Abstract
We spend about one-third of our lives either sleeping or attempting to sleep. Therefore, the socioeconomic implications of sleep disorders may be higher than expected. However, the fundamental mechanisms and functions of sleep are not yet fully understood. Neuroimaging has been utilized to reveal the connectivity between sleep and the brain, which is associated with the physiology of sleep. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) imaging studies have become increasingly common in sleep research. Recently, significant progress has been made in understanding the physiology of sleep through neuroimaging and the use of various radiopharmaceuticals, as the sleep–wake cycle is regulated by multiple neurotransmitters, including dopamine, adenosine, glutamate, and others. In addition, the characteristics of rapid eye and non-rapid eye movement sleep have been investigated by measuring cerebral glucose metabolism. The physiology of sleep has been investigated using PET to study glymphatic function as a means to clear the amyloid burden. However, the basic mechanisms and functions of sleep are not yet fully understood. Further studies are needed to investigate the effects and consequences of chronic sleep deprivation, and the relevance of sleep to other diseases.
Keywords: Sleep, Brain, Positron emission tomography, Single-photon emission computed tomography
Introduction
Each day, we sleep and then wake up. However, we do not know why we sleep everyday. In addition, sleep duration varies from individual to individual, and we spend around one-third of our life either sleeping or attempting to sleep [1]. Sleep disorders are associated with hypertension [2] and obesity [3], and sleep–wake disturbance is frequent in neurodegenerative disorders such as Alzheimer’s disease [4] and Parkinson’s disease [5]. The socioeconomic implications of sleep disorders may also be greater than expected [6]. However, the basic mechanisms and functions of sleep are not fully understood. Traditionally, sleep has been considered a restorative process [7].
Neuroimaging has been utilized to reveal the connectivity between sleep and the brain, which is associated with the physiology of sleep [8]. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) imaging studies have become increasingly common in sleep research [8]. Information can be gathered at the molecular level using PET, and specific molecules can be labeled to detect their targets [9]. In addition, PET scanning can be performed repeatedly over time with minimal invasiveness and strong reproducibility [9]. However, it is limited by low spatial/temporal resolution and nonspecific binding of radiopharmaceuticals [10]. Other disadvantages include restricted availability, exposure to radiation, and high cost [11].
Recently, significant progress has been made in understanding the physiology of sleep through neuroimaging and the use of various radiopharmaceuticals, as the sleep–wake cycle is regulated by multiple neurotransmitters. Therefore, this review provides an overview of brain imaging under conditions of sleep and sleep deprivation.
Sleep, Wakefulness, and Neuroimaging
Sleep is not the opposite of wakefulness, rather it is a more active, metabolically distinct state than previously expected [12]. The hypothalamus and the brain stem control the balance between sleep and wakefulness [13]. Several neurotransmitters such as histamine, acetylcholine, norepinephrine, serotonin, orexin, adenosine, glutamate, gamma aminobutyric acid (GABA), and dopamine are involved in regulating sleep and wakefulness [13].
Sleep consists of two fundamentally distinct cycles: rapid eye movement (REM) and non-rapid eye movement (NREM) sleep [12]. NREM sleep consists of three stages that progressively deepen from stage 1 to stage 3 [12]. Stage 1 of NREM sleep usually occurs at the beginning of sleep with slow eye movement and is the lightest stage of NREM sleep [12]. During stage 2, body temperature begins to decrease, and the heart rate slows. In stage 3, delta waves are common in electroencephalograms [12]. REM sleep is characterized by rapid eye movements, muscle atonia, and increased heart rate, blood pressure, sympathetic nerve activity, and respiration [12]. Throughout the night, there are four to six cyclical switches between NREM and REM sleep every 1–1.5 h [12, 14].
PET neuroimaging was first used to study sleep in 1985 by Heiss et al. [15], who used 18F-fluorodeoxyglucose (FDG) to compare cerebral glucose metabolism between wakefulness, sleep, and dreaming. During sleep, there was a significant decrease in the cerebral metabolic rate of glucose (CMRglu) compared to wakefulness, which could be interpreted as less CMRglu being needed while asleep than during wakefulness. NREM sleep in particular tends to show a significantly lower CMRglu than REM sleep and wakefulness [16]. The decrease in CMRglu during NREM sleep is greater in the frontal and temporal lobes than in other parts of the brain [16] (Fig. 1). In addition, the number of eye movements during REM sleep correlates with CMRglu in areas that correspond to the neural system that involves oculomotor control and visual spatial attention, i.e., the frontal eye fields, dorsolateral prefrontal cortex, superior/inferior parietal lobules, and occipital cortex [10]. However, 18F-FDG has a half-life of 109.7 min, which may limit repeated measurements within the same night, resulting in inaccuracy in investigating each sleep phase [17]. Studies that have used 15O-H2 PET to measure cerebral blood flow (CBF) [18, 19] have reported similar results to those that have used CMRglu. CBF in the cerebellum, thalamus, basal ganglia, prefrontal cortex, anterior cingulate cortex, precuneus, and mesial temporal lobes is significantly decreased during slow wave sleep, which is referred to as deep sleep of NREM, compared to wakefulness and REM sleep [18, 19].
Fig. 1.
PET scans at the mid-ventricular level show an overall decrease in the metabolic rate during NREM sleep (a) and right basal ganglia elevation in REM sleep (b) compared with wakefulness (c). Scale bar is in metabolic rate in micromoles glucose/100 g/min. Reprinted from Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography, Life Sci. 1989;45(15):1349–56. Buchsbaum et al., with permission from Elsevier
Although the connection between sleep and the amyloid β (Aβ) burden is not clear, sleep disorders are one of the most common comorbidities associated with Alzheimer’s disease [20]. In addition, diurnal physiological fluctuations in Aβ levels in the cerebrospinal fluid have been reported to increase during wakefulness and decrease during sleep, particularly during NREM sleep [21]. In a study that used 11C-Pittsburgh compound B PET in healthy older adults, self-reported shorter sleep duration and poor sleep quality were associated with a greater Aβ burden [22]. Similarly, in another self-report study that used 18F-FDG PET, longer sleep latency and poor sleep quality were associated with a greater Aβ burden but not with glucose metabolism [23]. In another study, sleep duration did not show any significant association with Aβ burden [24]. Amyloid is released during synaptic activity [25], which is decreased during sleep [26]. Therefore, subjects with poor sleep quality can show higher Aβ levels due to having elevated neuronal activity [24], in addition to reduced clearance of waste products such as Aβ aggregations [27].
Dopamine agonists cause wakefulness via the dopamine receptor (DR) [28], and poor sleep maintenance is the most frequent sleep disorder in Parkinson’s disease, a neurodegenerative disorder of the dopaminergic neurons [29]. However, there are no significant differences between good sleepers and poor sleepers in terms of dopamine transporter (DAT) availability [30] and DR availability [31]. In good sleepers, sleep duration is negatively correlated with striatal DR availability; however, this is not the case in poor sleepers [31].
Sleep Deprivation and Neuroimaging
Studies on sleep deprivation have been conducted to understand the functions and physiology of sleep. Sleep deprivation can induce behavioral, hormonal, and neurochemical changes [32]. In acute sleep deprivation studies, subjects are kept awake continuously for 24–72 h while being closely monitored [33].
The first 18F-FDG PET study that investigated the effects of sleep deprivation was carried out in 1991 and included eight normal controls over approximately 32 h of sleep deprivation [34]. After sleep deprivation, CMRglu of the thalamus, basal ganglia, cerebellum, and frontal/temporal lobes decreased, while that of the occipital lobe increased. As sleep deprivation has an antidepressant effect [35], a study investigated the effects of sleep deprivation by means of CMRglu. Subjects who showed a > 40% decrease in their Hamilton depression scores following sleep deprivation were considered responders to sleep deprivation, and the baseline CMRglu of the cingulate cortex and amygdala of responders was higher than in those of non-responders or normal controls [36]. In addition, after sleep deprivation, CMRglu of the cingulate cortex and amygdala of the responders decreased significantly, while that of non-responders or normal controls showed minimal change [36]. Interestingly, overactivation of CMRglu in the limbic system has been reported in patients with depression, and in these cases, sleep deprivation might reduce depression by reducing overactivity in the limbic system [36].
The glymphatic pathway, which clears toxic metabolites including Aβ from the brain, was first identified in 2012 [37]. Glymphatic function increases during sleep, whereas the accumulation of metabolic byproducts occurs during wakefulness [27]. An accumulation of Aβ in the brain is associated with cognitive impairment [38]. The effects of sleep deprivation on Aβ clearance in the human brain were reported by Shokri-Kojori et al. in 2018 [39]. After 31 h of sleep deprivation in 20 normal controls, a significantly higher Aβ burden was observed in the hippocampus, parahippocampus, and thalamus, with a mean increase of 5%, although the magnitude of Aβ changes varied widely regardless of age, sex, and apolipoprotein E status [39] (Fig. 2). Hippocampal Aβ increases following sleep deprivation may reflect either the reduced glymphatic function in clearing the Aβ or the increased synthesis of Aβ following neuronal activity during wakefulness [39]. In addition, the reported sleep hours from the normal controls in that study were negatively correlated with the baseline Aβ burden, which might imply an effect of chronic sleep deprivation [39].
Fig. 2.
Effects of one-night sleep deprivation (SD) on Aβ. a Voxel-wise paired t test between rested-wakefulness (RW) and SD conditions highlighting the hippocampus and other subcortical structures (PFWE < 0.05, cluster-size corrected). b Subject-level changes in florbetaben (FBB) standardized uptake value ratio (SUVr) (in the red cluster identified in a) from RW to SD. There was no significant effect of gender or gender × sleep interaction (p > 0.15). c Association between changes in mood from RW to SD and changes in the FBB SUVr for the cluster identified in a. Mood change was quantified using the principal component of the changes in self-reported measures from RW to SD, which accounted for 35.5% of the variance. Self-reported measures of alert, friendly, happy, social, and energetic significantly decreased, and measures of tiredness and difficulty staying awake significantly increased from RW to SD (p < 0.001, two-tailed). d Average FBB SUVr in the a priori hippocampus region of interest across all subjects. Error bars show the standard deviation. Reprinted from β-amyloid accumulation in the human brain after one night of sleep deprivation, Proc Natl Acad Sci USA. 2018;115(17):4483–4488. Shokri-Kojori et al., with permission from PNAS
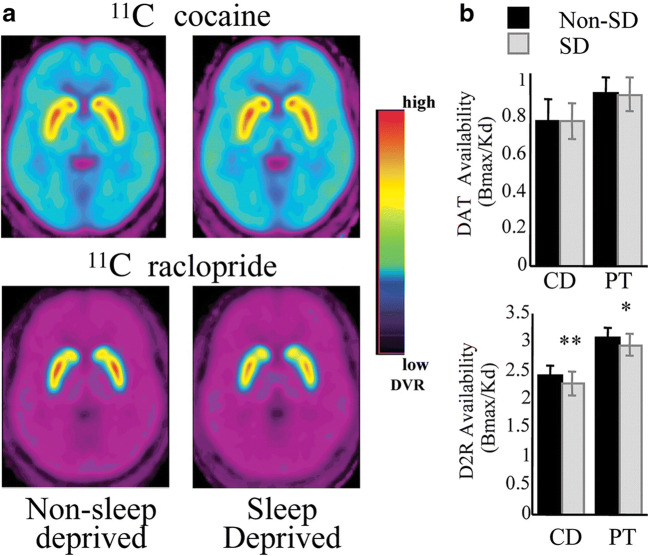
Amphetamine promotes wakefulness by enhancing dopamine release and blocking the reuptake of dopamine, which suggests that dopaminergic neurons are relevant to the sleep–wake cycle [40]. A study that used 11C-cocaine PET showed that approximately 30 h of sleep deprivation did not change DAT availability in 15 normal controls [41]. In Martins et al. [42], four nights of REM sleep deprivation and two nights of sleep deprivation did not significantly change DAT availability measured using 99mTc-TRODAT-1. However, in another study, sleep deprivation decreased DR availability measured using 11C-raclopride PET in both the caudate nucleus and putamen, which correlated with the “desire to sleep” and “tiredness” [41] (Fig. 3). However, the decrease in DR availability suggests either a decreased number of DRs or a decreased receptor affinity [43]. Volkow et al. [44] tested whether methylphenidate, a DAT blocker, could induce changes in DR availability after sleep deprivation, as 11C-raclopride competes with dopamine for DR binding. They concluded that the downregulation of DR under sleep deprivation might contribute to the associated decreased wakefulness through the action of adenosine, which internalizes the DR [44]. In that same study, microdialysis in rats showed no significant change in dopamine after sleep deprivation, consistent with human studies.
Fig. 3.
a Averaged brain images of the distribution volume ratio for 11C-cocaine and 11C-raclopride at the level of the striatum for non-SD and SD conditions. b Bmax/Kd in the caudate (CD) and putamen (PT) for 11C-cocaine (measure of DAT availability) and for 11C-raclopride (measure of D2 receptor availability) for non-SD and SD. Values represent mean ± standard deviation. Comparisons correspond to paired t tests: *p < 0.05; **p < 0.01. Reprinted from sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain, J Neurosci. 2008;28(34):8454–61. Volkow et al., with permission from J Neurosci
Coffee can make people alert through the action of caffeine, which is a non-selective adenosine antagonist for the adenosine receptor (AR) [45], which is an attractive candidate for the sleep switch [12]. Two previous studies have reported the effects of sleep deprivation on the AR using 18F-CPFPX PET [46, 47]. Twenty-four-hour sleep deprivation led to a significant increase in AR availability, ranging from 10.6% in the cerebellum to 15.3% in the orbitofrontal cortex [46] (Fig. 4). In a follow-up study, 14 h recovery sleep after 52 h sleep deprivation decreased elevated AR availability, which was evident in the striatum, thalamus, orbitofrontal cortex, amygdala, occipital cortex, frontal cortex, anterior cingulate cortex, insula, parietal cortex, and temporal cortex [47]. Fourteen-hour recovery sleep restored AR availability to the levels of the control groups.
Fig. 4.
Average images of the sleep deprivation group (n = 12) after spatial normalization. a Magnetic resonance imaging. b Parametric image of binding potential (BP2) before sleep deprivation. c Image after sleep deprivation. Reprinted from Sleep deprivation increases A1 adenosine receptor binding in the human brain: A positron emission tomography study, J Neurosci. 2007;27(9):2410–5. Elmenhorst et al., with permission from J Neurosci
Both GABA and glutamate neurons regulate sleep and wakefulness [48]. Previous studies have investigated the action of metabotropic glutamate receptor subtype 5 (mGluR5) during sleep deprivation [49, 50]. In one study, 40 h sleep deprivation in 22 normal controls increased mGluR5 availability by 3.5% [49]. The increase in mGluR5 availability was significant in the anterior cingulate cortex, insula, medial temporal lobe, parahippocampus, striatum, and amygdala, with no regions of decrease (Fig. 5). In addition, sleep deprivation-induced change in subjective sleepiness was correlated with an increase in mGluR5 availability. As subjects with major depression have reduced mGluR5 availability [51], sleep deprivation may have a rapid antidepressant effect [49]. The increase in mGluR5 availability after sleep deprivation is more pronounced in subjects with a lower baseline mGluR5 availability [50]. However, it is uncertain whether increased mGluR5 availability is a compensatory mechanism to induce wakefulness or signals the need for sleep [50].
Fig. 5.
Axial, sagittal, and coronal views of 11C-ABP688 binding in a representative individual. a Magnetic resonance image template for anatomical reference. b Color-coded normalized volumes of the distribution (Vnorm) of 11C-ABP688 after 9 h of wakefulness (sleep control condition). c Color-coded Vnorm of 11C-ABP688 after 33 h of wakefulness (sleep deprivation condition). The crosshair was placed in the right caudate nucleus. Reprinted from increased metabotropic glutamate receptor subtype 5 availability in human brain after one night without sleep, Biol Psychiatry. 2013;73(2):161–8. Hefti et al., with permission from Elsevier
Conclusions
Sleep involves a more active, metabolically distinct state than previously believed. The basic mechanisms and functions of sleep remain to be fully understood. Neuroimaging could improve our understanding of sleep and help to develop a new treatment for sleep-related disorders. However, further studies are needed to investigate the effects and consequences of chronic sleep deprivation and the relevance of sleep to other diseases.
Compliance with Ethical Standards
Conflict of Interest
Kyoungjune Pak, Jiyoung Kim, Keunyoung Kim, Seong Jang Kim, and In Joo Kom declare that they have no conflict of interest. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (2017R1D1A1B03029352, 2017R1D1A1B03033235).
Ethical Statement
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Not applicable.
Footnotes
The original online version of this article was revised:
The authors regret that there was a mistake in an affiliation of authors.
The original article has been corrected.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/4/2023
A Correction to this paper has been published: 10.1007/s13139-023-00807-4
Contributor Information
Kyoungjune Pak, Email: ilikechopin@me.com.
Jiyoung Kim, Email: bijoukim78@gmail.com.
Keunyoung Kim, Email: 4mura2@hanmail.net.
Seong Jang Kim, Email: growthkim@daum.net.
In Joo Kim, Email: injkim@pusan.ac.kr.
References
- 1.Aminoff MJ, Boller F, Swaab DF. We spend about one-third of our life either sleeping or attempting to do so. Handb Clin Neurol. 2011;98:vii. doi: 10.1016/B978-0-444-52006-7.00047-2. [DOI] [PubMed] [Google Scholar]
- 2.Feng X, Liu Q, Li Y, Zhao F, Chang H, Lyu J. Longitudinal study of the relationship between sleep duration and hypertension in Chinese adult residents (CHNS 2004-2011) Sleep Med. 2019;58:88–92. doi: 10.1016/j.sleep.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Sluggett L, Wagner SL, Harris RL. Sleep duration and obesity in children and adolescents. Can J Diabetes. 2019;43:146–152. doi: 10.1016/j.jcjd.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Cordone S, Annarumma L, Rossini PM, De Gennaro L. Sleep and beta-amyloid deposition in Alzheimer disease: insights on mechanisms and possible innovative treatments. Front Pharmacol. 2019;10:695. doi: 10.3389/fphar.2019.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann CR. Sleep-wake and circadian disturbances in Parkinson disease: a short clinical guide. J Neural Transm (Vienna). 2019. [DOI] [PubMed]
- 6.Research IoMUCoSMa . In: Sleep disorders and sleep deprivation: an unmet public health problem. Colten HR, Altevogt BM, editors. Washington (DC): The National Academies Collection: Reports funded by National Institutes of Health; 2006. pp. 67–166. [PubMed] [Google Scholar]
- 7.Maquet P, Dive D, Salmon E, Sadzot B, Franco G, Poirrier R, et al. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res. 1990;513:136–143. doi: 10.1016/0006-8993(90)91099-3. [DOI] [PubMed] [Google Scholar]
- 8.Dang-Vu TT, Schabus M, Desseilles M, Sterpenich V, Bonjean M, Maquet P. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33:1589–1603. doi: 10.1093/sleep/33.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Ann N Y Acad Sci. 2010;1191:133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- 10.Hong CC, Gillin JC, Dow BM, Wu J, Buchsbaum MS. Localized and lateralized cerebral glucose metabolism associated with eye movements during REM sleep and wakefulness: a positron emission tomography (PET) study. Sleep. 1995;18:570–580. doi: 10.1093/sleep/18.7.570. [DOI] [PubMed] [Google Scholar]
- 11.Michaelides M, Thanos PK, Volkow ND, Wang GJ. Translational neuroimaging in drug addiction and obesity. ILAR J. 2012;53:59–68. doi: 10.1093/ilar.53.1.59. [DOI] [PubMed] [Google Scholar]
- 12.Carley DW, Farabi SS. Physiology of sleep. Diabetes Spectr. 2016;29:5–9. doi: 10.2337/diaspect.29.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel JM. The neurotransmitters of sleep. J Clin Psychiatry. 2004;65(Suppl 16):4–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol. 2008;100:1160–1168. doi: 10.1152/jn.90592.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heiss WD, Pawlik G, Herholz K, Wagner R, Wienhard K. Regional cerebral glucose metabolism in man during wakefulness, sleep, and dreaming. Brain Res. 1985;327:362–366. doi: 10.1016/0006-8993(85)91537-9. [DOI] [PubMed] [Google Scholar]
- 16.Buchsbaum MS, Gillin JC, Wu J, Hazlett E, Sicotte N, Dupont RM, et al. Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography. Life Sci. 1989;45:1349–1356. doi: 10.1016/0024-3205(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 17.Peigneux P. Neuroimaging studies of sleep and memory in humans. Curr Top Behav Neurosci. 2015;25:239–268. doi: 10.1007/7854_2014_326. [DOI] [PubMed] [Google Scholar]
- 18.Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 19.Andersson JL, Onoe H, Hetta J, Lidstrom K, Valind S, Lilja A, et al. Brain networks affected by synchronized sleep visualized by positron emission tomography. J Cereb Blood Flow Metab. 1998;18:701–715. doi: 10.1097/00004647-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4:150ra22. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branger P, Arenaza-Urquijo EM, Tomadesso C, Mezenge F, Andre C, de Flores R, et al. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging. 2016;41:107–114. doi: 10.1016/j.neurobiolaging.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36:2568–2576. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27:1181–1194. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- 29.Stocchi F, Barbato L, Nordera G, Berardelli A, Ruggieri S. Sleep disorders in Parkinson’s disease. J Neurol. 1998;245(Suppl 1):S15–S18. doi: 10.1007/PL00007731. [DOI] [PubMed] [Google Scholar]
- 30.Chiu NT, Lee BF, Yeh TL, Chen PS, Lee IH, Chen KC, et al. Relationship between striatal dopamine transporter availability and sleep quality in healthy adults. Mol Imaging Biol. 2011;13:1267–1271. doi: 10.1007/s11307-010-0442-6. [DOI] [PubMed] [Google Scholar]
- 31.Lee BF, Chiu NT, Kuang Yang Y, Lin CC. The relation between striatal dopamine D2/D3 receptor availability and sleep quality in healthy adults. Nucl Med Commun. 2007;28:401–406. doi: 10.1097/MNM.0b013e3280bad8b6. [DOI] [PubMed] [Google Scholar]
- 32.Tufik S, Lindsey CJ, Carlini EA. Does REM sleep deprivation induce a supersensitivity of dopaminergic receptors in the rat brain? Pharmacology. 1978;16:98–105. doi: 10.1159/000136753. [DOI] [PubMed] [Google Scholar]
- 33.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553–567. [PMC free article] [PubMed] [Google Scholar]
- 34.Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Hazlett E, Sicotte N, et al. The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14:155–162. [PubMed] [Google Scholar]
- 35.Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- 36.Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Johnson JC, Bunney WE., Jr Effect of sleep deprivation on brain metabolism of depressed patients. Am J Psychiatry. 1992;149:538–543. doi: 10.1176/ajp.149.4.538. [DOI] [PubMed] [Google Scholar]
- 37.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra11. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krell-Roesch J, Vassilaki M, Mielke MM, Kremers WK, Lowe VJ, Vemuri P, et al. Cortical beta-amyloid burden, neuropsychiatric symptoms, and cognitive status: the Mayo Clinic study of aging. Transl Psychiatry. 2019;9:123. doi: 10.1038/s41398-019-0456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, et al. Beta-amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115:4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Wong C, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–8461. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martins RC, Andersen ML, Garbuio SA, Bittencourt LR, Guindalini C, Shih MC, et al. Dopamine transporter regulation during four nights of REM sleep deprivation followed by recovery--an in vivo molecular imaging study in humans. Sleep. 2010;33:243–251. doi: 10.1093/sleep/33.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gjedde A, Wong DF, Rosa-Neto P, Cumming P. Mapping neuroreceptors at work: on the definition and interpretation of binding potentials after 20 years of progress. Int Rev Neurobiol. 2005;63:1–20. doi: 10.1016/S0074-7742(05)63001-2. [DOI] [PubMed] [Google Scholar]
- 44.Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, et al. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32:6711–6717. doi: 10.1523/JNEUROSCI.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark I, Landolt HP. Coffee, caffeine, and sleep: a systematic review of epidemiological studies and randomized controlled trials. Sleep Med Rev. 2017;31:70–78. doi: 10.1016/j.smrv.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elmenhorst D, Elmenhorst EM, Hennecke E, Kroll T, Matusch A, Aeschbach D, et al. Recovery sleep after extended wakefulness restores elevated A1 adenosine receptor availability in the human brain. Proc Natl Acad Sci U S A. 2017;114:4243–4248. doi: 10.1073/pnas.1614677114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu X, Li W, Ma Y, Tossell K, Harris JJ, Harding EC, et al. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci. 2019;22:106–119. doi: 10.1038/s41593-018-0288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hefti K, Holst SC, Sovago J, Bachmann V, Buck A, Ametamey SM, et al. Increased metabotropic glutamate receptor subtype 5 availability in human brain after one night without sleep. Biol Psychiatry. 2013;73:161–168. doi: 10.1016/j.biopsych.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Holst SC, Sousek A, Hefti K, Saberi-Moghadam S, Buck A, Ametamey SM, et al. Cerebral mGluR5 availability contributes to elevated sleep need and behavioral adjustment after sleep deprivation. Elife. 2017;6. [DOI] [PMC free article] [PubMed]
- 51.Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, et al. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry. 2011;168:727–734. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]