Abstract
Recent epidemiological studies link Periodontal disease(PD) to age-related macular degeneration (AMD). We documented earlier that Porphyromonas gingivalis(Pg), keystone oral-pathobiont, causative of PD, efficiently invades human gingival epithelial and blood-dendritic cells. Here, we investigated the ability of dysbiotic Pg-strains to invade human-retinal pigment epithelial cells(ARPE-19), their survival, intracellular localization, and the pathological effects, as dysfunction of RPEs leads to AMD. We show that live, but not heat-killed Pg-strains adhere to and invade ARPEs. This involves early adhesion to ARPE cell membrane, internalization and localization of Pg within single-membrane vacuoles or cytosol, with some nuclear localization apparent. No degradation of Pg or localization inside double-membrane autophagosomes was evident, with dividing Pg suggesting a metabolically active state during invasion. We found significant downregulation of autophagy-related genes particularly, autophagosome complex. Antibiotic protection-based recovery assay further confirmed distinct processes of adhesion, invasion and amplification of Pg within ARPE cells. This is the first study to demonstrate invasion of human-RPEs, begin to characterize intracellular localization and survival of Pg within these cells. Collectively, invasion of RPE by Pg and its prolonged survival by autophagy evasion within these cells suggest a strong rationale for studying the link between oral infection and AMD pathogenesis in individuals with periodontitis.
Subject terms: Cell biology, Immunology, Microbiology, Diseases, Pathogenesis
Introduction
Periodontal disease (PD or periodontitis) is a highly prevalent (50%)1 inflammatory oral disease in the US. PD destroys the supportive connective tissue of teeth and alveolar bone and thereby is a primary cause of tooth loss. Over the years, studies have proven that chronic inflammation instigated in the oral cavity has implications for initiation and/or progression of many systemic diseases2–11. Markedly, type-2 diabetes3, cardiovascular disease12, stroke7, respiratory infections like pneumonia5, pre-term birth9, Alzheimer’s disease6,10, Parkinson’s disease13, liver disease14, rheumatoid arthritis8, intra- and extra-oral cancers15 are linked to periodontitis.
P. gingivalis16,17, an effective late colonizer of oral tissues and a red complex bacterium is highly associated with PD. This gram-negative anaerobe expresses gingipain proteinase (kgp/rgp), fimbriae (major and minor), lipopolysaccharides (LPS), collagenase, and endotoxin (TLR2/TLR4 agonist/antagonist), all of which contribute to its virulence. The pathogenicity of P. gingivalis, including its ability to colonize tooth surfaces, moderate host cytokine responses, cause tissue damage and inhibit cell apoptosis18 have been studied. Recent studies emphasize the ability of P. gingivalis to hijack host autophagy pathways to establish a successful replicative niche for extended survival in gingival epithelial cells (GECs)19. The major FimA and minor Mfa1 fimbriae facilitate invasion of host epithelial cells, endothelial cells, and dendritic cells (DCs) by P. gingivalis, enabling it to avoid the host defense mechanism20 and in the case of DCs, disseminate throughout the body. The tissue destruction caused by PD is not directly by the bacterial byproducts but by the cascade of host inflammatory response. Therefore, the “mobile microbiome” is capable of inhabiting extra-oral sites culminating in distant infections and persistent general inflammation21.
Age-related Macular Degeneration (AMD) is a highly prevalent eye disease that imposes significant impact on public health, causing irreversible loss of central vision in the elderly22,23. AMD occurs through a combined non-neovascular and neovascular derangement24. The hallmark of dry or nonexudative form of AMD is the deposition of extracellular material and drusen formation underneath the retinal pigment epithelial (RPE) layer, leading to loss of retinal photoreceptors25. Alternately, the exudative or wet type is characterized by choroidal neovascularization underneath the RPE. There are strong evidences stipulating that inflammation plays a major role in AMD26. Many studies suggest that drusen formation may initiate an inflammatory cascade that directs AMD progression25. It is reported that sections of drusen contain increased levels of autophagic markers27. Elevated oxidative stress and dysregulated autophagy induce RPE degeneration and subsequently chronic inflammation in the retina25,28 which is pathognomonic of AMD. The sequelae involves RPE degeneration ensuing in disrupted Bruch’s membrane, increased vascular permeability and retinal neovascularization, endangering central vision26. A complex etiology and unknown triggers limit a clear understanding of AMD pathophysiology and effective cure.
Chronic oral inflammation in PD is sustained by dysbiosis of the “proximal gut”, i.e. the oral cavity, and a leaky attachment apparatus. Recent epidemiological evidence revealed a 2-fold higher risk of AMD in PD patients with alveolar bone loss11,29 although the mechanisms are unidentified. Pathologic shifts in oral microbiota is also linked to higher mortality rates30. Specific microbiota of human nasopharynx is linked to AMD31. Despite these observations, a limited understanding exists about the role of dysbiotic oral pathobionts in AMD pathology indicating a critical knowledge gap and an imperative need to probe the underlying molecular mechanisms. Moreover, the dissemination of oral microbes to the eye structure and their interactions that could contribute to the development of AMD have not been investigated yet. This may represent a novel opportunity to understand the mechanisms underlying the pathological association of AMD in coexisting chronic oral inflammation.
The anatomical, cellular and molecular specializations of the eye are thought to maintain an immune-privileged state32 as the entry of immune cells to this organ was thought to be nonexistent33. However, recent studies show recruitment of immune cells to the eye following retinal injury through infections or inflammation. The low-grade inflammation, sustained by dysbiosis and a leaky gut34, has been identified to contribute to the development of AMD.
Previously, we observed mechanisms for how P. gingivalis and its fimbrial-mutant strains invade and survive in human DCs35, however, the ability of P. gingivalis or other oral microbes to invade RPE have not been demonstrated. The RPE is a highly specialized, metabolically active layer which continuously recycles the shed photoreceptor cells and processes the metabolic wastes by autophagy and support the visual function36,37. Moreover, an intact blood retinal barrier (BRB) is pivotal to maintain a homeostatic retinal microenvironment. The BRB consists of dual layer with inner (tight junctions between retinal capillary endothelial cells) and the outer (tight junctions between RPE cells) compartments. Breakdown of the inner endothelial BRB is reported in diabetic retinopathy and that of outer BRB in case of AMD38.
Therefore, our goal is to examine the hypothesis that the dysbiotic oral pathogen P. gingivalis and its isogenic mutants, at different multiplicities of infection, are capable of invading human RPE cells (ARPE-19) in-vitro and surviving within as an intracellular pathogen. Using a combination of immunofluorescence, SEM, TEM, confocal microscopy, qPCR, antibiotic protection and survival assay, we show that P. gingivalis adheres to and invades RPEs, with the latter being an active process, requiring that the invading strain be viable and express fimbriae to evade autophagy, as an intracellular pathogen of RPEs. So, this will be the first study to demonstrate the invasion and internalization of the oral pathogen Pg and its mutant strains in RPE cells in-vitro. These emanating findings will provide a platform to get a new insight into the pathogenic role of P. gingivalis, comprehend the underlying mechanisms and plausibly expand the rudimentary knowledge of the association of AMD and PD.
Results
Invasion byP. gingivalis(Pg381) of Human Retinal Pigment Epithelial (ARPE-19) cells
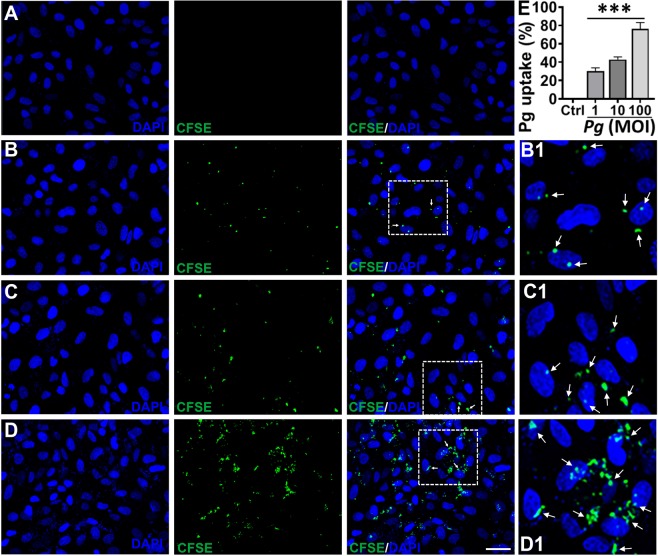
To determine whether P. gingivalis invades human RPE, ARPE-19 cells were cocultured with CFSE-labeled Pg381 at 1, 10 and 100 multiplicity of infection (MOI) (Fig. 1B–D) and uptake quantified (Fig. 1E), compared with uninfected control (Fig. 1A) after 24 hours. Observed was substantial adhesion and uptake of Pg381 by ARPE cells in a dose (MOI)-dependent manner as detected by confocal imaging analysis (Fig. 1B–D). It was interesting to note that Pg381 is capable of invading ARPE cells even at 1 MOI (Fig. 1B), which can be clearly observed in the cytosol and around the cell nuclei. Quantitative analysis showed significant uptake of Pg381 by ARPE-19 cells at 1, 10 and 100 MOI (Fig. 1E). This data also demonstrates that there was considerable increase in the invasion efficiencies of P. gingivalis with increasing MOI.
Figure 1.
Uptake of P. gingivalis by Human Retinal Pigment Epithelial (ARPE-19) cells. (A–D) Confocal images of human retinal pigment epithelial cells infected with CFSE-pre-labeled P. gingivalis (Pg381) after 24 hours at 1 (B), 10 (C) and 100 (D) MOI compared with uninfected control (A) group. The results show the apparent uptake of Pg381 by ARPE-19 cells at 1, 10, and 100 MOI as detected by confocal microscopy (white arrows). Boxed areas in B, C and D show an enlarged region as B1, C1 and D1, respectively. (Green-CFSE; Blue-DAPI). Scale bar: 20 µm. Images are representative of three independent experiments from three different passages in a double-blind manner. (E) The quantification analysis shows the uptake of Pg relative to the uninfected control and plotted as percentage. Analysis of fluorescent levels using IMAGEJ software revealed significant uptake of CFSE labeled P. gingivalis in all 1, 10 and 100 MOI groups compared with control group. The intensity of CFSE-labeled Pg were measured from six randomly selected images from three independent experiments. The data shown represent the mean ± standard error of the mean of three experiments (n = 3). One-way ANOVA analysis was used to compare the means of intensity of different groups/concentrations and Tukey’s multiple comparisons test with three different experiments (n = 3). ***P < 0.001. MOI - Multiplicity of Infection.
Live but not heat killedP. gingivalisinvade retinal epithelial cells
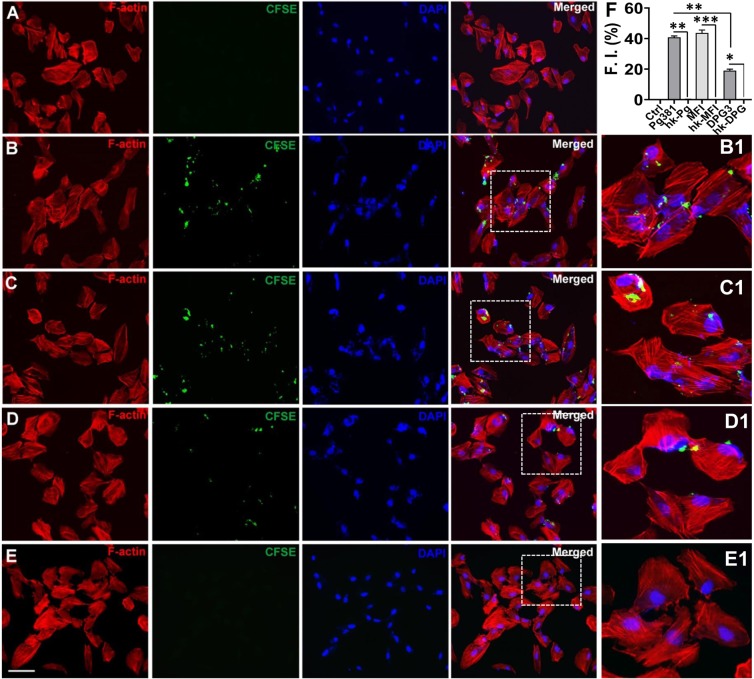
Next, we tested if heat-killed Pg381 or the isogenic fimbriae mutant strains MFI and DPG3 can invade the retinal epithelial cells. ARPE cells infected with CFSE labeled live Pg381 and its mutant strains at 10 MOI for 24 hours showed bacterial invasion upon examination by confocal imaging (Fig. 2A–E). Invasion was confirmed by the presence of P. gingivalis within the ARPE cell boundary surrounded by actin filaments through several consecutive z-sections. In the ARPE cells infected with live Pg381, MFI and DPG3, many were clearly visible around the cell membrane and nuclei as well cytosol at 10 MOI (Fig. 2B–D) compared with uninfected control (Fig. 2A). Interestingly, DPG3 strain, which lacks the major or FimA fimbriae was less able to invade ARPE cells (Fig. 2D). However, none of the heat-killed Pg381 (Fig. 2E and S1A) and its mutant strains (Fig. S1B,C) invaded ARPE-19 cells or were membrane-bound. The quantification analysis demonstrated significant invasion of Pg381, MFI and DPG3 compared to their respective heat-killed bacteria and uninfected control as well (Fig. 2F). Percentage of invasion of Pg381 is comparable with MFI, both of which express the major FimA, but MFI lacks minor or Mfa1 fimbriae, suggesting Mfa1 is not required for invasion of ARPE as it is for DCs. These results suggest that only live P. gingivalis and its mutant strains can efficiently invade retinal epithelial cells and that the major FimA fimbriae is required for optimum invasion.
Figure 2.
Live P. gingivalis and its isogenic mutant strains invades Human Retinal Pigment Epithelial (ARPE-19) cells. (A–E) ARPE-19 cells were co-cultured with live CFSE-labeled Pg381, MFI and DPG3 (10 MOI) or heat killed (HK) all fimbriated Pg strains for 24 hours and compared to uninfected control. After fixation and permeabilization, the infected ARPE cells were stained with rhodamine-phalloidin (F-actin for cell surface) and DAPI (nuclear stain) and then examined by confocal microscopy. Representative images show the live Pg381 (B), MFI (C) and DPG3 (D) can enter ARPE-19 cells but not the heat-killed Pg381 (E), HK-MFI and HK-DPG3 (refer Fig. S1A–C). Boxed areas in B, C, D and E show an enlarged region as B1, C1, D1 and E1, respectively. Red - F-actin; Green - CFSE; Blue - DAPI. The data shown are representative of three similar results. Scale bar: 20 µm. (F) The quantification analysis shows significant invasion of all fimbriated live Pg strains compared to the uninfected control as well as their respective heat-killed bacteria and plotted as percentage. The fluorescence intensity of CFSE-labeled Pg strains were measured as shown in Fig. 1. There were no significant differences between Pg381 and MFI groups. The analysis of the intensity used Kruskal-Wallis test of different groups and Dunn’s test for multiple comparisons 3 different experiments. *P < 0.05; **P < 0.01; ***P < 0.001. The error bars indicate ± SEM (n = 3). F.I. – Fluorescent Intensity.
Primary interaction ofP. gingivaliswith human retinal epithelial cell membrane
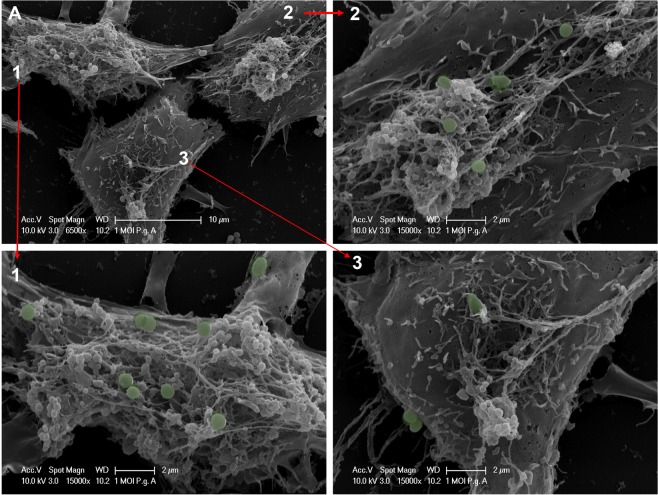
To examine early interactions of P. gingivalis with ARPE membrane at 1-hour, fixed cells were examined by scanning electron microscopy (SEM) as described earlier39. SEMs show initial interaction of Pg with the outer membrane of ARPE after 1-hour of infection (Fig. 3 and S2). Pg381 was able to adhere and tightly engage the human ARPE cell surface at 1 hour at both 1 (Fig. 3A 1–3 and S2A,B) and 10 (Fig. S2C,D) MOI. In addition, we also observed P. gingivalis highly clustered and engaged with cell membrane at 10 MOI (Fig. S2C,D).
Figure 3.
Primary interaction of P. gingivalis with ARPE cell membrane. (A) Adherence of P. gingivalis to ARPE cells examined by scanning electron microscopy (SEM). The sections show the primary interaction of Pg381 infected human retinal epithelial cells for 1 hour with 1 MOI. It clearly demonstrates apical and basolateral membrane engagement of Pg. Images A1, A2 and A3 (green stains for Pg381 are computer generated) shows the high magnification of 3 A. Scale bar: – A: 10 µm; 1, 2 and 3: - 2 µm. Refer fig. S2 for different images of SEM at 1 (Fig. 2A,B) and 10 (Fig. 2C,D) MOI of Pg381 for 24 hours infection.
High interaction and invasion ofP. gingivaliswithin ARPE-19 cells
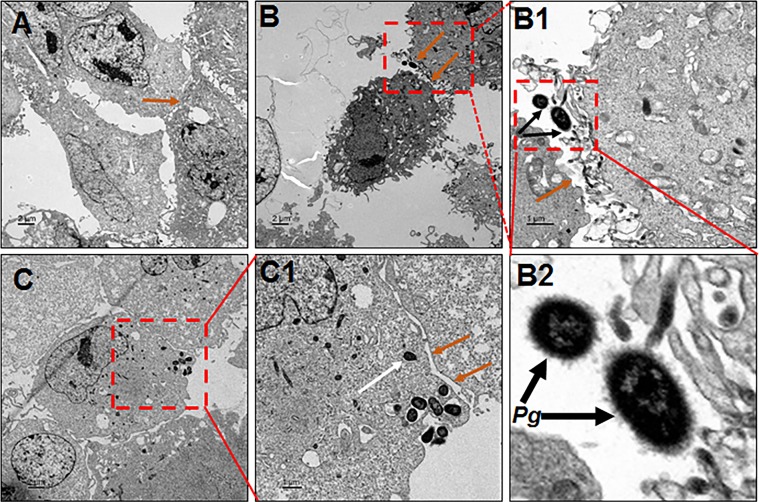
At 1-hour after bacterial co-culture with ARPE cells, the ARPEs were imaged for interaction and intracellular P. gingivalis by transmission electron microscopy (TEM). TEM delineated the intimate surface interaction, and adhesion of P. gingivalis, leading to invasion of Pg in ARPE cells after exposure at 1 (Fig. 4B,B1,B2) and 10 MOI (Fig. 4C,C1 and S3C,C1) for 1-hour, relative to uninfected control (Fig. 4A). It’s interesting to note at high power the visible Pg381 fimbriae and their interaction with ARPE cells (Fig. 4B2), particularly aggregated invasion at 10 MOI (Fig. 4C-C1 and S3C,C1), which is consistent with results observed in SEM images (Fig. S2C,D). These results confirm that the fimbriated Pg381 was able to interact and invade the ARPE cells.
Figure 4.
High interaction and invasion of P. gingivalis within ARPE cells. (A–C) Transmission electron microscopy (TEM) of human retinal pigment epithelial (ARPE) cells infected with P. gingivalis at 1 (B–B2) and 10 (C and C1) MOI for 1 hour compared with uninfected control (A). The sections show the clear host pathogen interaction and invasion of Pg381 (B,C) in ARPE cells relative to uninfected control (A). Boxed areas in B, B1 and C show an enlarged/magnified region B1, B2 and C1, respectively. (B1) P. gingivalis adhering to ARPE surface (black arrow). (B2) Enlarged section (of B1) showing the Pg fimbriae-mediated adhesion and their interaction with ARPE cells clearly. (C) P. gingivalis internalized and freely occupied the cytoplasm of ARPE cells (white arrow). Orange arrows indicate several epithelial junctions such long and short tight junctions between ARPEs, particularly, B, B1,C1- shows the disintegrated or altered tight junctions (orange arrows) and basolateral entry of Pg. Scale bars:- A-C: 2μm, B1, C1: 1μm), (n = 4). Note:- Refer Fig. S3 for another set of representative images.
P. gingivalisaccesses single membrane vesicles, cytosol and nucleus of ARPE cells
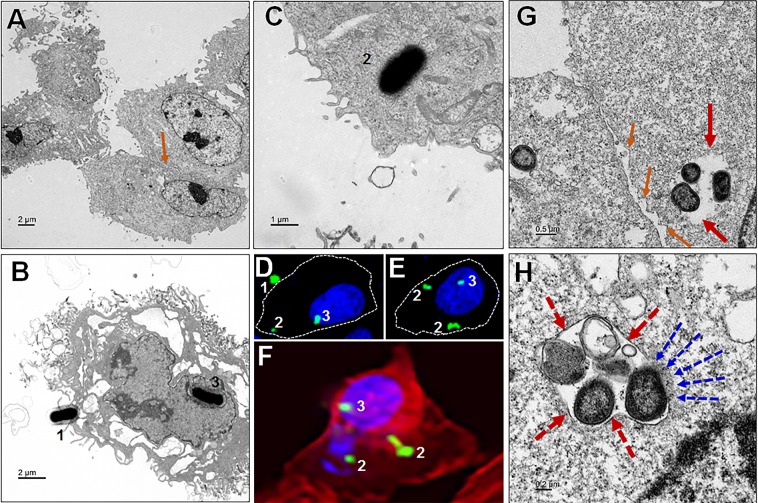
We next performed TEM imaging analysis to assess the intracellular localization P. gingivalis within ARPE cells. Available evidence suggests that the intracellular environment can protect certain microbes until they develop a full complement of virulence factors40. Here it is shown that Pg381 enters single membrane vacuole structures, as well as freely occupying the cytoplasm and reaching the cell nucleus (Fig. 5B–H) compared to uninfected control (Fig. 5A). Confocal imaging analysis also demonstrates the three stages of entry from adhesion to invasion and intracellular localization to cytoplasm and nucleus (Fig. 5D–F), consistent with TEM results (Fig. 5B,C and S4B). The formation of single cell vesicles enclosing P. gingivalis was evident, as was lack of visible degradation (Fig. 5G,H and S4C,D). The presence of Pg in the cytosol, without vesicular membrane surrounding them, suggests escape from the vacuole. Also evident is some disintegration of vacuole membrane and Pg re-localization to the cytosol (Fig. 5H).
Figure 5.
P. gingivalis manipulation of ARPE cell entry and escape to cytosol. (A) Uninfected control (orange arrow shows the tight junction). (B) Pg interact with ARPE cells (1) and reached the cell nucleus (3). (C) Invaded Pg freely occupy the cytoplasm of ARPE cells (2). (D–F) Confocal images shows the three stages (1. host-pathogen interaction to enter, 2. Invasion and 3, Internalization) and invasion location of the Pg into ARPE cells (membrane, cytoplasm and nucleus), which is consistent to the TEM as shown in (B,C) as well. (D,E) White dotted lines indicates the cell membrane boundary as visualized by F-actin (F). (G,H) The adhesive properties of fimbriae allow P. gingivalis to invade host cells and escape the host immune surveillance. (G) Bacteria in vacuole to evade the host immune defense and survive long (red arrows). Intracellular Pg is found within vesicular structures with single enclosed membranes (dotted red arrows); also noticed Pg organisms inside the retinal epithelial cell, without endocytic vacuoles surrounding them, probably escaped from the vacuole, is evident (H). Also observed loss of integrity of tight junctions in between RPEs (G), compared to uninfected control (A) - orange arrows. Note:- the vacuole membrane breaking and Pg escape to the cytosol (dotted blue arrows). (Scale bar:- A-B: 2 µm, C: 1 µm, G: 0.5 µm, H: 0.2 µm; D, E and F: enlarged region from Figs. 1B and 2B, respectively). Refer the low magnification in Fig. S4 for the complete view.
High internalization and intracellular content ofPgstrains within ARPE-19 cells
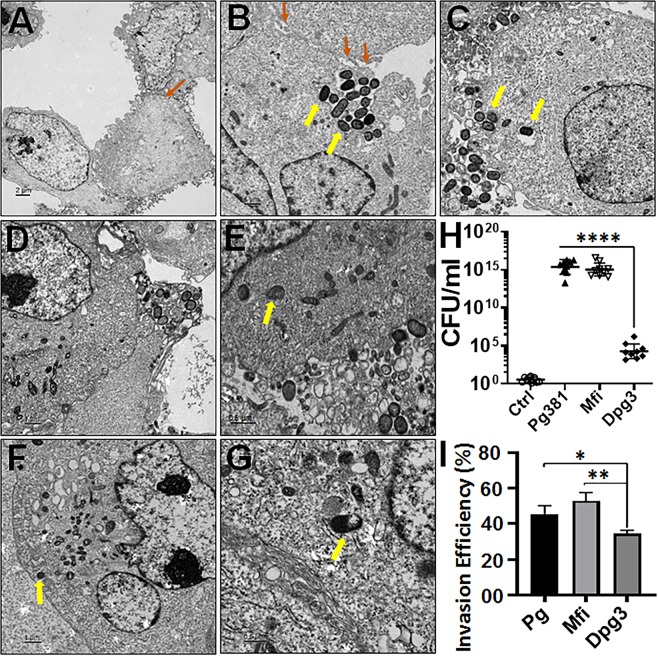
Next, we tested whether the Pg mutant strains MFI and DPG3 were also able to invade ARPE-19 cells. The TEM results show the intimate interaction and internalization of Pg strains and presence of nano-sized cellular vesicles, presently undefined, in ARPE cells after exposure to Pg381, MFI and DPG3 at 10 MOI at 24-hours (Fig. 6B–G) compared with uninfected control (Fig. 6A). The uptake of all Pg strains by ARPE cells is more obvious and most evident with higher numbers of Pg381 (Fig. 6B,C and S5 A–D) and MFI (Fig. 6D,E and S5E,F) intra- and extracellularly compared with DPG3 (Fig. 6F,G and S5G,H). We also observed most of the Pg strains freely occupied the cytoplasm of ARPE cells (Fig. 6B,C,E–G) and released their intracellular content (Fig. S5C).
Figure 6.
High internalization and intracellular content of Pg strains within ARPE cells. (A–G) Transmission electron microscopy (TEM) of human ARPE cells infected with all fimbriated Pg strains (10 MOI) for 24 hours. P. gingivalis and mutant strains internalized retinal pigment epithelial cells. The sections show the intra-and extra-cellular contents of un-infected control (A), Pg381 (B,C), MFI (DE) and DPG3 (F,G) infected ARPE cells for 24 hours with 10 MOI (2 sets of representative images from each group). Orange arrows indicate several epithelial junctions such as long and short tight junctions between ARPEs and loss of integrity (B). Yellow arrows indicate Pg strains freely occupy the cytoplasm (B,C,E–G). The top and bottom sections show the different magnifications of randomly selected sections (Scale bar:- A-B: 2 µm, C-E, G: 1 µm, F, H: 0.5 µm). Refer the low magnification in Fig. S5 for the complete view. (H,I) Antibiotic Protection Assay: The figure shows the survival of both adhered and internalized (H), and only internalized (I) P. gingivalis strains after 24 hours of infection. ARPE cells infected with Pg381 or isogenic mutant strains and incubated with or without antibiotics were lysed and the survived extra (cell surface/membrane attached) and intracellular bacteria were re-suspended and maintained in anaerobic broth for 3 days. (H) The data represents CFU within and on the surface of ARPEs harvested from biological triplicates before antibiotic treatment. The experiments were repeated thrice with three technical replicates (n = 9). The means ±standard deviation (in triplicates) were analyzed by One-way ANOVA of different groups and Tukey’s test for multiple group comparisons within three different experiments (n = 3) with 3 technical replicates (n = 3) from each experiments. (n = 9; ****P < 0.0001). CFU - Colony Forming Unit. (I) Invasion efficiency of Pg strains were expressed as the percentage of the initial inoculum recovered after antibiotic treatment. The means ± SD were analyzed by One-way ANOVA of different groups and Dunn’s test for multiple group comparisons with three different experiments with three technical replicates from each experiments. (n = 9; *P < 0.05; **P < 0.001). Refer Fig. S9 for adhesion and intracellular survival of Pg strains in AEPE cells.
Adhesive and invasive abilities ofP. gingivalisstrains in the ARPEs
The abilities of Pg381 and its isogenic mutant strains (MFI and DPG3) to adhere and/or invade ARPE cells was examined and further confirmed by an antibiotic protection-based recovery assay. Survival and persistence of viable adherent and/or intracellular bacteria (CFU) was then assessed quantitatively by lysing RPE cells and growing bacteria in broth cultures and on anaerobic blood agar plates (Fig. S9). Pg381 and MFI was significantly recovered at higher numbers from RPEs lysates in broth and on blood agar compared to DPG3 (Fig. 6H). This difference was most apparent after 24-hours, with large numbers of surface and intracellular bacteria present (Fig. S9). Consistent with their adherence capabilities, Pg381 and MFI, both of which express major FimA fimbriae, showed an increased invasive ability (Fig. 6H), suggesting a role for FimA in invasion of the RPE cells. Next, we estimated the invasion efficiency of viable bacteria recovered for each strain (Fig. 6I). Interestingly, measurement of the survival of internalized Pg (45%) and major fimbria mutant MFI (52%) showed significant invasive efficiency when comparing with the major fimbriae-lacking strain DPG3 (Fig. 6I), which showed approximately 30% survival within ARPE cells. These results imply an involvement of both Pg381, and MFI (FimA), in recognition of specific receptors on the surface of retinal pigmented epithelial cells. However, further investigation needed to elucidate the exact mechanisms.
P. gingivalismicroorganisms massively clustered along the ARPE cell surface adherent and cellular protrusions
The TEM showed evidence of clustered P. gingivalis microorganisms along the ARPE cell surface, both at the apical and basolateral sides (Fig. S6A,B). Higher magnification showed adherent Pg381 (Fig. S6A1) and subsequent cellular protrusions (Fig. S6B1). We also observed the apparent massive contact between micro-filamentous cellular components and surface-adhering P. gingivalis (Figs. S6B1 and S7). As demonstrated in Fig. 6, numerous P. gingivalis organisms were evident extra and intracellularly. Most extracellular P. gingivalis, appear to be clustered at certain spots along the cellular membrane, while the rest of the membrane remained relatively free of bacteria (Fig. S6B). This could be due to Pg aggregation by fimbrial interaction with the presence of cellular receptors that are expressed only at certain areas on the surfaces of the cellular membrane and subsequent attachment and/or invasion by the aggregate as a whole.
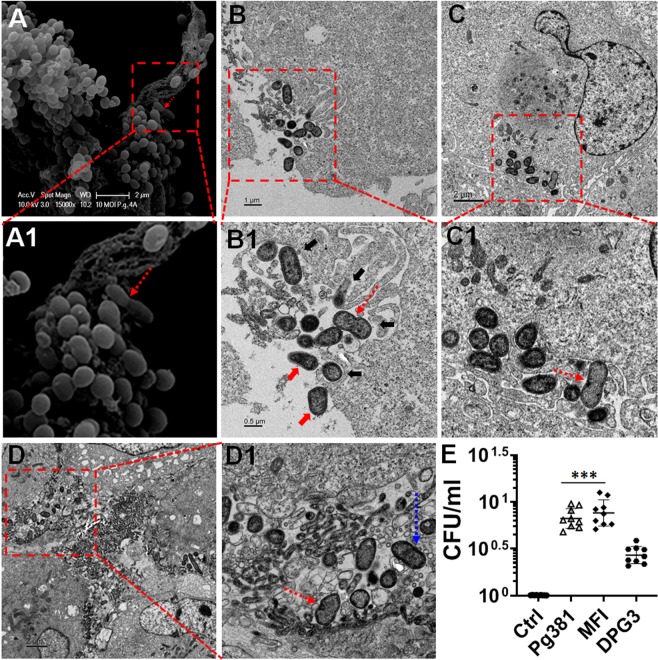
InternalizedP. gingivalismicroorganism initiation or undergoing division and intracellular survival in ARPE-19 cells
SEM demonstrated adherent Pg381 (10 MOI) microorganisms undergoing apparent division in ARPE for 1 hour (Fig. 7A,A1), we thus further analyzed the TEM for Pg-fimbriated mutant’s multiplication at 1 and 24-hours. TEM confirmed that internalized Pg381 and MFI (10 MOI) microorganism initiation or undergoing division in ARPE for 1 (Fig. 7B-B1) and 24 (Fig. 7C,D) hours respectively, however, no cell division of DPG3 observed. There was apparent contact between micro-filamentous cellular components and surface-adhering P. gingivalis while undergoing division at 1 hour as visualized by SEM (Fig. 7A-A1) and TEM (Fig. 7B-B1).
Figure 7.
Internalized Pg381 and MFI microorganism initiation or undergoing division and intracellular survival in ARPE-19 cells. (A–A1) SEM showed evidence of Pg undergoing division on the surface of infected ARPEs at 10 MOI for 1 hour. (B–D) TEM images illustrate the internalized Pg381 and MFI (10 MOI) microorganism initiation (dotted blue arrow) or undergoing (dotted red arrows) division and survival in ARPE for 1 (B, B1) and 24 (C-C1 & D-D1) hours is evident. We observed the apparent contact between micro-filamentous cellular components (black arrows) and surface-adhering P. gingivalis (red arrows) while undergoing division (B-B1) at 1 hour. Evidently, several of the TEM images show, Pg381 (C-C1) and MFI (D-D1) dividing within the ARPE cells at 24 hours. This indicates that the bacteria are metabolically active during invasion and may be able to persist in the ARPE cells for at least 24 hours. Note- Massive extracellular/intracellular bacteria, especially in the case of Pg381 and MFI, appear to be aggregated at certain spots along the cellular membrane and cytosol. Also refer the second set of low to high magnified TEM images in Fig. S7 for the complete view and confirmation. A1, B1, C1 and D1 shows a magnified/enlarged region of Boxed areas in A, B, C and D, respectively. (Scale bar:- A-D: 2 µm, B: 1 µm, B1: 0.5 µm and A1, C1, D1: enlarged from A, C and D, respectively). (E) The figure shows the replication of Pg-strains at 1-hour infection and 5 hours post invasion; the cells were lysed, Pg381, MFI and DPG3 recovered and CFU enumerated as described in the methods. The data represents CFU within ARPE cells harvested from biological triplicates. The mean ± SD were analyzed by One-way ANOVA of different groups and Tukey’s test for multiple group comparisons within 3 different experiments with 3 technical replicates from each experiments. (n = 9; ***P < 0.001). CFU- Colony Forming Unit. Refer Fig. S9 for Pg strains replication/colony formation.
We observed most (~60–70%) of the bacteria located at the apical membrane (Fig. 7A-A1) and the remaining near the basolateral margins (Fig. 7B-B1). Evident in several of the TEM images, are Pg381 (Fig. 7C-C1 and S7) and MFI (Fig. 7D-D1) dividing within the ARPE cells at 24-hours, which indicates that the bacteria were metabolically active during invasion and plausibly surviving inside the ARPE cells for at least 24-hours. Note that, at certain spots enormous extracellular/intracellular bacteria, especially Pg381 and MFI, appear to be aggregated along the cellular membrane and cytosol.
Intracellular replication ofPgstrains within ARPE cells
In order to study the replication of the intracellular Pg and its mutants within RPE cells, we harvested intracellular P. gingivalis using the antibiotic protection assay at 1-hour infection and 5-hours post invasion. The result shows significant replication of Pg and MFI compared with DPG3 (Fig. 7E). We also observed a moderate increase in the MFI counts compared with Pg, but no significant difference. To our surprise, we detected intracellular DPG3 (Mfa1) amplification at 24-hours infection (Figs. 6I) and 5-hours post invasion (Fig. 7E), whilst we could not observe any dividing cells by TEM analysis as shown for Pg and MFI (Fig. 7). However, this data is consistent with all other imaging analysis (Fig. 6H,I and Fig. 8I), including confocal quantifications (Fig. 2F). Thus, P. gingivalis and its mutant strains appears to be capable of replication within the RPE cells, increasing in numbers significantly over a 5-hours period (Fig. 7E). This could be one of the potential strategy of escape mechanisms employed by Pg to defend from RPE cell immune responses.
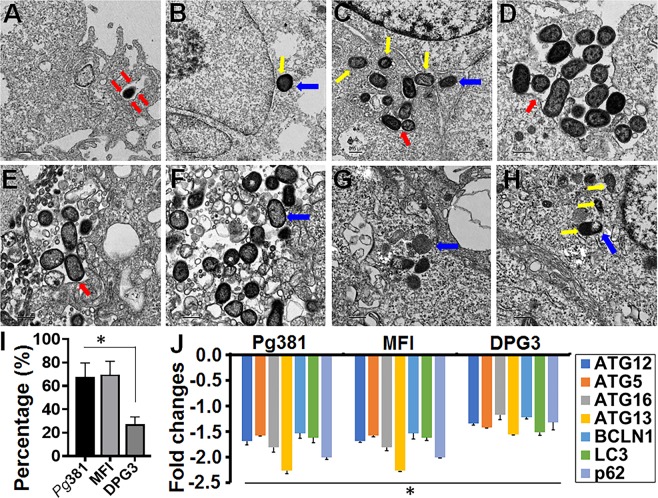
Figure 8.
P. gingivalis enclosed within single membrane structures or freely occupy the cytoplasm of ARPEs and escaped the autophagic vesicles. (A–H) Transmission electron microscopy (TEM) of single membrane structures within ARPEs infected with Pg381 (1 & 10 MOI), MFI and DPG3 (10 MOI) for 1 and 24 hours. All Pg strains shows mostly escaped the autophagic (double membrane) vesicles and enclosed within single membrane structures (red arrows) or freely occupy the cytoplasm (blue arrows). Yellow arrows show the bacteria in the cytosol around the nucleus. (A) Pg381 invaded into ARPEs enclosed within single membrane structure is evident at 1 MOI for 1 hour, whereas it reached the nucleus and lived-in the cytoplasm at 24 hours (B). As hypothesized, Pg381 (C,D), and MFI (E,F) were consistently detected within single membrane vesicles or freely occupy the cytoplasm of retinal epithelial cells, in contrast to our hypothesis that the DPG3 (G, H) also enclosed within the characteristics single membrane structures and apparently observed in the cytoplasm freely and close to the nucleus (yellow arrows) at 10 MOI for 24 hours. We also observed smaller numbers of DPG3 than the Pg381 and MFI both intra and extra cellularly (2 sets of representative images from each group). (I) The ratio of intracellular bacteria included in the single membrane were compared to total number of bacteria within ARPEs and plotted as percentage. Counting of the bacteria included in single membrane vesicles, freely occupy the cytoplasm or vacuoles after 24 hours of infection. Each strain was counted in six randomly selected grids for each sample. The ratio of DPG3 trapped in single membrane relative to the total intracellular bacteria was significantly lower than Pg381 and MFI. Refer the low magnification in Fig. S8 for the complete view. *P < 0.02. The analysis of the bacterial counts used Kruskal-Wallis test of different groups and Dunn’s test for multiple comparisons with 3 different experiments. (Scale bar:- A-H: 0.5 µm). J) TaqMan quantitative PCR results shows the genes related to autophagy machinery are significantly decreased by Pg381 (Mfa-1/FimA), MFI (FimA) and DPG3 (Mfa-1) infected ARPE-19 cells for 24 hours compared with uninfected control. This data is consistent with TEM imaging analysis (Figs. 5–8A–I) and confirm the lysosomal/vacuolar escape mechanism of P. gingivalis. The experiments were repeated thrice (biological) with three technical replicates (n = 9). Fold change in gene expression was normalized to the control and ≥ ±1.5 fold was considered as significant (*P < 0.05).
P. gingivalisescapes the autophagic vesicles and enclosed within single membrane structures or freely occupy the cytoplasm of retinal epithelial cells
The presence of autophagic double membrane vesicles was examined by TEM analysis in ARPEs infected with Pg and fimbria mutant strains. Tracking of Pg381, MFI and DPG3 (10 MOI) within ARPEs after 24-hours of infections demonstrated that the majority of bacteria were contained in single membrane structures (Fig. 8A–H), with notable absence of autophagic vesicles. All Pg strains exhibited a cell wall structure with an outer and inner cell membrane typical of Gram-negative bacteria. The results illustrate that Pg381 invades into RPEs, enclosed within single membrane structures, evident at 1 MOI for 1-hour (Fig. 8A and S8A), while it reached the nucleus and was not degraded in the cytoplasm at 24-hours (Fig. 8B and S8B).We also noted, greater numbers of Pg381 (Fig. 8C,D), MFI (Fig. 8E,F) consistently detected within single membrane vesicles or freely occupying the cytoplasm of ARPEs compared with DPG3 (Fig. 8G,H). However, all the Pg strains demonstrated that they were freely occupying the cytoplasm (Fig. 6B–G). Quantification of the single membrane structures that contained bacteria in or freely occupied the cytoplasm were carried out in six randomly selected TEM images of each sample. The ratio of Pg381 and MFI trapped in single membrane relative to the total intracellular bacteria were significantly higher than DPG3 (Fig. 8I), which is consistent with confocal imaging (Fig. 2) analysis.
Autophagy escape mechanism ofPg-strains in ARPE-19 cells
Our previous study has demonstrated the role of Pg-Mfa1 and FimA in dendritic cells35, in killing or survival by autophagy evasion39. Here, TEM showed autophagy vesicle evasion of Pg-strains in ARPE cells (Fig. 5 and 8). To further corroborate the lysosome/vacuolar escape mechanism of Pg-strains through the deactivation of autophagy signaling pathway in RPEs cells, we assessed the expression of autophagy related (ATG) genes by qPCR. Expectedly, our results show that Pg-strains significantly (P < 0.01; ≥±1.5 fold) downregulated the family of ATG genes including those of the autophagosome and controlling complex ATG12-ATG5:ATG16L1, and LC3 (autophagy marker), ATG13, cargo selection molecule or ubiquitin-binding protein p62 (or Sequestosome-1/SQSTM1) and beclin-1, compared with uninfected control (Fig. 8J). This leads to deformed or immature autophagosome and reduced clearance of autophagic substrates in RPE by Pg-strains, which may cause RPE dysfunction and damage due to chronic exposure to this dysbiotic pathogen. Collectively, these data revealed that ARPEs infected with Pg-strains induced downregulation of autophagy related genes, supporting a mechanism for how Pg escapes host immune response and immune homeostasis disruption.
Discussion
To our knowledge, this is the first report on the invasion of human retinal pigment epithelial cells by a dysbiotic oral-pathogen P. gingivalis in-vitro and its intracellular fate within the cytosol and single-membrane vacuolar compartments via autophagy evasion. Evidences indicate that P. gingivalis can invade several cell lines of diverse origin including gingival epithelial cells (GECs)41, endothelial cells42, dendritic cells43, macrophages44, osteoblasts45 and fibroblasts46. Studies suggest that there are positive relationships between periodontitis and tumors of Oro-digestive tract, pancreas and lung47. Our previous publication reported the virulence trait of Pg in potential oral epithelial-cell proliferation35 and other studies confirmed tumor-like transformation with long-term infection of Pg48. Active invasion of aortic tissues by Pg in mice causally links experimental periodontitis and atherosclerosis in-vivo, as Pg was found to be the most abundant(80%) species in atherosclerotic plaque49. Chronic systemic inflammation instigated by PD could be correlated with neuroinflammation as in Alzheimer’s disease, where Pg has been detected invading brain tissue through compromising the blood-brain-barrier50–53. In patients with both rheumatoid arthritis and PD, Pg DNA was detected by PCR in the synovial fluid8,54. This prompted us to surmise that periodontopathogens can instigate chronic inflammation at non-oral sites like eye by subversion of innate immune elimination through cellular invasion and activation of host-signaling pathways35,55. Hence, we intended to investigate the potential pathophysiological link between AMD and oral dysbiosis, using P. gingivalis as a prototype dysbiotic species56. This in-vitro study has demonstrated significant interaction of fimbriated-Pg381 with membrane of human retinal pigment epithelial (ARPE-19) cells (RPE in the following text), leading to invasion without obvious degradation of Pg381 within the cytosol and vacuolar structures.
P. gingivalis employ a variety of virulence factors, especially through the adhesive properties of its fimbriae, to invade the host cells and escape host immune surveillance57,58. The major FimA and minor Mfa1 fimbriae of Pg play a pivotal role in adhesion to host cell surface. Immunofluorescence confocal imaging demonstrated significant uptake of Pg381 by RPE cells at different multiplicity of infection (MOI) which were apparently distributed in the cytosol and around the cell nuclei. Uptake of Pg381 occurred in a concentration dependent manner, with intracellular P. gingivalis observed into RPE cells even at 1 MOI. P. gingivalis adhered and tightly engaged the human retinal pigmented epithelial cell surface at 1-hour at both 1 and 10 MOI, while highly clustered and engaged at 10MOI. Significantly, live Pg invaded retinal epithelial cells but not heat-killed bacteria, indicating an active invasion as opposed to phagocytosis. We assume that, in the living animal, the apical side of the RPE is in contact with the subretinal space (minimal space just between the apical villi of the RPE and the ends of the interdigitating outer segments of the photoreceptor cells), which could be varying with the mode of potential interaction of RPE with P. gingivalis or their endotoxins released would likely come in contact with the basolateral/choroidal side of the RPE. Further, in a chronic inflammatory state, as is said to exist in AMD and would certainly arise congruent with prolonged exposure to the fimbriated dysbiotic pathogen Pg, the outer blood-retinal barrier may become compromised. Under this condition, the subretinal space (apical surface of RPE) could additionally become susceptible.
Certain bacteria exploit the intracellular environment as a type of ‘armor’ during initial stages of infection or until they develop strong virulence against the host tissue40. Consistent with these observations and as an evidence of early host-pathogen interaction or engagement, scanning electron microscope (SEM) showed P. gingivalis adhering and tightly engaging the human RPE cell surface at 1 hour (1 and 10 MOI) and highly clustered at 10MOI. Earlier studies have shown that Pg express vesicles on their cell surface which are released into its environment. P. gingivalis may employ these vesicles as a vehicle for imperative virulence factors and proteolytic enzymes, major components of outer membrane proteins which are responsible for destruction of periodontal tissues, initiating host immune response and host cell invasion59. This was apparent from our results as the intracellular Pg was found within vesicular structures with single enclosed membranes inside the retinal epithelial cell, without endocytic vacuoles surrounding them.
P. gingivalis is an unique periodontopathic bacterium capable of altering its local environment to its favor, modifying the host immune systems and facilitating other colonizers while setting up the platform for pathogenesis20. Evidently, our SEM and TEM (transmission electron microscopy) data together confirmed that not only Pg381 but also the mutant strains were able to enter RPE cells. P. gingivalis showed signs of division as there was apparent contact between surface adhering Pg and micro-filamentous cellular components. Previous studies have shown that Pg in GECs, make use of actin-dependent protrusions to spread to the new host cells and propagate efficiently evading the host defense60. P. gingivalis promotes its adaptive fitness by evading clearance in-vitro and in-vivo. The unique ability of pathogens to evade lysosomal degradation is crucial to survive inside the host cells and induce persistent infections. A direct evidence of the intracellular survival of Pg has also been demonstrated in normal and immortalized human GECs19. Consistent with these previous findings, TEM revealed that Pg and its isogenic mutants (MFI and DPG3) invaded human RPE cells. The intracellular entry and survival of P. gingivalis and its isogenic mutants was quantitated by antibiotic protection/survival assays, which further confirmed the invasion efficiency and amplification of these strains. We found that viable Pg strains were recoverable in large numbers post-infection over a 5 and 24-hours period. We predict that this could be an escape mechanism employed by Pg to evade the cell immune responses. Thus, our data suggest that Pg not only survives in the cytosol of epithelial cells but also undergoes division. Furthermore, it has been previously shown that Pg is able to survive within DCs by subversion of canonical pathway via its Mfa-1 fimbriae39. However, presently unclear is how the minor fimbriated strain DPG3 invades the retinal epithelial cells, and if the entry is fimbria-receptor mediated or through engulfment by cellular protrusion; further study is warranted in this regard.
Our previous studies35,39 and the current TEM data, lead us to test our hypothesize that the lysosome/vacuolar escape or survival mechanism of Pg-strains might be through the deactivation of autophagy signaling molecules in RPEs cells. Expectedly, qPCR results are consistent, showing significantly reduced expression of autophagy related genes upon infecting RPEs with Pg-strains, suggesting inhibition of the autophagy pathway. Autophagy has essential role in periodontal cell biology, bacterial internalization and elimination and immune-suppression, thereby inhibiting apoptosis in the periodontal cells, thus having a major role in the pathogenesis of PD61,62. Studies have determined the critical function of autophagy-lysosome system in the maintenance of normal homeostasis in the aging or senescent RPE63. Any disparity in this regulated cell clearance process is associated with age-dependent degeneration of RPE cells along with secondary degeneration of photoreceptors, critical for visual function64. Experimental AMD models have illustrated the formation of retinal atrophic patches, subretinal migration of microglial cells, sub-RPE drusen and drusenoid deposition, complement activation and sites of choroidal neovascularization65, typical of AMD. It is observed that autophagic potential declines with aging and is often found to be associated with down-regulation of autophagy-induction proteins namely ATGs, Beclin-1 and LC3 (most widely used marker of autophagosomes)63. Interestingly, Atg13 required for phagophore formation, was significantly downregulated in Pg (Mfa-1/FimA), MFI (FimA) and DPG3 (Mfa-1) infected RPEs. The conjugated Atg5–Atg12 complex that pairs with Atg16L dimers to form a multimeric Atg5–Atg12–Atg16L complex and associates with the extending phagophore, were also significantly downregulated by Pg-strains. In addition, beclin-1, an autophagic protein, important for nucleation of autophagy and regulation of apoptosis process66 was downregulated. The sequestosome-1 (p62/SQSTM1) is a significant factor in inflammatory response, autophagy and cellular senescence and p62/SQSTM1 can be a target for inhibition of retinal inflammation, improvement of autophagy and the antiaging of RPE cells64. p62/SQSTM1 binds autophagy substrates to the autophagosome by interacting with ubiquitinated proteins via its ubiquitin-associated domain67 and LC3 with its LC3‐interacting region (LIR)68. While p62 accumulation and association with protein aggregates is related to autophagy defect, p62 mutations is directly linked to selective autophagy impairment and in turn to neurodegeneration. Notably, our data demonstrated that Pg-strains significantly downregulated both p62 and LC3 genes in RPEs. These data collectively suggests that Pg applies lysosomal/vacuolar escape mechanism through dysfunctional autophagy for its persistent survival inside the RPE and may further mediate pathological effects i.e. immune homeostasis disruption in the retina upon chronic exposure, yet more investigation needed.
In conclusion, these findings altogether indicate that the dysbiotic periodontal pathogen P. gingivalis efficiently invade retinal epithelial cells in high levels, replicate and are sustained within them. This invasion and autophagy evasion by the keystone species may be one of the contributing elements in the pathogenesis of retinal degenerative diseases. However, it remains to be verified whether P. gingivalis invade RPE cells in-vivo in patients with AMD and PD, and via what route.
Materials and Methods
Reagents
Human Retinal Pigment Epithelial (ARPE-19) cell lines were obtained from ATCC (Manassas, VA). DMEM/F12 culture medium, fetal bovine serum, penicillin and streptomycin were from Life Technologies (Grand Island, NY). Gentamicin, Metronidazole, 5(6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes), Rhodamine-phalloidin (F-actin), Trypsin (0.25%)/EDTA (0.1%), TRIzol reagent, High-Capacity cDNA Reverse Transcription Kit, TaqMan gene expression assay (primers/probes) and 8-well chamber slides were obtained from Thermo fisher scientific (Waltham, MA). RNeasy kit was from Qiagen (Germantown, MD). The universal power block was obtained from Biogenex Laboratories (Fremont, CA). DAPI (4′,6-Diamidino-2′-phenylindole dihydrochloride) from Sigma-Aldrich Co. LLC (St. Louis, MO).
Porphyromonas gingivalisand mutant strains culture
Porphyromonas gingivalis 381 (wild-type Pg381), which expresses both minor (Mfa1) and major (FimA) fimbriae, isogenic major fimbria-deficient mutant Pg-DPG3 (DPG3), which expresses only the minor fimbriae (Mfa1+/FimA−) and the isogenic minor fimbria-deficient mutant Pg-MFI (MFI), which expresses only the major fimbriae (Mfa1−/FimA+) were maintained anaerobically (10% H2, 10% CO2 and 80% N2) in a Coy Laboratory vinyl anaerobic system glove box at 37 °C in Difco anaerobe broth MIC as we described earlier35,69. Erythromycin (5 µg/ml) and tetracycline (2 µg/ml) were added as per the selection requirements of the strains35,39,70.
Human Retinal Pigment Epithelial (ARPE-19) cell culture
Human ARPE-19 has structural and functional properties distinctive of RPE cells in-vivo and this cell line will be valuable for in-vitro studies of retinal pigment epithelium physiology71. ARPE-19 cell lines were cultured and maintained in DMEM/F12K complete growth medium supplemented with 10% fetal bovine serum, 100 units mL−1 penicillin, and 100 μg mL−1 streptomycin and grown for 4–6 weeks and the cells were used after fifth passage for experiments as we described previously72. Briefly, the cells were grown to ~70–80% confluence then in serum-starved media per experimental conditions. After serum-starvation, fresh culture medium was then added to cells with and without bacterial strains at different multiplicity of infection (MOI) and different time points based on the experimental conditions.
P. gingivalisstrains labeling and Human ARPE-19 Infection
Bacterial suspensions were washed thrice and re-suspended in PBS for spectrophotometer reading of 0.11 for optical density at 660 nm (OD660), which was previously determined to be equal to 5 × 107 colony-forming units69,73. Corresponding bacterial counts were calculated and dilutions were prepared to infect ARPEs at different multiplicity of infection (MOI). To inactivate (heat-killed) bacteria, P. gingivalis was incubated for 1 hour at 95 °C prior to the experiment. For bacterial CFSE staining, the suspension was washed thrice and re-suspended in 5 μM of CFSE in PBS before being used as described earlier39. The bacteria were incubated for 30 min at 37 °C in the dark. Then the labelled Pg strains were washed twice with PBS and the residual CFSE dyes removed. Confluent (~70%) monolayers of ARPE-19 cells were then infected with Pg strains and placed into the anaerobic chamber at 37 °C and the pre-labeled Pg strains allowed to interact with host cells for 30–60 min. For infection assay, ARPEs were co-cultured with pre-labeled Pg381, Mfa1+Pg, and FimA+Pg at 1, 10 and 100 MOI in DMEM/F12K medium without antibiotics at 37 °C in a water-saturated atmosphere and incubated with the ARPEs for 1 or 24 hours and each experimental condition were performed in triplicate and repeated thrice.
Immunofluorescence and Confocal Imaging Analysis
Human ARPE-19 cells were plated at a density of 1 ×104 cells/well onto 8-well chamber slides 1 day before infection. At 70% confluence, the cells were infected with Pg and its isogenic mutant strains. 1). To test the uptake of bacteria by ARPE cells were infected with Pg at 1, 10, and 100 MOI (place chamber slides with infected ARPEs into anaerobic incubator and allowed bacteria to interact with host cells for 30/60 min) and incubated for 24 hours. 2). To determine the presence, internalization, colocalization and persistence of intracellular bacteria, the ARPE cells were infected with live or heat-killed CFSE labeled Pg, MFI and DPG3 for 24 hours and then stained with rhodamine-phalloidin (F-actin) for cytoskeleton visualization and DAPI, as described74. Briefly, after fixing and permeabilizing, the cells were blocked with 1X power block and incubated for 30 min at room temperature. To examine the colocalization of CFSE-labeled bacteria, ARPE-19 cells were stained with F-actin for 60 min before examination. After washing with PBS twice, subsequently, the slides were mounted after staining with the nuclear probe DAPI and the cells were examined by live-imaging by laser-scanning confocal microscopy (Carl Zeiss, Germany). Images were taken at different focal points (z-sections) for temporal-spatial visualization of bacteria.
Adhesion and invasion antibiotic protection assay
The adhesive and invasive ability of P. gingivalis and its isogenic mutants in ARPE-19 cells were determined by the standard antibiotic protection assay as described41,75,with some modification. Briefly, cells were seeded in 6-well flat-bottom culture plates at a cell density of 1 × 106 cells per well and grown overnight. Cells were infected with Pg381, MFI and DPG3 at 10 MOI and incubated for 24 hours. For determining total adhesion and invasion levels of Pg strains, the cells were washed thrice in PBS, re-suspended in sterile water on ice for 20 min to lyse the cells, with mechanical scraping and agitation to release the internalized bacteria. For invasion assay, external, nonadherent bacteria were removed by washing three times in anaerobic PBS, and external adherent bacteria were then killed by incubating for 1 hour with DMEM:F12 media containing 300 µg/ml of gentamicin and 200 µg/ml of metronidazole, and the cells lysed. Cell lysates were re-suspended in anaerobic broth for three days. After broth incubation, bacterial suspensions were washed thrice in PBS and re-suspended for spectrophotometer reading at OD660 in triplicate. The experiments were repeated thrice with three technical replicates. Viable counts (CFU) were enumerated based on a plate count serial dilution versus OD readings. For confirming the identity of the P. gingivalis (black pigmented Gram-negative coccobacilli), suspensions were cultured on 5% blood agar plates in triplicate under anaerobic conditions (10% H2, 5% CO2 and 85% nitrogen). Plates were then incubated in anaerobic conditions at 35 °C for 5 days until black colonies were detected.
Replication and survival assay ofP. gingivalisstrains in human ARPE-19 cells
Additional experiments were conducted to address the possibility of intracellular replication and survival as described41,76 with minor modification. Briefly, P. gingivalis strains were incubated with ARPE cells for 1 hour and the extracellular bacteria were killed with antibiotics (300 µg/ml of gentamicin and 200 µg/ml of metronidazole). After washing to remove the antibiotics, the cells were maintained for a further 5 hours to allow replication of intracellular bacteria. Antibiotics were added again to kill any released extracellular bacteria and cells were washed thrice in PBS and re-suspended in sterile water on ice for 20 min to lyse the cells. Cell lysates were re-suspended in anaerobic broth for three days. Bacterial suspensions were washed three times in PBS and re-suspended for spectrophotometer reading at OD660 in triplicate. The experiments were repeated thrice with three technical replicates. Corresponding CFU counts were calculated based on a linear regression of plate count in serial dilution versus OD readings. Black colonies were confirmed in blood agar plate under anaerobic conditions (10% H2, 5% CO2 in nitrogen).
Transmission Electron Microscopy (TEM)
ARPE-19 cells were plated into 6 wells 1 day before infection and when at ~70% confluence cells were infected with P. gingivalis or its isogenic mutant strains at 1 or 10 MOI for 1 and 3 hrs. The infected cells were then harvested and fixed or after further culturing in fresh medium without antibiotics for 24 hours. After ARPEs fixation, the procedures were carried out as described ealier39. Briefly, following incubation with bacteria, monolayers were washed three times in PBS and detached by trypsinization. The cell pellets were washed with PBS thrice and fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate (NaCac) buffer, pH 7.4, post-fixed in 2% osmium tetroxide in 0.1 M NaCac, stained en bloc with 2% uranyl acetate, dehydrated with a graded ethanol series and embedded in Epon-Araldite resin. Thin sections were cut with a diamond knife and stained with uranyl acetate and lead citrate. Cells were observed in transmission electron microscope (JEM 1230—JEOL USA Inc.) at 110 kV and imaged with a CCD camera and first light digital camera controller (Gatan Inc.). ARPE cells containing Pg strains were photographed at each time point.
Scanning Electron Microscopy (SEM)
ARPE-19 (1 × 105) cells were grown on cover-glass in 24-well plates, incubated with Pg at 1 and 10 MOI for 1 hour, and bacteria attached to retinal epithelial cells were observed with SEM as we previously described39. Briefly, the cells were fixed for 30 min in 4% paraformaldehyde, 2% glutaraldehyde in 0.1 M sodium cacodylate (NaCac) buffer, pH 7.4, post-fixed in 2% osmium tetroxide in NaCac buffer, dehydrated with a graded ethanol series (25–100% for 5 min each), followed by a graded alcohol hexamethyldisilazane (HMDS), and HMDS was allowed to evaporate overnight in a fume hood. The dried discs were mounted on aluminum stubs with carbon adhesive tabs and sputter coated with gold-palladium. Discs were observed and imaged in a FEI XL30 scanning electron microscope (FEI, Hillsboro, OR) at 10 kV.
RNA isolation and TaqMan-qPCR Assay
Total RNA was isolated as we described previously35,69 using TRIzol reagent and purified with RNeasy kit from uninfected control, and Pg, DPG3 & MFI (at 10MOI) infected ARPEs were cultured in 6-wells plates for 24 hours. RNA quantity and integrity were tested and only ratios of absorbance at 260 and 280 nm of 1.8–2.0, were included in this study. Analysis of gene expression in ARPEs induced by Pg-strains was performed using RT-PCR as we described previously35. Complementary DNA was synthesized from 1.0 μg RNA through a reverse-transcription reaction (Applied Biosystems). Real-time quantitative PCR was performed on TaqMan gene expression primers fast plates in triplicates for selected genes: ATG5 (Assay ID: HS00169468_m1), ATG12 (Assay ID: HS1047860_g1), ATG13 (Assay ID: HS00521135_m1), ATG16L1 (Assay ID: HS01003142_m1), LC3 (Assay ID: HS01061917_g1), Beclin-1 (Assay ID: HS1007018_m1), p62/SQSTM1 (Assay ID: HS01061917_g1) and GAPDH (Assay ID: HS02758991_g1). Three experimental (technical) replicates were analyzed for each biological sample and repeated thrice. The calculations for fold regulation used the 2−ΔΔCt method as described in our previous report69 and GAPDH were used as internal controls.
Supplementary information
Acknowledgements
The authors thank Libby Perry, and Marshall Brendan (The Electron Microscopy & Histology Core, Medical College of Georgia, AU) for preparation of the SEM/TEM and consistent support for the imaging. The authors also thank Dr. Tong Wang (Dental College of Georgia, AU) for assisting with preparation of samples for SEM/TEM. These studies were funded by the DCG startup and AU intramural grants (to P.A) and the Carlos and Marguerite Mason Trust Foundation (to C.W.C).
Author contributions
P.A. designed research and analyzed data; P.A., R.S. and J.Y. did experiments and analyzed data; P.M.M., D.G.E., J.N. and M.A. provided key scientific input; P.A., R.S. and C.W.C. analyzed data and wrote the paper.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64449-8.
References
- 1.Eke PI, et al. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009-2014. Journal of the American Dental Association (1939) 2018;149:576–588.e576. doi: 10.1016/j.adaj.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winning L, Linden GJ. Periodontitis and Systemic Disease: Association or Causality? Current oral health reports. 2017;4:1–7. doi: 10.1007/s40496-017-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artese HP, et al. Periodontal therapy and systemic inflammation in type 2 diabetes mellitus: a meta-analysis. PloS one. 2015;10:e0128344. doi: 10.1371/journal.pone.0128344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astafurov K, et al. Oral microbiome link to neurodegeneration in glaucoma. PloS one. 2014;9:e104416. doi: 10.1371/journal.pone.0104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, et al. Porphyromonas gingivalis induced inflammatory responses and promoted apoptosis in lung epithelial cells infected with H1N1 via the Bcl-2/Bax/Caspase-3 signaling pathway. Molecular medicine reports. 2018;18:97–104. doi: 10.3892/mmr.2018.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding Y, Ren J, Yu H, Yu W, Zhou Y. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immunity & ageing: I & A. 2018;15:6. doi: 10.1186/s12979-017-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghizoni JS, et al. Increased levels of Porphyromonas gingivalis are associated with ischemic and hemorrhagic cerebrovascular disease in humans: an in vivo study. Journal of applied oral science: revista FOB. 2012;20:104–112. doi: 10.1590/s1678-77572012000100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung H, et al. Arthritic role of Porphyromonas gingivalis in collagen-induced arthritis mice. PloS one. 2017;12:e0188698. doi: 10.1371/journal.pone.0188698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyauchi M, et al. Galectin-3 Plays an Important Role in Preterm Birth Caused by Dental Infection of Porphyromonas gingivalis. Scientific reports. 2018;8:2867. doi: 10.1038/s41598-018-21072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pritchard AB, Crean S, Olsen I, Singhrao SK. Periodontitis, Microbiomes and their Role in Alzheimer’s Disease. Frontiers in aging neuroscience. 2017;9:336. doi: 10.3389/fnagi.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagley S, et al. PERIODONTAL DISEASE AND AGE-RELATED MACULAR DEGENERATION: Results From the National Health and Nutrition Examination Survey III. Retina (Philadelphia, Pa.) 2015;35:982–988. doi: 10.1097/iae.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich T, et al. Evidence summary: the relationship between oral and cardiovascular disease. British Dental Journal. 2017;222:381–385. doi: 10.1038/sj.bdj.2017.224. [DOI] [PubMed] [Google Scholar]
- 13.Kaur T, Uppoor A, Naik D. Parkinson’s disease and periodontitis - the missing link? A review. Gerodontology. 2016;33:434–438. doi: 10.1111/ger.12188. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa M, et al. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3beta signaling pathway. Biochim Biophys Acta. 2013;1832:2035–2043. doi: 10.1016/j.bbadis.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10:e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mysak J, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. Journal of immunology research. 2014;2014:476068. doi: 10.1155/2014/476068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 18.Jia L, et al. Pathogenesis of Important Virulence Factors of Porphyromonas gingivalis via Toll-Like Receptors. Frontiers in cellular and infection microbiology. 2019;9:262. doi: 10.3389/fcimb.2019.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K, Roberts JS, Choi CH, Atanasova KR, Yilmaz O. Porphyromonas gingivalis traffics into endoplasmic reticulum-rich-autophagosomes for successful survival in human gingival epithelial cells. Virulence. 2018;9:845–859. doi: 10.1080/21505594.2018.1454171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nature reviews. Immunology. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J. Dent. Res. 2013;92:485–491. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman DS, et al. Prevalence of age-related macular degeneration in the United States. Archives of ophthalmology (Chicago, Ill.: 1960) 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 23.Wong WL, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet. Global health. 2014;2:e106–116. doi: 10.1016/s2214-109x(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 24.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet (London, England) 2012;379:1728–1738. doi: 10.1016/s0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 25.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol. Life Sci. 2016;73:1765–1786. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss O. The retinal pigment epithelium in visual function. Physiological reviews. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 27.Wang AL, et al. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PloS one. 2009;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaarniranta K, Tokarz P, Koskela A, Paterno J, Blasiak J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell biology and toxicology. 2017;33:113–128. doi: 10.1007/s10565-016-9371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karesvuo P, et al. Alveolar bone loss associated with age-related macular degeneration in males. Journal of periodontology. 2013;84:58–67. doi: 10.1902/jop.2012.110643. [DOI] [PubMed] [Google Scholar]
- 30.Chiu CJ, Chang ML, Taylor A. Associations between Periodontal Microbiota and Death Rates. Scientific reports. 2016;6:35428. doi: 10.1038/srep35428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho EXP, et al. Human pharyngeal microbiota in age-related macular degeneration. PloS one. 2018;13:e0201768. doi: 10.1371/journal.pone.0201768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. Journal of leukocyte biology. 2003;74:179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 33.Benhar I, London A, Schwartz M. The privileged immunity of immune privileged organs: the case of the eye. Frontiers in immunology. 2012;3:296. doi: 10.3389/fimmu.2012.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinninella, E. et al. The Role of Diet, Micronutrients and the Gut Microbiota in Age-Related Macular Degeneration: New Perspectives from the Gut(-)Retina Axis. Nutrients10, 10.3390/nu10111677 (2018). [DOI] [PMC free article] [PubMed]
- 35.Arjunan P, et al. Oral Pathobiont Activates Anti-Apoptotic Pathway, Promoting both Immune Suppression and Oncogenic. Cell Proliferation. Scientific Reports. 2018;8:16607. doi: 10.1038/s41598-018-35126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nature reviews. Immunology. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Progress in retinal and eye research. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 39.El-Awady AR, et al. Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS Pathog. 2015;10:e1004647. doi: 10.1371/journal.ppat.1004647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uribe-Querol E, Rosales C. Control of Phagocytosis by Microbial. Pathogens. Frontiers in immunology. 2017;8:1368. doi: 10.3389/fimmu.2017.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamont RJ, et al. Porphyromonas gingivalis invasion of gingival epithelial cells. Infection and immunity. 1995;63:3878–3885. doi: 10.1128/IAI.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsen I, Progulske-Fox A. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J. Oral Microbiol. 2015;7:28788. doi: 10.3402/jom.v7.28788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jotwani R, Cutler CW. Fimbriated Porphyromonas gingivalis is more efficient than fimbria-deficient P. gingivalis in entering human dendritic cells in vitro and induces an inflammatory Th1 effector response. Infection and immunity. 2004;72:1725–1732. doi: 10.1128/iai.72.3.1725-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, et al. Fimbrial proteins of porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Ju J, Rigney T, Tribble G. Porphyromonas gingivalis infection increases osteoclastic bone resorption and osteoblastic bone formation in a periodontitis mouse model. BMC Oral Health. 2014;14:89. doi: 10.1186/1472-6831-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amornchat C, Rassameemasmaung S, Sripairojthikoon W, Swasdison S. Invasion of Porphyromonas gingivalis into human gingival fibroblasts in vitro. J. Int. Acad. Periodontol. 2003;5:98–105. [PubMed] [Google Scholar]
- 47.Mai X, et al. History of periodontal disease diagnosis and lung cancer incidence in the Women’s Health Initiative Observational Study. Cancer Causes Control. 2014;25:1045–1053. doi: 10.1007/s10552-014-0405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geng, F. et al. Persistent Exposure to Porphyromonas gingivalis Promotes Proliferative and Invasion Capabilities, and Tumorigenic Properties of Human Immortalized Oral Epithelial Cells. Frontiers in cellular and infection microbiology7, 10.3389/fcimb.2017.00057 (2017). [DOI] [PMC free article] [PubMed]
- 49.Velsko IM, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PloS one. 2014;9:e97811. doi: 10.1371/journal.pone.0097811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dioguardi M, et al. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2020;9:E495. doi: 10.3390/jcm9020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olsen I, Singhrao SK. Is there a link between genetic defects in the complement cascade and Porphyromonas gingivalis in Alzheimer’s disease? J. Oral. Microbiol. 2020;12:1676486. doi: 10.1080/20002297.2019.1676486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singhrao SK, Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J. Oral. Microbiol. 2019;11:1563405. doi: 10.1080/20002297.2018.1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers. Dis. 2013;36:665–677. doi: 10.3233/jad-121918. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Martinez RE, et al. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J. Clin. Periodontol. 2009;36:1004–1010. doi: 10.1111/j.1600-051X.2009.01496.x. [DOI] [PubMed] [Google Scholar]
- 55.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends in molecular medicine. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hajishengallis G, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS microbiology letters. 2012;333:1–9. doi: 10.1111/j.1574-6968.2012.02579.x. [DOI] [PubMed] [Google Scholar]
- 58.Nakhjiri SF, et al. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS microbiology letters. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- 59.Nakao R, et al. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microbes and infection. 2014;16:6–16. doi: 10.1016/j.micinf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infection and immunity. 2006;74:703–710. doi: 10.1128/iai.74.1.703-710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lorenzo-Pouso AI, Castelo-Baz P, Perez-Sayans M, Lim J, Leira Y. Autophagy in periodontal disease: Evidence from a literature review. Arch. Oral Biol. 2019;102:55–64. doi: 10.1016/j.archoralbio.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 62.Darios, F. & Stevanin, G. Impairment of lysosome function and autophagy in rare neurodegenerative diseases. J Mol Biol, 10.1016/j.jmb.2020.02.033 (2020). [DOI] [PMC free article] [PubMed]
- 63.Blasiak J, et al. Cellular Senescence in Age-Related Macular Degeneration: Can Autophagy and DNA Damage Response Play a Role? Oxid Med Cell Longev. 2017;2017:5293258. doi: 10.1155/2017/5293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang S, et al. Autophagy Dysfunction, Cellular Senescence, and Abnormal Immune-Inflammatory Responses in AMD: From Mechanisms to Therapeutic Potential. Oxid Med Cell Longev. 2019;2019:3632169. doi: 10.1155/2019/3632169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao J, et al. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy. 2015;11:939–953. doi: 10.1080/15548627.2015.1041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han D, Wu X, Liu L, Shu W, Huang Z. Sodium tanshinone IIA sulfonate protects ARPE-19 cells against oxidative stress by inhibiting autophagy and apoptosis. Sci. Rep. 2018;8:15137. doi: 10.1038/s41598-018-33552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rea SL, Walsh JP, Layfield R, Ratajczak T, Xu J. New insights into the role of sequestosome 1/p62 mutant proteins in the pathogenesis of Paget’s disease of bone. Endocr. Rev. 2013;34:501–524. doi: 10.1210/er.2012-1034. [DOI] [PubMed] [Google Scholar]
- 68.Rogov V, Dotsch V, Johansen T, Kirkin V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell. 2014;53:167–178. doi: 10.1016/j.molcel.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 69.Arjunan P, El-Awady A, Dannebaum RO, Kunde-Ramamoorthy G, Cutler CW. High-throughput sequencing reveals key genes and immune homeostatic pathways activated in myeloid dendritic cells by Porphyromonas gingivalis 381 and its fimbrial mutants. Mol. Oral. Microbiol. 2016;31:78–93. doi: 10.1111/omi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Njoroge T, Genco RJ, Sojar HT, Hamada N, Genco CA. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infection and immunity. 1997;65:1980–1984. doi: 10.1128/IAI.65.5.1980-1984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Experimental eye research. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 72.Arjunan P, et al. Increased Retinal Expression of the Pro-Angiogenic Receptor GPR91 via BMP6 in a Mouse Model of Juvenile Hemochromatosis. Investigative ophthalmology & visual science. 2016;57:1612–1619. doi: 10.1167/iovs.15-17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cutler CW, Kalmar JR, Arnold RR. Phagocytosis of virulent Porphyromonas gingivalis by human polymorphonuclear leukocytes requires specific immunoglobulin G. Infection and immunity. 1991;59:2097–2104. doi: 10.1128/IAI.59.6.2097-2104.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji S, Shin JE, Kim YC, Choi Y. Intracellular degradation of Fusobacterium nucleatum in human gingival epithelial cells. Molecules and cells. 2010;30:519–526. doi: 10.1007/s10059-010-0142-8. [DOI] [PubMed] [Google Scholar]
- 75.Inaba H, et al. Adhesion and invasion of gingival epithelial cells by Porphyromonas gulae. PloS one. 2019;14:e0213309. doi: 10.1371/journal.pone.0213309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li L, Michel R, Cohen J, Decarlo A, Kozarov E. Intracellular survival and vascular cell-to-cell transmission of Porphyromonas gingivalis. BMC microbiology. 2008;8:26. doi: 10.1186/1471-2180-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.