Abstract
Background: Plasmodium tryptophan-rich (TR) proteins have been proposed as potential vaccine candidate antigens. Among them, P. vivax tryptophan-rich antigens (PvTR-Ags), which have positionally conserved tryptophan residues in a TR domain, are highly antigenic in humans. Several of these antigens, including PvTRAg-26, have exhibited erythrocyte-binding activities.
Methods: Subclasses of IgG antibodies against PvTRAg-26 were detected by enzyme-linked immunosorbent assay in 35 P. vivax infected patients and mice immunized with the recombinant antigen to characterize its antigenicity and immunogenicity. Moreover, the antigen-specific immune responses and Th1/Th2-type cytokine patterns of splenocytes from the immunized animals were determined in vitro. The subcellular localization of PvTRAg-26 in ring-stage parasites was also detected by indirect immunofluorescence assay.
Results: The IgG1 and IgG3 levels in P. vivax-infected patients were significantly higher than those in uninfected individuals. In the PvTRAg-26-immunized mice, elevated levels of antigen-specific IgG antibodies were observed, dominated by the IgG1 subclass, and Th1-type cytokines were remarkably increased compared with Th2-type cytokines. Additionally, the subcellular location of the PvTRAg-26 protein was closely associated with the caveola-vesicle complex on the infected-erythrocyte membrane in the early ring stage of P. vivax.
Conclusions: PvTRAg-26, a P. vivax TR antigen, with high antigenicity and immunogenicity, induces Th1-cytokine response and increases production of IgG1 antibodies. This immune profiling study provided a substantial evidence that PvTRAg-26 may be a potential candidate for P. vivax vaccine development.
Keywords: malaria, Plasmodium vivax, tryptophan-rich antigens, immunogenicity, vaccine candidate
Introduction
Plasmodium vivax is the predominant malaria parasite epidemic in Asian and South American countries, which affects millions of people each year (1). In most cases, the parasite causes benign malaria. However, it may give rise to a severe, even fatal infection (2–4). It has been well established that malaria parasites have presented relative resistance to commonly used anti-malarial drugs. Thus the identification of novel anti-malarial drugs and the development of vaccines are urgently needed for effective control of the disease.
In a long time, development of P. vivax vaccines has been hindered by the absence of a continuous in vitro culture system and low-level parasitemia of patients (5). Therefore, the majority of P. vivax vaccine studies are focused on orthologous antigens of P. falciparum; circumsporozoite surface proteins (CSPs), thrombospondin-related adhesive protein (TRAP) of the pre-erythrocyte stage, apical membrane antigen-1 (AMA-1), Duffy-binding protein (DBP), rhoptry-associated proteins, merozoite surface proteins of the erythrocyte stage, and Pvs25 and Pvs28 from sexual stage of the parasite (6–10). Among them, only three antigens of CSPs, TRAP, and Pvs25 of P. vivax have been extensively investigated in clinical vaccine trials (11). However, the novel practical vaccine molecules of P.vivax remain undiscovered.
Plasmodium tryptophan-rich antigens (TR-Ags) have been proposed as a group of potential vaccine candidates. The TR-Ags were first identified in the murine malaria parasite of P. yoelii. Mice immunized with the recombinant TR-Ags produced highly protective immunity against P. yoelii infection (12, 13). Similarly, TR-Ags of P. falciparum could inhibit the invasion of erythrocytes by its merozoites (14). The genome of P. vivax encodes more TR-Ags than that of any other Plasmodium species. So far, fifteen TR-Ags have been found to be able to evoke significant cellular and humoral immune responses in P. vivax-exposed individuals (15). A recent study showed that TR-Ags could bind to normal human erythrocytes and the process could be inhibited by the sera of malaria patients (16). Our previous research also revealed that the conserved TR motifs exist in most PvTR-Ags which have high antigenicity in P. vivax infection, even in patients from low-endemic regions. We recently demonstrated that there are five proteins that are associated with the caveola-vesicle complex (CVC) structure, a unique structure of P. vivax-infected erythrocytes (17).
Among the five PvTR-Ags, PvTRAg-26 is an erythrocyte-binding protein (16). Although the antigenicity of PvTRAg-26 was partially tested in the previous study in P. vivax patients, the nature of the IgG subclass response to PvTRAg-26 in patients and the immunogenicity of PvTRAg-26 remain unclarified either in vitro cell experiments or in vivo animal experiments. Moreover, the membrane-associated subcellular localization needs to be investigated. In the present study, we tested the antigenicity and immunogenicity of PvTRAg-26 in the serum samples collected from symptomatic P. vivax patients as well as PvTRAg-26 immunized mice. Total IgG antibody and its subclasses were detected in the blood and the antigen-specific immune response and Th1/Th2-type cytokines of splenocytes were measured. Additionally, the subcellular localization of the PvTRAg-26 antigen on the membrane of P. vivax-infected erythrocytes was also performed.
Materials and Methods
Human Serum Samples
Serum samples of 35 malaria patients were collected in the hospitals of Bengbu and Hefei, Anhui province of China, all of them showing positive P. vivax parasite by microscopy. Simultaneously, fifteen serum samples of the individuals from malaria non-endemic areas were taken as control. The positive or negative sera were confirmed by both microscopy and nested PCR methods (18).
Expression and Purification of Recombinant PvTRAg-26
Genomic DNAs were prepared from P. vivax isolates and used as templates for PCR amplifications. PvTRAg-26 coding genes were amplified with primers of PvTRAg-26-F (5′-CCTTCACTTATAGATAAGTACGATGCT-3′) and PvTRAg-26-R (5′-TTATATTTTTGAATTCTTCCACTGAATCC-3′) and inserted into pET-28a (+)-His vector (Sango Biotech, Shanghai, China). The inserted DNA fragments were sequenced on an ABI 3730 X 1 DNA Analyzer (Applied Biosystems, Foster City, CA, USA) by Sango Biotech Co. Ltd. Purified plasmid DNAs were prepared with a TIANprep Mini Plasmid Kit (TIANGEN, Beijing, China). The recombinant protein was affinity-purified by using a Ni-Sepharose column (Sango Biotech) as described previously (17). Recombinant PvTRAg-26 was then denatured with β-mercaptoethanol in sample buffer and analyzed by 13% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting assay with an anti-His tag antibody (Qiagen, Hilden, Germany).
Animal Immunization With Recombinant PvTRAg-26
Female BALB/c mice, 6-8 weeks old, were purchased from Vital River Laboratory Animal Technology Co, Ltd (Beijing, China). The mice were treated following the Guidelines for the Care and Use of Research Animals established by Anhui Medical University. Two groups of mice, 5 in each, were immunized subcutaneously (SC) with 50 μg of PvTRAg-26 in phosphate-buffered saline (PBS) or Freund's complete adjuvant (Sigma-Aldrich, San Francisco, CA, USA), for four times in a 3-wk interval. Boost injections were given after 3, 6, and 9 weeks of the priming with the same amount of antigen together with Freund's incomplete adjuvant (Sigma-Aldrich). The mouse sera were collected 2 weeks after the final boost and antibodies against PvTRAg-26 were measured as described previously (19).
Enzyme-Linked Immunosorbent Assay (ELISA)
To investigate the prevalence of IgG subclasses against PvTRAg-26, serum samples from 35 P. vivax-infected patients and 15 uninfected individuals were selected. The ELISA was performed following the manufacture's instructions. Briefly, 5 μg/mL of PvTRAg-26 in coating buffer (0.05 M NaHCO3, pH 9.6) was incubated in 96-well ELISA plates (Corning-Costar, Corning, NY, USA) overnight at 4°C. The plates were incubated with 5% skimmed milk in PBS/T (0.05% Tween-20) for 1 h at 37°C to block nonspecific binding sites, and then incubated with 100 μL of individual sera diluted 1:50 in PBS/T. For IgG subclasses, the plates were washed and then incubated with horseradish peroxidase (HRP)-conjugated anti-human IgG1, IgG2, IgG3, and IgG4 antibodies (ImmunoWay, Plano, TX, USA) in dilution of 1:2000 in PBS/T. Chromogenic reactions were developed and measured based on the previous description (19).
To identify and compare the levels of total and subclasses of IgG antibodies against PvTRAg-26 in immunized mice sera, we coated ELISA plates with the recombinant antigen (1.25 μg/mL). The plates were blocked and incubated with mouse sera (1:2000 dilution in PBS/T) at 37°C for 45 min. For total IgG antibody measurements, the plates were washed and then incubated with HRP-conjugated anti-mouse IgG (H + L) (Invitrogen, Waltham, MA, USA) at a 1:50,000 dilution at 37°C for 45 min, whereas for IgG subclasses the plates were incubated with HRP-conjugated anti-mouse IgG1 (Invitrogen, MA, USA), IgG2a (Invitrogen), IgG2b (Abcam, Cambridge, MA, USA), and IgG3 (Abcam) antibodies at 1:30,000, 1:1000, 1:2000, and 1:1000 dilutions, respectively. Chromogenic reactions were developed and determined as previously described (19).
Splenocyte Proliferation and Cytokine Assays
Spleens were removed from mice 2 weeks after the fourth immunization. Splenocytes obtained from PvTRAg-26 immunized or control mice were resuspended at concentrations of 5 × 106 cells/mL in complete RPMI 1640 supplemented with 10% FBS. One hundred microliters of the cell suspension, and 100 μL of PvTRAg-26 proteins were added to 96-well culture plates at final concentrations of 2.5, 5, 10, or 20 μg/mL, respectively. Concanavalin A (Con A; Sigma-Aldrich) or lipopolysaccharide (LPS; Sigma-Aldrich) at final concentrations of 5 μg/mL or 10 μg/mL were used as positive control and PBS, as negative control. After a 72 h culture (37°C and 5% CO2), 100 μL of supernatants per well was collected and stored at −20°C for cytokine assays. Viable cells were measured using a Cell Counting Kit-8 (CCK-8 or WST-8) assay following the commercial kit protocols. Cytokines of interferon (IFN)-γ, interleukin (IL)-2, IL-4, and IL-10 were examined in culture supernatants of immunized mice using BD Cytometric Bead Array (CBA) Flex Set kit (BD Biosciences, San Diego, CA, USA) according to the manufacturer's instructions. The results were obtained by flow cytometry (FACS Calibur, BD Biosciences, San Jose, CA, USA) and analyzed using Flow Cytometric Analysis Program (FCAP) array software (Soft Flow, Kedves, Hungary).
Indirect Immunofluorescence Assay (IFA)
IFA was performed with 4% paraformaldehyde-fixed parasites (17). Slides were incubated with the following primary antibodies: mouse anti-PvTRAg-26 sera (1:100) and rabbit anti-PvPHIST/CVC-8195 sera (1:100) or mouse anti-PvTRAg-26 sera (1:100) and rabbit anti-Band 3 antibody (1:200). After primary antibody reactions, the samples were then treated with secondary antibodies, Alexa Fluor 488-conjugated goat anti-mouse IgG (1:500, Invitrogen) or Alexa Fluor 568-conjugated goat anti-mouse IgG (1:500, Invitrogen), and 4, 6-diamidino-2-phenylindole (DAPI) (1:1000, Invitrogen) was used to stain the nuclei. The slides were then mounted with Prolong Gold anti-fade reagent (Invitrogen), and visualized with confocal laser-scanning microscopy (FV1000; Olympus, Tokyo, Japan) under oil immersion. Images were edited using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA, USA).
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Student's t-tests were used for comparing the difference between the means in each group. P < 0.05 was considered statistically significant.
Ethical Considerations
The protocols of the study were approved by and carried out following the recommendations of the Life Ethics Committee of Anhui Medical University (No. 20160118) and the Animal Ethics Committee of Anhui Medical University (LLSC20160161). All subjects gave their written informed consents as per the Declaration of Helsinki.
Results
Expression of Recombinant PvTRAg-26
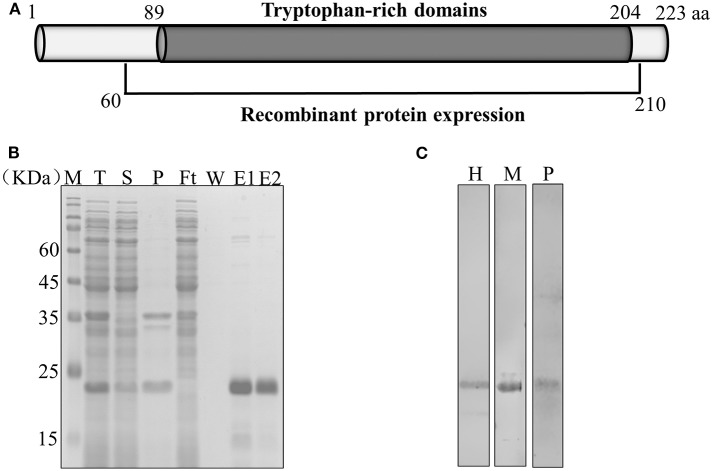
The complete PvTRAg-26 (PlasmoDB accession No. PVX_112660) protein sequence in the sal-1 strain consists of 223 amino acids (26 kDa), rich in tryptophan residues (5.8%). The entire exon-2 (450 bp, encoding the tryptophan-rich domain) of pvtrag-26 was amplified, cloned, and expressed in Escherichia coli (Figure 1A). The recombinant protein was purified under non-denaturing conditions, as shown in Figure 1B. The corresponding immunoblots were probed with an anti-His tag monoclonal antibody. Sera of P. vivax-infected patients and PvTRAg-26-immunized mice revealed a similar and specific migration pattern in PvTRAg-26 blotting (Figure 1C). Serum samples from uninfected individuals or normal mice were used as negative controls (data not shown).
Figure 1.
Schematic diagram showing the expression of PvTRAg-26. (A) Diagram of the gene structure of pvtrag-26; aa, amino acids. (B) Expression and purification of recombinant PvTRAg-26. T: total translation mix, S: supernatant, P: precipitate, Ft: flow through, W: wash; E: elution treated with reducing buffer. (C) Recombinant PvTRAg-26 protein under reducing conditions was probed with an anti-His tag antibody, P. vivax malaria patient serum and immune mouse serum. H: anti-His tag antibody, M: immune mouse serum, P: P. vivax-infected patients' pooled sera.
IgG Subclasses Recognizing PvTRAg-26 in Malaria Patients
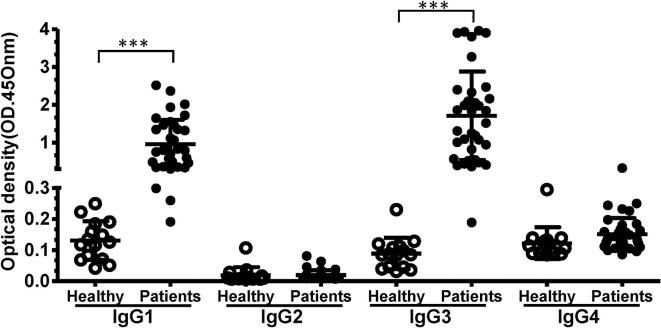
We evaluated the prevalence of each IgG subclass antibodies against PvTRAg-26. The results showed that the mean levels and the hierarchy of IgG subclasses were as follows: IgG3 > IgG1 > IgG4 > IgG2 (Figure 2). IgG1 and IgG3 subclasses were the predominant antibodies compared to the others (P < 0.0001). Titres of the cytophilic antibodies (IgG3 and IgG1) were significantly higher than the non-cytophilic antibodies (IgG2 and IgG4) (P < 0.05). The concentrations of IgG1 and IgG3 in P. vivax patients were markedly elevated in comparison with those of uninfected controls.
Figure 2.
The levels of IgG subclasses recognizing PvTRAg-26 in sera from P. vivax-infected patients and uninfected individuals. Differences between IgG subclass levels in the negative and positive groups were analyzed using Student's t-tests. ***P < 0.001. The P values for IgG1 and IgG3 were <0.001, and those for IgG2 or IgG4 were >0.05. P < 0.05 was considered to indicate a significant difference.
Anti-PvTRAg-26 IgG and Its Subclasses in the Sera of Immunized Mice
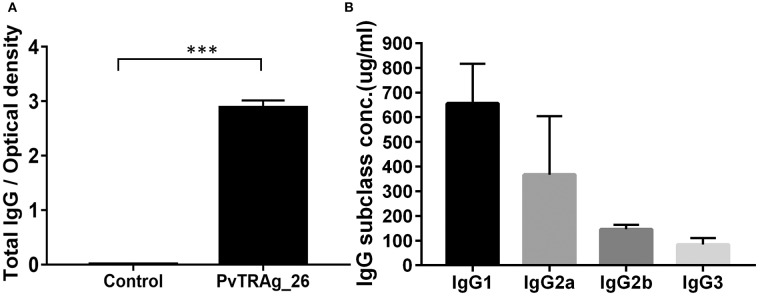
The serum levels of antigen-specific total IgG and its subclasses in response to PvTRAg-26 were determined. The level of total PvTRAg-26 specific IgG antibodies in antigen-immunized mice was notably increased in comparison with that in the control (P < 0.001) (Figure 3A). IgG1 subclass dominated compared to IgG2b and IgG3 in the immunized mice (Figure 3B).
Figure 3.
Levels of serum IgG and IgG subclasses in immunized mice. (A) Antigen-specific IgG levels were detected by ELISA in the sera of mice, as indicated after the final immunizations with PvTRAg-26, ***P < 0.001. (B) Serum IgG subclass pattern in PvTRAg-26-immunized mice, ***P < 0.001.
Antigen-Specific Immune Cell Responses and Cytokine Release
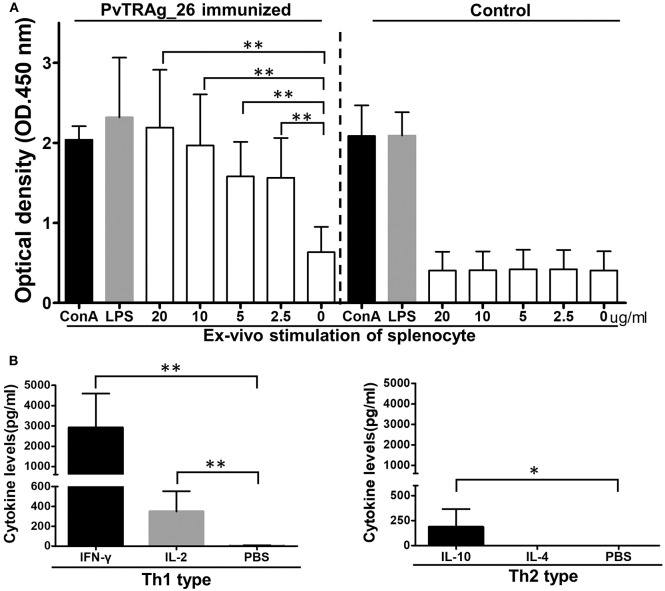
Splenocyte proliferation assays and CBAs were performed to assess the antigen-specific response and the secretion of cytokines in immunized BALB/c mice. Splenocytes from the animals immunized with PvTRAg-26 and controls were stimulated with various concentrations of the PvTRAg-26 antigen, Con A, or LPS for 72 h. The cultural supernatants were harvested for CBA and cell proliferation assay. The splenocyte proliferation in PvTRAg-26-immunized mice showed a notable proliferative response compared to those of the control group (P < 0.01, Figure 4A). Additionally, cytokine determinations demonstrated a biased Th1-type response, with an elevated level of IFN-γ and IL-2 secretions in the splenocytes of mice immunized with PvTRAg-26. By contrast, production of Th2-type cytokines of IL-4 and IL-10 were remarkably dampened (Figure 4B).
Figure 4.
Proliferation index and cytokine secretion of splenocytes from mice immunized with PvTRAg-26. (A) Splenocytes from mice immunized with or without PvTRAg-26 were stimulated with various concentrations of PvTRAg-26 for 72 h before testing, as indicated. The splenocytes were stimulated with Con A or LPS as positive controls, as indicated, *P < 0.05, **P < 0.01. (B) Cytokine secretion profile of the splenocytes from the antigen-immunized mice.
Association of PvTRAg-26 With CVC and the Erythrocyte Membrane in the Early Ring Stage
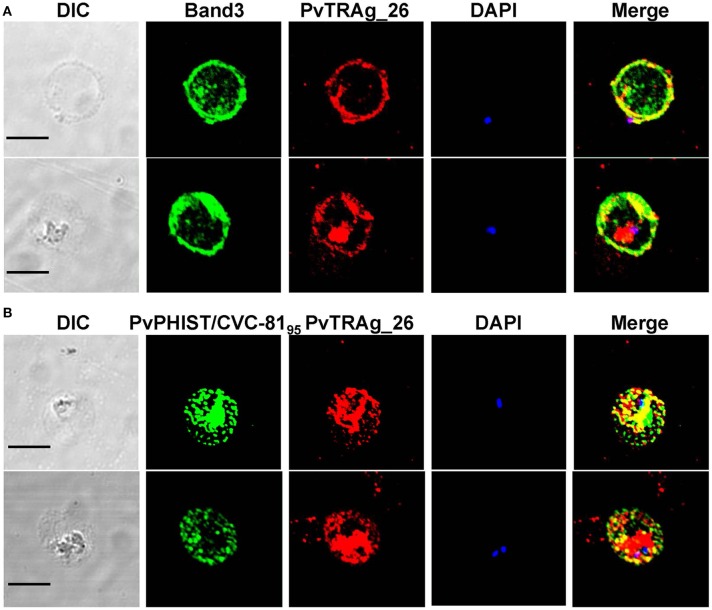
Immunofluorescent assay was conducted by using anti-Band 3 (an erythrocyte membrane marker) and anti-PvPHIST/CVC-8195 (a CVC marker) sera. In the early ring or trophozoite stage of the parasite, PvTRAg-26 signals were merged (at least partially) with Band 3 and PvPHIST/CVC-8195. Specific fluorescence was visualized on the parasitophorous vacuolar membrane (PVM) (Figure 5) but the pre-immunized mouse sera did not show any signals (data not shown).
Figure 5.
Localization of PvTRAg-26 in the early ring/trophozoite stage. (A) Ring stage parasites were double-labeled with mouse antisera against PvTRAg-26 (red) and a rabbit anti-Band 3 antibody (green). (B) Ring/trophozoite stage parasites were dual-labeled with mouse antisera against PvTRAg-26 (red) and rabbit antisera against PvCVC8195 (CVC marker, green). Nuclei were visualized with DAPI in merged images. The bar represents 5 μm.
Discussion
The TR-Ags of different Plasmodium species have been noted to have potential in malaria vaccine candidate screening (12, 16, 20–22) due to their parasite growth inhibition activity (23–25). P. vivax contains more abundant TR-Ags than any other human malaria parasites. Among them, PvTRAg-26, which contains positionally conserved tryptophan residues in a TR domain, could elicit a high level of protective IgG antibodies even in low malaria-endemic areas (17). Studies demonstrated that PvTRAg-26 possesses erythrocyte-binding ability (16). However, its immunogenic properties have not been fully explored. Here, we analyzed the cellular and humoral immune responses to PvTRAg-26 antigen in patients and immune mouse serum samples.
Antibodies play a crucial role in mediating acquired immunity to malaria during the intra-erythrocytic development stage of the parasites (26). The limited polymorphism of PvTR-Ags may contribute to their high immunogenicity. Our previous study exhibited that five PvTR-Ags, including PvTRAg-26, produced an elevated level of IgG antibodies in the sera of vivax patients (17). Definition of the IgG subclass response to PvTRAg-26 is important because the function of immune effectors varies in different subclasses (27, 28). Investigation of the antibody subclass response may provide further insight into the functions of antibodies and their roles in immune protection. Previous studies reported that cytophilic subclasses of IgG1 and IgG3 promote opsonic phagocytosis of merozoites or neutrophil-mediated killing, inducing a protection from malaria (29–31). Similarly, we noted that cytophilic antibody subclasses, IgG1 and IgG3, were predominant in host response to PvTRAg-26 antigen stimulation. Augmentation of humoral immune response mediated by IgG1 and IgG3 antibodies has been believed to play a pivotal role in reducing the risk of clinical malaria and parasitemia (32). During this process, complement activation mediated by IgG1 and IgG3 would be essential for inhibition of parasite invasion to host erythrocytes (33). Thus, further studies are needed to elucidate the functional activities of antibodies and their relationship with host protective immunity to vivax malaria.
Immunization with recombinant PvTRAg-26 resulted in a high level of IgG antibody response in mice, in which IgG1 subclass was predominant, followed by IgG2a. Similar results were also seen in the studies of mice with other malaria vaccine candidates, such as PvMSP119, PfMSP119, and PvMSP9 (34–36). The IgG1 and IgG3 antibodies are non-cytophilic and responsible for Th2-biased response (37), while the IgG2a and IgG2b are cytophilic and link to Th1 response in mice. Importantly, IgG2a antibodies in mice are considered to be most efficacious in complement activation and in activation of antibody-dependent cellular cytotoxic mechanism, in addition to ameliorating parasitemia caused by P. yoelii (31). We speculate that PvTRAg-26 antigen may induce a comprehensive Th1-Th2 protective response. Other studies have also shown the protective immunity in the presence of elevated levels of IgG1 and IgG2a with a combined Th1-Th2 immune responses to P. yoelii infection (38–44).
The mechanism of cellular immunity is closely associated with activation of phagocytes, antigen-specific cytotoxic T-lymphocytes and release of cytokines against infectious protozoan parasites (45). Cytokines are generally responsible for direct or indirect restriction of pathogenesis of infectious diseases (15, 46–48). It has been known that CD4+ T cells play a crucial role in protection against Plasmodium infection both in humans and in animals (49–51). The phenotype indicators of CD4+ T cells mainly include IFN-γ and IL-2 for Th1 response, and IL-4 and IL-10 for Th2 response (52, 53). Several studies demonstrated that PvTR-Ags elicit a combined Th1 and Th2 response in vivax malaria patients (51, 54). Here, we also observed a simultaneous up-regulation of cytokines of Th1 (IFN-γ and IL-2) and Th2 (IL-10) types, suggesting a systemic immune response of mice to PvTRAg-26 stimuation. The Th1 cytokines, e.g., IFN-γ, play an essential part in controlling malaria parasitemia during the early stages of infection (48, 55) and provide host with an effective protection from malaria (56, 57). IL-2 is a crucial T cell cytokine associated with proliferation, homeostasis, and differentiation of CD4+ and CD8+ T cells (58), and regulates the balance between effector Th1 cells and regulatory T cells in control of blood-stage malaria infection (59, 60). Contrarily, IL-10, a Th2 type cytokine, is known to be able to modulate the immune response to malaria parasites and to be involved in deterioration of parasitemia in Plasmodium infection (61, 62).
As visualized by IFA, PvTRAg-26 was transported from the parasite to the erythrocyte membrane through the CVC structure in the early ring stage. PvTRAg-26 was detectable on the PVM and its signal might be merged with Band 3 and CVC proteins. The function of the CVC largely remains unknown. It is hypothesized that the CVC may link to the transportation of materials from the parasite to the outside medium through the red blood cell cytoplasm (63–65). Similar to binding of PypAg-1/PypAg-3 to the membrane of red blood cells in rosette formation of P. yoelii (12), the co-localization of PvTRAg-26 with the CVC on the surface of infected erythrocytes suggests the transportation of PvTRAg-26 to the surface of the host cells, which may help promote the invasion process of P. vivax parasites. Further approaches in vivo are needed to determine the efficacy of PvTRAg-26 as a promising vaccine candidate.
Conclusions
PvTRAg-26 possesses high antigenicity and immunogenicity and can induce potent Th1 and Th2 responses in patients and immunized mice. The recombinant PvTRAg-26 antigen has the potential in development of a novel molecular vaccine for prevention of P. vivax infection.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Anhui Medical University (20160118), Anhui, China. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by the Animal Ethics Committee, Anhui Medical University (LLSC20160161).
Author Contributions
BW, YX, and E-TH conceived and designed the experiments. LF, JX, and MZ designed the research protocol and performed the experiments. LF, JS, HX, and QF performed data acquisition and analysis. LF, JX, BW, YX, JS, J-HH and E-TH contributed to the interpretation of results and assisted in writing the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Patchanee Chootong, and Mr. Obed Cudjoe and Mr. Paresh Vishwasrao for critical review of the manuscript.
Glossary
Abbreviations
- P. vivax
Plasmodium vivax
- TRAg
tryptophan-rich antigen
- CVC
caveola-vesicle complex
- PCR
polymerase chain reaction
- PBS
phosphate-buffered saline
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- ELISA
enzyme-linked immunosorbent assay
- Con A
concanavalin A
- LPS
lipopolysaccharide
- IFN-γ
interferon-γ
- IL
interleukin
- IFA
indirect immunofluorescence assay
- PHIST
helical interspersed subtelomeric
- PVM
parasitophorous vacuolar membrane.
Footnotes
Funding. This study was funded by the National Natural Science Foundation of China (No. 81601446) (BW), the Natural Science Foundation of Anhui Province (1708085QH210) (BW), the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (NRF-2017R1A2A2A05069562) (E-TH), (2015R1A4A1038666) (E-TH), and the Foundation of the Anhui Science and Technology Department (201904a07020049) (YX). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO World Malaria Report 2018. (2018). [Google Scholar]
- 2.Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A. Plasmodium vivax malaria. Emerg Infect Dis. (2005) 11:132–4. 10.3201/eid1101.040519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. (2009) 80:194–8. 10.4269/ajtmh.2009.80.194 [DOI] [PubMed] [Google Scholar]
- 4.Mehndiratta S, Rajeshwari K, Dubey AP. Multiple-organ dysfunction in a case of Plasmodium vivax malaria. J Vector Borne Dis. (2013) 50:71-3. [PubMed] [Google Scholar]
- 5.Pico de Coana Y, Rodriguez J, Guerrero E, Barrero C, Rodriguez R, Mendoza M, et al. A highly infective Plasmodium vivax strain adapted to Aotus monkeys: quantitative haematological and molecular determinations useful for P. vivax malaria vaccine development. Vaccine. (2003) 21:3930-7. 10.1016/S0264-410X(03)00278-0 [DOI] [PubMed] [Google Scholar]
- 6.Zakeri S, Abouie Mehrizi A, Djadid ND, Snounou G. Circumsporozoite protein gene diversity among temperate and tropical Plasmodium vivax isolates from Iran. Trop Med Int Health. (2006) 11:729–37. 10.1111/j.1365-3156.2006.01613.x [DOI] [PubMed] [Google Scholar]
- 7.Bueno LL, Fujiwara RT, Soares IS, Braga EM. Direct effect of Plasmodium vivax recombinant vaccine candidates AMA-1 and MSP-119 on the innate immune response. Vaccine. (2008) 26:1204–13. 10.1016/j.vaccine.2007.12.031 [DOI] [PubMed] [Google Scholar]
- 8.Tran TM, Oliveira-Ferreira J, Moreno A, Santos F, Yazdani SS, Chitnis CE, et al. Comparison of IgG reactivities to Plasmodium vivax merozoite invasion antigens in a Brazilian Amazon population. Am J Trop Med Hyg. (2005) 73:244–55. 10.4269/ajtmh.2005.73.244 [DOI] [PubMed] [Google Scholar]
- 9.Wickramarachchi T, Illeperuma RJ, Perera L, Bandara S, Holm I, Longacre S, et al. Comparison of naturally acquired antibody responses against the C-terminal processing products of Plasmodium vivax Merozoite Surface Protein-1 under low transmission and unstable malaria conditions in Sri Lanka. Int J Parasitol. (2007) 37:199–208. 10.1016/j.ijpara.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 10.Xainli J, Baisor M, Kastens W, Bockarie M, Adams JH, King CL. Age-dependent cellular immune responses to Plasmodium vivax Duffy binding protein in humans. J Immunol. (2002) 169:3200–7. 10.4049/jimmunol.169.6.3200 [DOI] [PubMed] [Google Scholar]
- 11.Mueller I, Shakri AR, Chitnis CE. Development of vaccines for Plasmodium vivax malaria. Vaccine. (2015) 33:7489–95. 10.1016/j.vaccine.2015.09.060 [DOI] [PubMed] [Google Scholar]
- 12.Burns JM, Adeeku EK, Belk CC, Dunn PD. An unusual tryptophan-rich domain characterizes two secreted antigens of Plasmodium yoelii-infected erythrocytes. Mol Biochem Parasitol. (2000) 110:11–21. 10.1016/S0166-6851(00)00252-8 [DOI] [PubMed] [Google Scholar]
- 13.Burns JM, Jr, Dunn PD, Russo DM. Protective immunity against Plasmodium yoelii malaria induced by immunization with particulate blood-stage antigens. Infect Immun. (1997) 65:3138–45. 10.1128/IAI.65.8.3138-3145.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ntumngia FB, Bouyou-Akotet MK, Uhlemann AC, Mordmuller B, Kremsner PG, Kun JF. Characterisation of a tryptophan-rich Plasmodium falciparum antigen associated with merozoites. Mol Biochem Parasitol. (2004) 137:349–53. 10.1016/j.molbiopara.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 15.Zeeshan M, Bora H, Sharma YD. Presence of memory T cells and naturally acquired antibodies in Plasmodium vivax malaria exposed individuals against a group of tryptophan-rich antigens with conserved sequences. J Infect Dis. (2013) 207:175–85. 10.1093/infdis/jis650 [DOI] [PubMed] [Google Scholar]
- 16.Zeeshan M, Tyagi RK, Tyagi K, Alam MS, Sharma YD. Host-parasite interaction: selective numbers of 'pv-fam-a' family proteins of Plasmodium vivax Bind to a restricted number of human erythrocyte receptors. J Infect Dis. (2015) 211:1111–20. 10.1093/infdis/jiu558 [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Lu F, Cheng Y, Chen JH, Jeon HY, Ha KS, et al. Immunoprofiling of the tryptophan-rich antigen family in Plasmodium vivax. Infect Immun. (2015) 83:3083–95. 10.1128/IAI.03067-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang B, Han SS, Cho C, Han JH, Cheng Y, Lee SK, et al. Comparison of microscopy, nested-PCR, and real-Time-PCR assays using high-throughput screening of pooled samples for diagnosis of malaria in asymptomatic carriers from areas of endemicity in Myanmar. J Clin Microbiol. (2014) 52:1838–45. 10.1128/JCM.03615-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y, Shin EH, Lu F, Wang B, Choe J, Tsuboi T, et al. Antigenicity studies in humans and immunogenicity studies in mice: an MSP1P subdomain as a candidate for malaria vaccine development. Microbes Infect. (2014) 16:419–28. 10.1016/j.micinf.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 20.Curtidor H, Ocampo M, Rodriguez LE, Lopez R, Garcia JE, Valbuena J, et al. Plasmodium falciparum TryThrA antigen synthetic peptides block in vitro merozoite invasion to erythrocytes. Biochem Biophys Res Commun. (2006) 339:888–96. 10.1016/j.bbrc.2005.11.089 [DOI] [PubMed] [Google Scholar]
- 21.Tyagi RK, Sharma YD. Erythrocyte binding activity displayed by a selective group of Plasmodium vivax tryptophan rich antigens is inhibited by patients' antibodies. PLoS ONE. (2012) 7:e50754. 10.1371/journal.pone.0050754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhlemann AC, Oguariri RM, McColl DJ, Coppel RL, Kremsner PG, Anders RF, et al. Properties of the Plasmodium falciparum homologue of a protective vaccine candidate of Plasmodium yoelii. Mol Biochem Parasitol. (2001) 118:41–8. 10.1016/S0166-6851(01)00370-X [DOI] [PubMed] [Google Scholar]
- 23.Alam MS, Choudhary V, Zeeshan M, Tyagi RK, Rathore S, Sharma YD. Interaction of Plasmodium vivax tryptophan-rich antigen PvTRAg38 with band 3 on human erythrocyte surface facilitates parasite growth. J Biol Chem. (2015) 290:20257–72. 10.1074/jbc.M115.644906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rathore S, Dass S, Kandari D, Kaur I, Gupta M, Sharma YD. Basigin Interacts with Plasmodium vivax tryptophan-rich antigen PvTRAg38 as a second erythrocyte receptor to promote parasite growth. J Biol Chem. (2017) 292:462–76. 10.1074/jbc.M116.744367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam MS, Zeeshan M, Mittra P, Choudhary V, Sharma YD. Receptor specific binding regions of Plasmodium vivax tryptophan rich antigens and parasite growth inhibition activity of PvTRAg35.2. Microbes Infect. (2016) 18:550–8. 10.1016/j.micinf.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 26.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. (1961) 192:733–7. 10.1038/192733a0 [DOI] [PubMed] [Google Scholar]
- 27.Wiener E, Atwal A, Thompson KM, Melamed MD, Gorick B, Hughes-Jones NC. Differences between the activities of human monoclonal IgG1 and IgG3 subclasses of anti-D(Rh) antibody in their ability to mediate red cell-binding to macrophages. Immunology. (1987) 62:401–4. [PMC free article] [PubMed] [Google Scholar]
- 28.Irani V, Guy AJ, Andrew D, Beeson JG, Ramsland PA, Richards JS. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol. (2015) 67(2 Pt A):171–82. 10.1016/j.molimm.2015.03.255 [DOI] [PubMed] [Google Scholar]
- 29.Hill DL, Eriksson EM, Li Wai Suen CS, Chiu CY, Ryg-Cornejo V, Robinson LJ, et al. Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS ONE. (2013) 8:e74627. 10.1371/journal.pone.0074627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osier FH, Mackinnon MJ, Crosnier C, Fegan G, Kamuyu G, Wanaguru M, et al. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med. (2014) 6:247ra102. 10.1126/scitranslmed.3008705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White WI, Evans CB, Taylor DW. Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with Plasmodium yoelii. Infect Immun. (1991) 59:3547–54. 10.1128/IAI.59.10.3547-3554.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. (1992) 60:1473–81. 10.1128/IAI.60.4.1473-1481.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. (2015) 42:580–90. 10.1016/j.immuni.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutta S, Ware LA, Barbosa A, Ockenhouse CF, Lanar DE. Purification, characterization, and immunogenicity of a disulfide cross-linked Plasmodium vivax vaccine candidate antigen, merozoite surface protein 1, expressed in Escherichia coli. Infect Immun. (2001) 69:5464–70. 10.1128/IAI.69.9.5464-5470.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Basagoudanavar SH, Gowda DC. Effect of GPI anchor moiety on the immunogenicity of DNA plasmids encoding the 19-kDa C-terminal portion of Plasmodium falciparum MSP-1. Parasite Immunol. (2008) 30:315–22. 10.1111/j.1365-3024.2008.01027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira-Ferreira J, Vargas-Serrato E, Barnwell JW, Moreno A, Galinski MR. Immunogenicity of Plasmodium vivax merozoite surface protein-9 recombinant proteins expressed in E. coli. Vaccine. (2004) 22:2023–30. 10.1016/j.vaccine.2003.07.021 [DOI] [PubMed] [Google Scholar]
- 37.Grey HM, Hirst JW, Cohn M. A new mouse immunoglobulin: IgG3. J Exp Med. (1971) 133:289–304. 10.1084/jem.133.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahlborg N, Ling IT, Holder AA, Riley EM. Linkage of exogenous T-cell epitopes to the 19-kilodalton region of Plasmodium yoelii merozoite surface protein 1 [MSP1(19)] can enhance protective immunity against malaria and modulate the immunoglobulin subclass response to MSP1(19). Infect Immun. (2000) 68:2102–9. 10.1128/IAI.68.4.2102-2109.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Near KA, Stowers AW, Jankovic D, Kaslow DC. Improved immunogenicity and efficacy of the recombinant 19-kilodalton merozoite surface protein 1 by the addition of oligodeoxynucleotide and aluminum hydroxide gel in a murine malaria vaccine model. Infect Immun. (2002) 70:692–701. 10.1128/IAI.70.2.692-701.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirunpetcharat C, Wipasa J, Sakkhachornphop S, Nitkumhan T, Zheng YZ, Pichyangkul S, et al. CpG oligodeoxynucleotide enhances immunity against blood-stage malaria infection in mice parenterally immunized with a yeast-expressed 19 kDa carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein-1 [MSP1(19)] formulated in oil-based Montanides. Vaccine. (2003) 21:2923–32. 10.1016/S0264-410X(03)00132-4 [DOI] [PubMed] [Google Scholar]
- 41.Tamborrini M, Stoffel SA, Westerfeld N, Amacker M, Theisen M, Zurbriggen R, et al. Immunogenicity of a virosomally-formulated Plasmodium falciparum GLURP-MSP3 chimeric protein-based malaria vaccine candidate in comparison to adjuvanted formulations. Malar J. (2011) 10:359. 10.1186/1475-2875-10-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dodoo D, Theisen M, Kurtzhals JA, Akanmori BD, Koram KA, Jepsen S, et al. Naturally acquired antibodies to the glutamate-rich protein are associated with protection against Plasmodium falciparum malaria. J Infect Dis. (2000) 181:1202–5. 10.1086/315341 [DOI] [PubMed] [Google Scholar]
- 43.Oeuvray C, Theisen M, Rogier C, Trape JF, Jepsen S, Druilhe P. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun. (2000) 68:2617–20. 10.1128/IAI.68.5.2617-2620.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hisaeda H, Saul A, Reece JJ, Kennedy MC, Long CA, Miller LH, et al. Merozoite surface protein 3 and protection against malaria in Aotus nancymai monkeys. J Infect Dis. (2002) 185:657–64. 10.1086/339187 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki Y. The immune system utilizes two distinct effector mechanisms of T cells depending on two different life cycle stages of a single pathogen, Toxoplasma gondii, to control its cerebral infection. Parasitol Int. (2019) 76:102030. 10.1016/j.parint.2019.102030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips S. Effector mechanisms against asexual erythrocytic stages of Plasmodium. Immunol Lett. (1994) 41:109–14. 10.1016/0165-2478(94)90117-1 [DOI] [PubMed] [Google Scholar]
- 47.Su Z, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect Immun. (2000) 68:4399–406. 10.1128/IAI.68.8.4399-4406.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angulo I, Fresno M. Cytokines in the pathogenesis of and protection against malaria. Clin Diagn Lab Immunol. (2002) 9:1145–52. 10.1128/CDLI.9.6.1145-1152.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonseca L, Seixas E, Butcher G, Langhorne J. Cytokine responses of CD4+ T cells during a Plasmodium chabaudi chabaudi (ER) blood-stage infection in mice initiated by the natural route of infection. Malar J. (2007) 6:77. 10.1186/1475-2875-6-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira GA, Kumar KA, Calvo-Calle JM, Othoro C, Altszuler D, Nussenzweig V, et al. Class II-restricted protective immunity induced by malaria sporozoites. Infect Immun. (2008) 76:1200–6. 10.1128/IAI.00566-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeeshan M, Tyagi K, Sharma YD. CD4+ T cell response correlates with naturally acquired antibodies against Plasmodium vivax tryptophan-rich antigens. Infect Immun. (2015) 83:2018–29. 10.1128/IAI.03095-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scholzen A, Mittag D, Rogerson SJ, Cooke BM, Plebanski M. Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog. (2009) 5:e1000543. 10.1371/journal.ppat.1000543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torre D, Speranza F, Giola M, Matteelli A, Tambini R, Biondi G. Role of Th1 and Th2 cytokines in immune response to uncomplicated Plasmodium falciparum malaria. Clin Diagn Lab Immunol. (2002) 9:348–51. 10.1128/CDLI.9.2.348-351.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mittra P, Singh N, Sharma YD. Plasmodium vivax: immunological properties of tryptophan-rich antigens PvTRAg 35.2 and PvTRAg 80.6. Microbes Infect. (2010) 12:1019–26. 10.1016/j.micinf.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 55.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. (2008) 9:725–32. 10.1038/ni.f.205 [DOI] [PubMed] [Google Scholar]
- 56.Patterson PS, Bosshardt SC, Udhayukumar V, Xiao L, Kidd M, Hunter RL, et al. Prolonged expression of IFNgamma induced by protective blood-stage immunization against Plasmodium yoelii malaria. Vaccine. (1999) 18:173–80. 10.1016/S0264-410X(99)00217-0 [DOI] [PubMed] [Google Scholar]
- 57.Coccia M, Collignon C, Herve C, Chalon A, Welsby I, Detienne S, et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFN gamma response promoting vaccine immunogenicity. Npj Vaccines. (2017) 2 10.1038/s41541-017-0027-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith KA. Interleukin-2: inception, impact, and implications. Science. (1988) 240:1169–76. 10.1126/science.3131876 [DOI] [PubMed] [Google Scholar]
- 59.Finney OC, Riley EM, Walther M. Regulatory T cells in malaria–friend or foe? Trends Immunol. (2010) 31:63–70. 10.1016/j.it.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 60.Scholzen A, Minigo G, Plebanski M. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol. (2010) 26:16–25. 10.1016/j.pt.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 61.Niikura M, Inoue S, Kobayashi F. Role of interleukin-10 in malaria: focusing on coinfection with lethal and nonlethal murine malaria parasites. J Biomed Biotech. (2011) 2011:383962. 10.1155/2011/383962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakamae S, Kimura D, Miyakoda M, Sukhbaatar O, Inoue SI, Yui K. Role of IL-10 in inhibiting protective immune responses against infection with heterologous Plasmodium parasites. Parasitol Int. (2019) 70:5–15. 10.1016/j.parint.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 63.Aikawa M, Miller LH, Rabbege J. Caveola–vesicle complexes in the plasmalemma of erythrocytes infected by Plasmodium vivax and P cynomolgi. Unique structures related to Schuffner's dots. Am J Pathol. (1975) 79:285–300. [PMC free article] [PubMed] [Google Scholar]
- 64.Aikawa M. Morphological changes in erythrocytes induced by malarial parasites. Biol Cell. (1988) 64:173–81. 10.1016/0248-4900(88)90077-9 [DOI] [PubMed] [Google Scholar]
- 65.Haldar K, Samuel BU, Mohandas N, Harrison T, Hiller NL. Transport mechanisms in Plasmodium-infected erythrocytes: lipid rafts and a tubovesicular network. Int J Parasitol. (2001) 31:1393–401. 10.1016/S0020-7519(01)00251-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.