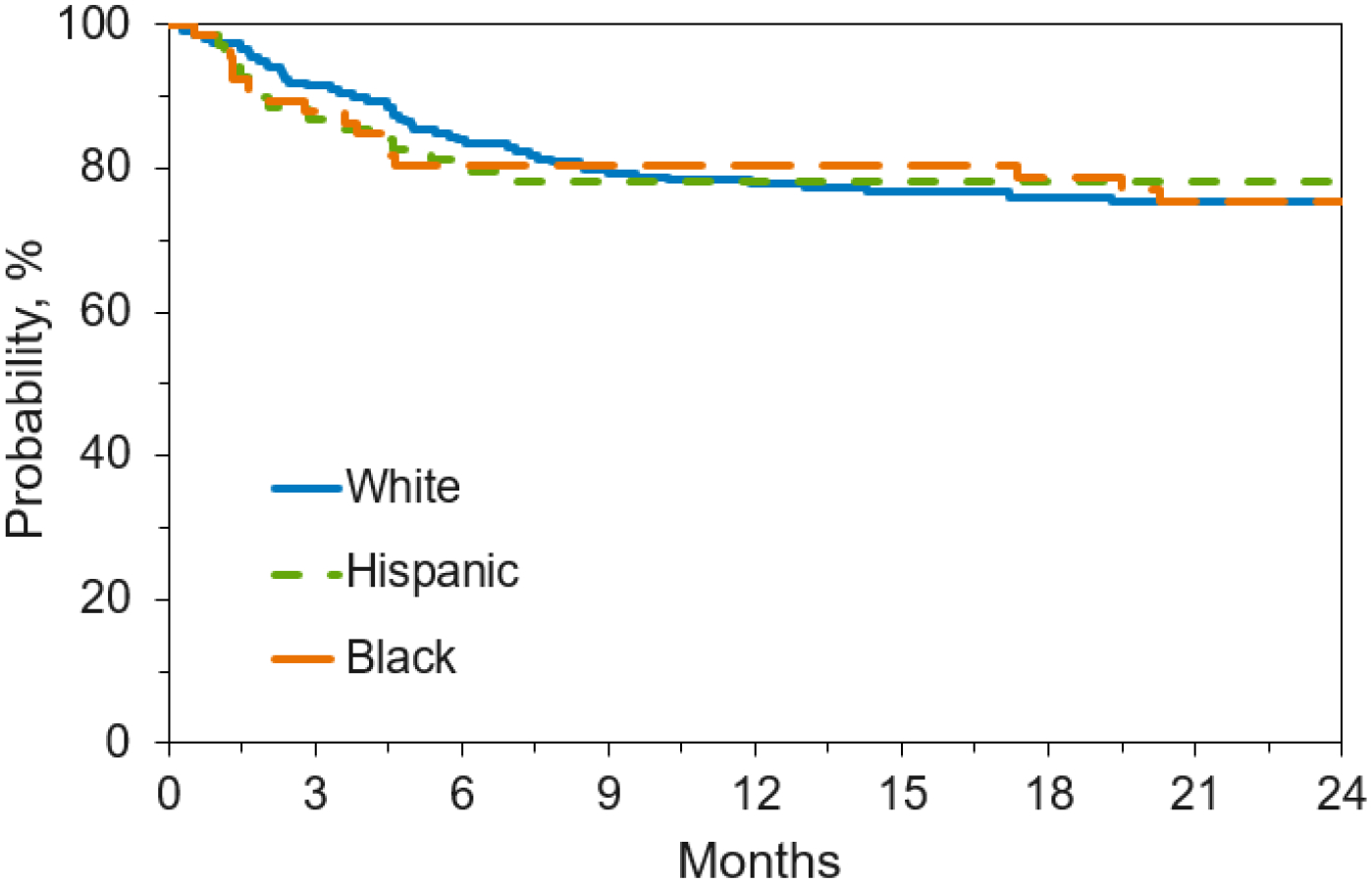
Figure 4a. Overall Survival by Race/Ethnicity for Adult Patients with Malignant Disease Receiving Unlicensed Umbilical Cord Blood Transplant
Figure 4b. Overall Survival by Race/Ethnicity for Pediatric Patients with Malignant Disease Receiving Unlicensed Umbilical Cord Blood Transplant
Figure 4c. Overall Survival by Race/Ethnicity for Pediatric Patients with Nonmalignant Disease Receiving Unlicensed Umbilical Cord Blood Transplant
Table 3b.
Pediatric Recipients with Malignant Diseases
| Neutrophil engraftment by day 42 | ||||
| (MAC only) | ||||
| Disease | 0.002 | |||
| ALL | 241 | 1.00 | ||
| AML | 214 | 0.37 | (0.20–0.70) | 0.002 |
| MDS/MPN | 58 | 0.81 | (0.28–2.34) | 0.704 |
| Other | 30 | 0.21 | (0.08–0.57) | 0.002 |
| GVHD Prophylaxis | 0.005 | |||
| CSA+MMF+/− other | 304 | 1.00 | ||
| FK+MMF+/− other | 124 | 0.68 | (0.34–1.35) | 0.268 |
| Other | 115 | 0.36 | (0.19–0.66) | 0.001 |
| Overall survival | ||||
| Disease | 0.005 | |||
| ALL | 242 | 1.00 | ||
| AML | 223 | 1.37 | (1.03–1.84) | 0.033 |
| MDS/MPN | 60 | 0.60 | (0.35–1.05) | 0.076 |
| Other | 31 | 0.73 | (0.37–1.46) | 0.379 |
| Disease risk (AML, ALL, CML, MDS, MPN) | 0.031 | |||
| Early | 302 | 1.00 | ||
| Intermediate | 190 | 0.94 | (0.68–1.29) | 0.712 |
| Advanced | 64 | 1.61 | (1.08–2.41) | 0.02 |
| Karnofsky / Lansky Performance Score | ||||
| 90–100 | 474 | 1.00 | ||
| 10–80 | 82 | 1.94 | (1.37–2.74) | <0.001 |
| Recipient sex | ||||
| Female | 231 | 1.00 | ||
| Male | 325 | 1.38 | (1.04–1.83) | 0.027 |
| GVHD Prophylaxis | 0.009 | |||
| CSA+MMF+/− other | 307 | 1.00 | ||
| TAC+MMF+/− other | 130 | 1.66 | (1.20–2.31) | 0.002 |
| Other | 119 | 1.20 | (0.85–1.71) | 0.302 |
| Year of transplant | 0.024 | |||
| 2011–2012 | 137 | 1.00 | ||
| 2013–2014 | 210 | 0.71 | (0.51–0.99) | 0.047 |
| 2015–2016 | 209 | 0.06 | (0.41–0.88) | 0.008 |
| Recipient race/ethnicity | <0.001 | |||
| Caucasian | 276 | 1.00 | ||
| Hispanic | 120 | 1.72 | (1.21–2.46) | 0.003 |
| Black or African American | 92 | 2.09 | (1.46–3.01) | <0.001 |
| Other/Unknown | 68 | 1.69 | (1.11–2.58) | 0.014 |
| Acute GVHD II-IV | ||||
| Use of ATG | ||||
| No ATG | 336 | 1 | ||
| ATG | 215 | 0.62 | (0.4–0.95) | 0.028 |
| HLA Matching | 0.005 | |||
| >6/8 | 136 | 1 | ||
| 6/8 | 138 | 1.16 | (0.62–2.19) | 0.638 |
| <6/8 | 244 | 2.29 | (1.35–3.89) | 0.002 |
| Unknown | 33 | 1.89 | (1.77–4.65) | 0.165 |
| Disease risk | 0.041 | |||
| Early | 299 | 1 | ||
| Intermediate | 189 | 1.72 | (1.13–2.64) | 0.012 |
| Advanced | 63 | 1.15 | (0.59–2.24) | 0.688 |
| Chronic GVHD | ||||
| Use of ATG | ||||
| No ATG | 334 | 1 | ||
| ATG | 208 | 0.57 | (0.4–0.81) | 0.002 |
| GVHD Prophylaxis | 0.023 | |||
| CSA+MMF+/−other | 299 | 1 | ||
| TAC+MMF+/−other | 126 | 1.71 | (1.16–2.5) | 0.006 |
| Other | 117 | 1.19 | (0.78–1.82) | 0.411 |
OR for neutrophil engraftment and acute GVHD; HR for OS and chronic GVHD
Abbreviations: OR is odds ratio; HR is hazard ratio; CI is confidence interval; AML is acute myeloid leukemia; ALL is acute lymphoblastic leukemia; CML is chronic myeloid leukemia; MDS is myelodysplasia; MPN is myeloproliferative neoplasms; NHL is non-Hodgkin lymphoma; HL is Hodgkin lymphoma; TNC is total nucleated cell; MAC is myeloablative conditioning; CSA is cyclosporine A; TAC is tacrolimus; MMF is mycophenylate mofetil.
Highlights.
Unlicensed umbilical cord blood units can be safely and effectively used for hematopoietic cell transplantation (HCT) in adults and pediatric patients
Unlicensed umbilical cord blood expands access to racially/ethnically diverse patients in need of HCT
Further studies are required to compared licensed and unlicensed umbilical cord blood HCT
ACKNOWLEDGEMENTS
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200018C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-17-1-2388 and N0014-17-1-2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. - Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government. *Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE OF CONFLICTS OF INTEREST
None of the authors has any conflict of interest to declare.
REFERENCES
- 1.Ballen KK, King RJ, Chitphakdithai P, et al. The National Marrow Donor Program 20 Years of Unrelated Donor Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2008. September;14(9 Suppl):2–7. doi: 10.1016/j.bbmt.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Gragert L, Eapen M, Williams E, et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N Engl J Med. 2014. July 24;371(4):339–48. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012. June 7;119(23):5591–8. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner JE, Eapen M, Carter S, et al. One-Unit versus Two-Unit Cord-Blood Transplantation for Hematologic Cancers. N Engl J Med. 2014. October 30;371(18):1685–94. doi: 10.1056/NEJMoa1405584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tucunduva L, Ruggeri A, Sanz G, et al. Risk factors for outcomes after unrelated cord blood transplantation for adults with acute lymphoblastic leukemia: a report on behalf of Eurocord and the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014. July;49(7):887–94. doi: 10.1038/bmt.2014.72. [DOI] [PubMed] [Google Scholar]
- 6.Ballen KK, Joffe S, Brazauskas R, et al. Hospital Length of Stay in the First 100 Days after Allogeneic Hematopoietic Cell Transplantation for Acute Leukemia in Remission: Comparison among Alternative Graft Sources. Biol Blood Marrow Transplant. 2014. November;20(11):1819–27. doi: 10.1016/j.bbmt.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisdorf D, Eapen M, Ruggeri A, et al. Alternative Donor Transplantation for Older Patients with Acute Myeloid Leukemia in First Complete Remission: A Center for International Blood and Marrow Transplant Research-Eurocord Analysis. Biol Blood Marrow Transplant. 2014. June;20(6):816–22. doi: 10.1016/j.bbmt.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ustun C, Giannotti F, Zhang MJ, et al. Outcomes of UCB transplantation are comparable in FLT3+ AML: results of CIBMTR, EUROCORD and EBMT collaborative analysis. Leukemia. 2017. June;31(6):1408–1414. doi: 10.1038/leu.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunstein CG, Fuch EJ, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011. July 14;118(2):282–8. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YB, Aldridge J, Kim HT, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biology Blood Marrow Transplant. 2012. May;18(5):805–12. doi: 10.1016/j.bbmt.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Kosuri S, Wolff T, Devlin SM, et al. Prospective Evaluation of Unrelated Donor Cord Blood and Haploidentical Donor Access Reveals Graft Availability Varies by Patient Ancestry: Practical Implications for Donor Selection. Biol Blood Marrow Transplant. 2017. June;23(6):965–970. doi: 10.1016/j.bbmt.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boo M, Welte K, Confer DL, et al. Accreditation and regulation of cord blood banking Accreditation and regulation of cord blood banking Cord Blood Biology, Transplantation, Banking and Regulation. Bethesda, MD: AABB Press (Advancing Transfusion and Cellular Therapies Worldwide) 2011:663–672. http://www.aabb.org/resources/marketplace/documents/112055_toc.pdf [Google Scholar]
- 13.Lazarus EF. US FDA Regulations of Placental/Umbilical Cord Blood Broxmeyer HE, ed. Cord Blood: Biology, Transplantation, Banking, and Regulation. Bethesda: AABB Press (Advancing Transfusion and Cellular Therapies Worldwide) 2011: 685–691. http://www.aabb.org/resources/marketplace/documents/112055_toc.pdf [Google Scholar]
- 14.National Marrow Donor. Investigator Brochure: A Multicenter Access and Distribution Protocol for Unlicensed Cryopreserved Cord Blood Units for Transplantation in Pediatric and Adult Patients with Hematologic Malignancies and Other Indications. Minneapolis, MN, USA: 2016. https://www.cibmtr.org/Studies/ClinicalTrials/RCI_BMT/Documents/Study%200264%20CB%20IND%20Investigator%20Brochure%20version%203.pdf [Google Scholar]
- 15.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999. March 30;18(6):695–706. doi: [DOI] [PubMed] [Google Scholar]
- 16.Klein J, Moeschberger ML. Survival Analysis: Statistical Methods for Censored and Truncated Data. New York, NY: Springer-Vering; 2003. http://sistemas.fciencias.unam.mx/~ediaz/Cursos/Estadistica3/Libros/0a9X.pdf [Google Scholar]
- 17.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol Blood Marrow Transplant. 2009. December;15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association, Vol. 53, No. 282 (June, 1958), pp. 457–481. (Jun., 1958)1958;53(282):457–481. https://web.stanford.edu/~lutian/coursepdf/KMpaper.pdf [Google Scholar]
- 19.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014. June 5; 123(23):3664–71. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorror ML, Logan BR, Zhu X, et al. Prospective Validation of the Predictive Power of the Hematopoietic Cell Transplantation Comorbidity Index: A Center for International Blood and Marrow Transplant Research Study. Biology of Blood and Marrow Transplantation. 2015. August 21(8):1479–87. doi: 10.1016/j.bbmt.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 22.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001. June 14;344(24):1815–22. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 23.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010. July;11(7):653–60. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematpoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010. November 25;116(22):4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballen KK. Ahn KW, Chen M, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016. September;22(9):1636–1645. doi: 10.1016/j.bbmt.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995. October 24;92(22):10119–22. doi: 10.1073/pnas.92.22.10119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahi PB, Ponce DM, Devlin S, et al. “No Wash” Albumin-Dextran Dilution for Double-Unit Cord Blood Transplantation is Safe with High Rates of Sustained Donor Engraftment. Biol Blood Marrow Transplant. 2014. April;20(4):490–4. doi: 10.1016/j.bbmt.2013.12.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindemans CA, Chiesa R Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcomes. Blood. 2014. January 2;123(1):126–32. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 29.Brown JA, Stevenson K, Kim HT, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood. 2010. May 20;115(20):4111–9. doi: 10.1182/blood-2009-09-244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascal L, Tucunduva L, Ruggeri A, et al. Impact of ATG-containing reduced-intensity conditioning after single- or double-unit allogeneic cord blood transplantation. Blood. 2015. August 20;126(8):1027–32. doi: 10.1182/blood-2014-09-599241. [DOI] [PubMed] [Google Scholar]
- 31.Ballen KK, Klein JP, Pedersen TL, et al. Relationship of Race/Ethnicity and Survival after Single Umbilical Cord Blood Transplantation for Adults and Children with Leukemia and Myelodysplastic Syndromes. Biol Blood Marrow Transplant. 2012. June;18(6):903–12. doi: 10.1016/j.bbmt.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warlick ED, Peffault de Latour R, et al. Allogeneic Hematopoietic Cell Transplantation Outcomes in Acute Myeloid Leukemia: Similar Outcomes Regardless of Donor Type. Biol Blood Marrow Transplant. 2015. February;21(2):357–63. doi: 10.1016/j.bbmt.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballen KK, Gluckman E, Broxmeyer HE, et al. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013. July 25;122(4):491–8. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011. July 14;118(2):282–8. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010. July;11(7):653–60. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015. September;29(9):1891–900. doi: 10.1038/leu.2015.98. [DOI] [PubMed] [Google Scholar]
- 37.Terakura S, Atsuta Y, Tsukada N, et al. Comparison of Outcomes of 8/8 and 7/8 Allele–Matched Unrelated Bone Marrow Transplantation and Single-Unit Cord Blood Transplantation in Adults with Acute Leukemia. Biol Blood Marrow Transplant. 2016. February;22(2):330–338. doi: 10.1016/j.bbmt.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Milano F, Gooley T, Wood B, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med. 2016. December 1;375(22):2204–2205. doi: 10.1056/NEJMc1612872 [DOI] [PubMed] [Google Scholar]
- 39.Gutman JA, Ross K, Smith C, et al. Chronic graft vs host disease burden and late transplant complications are lower following adult cord blood versus matched unrelated donor peripheral blood transplantation. Bone Marrow Transplant. 2016. December;51(12):1588–1593. doi: 10.1038/bmt.2016.186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


