Abstract
The prevalence and genetic characteristics of Bartonella species in eastern bent-wing bats (Miniopterus fuliginosus) from Japan were investigated. Bartonella bacteria were isolated from 12/50 (24%) of bats examined. Analyses of sequence similarities of the citrate synthase gene (gltA) and RNA polymerase beta-subunit-encoding (rpoB) gene indicated that the isolates from M. fuliginosus were distinct from those present in known Bartonella species as the levels of similarity for both of the genes were lower than the cut-off values for species identification in Bartonella. A phylogenetic analysis of the gltA sequences revealed that the Miniopterus bat-associated strains fell into five genotypes (I to V). Though genotypes I to IV formed a clade with Bartonella from Miniopterus bats from Taiwan, genotype V made a monophyletic clade separate from other bat isolates. In a phylogenetic analysis with the concatenated sequences of the 16S rRNA, gltA, rpoB, cell division protein (ftsZ) gene, and riboflavin synthase gene (ribC), isolates belonging to genotypes I to IV clustered with Bartonella strains from Taiwanese Miniopterus bats, similar to the outcome of the phylogenetic analysis with gltA, whereas genotype V also made a monophyletic clade separate from other bat-associated Bartonella strains. The present study showed that M. fuliginosus in Japan harbor both genus Miniopterus-specific Bartonella suggesting to be specific to the bats in Japan.
Keywords: Bartonella, bat, Japan, Miniopterus fuliginosus
Highlights
-
•
The prevalence of Bartonella in Miniopterus fuliginosus was 24%(12/50).
-
•
M. fuliginosus in Japan harbored two novel Bartonella species in their blood.
-
•
One Bartonella species was the genus Miniopterus-specific Bartonella.
-
•
The other was distinct from other known bat-associated Bartonella.
1. Introduction
Bats (Order Chiroptera) are widely distributed and are found on all continents except the polar regions. Thirty-five species of bats are now recognized to be present in Japan (Matsue et al., 2006); the genera Pipistrellus, Myotis, Rhinolophus, Eptesicus, and Miniopterus inhabit throughout the country. Bats can serve as reservoirs for various viral zoonotic pathogens such as rabies virus in Desmodus bats in South America, Nipah virus and Hendra virus in Pteropus bats in India and Australia, and SARS coronavirus in Rhinolophus bats in China (Calisher et al., 2006). Other zoonotic bacteria, such as Campylobacter jejuni and Leptospira spp., have also been detected in Myotis bats in the Netherlands and in Artibeus, Phyllostomus, and Sturnira bats in Peru (Muhldorfer, 2013).
Bartonella bacteria are small, fastidious, and Gram-negative bacilli that parasitize erythrocytes and endothelial cells in many species of mammals. Several Bartonella spp. are known to cause human diseases including cat-scratch disease (B. henselae), trench fever (B. quintana), and Carrión's disease (B. bacilliformis). Blood-sucking ectoparasites have been shown to play an important role in the transmission of Bartonella species among their hosts: cat fleas transmit B. henselae between cats (Chomel et al., 1996) and lice transmit B. quintana between humans (Bonilla et al., 2009). There are evidences that bats also serve as a reservoir for pathogenic Bartonella species. B. mayotimonensis was first detected in the aortic valve of a patient with endocarditis in the USA (Lin et al., 2010); subsequently, it was detected in Myotis bats in Finland (Veikkolainen et al., 2014), the USA (Lilley et al., 2017), France, and Spain (Stuckey et al., 2017b). Interestingly, the citrate synthase gene (gltA) sequence of Bartonella strains from Myotis bats in Georgia is closely related to that of Bartonella DNA identified in forest workers in Poland (Urushadze et al., 2017).
Bartonella species have been detected in many bat genera, such as Pipistrellus, Myotis, Nyctalus, Rhinolophus, Eptesicus, Miniopterus, Hipposideros, Megaderma, Cynopterus, Coleura, Triaenops, Epomophorus, Rousettus, Eidolon, and Megaerops (Stuckey et al., 2017a). Miniopterus bats have been found to show up to 56.3% (49/87) Bartonella prevalence in Kenya (Kosoy et al., 2013), 88.9% (24/27) in Georgia (Urushadze et al., 2017), and 42.9% (6/14) in Taiwan (Lin et al., 2012). To date, however, no information on the prevalence and genetic properties of Bartonella species is available in any bat species in Japan. The present study was conducted to determine the prevalence and genetic characteristics of Bartonella species in Miniopterus fuliginosus bats in Japan.
2. Materials and methods
2.1. Collection of blood and ectoparasites from bats
In March 2013, fifty M. fuliginosus were captured at a headrace tunnel in Wakayama Prefecture located in the western part of Japan (33°40′N, 135°23′E) after gaining the permission (license # Nishi 4 and 5) of sample collection from the local government. Blood samples were aseptically collected via cardiac puncture from bats euthanized following the guidelines for euthanasia of the Japan Veterinary Medical Association. The blood samples were transferred to a blood collection tube containing EDTA, the samples immediately frozen in dry ice, and transported to the Laboratory of Veterinary Public Health, the Department of Veterinary Medicine, College of Bioresource Sciences, Nihon University. The blood samples were stored at −70 °C until examined. Ectoparasites were also collected from the bat carcasses during autopsy in the laboratory.
2.2. Isolation of Bartonella bacteria from bats and identification of ectoparasites
Frozen blood samples were thawed at room temperature and 100 μl of each blood sample was separately transferred to sterile 1.5 ml conical tubes. The blood was mixed with 100 μl of medium 199 supplemented with 1 mM sodium pyruvate solution and 20% fetal bovine serum (Life Technologies, Carlsbad, CA, USA). Aliquots (100 μl) of the mixture were placed on two heart infusion agar plates (Difco, Sparks Glencoe, MI, USA) containing 5% rabbit blood. The inoculated plates were incubated at 35 °C in a moist atmosphere under 5% CO2 for up to 4 weeks. Bartonella bacteria were tentatively identified by colony morphology (small, gray or cream-yellow, round shape); three colonies were picked from each positive sample and sub-cultured on a fresh blood agar plate using the same conditions as the primary culture.
The identification of ectoparasites on the bats was performed by morphological characteristics under a stereomicroscope SZX16 (Olympus, Tokyo, Japan) by the reference of bat flies in Japan (Sato and Mogi, 2008).
2.3. PCR amplification of the gltA and rpoB
Genomic DNA was extracted from each sub-culture colony using InstaGene Matrix (Bio-Rad Laboratories, Inc. Hercules, CA, USA). Genus-specific PCR targeting the gltA and the RNA polymerase beta-subunit-encoding gene (rpoB) was then performed to confirm Bartonella identification (Norman et al., 1995; Renesto et al., 2001). The genomic DNA of B. alsatica IBS382T and nuclease-free distilled water were used as positive and negative controls, respectively. The PCR amplicons were separated by electrophoresis on 3% agarose gels and visualized by staining with ethidium bromide. Samples showing product sizes of approximately 390 bp for the gltA and approximately 900 bp for the rpoB were considered to be positive for the genus Bartonella.
2.4. DNA sequencing and genotyping
PCR amplicons were purified using the Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA). Subsequently, the nucleotide sequences of the amplicons were determined using a Genetic Analyzer model 3130 (Applied Biosystems, Foster City, CA, USA). The gltA genotype was determined when unique sequence variants with ≥1 nucleotide difference were found in each isolate by comparing the gene sequences by using Genetyx software Ver 12 (Genetyx Corporation, Tokyo, Japan). Representative isolates from each genotype were further analyzed by sequencing of the rpoB, the 16S rRNA, the cell division protein gene (ftsZ), and the riboflavin synthase gene (ribC). The PCR protocol used for amplification of the 16S rRNA (Heller et al., 1997), ftsZ (Zeaiter et al., 2002), and ribC (Johnson et al., 2003) was based on the conditions described in previous reports on Bartonella.
2.5. Sequence homology analysis
Nucleotide sequences of the gltA, rpoB and 16S rRNA were compared between the representative bat isolates and the strains of prokaryotes registered in the GenBank/EMBL/DDBJ database using the BLAST program. Sequence similarities in the gltA and rpoB genes were additionally compared with the type strains of Bartonella species.
2.6. Phylogenetic analysis
Based on evolutionary model selection using JModelTest2 (Darriba et al., 2012) with Akaike's information criterion corrected for finite sample sizes (AICc; Burnham and Anderson, 2004), the generalized time-reversible substitution model with four gamma-distributed categories and a proportion of invariant sites (GTR + G + I) model was the best available model in the phylogenetic analyses based on the gltA sequences and the concatenated sequences with the gltA, rpoB, ribC, ftsZ, and 16S rRNA.
A phylogenetic tree based on the gltA sequences of the Bartonella isolates was constructed using the Maximum Likelihood method based on the GTR + G + I model in MEGA 7 (Kumar et al., 2016). Known Bartonella species (N = 41) and bat-associated Bartonella strains (N = 249) derived from Taiwan (Lin et al., 2012), China (Han et al., 2017), Vietnam (Anh et al., 2015), Thailand (Mckee et al., 2017), France (Stuckey et al., 2017b), UK (Concannon et al., 2005), USA (Lilley et al., 2017), Guatemala (Bai et al., 2011), Mexico (Stuckey et al., 2017c), Peru (Bai et al., 2012; Becker et al., 2018), Belize (Becker et al., 2018) Madagascar (Brook et al., 2015), Kenya (Kosoy et al., 2013), and Georgia (Urushadze et al., 2017) were included in this analysis. Strain names, host species, countries where the bats were collected, and the gltA accession numbers are summarized in Supplementary Table 1.
A phylogenetic tree based on the concatenated sequences of the 16S rRNA, gltA, rpoB, ftsZ, and ribC was constructed using the Maximum Likelihood based on the GTR + G + I model in MEGA 7 (Kumar et al., 2016). The representative isolates in the present study were also compared with six Taiwanese Bartonella strains (No. 5, No. 6, No. 7, No. 8, No. 15, and No. 16) from M. schreibersii (Lin et al., 2012) in this phylogenetic analysis. Support for nodes in both trees was assessed by bootstrapping with 1000 replicates.
3. Results
3.1. Isolation of Bartonella bacteria from bats, genotyping the bat isolates based on the gltA sequences, and identification of ectoparasites on bats
Bartonella bacteria were isolated from 12/50 (24%) bats examined and totally 36 bacterial isolates were obtained. The gltA sequences of the isolates were classified into five genotypes (I to V, Table 1 ). Eleven of the bats were infected by a single Bartonella genotype, but the other bat was co-infected with two genotypes (I and III). The gltA, rpoB, 16S rRNA, ftsZ, and ribC sequences of all genotypes were deposited in the GenBank/EMBL/DDBJ database (Table 1).
Table 1.
Genotyping of bat isolates based on the gltA sequences and GenBank accession numbers for five housekeeping genes.
| Genotype | Representative strain | Accession numbers in: |
||||
|---|---|---|---|---|---|---|
| gltA | rpoB | 16S rRNA | ftsZ | ribC | ||
| I | bat2-1 | LC483820 | LC483825 | LC483830 | LC483835 | LC483841 |
| II | bat23-1 | LC483822 | LC483827 | LC483832 | LC483837 | LC483844 |
| III | bat8-3 | LC483821 | LC483826 | LC483831 | LC483836 | LC483843 |
| IV | bat43-1 | LC483824 | LC483829 | LC483833 | LC483838 | LC483842 |
| V | bat24-1 | LC483823 | LC483828 | LC483834 | LC483839 | LC483840 |
In total, 52 ectoparasites were collected from the bats and identified as the bat flies, genus Nycteribia (N = 34; species was not identified) and as Penicillidia jenynsii (N = 18).
3.2. Sequence homology of the gltA, rpoB and 16S rRNA
Sequence similarities among representative genotypes I (Strain bat2-1), II (Strain bat23-1), III (Strain bat8-3), and IV (Strain bat43-1) ranged from 99.0 to 99.7% for the gltA and 97.2 to 100% for the rpoB. Sequence similarities between genotype V and genotypes I to IV ranged from 84.4 to 85.4% for the gltA and 87.6 to 87.9% for the rpoB (Table 2 ).
Table 2.
Sequence similarities of the gltA and rpoB genes among strains from M. fuliginosus.
| Genotype (strain) | Sequence similarities (%) by strain: |
||||
|---|---|---|---|---|---|
| I (bat2-1) | II (bat23-1) | III (bat8-3) | IV (bat43-1) | V (bat24-1) | |
| I (bat2-1) | – | 99.4a | 99.4a | 99.0a | 84.4a |
| II (bat23-1) | 97.7b | – | 99.4a | 99.7a | 85.1a |
| III (bat8-3) | 97.2b | 99.3b | – | 99.7a | 85.1a |
| IV (bat43-1) | 97.7b | 100b | 99.3b | – | 85.4a |
| V (bat24-1) | 87.9b | 87.7b | 87.6b | 87.7b | – |
Sequence similarities of gltA.
Sequence similarities of rpoB.
Sequence similarities between representative genotypes I (Strain bat2-1), II (Strain bat23-1), III (Strain bat8-3), and IV (Strain bat43-1) and strains No. 5, No. 7, and No. 15 from Miniopterus bats in Taiwan ranged from 99.7 to 100% for the gltA and 99.2 to 100% for the rpoB. In contrast, representative genotype V (Strain bat24-1) showed 89.0% similarity for the gltA with strain 44,544 from Georgian mouse-eared bats (Myotis blithii) and 89.1% similarity for the rpoB with Bartonella quintana strain FullerT (Table 3 ).
Table 3.
Sequence similarities of the gltA and rpoB genes between representative isolates from M. fuliginosus and their closest strains.
| Geno type | Strain |
gltA |
rpoB |
||||
|---|---|---|---|---|---|---|---|
| Closest strain | Host (country) | % similarity | Closest strain | Host (country) | % similarity | ||
| I | bat2-1 | No. 5 | Miniopterus schreibersii (Taiwan) | 100% | No. 15 | Miniopterus schreibersii (Taiwan) | 99.4% |
| II | bat23-1 | No. 7 | 100% | No. 5 | 100% | ||
| III | bat8-3 | No. 7 | 99.7% | No. 5 | 99.3% | ||
| IV | bat43-1 | No. 7 | 99.7% | No. 5 | 100.0% | ||
| V | bat24-1 | 44,544 | Myotis blithii (Georgia) | 89.0% | B. quintana FullerT | Human (Yugoslavia) | 89.1% |
In addition, 16S rRNA sequences of genotypes I to IV were identical to that of Bartonella sp. strain No.5 from Miniopterus bats in Taiwan. On the other hand, genotype V showed 97.9% similarity with Bartonella sp. strain F2 isolated from Pteronotus parnellii in Mexico (data not shown).
Sequence similarities for the gltA and rpoB between representative isolates and type strains of existing Bartonella species ranged from 87.2 to 91.3% and 88.2 to 89.1%, respectively (Table 4 ).
Table 4.
Sequence similarities of the gltA and rpoB genes in representative isolates from M. fuliginosus in Japan and closest type strains of Bartonella species.
| Genotype | Strain |
gltA |
rpoB |
||
|---|---|---|---|---|---|
| Closest species | % similarity | Closest species | % similarity | ||
| I | bat2-1 | B. quintana FullerT | 91.0% | B. callosciuri BR11-1T | 88.7% |
| II | bat23-1 | B. capreoli IBS193T | 91.0% | B. melophagi K-2CR | 88.4% |
| III | bat8-3 | B. quintana FullerT | 91.0% | B. melophagi K-2CR | 88.5% |
| IV | bat43-1 | B. capreoli IBS193T | 91.3% | B. melophagi K-2CR | 88.4% |
| V | bat24-1 | B. rochalimae BMGH T | 87.2% | B. quintana FullerT | 89.1% |
3.3. Phylogenetic analysis based on the gltA sequences and concatenated sequences of five genes
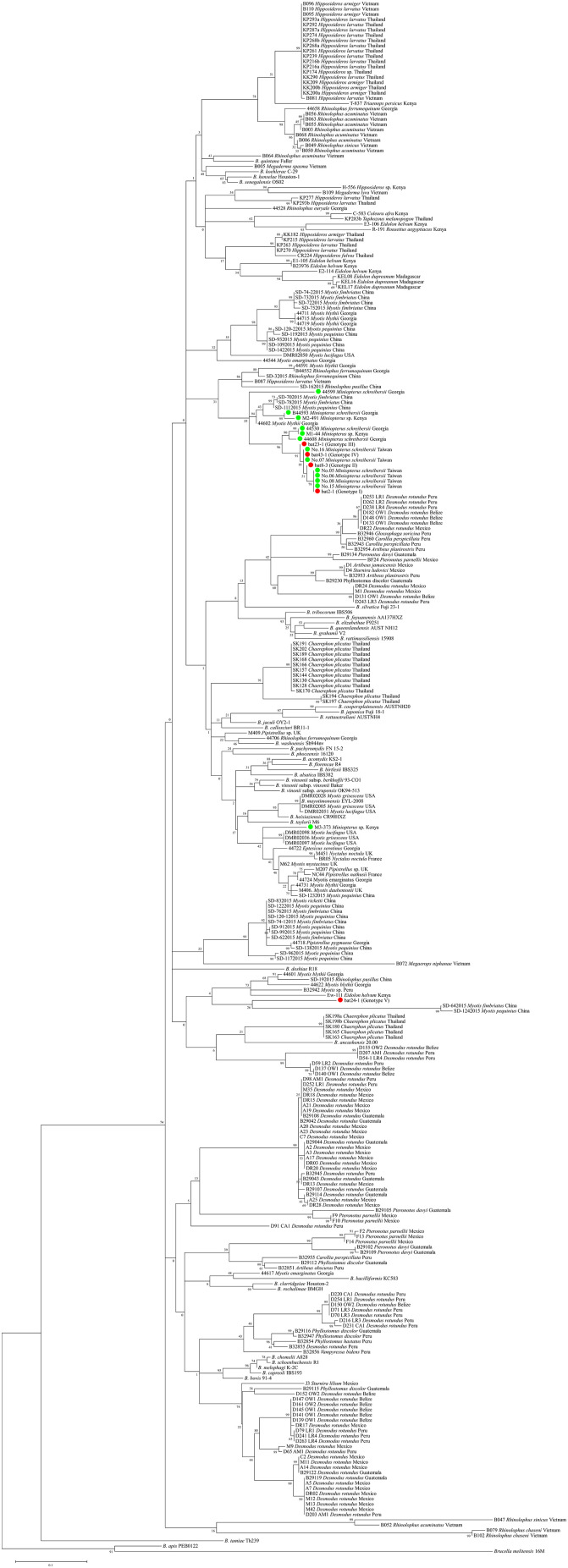
In the phylogenetic analysis based on the gltA sequences, four representative isolates derived from genotypes I (Strain bat2-1), II (Strain bat23-1), III (Strain bat8-3), and IV (Strain bat43-1) clustered with the isolates from Miniopterus bats in Taiwan (No. 5, No. 6, No. 7, No. 8, No. 15, and No. 16). Genotype V (Strain bat24-1) made a monophyletic clade distinct from the other known Bartonella strains (Fig. 1 ).
Fig. 1.
Phylogenetic relationship of bat-associated Bartonella strains based on the gltA sequences. Evolutionary distances were calculated by Maximum Likelihood method with GTR + G + I model in MEGA 7. The analysis included five representative isolates from M. fuliginosus, 249 bat-associated Bartonella strains, and 41 known type strains of Bartonella species. Bartonella isolates from M. fuliginosus in Japan are indicated by red circles and isolates from Miniopterus bats in Taiwan, Kenya and Georgia are shown by green circles. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
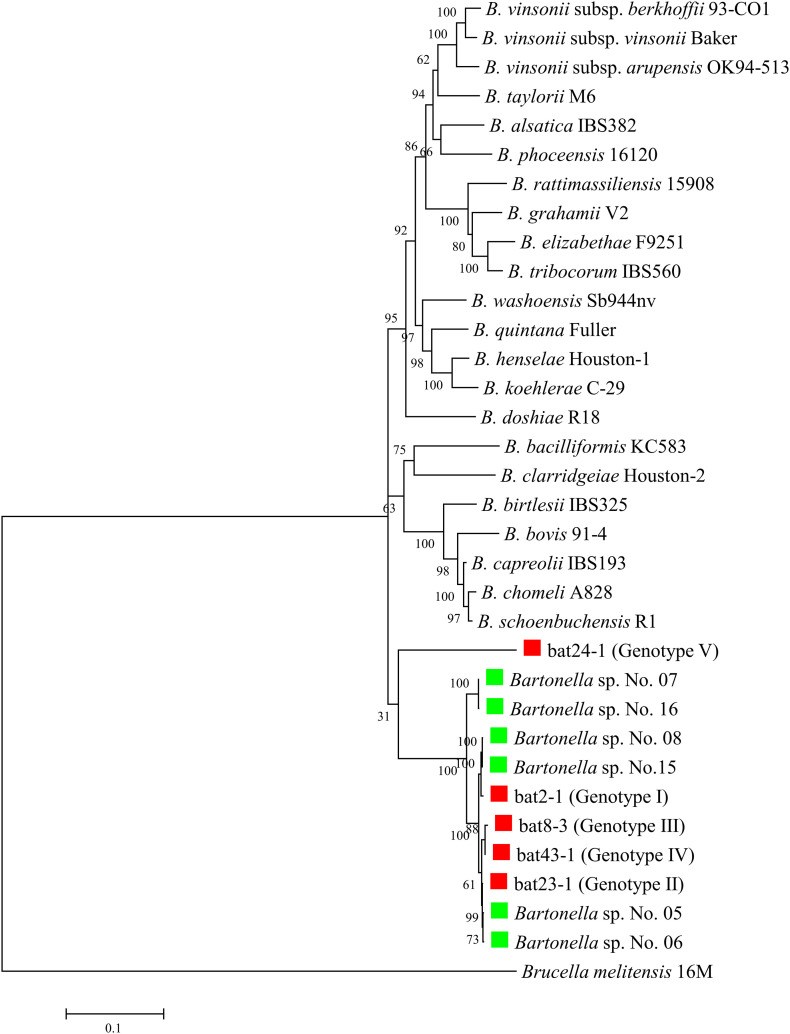
In the phylogenetic analysis with the concatenated sequences of five genes (16S rRNA, gltA, rpoB, ftsZ, and ribC), four representative isolates from genotypes I–IV grouped in a lineage with strains No. 5, No. 6, No. 8, and No. 15 from Miniopterus bats in Taiwan; no known Bartonella species was present in the lineage. Strain bat24-1 of genotype V made a monophyletic clade, consistent with the result of the phylogenetic analysis using the gltA sequences and was clearly different from known Bartonella species (Fig. 2 ).
Fig. 2.
Phylogenetic relationship based on concatenated sequences of bat-associated Bartonella strains. Evolutionary distances were calculated by Maximum Likelihood method with the GTR + G + I model and are shown as number of base substitutions per site. The analysis includes five representative isolates from M. fuliginosus, six Bartonella strains (No. 5, No. 6, No. 7, No. 8, No. 15, and No. 16) from Miniopterus bats in Taiwan, and 22 known type strains of Bartonella species. Evolutionary analyses were conducted in MEGA7. Bartonella isolates from M. fuliginosus in Japan are indicated by the red squares on the left and isolates from Miniopterus bats in Taiwan are shown by green squares. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The present study showed that 24% (12/50) of M. fuliginosus in Japan harbored Bartonella bacteria in their blood. The genus Miniopterus is insectivorous and is distributed worldwide, with the exception of polar regions (IUCN, 2019). In previous studies, Bartonella bacteria have been isolated from Miniopterus bats: 49/87 (56.3%; Kosoy et al., 2013) in Kenya; 24/27 (88.9%; Urushadze et al., 2017) in Georgia; and 6/14 (42.9%; Lin et al., 2012) in Taiwan. Although we used almost similar procedure for the isolation of Bartonella as reported in the previous studies, the prevalence in Miniopterus bats in Japan found to be lower than those in Kenya, Georgia, and Taiwan. It is suggested that the prevalence of Bartonella in Japanese Miniopterus bats examined may be basically lower than other countries. With regard to other bat genera, Bartonella has been reported in 140/445 (31.5%) of Myotis bats, 10/172 (5.8%) of Pipistrellus bats, 5/53 (9.4%) of Eptesicus bats, and 47/120 (39.2%) of Rhinolophus bats (Stuckey et al., 2017a). Thus, the rate of Miniopterus individuals carrying Bartonella bacteria is relatively high compared with other insectivorous bat genera. Miniopterus form large colonies, especially in winter, and show considerable inter-individual contact (Serra-Cobo and López-Roig, 2017). In the present study, we found many colonies consisted of several dozens to hundreds of individuals in the sampled tunnel. Furthermore, ectoparasitic bat flies (Nycteribia sp. and P. jenynsii) were recovered from the bats. The high colony density of Miniopterus and the presence of ectoparasitic bat flies may have contributed to the higher prevalence and horizontal transmission of Bartonella bacteria within the population.
Only one bat was co-infected with two genotypes (I and III) of Bartonella bacteria. Co-infection with different species and genotypes of Bartonella has been reported in various wild mammals such as bats (Urushadze et al., 2017; Han et al., 2017), rodents (Gutiérrez et al., 2015), carnivores (Henn et al., 2009), and ruminants (Sato et al., 2012). Urushadze et al. (2017) reported that the co-infection rate of Bartonella in Miniopterus bats was 29.2% (7/24) of the positive samples by analyzing all morphologically unique colonies. Since we examined three colonies per positive samples in the present study, more colonies are needed to detect detailed co-infection status in the bats.
La Scola et al. (2003) suggested that newly encountered Bartonella isolates should be considered as new species if the gltA fragment (327 bp) and rpoB fragment (825 bp) showed <96.0% and < 95.4% sequence similarities with those of validated Bartonella type strains, respectively. Here, the comparisons of the gltA and rpoB sequences between bat isolates and type strains of Bartonella species yielded similarities for both genes that were lower than the suggested cut-off values for species identification in Bartonella. In the homology analysis among representative strains, genotypes I to IV showed high sequence similarities for the gltA and rpoB, whereas genotype V showed low similarities with other genotypes. From the BLAST search, the gltA and rpoB sequences of genotypes I to IV showed the highest similarity (>99.7% for gltA and >99.2% for rpoB) with three strains from Miniopterus bats in Taiwan. However, genotype V showed low similarity (89.0%) with strain 44,544 from Myotis for the gltA and 89.1% with B. quintana FullerT for the rpoB sequence.
In the phylogenetic analysis based on the gltA sequences, the representative isolates from M. fuliginosus fell into two lineages. Urushadze et al. (2017) reported that bat-associated Bartonella strains tended to be grouped by genus and/or family of the bat in a phylogenetic analysis of the gltA. Corduneanu et al. (2018) also showed that Miniopterus bat-associated Bartonella clustered in the same clade in a phylogenetic analysis of gltA. Genotypes I to IV found in the present study formed a lineage with strains from Miniopterus in Taiwan, suggesting that these strains are specific to Miniopterus living in Japan and Taiwan. However, genotype V formed a monophyletic clade clearly separate from any bat-associated Bartonella strains. Thus, Japanese M. fuliginosus may harbor a unique Bartonella lineage which has not been found in any other bat species.
As reported by the ad hoc committee for the re-evaluation of species definition in bacteriology, the description of a new species should be based on the sequence analysis of housekeeping genes using at least five genes (Stackebrandt et al., 2002). The phylogenetic tree based on the concatenated sequences of five genes showed that genotypes I to IV were closely related to the isolates from Miniopterus in Taiwan, as in the phylogenetic tree for the gltA. These data suggest that Miniopterus bats in both Japan and Taiwan harbor Bartonella bacteria with high genetic similarities. However, strain bat 24-1 of genotype V formed a clearly independent clade that was separate from other bat isolates and known Bartonella species. These results suggest that M. fuliginosus in Japan harbor two novel Bartonella species: one is similar to species found in Taiwanese Miniopterus and another is specific to Japanese bats.
Previous studies have shown that some of the blood-sucking arthropods that infest bats may be involved in Bartonella transmission among bats (Brook et al., 2015; Muhldorfer, 2013). Bat flies have been suggested to be a potential vector for Bartonella transmission in bat population in Africa, South America, and Asia as Bartonella DNAs are often detected in these ectoparasites (Stuckey et al., 2017a). In the present study, we identified bat flies belonging to Nycteribia sp. and P. jenynsii on Miniopterus bats; these bat fly species are known as blood-sucking obligate ectoparasites. Further studies are necessary to clarify the role of bat flies as transmission vectors of Bartonella bacteria among M. fuliginosus.
5. Conclusion
In the present study, we showed that 24% (12/50) of eastern bent-wing bats (Miniopterus fuliginosus) harbored Bartonella bacteria in their blood for the first time in Japan. Phylogenetic analyses based on the gltA and the concatenated sequences with the gltA, rpoB, ribC, ftsZ, and 16S rRNA of the isolates clarified that two novel Bartonella species are present in the bats; one is close to the isolates from Taiwanese Miniopterus bats, and another is distinct from other known bat-associated Bartonella suggesting to be specific to the bats in Japan.
The following are the supplementary data related to this article.
Information of Bartonella strains used in the phylogenetic analysis of the gltA.
Acknowledgments
Acknowledgement
This work was supported by Grant-in Aid for Scientific Research from the Japan Society for the Promotion of Science [grant number 18K06003].
Declaration of Competing Interest
The authors have no conflict of interest.
References
- Anh P.H., Van Cuong N., Son N.T., Tue N.T., Kosoy M.Y., Woolhouse M.E., Baker S., Bryant J.E., Thwaites G., Carrique-Mas J.J., Rabaa M.A. Diversity of Bartonella spp. in bats, southern Vietnam. Emerg. Infect. Dis. 2015;21:1266. doi: 10.3201/eid2107.141760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Kosoy M.Y., Recuenco S., Alvarez Castillo D., Moran D., Turmelle A.S., Ellison J., Garcia D.L., Estevez A., Lindblade K., Rupprecht C.E. Bartonella spp. in bats, Guatemala. Emerg. Infect. Dis. 2011;17:1269–1272. doi: 10.3201/eid1707.101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Recuenco S., Gilbert A.T., Osikowicz L.M., Gómez J., Rupprecht C., Kosoy M.Y. Prevalence and diversity of Bartonella spp. in bats in Peru. Am. J. Trop. Med. Hyg. 2012;87:518–523. doi: 10.4269/ajtmh.2012.12-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D.J., Bergner L.M., Bentz A.B., Orton R.J., Altizer S., Streicker D.G. Genetic diversity, infection prevalence, and possible transmission routes of Bartonella spp. in vampire bats. PLoS Negl. Trop. Dis. 2018;12:e0006786. doi: 10.1371/journal.pntd.0006786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla D.L., Kabeya H., Henn J., Kramer V.L., Kosoy M. Bartonella quintana in body lice and head lice from homeless persons, San Francisco, California, USA. Emerg. Infect. Dis. 2009;15:912–915. doi: 10.3201/eid1506.090054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook C.E., Bai Y., Dobson A.P., Osikowicz L.M., Ranaivoson H.C., Zhu Q., Kosoy M., Dittmar K. Bartonella spp. in fruit bats and blood-feeding ectoparasites in Madagascar. PLoS Negl. Trop. Dis. 2015;9:e0003532. doi: 10.1371/journal.pntd.0003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham K.P., Anderson D.R. Multimodel inference: understanding AIC and BIC in model selection. Sociol. Methods Res. 2004;33:261–304. [Google Scholar]
- Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel B.B., Kasten R.W., Floyd-Hawkins K., Chi B., Yamamoto K., Roberts-Wilson J., Gurfield A.N., Abbott R.C., Pedersen N.C., Koehler J.E. Experimental transmission of Bartonella henselae by the cat flea. J. Clin. Microbiol. 1996;34:1952–1956. doi: 10.1128/jcm.34.8.1952-1956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon R., Wynn-Owen K., Simpson V.R., Birtles R.J. Molecular characterization of haemoparasites infecting bats (Microchiroptera) in Cornwall, UK. Parasitology. 2005;131:489–496. doi: 10.1017/S0031182005008097. [DOI] [PubMed] [Google Scholar]
- Corduneanu A., Sándor A.D., Ionică A.M., Hornok S., Leitner N., Bagó Z., Stefke K., Fuehrer H.P., Mihalca A.D. Bartonella DNA in heart tissues of bats in central and eastern Europe and a review of phylogenetic relations of bat-associated bartonellae. Parasit. Vectors. 2018;11:489. doi: 10.1186/s13071-018-3070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez R., Krasnov B., Morick D., Gottlieb Y., Khokhlova I.S., Harrus S. Bartonella infection in rodents and their flea ectoparasites: an overview. Vector Borne Zoonotic Dis. 2015;15:27–39. doi: 10.1089/vbz.2014.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H.J., Wen H.L., Zhao L., Liu J.W., Luo L.M., Zhou C.M., Qin X.R., Zhu Y.L., Zheng X.X., Yu X.J. Novel Bartonella species in insectivorous bats, northern China. PLoS One. 2017;12 doi: 10.1371/journal.pone.0167915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R., Artois M., Xemar V., De Briel D., Gehin H., Jaulhac B., Monteil H., Piemont Y. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J. Clin. Microbiol. 1997;35:1327–1331. doi: 10.1128/jcm.35.6.1327-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn J.B., Chomel B.B., Boulouis H.J., Kasten R.W., Murray W.J., Bar-Gal G.K., King R., Courreau J.F., Baneth G. Bartonella rochalimae in raccoons, coyotes, and red foxes. Emerg. Infect. Dis. 2009;15:1984. doi: 10.3201/eid1512.081692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN The IUCN Red List of Threatened Species. 2019. https://www.iucnredlist.org/ (accessed 8 January 2020)
- Johnson G., Ayers M., McClure S.C., Richardson S.E., Tellier R. Detection and identification of Bartonella species pathogenic for humans by PCR amplification targeting the riboflavin synthase gene (ribC) J. Clin. Microbiol. 2003;41:1069–1072. doi: 10.1128/JCM.41.3.1069-1072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy M., Bai Y., Lynch T., Kuzmin I.V., Niezgoda M., Franka R., Agwanda B., Breiman R.F., Rupprecht C.E. Bartonella spp. in bats, Kenya. Emerg. Infect. Dis. 2013;16:1875–1881. doi: 10.3201/eid1612.100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scola B., Zeaiter Z., Khamis A., Raoult D. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends Microbiol. 2003;11:318–321. doi: 10.1016/s0966-842x(03)00143-4. [DOI] [PubMed] [Google Scholar]
- Lilley T.M., Wilson C.A., Bernard R.F., Willcox E.V., Vesterinen E.J., Webber Q.M., Kurpiers L., Prokkola J.M., Ejotre I., Kurta A., Field K.A., Reeder D.M., Pulliainen A.T. Molecular detection of candidatus Bartonella mayotimonensis in North American bats. Vector Borne Zoonotic Dis. 2017;17:243–246. doi: 10.1089/vbz.2016.2080. [DOI] [PubMed] [Google Scholar]
- Lin E.Y., Tsigrelis C., Baddour L.M., Lepidi H., Rolain J.M., Patel R., Raoult D. Candidatus Bartonella mayotimonensis and endocarditis. Emerg. Infect. Dis. 2010;16:500–503. doi: 10.3201/eid1603.081673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.W., Hsu Y.M., Chomel B.B., Lin L.K., Pei J.C., Wu S.H., Chang C.C. Identification of novel Bartonella spp. in bats and evidence of Asian gray shrew as a new potential reservoir of Bartonella. Vet. Microbiol. 2012;156:119–126. doi: 10.1016/j.vetmic.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsue M., Fujiwara N., Oshio T., Iitsuka Y., Uchiyama T. A Draft of the Guideline for Ecological Surveys on Bat Species (in Japanese) 2006. http://www.nilim.go.jp/lab/bcg/siryou/tnn/tnn0354.htm Retrieved from. (accessed 8 January 2020)
- McKee C.D., Kosoy M.Y., Bai Y., Osikowicz L.M., Franka R., Gilbert A.T., Boonmar S., Peruski L.F. Diversity and phylogenetic relationships among Bartonella strains from Thai bats. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhldorfer K. Bats and bacterial pathogens: a review. Zoonoses Public Health. 2013;60:93–103. doi: 10.1111/j.1863-2378.2012.01536.x. [DOI] [PubMed] [Google Scholar]
- Norman A.F., Regnery R., Jameson P., Greene C., Krause D.C. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renesto P., Gouvernet J., Drancourt M., Roux V., Raoult D. Use of rpoB gene analysis for detection and identification of Bartonella species. J. Clin. Microbiol. 2001;39:430–437. doi: 10.1128/JCM.39.2.430-437.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Mogi M. Records of some blood-sucking flies from birds and bats of Japan (Diptera: Hippoboscidae, Nycteribiidae and Streblidae) Rishiri Stud. 2008;27:41–48. [Google Scholar]
- Sato S., Kabeya H., Yamazaki M., Takeno S., Suzuki K., Kobayashi S., Souma K., Masuko, T. Chomel. B.B. and Maruyama, S. Prevalence and genetic diversity of Bartonella species in sika deer (Cervus nippon) in Japan. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:575–581. doi: 10.1016/j.cimid.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Serra-Cobo J., López-Roig M. Bats and emerging infections: an ecological and virological puzzle. Adv. Exp. Med. Biol. 2017;972:35–48. doi: 10.1007/5584_2016_131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E., Frederiksen W., Garrity G.M., Grimont P.A., Kampfer P., Maiden M.C., Nesme X., Rossello-Mora R., Swings J., Truper H.G., Vauterin L., Ward A.C., Whitman W.B. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 2002;52:1043–1047. doi: 10.1099/00207713-52-3-1043. [DOI] [PubMed] [Google Scholar]
- Stuckey M.J., Chomel B.B., de Fleurieu E.C., Aguilar-Setién A., Boulouis H.J., Chang C.C. Bartonella, bats and bugs: a review. Comp. Immunol. Microbiol. Infect. Dis. 2017;55:20–29. doi: 10.1016/j.cimid.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Stuckey M.J., Boulouis H.J., Cliquet F., Picard-Meyer E., Servat A., Arechiga-Ceballos N., Echevarria J.E., Chomel B.B. Potentially zoonotic Bartonella in bats from France and Spain. Emerg. Infect. Dis. 2017;23:539–541. doi: 10.3201/eid2303.160934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey M.J., Chomel B.B., Galvez-Romero G., Olave-Leyva J.I., Obregón-Morales C., Moreno-Sandoval H., Aréchiga-Ceballos N., Salas-Rojas M., Aguilar-Setién A. Bartonella infection in hematophagous, insectivorous, and phytophagous bat populations of Central Mexico and the Yucatan Peninsula. Am. J. Trop. Med. Hyg. 2017;97:413–422. doi: 10.4269/ajtmh.16-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushadze L., Bai Y., Osikowicz L., McKee C., Sidamonidze K., Putkaradze D., Imnadze P., Kandaurov A., Kuzmin I., Kosoy M. Prevalence, diversity, and host associations of Bartonella strains in bats from Georgia (Caucasus) PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veikkolainen V., Vesterinen E.J., Lilley T.M., Pulliainen A.T. Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis. Emerg. Infect. Dis. 2014;20:960–967. doi: 10.3201/eid2006.130956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeaiter Z., Liang Z., Raoult D. Genetic classification and differentiation of Bartonella species based on comparison of partial ftsZ gene sequences. J. Clin. Microbiol. 2002;40:3641–3647. doi: 10.1128/JCM.40.10.3641-3647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information of Bartonella strains used in the phylogenetic analysis of the gltA.