Abstract
Purpose
PASylation® offers the ability to systematically tune and optimize the pharmacokinetics of protein tracers for molecular imaging. Here we report the first clinical translation of a PASylated Fab fragment (89Zr∙Df-HER2-Fab-PAS200) for the molecular imaging of tumor-related HER2 expression.
Methods
A patient with HER2-positive metastatic breast cancer received 37 MBq of 89Zr∙Df-HER2-Fab-PAS200 at a total mass dose of 70 μg. PET/CT was carried out 6, 24, and 45 h after injection, followed by image analysis of biodistribution, normal organ uptake, and lesion targeting.
Results
Images show a biodistribution typical for protein tracers, characterized by a prominent blood pool 6 h p.i., which decreased over time. Lesions were detectable as early as 24 h p.i. 89Zr∙Df-HER2-Fab-PAS200 was tolerated well.
Conclusion
This study demonstrates that a PASylated Fab tracer shows appropriate blood clearance to allow sensitive visualization of small tumor lesions in a clinical setting.
Keywords: Breast cancer (BCa), Fab fragment, Human epidermal growth factor receptor 2 (HER2), Imaging, PASylation, 89Zr
Introduction
Human epidermal growth factor receptor 2 (HER2) is a cell membrane receptor tyrosine kinase that plays a key role in cell development, proliferation, and differentiation [1]. Moreover, HER2 is overexpressed in a variety of cancers, including bladder; lung; gastric; ovarian; prostate; and, in particular, breast cancer (BCa) [2]. Overexpression of HER2 on tumor cells is associated with a high rate of proliferation and aggressive disease, poor prognosis, and short overall survival [3]. Notably, HER2-targeted therapies with monoclonal antibodies (mAbs) such as trastuzumab (Herceptin; Genentech, South San Francisco, CA) have significantly improved survival for up to 20% of patients suffering from BCa [4].
Precise determination of HER2 expression is the basis for success of HER2-targeted therapy. Routinely, HER2 status in BCa is determined on tissue biopsies either via immunohistochemistry (IHC) or by fluorescence in situ hybridization (FISH). However, both methods may be inaccurate in up to 20% of cases [5]. Also, due to the small size and limited number of tissue samples, tumor heterogeneity poses a challenge [2].
Molecular imaging using specific radiopharmaceuticals that target HER2 could offer a non-invasive option for better quantification and localization of HER2 overexpression and, thus, identify patients who may benefit from HER2-directed therapy. Several imaging tracers targeting HER2 have been reported, including radiolabeled mAbs, antibody fragments (Fab or F(ab)2), nanobodies, or affibodies [6]. One of the most well studied radiotracers for HER2 imaging is 89Zr-labeled trastuzumab, which allowed visualization and quantification of uptake in HER2-positive lesions for patients with metastatic BCa and other cancers in several clinical trials [7–10]. However, due to the large molecular size (150 kDa) and the intrinsically slow blood clearance of the full-length antibody, optimal detection of lesions is seen only 4–5 days after injection and accompanied by a considerable dose exposure to healthy organs, which is several-fold higher than for PET scans with 18F-fluorodeoxyglucose [11]. Furthermore, uptake of 89Zr-labeled trastuzumab has been found to be false-positive, i.e., to occur in the absence of clinically relevant HER2 expression, in a significant fraction of patients [8].
Generally, Fab fragments (48 kDa) offer rapid clearance and thereby better tumor contrast at early imaging time points. However, the fast elimination from the blood stream can also limit tumor uptake of such antibody fragments. In a preclinical study, we systematically examined the impact of plasma half-life of modified Fab fragments in a HER2-positive breast cancer model [12]. Tailoring of plasma half-life was conveniently achieved using the PASylation technology [12–14]. To this end, conformationally disordered 100–600 residue chains consisting of Pro, Ala, and Ser (PAS100–600) were genetically fused to the C-terminus of the light chain of the trastuzumab Fab (Fig. 1) and compared with the unmodified Fab in positron emission tomography (PET) and biodistribution experiments. In this preceding study, the radiotracer 89Zr∙Df-HER2-Fab-PAS200 revealed an optimal PET imaging contrast 24 h post injection (p.i.) in mice and, thus, appeared promising for clinical translation [15]. Here we report the first in-human PET imaging with 89Zr∙Df-HER2-Fab-PAS200 of a HER2-positive patient suffering from metastatic BCa.
Fig. 1.
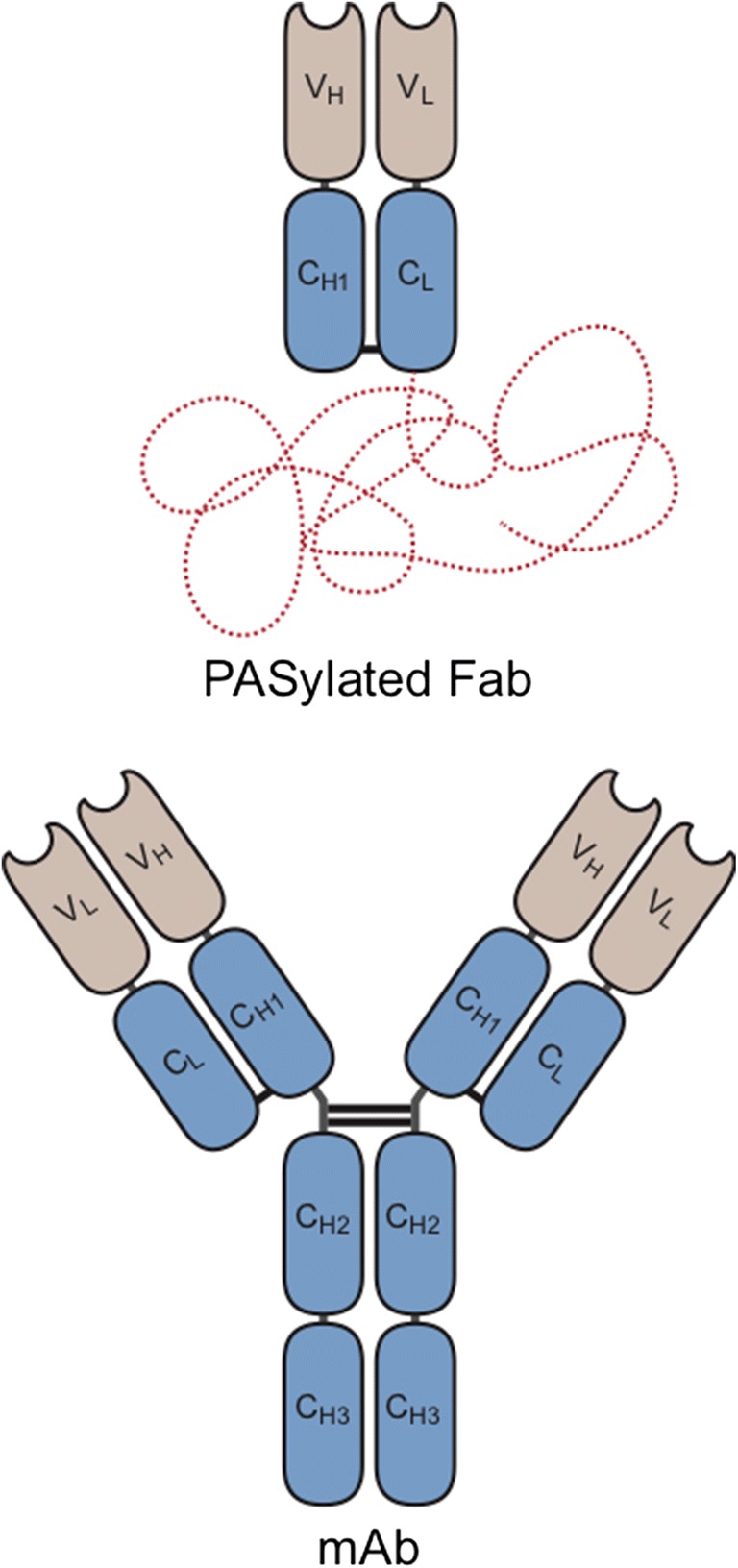
Structure of a PASylated Fab fragment in comparison with a full-size antibody
Materials and Methods
Production of HER2-Fab-PAS200
The HER2-Fab-PAS200 [12] was produced by bench top fermentation in E. coli as previously described [13]. Bacterial harvest and preparation of the periplasmic extract were performed under sterile conditions by crossflow filtration in a closed system, followed by chromatographic purification to homogeneity. Conjugation of p-SCN-Bn-Deferoxamine (Df; Macrocyclics, Plano, TX) was carried out according to a published protocol [15] following good manufacturing practice guidelines. On average, 2–3 Df chelates were coupled per Fab molecule as assessed by ESI-TOF mass spectrometry.
89Zr-Labeling, Formulation, and Quality Control
Radiolabeling of Df-HER2-Fab-PAS200 was performed according to a published procedure [15] using 89Zr as supplied by Perkin Elmer (Boston, MA). Briefly, 93 MBq of 89Zr in oxalic acid were neutralized with Na2CO3 and incubated with 260 μg of the purified Df-HER2-Fab-PAS200 in HEPES/NaOH buffer (pH 7.0) for 60 min at room temperature, followed by gel filtration on a PD-10 column (GE Healthcare, Munich, Germany). Radiolabeling efficiency was 92.4%, and the radiochemical purity was > 95%, as determined by instant thin-layer chromatography (TLC). Two milliliters of the isolated 89Zr∙Df-Her2-Fab-PAS200 was diluted with 9 ml sterile 0.9% saline and sterilized by filtration through a 0.2-μm Millex LG syringe filter (Merck Millipore, Darmstadt, Germany) under aseptic conditions (with only negligible amounts of radioactivity accumulating in the filter). The amount of protein was quantified by Bradford assay (Bio-Rad Laboratories, CA) using a dilution series of the unlabeled Df-HER2-Fab-PAS200 preparation as reference. As a further quality control, a radio-HPLC of a sample was performed, which revealed a single peak at the expected retention time. The final product (9.6 μg/ml) was documented to be sterile and free of particles at pH 7.0, and the bacterial endotoxin content was < 0.5 EU/ml.
For the toxicity study, Df-HER2-Fab-PAS200 was charged with non-radioactive zirconium (natZr) using the same protocol as for the radioisotope. The product was analyzed by ESI-TOF mass spectrometry, revealing successful complexation of 1–3 natZr ions per protein molecule.
Single-Dose Toxicity Study
To obtain information on the general toxicity of the PASylated Fab fragment, we performed a single-dose toxicity study in female CD1-Foxn1nu mice (7 months age, average weight 38.9 ± 5 g). Based on our preclinical findings [12], a maximum dose of 100 μg injected protein (“microdose”) was assessed as a starting point for the first clinical application of 89Zr∙Df-HER2-Fab-PAS200, corresponding to 1.4 μg/kg body weight for a 70-kg patient. Application of the same total protein amount to these mice was equal to a > 1000-fold dose, in line with the “ICH guideline M3(R2) on non-clinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals.” Therefore, two groups of mice (n = 11) were injected once intravenously with 100 μg of Df-HER2-Fab-PAS200 charged with natZr. The first group was sacrificed 24 h, and the second group was sacrificed 14 days after treatment with natZr∙Df-Her2-Fab-PAS200. Six mice (three per group) treated with saline served as reference. All tissues and organs were examined histologically by two veterinary pathologists, and findings were reported according to the INHAND criteria of the Society of Toxicologic Pathology (STP) in line with the most recent recommendations. Hematology, clinical chemistry, and urinalysis as well as analyses of organs and blood samples were performed as described elsewhere [16]. The animal experiments were approved by local authorities (General Administration of Upper Bavaria; license 55.2-1-54-2532-46-12) and in compliance with regulatory and institutional guidelines.
Patient
89Zr∙Df-HER2-Fab-PAS200 imaging was offered to support individual therapy planning and to identify the primary tumor under the German Pharmaceuticals Act (“Arzneimittelgesetz”, AMG), Sect. 13.2b, and with notification of the General Administration of Upper Bavaria. The patient was a 67-year-old woman with newly diagnosed HER2-positive metastatic BCa. Metastatic BCa had been proven by biopsy of an enlarged axillary lymph node, but no definitive abnormal findings were seen in both breasts on mammography. On immunohistochemistry, the tumor tissue in the axillary lymph node was positive for HER2 (score 3 +). An MRI scan of the brain showed multiple enhancing lesions, consistent with metastases (Fig. 2). Thus, the tumor stage was cTx pN1 cM1. The patient was treated with whole brain radiation therapy in combination with dexamethasone (4 mg per day) prior to the 89Zr∙Df-HER2-Fab-PAS200 PET/CT scans.
Fig. 2.
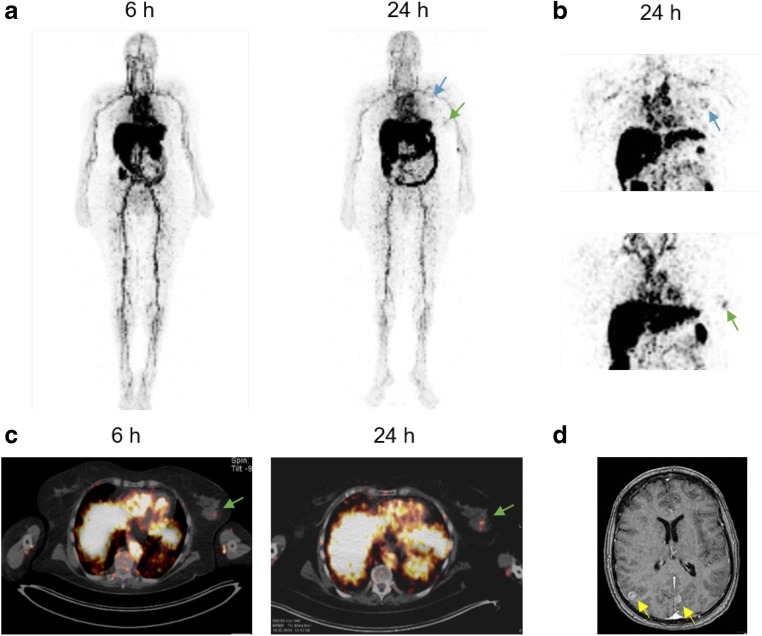
Biodistribution and lesion targeting of 89Zr∙Df-HER2-Fab-PAS200 in a mBCa patient. a Whole-body MIP images of 89Zr∙Df-HER2-Fab-PAS200. b Magnification of 89Zr∙Df-HER2-Fab-PAS200 accumulation in axillary lymph node metastases (blue arrow) and in the presumed primary tumor (green arrow) 24 h after injection. c Overlay of PET/CT images in the region of the presumed primary tumor in the left breast. d MRT scan of multiple brain metastases (yellow arrows)
89Zr∙Df-HER2-Fab-PAS200 Administration and PET Imaging
The radiolabeled Fab fragment (37 MBq, 70 μg) was administered intravenously over 1–2 min without premedication. The patient was monitored for at least 1 h after injection for any reactions or adverse events. During this period, the documentation of blood pressure and pulse did not show any relevant change.
Image Acquisition and Interpretation
Image acquisition was performed using a Biograph 128 PET/CT scanner (Siemens Molecular Imaging, Knoxville, TN). Whole-body PET/CT was conducted 6 and 24 h p.i. with 13 bed positions (5 min upper body, 2.5 min lower extremities). Images of the thorax and epigastrium were acquired 45 h p.i. with 2 bed positions. For quantitative evaluation, mean standardized uptake values (SUV) were calculated from attenuation corrected image data using the average intensity of the respective tissue as listed in Table 1.
Table 1.
Mean standardized uptake values (SUV) of 89Zr∙Df-HER2-Fab-PAS200 at different time points
| Organ | 6 h p.i. | 24 h p.i. | 45 h p.i. |
|---|---|---|---|
| Blood pool | 7 | 4.5 | 2.5 |
| Muscle | 0.5 | 0.6 | 0.02 |
| Bone | 0.8 | 0.6 | 1.1 |
| Liver | 10.4 | 8.3 | 7.9 |
| Kidney | 10.6 | 19.0 | 20.0 |
| Spleen | 3.7 | 6.2 | 3.8 |
| Lymph node filiae | |||
| SUVmax | 3.1 | 5.4 | 4.8 |
| SUVmean | 0.6 | 0.7 | 0.7 |
| Primarius | |||
| SUVmax | 2.8 | 4.2 | 3.8 |
| SUVmean | 0.4 | 0.7 | 0.6 |
Results
Single-Dose Toxicity Study
Non-toxicity of Df-HER2-Fab-PAS200 charged with natZr was substantiated in female mice prior to human application at > 1000-fold the clinical dose (on a mg/kg basis). No relevant histopathological findings for the main organs were detected after 24 h up to 14 days and natZr∙Df-HER2-Fab-PAS200 was well tolerated.
Biodistribution and Normal Organ Uptake
The mBCa patient underwent whole-body PET/CT imaging 6 and 24 h post injection (p.i.) with 89Zr∙Df-HER2-Fab-PAS200 (70 μg, 37 MBq). Additionally, PET images of the upper body were acquired 45 h p.i. Images showed a biodistribution typical for protein tracers, characterized by a prominent blood pool 6 h p.i., which decreased over time (Fig. 2 and Table 1). Increased uptake in the liver and kidneys was observed 24 h p.i., which—in case of the liver—decreased thereafter. Activity in the gastrointestinal tract was also prominent 24 h and 45 h p.i., suggesting hepatobiliary clearance. No significant activity was seen in the bladder, and no significant urinary excretion was noted. The 89Zr∙Df-HER2-Fab-PAS200 uptake in other non-tumor tissues (i.e., lung, muscle, bone) was low.
Lesion Targeting
Lesions were detectable as early as 24 h p.i. of 89Zr∙Df-HER2-Fab-PAS200 (Fig. 2). The cranial lymph node metastasis in the left axillary, earlier diagnosed by CT scan, was clearly visualized by PET imaging with this novel tracer (maximum standardized uptake value (SUVmax) = 5.4). Notably, a single lesion was also detectable in an area of dense parenchyma in the left breast, with an SUVmax of 4.2 at 24 h p.i., which was indicative of the putative primary tumor that had remained elusive at this time.
Of note, no accumulation of the protein tracer was visible in the known brain metastases. Furthermore, the second axillar lymph node revealed a prominent area of central necrosis with no uptake of 89Zr∙Df-HER2-Fab-PAS200.
Discussion
Molecular imaging should help to guide physicians to tailor individual treatment of patients and to monitor the therapeutic response. To this end, biodistribution and lesion targeting of 89Zr∙Df-HER2-Fab-PAS200 was evaluated in a patient with HER2-positive mBCa. Biodistribution of 89Zr∙Df-HER2-Fab-PAS200 was in accordance with expectation from preclinical studies in mice [12, 15]. Due to the genetic fusion of the Fab with the conformationally disordered PAS200 polypeptide and the resulting increased hydrodynamic volume, with an enlarged apparent MW ≈ 165 kDa, 89Zr∙Df-HER2-Fab-PAS200 showed moderately prolonged blood circulation compared with the unmodified Fab [12]. Similarly, a delayed blood clearance of 89Zr∙Df-HER2-Fab-PAS200 was observed in the patient (Fig. 2; cf. time points 6 and 24 h p.i.), confirming that PASylation is also effective in humans.
In the preclinical evaluation of 89Zr∙Df-HER2-Fab-PAS200, high tumor-to-background ratios were observed 24 h p.i. (tumor-to-blood, 3.4; tumor-to-muscle, 20) with even higher values at 48 h p.i. [15]. In the patient, blood pool activity was prominent 6 h p.i. and still seen at 24 and 45 h p.i. On the other hand, signals for the lesions became more evident 24 h p.i. compared with 6 h p.i., indicating ongoing accumulation in the tumor tissue. This is in agreement with the finding from our preceding preclinical study that a moderately prolonged plasma half-life can improve tumor uptake of a Fab-size protein tracer [12]. Furthermore, our present observations indicate a longer plasma half-life of the PASylated Fab in humans than in mice. Indeed, slower clearance rates of therapeutic antibodies in humans compared with mice have been reported, in line with the rules of allometric scaling [17]. An 111In-labeled Fab fragment of trastuzumab was previously investigated in a phase I trial of intraoperative detection of tumor margins in patients with HER2-positive carcinoma, revealing a terminal plasma half-life of 20.7 h [18]. However, it was not feasible to reliably detect the margins of disease in those patients due to the low uptake of the 111In∙DTPA-trastuzumab Fab in the tumor.
In contrast to the signal for the presumed primary tumor and the metastasis in the axillary lymph node seen with 89Zr∙Df-HER2-Fab-PAS200, no accumulation of radioactivity was visible in the diagnosed brain metastases. Of note, even large molecules (e.g., T-DM1, 89Zr-trastuzumab) were reported to penetrate HER2-positive breast cancer brain metastases [7, 19] due to local disruption of the blood-brain barrier at these sites [20]. Possibly, in the present case, the blood-brain barrier was stabilized by the pretreatment with dexamethasone during whole brain irradiation prior to the application of 89Zr∙Df-HER2-Fab-PAS200 [21].
Conclusions
Based on these first results, 89Zr∙Df-HER2-Fab-PAS200 has the potential to serve as a novel imaging agent to support individual therapy planning of HER2-positive BCa. PET imaging with 89Zr∙Df-HER2-Fab-PAS200 was feasible and tolerated well. This study indicates that PASylation technology is effective in a human patient and leads to delayed blood clearance of a radiolabeled anti-HER2 Fab fragment and sensitive tumor accumulation. However, the pharmacokinetics of 89Zr∙Df-HER2-Fab-PAS200 was longer than expected and may be further optimized, e.g., by use of a shorter PAS polypeptide or, possibly, by co-injection of trastuzumab in order to scavenge circulating shedded HER2 ectodomain and to effect more rapid clearance of immune complexes [22].
Acknowledgments
The authors wish to thank XL-protein GmbH for providing the PAS gene cassette and Uli Binder for critically reading the manuscript. Excellent technical assistance by Klaus Wachinger and Olga Seelbach, both at TUM, is gratefully acknowledged. We also thank Gabriel Buschner (MRI) for his helpful support.
Funding Information
Open Access funding provided by Projekt DEAL.
Compliance with Ethical Standards
Conflict of Interest
Martin Schlapschy and Arne Skerra are cofounders and shareholders of XL-protein GmbH, Germany. Wolfgang Weber is on advisory boards and receives compensation from Bayer, Blue Earth Diagnostics, Endocyte, and Pentixapharm and has received research support from BMS, Imaginab, Ipsen, and Piramal. Antonia Richter, Karina Knorr, Stephanie Robu, Volker Morath, Claudia Mendler, His-Yu Yen, Katja Steiger, Marion Kiechle, and Markus Schwaiger, declare that they have no conflict of interest. This work was partly funded by the Deutsche Forschungsgemeinschaft, Germany, in frame of the Collaborative Research Center 824.
Ethical Approval
In vivo imaging was offered to support individual therapy planning based on a clinical indication in compliance with the updated Declaration of Helsinki, § 37 “Unproven Interventions in Clinical Practice”, The German Medicinal Products Act (Arzneimittelgesetz) AMG §13 2b, and in accordance with the responsible regulatory authority (General Administration of Upper Bavaria, Germany).
Informed Consent
Informed consent regarding the experimental nature of 89Zr∙Df-HER2-Fab-PAS200 as an individual treatment concept was obtained from the patient.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Antonia Richter and Karina Knorr contributed equally to this work.
Contributor Information
Antonia Richter, Email: antonia.richter@tum.de.
Arne Skerra, Email: skerra@tum.de.
References
- 1.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebhart G, Flamen P, De Vries EG, Jhaveri K, Wimana Z. Imaging diagnostic and therapeutic targets: human epidermal growth factor receptor 2. J Nucl Med. 2016;57(Suppl 1):81S–88S. doi: 10.2967/jnumed.115.157941. [DOI] [PubMed] [Google Scholar]
- 3.Asif HM, Sultana S, Ahmed S, Akhtar N, Tariq M. HER-2 positive breast cancer–a mini-review. Asian Pac J Cancer Prev. 2016;17:1609–15. doi: 10.7314/APJCP.2016.17.4.1609. [DOI] [PubMed] [Google Scholar]
- 4.Gu G, Dustin D, Fuqua SA. Targeted therapy for breast cancer and molecular mechanisms of resistance to treatment. Curr Opin Pharmacol. 2016;31:97–103. doi: 10.1016/j.coph.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Phillips KA, Marshall DA, Haas JS, Elkin EB, Liang SY, Hassett MJ, et al. Clinical practice patterns and cost effectiveness of human epidermal growth receptor 2 testing strategies in breast cancer patients. Cancer. 2009;115:5166–5174. doi: 10.1002/cncr.24574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massicano AVF, Marquez-Nostra BV, Lapi SE. Targeting HER2 in nuclear medicine for imaging and therapy. Mol Imaging. 2018;17:1536012117745386. doi: 10.1177/1536012117745386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 8.Ulaner GA, Hyman DM, Ross DS, Corben A, Chandarlapaty S, Goldfarb S, et al. Detection of HER2-positive metastases in patients with HER2-negative primary breast cancer using 89Zr-trastuzumab PET/CT. J Nucl Med. 2016;57:1523–1528. doi: 10.2967/jnumed.115.172031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebhart G, Lamberts LE, Wimana Z, Garcia C, Emonts P, Ameye L, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27:619–624. doi: 10.1093/annonc/mdv577. [DOI] [PubMed] [Google Scholar]
- 10.O’Donoghue JA, Lewis JS, Pandit-Taskar N, Fleming SE, Schoder H, Larson SM, et al. Pharmacokinetics, biodistribution, and radiation dosimetry for 89Zr-trastuzumab in patients with esophagogastric cancer. J Nucl Med. 2018;59:161–166. doi: 10.2967/jnumed.117.194555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laforest R, Lapi SE, Oyama R, Bose R, Tabchy A, Marquez-Nostra BV, et al. [89Zr]Trastuzumab: evaluation of radiation dosimetry, safety, and optimal imaging parameters in women with HER2-positive breast cancer. Mol Imaging Biol. 2016;18:952–959. doi: 10.1007/s11307-016-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendler CT, Friedrich L, Laitinen I, Schlapschy M, Schwaiger M, Wester HJ, et al. High contrast tumor imaging with radio-labeled antibody Fab fragments tailored for optimized pharmacokinetics via PASylation. MAbs. 2015;7:96–109. doi: 10.4161/19420862.2014.985522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlapschy M, Binder U, Börger C, Theobald I, Wachinger K, Kisling S, et al. PASylation: a biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Eng Des Sel. 2013;26:489–501. doi: 10.1093/protein/gzt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebauer M, Skerra A. Prospects of PASylation® for the design of protein and peptide therapeutics with extended half-life and enhanced action. Bioorg Med Chem. 2018;26:2882–7. doi: 10.1016/j.bmc.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Mendler CT, Gehring T, Wester HJ, Schwaiger M, Skerra A. 89Zr-labeled versus 124I-labeled αHER2 Fab with optimized plasma half-life for high-contrast tumor imaging in vivo. J Nucl Med. 2015;56:1112–1118. doi: 10.2967/jnumed.114.149690. [DOI] [PubMed] [Google Scholar]
- 16.Düwel S, Hundshammer C, Gersch M, Feuerecker B, Steiger K, Buck A, et al. Imaging of pH in vivo using hyperpolarized 13C-labelled zymonic acid. Nat Commun. 2017;8:15126. doi: 10.1038/ncomms15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs. 2011;3:61–66. doi: 10.4161/mabs.3.1.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holloway CM, Scollard DA, Caldwell CB, Ehrlich L, Kahn HJ, Reilly RM. Phase I trial of intraoperative detection of tumor margins in patients with HER2-positive carcinoma of the breast following administration of 111In-DTPA-trastuzumab Fab fragments. Nucl Med Biol. 2013;40:630–637. doi: 10.1016/j.nucmedbio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Bartsch R, Berghoff AS, Vogl U, Rudas M, Bergen E, Dubsky P, et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin Exp Metastasis. 2015;32:729–737. doi: 10.1007/s10585-015-9740-3. [DOI] [PubMed] [Google Scholar]
- 20.Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 21.Rosa L, Galant LS, Dall’Igna DM, Kolling J, Siebert C, Schuck PF, et al. Cerebral oedema, blood-brain barrier breakdown and the decrease in Na+,K+-ATPase activity in the cerebral cortex and hippocampus are prevented by dexamethasone in an animal model of maple syrup urine disease. Mol Neurobiol. 2016;53:3714–3723. doi: 10.1007/s12035-015-9313-0. [DOI] [PubMed] [Google Scholar]
- 22.Bruno R, Washington CB, Lu JF, Lieberman G, Banken L, Klein P. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol. 2005;56:361–369. doi: 10.1007/s00280-005-1026-z. [DOI] [PubMed] [Google Scholar]