Abstract
Tuberculosis, a severe infectious disease caused by the Mycobacterium tuberculosis, arouses huge concerns globally. In this study, a total of 331,594 TB cases in Zhejiang Province were notified during the period of 2009–2018 with the gender ratio of male to female 2.16:1. The notified TB incidences demonstrated a continuously declining trend from 75.38/100,000 to 52.25/100,000. Seasonally, the notified TB cases presented as low in January and February closely followed an apparent rise in March and April. Further stratification analysis by both genders demonstrated the double peak phenomenon in the younger population (“15–35”) and the elders (“>55”) of the whole group. Results from the rate difference (RD) analysis showed that the rising TB incidence mainly presented in the young group of “15–20” and elder group of “65–70”, implying that some implementations such as the increased frequency of checkup in specific student groups and strengthening of elder health examination could be explored and integrated into available health policy. Finally, the SARIMA (2,0,2) (0,1,1)12 was determined as the optimal prediction model, which could be used in the further prediction of TB in Zhejiang Province.
Subject terms: Diseases, Health care, Medical research
Introduction
Tuberculosis (TB), as a severe infectious disease, is still a leading cause of death worldwide, causing massive concern in public health continuously1. Although new diagnosis technology, novel drugs for treatment, relevant support service, and positive political commitment were promoted steadily in recent years, there are still more than 10 million newly diagnosed patients reported globally, and 5% of them were identified as rifampicin-resistant cases2. More seriously, based on the WHO’s release, only 64% of cases were diagnosed and recorded formally, an estimated 1.3 million TB cases resulted in death among HIV-positive people, and an additional 0.3 million deaths resulted among HIV-negative people in 20171. The majority of infected cases (87%) are found in 30 high-burden countries. China ranked as second place among high-burden countries, contributing to 9% of the estimated global totals. Additionally, according to a release by the World Health Organization (WHO) in 2018, drug-resistant TB, including resistance to rifampicin TB (RR-TB) and multi-drug resistant TB (MDR-TB), was deemed as the gravest crisis, and the estimated cases in China accounted for 13% of global totals, also ranked second. Thus, realizing the target of End TB by the control and prevention of TB epidemics has become urgent in China.
In China, there are a variety of epidemic characteristics due to different influential factors like socio-economic indices and diverse regions3. Zhejiang Province is a developed economy in the eastern region of China4. Although decreased TB incidence in this region was reported, the current velocity of reduction may not be adequate for reaching future demands of WHO’s End TB strategy and the UN Sustainable Development Goals’ target5. During the recent decade, Zhejiang Province reported nearly 300,000 notified cases. Thus, the exploration of the potential implications hidden in this information was essential. Based on previous studies, the time series model could be used to carry out short-term predictions effectively, providing useful clues and evidence for the control and prevention of TB in the future6,7. Among several time series models, the autoregressive integrated moving average (ARIMA) model, including the seasonal ARIMA model, takes several key variables into account including periodic variables, random factors, and actual fluctuation caused by epidemics8,9. The model also has distinct advantages like the requirement of limited data variables and high prediction accuracy10. ARIMA model has been widely used in the field of infectious diseases like hemorrhagic fever with renal syndrome (HFRS), hand foot and mouth disease (HFMD), avian influenza, and TB11–15.
Thus, this study aimed to explore the underlying burden of TB in the past ten years, find the regulation of TB among different genders, discern the target groups, and predict further epidemics in Zhejiang Province. This study might not just contribute to the advancement of further health policy for TB control at the regional level but also provide useful references for TB prevention in China.
Materials and Methods
Study area
Zhejiang Province is in the eastern region of China with a land area of nearly 101,800 square kilometers, accounting for 1.06% of China7. As an economically developed province with a GDP of 6 trillion RMB in 2019, it consists of 11 regional cities: Hangzhou, Ningbo, Wenzhou, Jiaxing, Huzhou, Shaoxing, Jinhua, Quzhou, Zhoushan, Taizhou, and Lishui. As the smallest province in China, it has two sub-provincial cities and is composed of nearly 90 counties. In 2018, there was a reported total of 57.37 million permanent people and a migrant population of approximately 26 million in Zhejiang Province, which contributed to the complexity in controlling and preventing TB4. The location of the Zhejiang province is shown in Fig. 1.
Figure 1.
Area of Zhejiang Province in China.
Data collection
All included data was collected by date of notification from the Web-based TB Information Management System (TBIMS) in China, which was established in 200516. In this system, all notified TB cases including new and relapse cases were recorded in the designated hospital at the levels of county, city and province then checked by the local Centers for Disease Control and Prevention (CDC) in Zhejiang Province. In this study, the details of TB cases including gender, year, date of notification, reported city, etc. were acquired and analyzed. The residential populations of both genders in Zhejiang Province and other sociodemographic information were obtained from the Chinese Information System for Disease Control and Prevention (CISDCP) and the Zhejiang Statistical Yearbook (free access from official website: http://tjj.zj.gov.cn/col/col1525563/index.html). Permissions of data access in CISDCP and TBIMS were approved by the Zhejiang Provincial Center for Disease Control and Prevention. In this study, some private information, such as patient name, identification number, address, and contact information were excluded. The data was checked and screened by two independents, respectively.
Case definition
Notified TB cases included in the TBIMS consisted of laboratory confirmed pulmonary tuberculosis (PTB), clinical diagnostic PTB, and extrapulmonary tuberculosis (EPTB). All TB cases were classified based on the National Diagnostic Criteria for Pulmonary Tuberculosis (WS288–2008, WS196-2001, and WS 288-2017) and Classification of Tuberculosis (WS196-2017)17–19. The confirmed PTB cases were denoted as people with possible PTB symptoms such as continuous cough for more than two weeks, hemoptysis, night sweat, etc. and confirmed by sputum smear and/or sputum culture with the result of detectable acid-fast bacilli or positive result from a rapid molecular diagnostic instrument (e.g., GeneXpert). Clinical diagnosis of PTB was defined as people with obviously abnormal chest radiography along with no curative effect from anti-inflammatory treatment under the circumstance of negative results from laboratory tests or related result absence20,21. EPTB was defined as people who were diagnosed as tuberculosis in other organs other than lung19.
Epidemiological characteristics of TB in Zhejiang Province from 2009–2018
The data were downloaded from the TBIMS. After the data cleaning in our study group, the distribution and epidemiological characteristics of notified TB were presented by year, age, gender, ethnics, occupation, season, reported region, and treatment type, respectively.
Stratified analysis by gender and rate difference (RD) before-after five years
Using the registered permanent population of both genders in Zhejiang Province, all included cases were categorized into 18 age groups, each consisting of 5 years. The notified incidences of TB in each group were calculated. We used the RD to examine alterations in notified TB incidence by comparing each group with its corresponding senior group. This was defined as the group five years into the future by age and years. If the RD value was positive, it implied a still rising risk in this age-specific group with the definition of positive RD. Otherwise, the RD value hinted at declined risk, denoting negative RD.
Time-series analysis of ARIMA model
The ARIMA model was first presented by Box & Jenkins in 1970 and consisted of three sections in the order of autoregression (p), the degree of difference (d), and the order of moving average (q)22. For seasonal trends, the model presented as ARIMA (p, d, q) × (P, D, Q) s, in which P denoted seasonal autoregression, D as the seasonal differencing degree, and Q as the seasonal moving average. Given the underlying seasonal feature of notified TB, the seasonal model was selected and performed in this study. As is common, the stationarity of data was tested in the first step; if the data was not stationary, the appropriate differencing and/or exponential transformation was conducted to convert the data into a stationary series. In addition, autocorrelation function (ACF) and the partial autocorrelation function (PACF) were used to identify the q and p23. Ljung-Box tests were also used to perform the white noise test, and indicators like Akaike’s Information Criterion (AIC) and Bayesian Information Criterion (BIC) were adopted to screen the optimal model24.
Ethics statement
This research was approved by the Ethics Committee of the Zhejiang Provincial Center for Disease Control and Prevention. All personal information in this study was kept confidential as required.
Statistical analysis
The descriptive analysis and Mann-Kendall test were performed by R software (version 3.5.3) and Microsoft Excel, and the map presentation used the ArcGIS software (version 10.2, SERI Inc.; Redlands, CA, USA). The time series model was determined using R software (TSstudio package). All results were considered statistically significant at P < 0.05 with two sides.
Results
General epidemiological characteristics of TB
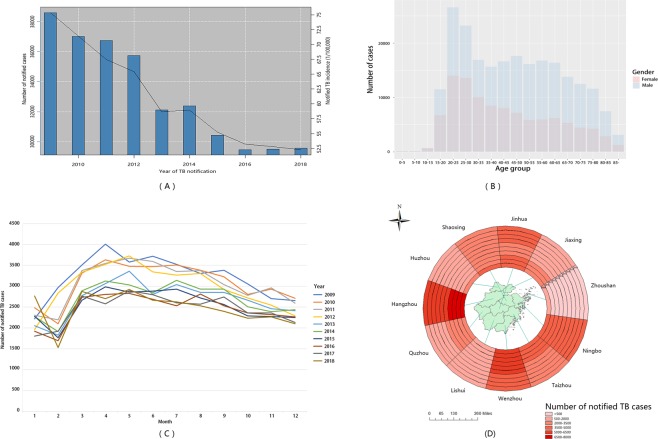
From 2009 to 2018, there was a total of 331,594 notified TB cases in the national surveillance system from Zhejiang Province, with a gender ratio of male to female 2.16:1. The number of males in all age groups was more than females. The top 10 ethnic groups were listed and the Han ethnic group accounted for more than 96% of notified TB cases. Also, a declining trend with significance by year was identified in Han, She and Mongolia ethnic groups, respectively (Supplement Table 1, determined by Mann-Kendall test). The sum of peasants and workers accounted for nearly 70% of notified cases in the study period. Additionally, the TB notification incidences showed a declining trend from 75.38/100,000 in 2009 to 52.25/100,000 in 2018. The nadir of notified TB cases was identified in February, and then the number of cases reached a peak in April with a persistent decline in the following months. For the regional distribution, Hangzhou, Ningbo, Wenzhou, and Jinhua had more TB cases than other cities, although recent decades witnessed decreasing case numbers in each prefecture. These are shown in Fig. 2. Furthermore, our results showed that the proportion of relapse cases accounted for nearly 7% of all notification TB cases in the study period (Supplement Table 2).
Figure 2.
General Epidemiological Characteristics of TB in Zhejiang Province between the Period 2009–2018. (A) Number of notified TB cases in Zhejiang Province during the period 2009–2018; (B) Number of notified TB cases for both genders among different age groups; (C) The seasonal distribution of notified TB cases; (D) The regional distribution of notified TB cases in Zhejiang Province. These were created by R software (3.5.3), Excel (Microsoft Excel 2016), and ArcGIS software (version 10.2, ESRI Inc.; Redlands, CA, USA). URL http://www.R-project.org; https://www.esri.com/.
The stratified analysis of notified TB by age groups in males and females
To find the accurate notified TB incidence in each age-specific group, the permanent population during the study period was used to standardize notified TB incidence. From Tables 1 and 2, the notification rate of different genders in each age group nearly all showed a declining trend, particularly in the groups of “20–50” and “>75”. Additionally, all age groups under 15 demonstrated a lower notified TB incidence in both male and female populations. Notified TB incidence rose sharply and reached its first peak within the age brackets of “15–35” in males and females. Following, the rate showed a slight decline and arrived at the second peak after “>55” for both genders. For the second peak, the incidence for males was higher than the female group by nearly 3–4 times. The details are shown in Tables 1 and 2.
Table 1.
Standardized Notified TB Incidence (1/100,000) in Males from 2009 to 2018.
| Age groups | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| 0- | 1.87 | 1.49 | 0.89 | 0.78 | 0.55 | 0.84 | 0.75 | 0.22 | 0.37 | 0.79 |
| 5- | 1.34 | 1.01 | 1.78 | 1.46 | 0.6 | 1.25 | 0.26 | 0.81 | 0.47 | 0.53 |
| 10- | 3.56 | 4.25 | 4.59 | 5.92 | 4.73 | 3.62 | 5.19 | 5.33 | 4.87 | 7.38 |
| 15- | 69.78 | 69.01 | 62.77 | 72.77 | 75.67 | 68.34 | 63.8 | 66.15 | 57.97 | 51.96 |
| 20- | 167.55 | 172.27 | 166.75 | 122.13 | 105.33 | 98.75 | 83.56 | 88.94 | 83.08 | 78.2 |
| 25- | 156.39 | 150.19 | 131.05 | 108.11 | 97.47 | 98.14 | 93.3 | 95.28 | 89.71 | 86.72 |
| 30- | 128.41 | 118.35 | 102.42 | 81.81 | 76.24 | 70.6 | 64.54 | 68.58 | 68.52 | 68.7 |
| 35- | 96.75 | 97.25 | 81.83 | 70.79 | 62.06 | 59.04 | 52.31 | 48.53 | 46.85 | 48.06 |
| 40- | 88.93 | 84.88 | 76.92 | 72.01 | 60.87 | 63.42 | 53.57 | 50.59 | 49.61 | 46.37 |
| 45- | 101.55 | 89.54 | 84.53 | 75.38 | 59.91 | 62.31 | 56.42 | 53.53 | 53.8 | 52.75 |
| 50- | 87.83 | 76.13 | 65.15 | 84.76 | 81.69 | 84.79 | 82.91 | 86.6 | 88.7 | 90.88 |
| 55- | 107.74 | 96.76 | 86.18 | 108.46 | 99.06 | 93.49 | 85.71 | 76.96 | 67.65 | 79.94 |
| 60- | 141.29 | 121.09 | 103.66 | 122.53 | 109.58 | 107.06 | 105.48 | 104.48 | 116.9 | 120.59 |
| 65- | 191.14 | 157.61 | 139.53 | 167.4 | 160.04 | 158.09 | 166.66 | 146.3 | 156.67 | 140.61 |
| 70- | 239.97 | 218.54 | 167.21 | 191.05 | 156.94 | 175.58 | 169.62 | 142.83 | 163.5 | 156.43 |
| 75- | 303.78 | 276.29 | 225.56 | 210.55 | 200.09 | 194.77 | 175.77 | 146.54 | 137.21 | 115.38 |
| 80- | 299.49 | 284.43 | 236.66 | 211.83 | 207.66 | 217.46 | 220.05 | 176.56 | 191.65 | 175.42 |
| >85 | 398.15 | 370.47 | 337.9 | 163.39 | 167.94 | 153.51 | 200.05 | 176.42 | 193.19 | 172.44 |
Table 2.
Standardized Notified TB Incidence (1/100,000) in Females from 2009 to 2018.
| Age groups | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| 0- | 0.94 | 0.74 | 1.17 | 0.63 | 0.73 | 0.36 | 0.27 | 0.26 | 0.26 | 0.17 |
| 5- | 1.03 | 1.09 | 1.29 | 0.82 | 0.74 | 0.52 | 0.68 | 0.6 | 0.6 | 0.59 |
| 10- | 5.99 | 5.41 | 5.62 | 6.66 | 5.37 | 4.61 | 6.83 | 8.84 | 5.87 | 6.03 |
| 15- | 49.78 | 53.15 | 46.86 | 41.85 | 42.07 | 47.83 | 39.55 | 35.69 | 36.89 | 31.92 |
| 20- | 103.51 | 105.63 | 97.17 | 70.93 | 60.34 | 57.02 | 48.68 | 44.84 | 43.3 | 41.04 |
| 25- | 88.66 | 88.41 | 81.39 | 68.61 | 61.03 | 61.4 | 61.9 | 59.22 | 56.78 | 50.61 |
| 30- | 65.03 | 67.33 | 58.85 | 50.27 | 50.81 | 47.1 | 45.42 | 44.4 | 45.67 | 46.54 |
| 35- | 49.47 | 51.57 | 44.37 | 38.03 | 33.56 | 34.74 | 30.64 | 29.56 | 26.54 | 30.57 |
| 40- | 42.15 | 39.81 | 37.06 | 36.82 | 29.14 | 32.15 | 26.52 | 27.48 | 24 | 26.04 |
| 45- | 39.8 | 35.25 | 36.32 | 33.25 | 23.59 | 26.82 | 24.27 | 24.13 | 23.73 | 22.84 |
| 50- | 30.17 | 26 | 23.83 | 30.45 | 32.66 | 33.99 | 35.63 | 34.8 | 38.29 | 38.36 |
| 55- | 43.61 | 34.58 | 32.06 | 43.02 | 33.31 | 33.65 | 30.19 | 27.8 | 25.5 | 28.56 |
| 60- | 50.83 | 49.06 | 41.76 | 49.22 | 41.59 | 43.22 | 43.67 | 41.38 | 43.18 | 45.8 |
| 65- | 67.29 | 64.51 | 50.79 | 68.53 | 66.83 | 68.81 | 68.85 | 63.43 | 64.52 | 54.64 |
| 70- | 68.66 | 70.54 | 60.95 | 68.61 | 66.66 | 68.25 | 68.54 | 66.71 | 64.77 | 64.03 |
| 75- | 85.72 | 82.42 | 72.02 | 70.63 | 68.49 | 74.4 | 68.96 | 53.29 | 52.97 | 47.26 |
| 80- | 74.4 | 67.86 | 70.19 | 67.65 | 67.72 | 81.58 | 72.54 | 63.05 | 69.53 | 57.92 |
| >85 | 75.27 | 87.63 | 69.35 | 42.53 | 50.87 | 52.01 | 56.79 | 49.55 | 48.77 | 49.21 |
Rate difference (RD) of notified TB before-after five years among the study population
In this study, all notified TB cases were classified into five-year age groups. We considered age groups in 2014–2018 to be the follow-up of age groups younger by five years in 2009–2013. For these five comparison groups, the total trend of RD in each year (2014–2018) was nearly the same. For males, the positive RD of notified TB incidence focused on the age groups of “5–25” and “50–70”, which was similar to females. The age groups of “25–50” and “>75” presented negative RD of notified TB incidence while the differences were diminishing in both genders. These results are shown in Fig. 3.
Figure 3.
RD of Notified TB Incidence Before-After Five Years in the Study Period. The RD in different age groups of males (A) and female (B); the pink histogram above the horizon represents increased incidence, while the light blue histogram under the horizon indicates the decreased incidence of TB. These were created by R software (3.5.3). URL http://www.R-project.org.
Time-series analysis of notified TB cases
In this study, we used R software to identify the predicted model of notified TB cases. Given the obvious periodic feature of TB occurrence, the seasonal ARIMA model was constructed in this study. Ultimately, SARIMA (2,0,2) (0,1,1)12 (AIC = 1465.9, BIC = 1484.7) was determined as the optimal one. The further prediction of notified TB cases in 2019 is shown in Fig. 4 and Table 3. Additionally, the estimated parameters for this SARIMA model are presented in Table 4.
Figure 4.
Periodic Identification of Notified TB Cases and Trend Prediction of TB in Zhejiang Province. (A) The identification of potential periodicity of TB notification. With the change of different lag, the scatter diagram showed an obvious linear trend at lag 12, implying a potential periodicity of 12 months; (B) Number of notified TB cases predicted by the SARIMA model. The TBIMS data in the study period was used as the training dataset to construct the model and predict notified TB cases with an 80% confidence interval (CI) and 95% CI in 2019. These figures were created by R software (3.5.3). URL http://www.R-project.org.
Table 3.
Predicted Notified TB Cases by SARIMA (2,0,2) (0,1,1)12 and the Actual Notified Number in 2019.
| Time | Point estimation | The upper value of 95% CI | The lower value of 95% CI | The notification number |
|---|---|---|---|---|
| Jan-19 | 2068 | 1674 | 2461 | 2154 |
| Feb-19 | 1574 | 1179 | 1969 | 1626 |
| Mar-19 | 2646 | 2239 | 3054 | 2628 |
| Apr-19 | 2594 | 2172 | 3016 | 2707 |
| May-19 | 2733 | 2304 | 3163 | 2662 |
| Jun-19 | 2546 | 2114 | 2978 | 2397 |
| Jul-19 | 2479 | 2046 | 2911 | 2620 |
| Aug-19 | 2434 | 2001 | 2867 | 2428 |
| Sep-19 | 2356 | 1923 | 2789 | 2292 |
| Oct-19 | 2108 | 1675 | 2541 | 2110 |
| Nov-19 | 2098 | 1665 | 2530 | 1942 |
| Dec-19 | 1961 | 1529 | 2394 | 1950 |
Table 4.
Estimated Parameters of SARIMA for the Prediction of Notified TB Cases.
| Parameter* | RMSE | MAE | MPE | MAPE | MASE |
|---|---|---|---|---|---|
| Value | 185.17 | 134.78 | −0.28 | 5.17 | 0.75 |
*RMSE: root mean square error; MAE: mean absolute error; MPE: mean percentage error; MAPE: mean absolute percentage error; MASE: mean absolute square error.
Discussion
Although TB is deemed as a preventable and treatable disease, it still causes a considerable global disease burden25. According to the release of the Global Burden of Disease (GBD) in 2016, the annualized age-standardized change in TB incidence among HIV-negative individuals was −1.3% [−1.5 to −1.2] and −4.0% [−4.5 to −3.7] in HIV-positive individuals, rates that would not meet the demand of the End TB strategy by 203025. Additional analysis of China in GBD 2015 demonstrated a slow decline in TB mortality and DALYs in recent years26. That is to say, some existing prevention, control strategies, and policies should still be improved. Zhejiang Province, as a developed area in China with a GDP like the Netherlands, recently undertook numerous explorations and endeavors to accelerate the realization of the End TB goal such as improving etiology diagnosis by popularizing Gene-Xpert technology, promoting treatment compliance through the implementation of electronic pillbox, and implementing health insurance reform for TB patients through payment reform, etc. Also, International Cooperation Projects like the Global Fund TB Program and China-Bill Melinda Gate Phase III provided a new horizon to control TB epidemics in local regions. Thus, the identification and exploration of regulation in the past decade was of importance in summarizing the previous practices, identifying existing insufficiencies, and providing advice for optimizing available health policy.
In this study, we included nearly 332,000 notified TB cases in the recent decade. In total, the incidence of notified TB declined by about 30%, which was inseparable with the actions above in Zhejiang Province. However, there was an obvious higher proportion of cases in the male population. According to the annual monitoring report in China, the average proportion of male to female in the whole population was about 2.19:1, which was similar to the proportion in Zhejiang Province. In different countries and regions, the ratio of male to female also demonstrated disparities. Previous TB prevalence surveys demonstrated a ratio of 1.2 in Ethiopia and 4.5 in Vietnam27,28. Given the average sex ratio of 1.9:1 around the globe, this implies a higher burden in the male population in China and Zhejiang Province29. This appearance has also presented in some low-burden countries30,31. Interestingly, one study in Germany demonstrated that the prevalence of latent TB infection had no difference in gender distribution while active TB in males showed an apparent dominance32. Thus, further study should be considered in the field of immune responses and inflammatory responses to find its potential mechanism28; meanwhile, more specific public health interventions, community health education and policy support should be considered to lower TB transmission20. The Han ethnic group accounted for the majority in Zhejiang Province, and the continuous decline of notified TB cases in this group was consistent with the trend of our findings in the whole population. Similar to the previous findings, the peasants and workers were the commonly vulnerable population for TB occurrence in Zhejiang Province, implying these occupation populations should still be prioritized in the developed area33.
The phenomenon of declined notification cases in January and February accompanied with the rapid rise around March and April was attributable to two factors. The first one, the Spring Festival, also known as the Lunar New Year in China, occurred in January or February. More patients seek medical care after this grand festival. Due to the concentration of family gatherings in the spring festival, delayed medical presentation might further aggravate potential TB infection and transmission. Therefore, more health education should be carried out before this period. Furthermore, health checkups for college enrollment examinations around March and April might also contribute to the increased identification of active TB cases with no/mild symptoms.
In spatial distribution, more notified cases were reported in Hangzhou, Ningbo and Wenzhou city, which were ascribed to their large population in some part, but also associated with the developed economic level that attracted more migrant populations in other regions of Zhejiang Province and outside the province33. Therefore, combined with a previous study, more holistic policies such as comprehensive health-care policy should be put forward to give full coverage to all people in the community, which could reduce the risk of treatment interruption in patient groups, particularly in migrant groups with low socio-economic conditions34.
The stratified analysis was performed in both gender populations. Broadly speaking, the notified TB incidence in males was higher than that in females. For both genders, the low occurrences were concentrated in ages under 15, which might be attributable to the efficacy of Bacillus Calmette-Guerin (BCG) in protection against childhood and disseminated TB35. This finding also proved to some extent that the preventive effect of BCG might cover the first decade of life, which was consistent with previous long-term results36. For both genders, age groups from “15–35” and “>55” showed a high notification incidence, especially in the senior age group, while the trend for females was relatively flat. The rising incidence in the “15–35” age group might be correlated with the attenuation of protective efficacy and the increased exposure of environmental mycobacteria that reduced reactivity to BCG37–40. Thus, for the student population during the age period of “15–35”, it is suggested that the school infirmary provide additional care to students with TB symptoms, especially for students residing in the same dormitory, which might imply the possibility of TB clustering. For manual workers, workplaces should provide regular physical examinations and necessary promotion and education to enhance early TB findings and reduce clustered epidemics. For the age group of “>55”, the increased TB incidence might be attributed to the low immunity in this specific population, particularly in the diabetic population with limited control levels of blood glucose20. Our previous study also demonstrated that active case findings could decrease the active TB incidence in some target populations20. In the future, more elaborated and comprehensive actions should be formulated and explored to reverse this TB epidemic in Zhejiang Province.
RD of notified TB incidence before-after five years were analyzed in our study. To our knowledge, the notification rate of TB in each age group might be influenced by several factors such as internal immune levels and existing supervision (like underdiagnosis and underreporting, etc.) combined with corresponding external health policy (medical insurance and special government subsidies, etc.)16,41,42. Thus, we used the RD to offset some internal effects such as the efficacy of BCG and identified the underlying external reasons, providing evidence for strengthening health strategies and advancing health policies. Based on the available results, although the overall notified TB incidence experienced a decline, we still found increased notification of TB among age groups “15–20” and “65–70” in both genders. For the age group of “15–20”, the majority was a student population around the period of high school to college and the relative risk ranged from 19.2 to 10.9 from 2014 to 201843. Despite a successive decline of TB risk in this specific group, the rate of descent was insufficient under the existing health policy. Given that the only uniform medical checkup for students occurs during college enrollment, we appealed to have routine checkups conducted annually for students in this age group to enhance TB identification and integrate this strategy into further policies of TB control in Zhejiang Province. For the age group of “65–70”, the increased TB notification incidence was attributable to recent efforts in health examination for the elderly, which improved the finding of active TB in this age group and correlated with the further decline in the older age group20,44,45. Thus, it is suggested that more comprehensive physical examination involving TB identification such as GeneXpert should be considered in some developed areas with a high TB incidence. Moreover, other age groups demonstrated declining notified TB incidence, implying the effectiveness of current health control and prevention strategies. Yet, the diminishing negative value of RD and absolutely high incidence in these groups might illustrate that novel implementations and strategy combined with the integration of early identification, clinical treatment and community management for TB control should be explored.
Ultimately, the SARIMA (2,0,2) (0,1,1)12 model was chosen and applied to the prediction of notified TB cases in Zhejiang Province. Comparing our predictions with actual notified TB number from the TBIMS system in 2019 demonstrated the accuracy in our model’s fit. Previous studies in other regions also used the SARIMA model to give a short-term prediction of TB epidemics with high predictive precision, which was consistent with our findings46,47.
Limitations
Some limitations should be listed in this study. Firstly, the data we used was notification records. Due to differences in notification quality amid different regions, some bias in the results might be unavoidable. Due to the paucity of details involving socio-economic parameters, TB latent infection data, and drug-resistance information throughout the study period, we did not analyze these factors in our study, which might not reveal the comprehensiveness of TB occurrence. Besides this, although we had prudently drawn some conclusions, we did not take the potential influence of the migrant population from other provinces into account, which might also have had an effect in our available results. In addition, we tried to explore the possible epidemiological characteristics of TB notification in eastern China while the data from one province might not give a full description. Finally, the model we had chosen was a common one with the possibility of overfitting, and another more suitable integrated model such as the ARIMA-NAR hybrid model was not considered in this study.
Conclusion
In general, the notification of TB incidence in Zhejiang Province was declining in the past decade while the male population and critical months from January to April still need special attention. Some implementations such as the increased frequency of checkups in specific student groups and strengthening of elder health examination could be explored and integrated into available health policy. Ultimately, the SARIMA model can be used to fit trends in TB notification cases well, and can be used in the further prediction of TB in Zhejiang Province.
Supplementary information
Acknowledgements
This work was supported by the Project of China-Bill Melinda Gate Phase III, Zhejiang Provincial Science and Public Welfare (LGF19H260004), Key Laboratory of Public Health Safety (Fudan University), Ministry of Education, China (GW2019-3), Philosophy and Social Science Project of Zhejiang Province (19NDJC243YB). We would like to thank the National Center for Tuberculosis Control and Prevention for their support and assistance. Also, we appreciated the local center for disease control and prevention (CDCs), community healthcare centers, and TB-designated hospitals for the implementation of TB’s report and notification in the daily work.
Author contributions
L.K. drafted the manuscript. L.T. revised the manuscript. V.A. revised the manuscript. P.Y. collected and screened the data. Z.Y. interpreted the manuscript. W.F. collected and screened the data. W.W. analyzed the data. C.C. revised the manuscript. C.S. analyzed the data. Z.L. revised the manuscript. C.X. revised the manuscript. B.Q. analyzed the data. B.C. designed and revised the manuscript. W.X. designed and revised the manuscript. J.J. designed and revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kui Liu and Tao Li.
Contributor Information
Bin Chen, Email: bchen@cdc.zj.cn.
Xiaomeng Wang, Email: xmwang@cdc.zj.cn.
Jianmin Jiang, Email: jmjiang@cdc.zj.cn.
Supplementary information
is available for this paper at 10.1038/s41598-020-64387-5.
References
- 1.World Health Organization. Geneva. Global tuberculosis report 2018, http://www.who.int/tb/publications/global_report/en/ (2018).
- 2.Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393:1642–1656. doi: 10.1016/s0140-6736(19)30308-3. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Yao H, Liu E. Analysis of factors affecting the epidemiology of tuberculosis in China. Int. J. Tuberc. Lung Dis. 2005;9:450–454. [PubMed] [Google Scholar]
- 4.Ge E, Zhang X, Wang X, Wei X. Spatial and temporal analysis of tuberculosis in Zhejiang Province, China, 2009–2012. Infect. Dis. Poverty. 2016;5:11. doi: 10.1186/s40249-016-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, et al. Epidemiological characteristics of tuberculosis in Zhejiang Province, 2010. Dis. Surveill. 2011;26:601–603. [Google Scholar]
- 6.Zeng Q, et al. Time series analysis of temporal trends in the pertussis incidence in Mainland China from 2005 to 2016. Sci. Rep. 2016;6:32367. doi: 10.1038/srep32367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, et al. Identification of distribution characteristics and epidemic trends of hepatitis E in Zhejiang Province, China from 2007 to 2012. Sci. Rep. 2016;6:25407. doi: 10.1038/srep25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang F, et al. Spatio-temporal trends and risk factors for Shigella from 2001 to 2011 in Jiangsu Province, People’s Republic of China. PloS One. 2014;9:e83487. doi: 10.1371/journal.pone.0083487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aryee G, et al. Estimating the incidence of tuberculosis cases reported at a tertiary hospital in Ghana: a time series model approach. BMC Public Health. 2018;18:1292. doi: 10.1186/s12889-018-6221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q, et al. Forecasting the seasonality and trend of pulmonary tuberculosis in Jiangsu Province of China using advanced statistical time-series analyses. Infect. Drug Resist. 2019;12:2311. doi: 10.2147/IDR.S207809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Rf, et al. Forecasting incidence of intestinal infectious diseases in mainland China with ARIMA model and GM (1, 1) model [J] Fudan University Journal of Medical Sciences. 2008;35:675–680. [Google Scholar]
- 12.Liu Q, Liu X, Jiang B, Yang W. Forecasting incidence of hemorrhagic fever with renal syndrome in China using ARIMA model. BMC Infect. Dis. 2011;11:218. doi: 10.1186/1471-2334-11-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Luan RS, Yin F, Zhu XP, Lü Q. Predicting the incidence of hand, foot and mouth disease in Sichuan province, China using the ARIMA model. Epidemiol. Infect. 2016;144:144–151. doi: 10.1017/S0950268815001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane MJ, Price N, Scotch M, Rabinowitz P. Comparison of ARIMA and Random Forest time series models for prediction of avian influenza H5N1 outbreaks. BMC Bioinformatics. 2014;15:276. doi: 10.1186/1471-2105-15-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moosazadeh M, Khanjani N, Nasehi M, Bahrampour A. Predicting the incidence of smear positive tuberculosis cases in Iran using time series analysis. Iran. J. Public Health. 2015;44:1526–1534. [PMC free article] [PubMed] [Google Scholar]
- 16.Li T, et al. Patient and health system delays before registration among migrant patients with tuberculosis who were transferred out in China. BMC Health Serv. Res. 2018;18:786. doi: 10.1186/s12913-018-3583-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinese Ministry of Health. Diagnostic Criteria for Pulmonary Tuberculosis, http://tb.chinacdc.cn/zcfg/dfzcfg/201208/P020120814448115268151.pdf (2008).
- 18.Gao MQ. Interpretation of clinical diagnosed pulmonary tuberculosis case in new national diagnostic standard on pulmonary tuberculosis. Chin. J. Antitubercul. 2018;40:243–246. [Google Scholar]
- 19.National Health and Family Planning Commission. Classification of Tuberculosis, https://www.sific.com.cn/Scripts/ueditor/asp/upload/file/20171129/15119447388882383.pdf (2018).
- 20.Liu K, et al. Assessment of active tuberculosis findings in the eastern area of China: A 3-year sequential screening study. Int. J. Infect. Dis. 2019;88:34–40. doi: 10.1016/j.ijid.2019.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Li T, et al. Evidence for heterogeneity in China’s progress against pulmonary tuberculosis: uneven reductions in a major center of ongoing transmission, 2005–2017. BMC Infect. Dis. 2019;19:615. doi: 10.1186/s12879-019-4262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Box, G. E., Jenkins, G. M., Reinsel, G. C. & Ljung, G. M. Time Series Analysis: Forecasting and Control (John Wiley & Sons, 2015).
- 23.Wang K, et al. Hybrid methodology for tuberculosis incidence time-series forecasting based on ARIMA and a NAR neural network. Epidemiol. Infect. 2017;145:1118–1129. doi: 10.1017/S0950268816003216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ljung GM, Box GE. On a measure of lack of fit in time series models. Biometrika. 1978;65:297–303. doi: 10.1093/biomet/65.2.297. [DOI] [Google Scholar]
- 25.Kyu HH, et al. Global, regional, and national burden of tuberculosis, 1990–2016: results from the Global Burden of Diseases, Injuries, and Risk Factors 2016 Study. Lancet Infect. Dis. 2018;18:1329–1349. doi: 10.1016/S1473-3099(18)30625-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu S, Xia L, Yu S, Chen S, Zhang J. The burden and challenges of tuberculosis in China: findings from the Global Burden of Disease Study 2015. Sci. Rep. 2017;7:14601. doi: 10.1038/s41598-017-15024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Geneva. Global tuberculosis report 2017, http://apps.who.int/iris/bitstream/handle/10665/259366/9789241565516-eng.pdf?sequence=1 (2017).
- 28.Hertz D, Schneider B. Sex differences in tuberculosis. Semin. Immunopathol. 2019;41:225–237. doi: 10.1007/s00281-018-0725-6. [DOI] [PubMed] [Google Scholar]
- 29.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir. Med. 2018;6:299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- 30.Brodhun, B., Altmann, D., Hauer, B., Fiebig, L. & Haas, W. Bericht zur Epidemiologie der Tuberkulose in Deutschland für 2016 (2017). [DOI] [PubMed]
- 31.Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2016. Atlanta: US Department of Health and Human Services, https://www.cdc.gov/mmwr/volumes/66/wr/mm6611a2.htm#suggestedcitation (2017).
- 32.Herzmann C, et al. Risk for latent and active tuberculosis in Germany. Infection. 2017;45:283–290. doi: 10.1007/s15010-016-0963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying-zhou Y. Focus on tuberculosis control and prevention among vulnerable populations. Chin. J. Antitubercul. 2013;35:868–870. [Google Scholar]
- 34.Narasimhan P, Wood J, MacIntyre CR, Mathai D. Risk factors for tuberculosis. Pulm. Med. 2013;2013:1–11. doi: 10.1155/2013/828939. [DOI] [Google Scholar]
- 35.Moliva JI, Turner J, Torrelles JB. Prospects in Mycobacterium bovis Bacille Calmette et Guerin (BCG) vaccine diversity and delivery: why does BCG fail to protect against tuberculosis? Vaccine. 2015;33:5035–5041. doi: 10.1016/j.vaccine.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aronson NE, et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. JAMA. 2004;291:2086–2091. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]
- 37.Colditz GA, et al. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA. 1994;271:698–702. doi: 10.1001/jama.1994.03510330076038. [DOI] [PubMed] [Google Scholar]
- 38.Mangtani P, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin. Infect. Dis. 2013;58:470–480. doi: 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 39.Moliva JI, Turner J, Torrelles JB. Immune responses to bacillus Calmette–Guérin vaccination: why do they fail to protect against Mycobacterium tuberculosis? Front. Immunol. 2017;8:407. doi: 10.3389/fimmu.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–1345. doi: 10.1016/S0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 41.Tao L, Hui Z, Xia WL, Yu P, Xin. D. Description and factors affecting the referral of presumptive tuberculosis patients in China. Biomed. Environ. Sci. 2017;30:444–449. doi: 10.3967/bes2017.058. [DOI] [PubMed] [Google Scholar]
- 42.Pan Y, et al. Disparity in reimbursement for tuberculosis care among different health insurance schemes: evidence from three counties in central China. Infect. Dis. Poverty. 2016;5:7. doi: 10.1186/s40249-016-0102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao HD, et al. Tuberculosis outbreaks among students in mainland China: a systematic review and meta-analysis. BMC Infect. Dis. 2019;19:1–12. doi: 10.1186/s12879-019-4573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, et al. The national awareness survey on key TB messages in 2015. Chin. J. Antitubercul. 2017;39:282–288. [Google Scholar]
- 45.Yu L, et al. The national awareness survey on key TB messages in 2010. Chin. J. Antitubercul. 2013;35:60–64. [Google Scholar]
- 46.Yi J, Du C, Wang R, Liu L. Applications of multiple seasonal autoregressive integrated moving average (ARIMA) model on predictive incidence of tuberculosis. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.]. 2007;41:118–121. [PubMed] [Google Scholar]
- 47.Chen, Y. C. & Zhu, W. J. Application of Seasonal ARIMA Model in Forecasting Incidence of Tuberculosis. Journal of Taiyuan Normal University (Natural Science Edition). 2 (2012).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.