Dear Editor,
Parainfluenza virus 5 (PIV5), known as canine parainfluenza virus in the veterinary field, is a negative-sense, nonsegmented, single-stranded RNA virus belonging to the Paramyxoviridae family (Chen 2018). The virus was first reported in primary monkey kidney cells in 1954 (Hsiung 1972), then it has been frequently discovered in various hosts, including humans, dogs, pigs, cats, rodents, calves, and lesser pandas (Chatziandreou et al. 2004; Lee and Lee 2013; Liu et al. 2015; Zhai et al. 2017; Jiang et al. 2018). So far, PIV5 has not been reported in horse. In this study, using metagenomics analysis we have identified a novel equine PIV5.
Totally 148 fecal samples, 81 sera samples, and 115 nasal swabs were collected in six different cities of Xinjiang and Inner Mongolia, China in 2018 (Supplementary Figure S1). First, Fresh fecal samples were collected from 20 thoroughbred horses (mean 5.6 years) at an equestrian club in Hutubi County, north Xinjiang, China. The cDNA library of fecal specimens was prepared as previously described (He et al. 2013). Briefly, viral RNAs were extracted from fecal pooled samples, then reverse transcribed, and randomly amplified using PCR. The tagged and purified PCR products were subjected to Illumina sequencing (HiSeq X-ten, United States, Illumina) in a lane by Shanghai Personal Biotechnology Co., Ltd (Shanghai, China). In total, 76,986,684 reads were generated, of which 197,056 (0.26%) being annotated to mammalian viruses. Sequence analysis showed that 146 reads were closely related to human PIV5, sharing 99.8%–100% identity with the fusion protein (F) gene of AGS strain (GenBank no. KX060176).
To confirm the next-generation sequencing results, a pair of primers amplifying the equine PIV5 F gene were designed (Supplementary Table S1). RT-PCR results showed that eight fecal samples from 20 thoroughbred horses of the same equestrian club were positive for equine PIV5, indicating that the virus was likely circulating in the thoroughbred horses. Further studies revealed that eight fecal samples from 30 Yili horses in Yining city and five fecal samples from 11 Asian wild horses in Urumqi were positive for F gene (Table 1). However, the F gene was not detected in Yanqi, Sanhe and Wushi horses (Table 1). Further investigation found that Yili and Asia wild horses were co-bred with thoroughbred horses, but Yanqi, Sanhe and Wushi horses were not co-bred with thoroughbred horses. This suggested that equine PIV5 might be an exotic virus with high prevalence and different geographic distribution in north Xinjiang. In addition, no F gene was detected in equine sera and nasal swabs of all samples (Table 1), indicating intestinal cell tropism of equine PIV5. Sequence comparison showed that the F genes of all 21 equine PIV5 shared 100% nucleotide identity.
Table 1.
Information of samples included in this study.
| Sampling place | Breed | Samples type | Sample no. | Positive no. |
|---|---|---|---|---|
| Hutubi County | Thoroughbred horses | Feces/Sera/Nasal swabs | 20/40/80 | 8/0/0 |
| Yining City | Yili horses | Feces/Sera/Nasal swabs | 30/30/30 | 8/0/0 |
| Urumqi City | Asia wild horses | Feces/Sera/Nasal swabs | 11/11/5 | 5/0/0 |
| Hejing County | Yanqi horses | Feces/Sera/Nasal swabs | 44/0/0 | 0 |
| Manzhouli City | Sanhe horses | Feces/Sera/Nasal swabs | 11/0/0 | 0 |
| Wuhai City | Wushi horses | Feces/Sera/Nasal swabs | 32/0/0 | 0 |
The whole genomes of equine PIV5 were successfully obtained by RT-PCR using primers listed in the Supplementary Table S1. The obtained genome sequence is 15,246 nt and consists of genes encoding nucleocapsid protein (NP), V protein (V), membrane protein (M), fusion protein (F), hemagglutinin-neuraminidase protein (HN) and L protein (L) (GenBank no. MN604146). Sequence analysis indicated that the novel equine PIV5 shared 98.2%–99.9% homology with six Human PIV5, and 95.6%–98.0% homology with the animal source PIV5 strains (Table 2), indicating that equine PIV5 was closely related to human PIV5.
Table 2.
Homology analysis of the whole-genome sequences between equine PIV5 and PIV5 reference strains from various hosts.
| Reference strains | Nucleotide identity (%) |
|---|---|
| KX060176/Human PIV5/AGS /USA/1983 | 99.9 |
| JQ743322/Human PIV5/DEN/UK/1980 | 98.3 |
| JQ743324/Human PIV5/LN/UK/1980 | 98.2 |
| JQ743325/Human PIV5/MEL/UK/1980 | 98.2 |
| JQ743327/Human PIV5/RQ/UK/1976 | 98.2 |
| JQ743326/Human PIV5/MIL /UK/1980 | 98.2 |
| Other 11 PIV5 reference strains | 95.6–98.0 |
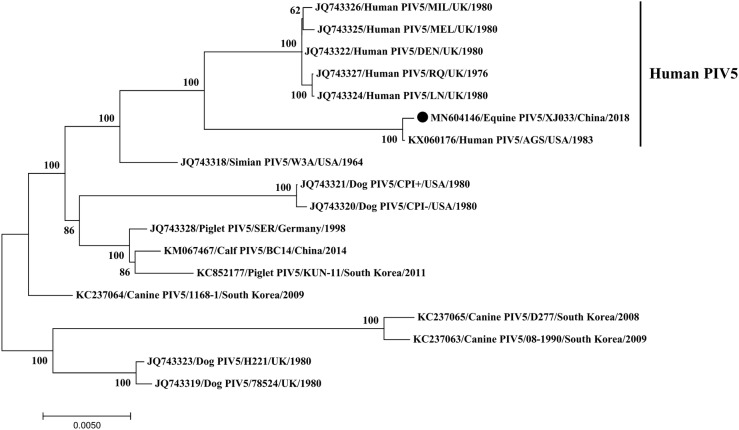
Phylogenetic analysis based on the whole genome sequences showed that the novel equine PIV5 and six human PIV5 reference strains formed a single clade and were closely related to the AGS strain, but diverging from the lineages of animal source PIV5 strains (Fig. 1), indicating it may be a human-source virus. Unfortunately, our attempts to isolate the novel equine PIV5 using Vero cells and chicken embryos failed.
Fig. 1.
Phylogenetic analysis of whole genome sequence of equine PIV5. The black circle indicates the virus identified in our study.
In summary, this study provides the first molecular evidence for equine PIV5. While our observations do not permit us to conclude that the virus associates with clinically symptomatic disease, we do indicate that equine PIV5 has a high prevalence and distributed in different geographic areas in Xinjiang, one of the major horse-producing regions in China. Further research is needed to develop new methods for virus isolation, and to investigate the serological epidemiology of PIV5 in horses in China.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by Natural Science Foundation of Xinjiang Uyghur Autonomous Region (2019D01A47), China Postdoctoral Science Foundation (2019M653901XB) and Prior Period Project of Xinjiang Agricultural University (XJAU201721). The authors thank Jingfei Wang and Chunguo Liu of Harbin Veterinary Research Institute for their support.
Compliance with Ethical Standards
Conflict of interest
The authors have declared no competing interests.
Ethics Statement
All experimental procedures involving animals were approved (animal protocol number: 2018005) by the Animal Care and Use Committee of Xinjiang Agricultural University.
Footnotes
Jinxin Xie and Panpan Tong have contributed equally to this work.
References
- Chatziandreou N, Stock N, Young D, Andrejeva J, Hagmaier K, McGeoch DJ, Randall RE. Relationships and host range of human, canine, simian and porcine isolates of simian virus 5 (parainfluenza virus 5) J Gen Virol. 2004;85:3007–3016. doi: 10.1099/vir.0.80200-0. [DOI] [PubMed] [Google Scholar]
- Chen ZH (2018) Parainfluenza virus 5-vectored vaccines against human and animal infectious diseases. Rev Med Virol 28. 10.1002/rmv.1965 [DOI] [PMC free article] [PubMed]
- He B, Yang F, Yang W, Zhang Y, Feng Y, Zhou J, Xie J, Feng Y, Bao X, Guo H, Li Y, Xia L, Li N, Matthijnssens J, Zhang H, Tu C. Characterization of a novel G3P[3] rotavirus isolated from a lesser horseshoe bat: a distant relative of feline/canine rotaviruses. J Virol. 2013;87:12357–12366. doi: 10.1128/JVI.02013-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung GD. Parainfluenza-5 virus. Infection of man and animal. Prog Med Virol. 1972;14:241–274. [PubMed] [Google Scholar]
- Jiang N, Wang EY, Guo DH, Wang X, Su MJ, Kong FZ, Yuan D, Zhai J, Sun DB. Isolation and molecular characterization of parainfluenza virus 5 in diarrhea-affected piglets in China. J Vet Med Sci. 2018;80:590–593. doi: 10.1292/jvms.17-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YN, Lee C. Complete genome sequence of a novel porcine parainfluenza virus 5 isolate in Korea. Arch Virol. 2013;158:1765–1772. doi: 10.1007/s00705-013-1770-z. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li N, Zhang S, Zhang F, Lian H, Hu R. Parainfluenza virus 5 as possible cause of severe respiratory disease in calves, China. Emerg Infect Dis. 2015;21:2242–2244. doi: 10.3201/eid2112.141111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai JQ, Zhai SL, Lin T, Liu JK, Wang HX, Li B, Zhang H, Zou SZ, Zhou X, Wu MF, Chen W, Luo ML. First complete genome sequence of parainfluenza virus 5 isolated from lesser panda. Arch Virol. 2017;162:1413–1418. doi: 10.1007/s00705-017-3245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.