Abstract
18F-labeled prostate-specific membrane antigen (PSMA) PET tracers are increasingly used in preference to 68Ga-PSMA-11 for restaging biochemical recurrence (BCR) of prostate cancer. They are associated with longer half-lives, larger-scale production, and lower positron range than their 68Ga-labeled counterparts. Here, we describe the efficacy of an 18F-labeled radiohybrid PSMA, rhPSMA-7, a novel theranostic PSMA-targeting agent for imaging BCR of prostate cancer. Methods: Datasets from 261 consecutive patients with noncastrate BCR after radical prostatectomy who underwent 18F-rhPSMA-7 PET/CT at our institution between June 2017 and March 2018 were reviewed retrospectively. All lesions suspected of being recurrent prostate cancer were recorded. The detection rate for sites of presumed recurrence was correlated with patients’ prostate-specific antigen (PSA) level, primary Gleason score, and prior therapy (androgen deprivation therapy and external-beam radiation therapy). Results: The 261 patients had a median PSA level of 0.96 ng/mL (range, 0.01–400 ng/mL). The median injected activity of 18F-rhPSMA-7 was 336 MBq, with a median uptake time of 76 min. In total, 211 patients (81%) showed pathologic findings on 18F-rhPSMA-7 PET/CT. The detection rates were 71% (42/59), 86% (44/51), 86% (42/49), and 95% (76/80) at PSA levels of 0.2 to <0.5 ng/mL, 0.5 to <1 ng/mL, 1 to <2 ng/mL, and ≥2 ng/mL, respectively. In 32% patients (7/22) with a PSA of less than 0.2 ng/mL, suggestive lesions were present. 18F-rhPSMA-7 PET/CT revealed local recurrence in 43% of patients (113). Lymph node metastases were present in the pelvis in 42% of patients (110), in the retroperitoneum in 17% (45), and in a supradiaphragmatic location in 8.0% (21). Bone and visceral metastases were detected in 21% (54) and 3.8% (10), respectively. Detection efficacy was not influenced by prior external-beam radiation therapy (79.1% vs. 82.1%, P = 0.55), androgen deprivation therapy within the 6 mo preceding imaging (80.6% vs. 80.9%, P = 0.54), or primary Gleason score (77.9% for ≤7 vs. 82.6% for ≥8, P = 0.38). Conclusion: 18F-rhPSMA-7 PET/CT offers high detection rates in early BCR after radical prostatectomy, especially among patients with low PSA values.
Keywords: biochemical recurrence, hybrid imaging, PET, prostate cancer, prostate-specific membrane antigen
Prostate cancer relapse after curative-intent primary treatment remains a considerable clinical burden. Up to approximately one third of patients experience biochemical recurrence (BCR) of prostate cancer in the 10 y after initial treatment (1,2). The utility of standard imaging for the localization of recurrence is limited, especially in patients with low prostate-specific antigen (PSA) levels (3). In recent years, investigational prostate-specific membrane antigen (PSMA)–based radiotracers have demonstrated encouraging results in the early detection of prostate cancer recurrence. The expression of membrane-bound enzyme PSMA is significantly higher in prostate cancer cells than in healthy tissue (4), and the extracellular location of its catalytic site permits easy targeting with specific inhibitors that become internalized after ligand binding (5).
The PSMA-based radiotracer 68Ga-PSMA-11 compares favorably with an existing agent, choline, in terms of image contrast and detection rate (6). A recent large, retrospective study revealed 68Ga-PSMA-11 to detect recurrent prostate cancer with high specificity, and a metaanalysis of data from over 1,300 patients reported a pooled, subject-level 68Ga-PSMA detection rate of 76% for BCR of prostate cancer (7,8). Most recently, a prospective bicentric study confirmed the high sensitivity of 68Ga-PSMA-11 in patients with low PSA values and reported a high positive predictive value for 68Ga-PSMA-11–positive lesions (9). 18F-labeled PSMA radiotracers are also under clinical evaluation and offer several potential advantages such as a longer half-life, larger-batch production, and lower positron range than their 68Ga-labeled counterparts. 18F-DCFPyL is a second-generation small-molecule PSMA inhibitor that is currently under investigation in a phase III study (NCT03739684), and a further example, 18F-PSMA-1007, shows a favorable profile, with low bladder excretion (10).
Radiohybrid PSMA (rhPSMA) ligands are a new class of theranostic PSMA-targeting PET agents with several favorable features, including a fast process for radiolabeling with 18F and radiometals (11). The lead compound in this class, 18F-rhPSMA-7, has shown promising initial data for the detection and localization of recurrent prostate cancer, as well as rapid blood clearance (11). Given that accumulation of PET agents in the bladder and ureter can interfere with the diagnosis of recurrent disease and hamper assessment of primary disease (12–14), 18F-rhPSMA-7 has notably low bladder retention when imaging 1 h after injection (11).
Here, we present the results of a retrospective study investigating the efficacy of 18F-rhPSMA-7 for detection and localization of recurrent disease in a large, homogeneous series of noncastrate patients with BCR after radical prostatectomy.
MATERIALS AND METHODS
Patients
Data from patients with BCR of prostate cancer who underwent clinically indicated 18F-rhPSMA-7 PET/CT between June 2017 and March 2018 at our institution were reviewed retrospectively. Only patients who had undergone primary radical prostatectomy with curative intent or salvage radical prostatectomy after external-beam radiation therapy were included. Patients with documented castrate-resistant disease were excluded from the analysis. The patients’ serum PSA level at the time of the PET/CT was recorded along with details of prior therapy.
All patients gave written informed consent for the procedure. All reported investigations were conducted in accordance with the Helsinki Declaration and with national regulations. The retrospective analysis was approved by the local Ethics Committee (permit 290/18S). Administration of 18F-rhPSMA-7 complied with the German Medicinal Products Act, AMG §13 2b, and the responsible regulatory body (government of Oberbayern).
Synthesis and Administration of 18F-rhPSMA-7
18F-rhPSMA-7 was synthesized as described previously (11). A median activity of 336 MBq of 18F-rhPSMA-7 (mean, 333 ± 44 MBq; range, 191–417 MBq) was administered by intravenous bolus at a median of 76 min (mean, 82 ± 22 min; range, 50–220 min) before scanning. An uptake time of around 1 h (50–70 min) is recommended for future use, on the basis of an additional investigation of biodistribution at different time points (11).
Imaging Protocol
All patients underwent 18F-rhPSMA-7 PET/CT on a Biograph mCT flow scanner (Siemens Medical Solutions). A diagnostic CT scan was performed in the portal venous phase 80 s after intravenous injection of contrast agent (Iomeron 300), followed by the PET scan. All patients received diluted oral contrast medium (300 mg of ioxitalamate [Telebrix; Guerbet]). All PET scans were acquired in 3-dimensional mode with an acquisition time of 1.1 mm/s. Emission data were corrected for randoms, dead time, scatter, and attenuation and were reconstructed iteratively by an ordered-subsets expectation maximization algorithm (4 iterations, 8 subsets) followed by a postreconstruction smoothing gaussian filter (5 mm in full width at half maximum).
Image Analysis
Images were reviewed by an experienced, board-certified nuclear medicine physician and a board-certified radiologist. All lesions suspected of being recurrent prostate cancer were noted. Any focal tracer uptake higher than the surrounding background and not associated with physiologic uptake was considered suggestive of malignancy. Typical pitfalls in PSMA-ligand PET imaging were considered, such as low-to-moderate PSMA expression associated with osteoblastic changes (i.e., with fractures or degenerative changes) or the low uptake associated with celiac and other ganglia (15). All lesions suggestive of recurrent prostate cancer were noted and categorized as local recurrence (prostate bed), lymph node metastases (stratified further by location as pelvic, retroperitoneal, or supradiaphragmatic), bone metastases, or other metastases (e.g., lung or liver).
Statistical Analysis
The detection rate for sites of presumed recurrence was plotted against the baseline PSA value for both patient-level recurrence (number of patients with at least 1 positive finding) and regional level (local recurrence, lymph node metastases, bone metastases, and other metastases as detailed above). The patient-level detection rate was correlated with primary Gleason score and prior therapy (androgen deprivation therapy [ADT] and external-beam radiation therapy).
Two-sample t tests were used to evaluate differences between single groups (Gleason score, ADT), and Mann–Whitney U tests were used to evaluate differences in PSA values between groups with and without pathologic uptake. All tests were 2-sided and used a significance level of α = 5%. Statistical analyses were conducted with MedCalc software (version 13.2.0).
RESULTS
In total, 261 patients were included in this study. They had a median age of 72 y and a median prescan PSA level of 0.961 ng/mL; 67 (26%) had received ADT within the 6 mo preceding the scan (Table 1).
TABLE 1.
Patient Characteristics (n = 261)
| Characteristic | Data |
| Age at time of scan (y) | 72 (49–88) |
| Further treatment | |
| External radiation after RP | 105 (40) |
| Antihormonal treatment | 97 (3) |
| ADT in 6 mo preceding scan | 67 (26) |
| Gleason score | |
| ≤6 | 12 (4.6) |
| 7 | 110 (42) |
| ≥8 | 87 (33) |
| Unknown | 52 (20) |
| Pathologic primary tumor staging at RP | |
| pT2 | 76 (29) |
| pT3 | 138 (53) |
| pT4 | 6 (2.3) |
| Unknown | 41 (15) |
| Pathologic regional lymph node staging at RP | |
| pN0 | 146 (56) |
| pN1 | 66 (25) |
| pNx | 49 (19) |
| Positive margin at RP | |
| R0 | 94 (36) |
| R1 | 73 (28) |
| Unknown | 94 (36) |
| Initial PSA value (ng/mL) | 10.5 (0.09–290) |
| Time between surgery and PET (mo) | 56 (0–336) |
| Last PSA value before PET (ng/mL)* | 0.961 (0.01–400.0) |
| Injected activity (MBq) | 336 (191–417) |
| Uptake time (min) | 76 (50–220) |
PSA value obtained within 4 wk before 18F-rhPSMA-7 PET examination.
RP = radical prostatectomy.
Qualitative data are expressed as numbers followed by percentages in parentheses; continuous data are expressed as median followed by range in parentheses.
18F-rhPSMA-7 Detection Efficacy
Detection Rate
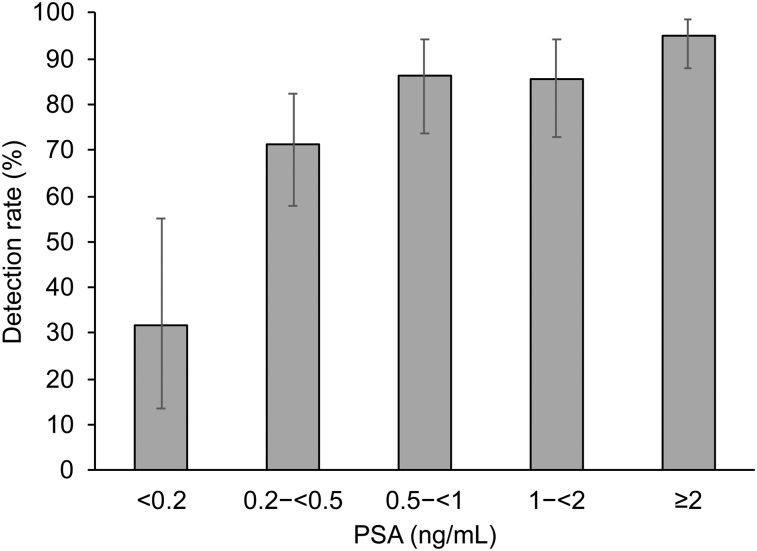
Of the 261 patients, 211 (81%) showed one or more localized areas suggestive of recurrent prostate cancer. The detection efficacy of 18F-rhPSMA-7 PET/CT correlated positively with PSA level and was 95% (76/80; 95% confidence interval [CI], 0.88–0.99) for a PSA value of ≥2 ng/mL, 86% (42/49; 95% CI, 0.73–0.95) for a PSA value of 1 to <2 ng/mL, 86% (44/51; 95% CI, 0.74–0.94) for a PSA value of 0.5 to <1 ng/mL, 71% (42/59; 95% CI, 0.58–0.82) for a PSA value of 0.2 to <0.5 ng/mL, and 32% (7/22; 95% CI, 13.7–54.9) for a PSA value of <0.2 ng/mL (Fig. 1). The mean PSA level was significantly lower among patients with negative results on 18F-rhPSMA-7 PET/CT than among those with positive results (P = 0.004; Table 2).
FIGURE 1.
Overall detection rate of 18F-rhPSMA-7 PET stratified by PSA value.
TABLE 2.
PSA Level in Patients with Positive Vs. Negative 18F-rhPSMA-7 PET/CT Results
| PSA, mean ± SD (ng/mL) |
||
| P | Positive results | Negative results |
| 0.004 | 8.18 ± 39.12 (n = 211) | 0.91223 ± 2.06 (n = 50) |
Lesion Location
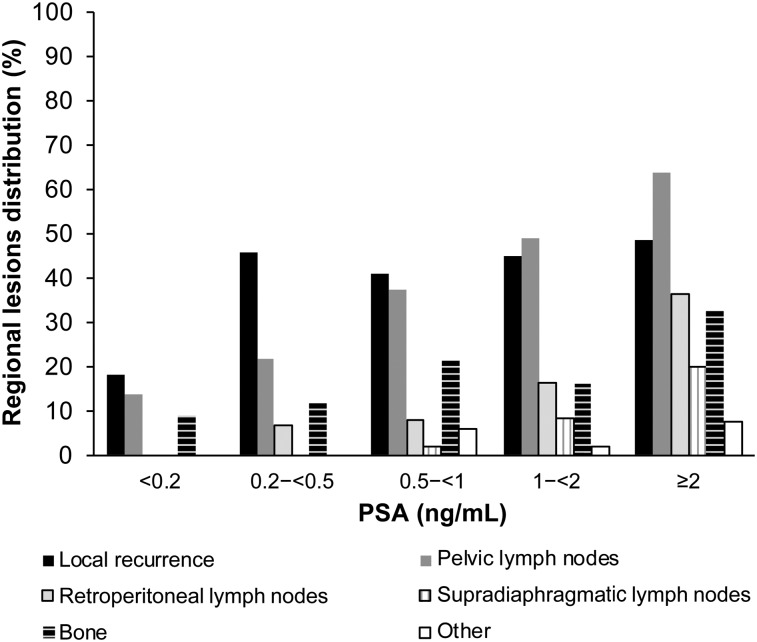
18F-rhPSMA-7–avid lesions were detected in both prostatic and extraprostatic regions as shown in Table 3. Regional positivity also broadly increased with increasing PSA level (Fig. 2). Local recurrence in the prostate bed ranged from 18% at PSA < 0.2 ng/mL to 49% at PSA ≥ 2 ng/mL, whereas pelvic lymph node metastases were present in 14% at PSA < 0.2 ng/mL to 64% at PSA ≥ 2 ng/mL. Although retroperitoneal lymph node metastases were rare at lower PSA levels, 36% of patients with PSA ≥ 2 ng/mL had positive retroperitoneal lymph nodes. Distant lymph node metastases were rare in very early BCR, with no supradiaphragmatic lymph node metastases observed at PSA < 0.5 ng/mL. However, 20% of patients with PSA ≥ 2 ng/mL were found to have positive supradiaphragmatic lymph nodes. Bone metastases were visible early in the recurrence timeline. 18F-rhPSMA-7–avid bone lesions were present in 9% of patients with PSA < 0.2 ng/mL and 33% of patients with PSA ≥ 2 ng/mL. Visceral metastases were absent or low across all PSA levels. Only 7.5% of patients with PSA ≥ 2 ng/mL were found to have visceral metastases. Figure 3 and Supplemental Figures 1 and 2 present example images from the study (supplemental materials are available at http://jnm.snmjournals.org).
TABLE 3.
Distribution of 18F-rhPSMA-7–Avid Lesions Stratified by PSA Value
| Metastatic site |
||||||
| PSA value (ng/mL) | Local recurrence | Pelvic lymph nodes | Retroperitoneal lymph nodes | Supradiaphragmatic lymph nodes | Bone | Viscera |
| <0.2 | 4/22 (18%) | 3/22 (13.6%) | 0/22 (0%) | 0/22 (0%) | 2/22 (9.1%) | 0/22 (0%) |
| 0.2–<0.5 | 27/59 (46%) | 13/59 (22.0%) | 4/59 (6.8%) | 0/59 (0%) | 7/59 (12%) | 0/59 (0%) |
| 0.5–<1 | 21/51 (41%) | 19/51 (37.3) | 4/51 (7.8%) | 1/51 (2.0%) | 11/51 (22%) | 3/51 (5.9%) |
| 1–<2 | 22/49 (45%) | 24/49 (49.0%) | 8/49 (16%) | 4/49 (8.2%) | 8/49 (16%) | 1/49 (2.0%) |
| ≥2 | 39/80 (49%) | 51/80 (63.8%) | 29/80 (36%) | 16/80 (20.0%) | 26/80 (33%) | 6/80 (7.5%) |
| All patients | 113/261 (43%) | 110/261 (42%) | 45/261 (17%) | 21/261 (8%) | 54/261 (21%) | 10/261 (4%) |
FIGURE 2.
Presence of 18F-rhPSMA-7–avid lesions stratified by PSA value.
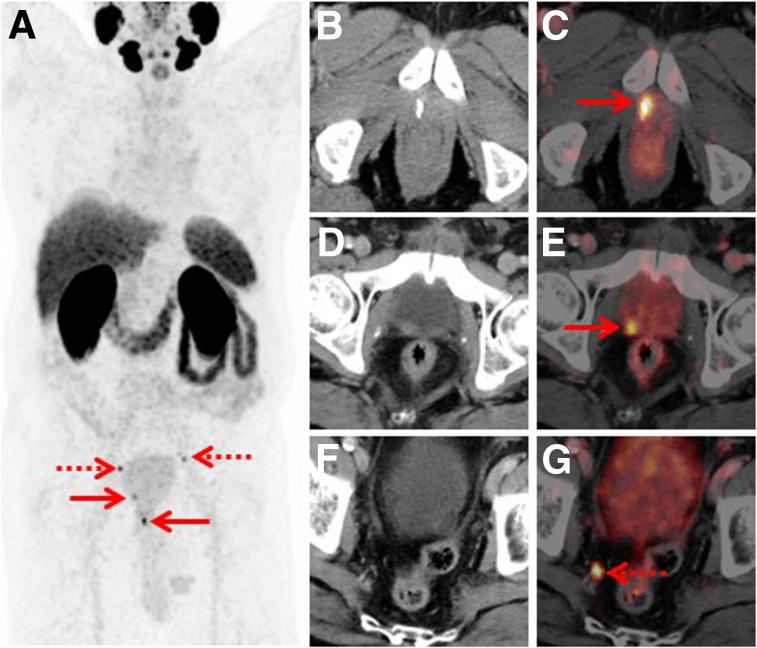
FIGURE 3.
Set of images from 77-y-old patient who underwent radical prostatectomy in 2015 (Gleason score of 9, pT3b, pN1) and was experiencing rising PSA (0.15 ng/mL). (A) Whole-body maximum-intensity projection shows 4 sites with focal PSMA-ligand uptake in pelvis (arrows). (B–G) Axial fused PET/CT and CT images demonstrate local recurrence at anastomosis (B and C, arrow), additional local recurrence at dorsal bladder wall (D and E, arrow), and tiny lymph node metastasis in right pelvis (F and G, arrow). Targeted external-beam radiation treatment lead to subsequent PSA drop.
Influence of Prior Therapy and Primary Histologic Differentiation
We observed no significant difference between the detection rate among patients who had previously received external-beam radiation therapy (79% [83/105]) and those who had not (82% [128/156], P = 0.55). Receiving ADT in the 6 mo preceding the scan also did not appear to affect the results (81% [54/67] for prior ADT compared with 81% [157/194] for no prior ADT, P = 0.54). When considering the histologic differentiation at the primary diagnosis, 18F-rhPSMA-7 PET/CT was positive in 78% (95/122) of patients with a Gleason score of 7 or less and in 83% (72/87) of patients with a Gleason score of at least 8 (P = 0.38).
DISCUSSION
A PSA level of greater than 0.2 ng/mL is the current definition of BCR of prostate cancer after radical prostatectomy (16–18). A rising PSA level after radical prostatectomy usually precedes a clinically detectable recurrence by years (19). However, as it cannot differentiate between local, regional, or systemic disease, precise imaging techniques are required to identify areas of involvement to facilitate the delivery of optimized therapy.
The performance of conventional imaging techniques, such as 11C-choline PET, is limited at low PSA values, and its use is not recommended for patients with a PSA level of below 1 ng/mL (3,20,21). PSMA-targeting tracers, particularly 68Ga-PSMA-11, have been shown to more effectively determine the site of disease and, as a result, have had a major impact on patient management (7,22). Although not currently approved by the European Medicines Agency or the U.S. Food and Drug Administration, 68Ga-PSMA-11 PET is in increasing use in research studies, where it has shown encouraging results.
In the present retrospective analysis investigating a large, homologous cohort of patients with BCR after prostatectomy, the novel PSMA-targeting radiotracer 18F-rhPSMA-7 was shown to be highly effective for prostate cancer restaging, with the site of disease recurrence being located in 81% of all patients and in 95% of those with a PSA level of 2 ng/mL or greater. Common with other PET tracers, the detection rate for 18F-rhPSMA-7 increases with increasing PSA level (23–25).
Previous data suggest that 18F-labeled PSMA tracers can achieve higher detection rates than reported for 68Ga-labeled PSMA, especially at low PSA values, and the present data corroborate this, notably for PSA levels of less than 0.5 ng/mL (26). The different energy profiles of 18F and 68Ga may play a role in the enhanced detection of 18F-labeled PSMA tracers; theoretically, the achievable resolution of 18F is higher than that of 68Ga (26,27). Our data indicate that, in general, 18F-rhPSMA-7 detects suspected BCR at a rate equivalent to that reported for 68Ga-PSMA-11, but in patients with very low PSA values (especially below 0.5 ng/mL) higher rates have been observed than reported for 68Ga-PSMA-11 (8,23). However, only limited conclusions can be drawn from comparisons with the literature. Patient cohorts can vary substantially between different reports, and sophisticated protocols (e.g., forced diuresis or additional delayed imaging) might further influence the performance of 68Ga-PSMA-11 by allowing for better assessment of local disease (28). The 18F-rhPSMA-7 detection rate in our retrospective analysis is similar to that recently published for 18F-PSMA-1007 (26).
The previously described low urinary retention of 18F-rhPSMA-7 at 1 h after injection might have an ancillary effect on the enhanced detection efficacy (11). High accumulation of 68Ga-PSMA-11 in the bladder during imaging is known to impair detection of small local recurrences, especially if near the bladder (14). In a recent study of a large cohort undergoing 68Ga-PSMA-11-imaging, local recurrence was present in 20% and 30% at PSA ranges of 0.2–0.5 and 0.5–1.0 ng/mL, respectively (29), compared with 46% and 41%, respectively, in the present study (Table 3). Differences in the patient cohorts may also have influenced results, and on the basis of different protocols, 68Ga-PSMA-11 retention in the bladder can be reduced (28).
Improved detection during very early recurrence is of great clinical importance to tailoring the salvage therapy approach. Salvage radiotherapy for patients with increasing PSA after prostatectomy provides the best chance for cure when delivered early (3,30,31). When salvage radiotherapy is delivered before the patient’s PSA reaches 0.5 ng/mL, more than 60% of patients will achieve an undetectable PSA level, and there is an approximately 80% chance of being progression-free at 5 y (32–36). Because of their ability to identify disease foci early in the recurrence timeline, multiple PET agents have been shown by recent studies to have clinical utility in influencing the future management of a patient (24,37,38). It was further shown that detection of lymph nodes and distant metastases has the highest impact on patient management (22).
Despite a largely homologous cohort, we evaluated the impact of primary histologic classification and prior treatment on the detection rate for 18F-rhPSMA-7. In our patient cohort, the detection rate for 18F-rhPSMA-7 was broadly consistent across a range of Gleason scores. Despite data indicating that PSMA overexpression increases with Gleason score (39), these reports focus mainly on the Gleason score of the primary tumor. In fact, a patient cohort presenting with BCR might already imply selection of more aggressive phenotypes of prostate cancer; the initial primary Gleason score might therefore be less relevant.
Some preliminary studies also suggest that the use of ADT may evoke overexpression of PSMA (40), but this may be a temporal relationship that requires further study. The use of ADT at the time of scanning has been shown to be associated with positive 68Ga-PSMA-11 PET results more frequently than does scanning of those not receiving ADT (8), and a recent case study demonstrated an increased number of lesions after 4 wk of treatment with ADT despite a lower PSA level (40). However, similar to our previous study with 68Ga-PSMA-11, we showed here that the use of ADT within the 6 mo preceding the scan did not significantly influence the detection rate with 18F-rhPSMA-7 (23). In addition, patients with prior ADT exposure are likely to have more advanced disease than those who have not received ADT, thus potentially highlighting a substantial confounding factor for such data in the literature. In general, the role of ADT in the uptake of PSMA-based tracers is highly controversial. At a cellular level, ADT appears to moderately increase PSMA expression, but the treatment may also cause a decrease in the number of tumor cells, constituting an effect in the opposite direction (40).
Similarly to recently published data for 18F-PSMA1007 (41), we observed PSMA-ligand uptake in the bones, which could partly be attributed to uptake not specific to prostate cancer. PSMA-ligand uptake in healing bone fractures, degenerative changes, or fibrocartilage lesions has been described previously (15,42,43). In this scenario, CT plays an important role, and its respective findings are essential for a correct differential diagnosis. The assessment of uptake not related to prostate cancer, using 18F-rhPSMA-7, is currently under investigation by our group.
Finally, we must emphasize that 18F-rhPSMA-7 yields a substantial logistical advantage over 68Ga-labeled counterparts, as the former can be produced with high yield (50%–70%) using automated radiosynthesizers within a short time frame (<1,000 s) at room temperature (11). The simple radiosynthesis is easily conducted in a manner compliant with good manufacturing practices, resulting in production of batches with activity suitable for distribution to offsite PET centers. In addition to logistical advantages, the true theranostic approach with rhPSMA ligands, including 18F labeling, is potentially beneficial for applications outside early BCR. In the field of prostate cancer, a highly prevalent disease, pretherapeutic dosimetry using PET imaging with 18F might become a relevant application of PSMA-targeted radioligand therapy.
Our retrospective analysis is subject to limitations. First, in common with most studies investigating PSMA-ligand PET imaging, we lacked histopathologic confirmation of the detected lesions. As known from other investigations, many recurrent lesions in prostate cancer are small and difficult to biopsy. However, when histopathologic validation has been used, results confirmed the high positive predictive value of PSMA-based PET agents (44). Second, we acknowledge that our study was retrospective, with the associated inherent limitations. Nevertheless, it provided substantial evidence for designing future prospective trials.
CONCLUSION
In this large population of patients with recurrent prostate cancer after radical prostatectomy, a novel PSMA-based PET tracer, 18F-rhPSMA-7, offered high detection rates that were at least equal to data reported for 68Ga-PSMA-11, especially at low PSA values. Such detection early in the recurrence timeline indicates the potential for 18F-rhPSMA-7 PET to guide future salvage therapy.
DISCLOSURE
Hans-Jürgen Wester, Alexander Wurzer, and Matthias Eiber are named as inventors on a patent application for rhPSMA. Hans-Jürgen Wester and Matthias Eiber received funding from the SFB 824 (DFG Sonderforschungsbereich 824, project B11) from the Deutsche Forschungsgemeinschaft, Bonn, Germany, and from Blue Earth Diagnostics (licensee for rhPSMA) as part of an academic collaboration. Hans-Jürgen Wester is a founder, shareholder, and advisory board member of Scintomics GmbH, Fuerstenfeldbruck, Germany. Matthias Eiber and Wolfgang Weber are consultants for Blue Earth Diagnostics. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the detection efficacy of 18F-rhPSMA7 PET/CT in noncastrate patients with biochemically recurrent prostate cancer?
PERTINENT FINDINGS: 18F-rhPSMA7 PET/CT offer high detection efficacy in biochemically recurrent prostate cancer—efficacy at least equal to data published for 68Ga-PSMA-11.
IMPLICATIONS FOR PATIENT CARE: If approved in the future, 18F-rhPSMA7 will allow patients easier and wider access to PSMA-ligand PET imaging.
Supplementary Material
REFERENCES
- 1.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–914. [DOI] [PubMed] [Google Scholar]
- 2.Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51:1175–1184. [DOI] [PubMed] [Google Scholar]
- 3.Mottet N, Bellmunt J, Briers E, et al. EAU-ESTRO-ESUR-SIOG guidelines on prostate cancer: 2017 update. European Association of Urology website. https://uroweb.org/wp-content/uploads/EAU-ESUR-ESTRO-SIOG-Guidelines-on-Prostate-Cancer-large-text-V2.pdf. Accessed April 7, 2020.
- 4.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 5.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. [DOI] [PubMed] [Google Scholar]
- 6.Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. [DOI] [PubMed] [Google Scholar]
- 8.Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giesel FL, Hadaschik B, Cardinale J, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wurzer A, Di Carlo D, Schmidt A, et al. Radiohybrid ligands: a novel tracer concept exemplified by 18F- or 68Ga-labeled rhPSMA-inhibitors. J Nucl Med. 2020;61: 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eiber M, Weirich G, Holzapfel K, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. 2016;70:829–836. [DOI] [PubMed] [Google Scholar]
- 13.Afshar-Oromieh A, Haberkorn U, Schlemmer HP, et al. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging. 2014;41:887–897. [DOI] [PubMed] [Google Scholar]
- 14.Freitag MT, Radtke JP, Afshar-Oromieh A, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imaging. 2017;44:776–787. [DOI] [PubMed] [Google Scholar]
- 15.Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–217. [DOI] [PubMed] [Google Scholar]
- 16.Bruce JY, Lang JM, McNeel DG, Liu G. Current controversies in the management of biochemical failure in prostate cancer. Clin Adv Hematol Oncol. 2012;10:716–722. [PubMed] [Google Scholar]
- 17.Goonewardene SS, Phull JS, Bahl A, Persad R. Interpretation of PSA levels after radical therapy for prostate cancer. Trends Urology Men’s Health. 2014;5:30–34. [Google Scholar]
- 18.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. [DOI] [PubMed] [Google Scholar]
- 19.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. [DOI] [PubMed] [Google Scholar]
- 20.Krause BJ, Souvatzoglou M, Tuncel M, et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2008;35:18–23. [DOI] [PubMed] [Google Scholar]
- 21.Castellucci P, Fuccio C, Rubello D, et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur J Nucl Med Mol Imaging. 2011;38:55–63. [DOI] [PubMed] [Google Scholar]
- 22.Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74:179–190. [DOI] [PubMed] [Google Scholar]
- 23.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 24.Andriole GL, Kostakoglu L, Chau A, et al. The impact of positron emission tomography with 18F-fluciclovine on the management of patients with biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol. 2019;201:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovacchini G, Picchio M, Briganti A, et al. [11C]choline positron emission tomography/computerized tomography to restage prostate cancer cases with biochemical failure after radical prostatectomy and no disease evidence on conventional imaging. J Urol. 2010;184:938–943. [DOI] [PubMed] [Google Scholar]
- 26.Giesel FL, Knorr K, Spohn F, et al. Detection efficacy of [18F]PSMA-1007 PET/CT in 251 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2019;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Crespo A. Comparison of gallium-68 and fluorine-18 imaging characteristics in positron emission tomography. Appl Radiat Isot. 2013;76:55–62. [DOI] [PubMed] [Google Scholar]
- 28.Schmuck S, Nordlohne S, von Klot CA, et al. Comparison of standard and delayed imaging to improve the detection rate of [68Ga]PSMA I&T PET/CT in patients with biochemical recurrence or prostate-specific antigen persistence after primary therapy for prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:960–968. [DOI] [PubMed] [Google Scholar]
- 29.Rauscher I, Duwel C, Haller B, et al. Efficacy, predictive factors, and prediction nomograms for 68Ga-labeled prostate-specific membrane antigen-ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. 2018;73:656–661. [DOI] [PubMed] [Google Scholar]
- 30.Emmett L, van Leeuwen PJ, Nandurkar R, et al. Treatment outcomes from 68Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med. 2017;58:1972–1976. [DOI] [PubMed] [Google Scholar]
- 31.Sterzing F, Kratochwil C, Fiedler H, et al. 68Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiegel T, Lohm G, Bottke D, et al. Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome: results of a retrospective study. Int J Radiat Oncol Biol Phys. 2009;73:1009–1016. [DOI] [PubMed] [Google Scholar]
- 33.Stish BJ, Pisansky TM, Harmsen WS, et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol. 2016;34:3864–3871. [DOI] [PubMed] [Google Scholar]
- 34.Pfister D, Bolla M, Briganti A, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol. 2014;65:1034–1043. [DOI] [PubMed] [Google Scholar]
- 35.Siegmann A, Bottke D, Faehndrich J, et al. Salvage radiotherapy after prostatectomy: what is the best time to treat? Radiother Oncol. 2012;103:239–243. [DOI] [PubMed] [Google Scholar]
- 36.Ohri N, Dicker AP, Trabulsi EJ, Showalter TN. Can early implementation of salvage radiotherapy for prostate cancer improve the therapeutic ratio? A systematic review and regression meta-analysis with radiobiological modelling. Eur J Cancer. 2012;48:837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein J, Even-Sapir E, Ben-Haim S, et al. Does choline PET/CT change the management of prostate cancer patients with biochemical failure? Am J Clin Oncol. 2017;40:256–259. [DOI] [PubMed] [Google Scholar]
- 38.Hope TA, Aggarwal R, Chee B, et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–1961. [DOI] [PubMed] [Google Scholar]
- 39.Ross JS, Sheehan CE, Fisher HA, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–6362. [PubMed] [Google Scholar]
- 40.Hope TA, Truillet C, Ehman EC, et al. 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: first human experience. J Nucl Med. 2017;58:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauscher I, Krönke M, König M, et al. Matched-pair comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J Nucl Med. 2020;61:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:2117–2136. [DOI] [PubMed] [Google Scholar]
- 43.Jochumsen MR, Dias AH, Bouchelouche K. Benign traumatic rib fracture: a potential pitfall on 68Ga-prostate-specific membrane antigen PET/CT for prostate cancer. Clin Nucl Med. 2018;43:38–40. [DOI] [PubMed] [Google Scholar]
- 44.Rauscher I, Maurer T, Beer AJ, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–1719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.