Abstract
Negative circumferential resection margins (CRM) are the cornerstone for the curative treatment of locally advanced rectal cancer (LARC). However, in up to 18.6% of patients, tumor-positive resection margins are detected on histopathology. In this proof-of-concept study, we investigated the feasibility of optical molecular imaging as a tool for evaluating the CRM directly after surgical resection to improve tumor-negative CRM rates. Methods: LARC patients treated with neoadjuvant chemoradiotherapy received an intravenous bolus injection of 4.5 mg of bevacizumab-800CW, a fluorescent tracer targeting vascular endothelial growth factor A, 2–3 d before surgery (ClinicalTrials.gov identifier: NCT01972373). First, for evaluation of the CRM status, back-table fluorescence-guided imaging (FGI) of the fresh surgical resection specimens (n = 8) was performed. These results were correlated with histopathology results. Second, for determination of the sensitivity and specificity of bevacizumab-800CW for tumor detection, a mean fluorescence intensity cutoff value was determined from the formalin-fixed tissue slices (n = 42; 17 patients). Local bevacizumab-800CW accumulation was evaluated by fluorescence microscopy. Results: Back-table FGI correctly identified a tumor-positive CRM by high fluorescence intensities in 1 of 2 patients (50%) with a tumor-positive CRM. For the other patient, low fluorescence intensities were shown, although (sub)millimeter tumor deposits were present less than 1 mm from the CRM. FGI correctly identified 5 of 6 tumor-negative CRM (83%). The 1 patient with false-positive findings had a marginal negative CRM of only 1.4 mm. Receiver operating characteristic curve analysis of the fluorescence intensities of formalin-fixed tissue slices yielded an optimal mean fluorescence intensity cutoff value for tumor detection of 5,775 (sensitivity of 96.19% and specificity of 80.39%). Bevacizumab-800CW enabled a clear differentiation between tumor and normal tissue up to a microscopic level, with a tumor-to-background ratio of 4.7 ± 2.5 (mean ± SD). Conclusion: In this proof-of-concept study, we showed the potential of back-table FGI for evaluating the CRM status in LARC patients. Optimization of this technique with adaptation of standard operating procedures could change perioperative decision making with regard to extending resections or applying intraoperative radiation therapy in the case of positive CRM.
Keywords: optical molecular imaging, vascular endothelial growth factor A, near-infrared fluorescence, back-table fluorescence-guided imaging
In today’s clinical practice, patients with locally advanced rectal cancer (LARC) receive long-course neoadjuvant chemoradiotherapy (nCRT) followed by surgical resection using total mesorectal excision (TME) principles. nCRT induces tumor downsizing and downstaging, which facilitate complete resection by TME, resulting in significantly reduced local recurrence rates and increased opportunity for sphincter-sparing resections (1–5).
Obtaining negative circumferential resection margins (CRM) is key in rectal cancer therapy. The CRM has proven to be one of the most important predictors for local recurrence and, to a lesser extent, the development of distant metastases and survival (6). Restaging after nCRT occurs through a high-resolution MRI scan for tumor staging and a CT scan of the thorax and abdomen for distant metastases and lymph node staging (7–9). Accurate restaging can be highly challenging, as differentiating between a desmoplastic reaction and viable tumor tissue on preoperative imaging modalities is often difficult after nCRT.
Intraoperatively, surgeons rely mainly on visual and tactile inspections for margin assessment and differentiation between tumor and healthy tissue. This approach is often inaccurate, especially after nCRT, as small tumor deposits are frequently present within fibrotic parts (10). In the presence of doubt, resection margins can be assessed intraoperatively by frozen-section pathologic evaluation, but this approach is time-consuming and costly and poses a high risk of sampling error (11).
Despite nCRT, TME surgery, and frozen-section analysis, a tumor-positive CRM, defined as the presence of tumor less than or equal to 1 mm from the CRM, is detected in up to 18.6% of primary LARC surgeries on final histopathology (7–9). Perioperative resection margin evaluation using optical molecular imaging could improve negative CRM rates by extending resections or allowing the application of intraoperative radiation therapy (IORT) or more innovative treatment modalities, such as photoimmunotherapy of the wound bed (12). In contrast, when a margin is evaluated as being tumor-negative, extended resections could be avoided.
The aim of this proof-of-concept study was to evaluate whether back-table fluorescence-guided imaging (FGI) using the near-infrared fluorescent tracer bevacizumab-800CW could aid in evaluating the CRM status in the surgical theater. Bevacizumab-800CW targets vascular endothelial growth factor A (VEGFA), which is overexpressed in LARC as well as many other solid tumors (13–15). We retrospectively analyzed back-table FGI data for fresh surgical specimens from LARC patients treated with nCRT. This technique may eventually allow real-time determination of CRM during surgery, which could aid in intraoperative clinical decision making with regard to extending resections or applying IORT in the case of a positive CRM, to improve the outcome of LARC patients.
MATERIALS AND METHODS
Study Design and Population
Postoperative fluorescence imaging data were collected from 25 LARC patients enrolled in a clinical trial evaluating VEGFA-targeted fluorescence molecular endoscopy (ClinicalTrials.gov identifier: NCT01972373). Eligibility criteria included histologically proven LARC, with the inferior margin within 16 cm from the anal verge, and treatment with long-course nCRT. For the determination of whether back-table FGI could aid in evaluation of the CRM status, patients were included if fluorescence imaging data were available from at least the anterior and posterior sides of the fresh surgical specimen. Furthermore, for the evaluation of local bevacizumab-800CW accumulation and determination of the sensitivity and specificity of bevacizumab-800CW for tumor detection using a fluorescence intensity cutoff value, patients were included if high-resolution fluorescence images of formalin-fixed tissue slices were available. The study was performed at the University Medical Center Groningen and approved by the institutional review board (METc 2013/067), and all subjects signed a written informed consent form.
Surgery
After nCRT, all patients received an intended curative resection by either low anterior resection for proximal rectum tumors or abdominal–perineal resection for distal rectum tumors. Resections were extended outside the TME planes in the case of tumor growth into adjacent organs. Patients received IORT only if judged necessary on the basis of preoperative suspicion of mesorectal fascia involvement or intraoperative evaluation by the surgeons.
Histopathologic Processing
Histopathologic processing of surgical specimens was performed by a board-certified gastrointestinal pathologist. After evaluation for gross pathology, CRM were inked black and staple lines were removed. Specimens were anteriorly opened from proximal to distal, except for specimens with an anterior lesion, which were opened until the rectal fold. All specimens were formalin-fixed for at least 48 h and serially sliced perpendicular to the rectum, from distal to proximal, into ±0.5-cm-thick tissue slices. Distal and proximal resection surfaces plus additional areas of interest (e.g., regions with suspected CRM involvement, perineural growth, vascular invasion, or lymph nodes) were included for paraffin embedding. Formalin-fixed paraffin-embedded tissue blocks were cut into 4-μm tissue sections and stained with hematoxylin–eosin for routine histopathologic examination. Immunohistochemistry was performed if required. A tumor-positive CRM was defined as the presence of tumor less than or equal to 1 mm from the inked CRM, in accordance with Dutch national guidelines.
Bevacizumab-800CW
All patients received a 4.5-mg intravenous bolus injection of bevacizumab-800CW (1 mg/mL) 2–3 days before surgery on the basis of microdosing regulations (Fig. 1). Bevacizumab-800CW was produced under current good manufacturing practice conditions at the University Medical Center Groningen as described previously (16).
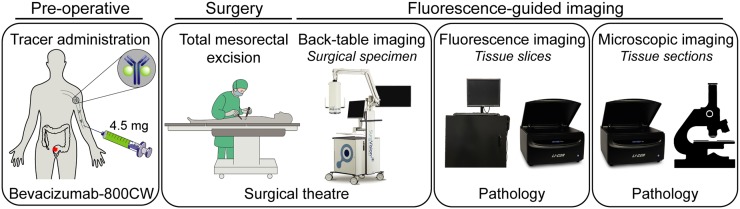
FIGURE 1.
Schematic overview of study design. Bevacizumab-800CW (4.5 mg) was administered intravenously 2–3 d before surgery. Fluorescence-guided imaging was performed at every step during pathologic processing: back-table of fresh surgical specimen, tissue slices, and tissue sections. Results were correlated with histopathology.
Back-Table FGI
For evaluation of the CRM status, back-table FGI of a fresh surgical specimen was performed in a light-tight room directly after surgery using an Explorer Air fluorescence camera (SurgVision BV) (Fig. 1). Areas with high fluorescence signals were marked with a pin and subsequently inked with a color different from that used for the CRM during pathologic processing to ensure an accurate correlation of fluorescence with histopathology.
Fluorescence Imaging of Tissue Slices and Sections
For the evaluation of local tracer accumulation, fluorescence imaging of both sides of all formalin-fixed tissue slices was performed using Explorer Vault, a standardized and light-tight fluorescence imaging system (SurgVision BV). Thereafter, 2 or 3 tissue slices containing tumor or normal rectal tissue were imaged per patient using a high-resolution Odyssey CLx fluorescence imaging system (LI-COR Biosciences Inc.) (Fig. 1). Additional areas of interest based on fluorescence imaging were also paraffin embedded, sliced into 4-μm tissue sections, and stained with hematoxylin–eosin. All formalin-fixed paraffin-embedded tissue blocks were subjected to fluorescence scanning using the Odyssey CLx (Fig. 1). To enable comparison of fluorescence images within each patient, a threshold was applied per imaging modality to all fluorescence images.
To broaden our understanding of the overall penetration, distribution, and accumulation of bevacizumab-800W in rectal cancer tissue, a combination of optical tissue clearing and light-sheet fluorescence microscopy was performed on selected tissue slices as previously described for preclinical tissue samples (Supplemental Video 1; supplemental materials are available at http://jnm.snmjournals.org) (17).
Fluorescence Grid Analysis
Finally, for evaluation of the sensitivity and specificity of bevacizumab-800CW for tumor detection, fluorescence grid analysis based on histopathology was used as a gold standard to determine a fluorescence cutoff value (supplemental materials).
Statistical Analysis
Normally distributed data are presented as mean values with SDs, and skewed data are presented as median values with interquartile ranges. A receiver operating characteristic curve was plotted to determine the median fluorescence intensity (MFI) cutoff value for tumor detection. P values of less than 0.05 were considered statistically significant. Statistical analyses were performed using Prism (version 7.0; GraphPad Software).
RESULTS
Patient Characteristics
In this retrospective proof-of-concept study, 8 of 25 patients met the criteria to determine the feasibility of back-table FGI for evaluation of the CRM status (Supplemental Fig. 1). For the second aim of the present study—evaluation of the local bevacizumab-800CW distribution and determination of the sensitivity and specificity of bevacizumab-800CW for tumor detection in high-resolution tissue slices, 17 of 25 patients met the inclusion criteria (Supplemental Fig. 1). Patient characteristics are depicted in Table 1. All patients received 4.5 mg of bevacizumab-800CW intravenously 2–3 d before surgery. Ten patients underwent low anterior resection, and 7 patients underwent abdominal–perineal resection. There were no tracer-related (serious) adverse events in any of the patients.
TABLE 1.
Patient and Tumor Characteristics
| Characteristic | CRM evaluation (n = 8)* | Fluorescence cutoff value determination (n = 17)* |
| Sex | ||
| Male | 5 (62.5) | 12 (70.6) |
| Female | 3 (37.5) | 5 (29.4) |
| Age (y)† | 56 (54–61) | 56 (31–76) |
| Duration between nCRT and surgery (d)‡ | 87 (76–111) | 87 (77–117) |
| Surgery | ||
| Low anterior resection | 5 (62.5) | 10 (58.8) |
| Including adjacent organs | 1 | 2 |
| Abdominal–perineal resection | 3 (37.5) | 7 (41.2) |
| Including adjacent organs | 3 | 5 |
| IORT | ||
| Not standby | 3 (37.5) | |
| Standby | 4 (50) | |
| Applied | 1 (12.5) | |
| Histopathologic staging | ||
| pT0N0M0 (pCR) | 1 (12.5) | 1 (5.9) |
| pT2N0M0 | 2 (25.0) | 2 (11.8) |
| pT3N0M0 | 1 (12.5) | 4 (23.5) |
| pT3N1M0 | 0 | 3 (17.6) |
| pT3N2M0 | 2 (25.0) | 5 (29.4) |
| pT4N0M0 | 2 (25.0) | 2 (11.8) |
| CRM | ||
| ≤1 mm (tumor-positive) | 2 (25.0) | 3 (17.6) |
| 1–2 mm | 1 (12.5) | 2 (11.8) |
| >2 mm | 4 (50.0) | 11 (64.7) |
| pCR | 1 (12.5) | 1 (5.9) |
| Distal resection margin | ||
| ≤1 mm (tumor-positive) | 0 | 0 |
| 1–2 mm | 0 | 1 (5.9) |
| >2 mm | 7 (87.5) | 15 (88.2) |
| pCR | 1 (12.5) | 1 (5.9) |
Data are reported as numbers of patients, with percentages in parentheses, unless otherwise indicated.
Reported as median, with range in parentheses.
Reported as median, with interquartile range in parentheses.
pCR = pathologic complete response.
Evaluation of CRM Status
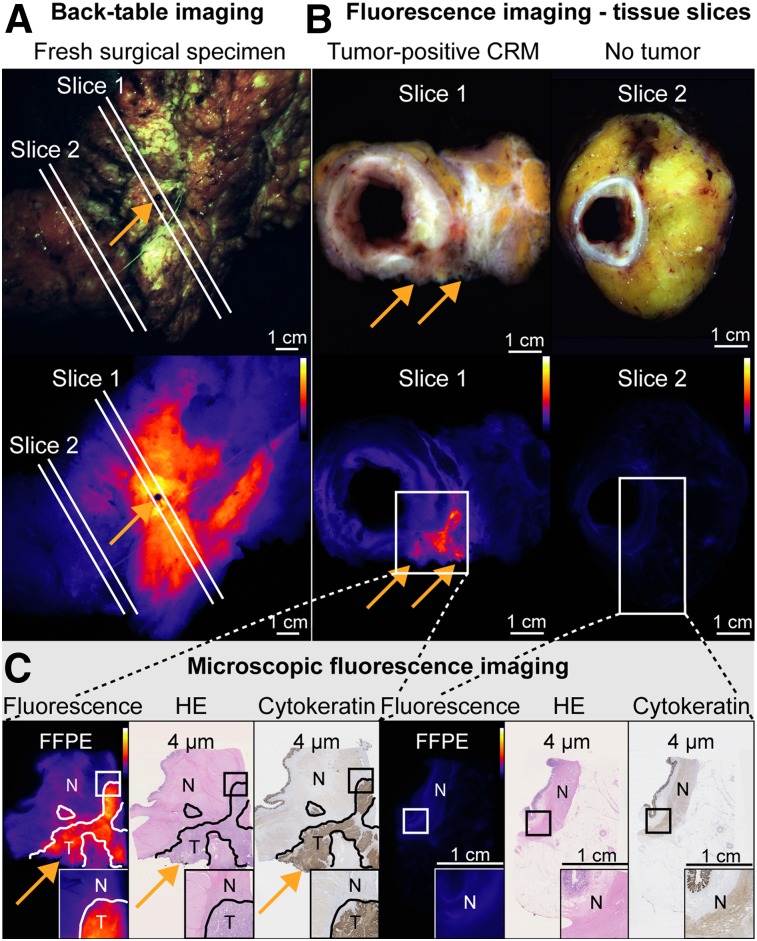
Of 8 evaluated patients, 2 (25%) had tumor-positive CRM on final histopathology. Back-table FGI correctly predicted the tumor-positive CRM in 1 of these patients because of increased fluorescence signals in the CRM (50%) (Fig. 2). This patient was treated with abdominal–perineal resection with en bloc resection of the sacrum and a wide perineal excision because of a fistula. Intraoperatively, 2 frozen-section analyses of the lateral resection surface were performed; both samples were benign. Interestingly, during back-table FGI of the fresh surgical specimen, high fluorescence signals were observed at the lateral resection margin (Fig. 2A). Fluorescence imaging of the corresponding tissue slice also showed high fluorescence signals that seemed to reach into the CRM, whereas the surrounding, nontumor tissue showed low fluorescence signals (Fig. 2B). Final histopathology proved that this exact location was a tumor-positive CRM (Fig. 2C, orange arrows).
FIGURE 2.
Back-table FGI of patient with tumor-positive CRM, with black pin at location showing increased fluorescence, to enable accurate correlation with histology (A, orange arrow). Fluorescence imaging of 2 corresponding tissue slices (B) and further microscopic fluorescence imaging and histologic correlation (C), with orange arrows indicating location of tumor-positive CRM. High fluorescence signals were observed at CRM of tissue slice 1, containing tumor-positive CRM, whereas low fluorescence signals were observed in nontumor tissue slice 2, corresponding to microscopy results. FFPE = formalin-fixed paraffin-embedded; HE = hematoxylin–eosin; N = nontumor; T = tumor.
The second patient with a tumor-positive CRM received low anterior resection. Back-table FGI of the fresh surgical specimen and subsequent fluorescence imaging of the tissue slices showed low fluorescence intensities in the CRM, apart from fluorescence in 2 enlarged suspect lymph nodes that proved to be tumor-positive (Supplemental Fig. 2A). However, the pathologist reported a tumor-positive CRM that was solely based on the presence of isolated microscopic tumor deposits that appeared vital and that were of (sub)millimeter size within 0.2–1 mm of the CRM (Supplemental Fig. 2B, orange arrow). The fact that the tumor volume was limited because of the (sub)millimeter size of the tumor deposits may explain the negative imaging results.
Six of 8 patients (75%) had tumor-negative CRM, which were correctly predicted in 5 of the patients by low fluorescence intensities during back-table FGI (a representative example is shown in Supplemental Fig. 3). Subsequent fluorescence imaging of tissue slices also showed low fluorescence intensities in relation to the CRM, although fluorescence was observed in the (intra)luminal tumor, except in the patient with a pathologic complete response. IORT was applied to 1 of these patients because of the macroscopic suggestion of a tumor-positive CRM, whereas histopathology showed a tumor-negative CRM (>1 cm).
The remaining patient with a tumor-negative CRM received low anterior resection with en bloc resection of the uterus, cervix, and adnexa and the distal right ureter because of tumor ingrowth. Increased fluorescence was observed at the cervix during back-table FGI and subsequent fluorescence imaging of the tissue slices, potentially indicating a tumor-positive CRM. Although the fluorescence colocalized with a tumor deposit on histopathology, the CRM was defined as tumor-negative, as the distance to the CRM was 1.4 mm.
Fluorescence Cutoff Value and Local Bevacizumab-800CW Accumulation
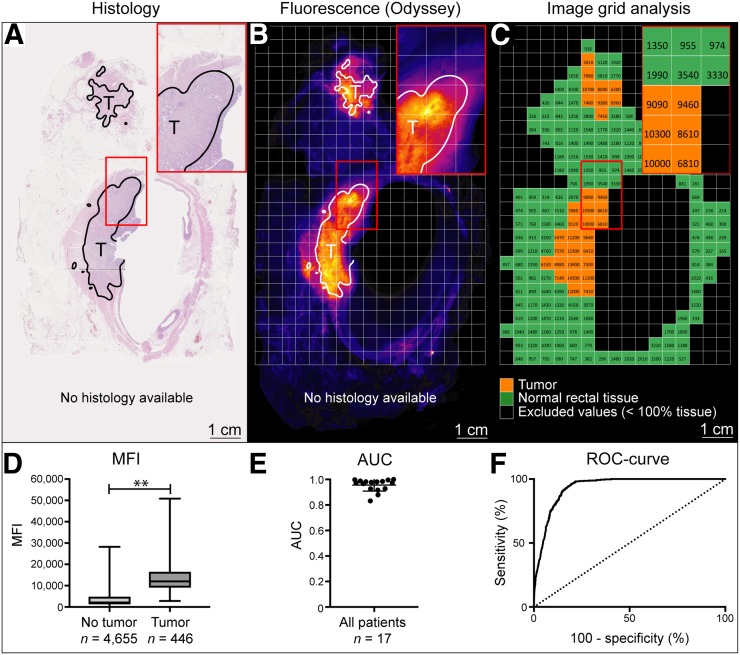
High-resolution fluorescence Odyssey scans of formalin-fixed tissue slices (n = 42) from 17 patients were used to determine a semiquantitative fluorescence cutoff value for tumor detection. A total of 5,101 grid squares were analyzed; of these, 446 were classified as tumor-positive and 4,655 were classified as tumor-negative (Figs. 3A–3C). Significantly higher fluorescence intensities were observed in tumor areas (median MFI: 12,000) than in surrounding nontumor tissue (median MFI: 2,140) (P < 0.0001) (Fig. 3D), resulting in a ratio of tumor to surrounding tissue of 4.7 ± 2.5 (mean ± SD). Receiver operating characteristic curves were plotted per patient (Fig. 3E) and for all patients combined, with an area under the curve of 0.94 (SE: 0.0039) (Fig. 3F). For our limited sample, an optimal MFI cutoff value of 5,775 was calculated using Youden J statistics (J = 0.77), with a sensitivity of 96.19% and a specificity of 80.39%.
FIGURE 3.
Fluorescence grid analysis. All tumor tissue was delineated on hematoxylin–eosin staining (A) and subsequently overlaid on high-resolution fluorescence images of tissue slices (B). Each 3- by 3-mm square was selected as tumor-positive (C, orange) or tumor-negative (C, green). MFIs were calculated per square, with significantly higher fluorescence intensities in tumor tissue than in surrounding nontumor tissue (12,000 vs. 2,140; P < 0.001) (D). Individual areas under curve (AUC) were determined per patient (E), and receiver operating characteristic (ROC) curve was plotted, showing AUC of 0.94 (F).
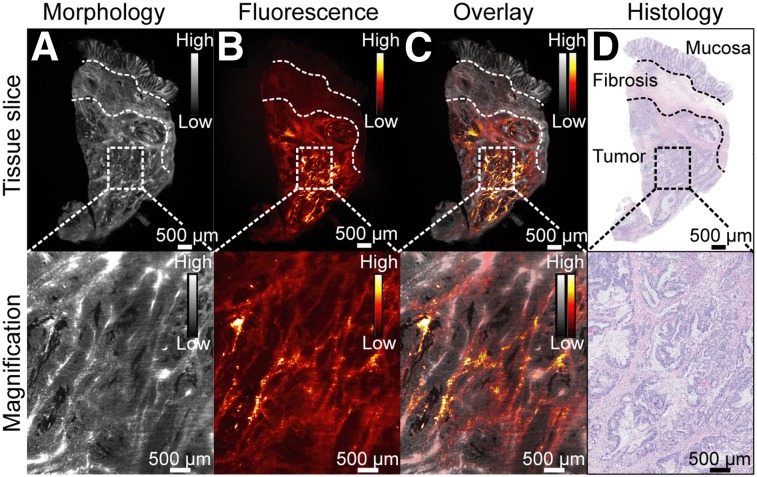
Finally, we evaluated the distribution and accumulation of bevacizumab-800CW on a microscopic level. Bevacizumab-800CW was localized mainly in the microenvironment of the tumor cells (Fig. 4), in line with the expected location of VEGFA (Supplemental Video 1).
FIGURE 4.
Representative example of light-sheet fluorescence microscopy for evaluating local tracer accumulation in rectal cancer tissue slice, showing tissue morphology based on autofluorescence (A), fluorescence (B), and morphology and fluorescence overlay (C) and corresponding histology (D). Magnified images are depicted in bottom row. Increased bevacizumab-800CW binding can be seen in microenvironment of tumor cells compared with surrounding normal mucosa and fibrosis.
DISCUSSION
Accurate perioperative evaluation of resection margins is highly important for the prognosis of LARC patients. In this explorative study, we demonstrated for the first time the feasibility of back-table FGI for the identification of tumor-positive resection margins in LARC patients. Our data suggest that FGI may have the potential to guide current clinical decision making with regard to additional targeted resections or the application of IORT. Future studies using a standardized imaging protocol with larger samples of patients should confirm these results.
During the past decade, optical molecular imaging has been applied predominantly to intraoperative guidance and surgical navigation. Two clinical studies have demonstrated the feasibility and potential benefit of fluorescence-guided surgery in colorectal cancer patients (18,19). However, fluorescence-guided surgery is subject to several limitations related to the currently available hardware, such as variation in image acquisition parameters and interference of ambient light. Moreover, homogeneous tracer excitation is difficult, especially in rectal cancer surgery, as the pelvic region is a confined surgical field often situated deep in the patient. Perhaps the development of fluorescence laparoscopes and robotic systems sensitive enough to detect microdoses of fluorescent tracers will enable a more reliable intraoperative evaluation of rectal cancer resection margins.
Back-table FGI circumvents these limitations, as it makes use of a controlled, standardized, and closed-field imaging environment that results in a consistent field of view, imaging distance, and image acquisition parameters (20). This approach enables a highly sensitive and semiquantitative evaluation of resection margins, within a maximum of 1 h after specimen excision in the surgical theater.
The potential added value of back-table FGI was clearly demonstrated for the patient in whom a tumor-positive CRM was detected in the surgical theater, whereas the results of surgical assessment and frozen-section analysis were false-negative. Although intraoperative frozen-section analysis is recommended for tumors in the low and middle rectum, it is prone to sampling error, is labor-intensive, and significantly prolongs anesthesia (21,22). By bringing pathology to the surgical theater using back-table FGI, both surgeons and pathologists may be guided in correctly assessing the CRM status and evaluating the need for extended surgery or application of IORT, thereby improving personalized treatment.
In rectal cancer surgery, caution should be taken regarding unnecessarily extending TME surgery to a partial or total resection of adjacent pelvic organs or applying IORT, as this approach can result in substantial postoperative complications and is associated with the need for reinterventions (23). Back-table FGI correctly predicted 5 of 6 tumor-negative CRM, perhaps preventing unnecessary IORT application in 1 patient. In contrast, 1 close margin of 1.4 mm was identified as tumor-positive on the basis of fluorescence. Although at present a tumor-positive margin is defined as tumor cells less than or equal to 1 mm from the CRM, this definition is under debate, as both patients with a CRM of 0.0–1.0 mm and those with a CRM of 1.1–2.0 mm have been shown to have an equally increased 2-y risk of local recurrence and distant metastases (8,24). The application of fluorescence may highlight the location of a (potentially) tumor-positive CRM, allowing the surgeon to perform more “targeted” intraoperative frozen-section analysis.
FGI may also support the pathologist by differentiating tumor from healthy tissue, that is, fluorescence-guided pathology. At present, tissue sampling is performed by gross examination of the surgical specimen and tissue slices; this approach can be challenging, as the tissue architecture is changed by nCRT. Using fluorescence grid analysis, we showed a tumor-to-background ratio of 4.7 ± 2.5 for tissue slices, with high sensitivity (96.19%) and specificity (80.39%) for tumor detection. A fluorescence cutoff value provides more objective information and may enable targeted tissue sampling, potentially saving labor, time, and money. In addition, fluorescence grid analysis can be used to evaluate tracer biodistribution and can easily be implemented on other closed-field imaging devices.
The present study has several limitations. First, the number of patients included for CRM evaluation was relatively low, as this was a retrospective proof-of-concept analysis of a clinical trial evaluating fluorescence molecular endoscopy (NCT01972373), and imaging techniques as well as ex vivo imaging procedures have developed throughout the study. Second, dose escalation was not incorporated in the design of the present study. Currently available evidence from 2 bevacizumab-800CW dose escalation studies suggests that increasing the dose above microdosing levels may result in higher levels of tracer accumulation without significantly increasing background fluorescence (25,26). This approach may further improve the detection of tumor-positive CRM that are based on small tumor deposits, which proved challenging in 1 of our patients.
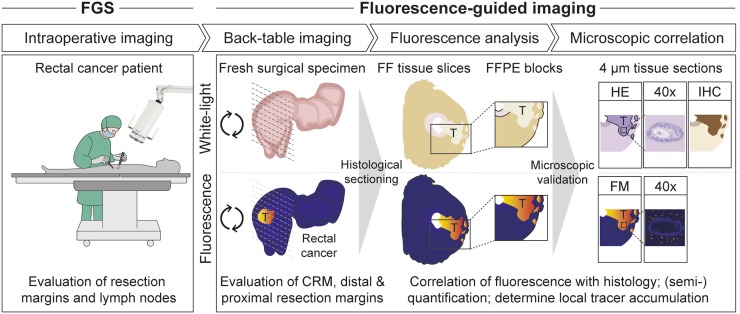
We propose a standardized imaging protocol that can be used in future research and for the development of FGI on the basis of our findings in the present study and our recently reported analytic workflow (Fig. 5) (26). Fluorescence imaging is performed at fixed time points. First, when feasible, intraoperative fluorescence-guided surgery is performed. After resection, back-table FGI of the fresh surgical specimen is performed to evaluate the resection margin status, preferably using a closed-field imaging system. If high fluorescence intensities are observed during surgery or back-table FGI, then several treatment options—such as frozen-section analysis, an additional or extended resection, IORT, or potentially more innovative treatment modalities, such as photo immunotherapy—can be considered (12). Subsequently, to correlate fluorescence with histology, fluorescence imaging is performed during histopathologic processing of the fresh surgical specimen, formalin-fixed tissue slices, and paraffin-embedded tissue sections. Additionally, fluorescence microscopy can evaluate local tracer accumulation. Altogether, this approach can provide more insight into tissue biodistribution, which is important for tracer or drug development, or can be used to evaluate potential off- and on-target effects of fluorescent tracers. We believe that such a standardized imaging protocol is widely applicable for the validation of FGI in different tumor types and with different fluorescent tracers.
FIGURE 5.
Proposed data collection and analysis design for resection margin evaluation, including intraoperative imaging to evaluate resection margins and identify potential lymph node or peritoneal metastases, back-table imaging of fresh surgical specimen to evaluate resection margins, and further fluorescence analysis of tissue slices, formalin-fixed paraffin-embedded (FFPE) tissue blocks, and tissue sections for cross-reference and correlation of fluorescence with histology. FF = formalin fixed; FGS = fluorescence-guided surgery; FM = fluorescence microscopy; HE = hematoxylin–eosin; IHC = immunohistochemistry; T = tumor.
CONCLUSION
The present study shows the potential of back-table FGI using the near-infrared fluorescent tracer bevacizumab-800CW (which targets VEGFA) for margin evaluation in the surgical theater for patients with LARC. The technique itself proved to be safe and feasible and showed potential for guiding perioperative clinical decision making with a high sensitivity in patients with the threat of a tumor-positive resection margin. A phase 2 study using a standardized imaging protocol is in development to confirm these results. Future studies will provide evidence of whether FGI will be beneficial for all patients or will be applicable only in selected cases to guide clinical decision making.
DISCLOSURE
The research leading to these results was supported by a personal grant from the Dutch Cancer Society (Wouter B. Nagengast; RUG2012-5416), by a grant from the European Community’s Seventh Framework Program (Marjory Koller and Gooitzen M. van Dam; FP7/2007-2013 BetaCure project; number 602812), and by unrestricted research grants from SurgVision B.V. (Gooitzen M. van Dam and Wouter B. Nagengast) and Boston Scientific (Wouter B. Nagengast). Gooitzen M. van Dam is a member of the scientific advisory board of SurgVision B.V. and is a founder, shareholder, and chief executive officer of TRACER Europe B.V. (Groningen, The Netherlands). No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Can back-table FGI be used as a tool to evaluate the CRM status in the surgical theater to improve tumor-positive resection margin rates in patients with LARC?
PERTINENT FINDINGS: In this explorative study, we demonstrated for the first time (to our knowledge) the potential of back-table FGI for the identification of tumor-positive resection margins in LARC patients. In addition, we provided a data collection and data analysis design for future studies examining the added value of optical molecular imaging for the evaluation of resection margins.
IMPLICATIONS FOR PATIENT CARE: Accurate intraoperative detection of tumor-positive resection margins by back-table FGI has the potential to improve clinical decision making with regard to extending resection margins or applying intraoperative radiation therapy for LARC patients.
Supplementary Material
Acknowledgments
We thank Wytske Boersma-van Ek for her technical assistance.
REFERENCES
- 1.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. [DOI] [PubMed] [Google Scholar]
- 3.Bosset J-F, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. [DOI] [PubMed] [Google Scholar]
- 4.Gérard J-P, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. [DOI] [PubMed] [Google Scholar]
- 5.van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–2545. [DOI] [PubMed] [Google Scholar]
- 6.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303–312. [DOI] [PubMed] [Google Scholar]
- 7.Simillis C, Baird DLH, Kontovounisios C, et al. A systematic review to assess resection margin status after abdominoperineal excision and pelvic exenteration for rectal cancer. Ann Surg. 2017;265:291–299. [DOI] [PubMed] [Google Scholar]
- 8.Nagtegaal ID, Marijnen CA, Kranenbarg EK, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350–357. [DOI] [PubMed] [Google Scholar]
- 9.MERCURY study group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka S, Martling A, Lindholm J, Holm T, Palmer G. Remaining cancer cells within the fibrosis after neo-adjuvant treatment for locally advanced rectal cancer. Eur J Surg Oncol. 2015;41:1204–1209. [DOI] [PubMed] [Google Scholar]
- 11.Khoury W, Abboud W, Hershkovitz D, Duek SD. Frozen section examination may facilitate reconstructive surgery for mid and low rectal cancer. J Surg Oncol. 2014;110:997–1001. [DOI] [PubMed] [Google Scholar]
- 12.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krajnović M, Marković B, Knežević-Ušaj S, et al. Locally advanced rectal cancers with simultaneous occurrence of KRAS mutation and high VEGF expression show invasive characteristics. Pathol Res Pract. 2016;212:598–603. [DOI] [PubMed] [Google Scholar]
- 14.Zlobec I, Vuong T, Compton CC, et al. Combined analysis of VEGF and EGFR predicts complete tumour response in rectal cancer treated with preoperative radiotherapy. Br J Cancer. 2008;98:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. [DOI] [PubMed] [Google Scholar]
- 16.Ter Weele EJ, Terwisscha van Scheltinga AGT, Linssen MD, et al. Development, preclinical safety, formulation, and stability of clinical grade bevacizumab-800CW, a new near infrared fluorescent imaging agent for first in human use. Eur J Pharm Biopharm. 2016;104:226–234. [DOI] [PubMed] [Google Scholar]
- 17.Dobosz M, Ntziachristos V, Scheuer W, Strobel S. Multispectral fluorescence ultramicroscopy: three-dimensional visualization and automatic quantification of tumor morphology, drug penetration, and antiangiogenic treatment response. Neoplasia. 2014;16:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boogerd LSF, Hoogstins CES, Schaap DP, et al. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. Lancet Gastroenterol Hepatol. 2018;3:181–191. [DOI] [PubMed] [Google Scholar]
- 19.Harlaar NJ, Koller M, de Jongh SJ, et al. Molecular fluorescence-guided surgery of peritoneal carcinomatosis of colorectal origin: a single-center feasibility study. Lancet Gastroenterol Hepatol. 2016;1:283–290. [DOI] [PubMed] [Google Scholar]
- 20.Tummers WS, Warram JM, van den Berg NS, et al. Recommendations for reporting on emerging optical imaging agents to promote clinical approval. Theranostics. 2018;8:5336–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alberda WJ, Verhoef C, Nuyttens JJ, et al. Intraoperative radiation therapy reduces local recurrence rates in patients with microscopically involved circumferential resection margins after resection of locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2014;88:1032–1040. [DOI] [PubMed] [Google Scholar]
- 22.Hyngstrom JR, Tzeng C-WD, Beddar S, et al. Intraoperative radiation therapy for locally advanced primary and recurrent colorectal cancer: ten-year institutional experience. J Surg Oncol. 2014;109:652–658. [DOI] [PubMed] [Google Scholar]
- 23.Vermaas M, Ferenschild FTJ, Verhoef C, et al. Total pelvic exenteration for primary locally advanced and locally recurrent rectal cancer. Eur J Surg Oncol. 2007;33:452–458. [DOI] [PubMed] [Google Scholar]
- 24.Trakarnsanga A, Gonen M, Shia J, et al. What is the significance of the circumferential margin in locally advanced rectal cancer after neoadjuvant chemoradiotherapy? Ann Surg Oncol. 2013;20:1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmans E, Tjalma JJJ, Linssen MD, et al. Potential red-flag identification of colorectal adenomas with wide-field fluorescence molecular endoscopy. Theranostics. 2018;8:1458–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koller M, Qiu S-Q, Linssen MD, et al. Implementation and benchmarking of a novel analytical framework to clinically evaluate tumor-specific fluorescent tracers. Nat Commun. 2018;9:3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.