Abstract
For effective clinical management of patients being treated with 223Ra, there is a need for radiographic response biomarkers to minimize disease progression and to stratify patients for subsequent treatment options. The objective of this study was to evaluate an automated bone scan index (aBSI) as a quantitative assessment of bone scans for radiographic response in patients with metastatic castration-resistant prostate cancer (mCRPC). Methods: In a multicenter retrospective study, bone scans from patients with mCRPC treated with monthly injections of 223Ra were collected from 7 hospitals in Sweden. Patients with available bone scans before treatment with 223Ra and at treatment discontinuation were eligible for the study. The aBSI was generated at baseline and at treatment discontinuation. The Spearman rank correlation was used to correlate aBSI with the baseline covariates: alkaline phosphatase (ALP) and prostate-specific antigen (PSA). The Cox proportional-hazards model and Kaplan–Meier curve were used to evaluate the association of covariates at baseline and their change at treatment discontinuation with overall survival (OS). The concordance index (C-index) was used to evaluate the discriminating strength of covariates in predicting OS. Results: Bone scan images at baseline were available from 156 patients, and 67 patients had both a baseline and a treatment discontinuation bone scan (median, 5 doses; interquartile range, 3–6 doses). Baseline aBSI (median, 4.5; interquartile range, 2.4–6.5) was moderately correlated with ALP (r = 0.60, P < 0.0001) and with PSA (r = 0.38, P = 0.003). Among baseline covariates, aBSI (P = 0.01) and ALP (P = 0.001) were significantly associated with OS, whereas PSA values were not (P = 0.059). After treatment discontinuation, 36% (24/67), 80% (54/67), and 13% (9/67) of patients demonstrated a decline in aBSI, ALP, and PSA, respectively. As a continuous variable, the relative change in aBSI after treatment, compared with baseline, was significantly associated with OS (P < 0.0001), with a C-index of 0.67. Median OS in patients with both aBSI and ALP decline (median, 134 wk) was significantly longer than in patients with ALP decline only (median, 77 wk; P = 0.029). Conclusion: Both aBSI at baseline and its change at treatment discontinuation were significant parameters associated with OS. The study warrants prospective validation of aBSI as a quantitative imaging response biomarker to predict OS in patients with mCRPC treated with 223Ra.
Keywords: automated bone scan index (aBSI), imaging biomarker, bone scan, 223Ra, mCRPC
Adenocarcinoma of the prostate is one of the most common malignancies among men, with an incidence of 450,000 cases per year (1). Metastases to bone are frequent, particularly to well-vascularized areas such as the vertebral column, skull, ribs, and proximal ends of the long bones. Approximately 80% of patients with metastatic castration-resistant prostate cancer (mCRPC) develop skeletal metastases (2), which frequently are symptomatic and cause bone pain, bone marrow suppression, leukopenia, pathologic fractures, and hypercalcemia. There are no direct means or methods available by which skeletal metastases can be objectively quantified and visualized. However, whole-body bone scintigraphy is a ubiquitous and vital tool for investigation of skeletal engagement of the disease. Such conventional bone scans can be used to visualize metastasis by increased uptake of 99mTc-labeled bisphosphonates, which are injected into the bloodstream and detected using a γ-camera.
An automated bone scan index (aBSI) was developed as a way to quantify the extent of skeletal tumor burden in bone scans as the percentage of total skeletal weight (3). It is therefore a valuable metric and a potentially helpful tool for estimating the total quantitative skeletal metastatic burden in patients with mCRPC. With rapid processing time, the automated methodology using artificial intelligence has been demonstrated to be accurate and reproducible (4,5). In a recent study, aBSI was clinically validated as a prognostic biomarker in a prospective multiinstitutional phase III study with 721 mCRPC patients (6).
223Ra-dichloride is an α-particle emitter that selectively targets bone metastases and induces double-stranded DNA breaks resulting in a localized cytotoxic effect in the target area. α-emitters produce radiation with a high-linear-energy transfer with a shorter range than β-emitters (7). For mCRPC patients with bone metastases and little or no soft-tissue involvement, 223Ra has been shown to be a significant life-prolonging (3.6 mo) therapeutic option with a patient-friendly side effect profile (8).
However, despite the mechanism of 223Ra targeting the disease in the bone, there has been little work to evaluate the radiologic response in patients being treated with 223Ra. One of the primary reasons is the limitation of bone scanning and sodium fluoride as an imaging modality that measures bone metabolism; the paradoxic increase in uptake could be a result of bone healing in response to an effective treatment. However, a delayed assessment of the bone scan after initiating 223Ra could be indicative of an efficacious response by patients and enable better stratification of patients for subsequent therapies. Several prior studies have evaluated aBSI as a prognostic imaging biomarker for 223Ra (9–12). The aim of this study was to evaluate aBSI as an imaging response biomarker for mCRPC patients treated with 223Ra.
MATERIALS AND METHODS
Study Design and Patient Population
Retrospectively, bone scans available in raw DICOM format, from patients with mCRPC treated with monthly injections of 223Ra, were collected from 7 hospitals in Sweden: Skåne University Hospital, Gothenburg University Hospital, Central Hospital Karlstad, Sundsvall-Härnösand County Hospital, Skövde Hospital, Umeå University Hospital, and Karolinska University Hospital. All patients with baseline bone scan images in raw DICOM format and baseline values of alkaline phosphatase (ALP) and prostate-specific antigen (PSA) were eligible for the study. Treatment follow-up bone scans and PSA and ALP values within 3 mo of the last injection of 223Ra were also obtained when available. Ethical permission for the multiinstitutional retrospective analysis on patients being clinically treated with 223Ra was obtained from Skåne University Hospital.
aBSI
Version 3.2 of the aBSI software program (EXINI BONE, developed by EXINI Diagnostic AB, Sweden) was used to analyze the retrospectively collected bone scans and to generate aBSIs. The methodology of the automated platform has been described in detail in a previous publication (3). In summary, the different anatomic regions of the skeleton are segmented, followed by detection and classification of the abnormal hot spots as metastatic lesions. The weight fraction of the skeleton for each metastatic hot spot is calculated, and the aBSI is calculated as the sum of all such fractions.
Statistical Analysis
As a hypothesis-generating retrospective study, no prior assumptions were made for the clinical utility of aBSI to render power calculations. To evaluate the aBSI as a distinct and independent variable, we used the nonparametric Spearman rank correlation coefficient to determine the association between aBSI and blood-based biomarkers. Univariate and multivariate Cox regression analysis was used to evaluate the association of aBSI, PSA level, and ALP level at baseline with overall survival (OS). Additionally, Cox regression analysis was used to evaluate the aBSI and ALP response, as a continuous variable, with OS. Kaplan–Meier analysis was performed to evaluate the difference in median survival time with regard to ALP level and aBSI response. The concordance index (C-index) was used to evaluate the discriminatory strength of the covariates, as continuous variables, in predicting OS. A strong concordance would indicate that the covariate is highly informative in predicting the relative risk of death between any 2 patients at a given time. χ2 testing was performed to evaluate the relationship between the decline in aBSI or ALP and the number of 223Ra injections. Statistical significance for each test was set at 0.05 (2-tailed). All statistical analyses were performed using R software, version 3.1.2.
RESULTS
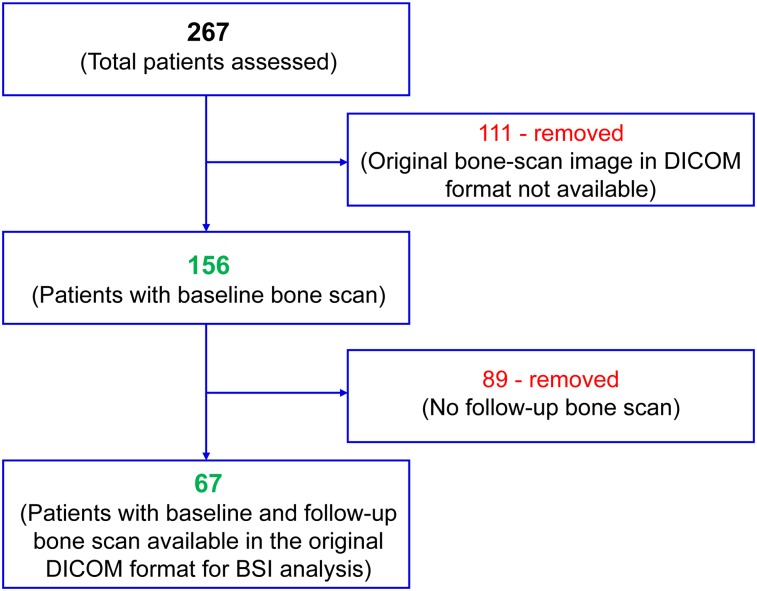
Of the 267 mCRPC patients screened in total, 156 at baseline and 67 at treatment follow-up (median, 5 injections; range, 1–6 injections) were deemed evaluable for the retrospective analysis. The Figure 1 flowchart illustrates the total number of patients at baseline and the final evaluable patients for posttreatment analysis. The demographics, prior treatment history, and baseline characteristics of the evaluable mCRPC patients are outlined in Table 1.
FIGURE 1.
Flowchart illustrating evaluable patients.
TABLE 1.
Patient Characteristics
| Characteristic | Evaluable patients for baseline assessment (n = 156) | Evaluable patients for response assessment (n = 67) |
| Median baseline age (y) | 68 (range, 62–77) | 70 (range, 64–77) |
| Median baseline PSA (ng/mL) | 189 (range, 0.67–6,359) | 159 (range, 0.67–1,425) |
| Median baseline ALP (U/L) | 2.9 (range, 0.19–28.6) | 2.9 (range, 0.19–20.3) |
| Median baseline aBSI | 4.5 (range, 0.01–22.4) | 4.0 (range, 0.01–19.7) |
| Metastasis to bone (n) | 156 (100%) | 91 (100%) |
| Deaths (n) | 104 (56%) | 39 (58%) |
| 223Ra doses (n) | 5 (1–6) | 5 (1–6) |
Baseline aBSI (median, 4.5; interquartile range, 2.4–6.5) was moderately correlated with baseline ALP (r = 0.60, P < 0.0001) and with baseline PSA (r = 0.38, P = 0.003). The univariate and multivariate Cox regression analysis is detailed in Table 2. On univariate analysis, both baseline aBSI and baseline ALP significantly correlated with OS, whereas PSA at baseline did not. In the bivariate model, consisting of baseline values of ALP and aBSI, the aBSI was not significantly associated with OS. The discriminatory strength of ALP (C-index, 0.67) in predicting OS was significantly higher than that of aBSI (C-index, 0.60; P = 0.03).
TABLE 2.
Univariate and Bivariate Cox Regression Analysis of aBSI and Levels of PSA and ALP as Continuous Variable at Baseline
| Baseline (log10) for… | Hazard ratio | 95% confidence interval | P |
| Univariate | |||
| BSI | 2.46 | 1.22–4.94 | 0.01 |
| PSA | 1.33 | 0.89–2.01 | 0.16 |
| ALP | 6.28 | 2.77–14.23 | <0.001 |
| Bivariate | |||
| BSI | 1.23 | 0.61–2.49 | 0.55 |
| ALP | 5.29 | 1.97–14.17 | <0.001 |
n = 156.
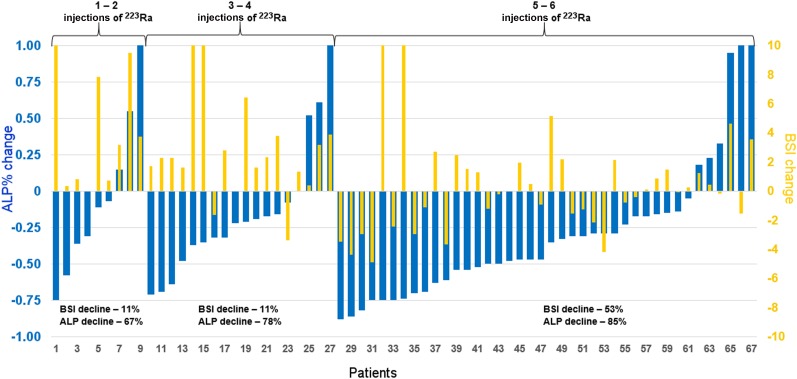
Table 3 demonstrates the change in ALP, PSA, and aBSI with respect to the number of 223Ra injections. After the last dose of 223Ra, 13% (9/67) of the patients demonstrated a decline in PSA and 80% (54/67) of the patients demonstrated a decline in ALP. Of the 54 patients with an ALP decline, 38 (57%) registered an ALP response with a decline of 25% of more. The median time of baseline bone scanning and the first cycle of 223Ra was 6 wk, compared with the 4-wk duration between the last treatment with 223Ra and follow-up bone scanning. In total, 24 of the 67 patients (37%) demonstrated an aBSI decline, and 21 of these 24 patients (88%) with aBSI decline had received 5 or more cycles of 223Ra treatment. The aBSI declines were more frequent in patients with 5 or more injections of 223Ra than in patients with less than 5 injections of 223Ra (P = 0.0006); no such significance was observed for changes in ALP (P = 0.8513). An example of aBSI decline with resolving lesions on a patient’s bone scan is demonstrated in Figure 2. Figure 3 demonstrates the percentage ALP change and aBSI change from baseline by number of doses of 223Ra. The subset of patients having received 5–6 doses of 223Ra demonstrated the largest relative decline in PSA (20%) and ALP (85%), as well as aBSI (53%).
TABLE 3.
Decline of PSA, ALP, and aBSI by Number of 223Ra Injections
| Doses of 223Ra | PSA decline | ALP decline | aBSI decline |
| 1–2 (n = 9) | 0/9 (0%) | 6/9 (67%) | 1/9 (11%) |
| 3–4 (n = 18) | 1/18 (5%) | 14/18 (78%) | 2/18 (11%) |
| 5–6 (n = 40) | 8/40 (20%) | 34/40 (85%) | 21/40 (53%) |
| Total | 9/67 (13%) | 54/67 (80%) | 24/67 (36%) |
n = 67.
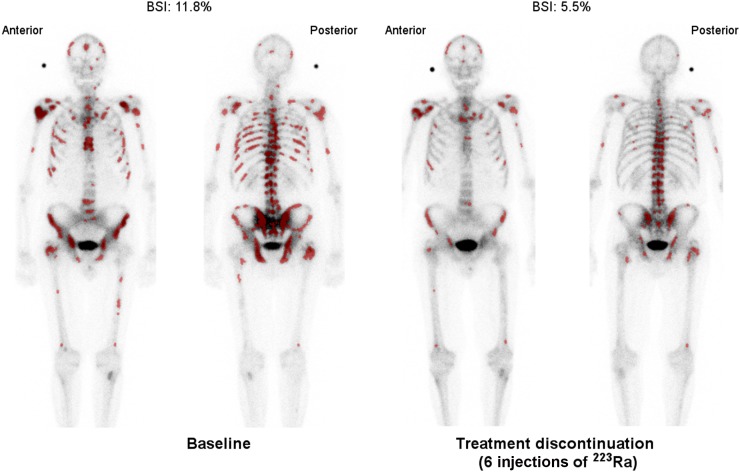
FIGURE 2.
Example of declining aBSI value after treatment with 223Ra. Artificial neural network has preselected hot spots (shown in dark red) used in calculation of aBSI.
FIGURE 3.
Waterfall plot of ALP (blue) and aBSI (yellow) change in each patient by number of 223Ra injections.
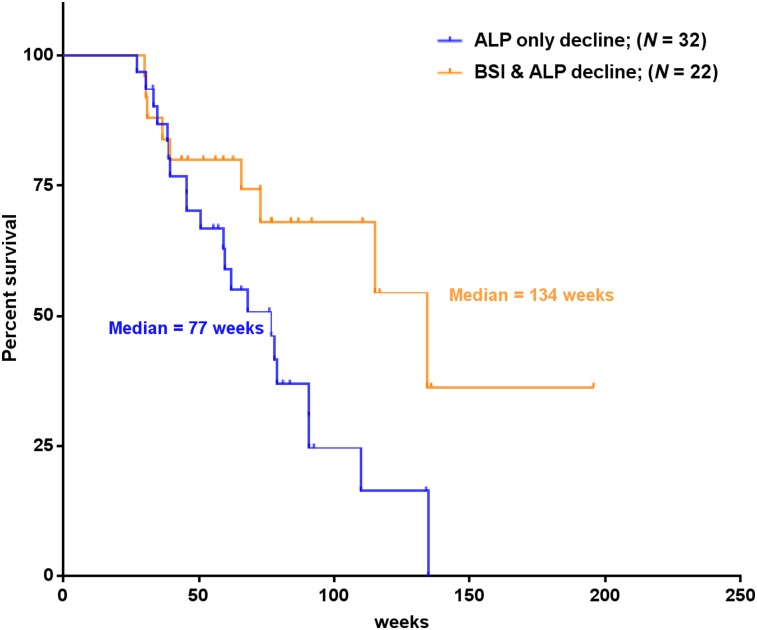
The Kaplan–Meier curve demonstrated that the median OS of patients with both aBSI decline and ALP decline (median OS, 134 wk) was significantly longer than that of patients with ALP decline only (median OS, 77 wk; P = 0.023), as illustrated in Figure 4. As a continuous variable, aBSI was the only covariate whose posttreatment change was significantly associated with OS on Cox regression analysis (Table 4). The discriminatory strength of a change in aBSI after 223Ra treatment (C-index, 0.66) in predicting OS was significantly higher than that of a change in ALP (C-index, 0.47; P < 0.001).
FIGURE 4.
Kaplan–Meier curves demonstrating median OS of patients with 223Ra treatment–induced decline in levels of ALP (n = 32), in comparison to patients with treatment-induced decline in both aBSI and ALP levels (n = 22).
TABLE 4.
Cox Regression Analysis of On-Treatment Change in aBSI, PSA, and ALP as Continuous Variables
| Percentage change in… | HR | 95%CI | P |
| BSI | 1.14 | 1.07–1.21 | <0.0001 |
| ALP | 0.95 | 0.71–1.27 | 0.713 |
| PSA | 1.05 | 0.95–1.18 | 0.329 |
n = 67.
DISCUSSION
The aim of this study was to investigate whether 223Ra treatment–induced changes in aBSI can be used as a response biomarker to predict OS in mCRPC patients. The lack of a response biomarker is a significant challenge in routine clinical management of patients being treated with 223Ra (13). These studies have also demonstrated a discrepancy in response regarding PSA and ALP levels during treatment with 223Ra. In daily clinical practice, there is no validated imaging biomarker for assessment of treatment response. aBSI has been shown to be a prognostic biomarker by several studies (9–12). As a fully quantitative method of assessing bone scans, aBSI may have the potential to be a radiographic imaging biomarker to assess the effectiveness of the responding metastatic lesions. An advantage of aBSI is that it is based on bone scan examinations, which are still the most common method to detect metastatic spread to the bone in patients with prostate cancer.
In this study, our baseline data confirmed the findings of others who have demonstrated the prognostic value of aBSI and ALP for OS in patients treated with 223Ra (9,10). On bivariate analysis, the baseline levels of ALP had a stronger association with OS than did aBSI value at baseline, with a greater discriminatory power for OS than that of aBSI. However, the posttreatment-change analysis demonstrated that change in aBSI was strongly associated with OS. These findings indicate that the treatment-induced changes in aBSI may serve as marker for response to treatment with 223Ra. The Kaplan–Meier analysis demonstrated an additive value for aBSI response, compared with ALP-only response, to 223Ra.
Our data also indicate a trend toward an association between number of doses of 223Ra and change in aBSI, because patients who received 5 or 6 doses of 223Ra had a significantly greater decrease in aBSI than did patients who received fewer doses (P = 0.0006). This finding supports previous reports that patients who received a higher number of 223Ra injections had a better outcome (14), including a better prognosis of survival, and disease progression. Additionally, these data also confirm that the aBSI assessment does not absolve the inherent limitations of bone scanning as a non–tumor-specific imaging modality. The uptake of 99mTc-methylene diphosphonate and 99mTc-hydroxydiphosphonate in hydroxyapatite in bone scans reflects the increase in the osteoblastic activity of bone. As a result, in response to the first few treatments of 223Ra, which also binds to hydroxyapatite, the bone scans may show a temporary increase in activity. This increase after treatment with 223Ra has been documented in a prior study (15). A clinically relevant change in aBSI may therefore be delayed, as observed in this study. As a quantitative parameter, the aBSI could capture this delayed response on bone scans and accurately stratify patients for subsequent treatment options.
Limitations are inherent in the scope and scale of our study’s retrospective design. The study also lacked control over the follow-up time for the end-of-treatment bone scan analysis. The aBSI response data are the first of their kind and need prospective validation. The discrepancy between our data and those of the ALSYMPCA study (16), regarding the association between OS and ALP change, could be attributed to the lower number of patients in our study. The ALP increase in our study was observed in 19.4% (13/67) of the 223Ra-treated population. The data were similar to those observed in the ALSYMPCA study, in which 20% (97/497) of patients treated with 223Ra demonstrated an ALP increase.
CONCLUSION
For an accurate and comprehensive understanding of disease status, a quantitative and reproducible radiographic assessment such as aBSI can potentially add clinical utility to existing CT evaluations of soft-tissue metastases and to the blood-based biomarkers. In this study, we demonstrated that aBSI at baseline and its change after treatment were significantly associated with OS and additive to the current predictor of response in mCRPC patients treated with 223Ra. Prospective studies are warranted to validate aBSI as a quantitative imaging biomarker of response to 223Ra.
DISCLOSURE
This study was funded by an unrestricted grant from Bayer and by grants from the Swedish Cancer Society and by government funding for clinical research within the National Health Service, Sweden, through Lund University (medical faculty ALF grants). Outside the submitted work, Lars Edenbrandt and Aseem Anand are employees of EXINI Diagnostics AB and Anders Bjartell is a consultant to EXINI Diagnostics AB. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is decline in aBSI indicative of response to 223Ra?
PERTINENT FINDINGS: In a retrospective analysis of 67 mCRPC patients treated with 223Ra, the decline in aBSI was predictive of OS and was additive to the ALP response.
IMPLICATIONS FOR PATIENT CARE: As a quantitative imaging parameter, aBSI can capture the radiographic response to 223Ra and stratify patients for more accurate management using treatment options subsequently available.
REFERENCES
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. [DOI] [PubMed] [Google Scholar]
- 2.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. [DOI] [PubMed] [Google Scholar]
- 3.Ulmert D, Kaboteh R, Fox JJ, et al. A novel automated platform for quantifying the extent of skeletal tumour involvement in prostate cancer patients using the bone scan index. Eur Urol. 2012;62:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand A, Morris MJ, Kaboteh R, et al. Analytic validation of the automated bone scan index as an imaging biomarker to standardize quantitative changes in bone scans of patients with metastatic prostate cancer. J Nucl Med. 2016;57:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand A, Morris MJ, Kaboteh R, et al. A preanalytic validation study of automated bone scan index: effect on accuracy and reproducibility due to the procedural variabilities in bone scan image acquisition. J Nucl Med. 2016;57:1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong AJ, Anand A, Edenbrandt L, et al. Phase 3 assessment of the automated bone scan index as a prognostic imaging biomarker of overall survival in men with metastatic castration-resistant prostate cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henriksen G, Breistol K, Bruland OS, Fodstad O, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting 223Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002;62:3120–3125. [PubMed] [Google Scholar]
- 8.Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15:1397–1406. [DOI] [PubMed] [Google Scholar]
- 9.Alva A, Nordquist L, Daignault S, et al. Clinical correlates of benefit from radium-223 therapy in metastatic castration resistant prostate cancer. Prostate. 2017;77:479–488. [DOI] [PubMed] [Google Scholar]
- 10.Fosbøl MØ, Petersen PM, Kjaer A, Mortensen J. 223Ra therapy of advanced metastatic castration-resistant prostate cancer: quantitative assessment of skeletal tumor burden for prognostication of clinical outcome and hematologic toxicity. J Nucl Med. 2018;59:596–602. [DOI] [PubMed] [Google Scholar]
- 11.McNamara MA, Oyekunle T, Chin BB, et al. Patterns of response and progression in bone and soft tissue during and after treatment with radium-223 for metastatic castrate-resistant prostate cancer. Prostate. 2019;79:1106–1116. [DOI] [PubMed] [Google Scholar]
- 12.Roque V, Jessop M, Pereira L, et al. Bone scan index as metastatic bone disease quantifier and predictor of radium-223-dichloride biochemical response. Nucl Med Commun. 2019;40:588–596. [DOI] [PubMed] [Google Scholar]
- 13.Heidegger I, Pichler R, Heidenreich A, Horninger W, Pircher A. Radium-223 for metastatic castration-resistant prostate cancer: results and remaining open issues after the ALSYMPCA trial. Transl Androl Urol. 2018;7(suppl):S132–S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etchebehere EC, Araujo JC, Milton DR, et al. Skeletal tumor burden on baseline 18F-fluoride PET/CT predicts bone marrow failure after 223Ra therapy. Clin Nucl Med. 2016;41:268–273. [DOI] [PubMed] [Google Scholar]
- 15.Isensee G, Peporte A, Muller J, Schmid S, Gillessen S, Omlin A. Is there a flare phenomenon on bone scintigraphy in men with advanced prostate cancer treated with radium-223? Clin Genitourin Cancer. 2018;16:349–354. [DOI] [PubMed] [Google Scholar]
- 16.Sartor O, Coleman RE, Nilsson S, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28:1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]