Introduction
Since emerging from Wuhan, China, in late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), has infected >3.2 million individuals worldwide and ∼1 million in the United States (as of April 29, 2020).1 , 2 Despite the institution of measures designed to “flatten the curve,” COVID-19 has claimed the lives of >225,000 individuals worldwide and >60,000 individuals in the United States alone (as of April 29, 2020).2 Of note, mortality estimates in some of the hardest hit regions have already or may need to be revised to account for a spike in sudden deaths occurring at home.3 , 4
Although most of these deaths are likely directly attributable to COVID-19 (eg, pulseless electrical activity/respiratory arrests), many of the same regions within the United States have also seen the outpatient prescription volume of COVID-19–directed heart rate–corrected QT interval (QTc)–prolonging drug(s), specifically hydroxychloroquine, rise by as much as 22,700% compared to the same time period (March 15–31) in 2019.5 Importantly, recent studies have shown that between 11% and 25% of hospitalized patients with COVID-19 treated with chloroquine or hydroxychloroquine and azithromycin had QTc values rise above the critical 500 ms threshold.6 , 7
As a result, a substantial number of patients with COVID-19, prescribed these so-called corona cocktails, may be at an increased risk of developing drug-induced long QT syndrome (DI-LQTS), which could deteriorate into drug-induced torsades de pointes (DI-TdP) or worse drug-induced sudden cardiac death (DI-SCD).8 , 9 Therefore, it stands to reason that the same phenomenon (DI-LQTS/DI-TdP) that has already halted 1 chloroquine/hydroxychloroquine clinical trial7 and led the Food and Drug Administration to caution against the use of chloroquine/hydroxychloroquine outside the hospital setting/clinical trial10 could also be contributing, at least in small part, to the increase in sudden deaths observed in some COVID-19 epicenters.
In addition, the COVID-19 pandemic has already highlighted alarming health disparities in the United States. For example, in Illinois where age, sex, and racial demographic data are reported for all COVID-19 cases, African Americans account for 26% of confirmed COVID-19 cases but 43% of COVID-19 deaths.11 A similar trend has been observed across the United States where COVID-19 mortality rates in predominantly black counties are 6-fold higher than that in predominantly white counties.12 Although this phenomenon is likely explained by the convergence of multiple cultural and socioeconomic factors,12 an underlying genetic susceptibility to SARS-CoV-2 infection, its sequelae (such as hypoxia and inflammation), or the potentially lethal side effects of COVID-19–directed therapies (ie, DI-TdP and DI-SCD), assuming equal exposure to these medications, could also contribute.
Current evidence supports the notion that the common ion channel variants p.Asp85Asn-KCNE1 and p.Ser1103Tyr-SCN5A confer an increased risk of DI-LQTS and DI-SCD (Table 1 ).13 Importantly, p.Ser1103Tyr-SCN5A is seen almost exclusively in individuals of African descent and its frequency in that population (∼8%) is higher than that of p.Asp85Asn-KCNE1 (0.2%–2.5% depending on ancestry; 0.2% in individuals of African origin) (Table 1).13 Furthermore, unlike p.Asp85Asn-KCNE1 whose proarrhythmic potential is largely limited to DI-LQTS risk, the modest increase in late/persistent sodium current generated by p.Ser1103Tyr-SCN5A is accentuated markedly by hypoxia/acidosis and has been linked to an increased risk of ventricular arrhythmia (VA) and sudden cardiac death (SCD) in African Americans across the age spectrum.13, 14, 15, 16
Table 1.
Epidemiological and functional data supporting a role for p.Ser1103Tyr-SCN5A and p.Asp85Asn-KCNE1 in DI-LQTS risk
| Gene | dbSNP ID | Amino acid change | Overall MAF (gnomAD) | African MAF (gnomAD) | Asian MAF (gnomAD) | European MAF (gnomAD)∗ | Latino MAF (gnomAD) | Electrophysiological phenotype (in vitro) | Odds ratio (95% confidence interval) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| SCN5A | rs7626962 | S1103Y† | 0.008 | 0.08 | 0.00003 | 0.0003 | 0.004 | Increased sustained/late INa secondary to altered inactivation gating that is accentuated by intracellular acidosis | 8.7 (3.2–23.9) | 16, 17 |
| KCNE1 | rs1805128 | D85N | 0.009 | 0.002 | 0.003 | 0.01 | 0.003 | Decreased IKs and/or IKr secondary to altered activation/inactivation kinetics | 9.0 (3.5–22.9) | 33 |
dbSNP = Single Nucleotide Polymorphism Database; DI-LQTS = drug-induced long QT syndrome; gnomAD = Genome Aggregation Consortium; IKs = slowly activating delayed rectifier potassium current; IKr = rapidly activating delayed rectifier potassium current; INa = sodium current; MAF = minor allele frequency.
Adapted from Giudicessi et al,13 with permission. Copyright © Lippincott, Williams and Wilkins, 2018.
Includes individuals of both Finnish and non-Finnish European ancestry.
Based on the reference transcript used. p.Ser1103Tyr-SCN5A is also occasionally listed as p.Ser1102Tyr-SCN5A.
Given the potential of p.Ser1103Tyr-SCN5A to exacerbate outcome-related health disparities in the COVID-19 pandemic, this review was assembled to raise awareness of the multiple mechanism(s) whereby the proarrhythmic p.Ser1103Tyr-SCN5A common variant may increase the risk of VA/SCD in those of African descent as well as the importance of identifying modifiable risk factors for mortality during the COVID-19 pandemic.
p.Ser1103Tyr-SCN5A and VA/SCD susceptibility in individuals of African descent
In individuals of African descent, the common SCN5A-encoded Nav1.5 sodium channel variant p.Ser1103Tyr-SCN5A has been associated with an increased risk of VA/SCD.14 , 15 , 17 Of note, this proarrhythmic potential has been reported to be enhanced by nongenetic risk factors known to reduce cardiac repolarization reserve (eg, hypokalemia and QTc-prolonging medication exposure; odds ratio 8.7; 95% confidence interval 3.2–23.9; P = .00003)17 and/or structural heart disease (relative risk 8.4; 95% confidence interval 2.1–28.6; P = .001) (Table 1).14 , 15 Furthermore, p.Ser1103Tyr-SCN5A was associated with potentiation of the QT-prolonging effect of hypokalemia in the 4476 participants of the Jackson Heart Study, suggesting that p.Ser1103Tyr-SCN5A contributes to decreased cardiac repolarization reserve at a population-specific level.18 In addition, 2 population-based studies reported that p.Ser1103Tyr-SCN5A is overrepresented in African American sudden infant death syndrome decedents,16 , 19 providing additional epidemiological evidence that p.Ser1103Tyr-SCN5A confers an increased risk of sudden death in African Americans regardless of age.
Besides this epidemiological evidence, in vitro functional characterization by 2 independent groups demonstrated that p.Ser1103Tyr-SCN5A alters the inactivation gating of the Nav1.5 sodium channel (negative shift in the voltage dependence of steady-state inactivation [V1/2]) and accentuates the sustained/late sodium current that is also the signature of the electrophysiological phenotype displayed by the type 3 long QT syndrome (LQT3) pathogenic variants.16 , 17 , 20 That said, the experimental conditions—physiological pH vs low intracellular pH—under which p.Ser1103Tyr-SCN5A increased the late sodium current differed in the studies of Splawski et al17 and Plant et al,16 respectively. Furthermore, neither study demonstrated an in vitro or in silico ability of p.Ser1103Tyr-SCN5A to prolong the action potential duration (APD) in the absence of a “second hit” such as blockade of the rapid component of the delayed rectifier potassium current or intracellular acidosis. This appears to be consistent with (1) the exaggerated QTc response to hypokalemia observed in p.Ser1103Tyr-SCN5A–positive participants of the Jackson Heart Study18 and (2) a circumstance-dependent proarrhythmic state in infants (respiratory acidosis caused by hypoxia/apnea)16 , 19 and older (DI-LQTS in the setting of ≥1 QTc-prolonging drug and advanced structural heart disease) p.Ser1103Tyr-SCN5A–positive African Americans.14, 15, 16
Implications of p.Ser1103Tyr-SCN5A during the COVID-19 pandemic
Direct and/or indirect myocardial injury/stress, as assessed by cardiac biomarkers such as troponin I and brain-type natriuretic peptide, has emerged as both a prominent and a prognostic feature in COVID-19.21, 22, 23 Of note, in an adjusted Cox regression model, the mortality risk associated with elevated cardiac biomarkers/acute cardiac injury was more significant than age and high-risk comorbid conditions such as chronic obstructive/fibrotic pulmonary disease, diabetes, and a history of cardiovascular disease.23 , 24 In addition, life-threatening VAs (eg, ventricular tachycardia/ventricular fibrillation) have been documented in ∼6% of hospitalized patients with COVID-19 and appear to be driven by underlying myocardial injury.25
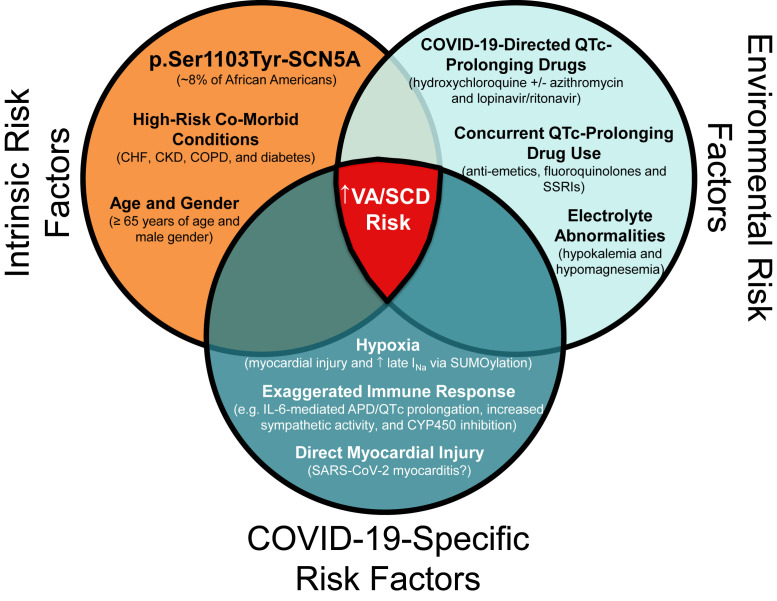
The proposed mechanisms of acute myocardial injury and resulting VA risk in patients with COVID-19 include (1) direct SARS-CoV-2 myocardial infection (eg, myocarditis), (2) myocardial stress induced by hypoxemic respiratory failure, and/or (3) an exaggerated immune response that results in high levels of circulating cytokines (ie, interleukin-6 [IL-6], tumor necrosis factor-α, etc) directly injuring cardiomyocytes (Figure 1 ).25, 26, 27 Notably, IL-6 may prolong the ventricular action potential via modulation of cardiac ion channel expression/function.27
Figure 1.
Potential gene-environment interactions leading to an increased risk of ventricular arrhythmias and sudden cardiac death in p.Ser1103Tyr-SCN5A–positive patients with COVID-19. APD = action potential duration; CHF = congestive heart failure; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; COVID-19 = coronavirus disease 2019; IL-6 = interleukin-6; INa = sodium current; QTc = heart rate–corrected QT; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SCD = sudden cardiac death; SSRI = selective serotonin reuptake inhibitor; VA = ventricular arrhythmia.
Under normal physiological circumstances, Nav1.5 cardiac sodium channels conduct a tiny amount of persistent/late sodium current that contributes minimally to the maintenance of the action potential plateau (ie ≤0.5% of the peak sodium current [INa]).28 However, in the setting of hypoxia, myocardial ischemia, heart failure, and LQT3-causative SCN5A gain-of-function variants, the relative contribution of the late sodium current can increase up to 10-fold to 4%–5% of the peak INa.16 , 20 , 28 , 29 In turn, this “pathological” late sodium current can prolong ventricular APD and predispose to VA/SCD.
As discussed previously, p.Ser1103Tyr-SCN5A Nav1.5 sodium channels function normally under physiological conditions (eg, intracellular pH of 6.9–7.1).16 However, when intracellular pH is decreased in vitro to levels consistent with respiratory acidosis that occurs secondary to hypoxia/prolonged apnea (eg, intracellular pH of 6.6–6.8), p.Ser1103Tyr-SCN5A Nav1.5 sodium channels generate a proarrhythmic, LQT3-like increase in persistent/late sodium current (∼5% of the peak INa).16 This mechanism was put forward initially to explain the gene-environment interaction(s) responsible for the association between p.Ser1103Tyr-SCN5A and sudden infant death syndrome.16 However, the profound hypoxia observed in many COVID-19 cases raises reasonable concern that p.Ser1103Tyr-SCN5A could produce a similar African American–specific susceptibility to hypoxia-induced VA/SCD in the setting of SARS-CoV-2 infection/COVID-19 (Figure 1).
Unfortunately, the increased risk of VA/SCD linked to the potentially proarrhythmic p.Ser1103Tyr-SCN5A common variant is likely not limited to the possibility of hypoxia-induced VA/SCD. As succinctly outlined in recent work by Lazzerini et al,27 the exaggerated immune response triggered by SARS-CoV-2 infection, specifically elevation of IL-6, likely increases arrhythmia risk via (1) modulation of cardiac ion channel expression/function leading to APD prolongation (eg, direct IL-6–mediated blockade of hERG/Kv11.1 potassium channels), (2) cardiac sympathetic nervous system hyperactivity, and (3) inhibition of cytochrome P450 enzymes involved in the metabolism of some QTc-prolonging drugs (eg, IL-6 and CYP3A4) (Figure 1). The latter effect of IL-6 is particularly important given that a number of COVID-19 pharmacotherapies (eg, hydroxychloroquine ± azithromycin and lopinavir/ritonavir) under investigation and/or in use clinically are known to prolong the QTc interval and predispose to DI-TdP/DI-SCD (Table 2 ).8 , 30
Table 2.
COVID-19–directed pharmacotherapies with DI-TdP/DI-SCD risk
| Possible COVID-19 therapy | In vitro inhibition of SARS-CoV-2 | CredibleMeds classification | VT/VF/TdP/LQTS in FAERS∗ | Cardiac arrest in FAERS∗ | References |
|---|---|---|---|---|---|
| Repurposed antimalarial agents | |||||
| Chloroquine | Yes | Known TdP risk | 72 | 54 | 34, 35, 36 |
| Hydroxychloroquine | Yes | Known TdP risk | 222 | 105 | 37,38 |
| Repurposed antiviral agents | |||||
| Lopinavir/ritonavir | Unknown† | Possible TdP risk | 27 | 48 | 39, 40, 41 |
| Adjunct agents | |||||
| Azithromycin | Unknown | Known TdP risk | 396 | 251 | 42,43 |
COVID-19 = coronavirus disease 2019; DI-SCD = drug-induced sudden cardiac death; DI-TdP = drug-induced torsades de pointes; FAERS = Food and Drug Administration Adverse Event Reporting System; LQTS = long QT syndrome; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; TdP = torsades de pointes; VF = ventricular fibrillation; VT = ventricular tachycardia.
Adapted from Giudicessi et al,8 with permission. Copyright © Elsevier, 2020.
Adverse event reporting from postmarketing surveillance does not account for prescription volume and is often subjected to significant bias from confounding variables, quality of reported data, duplication, and underreporting of events.
Lopinavir/ritonavir inhibits other SARS viruses in vitro. However, a recent randomized trial demonstrated no benefit in COVID-19.
Taken together, these data suggest that 1 in 13 African Americans may be at a substantially increased risk of potentially lethal VAs, most notably DI-TdP, during the COVID-19 pandemic because of the perfect storm of (1) intrinsic genetic susceptibility (ie, p.Ser1103Tyr-SCN5A), (2) modifiable environmental risk factors (eg, electrolyte abnormalities and concurrent QTc-prolonging drug use), and (3) COVID-19–specific risk factors (eg, profound hypoxemia and cytokine storm) (Figure 1). Whether population-specific genetic risk factors such as p.Ser1103Tyr-SCN5A are contributing to the spike in sudden deaths and racial health disparities observed in COVID-19 epicenters remains to be proven, and given the lack of banked DNA in these epicenters, this speculation may not even be testable.
Nevertheless, given the potential for COVID-19 to exacerbate known gene-environment interactions pertaining to the potentially proarrhythmic p.Ser1103Tyr-SCN5A common variant, it seems reasonable (1) to avoid using COVID-19–directed QTc-prolonging drugs (eg, hydroxychloroquine ± azithromycin and lopinavir/ritonavir) unless careful, and preferably personal protective equipment–sparing, cardiac monitoring can be implemented (Figure 2 )8 , 31; (2) to explore the association between p.Ser1103Tyr-SCN5A and rates of sudden death and COVID-19–related mortality in areas with medical record–linked DNA biobanks (eg, UK Biobank); (3) to investigate further the feasibility of point-of-care p.Ser1103Tyr-SCN5A genetic testing; and (4) to determine the clinical utility of QTc-shortening agents such as late sodium current blockers (eg, mexiletine and lidocaine) and anti-IL-6–targeted therapies (eg, tocilizumab and sarilumab)27 , 32 to better protect at-risk individuals, especially African Americans in the context of the ongoing COVID-19 pandemic.
Figure 2.
Proposed approach to mitigating the risk of DI-TdP/DI-SCD in patients with COVID-19 treated with ≥1 QTc-prolonging drug. The estimated 99th percentile QTc values, derived from otherwise healthy individuals, that place a patient in the “green light” category are <460 ms before puberty, <470 ms in men, and <480 ms in women. We estimate that the baseline QTc assessment will place 90% in the “green light,” 9% in the “yellow light,” and 1% in the “red light” category. No randomized controlled trial data are available to support the clinical efficacy of any of the COVID-19–directed QTc-prolonging drugs despite the Food and Drug Administration’s Emergency Use Approval of hydroxychloroquine. COVID-19 = coronavirus disease 19; CV = cardiovascular; DI-SCD = drug-induced sudden cardiac death; DI-TdP = drug-induced torsades de pointes; ECG = electrocardiogram; QTc = heart rate–corrected QT; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; TdP = torsades de pointes. Adapted from Giudicessi et al,8 with permission. Copyright © Elsevier, 2020.
Conclusion
A potentially pro-arrhythmic common variant, p.Ser1103Tyr-SCN5A, present in 1 out of 13 individuals of African descent has the potential to increase the risk of drug- and hypoxia-induced ventricular arrhythmias/sudden cardiac death and contribute to observed racial health disparities in the COVID-19 pandemic. As such, the use of unproven, QTc-prolonging COVID-19-directed therapies, most notably the combination of hydroxychloroquine and azithromycin, should be limited to settings where careful cardiac monitoring can be implemented (e.g. hospital setting or clinical trial).
Footnotes
This work was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program.
Dr Ackerman is a consultant for Abbott, Audentes Therapeutics, Boston Scientific, Invitae, LQT Therapeutics, Medtronic, MyoKardia, and UpToDate. Dr Ackerman and Mayo Clinic are involved in an equity/royalty relationship with AliveCor. However, AliveCor was not involved in this study. The rest of the authors report no conflicts of interest.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deaths from cardiac arrests have surged in New York City. The Economist. https://www.economist.com/graphic-detail/2020/04/13/deaths-from-cardiac-arrests-have-surged-in-new-york-city Published April 13, 2020. Accessed April 29, 2020.
- 4.Katz J., Lu D., Sanger-Katz M.U.S. coronavirus death toll is far higher than reported, C.D.C. data suggests. The New York Times. https://www.nytimes.com/interactive/2020/04/28/us/coronavirus-death-toll-total.html Published April 28, 2020. Accessed April 29, 2020.
- 5.Williams M. Hydroxychloroquine prescription volume changes during COVID-19. Swoop Web site. https://www.swoop.com/news/hydroxychloroquine-prescription-volume-changes-during-covid-19
- 6.Chorin E, Dai M, Shulman E, et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin [published online ahead of print April 24, 2020]. Nat Med. 10.1016/j.hrthm.2020.05.014. [DOI] [PubMed]
- 7.Borba M.G.S., Val F.F.A., Sampaio V.S. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 8.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C.-I., Postema P.G., Arbelo E. SARS-CoV-2, COVID-19 and inherited arrhythmia syndromes. Heart Rhythm. 2020;17:1456–1462. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. U.S. Food and Drug Administration. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. Accessed April 26, 2020.
- 11.COVID-19 statistics Illinois Department of Health Web site. https://www.dph.illinois.gov/covid19/covid19-statistics Accessed April 29, 2020.
- 12.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 13.Giudicessi J.R., Roden D.M., Wilde A.A.M., Ackerman M.J. Classification and reporting of potentially proarrhythmic common genetic variation in long QT syndrome genetic testing. Circulation. 2018;137:619–630. doi: 10.1161/CIRCULATIONAHA.117.030142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke A., Creighton W., Mont E. Role of SCN5A Y1102 polymorphism in sudden cardiac death in blacks. Circulation. 2005;112:798–802. doi: 10.1161/CIRCULATIONAHA.104.482760. [DOI] [PubMed] [Google Scholar]
- 15.Sun A.Y., Koontz J.I., Shah S.H. The S1103Y cardiac sodium channel variant is associated with implantable cardioverter-defibrillator events in blacks with heart failure and reduced ejection fraction. Circ Cardiovasc Genet. 2011;4:163–168. doi: 10.1161/CIRCGENETICS.110.958652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plant L.D., Bowers P.N., Liu Q. A common cardiac sodium channel variant associated with sudden infant death in African Americans, SCN5A S1103Y. J Clin Invest. 2006;116:430–435. doi: 10.1172/JCI25618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Splawski I., Timothy K.W., Tateyama M. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 18.Akylbekova E.L., Payne J.P., Newton-Cheh C. Gene-environment interaction between SCN5A-1103Y and hypokalemia influences QT interval prolongation in African Americans: the Jackson Heart Study. Am Heart J. 2014;167:116–122.e111. doi: 10.1016/j.ahj.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Norstrand D.W., Tester D.J., Ackerman M.J. Overrepresentation of the proarrhythmic, sudden death predisposing sodium channel polymorphism S1103Y in a population-based cohort of African-American sudden infant death syndrome. Heart Rhythm. 2008;5:712–715. doi: 10.1016/j.hrthm.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett P.B., Yazawa K., Makita N., George A.L., Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 21.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akhmerov A., Marban E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk and inflammation: mind the gap! Circulation. 2020;142:7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 28.Makielski J.C. Late sodium current: a mechanism for angina, heart failure, and arrhythmia. Trends Cardiovasc Med. 2016;26:115–122. doi: 10.1016/j.tcm.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belardinelli L., Giles W.R., Rajamani S., Karagueuzian H.S., Shryock J.C. Cardiac late Na(+) current: proarrhythmic effects, roles in long QT syndromes, and pathological relationship to CaMKII and oxidative stress. Heart Rhythm. 2015;12:440–448. doi: 10.1016/j.hrthm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Roden D.M., Harrington R.A., Poppas A., Russo A.M. Considerations for drug interactions on QTc in exploratory COVID-19 treatment. Circulation. 2020;141:e906–e907. doi: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 31.Giudicessi J.R., Noseworthy P.A., Ackerman M.J. The QT interval: an emerging vital sign for the precision medicine era? Circulation. 2019;139:2711–2713. doi: 10.1161/CIRCULATIONAHA.119.039598. [DOI] [PubMed] [Google Scholar]
- 32.Mitra R.L., Greenstein S.A., Epstein L.M. An algorithm for managing QT prolongation in coronavirus disease 2019 (COVID-19) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: possible benefits of intravenous lidocaine. HeartRhythm Case Rep. 2020;6:244–248. doi: 10.1016/j.hrcr.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaab S., Crawford D.C., Sinner M.F. A large candidate gene survey identifies the KCNE1 D85N polymorphism as a possible modulator of drug-induced torsades de pointes. Circ Cardiovasc Genet. 2012;5:91–99. doi: 10.1161/CIRCGENETICS.111.960930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traebert M., Dumotier B., Meister L., Hoffmann P., Dominguez-Estevez M., Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484:41–48. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Stas P., Faes D., Noyens P. Conduction disorder and QT prolongation secondary to long-term treatment with chloroquine. Int J Cardiol. 2008;127:e80–e82. doi: 10.1016/j.ijcard.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 37.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C.Y., Wang F.L., Lin C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila) 2006;44:173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 39.Chen F., Chan K.H., Jiang Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soliman E.Z., Lundgren J.D., Roediger M.P., INSIGHT SMART Study Group Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS. 2011;25:367–377. doi: 10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giudicessi J.R., Ackerman M.J. Azithromycin and risk of sudden cardiac death: guilty as charged or falsely accused? Cleve Clin J Med. 2013;80:539–544. doi: 10.3949/ccjm.80a.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arellano-Rodrigo E., Garcia A., Mont L., Roque M. Torsade de pointes and cardiorespiratory arrest induced by azithromycin in a patient with congenital long QT syndrome [in Spanish] Med Clin (Barc) 2001;117:118–119. doi: 10.1016/s0025-7753(01)72036-2. [DOI] [PubMed] [Google Scholar]