Abstract
The aim of the present study was to evaluate intra-day (test) and inter-day (re-test) reliability of surface electromyography (sEMG) signals of the masseter and temporal muscles in patients with Down syndrome (DS). We determined the reliability of sEMG variables in 33 patients with DS. EMG signals were recorded at rest as well as during maximum voluntary clenching and maximum habitual intercuspation (MHI). The signals were analyzed considering the amplitude in the root mean square (RMS), mean frequency (MNF), median frequency (MDF) and approximate entropy (ApEn). The intraclass correlation (ICC2,1) for the three trials recorded during MHI in the two sessions (test and retest) revealed excellent intra-session and inter-session reliability (ICC2,1 = 0.76 to 0.97) for all sEMG variables and muscles. In the rest position, excellent reliability was found for RMS and ApEn (ICC2,1 = 0.75 to 1.00) and good to excellent reliability was found for MDF and MNF (ICC2,1 = 0.64 to 0.93). The intra-session (test) and inter-session (re-test) analyses demonstrated the reliability of nonlinear sEMG variables of the masticatory muscles in adults with Down Syndrome.
Subject terms: Electromyography - EMG, Rehabilitation
Introduction
Down syndrome (DS), also known as trisomy of chromosome 21 (HSA21), is the most common genetic alteration, the prevalence of which ranges from six to 13 per every 10000 people in the general populatio1,2. This condition is associated with cognitive impairment, several comorbidities and emotional-social limitations, leading to substantial medical and social costs3–6.
Generalized muscular hypotonia is one of characteristics of DS, which, along with oral abnormalities, directly affects oral functions, such as swallowing, speech and breathing (even during sleep, leading to obstructive sleep apnea)7. The combination of abnormal masticatory muscle function (due to hypotonia) and altered skeletal developmental (e.g., discrepancy between alveolar arches, reduced maxillary length and midface retrusion) has several consequences during the growth phase, such as oromotor incoordination (weak jaw-closing muscles, hypotonicity of the tongue and inefficient lingual lateralization), difficulties during meals (choking, belching and food spillage from the mouth), uncontrolled facial movements, mouth open at rest and mouth-breathing due to poor muscle tonicity8–10.
Some of these abnormal muscle functions, such as those in the muscles responsible for the sustaining and moving the jaw (masseter and temporal) can be measured using surface electromyography (EMGs). However, the selection of a measure for research or clinical use depends on several factors, such as reliability. In clinical practice, the combination of linear and nonlinear measures is important to the characterization, classification and evaluation of the complexity of sEMG variables of the masticatory muscles and can enable a better understanding of neurophysiological conditions.
The root mean square (RMS) is a linear variable used to evaluate the excitability and activation of muscles before and after therapy11,12. Median frequency (MDF) is another linear variable widely used to interpret spectral characteristics of sEMG signals, reflecting muscle electrophysiology in different clinical conditions such as temporomandibular disordes13, cerebral palsy14 and low back pain15.
Nonlinear time-series analyses indicate features of motor control that are important for clinicians to measure16. Information entropy has been proposed as a measure of irregularity (nonlinear behavior) in biological signals16–19. The measurement of entropy is reported to be a reliable method for characterizing neuromuscular changes20 and approximate entropy (ApEn) enables a general understanding of the complexity of sEMG data21,22. In statistics, ApEn is used to establish the uncertainty or variability in a system. ApEn calculated from sEMG signals is dependent on the variability in both amplitude and frequency and better represents this aspect than amplitude or frequency alone21,22. As studies have demonstrated that nonlinear time-series analysis is more sensitive to changes in myoelectrical signals in comparison to linear methods23,24, the use of ApEn to quantify the irregularity or complexity of sEMG signals of the masseter and anterior temporal muscles in patients with DS may be important to the quantification and understanding of the neurophysiological conditions of these muscles.
However, the usefulness of sEMG data is dependent on the reproducibility of the signal detection method both within and between recording sessions25. This is a key factor to evaluating sEMG data in rehabilitation programs and clinical trials. Factors such as the preparation of the skin and positioning of the electrodes can exert an influence on the results when sEMG data are collected on different days26,27, as occurs when signals are collected before and after a clinical intervention.
Although a number of studies have demonstrated the reliability and reproducibility of sEMG signals for the evaluation of the masticatory muscles in both healthy and disabled patients17,21,28, to the best of our knowledge, no studies have assessed the reliability of sEMG signals of the masticatory muscles or the reproducibility of sEMG variables (linear and nonlinear) of the masseter and anterior temporal muscles in adults with DS. It is important to determine the reproducibility of measuring sEMG signals (intra-session and inter-session reliability) prior to suggesting the use of this technique as a tool for evaluating the efficacy of therapy designed to improve masticatory muscle function in this population.
Therefore, the aim of the present study was to determine the intra-session (test) and inter-session (re-test) reliability of sEMG signals of the masseter and anterior temporal muscles in adults with DS.
Results
The sample was composed of 23 adults with DS (15 men and eight women) with a mean age of 22.7 ± 6.5 years, mean body mass index (BMI) of 28.5 ± 6.8 kg/m2 and mean neck circumference of 40.3 ± 4.2 cm (Table 1).
Table 1.
Demographic and anthropometric data of 23 Down Syndrome patients.
| Variables | Baseline mean/SD |
|---|---|
| Age (years) | 22.7 ± 6.5 |
| Gender (female/male) | 8/15 |
| BMI (Kg/cm2) | 28.5 ± 6.8 |
| Neck circumference (cm) | 40.3 ± 4.2 |
| Abdomen circumference (cm) | 93.7 ± 16.5 |
Note: BMI = body mass index.
The reliability of the sEMG variables was determined in all participants. Tables 2 and 3 displays the mean (SD), ICC and SEM values for each sEMG measurement during MIH and in the rest position for each muscle. The ICCs for all sEMG parameters and muscles during MHI in the two sessions (test and retest) revealed excellent intra-session and inter-session reliability (range: 0.76 to 0.97) (Table 2). In the rest position, excellent reliability was found for RMS and ApEn (range: 0.75 to 1.00), whereas good to excellent reliability was found for MDF and MFN (range: 0.64 to 0.93).
Table 2.
Intra-day (Test and Retest) and between-day (Test/Retest) reliability of EMG signal measurements in MHI position.
| Test | Retest | Test/Retest | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | ICC2,1 (95%CI) | SEM | Mean (SD) | ICC2,1 (95%CI) | SEM | Mean (SD) | ICC2,1 (95%CI) | SEM | |
| RMS MHI | |||||||||
| RT | 57.88 (33.26) | 0.97 (0.93 to 0.99) | 5.73 | 57.59 (36.46) | 0.76 (0.51 to 0.89) | 30.05 | 3.17 (3.81) | 0.67 (0.36 to 0.85) | 21.25 |
| RM | 57.55 (25.99) | 0.90 (0.78 to 0.96) | 8.27 | 57.27 (26.54) | 0.84 (0.66 to 0.93) | 10.60 | 2.92 (3.61) | 0.78 (0.55 to 0.90) | 12.28 |
| LT | 44.26 (32.79) | 0.91 (0.90 to 0.96) | 10.04 | 41.26 (26.26) | 0.93 (0.84 to 0.97) | 6.99 | 2.73 (2.32) | 0.89 (0.76 to 0.95) | 9.87 |
| LM | 48.29 (30.65) | 0.86 (0.70 to 0.94) | 11.45 | 48.56 (26.33) | 0.85 (0.68 to 0.93) | 10.14 | 3.64 (2.97) | 0.82 (0.62 to 0.92) | 11.99 |
| FDM MHI | |||||||||
| RT | 164.82 (40.40) | 0.93 (0.84 to 0.97) | 10.31 | 165.49 (42.50) | 0.95 (0.89 to 0.98) | 9.00 | 71.82 (26.49) | 0.95 (0.89 to 0.98) | 8.97 |
| RM | 155.82 (39.24) | 0.96 (0.91 to 0.98) | 8.17 | 162.10 (38.18) | 0.89 (0.76 to 0.95) | 12.35 | 87.58 (26.84) | 0.95 (0.89 to 0.98) | 8.88 |
| LT | 119.14 (40.73) | 0.92 (0.82 to 0.97) | 11.77 | 124.54 (42.73) | 0.95 (0.89 to 0.98) | 10.35 | 72.01 (31.24) | 0.94 (0.85 to 0.97) | 10.53 |
| LM | 118.46 (43.44) | 0.94 (0.86 to 0.97) | 10.89 | 125.90 (40.78) | 0.93 (0.84 to 0.97) | 10.75 | 34.97 (29.99) | 0.94 (0.86 to 0.97) | 10.21 |
| FM MHI | |||||||||
| RT | 182.56 (37.77) | 0.95 (0.89 to 0.98) | 8.63 | 181.69 (40.08) | 0.97 (0.92 to 0.99) | 7.33 | 105.28 (30.85) | 0.96 (0.91 to 0.98) | 7.59 |
| RM | 177.32 (36.90) | 0.95 (0.88 to 0.98) | 8.31 | 180.58 (36.27) | 0.91 (0.90 to 0.96) | 11.00 | 132.41 (37.19) | 0.95 (0.89 to 0.98) | 8.36 |
| LT | 139.66 (42.90) | 0.94 (0.86 to 0.97) | 10.48 | 148.83 (46.37) | 0.95 (0.89 to 0.98) | 10.69 | 116.33 (31.25) | 0.94 (0.86 to 0.97) | 10.41 |
| LM | 138.88 (38.68) | 0.95 (0.89 to 0.98) | 8.85 | 143.39 (40.39) | 0.94 (0.86 to 0.97) | 9.48 | 65.43 (4.83) | 0.95 (0.89 to 0.98) | 8.53 |
| ApEn MHI | |||||||||
| RT | 1.20 (0.20) | 0.86 (0.70 to 0.94) | 0.08 | 1.20 (0.23) | 0.85 (0.68 to 0.93) | 0.09 | 1.39 (0.25) | 0.89 (0.76 to 0.95) | 0.07 |
| RM | 1.20 (0.19) | 0.82 (0.62 to 0.92) | 0.08 | 1.24 (0.20) | 0.78 (0.55 to 0.90) | 0.13 | 1.48 (0.28) | 0.83 (0.64 to 0.92) | 0.08 |
| LT | 1.09 (0.28) | 0.88 (0.74 to 0.95) | 0.10 | 1.15 (0.26) | 0.89 (0.76 to 0.95) | 0.09 | 1.60 (0.22) | 0.88 (0.74 to 0.95) | 0.10 |
| LM | 1.10 (0.22) | 0.88 (0.74 to 0.95) | 0.08 | 1.08 (0.24) | 0.87 (0.77 to 0.94) | 0.09 | 1.38 (0.22) | 0.90 (0.77 to 0.96) | 0.07 |
ApEn: approximate entropy. EMG: electromyographic. ICC: intraclass correlation coefficient. LT: left temporal. LM: left masseter. MHI: maximum habitual intercuspation. MVC: maximal voluntary contraction. MDF: median frequency. MNF: mean frequency. RT: right temporal. RM: right masseter. RMS: root mean square. SEM: standard error of measurement. ZC: zero crossing. 95%CI: 95% confidence interval of the mean.
Table 3.
Intra-day (Test and Retest) and between-day (Test/Retest) reliability of EMG signal measurements in rest position.
| Test | Retest | Inter day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | ICC2,1 (95%CI) | SEM | Mean (SD) | ICC2,1 (95%CI) | SEM | Mean (SD) | ICC | SEM | |
| RMS_rest | |||||||||
| RT | 3.31 (5.25) | 0.99 (0.98 to 1.00) | 0.41 | 3.03 (2.38) | 0.98 (0.95 to 0.99) | 0.35 | 3.17 (3.81) | 0.95 (0.89 to 0.98) | 0.91 |
| RM | 2.28 (1.19) | 0.83 (0.64 to 0.92) | 0.50 | 3.57 (6.04) | 0.99 (0.98 to 1.00) | 0.31 | 2.925 (3.61) | 0.88 (0.74 to 0.95) | 1.52 |
| LT | 2.96 (2.50) | 0.87 (0.77 to 0.94) | 0.89 | 2.51 (2.15) | 0.99 (0.98 to 1.00) | 0.23 | 2.73 (2.32) | 0.95 (0.89 to 0.98) | 0.53 |
| LM | 3.51 (2.39) | 0.80 (0.58 to 0.91) | 1.10 | 3.77 (3.56) | 0.99 (0.98 to 1.00) | 0.15 | 3.64 (2.97) | 0.91 (0.89 to 0.96) | 0.89 |
| FDM rest | |||||||||
| RT | 69.18 (25.41) | 0.80 (0.58 to 0.91) | 11.59 | 74.46 (27.57) | 0.86 (0.70 to 0.94) | 10.69 | 71.82 (26.49) | 0.66 (0.35 to 0.84) | 15.97 |
| RM | 93.86 (26.07) | 0.64 (0.32 to 0.83) | 16.00 | 81.31 (27.62) | 0.90 (0.78 to 0.96) | 8.66 | 87.58 (26.84) | 0.77 (0.53 to 0.90) | 13.24 |
| LT | 72.29 (28.21) | 0.85 (0.68 to 0.93) | 11.16 | 71.74 (34.28) | 0.89 (0.76 to 0.95) | 11.30 | 72.01 (31.24) | 0.77 (0.53 to 0.90) | 14.95 |
| LM | 37.30 (29.84) | 0.76 (0.51 to 0.89) | 15.54 | 32.65 (30.15) | 0.86 (0.69 to 0.94) | 11.32 | 34.97 (29.99) | 0.69 (0.40 to 0.86) | 16.69 |
| FM rest | |||||||||
| RT | 105.89 (29.29) | 0.88 (0.74 to 0.95) | 10.17 | 104.67 (32.42) | 0.91 (0.90 to 0.96) | 10.12 | 105.28 (30.85) | 0.83 (0.64 to 0.92) | 12.91 |
| RM | 137.29 (35.92) | 0.82 (0.62 to 0.92) | 15.38 | 127.53 (38.47) | 0.93 (0.84 to 0.97) | 132.41 (37.19) | 0.75 (0.50 to 0.89) | 19.02 | |
| LT | 115.18 (27.87) | 0.76 (0.51 to 0.89) | 14.03 | 117.48 (34.63) | 0.90 (0.78 to 0.96) | 10.85 | 116.33 (31.25) | 0.69 (0.40 to 0.86) | 17.45 |
| LM | 64.28 (22.20) | 0.68 (0.38 to 0.85) | 12.60 | 66.59 (27.46) | 0.90 (0.78 to 0.96) | 8.90 | 65.43 (4.83) | 0.75 (0.50 to 0.89) | 12.39 |
| ApEn rest | |||||||||
| RT | 1.40 (0.22) | 0.83 (0.63 to 0.92) | 0.09 | 1.38 (0.28) | 0.91 (0.90 to 0.96) | 0.08 | 1.39 (0.25) | 0.83 (0.63 to 0.92) | 0.10 |
| RM | 1.49 (0.28) | 0.86 (0.70 to 0.94) | 0.11 | 1.47 (0.29) | 0.89 (0.76 to 0.95) | 0.09 | 1.48 (0.28) | 0.76 (0.51 to 0.89) | 0.17 |
| LT | 1.59 (0.21) | 0.75 (0.50 to 0.89) | 0.11 | 1.62 (0.24) | 0.89 (0.76 to 0.95) | 0.08 | 1.60 (0.22) | 0.82 (0.62 to 0.92) | 0.10 |
| LM | 1.35 (0.18) | 0.91 (0.90 to 0.96) | 0.05 | 1.42 (0.27) | 0.92 (0.82 to 0.97) | 0.08 | 1.38 (0.22) | 0.84 (0.66 to 0.93) | 0.09 |
ApEn: approximate entropy. EMG: electromyographic. ICC: intraclass correlation coefficient. LT: left temporal. LM: left masseter. MHI: maximum habitual intercuspation. MVC: maximal voluntary contraction. MDF: median frequency. MNF: mean frequency. RT: right temporal. RM: right masseter. RMS: root mean square. SEM: standard error of measurement. ZC: zero crossing. 95%CI: 95% confidence interval of the mean.
Discussion
The present study confirmed the reliability (calculated using the ICC and SEM) of different sEMG variables of the masseter and anterior temporal muscles recorded during MHI and in the rest position in adults with DS. The results of the linear (amplitude and frequency) and non-linear (ApEn) analyses revealed excellent intra-session and inter-session reliability of sEMG signal readings during MIH (Table 2) as well as good to excellent reliability in the rest position (Table 3).
These results are similar to those reported in a previous study involving patients with cerebral palsy, i.e., high reproducibility for the data recorded during MIH and good to excellent reproducibility for data recorded in the rest position17. A high degree of reliability during MHI has also been found for the anterior temporal and masseter muscles in healthy patients29,30. These results demonstrate that sEMG activity recorded during MIH and at rest can be used to quantify the effects of clinical interventions on the masseter and anterior temporal muscles.
The sEMG evaluation is diagnostically useful in identifying patients with pain-related temporomandibular disorders31,32 or for the observation of muscle activity in the clinical setting30. Multiparameter sEMG analysis has been used as a strategy to investigate the performance of the masticatory muscles following a stroke33,34, assess pain patterns in the masticatory muscles35 and investigate the reproducibility of the evaluation of the masticatory muscles in patients with cerebral palsy17.
Electromyographic components calculated in the time and frequency domains are the most widely used variables for the study of masseter and anterior temporal muscle activity, whereas nonlinear components, such as ApEn, remain under-investigated in clinical studies. This is an issue that needs to be better explored, as a change in the entropy of the signal also reflects a qualitative change in the physiological system22. Specifically for ApEn, the result is given between 0 and 2, with higher values reflecting greater irregularity within the time-series36–38. Thus, values near 2 indicate healthy muscle function. The excellent reliability found for ApEn under both test conditions (MIH and rest) could be an important stimulus for future studies to use this sEMG parameter in the evaluation of the effects of therapeutic interventions performed on the masseter and anterior temporal muscles.
Conclusion
The present findings demonstrate the excellent reliability of surface electromyography variables (root mean squared, mean frequency, median frequency and approximate entropy) of the masseter and anterior temporal muscles in adults with Down Syndrome during maximum clenching effort as well as good to excellent reliability of the median and mean frequency recorded in the rest position.
Materials and Methods
Subjects
A convenience sample of adult patients with Down syndrome were evaluated at the Center of Bioscience Applied to Persons with Special Care Needs (CEBAPE), Institute of Science and Technology – UNESP/SJC, Brazil. The inclusion criteria were adult patients aged between 18–35 years-old, presenting snoring and/or mild to moderate apnea/hypopnea index, preserved cognitive function (ability to understand and respond to verbal commands necessary to perform this study, such as “open the mouth”, “close the mouth”, “ bite”, “relax”), agreed to participate by free will and, the Awareness Term along with signed by patient or patient’s responsible. The exclusion criteria were body mass index (BMI) greater than 30, unsatisfactory dental health, not able to reach the University when is needed, present psychiatric disease and have been submitted to physiotherapy, speech therapy and/or orthodontic treatment at least 6 months before the beginning of this study.
This study received approval from the Human Research Ethics Committees of the Institute of Science and Technology– UNESP/SJC, Brazil, CEPh 2.127.141 (process number CAAE 64173616.4.0000.0077).
Instrumentation
The device used for the EMG acquisition was an eight-channel module (EMG System do Brasil Ltda®) consisting of a conditioner with a band pass filter with cut-off frequencies at 20 to 500 Hz, an amplifier gain of 1000 times and a common mode rejection ratio >120 dB. All data were acquired and processed using a 16-bit analog to digital converter (EMG System do Brasil Ltda®) with a sampling frequency 2 kHz. Active bipolar electrodes with a pre-amplification gain of 20x are included.
Procedures
The participants visited the Physiology Laboratory on two different occasions, with a one-week interval between visits. Sessions were held in a silent, well-lit recording room. The participant was instructed to remain seated comfortably erect on a chair with eyes open, feet apart, shoulders relaxed and hands resting on thighs, with the head on the Frankfort plane parallel to the ground. During the procedures, the participants did not receive any visual feedback of the signals displayed on the computer.
A short training period was conducted prior to the tests to familiarize the participant with the tasks. Explanations concerning the procedures and electrode placement were given and the participant was trained to bite as hard as possible with maximum voluntary clenching (MVC) effort with a cotton role between the arches and maximum habitual intercuspation (MHI) effort with no material interposed between the teeth.
The sites for the placement of the electrodes were shaved and cleaned with a cotton ball soaked in 70% alcohol to diminish impedance between the skin and electrode. Pre-gelled, self-adhesive, bipolar, disposable, Ag/AgCl surface electrodes (MediTrace®) measuring 10 mm in diameter were positioned over the right masseter (RM), left masseter (LM), right temporal (RT) and left temporal (LT) muscles with an inter-electrode distance of 20 mm in the region of greatest tonus after the participant performed moderate intercuspation13. Bandage tape was used to secure the electrodes further, with care taken to avoid micro movements. A rectangular metallic electrode measuring 3 ×2 cm coated with Lectron II conductive gel (Pharmaceutical Innovations) to increase the conduction capacity and impede interference from external noise was attached to the left wrist as a ground reference.
Readings were performed under three conditions: at rest, during MVC and during MHI. In session 1 (test), three readings (10 seconds each) were performed in the rest position, followed by three readings (five seconds each) during MVC with a two-minute interval between readings. After three minutes, three readings (five seconds each) were performed during MIH with a two-minute interval between readings. The same procedures were repeated after one week in session 2 (retest).
Data processing
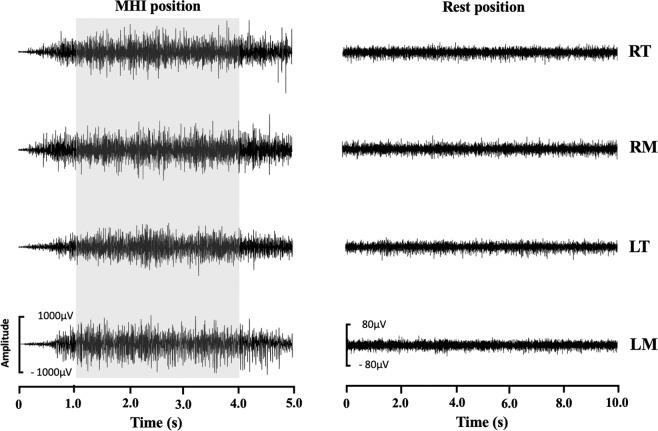
The sEMG signals obtained for the RT, RM, LT and LM muscles were processed using traditional linear analysis in the time (amplitude) and frequency domains as well as nonlinear analysis through the calculation of the complexity of the sEMG signal17. For the analysis of MVC and MIH, the first and last seconds of the five-second signals were discarded, resulting in three-second readings considered for each test. In contrast, all 10 seconds of the signals captured in the rest position were considered (Fig. 1).
Figure 1.
Surface electromyography signal of the right temporal (RT), right masseter (RM), left temporal (LT) and left masseter (LM) muscled during maximum habitual intercuspidation (MHI) and rest position. The grey band shows three-second period selected (Patient with Down Syndrome – mean age: 22.7 ± 6.5 years; body mass: 28.5 ± 6.8 kg/m2).
The amplitude of sEMG signal was determined considering root mean square (RMS) using a 200-ms moving window. The mean RMS values of the three readings at rest and during MIH were normalized by the highest RMS values obtained during the three MVC readings (µV/µV × 100: % MVC).
The power spectral density of the sEMG signals was calculated using Welch’s averaged periodogram with a Hamming window length zero-padded to the length of 2048 points. Overlap was 50% of the window length. The mean frequency (MNF) and median frequency (MDF) of the power spectrum were calculated for each one-second window (total: three windows)17 and the average of the values among the three windows was used in the reliability tests.
ApEn (m, r, N) was calculated to quantify the regularity or complexity of the sEMG signals, with the embedding dimension (m) and tolerance distance (r) set to m = 2 and r = 0.20 of the standard deviation (± SD) of the data sequence and N = number of points to be analyzed36–38. This analysis returns a value between 0 and 2 (arbitrary units [au]), with higher values reflecting greater irregularity in the time series36–38.
The sEMG signals were processed using specific routines carried out in Matlab version 7.1 (The MathWorks Inc., Natick, MA, USA).
Data analysis
The Shapiro-Wilk test was used to determine the distribution of the data and data were expressed as mean and SD. Intraclass correlation coefficients (ICCs) and the standard error of the mean (SEM) was used to verify the reliability of each sEMG measure. Intra-session reliability was determined by computing the intraclass correlation coefficient (ICC2,1) using only the first and second of the three measurements. Inter-day reliability (ICC2,1) was determined by comparing the average (3 trails) of each sEMG activity index for each muscle between the two sessions (test and retest). SEM was estimated by subtracting the ICCs value from one, taking the square root of this value, and multiplying by the SD39. The ICCs was interpreted using the following criteria: 0.00 to 0.39 (poor), 0.40 to 0.59 (fair), 0.60 to 0.74 (good) and 0.75 to 1.00 excellent)40. The SEM was used to express reliability in absolute values, with a lower SEM denoting greater reliability of the measurement, whereas a high SEM indicates a high level of error and implies the non-reproducibility of the tested values.
All data were analyzed using the Statistical Package for the Social Sciences (SPSS) Version 17.
Ethic approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Human Research Ethics Committees of the Institute of Science and Technology– UNESP/SJC, Brazil, CEPh 2.127.141, process number CAAE 64173616.4.0000.0077) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consent, for adult with Down syndrome in this study, was obtained from their parent/caregiver.
Acknowledgements
This study was supported by Fundação De Amparo à Pesquisa do Estado de São Paulo- FAPESP, grant number 2017/06838-5.
Author contributions
All the authors contributed to the conception, design and performance of the study. L.C.G., F.P., M.A.C.S. and M.F.G. provided the idea for the study, established the hypothesis and wrote the original proposal. L.C.G., V.L.S.T., G.R.C.S., G.P.M. and D.B.S. performed the acquisition of sEMG signals, patient electrode placement and exported sEMG data. MTSD performed the data tabulation. J.B.O.A., L.C.G. and F.P. made the interpretation of sEMG data. F.P. significantly contributed to sEMG signal and statistical analysis. J.B.O.A., L.V.F.O., C.S.O. and L.C.G. were involved in critically revising the manuscript. M.A.C.S., L.C.G. and M.F.G. will supervise this study, participate in its design and coordination and, will revise the manuscript that will lead to the final approval of the current submission. All authors read and approved the final manuscript. The author J.C.R. will be responsible for the screening of the patients that will be included in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Graaf G, et al. Assessment of prevalence of persons with Down syndrome: a theory-based demographic model. Journal of Applied Research in Intellectual Disabilities. 2011;24:247–262. doi: 10.1111/j.1468-3148.2010.00593.x. [DOI] [Google Scholar]
- 2.Presson AP, et al. Current estimate of Down syndrome population prevalence in the United States. The Journal of Pediatrics. 2013;163:1163–1168. doi: 10.1016/j.jpeds.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lott IT. Neurological phenotypes for Down syndrome across the life span. Progress in Brain Research. 2012;197:101–121. doi: 10.1016/B978-0-444-54299-1.00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roizen NJ, et al. A community cross-sectional survey of medical problems in 440 children with Down syndrome in New York State. The Journal of Pediatrics. 2014;164:871–75. doi: 10.1016/j.jpeds.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Dierssen M. Down syndrome: The brain in trisomic mode. Nature Reviews Neuroscience. 2012;13:844–858. doi: 10.1038/nrn3314. [DOI] [PubMed] [Google Scholar]
- 6.Ballard C, Mobley W, Hardy J, Williams G, Corbett A. Dementia in down’s syndrome. The Lancet Neurology. 2016;15:622–636. doi: 10.1016/S1474-4422(16)00063-6. [DOI] [PubMed] [Google Scholar]
- 7.Lal C, White DR, Joseph JE, van Bakergem K, LaRosa A. Sleep-disordered breathing in Down syndrome. Chest. 2015;147:570–579. doi: 10.1378/chest.14-0266. [DOI] [PubMed] [Google Scholar]
- 8.Suharsini M, Glinka J, Djokosalamoen S. The effect of mastication muscular tone on facial size in patients with Down syndrome. Incisiva Dental Journal: Majalah Kedokteran Gigi Insisiva. 2006;39(4):161–64. [Google Scholar]
- 9.Faulks D, Collado V, Mazille MN, Veyrune JL, Hennequin M. Masticatory dysfunction in persons with Down’s syndrome. Part 1: a etiology and incidence. Journal of Oral Rehabilitation. 2008;35:854–62. doi: 10.1111/j.1365-2842.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- 10.Suri S, Tompson BD, Cornfoot L. Cranial base, maxillary and mandibular morphology in Down syndrome. The Angle Orthodontist. 2010;80:861–69. doi: 10.2319/111709-650.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianansi LC, et al. Effects of Neuromuscular Electrical Stimulation on the Masticatory Muscles and Physiologic Sleep Variables in Adults with Cerebral Palsy: A Novel Therapeutic Approach. PLoS One. 2015;6(10):e0128959. doi: 10.1371/journal.pone.0128959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva, A. C. O. et al. Effect of Osteopathic Visceral Manipulation on Pain, Cervical Range of Motion, and Upper Trapezius Muscle Activity in Patients with Chronic Nonspecific Neck Pain and Functional Dyspepsia: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Evidence-Based Compl and Alternative Medicine.6, e4929271, 1–9 (2018). [DOI] [PMC free article] [PubMed]
- 13.Politti F, Casellato C, Kalytczak MM, Garcia MB, Biasotto-Gonzalez DA. Characteristics of EMG frequency bands in temporomandibullar disorders patients. Journal of Electromyography and Kinesiology. 2016;31:119–125. doi: 10.1016/j.jelekin.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Lauer RT, Pierce SR, Tucker CA, Barbe MF, Prosser LA. Age and Electromyographic Frequency Alterations during Walking in Children with Cerebral Palsy. Gait & Poture. 2010;31:136–139. doi: 10.1016/j.gaitpost.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiou SY, Koutsos E, Georgiou P, Strutton PH. Association between spectral characteristics of paraspinal muscles and functional disability in patients with low back pain: a cohort study. BMJ Open. 2018;8:e017091. doi: 10.1136/bmjopen-2017-017091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stergiou N, Decker LM. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Human Movement Science. 2011;30:869–88. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannasi LC, et al. Test-retest reliability of electromyographic variables of masseter and temporal muscles in patients with cerebral palsy. Archives of Oral Biology. 2014;59:1352–8. doi: 10.1016/j.archoralbio.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Costa M, Goldberger AL, Peng CK. Multiscale entropy to distinguish physiologic and synthetic RR time series. Computing in Cardiology. 2000;29:137–40. [PubMed] [Google Scholar]
- 19.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis: a new measure of complexity loss in heart failure. J Electrocardiol. 2003;36(Suppl.):39–40. doi: 10.1016/j.jelectrocard.2003.09.011. [DOI] [Google Scholar]
- 20.Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiology of Aging. 2002;23:23–6. doi: 10.1016/S0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 21.Sung PS, Zurcher U, Kaufman M. Reliability difference between spectral and entropic measures of erector spinae muscle fatigability. Journal of Electromyography and Kinesiology. 2010;20:25–30. doi: 10.1016/j.jelekin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Sung, P. S. Nonlinear analysis of surface electromyography, EMG Methods for evaluating muscle and nerve function, Mr. Mark Schwartz (Ed.), InTech, 793–802 (2012).
- 23.Fattorini L, Felici F, Filligoi GC, Traballesi M, Farina D. Influence of high motor unit synchronization levels on non-linear and spectral variables of the surface EMG. Journal of Neuroscience Methods. 2005;143:133–9. doi: 10.1016/j.jneumeth.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Felici F, et al. Linear and non-linear analysis of surface electromyograms in weight-lifters. European Journal Applied Physiology. 2001;84:337–42. doi: 10.1007/s004210000364. [DOI] [PubMed] [Google Scholar]
- 25.Oskouei AH, Paulin MG, Carman AB. Intra-session and inter-day reliability of forearm surface EMG during varying hand grip forces. Journal Electromyography and Kinesiology. 2012;23:216–222. doi: 10.1016/j.jelekin.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Merletti, R. & Parker, P. A. Electromyography-physiology, engineering and noninvasive applications. New Jersey: John Wiley & Sons; 2004.
- 27.Veiersted KB. The reproducibility of test contractions for calibration of electromyographic measurements. European Journal of Appllied Physiology and Occupational Physiology. 1991;62:91–98. doi: 10.1007/BF00626762. [DOI] [PubMed] [Google Scholar]
- 28.Lucareli PR, et al. Repeatability of a 3D multi-segment foot model during anterior and lateral step-down tests. Gait & Posture. 2016;43:9–16. doi: 10.1016/j.gaitpost.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez Y, et al. Reliability of electromyographic activity vs. bite-force from human masticatory muscles. European Journal of Oral Sciences. 2011;119:219–224. doi: 10.1111/j.1600-0722.2011.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Felício CM, Sidequersky FV, Tartaglia GM, Sforza C. Electromyographic standardized indices in healthy Brazilian young adults and data reproducibility. Journal Oral Rehabilitation. 2009;36:558–577. doi: 10.1111/j.1365-2842.2009.01970.x. [DOI] [PubMed] [Google Scholar]
- 31.Szyszka-Sommerfeld L, Machoy M, Lipski M, Wozniak K. The diagnostic value of electromyography in identifying patients with pain-related temporomandibular disorders. Frontiers Neurology. 2019;10:180. doi: 10.3389/fneur.2019.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishi SE, Basri R, Alam MK. Uses of electromyography in dentistry: An overview with meta-analysis. European. Journal of Dentistry. 2016;10:419–25. doi: 10.4103/1305-7456.184156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jian C, et al. Multiparameter electromyographic analysis of the masticatory muscle activities in patients with brainstem stroke at different head positions. Frontiers Neurology. 2017;8:221. doi: 10.3389/fneur.2017.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang X, Zhang X, Gao X, Chen X, Zhou P. A novel interpretation of sample entropy in surface electromyographic examination of complex neuromuscular alterations in subacute and chronic stroke. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2018;26:1878–1888. doi: 10.1109/TNSRE.2018.2864317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Álvarez-Méndez AM, Exposto FG, Castrillon EE, Svensson P. Systematic mapping of pressure pain thresholds of the masseter and termporalis muscles and assessment of their diversity through the novel application of entropy. Journal Oral Facial Pain Headache. 2017;31:362–371. doi: 10.11607/ofph.1927. [DOI] [PubMed] [Google Scholar]
- 36.Inbar GF, Allin J, Paiss O, Kranz H. Monitoring surface EMG spectral changes by the zero crossing rate. Medical & Biological Engineering & Computing. 1986;24:10–8. doi: 10.1007/BF02441600. [DOI] [PubMed] [Google Scholar]
- 37.Pincus SM. Approximate entropy as a measure of system complexity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2297–301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? American Journal of Physiology. 1994;266:H1643–56. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- 39.Bruton A, Conway JH, Holgate ST. Reliability: what is it and how is it measured? Physiotherapy. 2000;86:94–9. doi: 10.1016/S0031-9406(05)61211-4. [DOI] [Google Scholar]
- 40.Cicchetti DV, Sparrow SS. Developing criteria for establishing inter-rater reliability of specific items: application to assessment of adaptive behavior. American Journal of Mental Deficiency. 1981;86:127–37. [PubMed] [Google Scholar]