Abstract
Parallel to the application of new PET radiopharmaceuticals for pheochromocytoma and paraganglioma (collectively named PPGLs) imaging, several studies have increased our understanding on their biology, genetics, metabolomics, and embryologic origin. In this review, we highlight the current relationship between genotypes and molecular imaging phenotypes. Additionally, we summarize the referral guidelines for imaging of PPGL patients with or without knowledge of their genetic background.
Keywords: Key Terms: pheochromocytoma, paragangliomas, PET/CT, radiopharmaceuticals, genetics, precision medicine
NOTEWORTHY
PPGLs are caused by inherited genetic mutations in more than 40% of cases.
Nuclear imaging phenotype is driven mainly by genotype.
The choice of the optimal radiopharmaceutical can be guided by tumor location (tightly linked to embryologic origin) and genetic background.
The revised 2019 European Association of Nuclear Medicine practice guideline/Society of Nuclear Medicine and Molecular Imaging procedure standard provides up-to-date information for nuclear physicians encountering patients with PPGLs.
Nuclear (molecular) imaging allows visualization of various pathophysiologic processes on a whole-body scale with subsequent detailed characterization of a specific area of interest. As a general concept, the main goal of proliferating cells is to take up and transform available nutrients into biomass powered by the energy generation needed to produce new cells. One of the best-known cancer phenotypes is the avid uptake of glucose via the (often upregulated) glucose transporter family. This feature has been attributed by Otto H. Warburg (Nobel prize received in 1931) to switch from cellular respiration to aerobic glycolysis, which, despite less efficacy for generating adenosine triphosphate, facilitates rapid cell division and new-cell maintenance (1). The nutrient demand is determined by several cell-intrinsic factors (e.g., the presence of specific tumor-promoting mutations, chromosomal abnormalities, phenotypic states, and the tumor tissue of origin), as well as extracellular factors (e.g., the cell microenvironment). A prime example of the relationship between genetics and imaging phenotype is given by pheochromocytomas and paragangliomas (collectively named PPGLs).
PPGLs arise either from the adrenal medulla, which is a main hormonal component of the autonomic nervous system, or from neurosecretory tissue called paraganglia, which is involved in various sensory reflex loops. PPGLs are related to genetic driver events in 70%–80% of cases, considering germline and somatic mutations together (in more than 20 genes with mutually exclusive events). The major genetic events involve hypoxia response genes (cluster 1) or kinase signaling and protein translation genes (cluster 2) (2,3).
Most PPGLs exhibit, even at the metastatic stage, a low growth pattern. Therefore, their nutrient demand is most likely oriented toward biosynthesis, storage, and secretion of catecholamines rather than rapid cell division. This is nicely illustrated on PET imaging by the highly elevated avidity of PPGLs for 6-18F-fluoro-l-dopa (18F-FDOPA), allowing catecholamine building blocks (amino acids) to enter a PPGL cell with a low-to-moderate 18F-FDG uptake to maintain slightly elevated but not accelerated cell growth and proliferation. Cluster 2 PPGLs (RET, NF1, MAX, TMEM127), which are almost always confined to the adrenal medulla and are well differentiated, usually follow this rule. In contrast, under certain circumstances referred to as pseudohypoxic states, imaging phenotypes can profoundly differ. Since the seminal discoveries by the 2019 Nobel laureates (William G. Kaelin, Jr., Peter J. Ratcliffe, and Gregg L. Semenza), it has been established that in the presence of oxygen, VHL targets hypoxia-inducible factor (HIF)-α for its subsequent proteasomal degradation. However, under specific circumstances, particularly related to PPGLs, decreased HIF-α degradation, causing enhanced stabilization, can be observed despite normoxia (so-called pseudohypoxia, when normally available oxygen cannot be properly used). Thus, HIF stabilization, viewed as its prolonged activation, contributes to an increase in cellular dopamine and norepinephrine content. This characteristic has been attributed to HIF-2α–mediated activation of tyrosine hydroxylase, the rate-limiting enzyme, and inhibition of phenylethanolamine-N-methyl-transferase, which converts norepinephrine to epinephrine (4). Altered gene expression, proliferation rate, and other cellular characteristics, as well as the presence of dopaminergic or noradrenergic phenotypes of cluster 1 compared with cluster 2 PPGLs, especially for VHL or EPAS1/HIF-2α mutations, is reflected by very avid 18F-FDOPA uptake, as well as high 18F-FDG uptake due to parallel activation of the glucose transporter family and glycolytic enzymes (5).
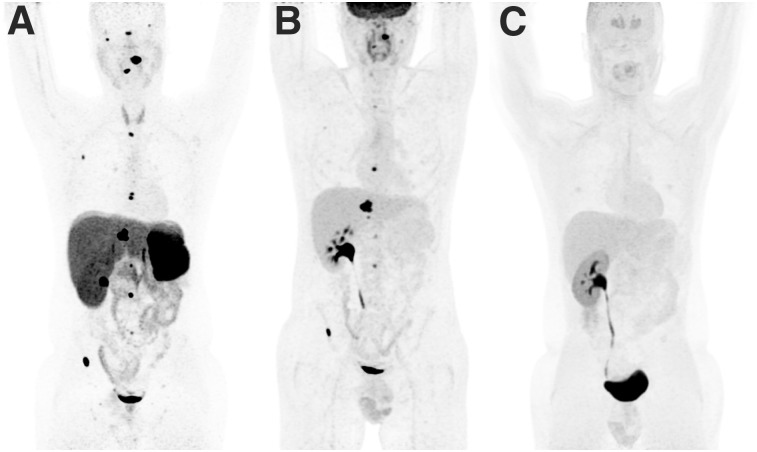
However, the situation becomes more complex for PPGLs linked to mutations in one of the genes encoding for the succinate dehydrogenase (SDH) enzyme complex (A–D, collectively named SDHx). SDH is at the crossroads for the tricarboxylic acid cycle, where it catalyzes the oxidation of succinate to fumarate, and for the respiratory electron transfer chain (complex II), where it functions mainly as an electron transporter. Although truncation of the succinate dehydrogenase complex is currently viewed as the cause of PPGLs and other tumors, clinical phenotypes can differ across various SDHx-related mutations and other unknown variables. These phenotypic differences are currently unexplained and conceptually challenging. SDH deficiency results in a partial tricarboxylic acid blockade, with accumulation of enormous concentrations of succinate that can be detected by in vitro and in vivo metabolomic studies. With regard to 18F-FDG uptake, these tumors share a marked 18F-FDG avidity (6). For many years, this profile has been attributed to a succinate-driven pseudohypoxic state due to its inhibitor effect on prolyl hydroxylases with subsequent stabilization of HIF-α, followed by upregulation of glucose transporters. Although there are conflicting results with certain molecular genetic studies that have failed to detect a glycolytic signature, this hypothesis remains appealing since there are some other mechanistic increases in enzymatic activity (e.g., for hexokinase) to be associated with this pathogenic mechanism. An alternative hypothesis relies on the extracellular effects of succinate on stroma cells. Fluxomic studies performed on mutated yeast have shown that succinate can efflux out of cells through specific mitochondria and plasma membrane transporters. Such an aberrant retrograde pathway is expected to prevent the potential detrimental effects of high levels of succinate on cytosolic metabolic processes. Interestingly, intratumoral injection of succinate in human xenografts, unlike fumarate, induces 18F-FDG uptake whereas succinate has no effect on tumor metabolism in vitro. By contrast, succinate induces 18F-FDG uptake by endothelial cells in vitro and can also induce muscular uptake after direct intramuscular injection in mice (7). These data suggest that together with the intracellular effects of succinate on HIF-α stabilization, succinate may also have extracellular effects on peritumoral stroma cells that contribute to the 18F-FDG phenotype in these tumors. SDH-related PPGLs also overexpress somatostatin receptors and are therefore targetable with somatostatin analogs labeled with diagnostic radionuclides (e.g., 68Ga-somatostatin analog). Regarding 18F-FDOPA, the phenotype depends largely on the tissue of origin, with positivity for head and neck paragangliomas (HNPGL, almost always) and less sensitivity in PPGLs of sympathetic origin (Fig. 1) (8). This phenomenon is currently unexplained and, in our opinion, needs further research in genetics and embryology. Until recently, paraganglia were thought to originate from freely sympathoadrenal neural crest migratory cells. However, several new sophisticated studies have shown that adrenal medulla and extraadrenal paraganglia are derived mainly from multifated Schwann cell precursors, pointing to the fact that Schwann cell precursors can be one of the initiating tumorigenic cell types (9). The acquisition of a mature catecholaminergic phenotype requires a subsequent conversion from a glia-to-chromaffin gene expression process. Interestingly, Schwann cell precursors can stay in their niche along the preganglionic nerves. Thus, the timing of development, tumor-initiating cells, and Schwann cell precursor-to-chromaffin fate may differ across locations and genotypes. In the future, beyond a better understanding of genotype-related imaging phenotypes, new PPGL classification (e.g., related to their specific developmental characteristics and microenvironment) may allow more selective and effective treatment.
FIGURE 1.
Typical imaging phenotype in patient with SDHB-related metastatic retroperitoneal paraganglioma (PGL): 68Ga-DOTATATE (A), 18F-FDG (B), and 18F-FDOPA (C). There was a previous history of surgery for large retroperitoneal PGL. 68Ga-DOTATATE identified more bone metastases than 18F-FDG, whereas 18F-FDOPA was negative.
This editorial discusses how genotype can be considered a critical determinant of imaging phenotype in PPGLs at the current time. A question often raised is whether we should wait for genetic screening before imaging PPGL patients. In an ideal situation, one would expect that genotype could guide the choice of an optimal radiopharmaceutical. However, the genetic testing process can take time. The revised European Association of Nuclear Medicine/Society of Nuclear Medicine and Molecular Imaging joint recommendations provide a personalized approach and are applicable to PPGLs with or without knowledge of the genetic background (Table 1) (10). Nuclear physicians should be aware of PPGL imaging phenotypes (location, multifocality, uptake pattern) since these may also suggest which mutation could be involved in the disease, further offering a specific imaging algorithm during follow-up. Finally, specific genotype imaging–related phenotypes may accelerate our thinking on which radiotherapeutic strategy can be used in some patients with metastatic PPGLs.
TABLE 1.
Proposed Clinical Algorithm for Nuclear Imaging Investigations in Cases of PPGL (10)
| Condition | First choice | Second choice |
| Pheochromocytoma (apparently sporadic) | 18F-FDOPA or 123I-MIBG | 68Ga-SSA |
| Inherited pheochromocytoma (except SDHx): NF1/RET/VHL/MAX | 18F-FDOPA | 123I-MIBG or 68Ga-SSA |
| HNPGL | 68Ga-SSA | 18F-FDOPA |
| Extraadrenal sympathetic or multifocality or metastatic disease or SDHx mutation | 68Ga-SSA | 18F-FDG and 18F-FDOPA |
MIBG = metaiodobenzylguanidine; SSA = somatostatin analog.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crona J, Taieb D, Pacak K. New perspectives on pheochromocytoma and paraganglioma: toward a molecular classification. Endocr Rev. 2017;38:489–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fishbein L, Leshchiner I, Walter V, et al. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechmann N, Poser I, Seifert V, et al. Impact of extrinsic and intrinsic hypoxia on catecholamine biosynthesis in absence or presence of Hif2α in pheochromocytoma cells. Cancers (Basel). 2019;11:E594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favier J, Briere JJ, Burnichon N, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4:e7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taïeb D, Timmers HJ, Shulkin BL, Pacak K. Renaissance of 18F-FDG positron emission tomography in the imaging of pheochromocytoma/paraganglioma. J Clin Endocrinol Metab. 2014;99:2337–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrigue P, Bodin-Hullin A, Balasse L, et al. The evolving role of succinate in tumor metabolism: an 18F-FDG-based study. J Nucl Med. 2017;58:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taïeb D, Pacak K. New insights into the nuclear imaging phenotypes of cluster 1 pheochromocytoma and paraganglioma. Trends Endocrinol Metab. 2017;28:807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furlan A, Dyachuk V, Kastriti ME, et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science. 2017;357:eaal3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taïeb D, Hicks RJ, Hindie E, et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2019;46:2112–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]