Graphical abstract
Keywords: Chinese patent medicines, COVID-19, Review, Current evidence
Abstract
The outbreak of emerging infectious pneumonia caused by 2019 Novel Coronavirus (2019-nCoV) has posed an enormous threat to public health, and traditional Chinese medicine (TCM) have made vast contribution to the prevention, treatment and rehabilitation of coronavirus disease 19 (COVID-19) among Chinese population. As an indispensable part of TCM, Chinese patent medicines (CPMs) are highly valued and critically acclaimed in their campaign to contain and tackle the epidemic, they can achieve considerable effects for both suspected cases under medical observation period, and confirmed individuals with serious underlying diseases or critical conditions. Given this, based on the Guideline on Diagnosis and Treatment of Coronavirus Disease 2019 in China, the present review summarized the basic information, clinical evidence and published literatures of recommended CPMs against COVID-19. The details were thoroughly introduced involving compositions, therapeutic effects, clinical indications, medication history of CPMs and the profiles of corresponding research. With regard to infected patients with different stages and syndrome, the preferable potentials and therapeutic mechanism of CPMs were addressed through the comprehensive collection of relevant literatures and on-going clinical trials. This study could provide an insight into clinical application and underlying mechanism of recommended CPMs against COVID-19, with the aim to share the Chinese experience in clinical practice and facilitate scientific development of TCM, especially CPMs in the fierce battle of COVID-19.
1. Introduction
From its beginning in December 2019, a cluster of unexplained pneumonia cases have witnessed and reported in Wuhan City, Hubei Province, China [1,2]. The cause of the novel coronavirus disease (COVID-19) is identified as a novel betacoronavirus, named the 2019 novel coronavirus (2019-nCoV), the spread of severe acute respiratory syndrome has been almost entirely driven by 2019-nCoV via respiratory droplets, human-to-human transmission, posing pandemic potential with unfortunate characteristics of strong infectiousness, rapid dissemination, long incubation period, and general susceptibility [[3], [4], [5]]. In subsequent months, the ongoing outbreak of COVID-19 is rapidly spreading globally and declared as a public health emergency by the World Health Organization (WHO) [[6], [7], [8]]. As of April 4, 2020, more than 200 countries sufferred from the expansive spread of the virus 2019-nCoV in a global pandemic with massive and urgent crisis on healthcare, economic and social systems [[9], [10], [11], [12]], there were over one million confirmed cases with COVID-19 worldwide, of these, a total of 277,965 subjects were from the United States [13,14]. According to official release on April 4, 2020 by National Health Commission of the People’s Republic of China, more than 80,000 cases had been confirmed as COVID-19 with 3326 death cases [15].
Currently, the global pandemic of COVID-19 continues to accelerate and escalate, the health-care systems of different countries are beavering away to contain the virus, therefore, there is urgent need to seek for effective or adjuvant therapies with excellent safety profiles against COVID-19 [[16], [17], [18]]. In the theory of traditional Chinese medicine (TCM), 2019-nCoV infected pneumonia was deemed to the category of “Pestilence”, and the characteristics of its pathogenesis was “dampness, toxin, stasis and closure” [19,20]. Over the past few months, TCM exhibited remarkable benefits against COVID-19 in China, and it was convinced that TCM achieved satisfactory therapeutic superiority for patients infected by 2019-nCoV with regard to preventive treatment of diseases, comprehensive therapies and rehabilitation by the accumulation of clinical experience and scientific evidence [21,22]. As recommended in the Guideline on Diagnosis and Treatment of Coronavirus Disease 2019 (Revised 7th version) which was officially released by National Health Commission of the People’s Republic of China, TCM could exert favorable effects for patients with different syndromes and distinct stages of COVID-19, contributing to infections in the periods of both medical observation and clinical treatment [23]. Recently, the latest official data showed that 91.5 % of confirmed subjects with COVID-19 (74,187 cases) received TCM in China, and increasing results of clinical observation indicated that the total effective rate of TCM reached more than 90 % [24]. These promising advantages were associated with its unique therapeutic principles including syndrome differentiation and treatment, boosting the individual's endogenous healing ability, balancing Yin and Yang, various therapies and personalized treatment in first-line clinic [25,26].
With the development of technology in TCM domain, Chinese herbal products were transformed into varied dosage forms, Chinese patent medicines (CPMs) were consumer-near and popular "folk medicine" that contributed to widely application in clinical practice among Chinese citizens [27,28]. Compared to herbal decoction, CPMs had the advantages of stable quality, curative efficacy, considerable safety, rapid absorption, high bioavailability, convenience of taking, carrying and storing [29,30]. Similarly, as an indispensable part of TCM, CPMs were substantial utilized in combination with western medicine for the management of COVID-19, and proposed as adjunctive and therapeutic options to fight the public health emergency of 2019-nCoV by national and provincial guidelines in China [[31], [32], [33]]. Given the paucity of published English research concerning CPMs against COVID-19 currently, the present review performed a descriptive analysis of recommended CPMs from both clinical trials and published literatures, based on the following aspects: information retrieved from their instructions (compositions, therapeutic effects, and clinical indications), medical evidence, pharmacodynamic mechanism, dominant components, applicable patients, clinical cautions and so on. As regarding to some types of CPMs without accessible published evidence for treating 2019-nCoV, we introduced the relevant results of infectious or health-threatening diseases. The aim of present review was to provide the scientific basis and share clinical experiences for promising choices of CPMs against COVID-19.
2. The profiles of current evidence
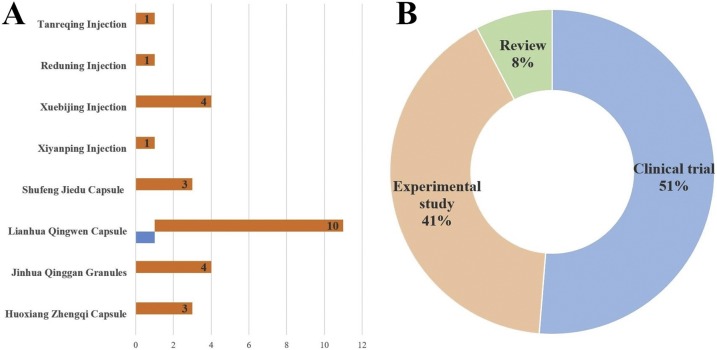
First, the comprehensive retrieval of electronic databases in both Chinese and English (the China National Knowledge Infrastructure Database, WangFang Database, Sinomed Database, PubMed and Embase) was performed for collecting the published research from inception to Apr 10, 2020. On the one hand, the following terms of COVID-19 were adopted: “COVID-19 [Supplementary Concept],” “2019 novel coronavirus”, “COVID19”, “SARS-CoV-2”, “2019-nCoV”, “coronavirus disease 2019”, “coronavirus disease-19”. On the other hand, the searching terms of CPMs mainly included their Mandarin Chinese and trade name. The results of literature search were displayed in Fig. 1 A, a total of 28 citations were yielded initially for eight CPMs. Only one eligible study was in English, focused on the anti-viral and anti-inflammatory activities of Lianhuaqingwen Capsule for treating COVID-19, which published by the team of distinguished Academician Nanshan Zhong.
Fig. 1.
The profiles of current evidence for CPMs against COVID-19.
Note: A: The retrieval results for eligible studies. B: The distribution of research types.
In addition, the on-going clinical trials concerning on CPMs anagist COVID-19 were supplemented to identify the potential evidence by utilizing the platform of Chinese Clinical Trial Register (www.chictr.org.cn/). Ultimately, there were 11 undergoing clinical trials that used CPMs for the treatment of 2019-nCoV infection, namely, Xiyanping injection (Registration Number: ChiCTR2000030218, ChiCTR2000030117, ChiCTR2000029756), Lianhua Qingwen Capsule/ Granules (ChiCTR2000029434, ChiCTR2000029433), Xuebijing Injection (ChiCTR2000030388, ChiCTR2000029381), Tanreqing Injection/Capsule (ChiCTR2000029432, ChiCTR2000029813), Reduning Injection (ChiCTR2000029589), Shenfu Injection (ChiCTR2000030043). Taken together, the research type of clinical trials occupied approximately half proportion in current evidence (Fig. 1B).
3. Basic information of recommended CPMs against COVID-19
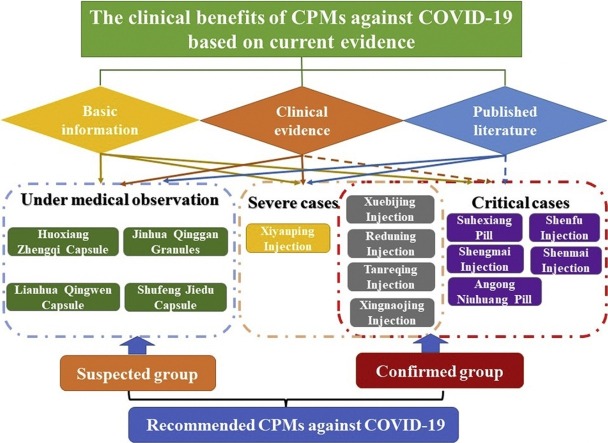
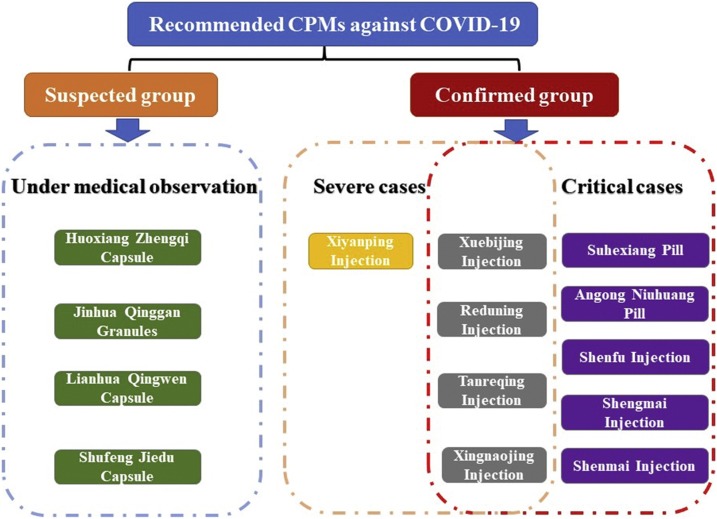
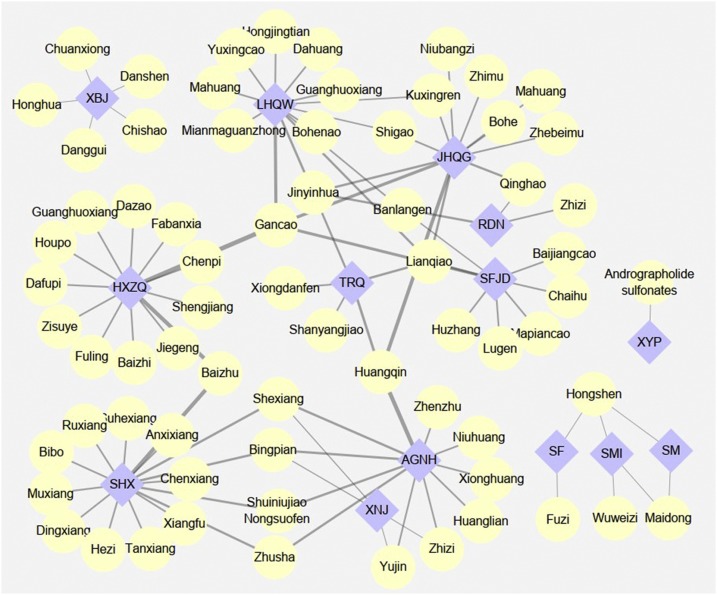
According to aforementioned guidelines of COVID-2019 in China, totally 14 types of CPMs were officially issued to prevent and treatment COVID-19, 57.14 % (8 types) of them were Chinese herbal injection, and others were oral dosage forms of TCM. As illustrated in Fig. 2 , there were different optimal choice among CPMs corresponding to infected individuals with different stages or TCM syndromes, for example, the suspected cases under medical observation could receive the CPMs including Huoxiang Zhengqi Capsule, Jinhua Qinggan Granules, Lianhua Qingwen Capsule, Shufeng Jiedu Capsule according to their specific clinical manifestations and syndrome differentiation in the theory of TCM. Remarkably, CPMs were often composed by many kinds of Chinese herbal materials, there was a vast difference between their compositions among enrolled CPMs, for instance, Xiyanping Injection contained sole active ingredient, whereas Suhexiang Pill was processed from 15 crude herbs. The relationship of CPMs and their compositions was depicted in Fig. 3 . Further, the details involving compositions, therapeutic effects, clinical indications for recommended CPMs were summarized in Table 1 , to provide the relative references for clinician and specialists to control the spread of this fatal disease.
Fig. 2.
Recommended CPMs and corresponding applicable patients with COVID-19.
Fig. 3.
The relationship of recommended CPMs and compositions.
Note: The purple nodes represented different CPMs, and compositions were labelling yellow. AGNH: Angong Niuhuang Pill; HXZQ: Huoxiang Zhengqi Capsule; JHQG: Jinhua Qinggan Granules; LHQW: Lianhua Qingwen Capsule; RDN: Reduning Injection; SF: Shenfu Injection; SFJD: Shufeng Jiedu Capsule; SHX: Suhexiang Pill; SM: Shenmai Injection; SMI: Shengmai Injection; TRQ: Tanreqing Injection; XBJ: Xuebijing Injection; XNJ: Xingnaojing Injection; XYP: Xiyanping Injection.
Table 1.
Summary of instructions for recommended CPMs in the Guidelines on Diagnosis and Treatment of COVID-2019.
| Name of CPMs | Compositions | Therapeutic effects | Clinical indications |
|---|---|---|---|
| Huoxiang Zhengqi Capsule | Guanghuoxiang (Pogostemonis Herba), Zisuye (Perillae Folium), Baizhi (Angelicae Dahuricae Radix), Baizhu (Atractylodis Macrocephalae Rhizoma), Chenpi (Citri Reticulatae Pericarpium), Fabanxia (Pinelliae Rhizoma Praeparatum), Houpo (Magnoliae Officinalis Cortex), Fuling (Poria), Jiegeng (Platycodonis Radix), Gancao (Glycyrrrhizae Radix Et Rhizoma), Dafupi (Arecae Pericarpium), Dazao (Jujubae Fructus), Shengjiang (Zingiberis Rhizoma Recens) | Relieving exterior and resolving dampness, regulating Qi and the middle warmer | Headache and dizziness, stuffiness of chest and diaphragm, abdominal distention and pain, vomiting and diarrhea induced by exogenous wind-cold and endogenous damp stagnation. |
| Jinhua Qinggan Granules | Jinyinhua (Lonicerae Japonicae Flos), Shigao (Gypsum Fibrosum), Mahuang (Ehedraep Herba), Kuxingren (Armeniacae Seman Amarum), Huangqin (Scutellariae Radix), Lianqiao (Forsythiae Fructus), Zhebeimu (Fritillariae Thunbergii Bulbus), Zhimu (Anemarrhenae Rhizoma), Niubangzi (Arctii Fructus), Qinghao (Artemisiae Annuae Herba), Bohe (Menthae Haplocalycis Herba), Gancao (Glycyrrrhizae Radix Et Rhizoma) | Dispelling wind and dispersing lung, clearing heat and detoxication | Mild symptom of simplex influenza, the syndrome differentiation of TCM belongs to wind-heat attacking the lung syndromes, including fever, headache, body aches, pharyngalgia, cough, aversion to wind or cold, nasal congestion and rhinorrhea, tongue texture with red, tongue coating with yellow-thin, rapid pulse. |
| Lianhua Qingwen Capsule | Lianqiao (Forsythiae Fructus), Jinyinhua (Lonicerae Japonicae Flos), Mahuang (Ehedraep Herba), Kuxingren (Armeniacae Seman Amarum), Shigao (Gypsum Fibrosum), Banlangen (Isatidis Radix), Mianmaguanzhong (Dryopteridis Crassirhizomatis Rhizoma), Guanghuoxiang (Pogostemonis Herba), Dahuang (Rhei Radix Et Rhizoma), Yuxingcao (Houttuyniae Herba), Hongjingtian (Rhodiolae Crenulatae Radix Et Rhizoma), Bohenao (l-Menthol), Gancao (Glycyrrrhizae Radix Et Rhizoma) | Clearing pestilence and detoxication, dispersing lung and dispersing heat | Influenza belongs to syndrome of noxious heat attacking lung, including fever or hyperthermia, aversion to cold, muscles aches, nasal congestion and rhinorrhea, cough, headache, pharyngeal dryness and pharyngalgia, tongue texture with red, tongue coating with yellow or yellow-greasy, etc. |
| Shufeng Jiedu Capsule | Huzhang (Polygoni Cuspidati Rhizoma Et Radix), Lianqiao (Forsythiae Fructus), Banlangen (Isatidis Radix), Chaihu (Bupleuri Radix), Baijiangcao (Patriniae Herba), Mapiancao (Verbenae Herba), Lugen (Phragmitis Rhizoma), Gancao (Glycyrrrhizae Radix Et Rhizoma) | Dispelling wind and clearing heat, detoxication and relieving sore throat | Acute upper respiratory infection belongs to wind-heat syndrome, including fever, aversion to wind, pharyngalgia, headache, nasal congestion and rhinorrhea, cough, etc. |
| Xiyanping Injection | Andrographolide sulfonates | Clearing heat and detoxication, relieving cough and stopping dysentery | Bronchitis, tonsillitis, bacillary dysentery, etc. |
| Xuebijing Injection | Honghua (Carthami Flos), Chishao (Paeoniae Radix Rubra), Chuanxiong (Chuanxiong Rhizoma), Danshen (Salviae Miltiorrhizae Radix Et Rhizoma), Danggui (Angelicae Sinensis Radix) | Removing blood stasis and detoxication | Diseases of warm febrile class, including fever, dyspnea with rapid and short breath, palpitation, fidgetiness and other toxin-stasis syndromes, systemic inflammatory response syndrome induced by infection, and it used as adjuvant therapy for multiple organ dysfunction syndrome. |
| Reduning Injection | Qinghao (Artemisiae Annuae Herba), Jinyinhua (Lonicerae Japonicae Flos), Zhizi (Gardeniae Fructus) | Clearing heat, dispelling wind, detoxication | Upper respiratory infection induced by exogenous wind-heat syndrome, including hyperthermia, slightly aversion to cold, headache, cough, yellow phlegm, etc. |
| Tanreqing Injection | Huangqin (Scutellariae Radix), Xiongdanfen (Bear Bile Powder), Shanyangjiao (Cornu Caprae Hircus), Jinyinhua (Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus) | Clearing heat, reducing phlegm, detoxication | Wind-warm diseases with lung heat and pulmonary retention of phlegmopyrexia syndrome, including fever, cough, ungratifying expectoration of phlegm, sore throat, thirst, tongue texture with red, tongue coating with yellow. Early stage of pneumonia, acute bronchitis, acute exacerbations of chronic bronchitis and upper respiratory tract infection belong to the above syndromes. |
| Xingnaojing Injection | Shexiang (Moschus), Yujin (Curcumae Radix), Bingpian (Borneolum Syntheticum), Zhizi (Gardeniae Fructus) | Clearing heat and detoxication, cooling blood and activating blood circulation, inducing resuscitation | Stroke coma, hemiplegia induced by Qi and blood adversity, cerebral channels stasis. Traumatic headache, delirium. Headache, vomiting and diarrhea, coma, convulsion induced by alcoholism. Cerebral embolism, acute stage of cerebral hemorrhage, craniocerebral trauma, acute alcoholism belongs to the above syndromes. |
| Suhexiang Pill | Suhexiang (Styrax), Anxixiang (Benzoinum), Bingpian (Borneolum Syntheticum), Shuiniujiao Nongsuofen (Powerered Buffalo Horn Extract), Shexiang (Moschus), Tanxiang (Santali Albi Lignum), Chenxiang (Aquilariae Lignum Resinatum), Dingxiang (Caryophylli Flos), Xiangfu (Cyperi Rhizoma), Muxiang (Aucklandiae Radix), Ruxiang (Olibanum), Bibo (Piperis Longi Fructus), Baizhu (Atractylodis Macrocephalae Rhizoma), Hezi (Chebulae Fructus), Zhusha (Cinnabaris) | Aromatic resuscitation, promoting Qi circulation and relieving pain | Phlegm syncope and coma, apoplectic hemiplegia, inconvenient activity of limbs, heatstroke induced by phlegm confusing heart syndrome. |
| Angong Niuhuang Pill | Niuhuang (Bovisc Alculus), Shuiniujiao Nongsuofen (Powerered Buffalo Horn Extract), Shexiang (Moschus), Zhenzhu (Margarita), Zhusha (Cinnabaris), Xionghuang (Realgar), Huanglian (Coptidis Rhizama), Huangqin (Scutellariae Radix), Zhizi (Gardeniae Fructus), Yujin (Curcumae Radix), Bingpian (Borneolum Syntheticum) | Clearing heat and detoxication, relieving convulsion and inducing resuscitation | Heat diseases including invasion of pericardium by evil, febrile convulsion, coma and delirium. Apoplectic coma, encephalitis, cephalomeningitis, toxic encephalopathy, hematencephalon, septicemia belongs to the above syndromes. |
| Shenfu Injection | Hongshen (Ginseng Radix Et Rhizoma Rubra), Fuzi (Aconiti Lateralis Radix Praeparaia) | Reviving yang for resuscitation, tonifying Qi and preventing exhaustion | Syncope syndrome induced by Yang-Qi deficiency (infectious, hemorrhagic, hypovolemic shock,etc.). Palpitation, asthma, cough, stomachache, diarrhea, arthralgia induced by Yang deficiency (Qi deficiency). |
| Shengmai Injection | Hongshen (Ginseng Radix Et Rhizoma Rubra), Maidong (Ophiopogonis Radix), Wuweizi (Schisandrea Chinensis Fructus) | Tonifying Qi and nourishing Yin, recovering pulse and preventing exhaustion | Palpitation, shortness of breath, limbs frosty, old sweat, barely palpable pulse induced by deficiency of Qi and Yin. Myocardial infarction, cardiogenic shock, septic shock and other belongs to the above syndromes. |
| Shenmai Injection | Hongshen (Ginseng Radix Et Rhizoma Rubra), Maidong (Ophiopogonis Radix) | Tonifying Qi and preventing exhaustion, nourishing Yin and generating body fluid, activating pulse | Shock, coronary heart disease, viral myocarditis, chronic cor pulmonale, granulocytopenia induced by deficiency of Qi and Yin. It can improve the immune function of patients with tumors. When combined with chemotherapeutic drugs, it has a certain synergistic effect and can reduce the side effects caused by chemotherapeutic drugs. |
4. Preferable benefits of CPMs for cases under medical observation
Based on the findings of related review, the CPMs including Huoxiang Zhengqi Capsule, Jinhua Qinggan Granules, Lianhua Qingwen Capsule, Shufeng Jiedu Capsule could be presumably as preventive measure for the patients in the medical observation period [34]. It was noteworthy that early treatment was essential for suspected or mild cases, accumulated evidence of bioinformatics, pharmacodynamics and clinical findings suggested that these multiple component Chinese herbal products also exerted effects on immune regulation, symptom improvement, anti-inflammation and so on during the treatment of COVID-19.
The results of previously pharmacological research indicated that Huoxiang Zhengqi Capsule or other dosage forms could improve gastrointestinal dysfunction, modulate immune responses, and anti-inflammation [35]. With regard to the national and provincial guidelines against COVID-19 in China, Huoxiang Zhengqi Capsule was recommended for patients with fatigue and gastrointestinal discomfort [36]. Through the techniques of network pharmacology and molecular docking, Huoxiang Zhengqi Oral Liquid could regulate multiple signaling pathways involving Hepatitis B, small cell lung cancer, non-small cell lung cancer and inhibit the replication of 2019-nCoV to exhibit the preventive or therapeutic effects on COVID-19, and its active compounds had definite affinity with angiotensin converting enzyme II (ACE2) and 3- chymotrypsin-like protease (3CLpro) [37,38].
Compared with other recommended CPMs, Jinhua Qinggan Granules posed the shorter period of medication history, nevertheless, it was proved that the application of Jinhua Qinggan Granules could significantly alleviate clinical symptoms such as fever, cough, fatigue, expectoration, and relieve psychological anxiety of mild cases suffered from COVID-19 [39]. The results of systems biology and bioinformatics revealed that the mechanism of Jinhua Qinggan granules in the treatment of COVID-19 involving multiple targets, namely MAPK1, CASP3, TP53, ALB, TNF, IL6, and multiple pathways, which might be related to antiviral, immune regulation, inflammation inhibition and apoptosis regulation via PI3K-Akt, HIF-1, TNF, MAPK, NF-κB pathways, and dominant principles including kaempferol, baicalein and oroxylin A could take participate in multiple signal pathways (such as PTGS2, HSP90AB1, PTGS2, BCL2 and CASP3) by binding with ACE2 [[40], [41], [42]].
Relieving typical symptoms and representative complications, diminishing inflammation and infection, the Chinese herbal products of Lianhua Qingwen Capsule was recognized as an excellent antidote in this anti-epidemic by adequate clinical and fundamental research [34,43]. According to preliminary clinical evidence of retrospective, multicenter study and cases reports, for general type patients and suspected cases with COVID-19, the scheme of Lianhua Qingwen Capsule combined with western medicine had considerable effective rate without obvious adverse reactions (ADRs), and it could not only improve clinical symptoms including fatigue, fever, cough, expectoration, shortness of breath, chest tightness, poor appetite, etc., but also control the progression of upper respiratory tract infection, reduce the rate of conversion from mild into serious status, shorten the duration of hospital stay [[44], [45], [46], [47], [48]]. Similarly, accumulated evidence displayed that its pharmacodynamic mechanisms were associated with the biological functions involving anti-inflammation, inhibition of 2019-nCoV replication, affection of virus morphology, activation of T-cell, molecular response to bacterial origin, broad-spectrum antivirus, immune regulation and so on [[49], [50], [51], [52]].
Meanwhile, compared with the control group that only receiving arbidol, Shufeng Jiedu Capsule combined with arbidol could achieve favorable effects in the treatment of COVID-19 and without reported drug-related adverse events, there were significant improvements in CD4+ and CD8+ T cell subpopulations, white blood cell and CT imaging, antipyretic time, the disappearance time of dry cough, nasal congestion, pharyngeal pain, fatigue, diarrhea, and the negative conversion of nucleic acid examination for 2019-nCoV [53,54]. The latest research investigated the potential targets and mechanisms of Shufeng Jiedu Capsule for treating COVID-19 via constructing the "drug-component-disease-target" network, the results demonstrated that therapeutic mechanism involved a variety of biological processes such as the interaction of viral proteins with cytokines, with critical proteins as IL-6, ALB, MAPK3 [55]. For patients infected 2019-nCoV, the large amounts of cytokines might be associated with rapidly provoking of acute respiratory distress syndrome (ARDS), single or multiple organ failure, and eventually death.
5. Therapeutic potentials for severe or critical patients
Depending on the differentiation of clinical syndromes, the severe or critical subjects could receive the Chinese herbal injections that prescribed by Chinese herbalists or doctors, including Xiyanping Injection, Xuebijing Injection, Reduning Injection, Tanreqing Injection, Xingnaojing Injection [56]. Among them, only Xiyanping Injection was applied for treating severe patients, whereas others were also recognized as the complementary choices for critical cases with COVID-19. Compared with oral administration of TCM, the Chinese herbal injections possessed the benefits of rapid onset, high bioavailability, and content accuracy [57], therefore, they were more suitable for the severe or critical patients with COVID-19.
Some scholars concluded that Xiyanping Injection was reputed as effective alternative to antibiotics in clinical practice [58]. Modern pharmacological studies showed that its active ingredient, sulfonated andrographolide had notable effects of antipyretic, anti-inflammation to treat various infectious diseases [59]. In addition, prevenient Chinese research pointed out its clinical advantages that were related to improve respiratory symptoms, inhibit concurrent bacterial infection, and regulate immune function, superior clinical safety, especially certain hepatoprotective effects, suggesting it might have potentials to relieve some drug-induced liver injury during the treatment of COVID-19 for serious cases [60]. Remarkably, it was reported that andrographolide sulfonate could ameliorate sepsis in mice through suppressing MAPK, STAT3 and NF-κB pathways, these pathways also played the important role in pulmonary diseases [[61], [62], [63]].
The prescription of Xuebijing Injection originated from therapeutic principles of TCM, that was proposed by famous integrative medicine emergency experts, Professor Jin-Da Wang, it was approved as second grade national new medicine for treating sepsis in China over 15 years [64,65]. In this clinical fight against COVID-19, considerable effects of Xuebijing injection had been displayed by retrospective study and relevant review, the results revealed that Xuebijing injection could promote the absorption of lung infection, and improve clinical efficacy and negative rate of nucleic acid [66,67]. Besides, some research demonstrated its characteristics of multi-target and multi-pathway in treating COVID-19 based on the approaches of network pharmacology and molecular docking. Among a series of involved signaling pathways, such as HIF-1 and PI3K-Akt were represented pathways against COVID-19 in terms of lung inflammation, virus infection and lung injury. Besides, core targets including TNF, MAPK1, JUN, IL6, STAT3, EGFR, etc. were closely correlation with the inhibition of cytokine storm in severe cases, and fatal risk of cytokine storms in the immune system of serious patients might result in organ failure and even death [[68], [69], [70]].
Reduning Injection was widely utilized to treat upper respiratory tract infection with multiple functions such as clearing heat, dispelling wind, and detoxification, and previous pharmacological research proposed that it could ameliorate paraquat-induced acute lung injury involved in regulating AMPK/MAPK/NF-κB signaling pathways [71]. In this regard to treating COVID-19, Reduning injection might be related to anti-inflammatory, immunoregulation of active compounds through multi-target and multi-pathway. On the one hand, the critical targets were namely PTGS2, PTSG1, CCL2, RELA, NOS2, HMOX1, CASP3, IL6 and MAPK1, some of them belonged to chemokines, which posed the immune activation profiles of 2019-nCoV. On the other hand, KEGG pathway enrichment analysis revealed 45 related pathways, mainly IL-17, C-type lectin receptor, HIF-1 and NF-κB signaling pathways [72,73].
A large number of clinical data had accumulated to confirm superior efficacy of Tanreqing Injection in the treatment of acute bronchitis disease, tuberculosis and so on [74,75]. In particular, the severe patients with COVID-19 suffered similar clinical manifestations of its dominant diseases. Meanwhile, the underlying mechanism and binding activity of Tanreqing Injection were elucidated, the results in molecular level showed that it might be potential as antiviral agent due to critical components (kaempferol, quercetin, baicalein luteolin, wogonin, etc.) had good affinity with 3CLpro of 2019-nCoV. The enrichment analysis of biological process indicated that the target of Tanreqing injection involved in inflammatory response, immune system, signal pathway and apoptosis process. Interesting, the core targets involved IL6, IL1B, MAPK1, IL10, IL4, CXCL8, IP10, etc. [76]. This finding was consistent with former clinical report regarding severe patients with higher level of IL2, IL7, IL10, GSCF, IP10 in the plasma [77].
The commercialized injectable product of Xingnaojing Injection was extracted and refined scientifically from classic Chinese emergency prescription "Angong Niuhuang Pill", it had the functions of clearing heat and detoxication, cooling blood and activating blood circulation, inducing resuscitation and widely used in the treatment of intracerebral haemorrhage, cerebral ischemia, and nervous system disorders in China [[78], [79], [80]]. In view of critical cases with COVID-19 might suffer from consciousness disturbance, Xingnaojing Injection had major biological effects to relax the cerebral vascular and protect the mature neuron, in vivo and in vitro research, it was confirmed that the mechanism of cerebrovascular protection might be relevant to the activation of PI3K/Akt/eNOS signaling pathways and the suppression of NLRP3 inflammasomes [81,82].
6. Only adjuvant rescue for critical infection
In aforementioned guidelines, five CPMs were recommended as adjuvant rescue just for critical infections with COVID-19, namely Suhexiang Pill, Angong Niuhuang Pill, Shenfu Injection, Shengmai Injection, Shenmai Injection. Currently, there were paucity of accessibly published evidence concerning on these CPMs and 2019-nCoV simultaneously, it was urgent and essential that subsequently clinical trials or pharmacological research to provide sufficient references for clinical recommendation. Herein, we brief summarized the findings in the field of infectious or health-threatening diseases to supplement the correlative knowledge.
Although both of them were famous Chinese emergency prescription and contained same resuscitation-inducing aromatic herbs as Bingpian (Borneolum Syntheticum), Suhexiang Pill and Angong Niuhuang Pill were used for patients with opposite syndromes. The former was adopted to seizures, infantile convulsions and stroke with cold syndromes [83], therefore, Suhexiang Pill might achieve therapeutic effectiveness for COVID-19 cases with critical conditions such as delirium, phlegm syncope, central nervous depression, and coma or worsen. Its neuroprotective, anticonvulsant and antioxidative effects had been proven by fundamental research both in vitro and in vivo, suggesting its pharmacological mechanism involved the suppression of JNK hyperactivation and apoptosis, inhibition of EGFR/ERK pathways and glial cell proliferation, decreasing ROS formation and restoring mitochondrial function [[84], [85], [86], [87]]. As a recipe of “Liangkai Sanbao” in TCM theory, the latter was recognized to treat heat diseases only, including acute ischemic stroke, viral encephalitis, acute hemorrhagic stroke, and trauma brain injury, Angong Niuhuang Pill could play desirable role for treating critical infections with 2019-nCoV in attenuating the negative symptoms including hyperthermia, stupor, coma, etc. [88]. Meanwhile, based on the research was conducted on a high-fat and vitamin D3-induced rodent model of atherosclerosis, the results presented that Angong Niuhuang Pill had antiplatelet aggregation, lipid regulatory, antioxidant, anti-inflammatory and anti-apoptotic properties contributing to robust ant-atherosclerosis and cardio-protective effects [89], which might be beneficial for critical cases with cardiovascular and cerebrovascular diseases. The mechanisms of Angong Niuhuang Pill exhibited the neuroprotection was related to depressed Bax/Bcl-2 ratio and caspase-3 level, resulting the inhibition of apoptotic cell [90].
Three ginseng containing formulations, Shenfu Injection, Shengmai Injection and Shenmai Injection had similar therapeutic effects of tonifying Qi and preventing exhaustion, they could be adjuvant rescue and alternative treatment of COVID-19 patients with septic shock, viral myocarditis, and cardiogenic shock in clinical practice [[91], [92], [93]]. For example, in rabbits with LPS-induced septic shock, Shenfu injection could increase mean arterial pressure, decrease the serum lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and glutamate transaminase (ALT) levels, improve the tissue morphology of heart, liver and kidney, and increase the contents of ATP and taurine in the heart tissue during septic shock [94]. Besides, previous studies demonstrated that Shenfu Injection, Shengmai Injection and Shenmai Injection were associated with protective effects on lung ischemia-reperfusion injury, reducing chemotherapy-induced adverse effects, and promoting cellular immunity and cognitive dysfunction and so on, presumably, these clinical functions might improve symptoms of critical patients with COVID-19 in terms of lung inflammation, virus infection, drug-induced disease lung injury [[95], [96], [97], [98]].
7. Discussion
Since the globally health-care-associated outbreaks of 2019-nCoV, in China, the implementing “attach equal importance to TCM and Western medicine” policy in clinical practice exerted essential effects for treating COVID-19 [99,100]. Based on unique guiding principles of syndrome differentiation and treatment in TCM, its clinical superiority had considerable recognition and public acclaim in the furious battle for 2019-nCoV, the cumulative number of research revealed that its curative advantages involved in whole treatment process, namely prevent disease progression, alleviate clinical symptoms, improve the hospital stay and negative results of nucleic acid detection, and promote the physical recovery [21,22,101]. Notably, the majority of enrolled CPMs possessed the medical history approximately 20 years in clinical practice among Chinese citizens, therefore, the clinical applications of CPMs to tackle the epidemic were with definite therapeutic effects, clinical basis, social recognition and other superior factors. For example, Huoxiang Zhengqi Capsule and Lianhua Qingwen Capsule was over-the-counter medicines, and some CPMs were covered by national lists of essential medicine and basic insurance program in China.
Regarding suspected and mild patients with COVID-19, we calculated the frequency of compositions for each CPMs, the results revealed that the crude herbs with the higher frequency of recommended CPMs were Jinyinhua (Lonicerae Japonicae Flos), Lianqiao (Forsythiae Fructus) and Gancao (Glycyrrrhizae Radix Et Rhizoma), and they all had therapeutic effects of clearing heat and detoxication, which could bring potential for the prevention and treatment of 2019-nCoV. For instance, phillyrin, the natural lignan of Lianqiao, had antibacterial, anti-quorum sensing and anti-inflammatory activities for the control of infectious pathogens via the regulation the MyD88/IκBα/NF-κB signalling pathway, decrease the virus titers and IκBα, IL-1β, IL-6, TNF-α levels, reduction the expression of influenza hemagglutinin protein, and attenuation of lung tissue damage [[102], [103], [104]]. With regard to severe cases, the Chinses herbal injections could obviously alleviate clinical symptoms and correct complications. In particular, Xuebijing injection was widely used for treating patients with critical diseases including sepsis, severe pneumonia, severe acute pancreatitis, infection-induced systemic inflammatory response syndrome, chronic obstructive pulmonary disease (COPD), ARDS, multiple organ dysfunction syndrome (MODS) and so on [104,105]. Moreover, our study highlighted that its clinical beneficial effects in severe cases was closely associated with the inhibition of cytokine storm, improve virus infection and lung injury, superior binding activities with 3CLpro and ACE2. In addition, three types of recommended herbal injectable dosage forms contained Hongshen (Ginseng Radix Et Rhizoma Rubra), ginseng was generally known for its tonic properties, and previous findings also suggested it might be a promising supplemental remedy against infectious diseases, this administration of ginseng could fast and accurate adjust excess or deficiency between Yin or Yang and restore their balance among critical patients [[106], [107], [108]].
When great attention was paid to the application of TCM in real-world, there were growing concerns related to the potential toxicity or inevitable ADRs of herbal medicines. The results of pharmacovigilance pointed out the risk factors of ADRs triggered by TCM including responsible Chinese materia medica, susceptible patients and clinical administration [109]. In this regard, our research should emphasize the following aspects, including poisonous composition, the safety status of Chinese herbal injection, special population, and irrational drug use. First, Angong Niuhuang Pill contained cinnabar (HgS) and realgar (As2S2) which were correlated to hepatorenal toxicity [110]. Fuzi (Aconiti Lateralis Radix Praeparaia) in Shenfu Injection was associated with narrow therapeutic window, its cardiac ADRs has been frequently observed that mainly manifested as palpitations, hypotension, arrhythmia, ventricular fibrillation, and even shock [111,112]. Second, compared with other dosage forms of TCM medications, Chinese herbal injections were associated with the higher risk of ADRs, especially serious side effects [113,114]. Therefore, during the treatment of COVID-19, corresponding measures including drug safety monitoring and risk management for CPMs should be strengthened to achieve optimal benefits and minimal hazards. Third, the available evidence highlighted that special population including children, gravida and elderly people were particularly vulnerable to unfavorable drug responses, because their pharmacokinetic and pharmacodynamic profiles differed from the general population [115]. Indeed, clinical medication was complicated, the irrational uses reflected in misuse or abuse application of CPMs, overdose, repeated medication, dissolvant mismatch, pharmacologic antagonism, pharmaceutical incompatibility, herb-drug interaction and inappropriate syndrome differentiations and so on, these factors all would be culminating in drug-induced risk. The selection of treatments for individuals infected 2019-nCoV should be consistent with the theory of TCM. For example, it was not suitable to receive tonic herbs and Lianhua Qiwen Capsule for cases with COVID-19 simultaneously. Besides, Huoxiang Zhengqi Liquid contained ethanol, which could cause disulfiram-like reaction combined with cephazolin, cefoperazone, cefoperazone and sulbactam, latamoxef and so on [116]. To overall contain the COVID-19 pandemic, except for support medication and equipment supply, we suggested that pharmacists should play important role in the guarantee of medication safety including prescription checking and the rational use of CPMs.
Several limitations of our study should be noted. Unsatisfactory, there were lack of published research concerning on both COVID-19 and some recommended CPMs, such as those were only adjuvant rescue for critical infection (Suhexiang Pill, Angong Niuhuang Pill, Shenfu Injection, Shengmai Injection, Shenmai Injection). Thus, clinical benefits and therapeutic mechanism of CPMs against COVID-19 should be substantiated further confirmed and illustrated by the well-designed fundamental studies. Present review only focused on the recommended CPMs for treating COVID-19, except for the enrolled CPMs, it was noteworthy that plenty of prescriptions in TCM that were crucial to the prevention and management of COVID-2019, such as QingFei Paidu decoction, the relevant retrospective study showed that Qingfei Paidu decoction combined with western medicine in the treatment of COVID-19 was more effective than patients receiving western medicine alone, which can significantly shorten the hospitalization, improve clinical symptom and imaging results [117].
In conclusion, the present study was devoted to summarize the comprehensive information and updated evidence of recommended CPMs for different suitable patients with COVID-19. As accessible, efficient and modern products of TCM, CPMs played an indispensable role in the prevention and treatment of this epidemic diseases, it could be an alternative approach against COVID-19 in both suspected cases and high-risk population. In order to share Chinese experiences in clinical practice and provide scientific references for the international health systems, the continued evidence was still needed to support existing therapies of CPMs for 2019-nCoV.
Declaration of Competing Interest
The authors of the manuscript that titled “The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence” declared that there are no conflicts of no financial/personal interest.
Acknowledgement
This work is supported by the Programs Foundation for Leading Talents in State Administration of Traditional Chinese Medicine of China-“Qihuang scholars” Project (10400633210004), the National Natural Science Foundation of China (Grant No. 81874349), and National special support plan for high-level talents (Plan of ten thousand people)-Famous Teacher Program to Professor Bing Zhang.
References
- 1.Lancet The. Emerging understandings of 2019-nCoV. Lancet. 2020;395:311. doi: 10.1016/S0140-6736(20)30186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymann D.L. COVID-19: what is next for public health? Lancet. 2020;395(10224):542–545. doi: 10.1016/S0140-6736(20)30374-3. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Health Commission of the People’s Republic of China, The guideline on diagnosis and treatment of coronavirus disease 2019 (Revised 6th version). Tianjin Journal of Traditional Chinese Medicine:1–5 2020 http://kns.cnki.net/kcms/detail/12.1349.R.20200304.1638.006.html [In Chinese].
- 4.Weiss P. Clinical course and mortality risk of severe COVID-19. Lancet. 2020 doi: 10.1016/S0140-6736(20)30633-4. pii: S0140-6736(20)30633-30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haffajee R.L. Thinking globally, acting locally - the U.S. Response to Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2006740. [DOI] [PubMed] [Google Scholar]
- 7.Musa T.H. Global outbreak of 2019-nCoV, a new challenge? J. Infect. Dev. 2020;14(3):244–245. doi: 10.3855/jidc.12530. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . 2020. Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV)https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
- 9.Gostin L.O. Governmental public health powers during the COVID-19 pandemic: stay-at-home orders, business closures, and travel restrictions. JAMA. 2020 doi: 10.1001/jama.2020.5460. [DOI] [PubMed] [Google Scholar]
- 10.Holshue M.L. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothe C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Coronavirus disease (COVID-19) Situation dashboard. https://www.who.int/redirect-pages/page/novel-coronavirus-(covid-19)-situation-dashboard.
- 13.Johns Hopkins University. COVID-19 Data Center. https://coronavirus.jhu.edu/?utm_source=jhu_properties&utm_medium=dig_link&utm_content=ow_jhuhomepage&utm_campaign=jh20 [Last accessed on 2020 Apr 4].
- 14.World Health Organization. Coronavirus disease (COVID-19) Pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Last accessed on 2020 Apr 4].
- 15.National Health Commission of the People’s Republic of China. The epidemic situation coronavirus disease (COVID-19) up to 4 April 2020. http://www.nhc.gov.cn/xcs/yqfkdt/202004/185a308e4c66426190da0c4f2f9ab026.shtml [Last accessed on 2020 Apr 4].
- 16.FitzGerald G.A. Misguided drug advice for COVID-19. Science. 2020;367(6485):1434. doi: 10.1126/science.abb8034. [DOI] [PubMed] [Google Scholar]
- 17.Sargiacomo C. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging. 2020 doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mégarbane B. Chloroquine and hydroxychloroquine to treat COVID-19: between hope and caution. Clin. Toxicol. 2020:1–2. doi: 10.1080/15563650.2020.1748194. [DOI] [PubMed] [Google Scholar]
- 19.Chen J. Thoughts on prevention and treatment of coronavirus disease 2019(COVID-19) by traditional Chinese medicine. Chinese Traditional and Herbal Drugs. 2020;51(5):1106–1112. [In Chinese] [Google Scholar]
- 20.You Y.N. Therapeutic strategy of traditional Chinese medicine for 2019 novel coronavirus pneumonia. Drug Evaluation Research. 2020:1–7. http://kns.cnki.net/kcms/detail/12.1409.r.20200318.1735.002.html [In Chinese] [Google Scholar]
- 21.Ren J.L. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo H. Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020 doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Health Commission of the People’s Republic of China. The guideline on diagnosis and treatment of coronavirus disease 2019 (Revised 7th version). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml [In Chinese] [Last accessed on 2020 Apr 4].
- 24.Publicity Department of the People’s Republic of China. Press conference of the important role and effective drugs of traditional Chinese medicine in the prevention and treatment of COVID-19 on Mar 23, 2020. http://www.gov.cn/xinwen/2020-03/23/content_5494694.htm [accessed Feb 23, 2020; In Chinese].
- 25.Yang Y. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du H.Z. Traditional Chinese Medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP) Chin. J. Nat. Med. 2020;18(3):206–210. doi: 10.1016/S1875-5364(20)30022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H. Traditional Chinese herbal injection: current status and future perspectives. Fitoterapia. 2018;129:249–256. doi: 10.1016/j.fitote.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z. Analysis on dosage form theory and current application situation of traditional Chinese medicine pill. Zhongguo Zhong Yao Za Zhi. 2017;42(12):2408–2412. doi: 10.19540/j.cnki.cjcmm.20170416.001. [In Chinese] [DOI] [PubMed] [Google Scholar]
- 29.Hsu E. Chinese propriety medicines: an “alternative modernity?” the case of the anti-malarial substance artemisinin in East Africa. Med. Anthropol. 2009;28(2):111–140. doi: 10.1080/01459740902848303. [DOI] [PubMed] [Google Scholar]
- 30.Kang T. Establishment of a quality marker (Q-marker) system for Chinese herbal medicines using burdock as an example. Phytomedicine. 2019;54:339–346. doi: 10.1016/j.phymed.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Chan K.W. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western medicine for the management of 2019 novel coronavirus disease. Am. J. Chin. Med. (Gard City N Y) 2020:1–26. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 32.Ni L. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19 in Wuhan. Front. Med. 2020 doi: 10.1007/s11684-020-0757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin R. Expert consensus on prescription comment of Chinese traditional patent medicine for promoting the rational use of drugs in Beijing. Zhongguo Zhong Yao Za Zhi. 2018;43(5):1049–1053. doi: 10.19540/j.cnki.cjcmm. [In Chinese] [DOI] [PubMed] [Google Scholar]
- 34.Runfeng L. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Y.H. Effects of huoxiangzhengqi liquid on enteric mucosal immune responses in mice with Bacillus dysenteriae and Salmonella typhimurium induced diarrhea. World J. Gastroenterol. 2006;12(45):7346–7349. doi: 10.3748/wjg.v12.i45.7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huo Z.P. Research progress on potential application of Huoxiang Zhengqi in prevention and treatment of coronavirus disease 2019. Drugs & Clinic. 2020;35(3):405–410. [In Chinese] [Google Scholar]
- 37.Deng Y.J. Study on active compounds from Huoxiang Zhengqi Oral Liquid for prevention of coronavirus disease 2019(COVID-19) based on network pharmacology and molecular docking. Chinese Traditional and Herbal Drugs. 2020;51(5):1113–1122. [In Chinese] [Google Scholar]
- 38.Du Ht., et al., Preliminary study on the effective components and mechanism of Huoxiang Zhengqi Decoction in inhibiting the replication of novel coronavirus. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology:1-7[2020-04-03]. http://kns.cnki.net/kcms/detail/11.5699.r.20200331.0834.002.html. [In Chinese].
- 39.Duan C. Clinical observation of novel coronavirus infected pneumonia treated by Jinhua Qinggan Granule. J. Tradit. Chinese Med. 2020:1–5. http://kns.cnki.net/kcms/detail/11.2166.R.20200323.0853.002.html [In Chinese] [Google Scholar]
- 40.Gong P.Y. Exploring active compounds of Jinhua Qinggan Granules for prevention of novel coronavirus pneumonia (COVID-19) based on network pharmacology and molecular docking. Chinese Traditional and Herbal Drugs. 2020:1–9. http://kns.cnki.net/kcms/detail/12.1108.R.20200326.1115.002.html [In Chinese] [Google Scholar]
- 41.Jimilihan S.M.Y. Study on the active components in the adjuvant treatment of novel coronavirus pneumonia (COVID-19) with Jinhua Qinggan Granules based on network pharmacology and molecular docking. Journal of Chinese Medicinal Materials. 2020:1–10. http://kns.cnki.net/kcms/detail/44.1286.R.20200323.1926.002.html [In Chinese] [Google Scholar]
- 42.Mao J. Discussion on the mechanism of Jinhua Qinggan Granule in the treatment of novel coronavirus pneumonia. Journal of Chinese Medicinal Materials. 2020:1–8. http://kns.cnki.net/kcms/detail/44.1286.R.20200408.1725.002.html [In Chinese] [Google Scholar]
- 43.Qi G.D. The efficacy of Lianhua Qingwen combined with western medicine scheme on COVID-19 general type patients : a systematic review. Clinical Journal of Traditional Chinese Medicine. 2020:1–9. http://kns.cnki.net/kcms/detail/34.1268.r.20200410.0909.002.html [In Chinese] [Google Scholar]
- 44.Cheng D.Z. Novel coronavirus pneumonitis patients treated with Chinese herbal medicine Lianhua Qingwen: a multicenter retrospective study of 51 cases. Tianjin Journal of Traditional Chinese Medicine. 2020:1–6. http://kns.cnki.net/kcms/detail/12.1349.R.20200310.1024.004.html [In Chinese] [Google Scholar]
- 45.Lv R.B. 63 suspected cases with Novel coronavirus pneumonia treated with Lianhua Qingwen Granules: a clinical observation. J. Tradit. Chinese Med. 2020:1–5. http://kns.cnki.net/kcms/detail/11.2166.R.20200215.1633.004.html [In Chinese] [Google Scholar]
- 46.Yao K.M. Retrospective clinical analysis on treatment of coronavirus disease 2019 with traditional Chinese medicine Lianhua Qingwen. Chinese Journal of Experimental Traditional Medical Formulae. 2020:1–7. doi: 10.13422/j.cnki.syfjx.20201099. [In Chinese] [DOI] [Google Scholar]
- 47.Wang S.Z. Lianhua Qingwen capsule and interferon-α combined with lopinavir/ritonavir for the treatment of 30 COVID-19 patients. Journal of Bengbu Medical College. 2020;45(2):154–155. [In Chinese] [Google Scholar]
- 48.Cheng D.Z. Clinical effectiveness and case analysis in 54 NCP patients treated with Lanhuaqingwen Granules. World Chinese Medicine. 2020;15(2):150–154. . [In Chinese] [Google Scholar]
- 49.Wang L. Study on the network pharmacology and preliminary evidence of Lianhua Qingwen in the treatment of novel coronavirus (2019-nCoV) Journal of Chinese Medicinal Materials. 2020;3:772–778. doi: 10.13863/j.issn1001-4454.2020.03.049. [In Chinese] [DOI] [Google Scholar]
- 50.Ling X.Y. Exploring material basis and mechanism of Lianhua Qingwen Prescription against coronavirus based on network pharmacology. Chinese Traditional and Herbal Drugs. 2020:1–8. http://kns.cnki.net/kcms/detail/12.1108.R.20200320.1650.006.html [In Chinese] [Google Scholar]
- 51.Wang F.C. Clinical efficacy and mechanism of Lianhua Qingwen Granule on COVID-19 based on network pharmacology research. Pharmacology and Clinics of Chinese Materia Medica. 2020:1–22. doi: 10.13412/j.cnki.zyyl.20200318.001. [In Chinese] [DOI] [Google Scholar]
- 52.Li H.R. Theoretical research basis and clinical efficacy of Lianhua Qingwen in treating novel coronavious pneumonica. World Chinese Medicine. 2020:1–5. http://kns.cnki.net/kcms/detail/11.5529.R.20200309.1642.036.html [In Chinese] [Google Scholar]
- 53.Xiao Q. Clinical value analysis of Shufeng Jiedu Capsule combined with abidol in treating mild cases of novel coronavirus pneumonia. Journal of Emergency in Traditional Chinese Medicine. 2020:1–3. http://kns.cnki.net/kcms/detail/50.1102.R.20200309.1528.004.html [In Chinese] [Google Scholar]
- 54.Qu X.K. Observation on clinical effect of Shufeng Jiedu Capsule combined with Arbidol Hydrochloride Capsule in treatment of COVID-19. Chinese Traditional and Herbal Drugs. 2020;51(5):1167–1170. [In Chinese] [Google Scholar]
- 55.Shen F. The potential targets and mechanisms of Shufeng Jiedu Capsule for novel coronavirus pneumonia(COVID-19) based on network pharmacology and molecular docking. Guiding Journal of Traditional Chinese Medicine and Pharmacy. 2020;26(5):8–15. [In Chinese] [Google Scholar]
- 56.Li Y. Traditional Chinese herbal medicine for treating novel coronavirus (COVID-19) pneumonia: protocol for a systematic review and meta-analysis. Syst. Rev. 2020;9(1):75. doi: 10.1186/s13643-020-01343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J.P. A comprehensive strategy to evaluate compatible stability of Chinese medicine injection and infusion solutions based on chemical analysis and bioactivity assay. Front. Pharmacol. 2017;8:833. doi: 10.3389/fphar.2017.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Q. Xiyanping plus azithromycin chemotherapy in pediatric patients with mycoplasma pneumoniae pneumonia: a systematic review and meta-analysis of efficacy and safety. Evid. Complement. Alternat. Med. 2019;2019:2346583. doi: 10.1155/2019/2346583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Q.W. Crystal structure and anti-inflammatory and anaphylactic effects of andrographlide sulphonate E in Xiyanping, a traditional Chinese medicine injection. J. Pharm. Pharmacol. 2019;71(2):251–259. doi: 10.1111/jphp.13028. [DOI] [PubMed] [Google Scholar]
- 60.Cai N. Theoretical basis and effect characteristics of andrographolide against COVID-19. Chinese Traditional and Herbal Drugs. 2020;51(5):1159–1166. [Google Scholar]
- 61.Guo W. Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3 and NF-κB pathways. Int. Immunopharmacol. 2012;14(4):613–619. doi: 10.1016/j.intimp.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Hernández-Aquino E. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J. Gastroenterol. 2018;24(16):1679–1707. doi: 10.3748/wjg.v24.i16.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He G. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21(1):159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li T. Xuebijing injection alleviates Pam3CSK4-induced inflammatory response and protects mice from sepsis caused by methicillin-resistant staphylococcus aureus. Front. Pharmacol. 2020;11:104. doi: 10.3389/fphar.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S. Therapeutic effect of Xuebijing, a traditional Chinese medicine injection, on rheumatoid arthritis. Evid. Complement. Alternat. Med. 2020;2020 doi: 10.1155/2020/2710782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang C.Y. Clinical observation of Xuebijing in the treatment of COVID-19. Chinese Journal of Hospital Pharmacy. 2020:1–5. http://kns.cnki.net/kcms/detail/42.1204.r.20200409.1637.002.html [In Chinese] [Google Scholar]
- 67.Li C.Y. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2020. Current evidence and research prospects of xuebijing injection in treating novel coronavirus-infected pneumonia (COVID-19) pp. 1–6.http://kns.cnki.net/kcms/detail/11.5699.R.20200217.1242.002.html [In Chinese] [Google Scholar]
- 68.Shi Y. Study on the overall regulation of Xuebijing injection in treating corona virus disease 2019. Shanghai Journal of Traditional Chinese Medicine. 2020:1–7. http://kns.cnki.net/kcms/detail/31.1276.R.20200228.1042.005.html [In Chinese] [Google Scholar]
- 69.He T.M. Potential mechanism of Xuebijing Injection in treatment of coronavirus pneumonia based on network pharmacology and molecular docking. Chinese Journal of Modern Applied Pharmacy. 2020;37(4):398–405. [In Chinese] [Google Scholar]
- 70.Mehta P. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang C. Reduning injection ameliorates paraquat-induced acute lung injury by regulating AMPK/MAPK/NF-κB signaling. J. Cell. Biochem. 2019;120(8):12713–12723. doi: 10.1002/jcb.28540. [DOI] [PubMed] [Google Scholar]
- 72.Sun X.Z. Study on mechanism of Reduning Injection in treating novel coronavirus pneumonia based on network pharmacology. Journal of Chinese Medicinal Materials. 2020:1–9. http://kns.cnki.net/kcms/detail/44.1286.R.20200331.1932.004.html [In Chinese] [Google Scholar]
- 73.Chu H. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa410. pii: ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang P. Tanreqing injection for acute bronchitis disease: a systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2016;25:143–158. doi: 10.1016/j.ctim.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Xiong L. Clinical efficacy and safety of Tanreqing Injection for pulmonary infection in patients with tuberculosis: a meta-qnalysis. J. Altern. Complement. Med. 2018;24(11):1051–1062. doi: 10.1089/acm.2018.0020. [DOI] [PubMed] [Google Scholar]
- 76.Kong Y. Mechanism of Tanreqing Injection on treatment of coronavirus disease 2019 based on network pharmacology and molecular docking. Chinese Traditional and Herbal Drugs. 2020:1–11. http://kns.cnki.net/kcms/detail/12.1108.R.20200318.1518.002.html [In Chinese] [Google Scholar]
- 77.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Y.M. Role of Xingnaojing combined with naloxone in treating intracerebral haemorrhage: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2018;97(43):e12967. doi: 10.1097/MD.0000000000012967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu L. Meta-analysis of the effects of Xingnaojing Injection on consciousness disturbance. Medicine. 2016;95(7):e2875. doi: 10.1097/MD.0000000000002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma X. Meta-analysis for clinical evaluation of Xingnaojing Injection for the treatment of cerebral infarction. Front. Pharmacol. 2017;8:485. doi: 10.3389/fphar.2017.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y.M. Xingnaojing Injection protects against cerebral ischemia reperfusion injury via PI3K/Akt-mediated eNOS phosphorylation. Evid. Complement. Alternat. Med. 2018;2018:2361046. doi: 10.1155/2018/2361046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qu X.Y. XingNaoJing injections protect against cerebral ischemia/reperfusion injury and alleviate blood-brain barrier disruption in rats, through an underlying mechanism of NLRP3 inflammasomes suppression. Chin. J. Nat. Med. 2019;17(7):498–505. doi: 10.1016/S1875-5364(19)30071-8. [DOI] [PubMed] [Google Scholar]
- 83.Jeon S. SuHeXiang Wan essential oil alleviates amyloid beta induced memory impairment through inhibition of tau protein phosphorylation in mice. Am. J. Chin. Med. (Gard City N Y) 2011;39(5):917–932. doi: 10.1142/S0192415X11009305. [DOI] [PubMed] [Google Scholar]
- 84.Hong Y.K. Neuroprotective effect of SuHeXiang Wan in Drosophila models of Alzheimer’s disease. J. Ethnopharmacol. 2011;134(3):1028–1032. doi: 10.1016/j.jep.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 85.Liu Q. Neuroprotective effects of Suhexiang Wan on the in vitro and in vivo models of Parkinson’s disease. J. Tradit. Chin. Med. 2019;39(6):800–808. [PubMed] [Google Scholar]
- 86.Park S.H. Suppressive effects of SuHeXiang Wan on amyloid-β42-induced extracellular signal-regulated kinase hyperactivation and glial cell proliferation in a transgenic Drosophila model of Alzheimer’s disease. Biol. Pharm. Bull. 2013;36(3):390–398. doi: 10.1248/bpb.b12-00792. [DOI] [PubMed] [Google Scholar]
- 87.Koo B.S. Inhibitory effects of the essential oil from SuHeXiang Wan on the central nervous system after inhalation. Biol. Pharm. Bull. 2004;27(4):515–519. doi: 10.1248/bpb.27.515. [DOI] [PubMed] [Google Scholar]
- 88.Guo Y. Use of angong niuhuang in treating central nervous system diseases and related research. Evid. Complement. Alternat. Med. 2014;2014:346918. doi: 10.1155/2014/346918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu W.J. Anti-atherosclerosis and cardio-protective effects of the Angong Niuhuang Pill on a high fat and vitamin D3 induced rodent model of atherosclerosis. J. Ethnopharmacol. 2017;195:118–126. doi: 10.1016/j.jep.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 90.Wang G.H. An-Gong-Niu-Huang Wan protects against cerebral ischemia induced apoptosis in rats: up-regulation of Bcl-2 and down-regulation of Bax and caspase-3. J. Ethnopharmacol. 2014;154(1):156–162. doi: 10.1016/j.jep.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 91.Mao Z.J. Shenfu Injection attenuates rat myocardial hypertrophy by up-regulating miR-19a-3p expression. Sci. Rep. 2018;8(1):4660. doi: 10.1038/s41598-018-23137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu L.Y. An overview of systematic reviews of shenmai injection for healthcare. Evid. Complement. Alternat. Med. 2014;2014:840650. doi: 10.1155/2014/840650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Z.L. Herbal medicines for viral myocarditis. Cochrane Database Syst. Rev. 2010;7 doi: 10.1002/14651858.CD003711.pub3. CD003711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu X. Effect of Shenfu injection on lipopolysaccharide (LPS)-induced septic shock in rabbits. J. Ethnopharmacol. 2019;234:36–43. doi: 10.1016/j.jep.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 95.Zhang H. Protective effect of Shenfu injection preconditioning on lung ischemia-reperfusion injury. Exp. Ther. Med. 2016;12(3):1663–1670. doi: 10.3892/etm.2016.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen G. Effects of Shenfu injection on chemotherapy-induced adverse effects and quality of life in patients with advanced nonsmall cell lung cancer: a systematic review and meta-analysis. J. Cancer Res. Ther. 2018;14(Supplement):S549–S555. doi: 10.4103/0973-1482.187299. [DOI] [PubMed] [Google Scholar]
- 97.Cai H. Comments on Shenfu injection for improving cellular immunity and clinical outcome in patients with sepsis or septic shock. Am. J. Emerg. Med. 2019;37(6):1207–1208. doi: 10.1016/j.ajem.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 98.Liu W.Y. Shenmai injection enhances the cytotoxicity of chemotherapeutic drugs against colorectal cancers via improving their subcellular distribution. Acta Pharmacol. Sin. 2017;38(2):264–276. doi: 10.1038/aps.2016.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W.L. Administrations of preoperative Shenmai injection and postoperative Shenfu Injection, two ginseng containing TCM formulas, improve cognitive dysfunction in aged rats. Am. J. Chin. Med. (Gard City N Y) 2018;46(5):1065–1078. doi: 10.1142/S0192415X18500556. [DOI] [PubMed] [Google Scholar]
- 100.Duan B. Effects of Shengmai injection add-on therapy to chemotherapy in patients with non-small cell lung cancer: a meta-analysis. Support. Care Cancer. 2018;26(7):2103–2111. doi: 10.1007/s00520-018-4167-4. [DOI] [PubMed] [Google Scholar]
- 101.Liu C.X. Interpretation of the Expert guidance on a comprehensive intervention program of Traditional Chinese Medicine for the recovery of Novel Coronavirus Pneumonia(Draft) Chinese Journal of Basic Medicine in Traditional Chinese Medicine. 2020:1–10. http://kns.cnki.net/kcms/detail/11.3554.R.20200318.1204.002.html [In Chinese] [Google Scholar]
- 102.Zhang D.H. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wan S. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang L. Protective effect of phillyrin on lethal LPS-induced neutrophil inflammation in zebrafish. Cell. Physiol. Biochem. 2017;43(5):2074–2087. doi: 10.1159/000484192. [DOI] [PubMed] [Google Scholar]
- 105.Zhou S. Phillyrin is an effective inhibitor of quorum sensing with potential as an anti-Pseudomonas aeruginosa infection therapy. J. Vet. Med. Sci. 2019;81(3):473–479. doi: 10.1292/jvms.18-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qu X.Y. Protective effects of phillyrin against influenza A virus in vivo. Arch. Pharm. Res. 2016;39(7):998–1005. doi: 10.1007/s12272-016-0775-z. [DOI] [PubMed] [Google Scholar]
- 107.Liu S. Efficacy of Xuebijing injection for sepsis (EXIT-SEP): protocol for a randomised controlled trial. BMJ Open. 2019;9:e028664. doi: 10.1136/bmjopen-2018-028664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin H. Microcirculatory disorders and protective role of Xuebijing in severe heat stroke. Sci. Rep. 2018;8:4553. doi: 10.1038/s41598-018-22812-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu H. Effects of radix ginseng on microbial infections: a narrative review. J. Tradit. Chin. Med. 2014;34(2):227–233. doi: 10.1016/s0254-6272(14)60083-2. [DOI] [PubMed] [Google Scholar]
- 110.Kim Y.R. Protective roles of ginseng against bacterial infection. Microb. Cell. 2018;5(11):472–481. doi: 10.15698/mic2018.11.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang D. The characteristics and regularities of cardiac adverse drug reactions induced by Chinese materia medica: a bibliometric research and association rules analysis. J. Ethnopharmacol. 2020;252:112582. doi: 10.1016/j.jep.2020.112582. [DOI] [PubMed] [Google Scholar]
- 112.Liu J. A review of cinnabar (HgS) and/or realgar (As4S4)-containing traditional medicines. J. Ethnopharmacol. 2018;210:340–350. doi: 10.1016/j.jep.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 113.Huang G. Study on cardiotoxicity and mechanism of “Fuzi” extracts based on metabonomics. Int. J. Mol. Sci. 2018;19(11) doi: 10.3390/ijms19113506. pii: E3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu S. A review of traditional and current methods used to potentially reduce toxicity of Aconitum roots in Traditional Chinese Medicine. J. Ethnopharmacol. 2017;207:237–250. doi: 10.1016/j.jep.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 115.Li H. Safety profile of traditional Chinese herbal injection: an analysis of a spontaneous reporting system in China. Pharmacoepidemiol. Drug Saf. 2019;28(7):1002–1013. doi: 10.1002/pds.4805. [DOI] [PubMed] [Google Scholar]
- 116.Tan L. Safety concerns of traditional Chinese medicine injections used in Chinese children. Evid. Complement. Alternat. Med. 2019;2019:8310368. doi: 10.1155/2019/8310368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li K.Y. Observation on clinical effect of modified Qingfeipaidu decoction in treatment of COVID-19. Chinese Traditional and Herbal Drugs. 2020:1–4. http://kns.cnki.net/kcms/detail/12.1108.R.20200407.1425.011.html [Google Scholar]