Abstract
The coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 that has significant implications for the cardiovascular care of patients. First, those with COVID-19 and pre-existing cardiovascular disease have an increased risk of severe disease and death. Second, infection has been associated with multiple direct and indirect cardiovascular complications including acute myocardial injury, myocarditis, arrhythmias, and venous thromboembolism. Third, therapies under investigation for COVID-19 may have cardiovascular side effects. Fourth, the response to COVID-19 can compromise the rapid triage of non-COVID-19 patients with cardiovascular conditions. Finally, the provision of cardiovascular care may place health care workers in a position of vulnerability as they become hosts or vectors of virus transmission. We hereby review the peer-reviewed and pre-print reports pertaining to cardiovascular considerations related to COVID-19 and highlight gaps in knowledge that require further study pertinent to patients, health care workers, and health systems.
Key Words: cardiovascular therapy, coronavirus, health system
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; ARB, angiotensin receptor blocker; ARDS, acute respiratory distress syndrome; CI, confidence interval; COVID-19, coronavirus disease 2019; CV, cardiovascular; CVD, cardiovascular disease; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; MI, myocardial infarction; PPE, personal protective equipment; RNA, ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Central Illustration
Highlights
-
•
Patients with pre-existing CVD appear to have worse outcomes with COVID-19.
-
•
CV complications include biomarker elevations, myocarditis, heart failure, and venous thromboembolism, which may be exacerbated by delays in care.
-
•
Therapies under investigation for COVID-19 may have significant drug-drug interactions with CV medications.
-
•
Health care workers and health systems should take measures to ensure safety while providing high-quality care for COVID-19 patients.
First appearing in Wuhan, China, coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1,2). Given the rapid spread of this virus with consequences on an international scale, COVID-19 was declared a pandemic by the World Health Organization on March 11, 2020 (2). It is imperative that health care workers and researchers across all disciplines be aware of the potential impact that this disease can have on their respective fields and the medical community at large (3).
Based on currently observed disease patterns, cardiovascular (CV) specialists will be actively engaged in the care of patients with COVID-19. The infection may directly affect cardiovascular disease (CVD). Pre-existing CVD may predispose to COVID-19 infection. Those with CVD who are infected by the virus have an elevated risk of adverse outcomes, and infection itself is associated with CV complications (4, 5, 6). Moreover, COVID-19 infection may also have numerous indirect effects relevant to CV health. The large numbers of infected people requiring care may affect optimal treatment delivery to patients with acute CV conditions. Therapeutics for COVID-19 have the potential for adverse CV effects and clinicians delivering CV care are at risk of developing the illness or becoming vectors for the infection. The objective of this review is to characterize the CV impact of COVID-19, its potential consequences in patients with established CVD, as well as considerations for individual patients (with and without COVID-19), health care workers, and health systems, as understanding and addressing these issues will be crucial to optimize outcomes during the current critical period and beyond.
Methodologic Considerations
Given the time-sensitive nature of the challenges associated with this outbreak, we reviewed the published reports (including multiple search strategies in MEDLINE with PubMed interface) and critically assessed early reports on medRxiv, a pre-print server (date of last search: March 16, 2020). Because the initial epicenter for this outbreak was from China, the majority of data on patients with COVID-19 are from this region. Although a systematic attempt was made to include reports and viewpoints from other heavily affected countries, data related to CV risk factors or presentation were limited. This is important, because the testing strategies, care-seeking behavior, and hospitalization thresholds vary in different settings and can bias numerators and denominators, influencing estimates of the impact of the virus. This selection bias in testing, care, and reporting can lead to differences in prevalence estimates of pre-existing risk factors and patient presentation across the reports from various countries. Furthermore, the majority of the existing analyses, including those related to CV complications of COVID-19 are based on retrospective and often single-center series. Accordingly, data elements were usually reported via chart review, without external prospective ascertainment. No published or completed prospective cohort studies or randomized controlled trials were present in this publications search. These issues have important implications for research priority setting, and for interpretations of the results reported herein. There is an urgent need for high-quality research in this area, but at this point it is useful to review the available data.
Pathophysiology, Epidemiology, and Clinical Features of COVID-19
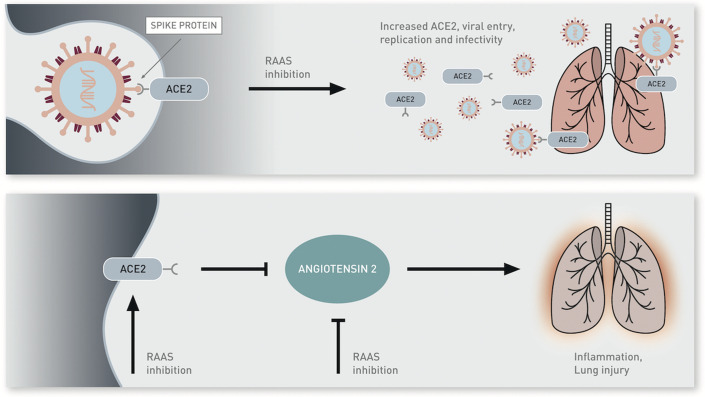
SARS-CoV-2, like other members of the Coronaviridae family, is an enveloped virus with nonsegmented, single-stranded, positive-sense ribonucleic acid (RNA) genome (1,7). A number of SARS-related coronaviruses have been discovered in bats, and a working theory is that bats may have been the initial zoonotic host for SARS-CoV-2 given that its genome is 96.2% identical to a bat coronavirus (8). Studies have demonstrated that SARS-CoV-2 as well as other coronaviruses can use the angiotensin-converting enzyme 2 (ACE2) protein for cell entry. ACE2 is a type I integral membrane protein that serves many important physiologic functions. It is highly expressed in lung alveolar cells, providing the main entry site for the virus into human hosts (8,9). After ligand binding, SARS-CoV-2 enters cells via receptor-mediated endocytosis in a manner akin to human immunodeficiency virus (10). ACE2 also serves a role in lung protection and therefore viral binding to this receptor deregulates a lung protective pathway, contributing to viral pathogenicity (11). Figure 1 depicts the potential mechanisms for ACE2 with regard to viral pathogenicity and lung protection, as well as the potential effects on this from renin-angiotensin-aldosterone inhibition as noted in the section on Drug Therapy and COVID-19.
Figure 1.
Postulated Relationship Between SARS-CoV-2 and ACE2
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds to angiotensin-converting enzyme 2 (ACE2) via spike protein, which facilitates entry into the cell. It is hypothesized that renin-angiotensin-aldosterone system (RAAS) inhibition may up-regulate ACE2 expression, thereby increasing viral entry and replication (top). ACE2 reduces levels of angiotensin II, which is a potent proinflammatory agent in the lungs and can contribute to lung injury. RAAS inhibitors may block the production or function of angiotensin II and potentially also increase levels of ACE2, thereby indirectly inhibiting angiotensin II (bottom).
Since initial identification, the disease has spread to over 100 countries across the world (1). As of March 16, 2020, at 11:53 am, there have been a total of 174,961 COVID-19 cases reported globally (3,813 in the United States) associated with 6,705 deaths thus far (69 in the United States), resulting in a crude case-fatality rate of 3.8% (12,13). Johns Hopkins University is making current data available (12,14). The infectivity of COVID-19 is greater than that of influenza, with an estimated R 0 value (the basic reproduction number, representing viral infectivity) of 2.28 (15). Notably, the death rate associated with COVID-19 is also considerably higher compared with the most recent World Health Organization estimate of seasonal influenza mortality rate of <0.1%, and may reach much higher rates in elderly patients, those with comorbidities, and in those for whom efficient intensive care support is absent (13). While other zoonotic coronaviruses, including the 2002 to 2003 SARS-CoV epidemic and the Middle East respiratory syndrome (MERS-CoV), had higher associated case fatality rates of 9.6% and 34.4%, respectively (16), COVID-19 has resulted in many more deaths than both of these prior outbreaks combined, an issue that is in part related to the greater infectivity and higher attack rate of this virus, leading to a larger number of infected patients (16,17). Uncertain and inconsistent disease ascertainment have resulted in variability in reported case fatality rates for several reasons, including: 1) the disease may be asymptomatic or mildly symptomatic in a large proportion of patients (16); 2) there are inadequate testing capabilities in most geographies, leading to frequent underdiagnosis, especially in patients with less serious illness; and 3) complications and death often ensue much later than contagion (typically between 2 and 3 weeks after infection). Notably, the appraisal of SARS-CoV-2 infection may be further complicated by asymptomatic infection in a sizable portion of individuals, which may significantly contribute to further spread of infection (18).
The clinical presentation for COVID-19 is quite variable. A large study from the Chinese Center for Disease Control and Prevention demonstrated that among 72,314 patients with COVID-19 (44,672 laboratory confirmed, 16,186 suspected, and 10,567 clinically diagnosed), the clinical severity was reported as mild in 81.4%, severe in 13.9%, and critical in 4.7% (16). The clinical characteristics of mild COVID-19 appear to include symptoms common to other viral infections (i.e., fever, cough, dyspnea, myalgias, fatigue, and diarrhea) as well as laboratory abnormalities such as lymphopenia (19), although knowledge of the clinical feature of the disease is evolving daily (1,20). In severe cases, COVID-19 may present as pneumonia, the acute respiratory distress syndrome (ARDS), with or without both distributive and cardiogenic shock, to which elderly populations with pre-existing medical comorbidities are the most vulnerable (1,6,20,21). Notably whereas rates of concomitant infections with other viruses and bacterial superinfections in preliminary data appear low (16), patients with the most severe clinical presentations are likely still at risk for coinfections, and unsurprisingly, worse outcomes have been noted in such cases (21,22). Children account for the minority of laboratory-confirmed cases of COVID-19 in China and appear to be less susceptible to severe disease, possibly due to stronger innate immunity, fewer comorbidities, differences in maturation of viral receptors, and/or prior exposure to other coronavirus species (23). However, moderate-to-severe illness has been described in children as well (24). Moreover, it is not clear how often children were being tested.
Because an extremely large and increasing number of patients have been diagnosed with COVID-19, identification of prognostic factors associated with morbidity and mortality are crucial. To date, no approved preventative vaccines or approved therapies are available for COVID-19, although several are being actively studied (25).
Prevalence of CVD in Patients With COVID-19
The lack of widespread testing, national surveillance, and standardized data collection, as well as the potential sampling bias in sicker, hospitalized patients with more comorbidities such as CVD has complicated efforts to accurately estimate the prevalence of CVD in patients with COVID-19. Moreover, there is marked variation in testing by country. A number of studies in the available reports suggest an association between pre-existing CVD and severe COVID-19, which are summarized in Tables 1 and 2 . A meta-analysis of 6 studies inclusive of 1,527 patients with COVID-19 examined the prevalence of CVD and reported the prevalence of hypertension, cardiac and cerebrovascular disease, and diabetes to be 17.1%, 16.4%, and 9.7%, respectively (4). Patients who required intensive care unit (ICU) admission were more likely to have these comorbidities than were non-ICU patients. Increased case-fatality rates in the previously referenced analysis of 44,672 confirmed COVID-19 cases from Wuhan, China, were noted in patients with CVD (10.5%), diabetes (7.3%), and hypertension (6.0%), all notably higher than the overall case-fatality rate of 2.3% (16). Several smaller cohort studies have yielded similar results, suggesting higher risk for adverse events in patients with CVD who contract COVID-19, although biases related to testing and standardized data apply here as well (1,20,26, 27, 28, 29). Notably, whereas reports outside of China are limited, data from Italy suggest similar mortality rates and an elevated risk for death in patients with comorbidities (30). As emerging international data become available, analysis from multinational cohorts can help inform risk stratification for severe disease especially for patients with prior CVD.
Table 1.
Relative Frequency of CV Risk Factors or Underlying CV Conditions in Available COVID-19 Cohorts and Representative Parent Populations
| First Author, Year (Ref. #) | CVD | Diabetes | Hypertension | Smoking | Coronary Artery Disease | Cerebrovascular Disease |
|---|---|---|---|---|---|---|
| Guan et al. 2020 (29) (N = 1,099) | — | 81 (7.3) | 165 (15.0) | 158 (14.4) | 27 (2.5) | 15 (1.4) |
| Zhou et al. 2020 (6) (N = 191) | — | 36 (18.8) | 58 (30.4) | 11 (5.8) | 15 (7.9) | — |
| Wang et al. 2020 (20) (N = 138) | 20 (14.5) | 14 (10.1) | 43 (31.2) | — | — | 7 (5.1) |
| Huang et al. 2020 (1) (N = 41) | 6 (14.6) | 8 (19.5) | 6 (14.6) | 3 (7.3) | — | — |
| Ruan et al. 2020 (22) (N = 150) | 13 (8.7) | 25 (16.7) | 52 (34.7) | — | — | 12 (8.0) |
| Wu et al. 2020 (28) (N = 201) | 8 (4.0) | 22 (10.9) | 39 (19.4) | — | — | — |
| Wu et al. 2020 (16)∗ (N = 44,672) | 4,690 (10.5)† | 3,261 (7.3) | 2,903 (6.5) | — | — | — |
| Fang et al. 2020 (100)∗ (N = 2,818) | 233 (8.3)† | 206 (7.3) | 376 (13.3) | — | — | — |
| Lu et al. 2018 (101)§ (N = 12,654) | 1,455 (11.5) | 2,125 (16.8) | 4,884 (38.6) | 4,985 (39.4) | — | 278 (2.2) |
Values are n (%). To date, no publications have described these statistics for COVID-19 patients from other areas including South Korea, Iran, Italy, Spain, and others. Therefore, the comparator parent population was chosen from China.
COVID-19 = coronavirus disease 2019; CV = cardiovascular; CVD = cardiovascular disease.
These studies by Wu et al. (16) and Fang et al. (98) include a large, population-based dataset and a systematic review, respectively, from China that are inclusive of the other displayed cohort studies.
Composite of CVD and cerebrovascular disease.
Chinese population prior to COVID-19 included for comparison. Please note that disease ascertainment was different in this study compared with studies of patients with COVID-19.
Table 2.
Association Among Underlying CV Risk Factors, Known CVD, and Outcomes in COVID-19
| Outcome Variable | Guan et al. 2020 (29) (N = 1,090) | Zhou et al. 2020 (6) (N = 191) | Wang et al. 2020 (20) (N = 138) | Huang et al. 2020 (1) (N = 41) | Ruan et al. 2020 (5) (N = 150) | Wu et al. 2020 (28)∗ (N = 201) | |
|---|---|---|---|---|---|---|---|
| CV Risk Factors | |||||||
| Diabetes | ICU vs. non-ICU | — | — | 8 (22.2) vs. 6 (5.9) | 1 (7.7) vs. 7 (25.0) | — | — |
| Severe vs. nonsevere | 28 (16.2) vs. 53 (5.7) | — | — | — | — | — | |
| Dead vs. alive | — | 17 (31.4) vs. 19 (13.9) | — | — | 12 (17.6) vs. 13 (15.9) | 11 (25.0) vs. 5 (12.5) | |
| Hypertension | ICU vs. non-ICU | — | — | 21 (58.3) vs. 22 (21.6) | 2 (15.4) vs. 4 (14.3) | — | — |
| Severe vs. nonsevere | 41 (23.7) vs. 124 (13.4) | — | — | — | — | — | |
| Dead vs. alive | — | 26 (48.1) vs. 32 (23.4) | — | — | 29 (42.6) vs. 23 (28.0) | 16 (36.4) vs. 7 (17.5) | |
| Smoking | ICU vs. non-ICU | — | — | — | 0 vs. 3 (10.7) | — | — |
| Severe vs. non-severe | 38 (22.0) vs. 130 (14.0) | — | — | — | — | — | |
| Dead vs. alive | — | 5 (9.3) vs. 6 (4.4) | — | — | — | — | |
| Known CVD | |||||||
| Coronary artery disease | ICU vs. non-ICU | — | — | 9 (25.0) vs. 11 (10.8) | — | — | — |
| Severe vs. nonsevere | 10 (5.8) vs. 17 (1.8) | — | — | — | — | — | |
| Dead vs. alive | — | 4 (7.4) vs. 2 (1.5) | — | — | — | — | |
| Cerebrovascular disease | ICU vs. non-ICU | — | — | 6 (16.7) vs. 1 (1.0) | — | — | — |
| Severe vs. nonsevere | 4 (2.3) vs. 11 (1.2) | — | — | — | — | — | |
| Dead vs. alive | — | — | — | — | 7 (10.3) vs. 5 (6.1) | — | |
| Cardiovascular disease | ICU vs. non-ICU | — | — | — | 3 (23.0) vs. 3 (10.7) | — | — |
| Severe vs. nonsevere | — | — | — | — | — | — | |
| Dead vs. alive | — | — | — | — | 13 (19.1) vs. 0 | 4 (9.1) vs. 4 (10.0) | |
Values are n (%). Only a few studies with single-center experience have presented data to date, which limits the generalizability of the findings, and the confidence in the point estimates.
ICU = intensive care unit; other abbreviations as in Table 1.
This study used multivariable modeling for outcome of death for each CV risk factor for CVD.
COVID-19 outcomes and CVD: Potential mechanisms of increased risk
Mechanisms that lead to CVD are increasingly recognized to overlap with pathways that regulate immune function. For instance, age is the strongest risk factor for CVD and the effect of aging on immune function may be equally important for COVID-19 susceptibility and severity. Exemplary of this, the effect of age on the immune system is exemplified by low protective titers among 50% of adults older than 65 years who receive the influenza vaccine (31,32). Other traditional CVD risk factors such as diabetes and hyperlipidemia affect immune function, and conversely, dysregulated immunologic status corresponds with elevated risk of incident CVD (33, 34, 35, 36). Thus, prevalent CVD may be a marker of accelerated immunologic aging/dysregulation and relate indirectly to COVID-19 prognosis. An increased frequency of adverse CVD events post-COVID-19 infection might also play a role in prognosis, similar to other viral infections such as influenza with mechanistic underpinnings that are complex, multifactorial, and bidirectional (37,38). In addition, COVID-19 infection may trigger pathways unique to this pathogen that contribute to outcomes in CVD patients. For instance, higher expression of ACE2 in patients with hypertension and CVD has been postulated to enhance susceptibility to SARS-CoV-2, although the data are conflicting and without clear suggestion for treatment (Figure 1) (5). Additional study is needed to understand the potential mechanistic relationships between CVD and COVID-19 outcomes.
Heart transplantation
In addition to the mechanisms by which COVID-19 can affect patients with CVD risk factors, it is also important to consider COVID-19 in the context of an especially vulnerable group of patients, such as individuals awaiting or who have undergone heart transplantation. There are now case reports of COVID-19 infection among heart transplant patients (39,40). Two heart transplant patients in China, 1 with mild and 1 with severe disease, presented with symptoms typical of COVID-19 disease. Both were managed by withholding baseline immunosuppressive regimens and treating aggressively with high-dose steroids, intravenous immunoglobulin, and antibiotics, and both survived without evidence of allograft rejection. Previous viral outbreaks have noted particularly severe infection in immunosuppressed solid organ transplant recipients (41). Formal treatment guidelines in these patients do not exist at this time. Heart allocation teams need to consider the optimal screening strategies to prevent severe infection in recipients including whether all donor hearts should be screened, given the existence of asymptomatic COVID-19, versus limiting screening to patients with a history of symptoms or exposure of COVID-19. During the influenza A subtype H1N1 pandemic, to prevent infection in the recipient or as an impetus to initiate prophylaxis if the donor was positive, potential donors were screened if they were symptomatic or had significant exposure history (42). Similarly, screening recipients for a history of symptoms or exposure of COVID-19 to avoid a post-transplant flare will be reasonable to be considered. Utmost precautions in infection control must be employed when interacting with these vulnerable immunosuppressed patients.
Cardiovascular Sequelae Associated With COVID-19
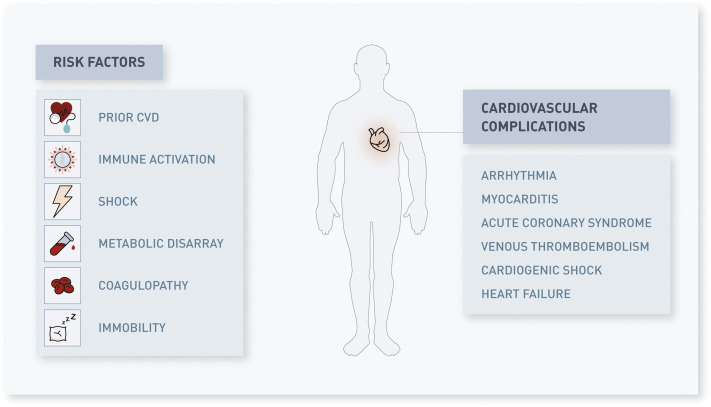
Figure 2 summarizes some of the potential CV sequelae that may result from COVID-19 infection. Pending larger studies, several existing reports are suggestive of SARS-CoV-2 infection leading to CV complications or exacerbation of pre-existing CVD (6,16,22).
Figure 2.
Risk Factors for Complications and Cardiovascular Sequelae of COVID-19
Risk factors for complications in patients afflicted with coronavirus disease 2019 (COVID-19) and potential cardiovascular issues that may result from this disease process. CVD = cardiovascular disease.
Myocardial injury, myocarditis, and acute coronary syndromes
Myocardial injury, as defined by an increased troponin level, can occur due to myocardial ischemia or nonischemic myocardial processes including myocarditis (6,43,44). With severe respiratory infection and hypoxia, especially in the setting of severe infection and ARDS due to COVID-19, it is likely that a number of patients will develop such injury. Elevated serum troponin levels have been described in many patients infected with COVID-19, with significant differences noted between patients who died and those who survived to discharge (22,45). In a meta-analysis of 4 studies including a total of 341 patients, standardized mean difference of cardiac troponin I levels were significantly higher in those with severe COVID-19-related illness compared with in those with nonsevere disease (25.6; 95% confidence interval [CI]: 6.8 to 44.5) (46). Reports have also suggested that acute cardiac injury—which includes not only elevation of cardiac biomarkers to >99th percentile of the upper reference limit, but also electrocardiographic and echocardiographic abnormalities—is highly prevalent in patients with COVID-19 and is associated with more severe disease and worse prognosis. Cohort studies from hospitalized patients in China estimate that such injury occurs in 7% to 17% of hospitalized patients with the disease (1,6,20) and is significantly more common in patients admitted to the ICU (22.2% vs. 2.0%; p < 0.001) and among those who died (59% vs. 1%; p < 0.0001) (6,8). However, troponin levels can be exacerbated in patients with renal insufficiency due to delayed excretion, which is common in patients with advanced disease. Given limited high-quality data, and the heterogeneity of definitions across the studies, standardized data collection methods are recommended using the most recent Universal Definition of Myocardial Infarction (MI) (44).
Prior studies in other coronavirus species (MERS-CoV) have demonstrated evidence of acute myocarditis using cardiac magnetic resonance imaging (47), and myocardial inflammation and damage have been reported with COVID-19 infection. Among 68 deaths in a case series of 150 patients with COVID-19, 7% were attributed to myocarditis with circulatory failure and 33% to cases in which myocarditis may have played a contributing role to the patient’s demise (22). Other reports have described fulminant myocarditis in the setting of high viral load with autopsy findings of inflammatory mononuclear infiltrate in myocardial tissue (27,48,49). While glucocorticoid therapy and other agents have been administered (50), the most effective treatment strategy for such patients is yet to be defined. Pericardial involvement has not yet been reported, but further study is needed. Pericardial involvement has not yet been reported but further study is needed. In addition, the extent to which supply and demand mismatch (type 2 MI) in patients with underlying CVD have contributed to the CV manifestations of the syndrome is uncertain.
Case reports of acute coronary syndromes (type 1 MI) in the setting of COVID-19 have yet to be published. Nonetheless, the profound inflammatory response and hemodynamic changes associated with severe disease may confer risk for atherosclerotic plaque rupture in susceptible patients (6). In this regard, analysis by Kwong et al. (37) demonstrated that patients with acute respiratory infections are at elevated risk for subsequently developing acute MI after influenza (incidence ratio: 6.1; 95% CI: 3.9 to 9.5) and after noninfluenza viral illnesses including other coronavirus species (incidence ratio: 2.8; 95% CI: 1.2 to 6.2). The development of care pathways and protocols for COVID-19 patients with ST-segment elevation MI suggest that both within and outside of China such a clinical scenario is highly probable (51).
Additionally, it is important to note potential overlapping symptomatology between acute coronary syndromes and COVID-19. Even though the predominant presenting symptoms of COVID-19 are respiratory, a case report described a patient in Italy with chest pain and electrocardiographic changes for which the cardiac catheterization lab was activated. Notably, the patient was found to be free of obstructive coronary artery disease but ultimately tested positive for COVID-19 (52). Moving forward as the virus continues to infect patients with significant CV risk factors or established CVD, cases of acute coronary syndromes in the setting of COVID-19 are likely to develop. The true prevalence in this setting may be under-reported given the logistical challenges associated with limited testing and cardiac catheterization laboratory availability in the setting of this outbreak. For further recommendations for the care and management of COVID-19 patients in the cardiac catheterization laboratory, please see the joint American College of Cardiology and Society of Cardiovascular Angiography and Intervention guidance statement (53).
Cardiac arrhythmia and cardiac arrest
Cardiac arrhythmias are another common CV manifestation described in patients with COVID-19 infection. Though nonspecific, heart palpitations were part of the presenting symptomology in 7.3% of patients in a cohort of 137 patients admitted for COVID-19 disease (27). In hospitalized COVID-19 patients, cardiac arrhythmia was noted in 16.7% of 138 patients in a Chinese cohort and was more common in ICU patients than in non-ICU patients (44.4% vs. 6.9%) (20). Unfortunately, specifics about the types of arrhythmias that occur in these patients are yet to be published or presented. High prevalence of arrhythmia might be, in part, attributable to metabolic disarray, hypoxia, or neurohormonal or inflammatory stress in the setting of viral infection in patients with or without prior CVD. However, new onset of malignant tachyarrhythmias in the setting of troponin elevation should raise suspicion for underlying myocarditis (45,54).
Cardiomyopathy and heart failure
Zhou et al. (6) reported that heart failure was observed in 23.0% of patients with COVID-19 presentations. Notably, heart failure was more commonly observed than acute kidney injury in this cohort and was more common in patients who did not survive the hospitalization than in those who did survive (51.9% vs. 11.7%). Whether heart failure is most commonly due to exacerbation of pre-existing left ventricular dysfunction versus new cardiomyopathy (either due to myocarditis or stress cardiomyopathy) remains unclear (55). Right heart failure and associated pulmonary hypertension should be also considered, in particular in the context of severe parenchymal lung disease and ARDS.
Cardiogenic and mixed shock
The predominant clinical presentation of COVID-19 is acute respiratory illness, which may lead to ARDS manifested as ground-glass opacities on chest imaging (56) and hypoxemia. However, similar features may be seen in the case of de novo or coexisting cardiogenic pulmonary edema. As such, it is important to consider cardiogenic or mixed cardiac plus primary pulmonary causes of respiratory manifestations in COVID-19. Historically, right heart catheterization was used to determine pulmonary capillary wedge pressure to aid in this distinction, although this has been removed from the Berlin criteria used for the diagnosis of ARDS. Rather, the Berlin criteria use timing of symptom onset, imaging with bilateral pulmonary opacities, and lack of volume overload to identify patients with ARDS (57). In many cases, serum brain natriuretic peptide and echocardiography can help clarify the diagnosis (58,59). However, if these tests are unclear and there remains concern for mixed presentation, pulmonary artery catheterization should be considered in select cases to assess filling pressures, cardiac output, and to guide clinical decision making, given the different management approaches for ARDS and cardiogenic shock. Finally, it is crucial to determine whether a concomitant cardiogenic component is present when considering mechanical respiratory and circulatory support with extracorporeal membranous oxygenation (ECMO) or other techniques, as this may lead to changes in device selection (e.g., venovenous vs. venoarterial ECMO cannulation). Regardless, in the most severe of infections with ARDS and necrotizing pneumonias, patient prognosis may be poor even with ECMO support. In a case series of 52 critically ill patients with COVID-19, 83.3% (5 of 6) of patients who were treated with ECMO did not survive. Further studies regarding the utility of ECMO support in advanced COVID-19, including which patients may (or may not) benefit and whether concomitant left ventricular venting should be done, are warranted (60).
Venous thromboembolic disease
COVID-19 infected patients are likely at increased risk of venous thromboembolism, and data from Klok et al. (61) suggests that rates of thrombotic complications may be as high as 31% in critically ill patients with COVID-19. Reports suggest abnormal coagulation parameters in hospitalized patients with severe COVID-19 disease (62,63). In a multicenter retrospective cohort study from China, elevated D-dimer levels (>1 g/l) were strongly associated with in-hospital death, even after multivariable adjustment (odds ratio: 18.4; 95% CI: 2.6 to 128.6; p = 0.003) (6). In another study comparing COVID-19 survivors to nonsurvivors, nonsurvivors had significantly higher D-dimer and fibrin degradation products levels, and 71.4% of nonsurvivors met clinical criteria for disseminated intravascular coagulation during the course of their disease (62). In addition to disseminated intravascular coagulation, critically ill patients with prolonged immobilization are inherently at high risk for venous thromboembolism. Vascular inflammation may also contribute to the hypercoagulable state and endothelial dysfunction in such patients. In the setting of critically ill COVID-19 patients who demonstrate clinical deterioration as evidenced by hypoxia or hemodynamic instability, thromboembolic disease should be considered. Retrospective data from 97 patients with severe COVID-19, the majority of whom received prophylactic dose low–molecular-weight heparin in the setting of sepsis-induced coagulopathy, suggests that anticoagulation in such patients may be associated with lower mortality rates (64). The optimal thromboprophylactic regimen for patients hospitalized with COVID-19-related illness is not known, however. As such, contemporary guideline-endorsed strategies should be observed (65). Given the drug-drug interactions between some antiviral treatments and direct oral anticoagulants, low molecular weight heparins or unfractionated heparin with or without mechanical prophylaxis are likely to be preferred in acutely ill hospitalized patients.
Drug Therapy and COVID-19: Interactions and Cardiovascular Implications
Data regarding antiviral therapies and other treatment strategies, as well as their potential interaction with CV medications and CV toxicities are summarized in Tables 3, 4, and 5 . Although currently there are no specific effective therapies for COVID-19, various pharmacologic agents are under active investigation. As these drugs are being studied, it is important to review the potential CV side effects and interactions with other CV medications.
Table 3.
Antiviral Therapies Currently Being Studied for COVID-19: Potential Cardiovascular Interactions and Toxicities
| Antiviral Therapy | ClinicalTrials.gov Identifiers | Mechanism of Action | CV Drug Class Interactions | CV Adverse Effects |
|---|---|---|---|---|
| Remdesevir |
NCT04302766 NCT04280705 NCT04292899 NCT04292730 NCT04315948 NCT04321616 |
Nucleotide-analog inhibitor of RNA-dependent RNA polymerases | N/A | Unknown |
| Ribavirin |
NCT04276688 NCT00578825 |
Inhibits replication of RNA and DNA viruses | Anticoagulants∗ | Drug-induced hemolytic anemia Avoid in patients with significant/unstable cardiac disease |
| Lopinavir/ritonavir |
NCT04252885 NCT04275388 NCT04276688 NCT04286503 NCT04307693 NCT04261907 NCT02845843 NCT04321174 NCT04295551 NCT04315948 NCT04251871 NCT00578825 NCT04328012 NCT04321993 |
Lopinavir is a protease inhibitor; ritonavir inhibits CYP3A metabolism increasing levels of lopinavir | Antiplatelets∗ Anticoagulants∗ Statin∗ Antiarrhythmics∗ |
Altered cardiac conduction: QTc prolongation, high-degree AV block, torsade de pointes; increased serum cholesterol |
Table 4.
Other Therapies Being Studied for COVID-19: Potential Cardiovascular Interactions and Toxicities
| Therapy | ClinicalTrials.gov Identifiers |
Mechanism of Action | CV Drug Interactions |
CV Adverse Effects |
|---|---|---|---|---|
| Anakinra |
NCT04341584 NCT04339712 NCT04330638 |
Recombinant human decoy IL-1Ra. Blocks IL-1α and IL-1β. IL-1β overproduction is associated with the pathogenesis of macrophage activation syndrome. | None | None |
| Bevacizumab | NCT04275414 | COVID-19 patients may have elevated VEGF levels. Inhibits VEGF and decreases vascular permeability and pulmonary edema. | None | Direct myocardial toxicity vs. exacerbation of underlying cardiomyopathy Severe hypertension Thromboembolic events |
| Chloroquine/hydroxychloroquine |
NCT04303507 NCT04307693 NCT04318444 NCT04341493 NCT04316377 NCT04333654 NCT04322123 NCT04307693 NCT04261517 NCT04341727 NCT04328961 NCT04321278 NCT04342169 NCT04332835 NCT04332991 NCT04315948 NCT04334967 NCT04321993 NCT04342221 NCT04321616 NCT04338698 NCT04315896 NCT04328012 NCT04330586 |
Alters endosomal pH required for virus/cell fusion and interferes with glycosylation of SARS-CoV-2 cellular receptors. | Antiarrhythmics∗ | Direct myocardial toxicity vs. exacerbation of underlying cardiomyopathy Altered cardiac conduction: AV block, bundle-branch block, QT prolongation, torsades de pointes, ventricular tachycardia/fibrillation |
| Colchicine |
NCT04322682 NCT04326790 NCT04322565 |
Anti-inflammatory properties to potentially inhibit the pathogenetic cycle of SARS-COV-2 by counteracting the assembly of the NLRP3 inflammasome and (less likely) to inhibit viral endocytosis in myocardial and respiratory cells. | Non-DHP CCB∗ Statins∗ Antiarrhythmics∗ |
None |
| Eculizumab | NCT04288713 | Inhibits complement activation and prevents the formation of the membrane attack complex. | None | Hypertension or hypotension, tachycardia, peripheral edema |
| Fingolimod | NCT04280588 | Inhibits lymphocytes through sphingosine-1 phosphate regulation. | Antiarrhythmics∗ | Hypertension, first and second degree AV block (contraindicated with high degree AV block and sick sinus syndrome), bradycardia, QTc prolongation (contraindicated if QTc ≥500 ms) Contraindicated after myocardial infarction, unstable angina, CVA/TIA, ADHF in previous 6 months |
| Interferon-alpha, beta |
NCT04275388 NCT04273763 NCT04276688 NCT04293887 NCT04251871 NCT04320238 NCT02845843 NCT04291729 NCT04315948 |
Immune activation. | Warfarin | Direct myocardial toxicity vs. exacerbation of underlying cardiomyopathy Reports of: hypotension, arrhythmia, cardiomyopathy, myocardial infarction |
| Pirfenidone | NCT04282902 | Antifibrotic ability, possible IL-1β and IL-4 inhibition to reduce cytokine storm and resultant pulmonary fibrosis. | None | None |
| Methylprednisolone |
NCT04273321 NCT04244591 NCT04323592 |
Alters gene expression to reduce inflammation. | Warfarin | Fluid retention Electrolyte disturbances Hypertension |
| Sarilumab |
NCT04324073 NCT04327388 NCT04322773 |
Binds to both soluble and membrane-bound IL-6Rs and inhibit signaling that could help mitigate cytokine storm. | Antiplatelets∗ Anticoagulants∗ Statins∗ Antiarrhythmics∗ |
Unknown |
| Tocilizumab |
NCT04306705 NCT04335071 NCT04331808 NCT04310228 NCT04339712 NCT04330638 |
Inhibits IL-6 receptor. | Antiplatelets∗ Anticoagulants∗ Statins∗ Evolocumab Beta blockers∗ Antiarrhythmics∗ |
Hypertension Increased serum cholesterol |
| Tranexamic acid |
NCT04338126 NCT04338074 |
Reduced conversion of plasminogen to plasmin could potentially reduce the infectivity and virulence of the virus. | None | Venous and arterial thrombosis and thromboembolism, including retinal artery/vein obstruction, has been reported Use caution in patients with uncorrected cardiovascular or cerebrovascular disease due to complications of thrombosis |
Table 5 summarizes specific recommendations in the setting of medication interactions.
ADHF = acute decompensated heart failure; CVA = cerebrovascular accident; IL = interleukin; NLRP3 = nucleoside-binding domain-like receptor protein 3; non-DHP CCB = non-dihydropyridine calcium-channel blocker; TIA = transient ischemic attack; VEGF = vascular endothelial growth factor.
Indicates drug class interactions.
Table 5.
Recommendations Regarding Dosing and Adjustment in the Setting of Medication Interactions
| Therapy | Specific Interaction | MOA of Drug Interaction and Specific Dose Adjustments | Other Notes |
|---|---|---|---|
| Chloroquine / hydroxychloroquine |
|
CYP2D6 inhibition:
|
|
Antiarrhythmics
|
Intensified QTc prolongation | Monitor ECG | |
|
P-glycoprotein inhibition:
|
Monitor digoxin levels | |
| Colchicine | Non-DHP CCB
|
CCB-Induced CYP3A4 and P-glycoprotein inhibition:
|
Monitor for signs of colchicine toxicity. |
Statins
|
Potential CYP3A4 inhibition or impaired excretion:
|
Monitor for signs of muscle pain and/or weakness with concomitant therapy | |
Antiarrhythmics
|
P-glycoprotein inhibition:
|
Monitor digoxin levels | |
| Fingolimod | Bradycardia-causing medications
|
Inhibition of sphingosine-1-phosphate on atrial myocytes can decrease AV conduction; if co-administration is necessary, overnight continuous ECG monitoring recommended after first dose | Stop AV nodal blocking medications if possible. |
QT-prolonging antiarrhythmics
|
Do not co-administer | Monitor ECG | |
| Interferon-alpha, beta | Warfarin | Unknown mechanism of action:
|
Monitor INR |
| Lopinavir/ritonavir | Anticoagulants
|
CYP3A4 and P-glycoprotein inhibition:
|
Dabigatran and warfarin can be administered with caution Monitor INR with warfarin |
Antiplatelets
|
CYP3A4 inhibition:
|
Based on limited evidence, recommend prasugrel if no contraindications | |
Statins
|
OATTP1B1 and BCRP inhibition:
|
Start at lowest possible dose of rosuvastatin and atorvastatin and titrate up Can consider pitavastatin and pravastatin |
|
Antiarrhythmics
|
Intensified QTc prolongation P-glycoprotein inhibition:
|
Monitor ECG Monitor digoxin level |
|
| Ivabradine | CYP3A4 inhibition:
|
||
| Methylprednisolone | Anticoagulants
|
Unknown mechanism:
|
Monitor INR |
| Remdesivir | N/A | Potential inducer of CYP1A2, CYP2B6 and CYP3A4 No dose adjustment recommended |
N/A |
| Ribavirin | Anticoagulants
|
Unknown mechanism of action:
|
Monitor INR |
| Sarilumab | Anticoagulants
|
Increased CYP3A4 expression:
|
Monitor INR Monitor ECG |
| Tocilizumab | Anticoagulants
|
Can increase expression of CYP1A2, 2B6, 2C9, 2C19, 2D6, 3A4 leading to increased metabolism. No dose adjustment recommendation | Monitor INR Monitor ECG |
AV = atrioventricular; BCRP = breast cancer resistance protein; ECG = electrocardiogram; INR = international normalized ratio; MOA = mechanism of action; non-DHP CCB = non-dihydropyridine calcium-channel blocker; OATP1B1 = organic anion transporting polypeptide 1B1.
Antiviral therapy
Antivirals are at the forefront of medications under study for the treatment COVID-19 and the clinical trial identifiers for each are listed in Table 3. Ribavirin and remdesivir are 2 such agents that bind to the active site on the RNA-dependent RNA polymerase on SARS-CoV-2 (66), whereas lopinavir/ritonavir inhibits replication of RNA virus and has evidence of a synergistic effect in vitro with ribavirin (67). Ribavirin and lopinavir/ritonavir are under investigation in clinical trials for COVID-19 and have been used for years as components of treatment for hepatitis C and human immunodeficiency virus, respectively (68,69). Whereas ribavirin has no characterized direct CV toxicity, lopinavir/ritonavir may result in QT- and PR-interval prolongation, especially in patients who have a baseline abnormality (long QT) or those who are at risk for conduction abnormalities including those taking other QT prolonging drugs (69). Both ribavirin and lopinavir/ritonavir have the potential to affect anticoagulant dosing: ribavirin has variable effects on warfarin dosing (70) and lopinavir/ritonavir may require dose reductions or avoidance of CYP3A-mediated drugs such as rivaroxaban and apixaban (71,72).
Lopinavir/ritonavir can also influence the activity of P2Y12 inhibitors through CYP3A4 inhibition, which results in decreased serum concentrations of the active metabolites of clopidogrel and prasugrel and increased serum concentrations of ticagrelor. The active metabolite for clopidogrel is mostly formed by CYP2C19, but several other CYP enzymes contribute, including CYP1A2, 2B6, and 3A (73,74). Given the increase in serum ticagrelor levels with such medications (75,76), concomitant use with ticagrelor is discouraged in the United States and Canada due to excess in bleeding risk. Conversely, there is evidence that clopidogrel may not always provide sufficient platelet inhibition in the setting of concomitant administration of ritonavir, whereas this was not the case with prasugrel as assessed by the VerifyNow P2Y12 assay (Accumetrics, San Diego, California) (73,74). If P2Y12 inhibition is needed during treatment with lopinavir/ritonavir, prasugrel can be used; however, if contraindicated (i.e., history of stroke or transient ischemic attack, low body mass index, or active pathological bleeding), a testing-guided approach (e.g., with P2Y12 platelet function assays) may be considered with alternate antiplatelet agents. Details about switching between P2Y12 inhibitors have been described elsewhere (77). Finally, metabolism of the intravenous P2Y12 inhibitor, cangrelor, is independent of hepatic function, therefore a drug interaction is not expected (78).
β-Hydroxy-β-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) also have the potential to interact with the combination of lopinavir/ritonavir and can result in myopathy due to elevated statin levels when administered together. Lovastatin and simvastatin, in particular, are contraindicated for coadministration with lopinavir/ritonavir due to risk of rhabdomyolysis. Other statins, including atorvastatin and rosuvastatin, should be administered at the lowest possible dose but not to exceed the maximum dose stated in the package insert while on lopinavir/ritonavir (69).
Remdesivir is an investigational drug previously evaluated in the Ebola epidemic and is now being studied in patients with COVID-19. The drug is currently available in clinical trials and through compassionate use from Gilead Sciences, Inc. (Foster City, California). Whereas extensive CV toxicities and medication interactions have yet to be reported, prior evaluation of this drug during the Ebola outbreak did note the development of hypotension and subsequent cardiac arrest after loading dose in 1 patient (among 175 total) (79).
Other treatments
Table 4 presents information on other treatments being studied for COVID-19 (including ClinicalTrials.gov identifiers). In addition to antiviral medications, numerous immune-modulating and secondary medications to prevent complications that could arise from COVID-19 are currently being investigated. Chloroquine, which has been used as an antimalarial agent, blocks virus infection by increasing the endosomal pH required for virus/cell fusion and has been demonstrated in vitro to have inhibitory activity in SARS-CoV-2 (80,81). Chloroquine and the closely related hydroxychloroquine have the potential for intermediate-to-delayed myocardial toxicity. Risk factors include long-term exposure (>3 months), higher weight-based dose, pre-existing cardiac disease, and renal insufficiency (82). Chloroquine cardiac toxicity presents as restrictive or dilated cardiomyopathy or conduction abnormalities thought to be due to intracellular inhibition of lysosomal enzymes in the myocyte (82,83). In addition, due to effects of chloroquine on CYP2D6 inhibition, beta-blockers metabolized via CYP2D6 (such as metoprolol, carvedilol, propranolol, or labetalol) can have increased concentration of drug requiring careful monitoring for heart rate and blood pressure shifts. Lastly, both agents are associated with a conditional risk of torsade des pointes in patients with electrolyte abnormalities or with concomitant use of QT-interval–prolonging agents. Short-term exposure to these agents, as would be expected in treatment of COVID-19, confers lower risk of these dose-duration–dependent side effects.
Methylprednisolone is another drug under investigation that is currently being used to treat severe cases of COVID-19 that are complicated by ARDS (49). A retrospective analysis in patients with COVID-19 who develop ARDS demonstrated that methylprednisolone use was associated with decreased mortality (28). This steroid is known to cause fluid retention, electrolyte derangement, and hypertension as direct CV effects, and it also may interact with warfarin via an undescribed mechanism. Clinicians are advised to observe for these drug interactions.
Ibuprofen, a nonsteroidal anti-inflammatory agent, is often used as part of treatment of patients with viral illnesses. However, recent anecdotal evidence has raised wider concerns that the use of ibuprofen can potentially contribute to severe disease in patients with COVID-19 (84). While data is limited, one of the working theories is that ibuprofen may interfere with the host’s immune response to infection. Therefore, at least preliminarily, this mechanism for worse outcomes in patients taking ibuprofen does not appear to involve the CV system.
Finally, patient debilitation from severe COVID-19 may pose challenges in administering routine CV medications, ranging from antiplatelet therapy to beta-blockers, thus putting patients with or at risk of ischemic heart disease or heart failure at risk of further deterioration of their clinical condition.
ACE2 and potential therapeutic implications
As the ACE2 receptor is the mechanism of entry for SARS-CoV-2, some data suggest that ACE inhibitors and angiotensin receptor blockers (ARBs) may up-regulate ACE2, thereby increasing susceptibility to the virus (Figure 1) (5). In contrast, other studies show that ACE inhibitors/ARBs may potentiate the lung protective function of ACE2, by reducing angiotensin II levels through its conversion to angiotensin (1, 2, 3, 4, 5, 6, 7,85, 86, 87). Thus, the therapeutic implications for ACE inhibitor/ARB therapy during COVID-19 infection is unclear. Overall, there are insufficient data to suggest any mechanistic connections involving ACE inhibitor/ARB therapy with contracting COVID-19 or with severity illness once infected.
Considerations for Health Care Workers
Protective equipment for CV health care workers
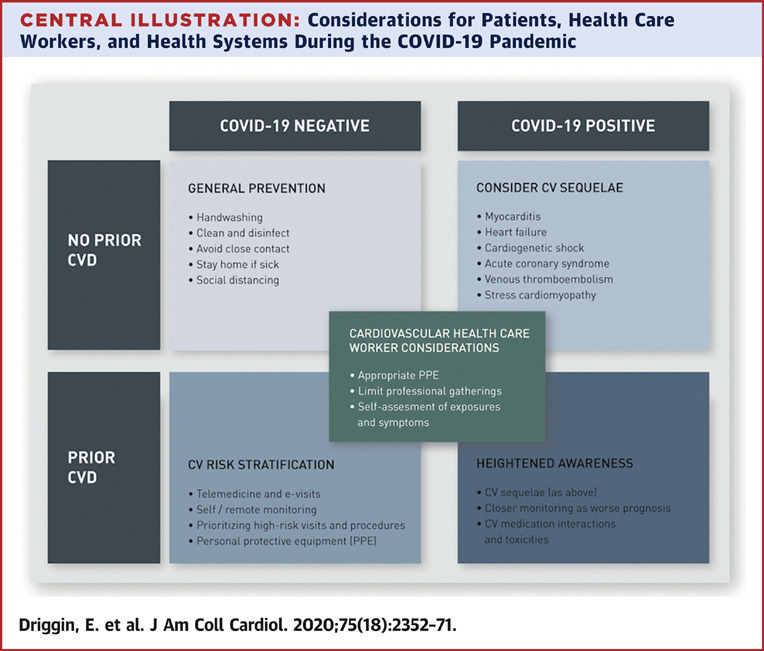
The Central Illustration demonstrates key considerations for treating patients in the current era of the COVID-19 pandemic. Early reports from the outbreak have suggested that transmission occurs most commonly via respiratory droplets that are produced when an infected individual coughs or sneezes. These droplets can land on exposed mucous membranes or be inhaled into the lungs of those within close proximity and the virus may remain active on surfaces for several days (88). Whereas the Centers for Disease Control and Prevention had previously recommended airborne precautions for the care of patients with COVID-19, this recommendation was recently changed such that only patients undergoing aerosol-generating procedures require airborne isolation. Recommendations made by the World Health Organization and Centers for Disease Control and Prevention for personal protective equipment (PPE) are in agreement that standard, contact precautions with face mask, eye protection, gown, and gloves are necessary (53). When available, respirators are preferred.
Central Illustration.
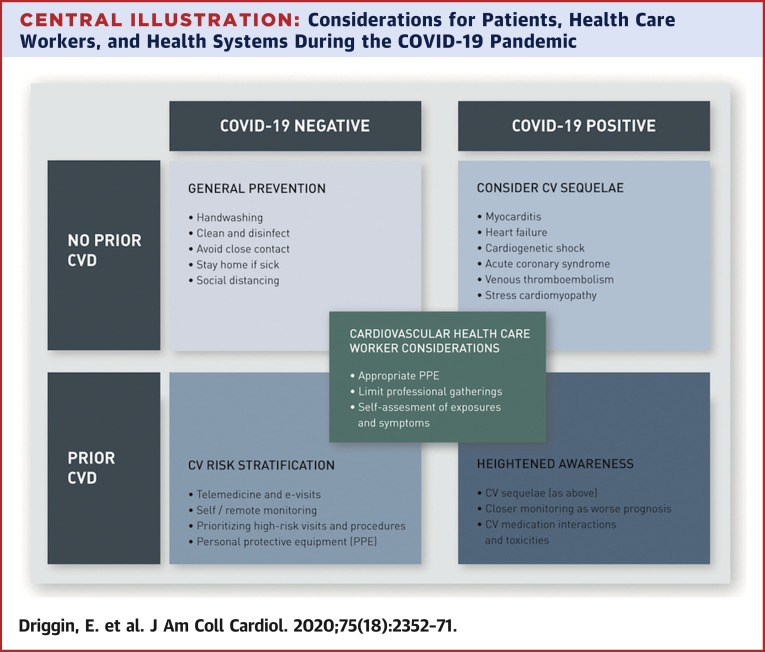
Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic
Key considerations for patients with established cardiovascular disease (CVD), patients without CVD, and for health care workers and health care systems in the setting of the coronavirus disease 2019 (COVID-19) outbreak. CV = cardiovascular; PPE = personal protective equipment.
In addition, when performing certain procedures that are aerosol-generating, such as transesophageal echocardiography, endotracheal intubation, cardiopulmonary resuscitation, and bag mask ventilation, additional PPE may be required, including controlled or powered air purifying respirators. Thorough infection prevention and control measures specific to the procedural cardiology specialties must be considered in light of the COVID-19 outbreak. Such procedures are associated with the small but quantifiable risk of complications and patient deterioration. In the event of a cardiac arrest, efforts at cardiopulmonary resuscitation causing aerosolized pathogens could result in the wide dissemination of virus particles to clinicians, health care workers, and other patients. One measure that may help protect health care workers in the setting of cardiac arrest and chest compressions is the use of external mechanical compression devices to minimize direct contact with infected patients. Another important consideration for the catheterization laboratory is appropriate post-intervention cleaning of all equipment potentially contaminated with SARS-CoV-2. The necessary downtime required for cleaning may seriously affect the availability of catheterization laboratory-based treatments for other patients. As such, many hospitals are minimizing or cancelling elective procedures during the growth phase of the outbreak. Another consideration is the fact that catheterization laboratories and operating rooms are typically configured with positive pressure ventilation, and there have been reports of centers in China converting such facilities to negative pressure isolation in the setting of COVID-19 (89). Guidance and recommendations in this space will be forthcoming from interventional communities, including the American College of Cardiology and Society of Cardiovascular Angiography and Intervention (53).
Figure 3 depicts key information summarizing considerations to prevent infection among CV health care workers as summarized in an infographic. Overall, as CV health care workers are on the front lines treating COVID-19-infected patients, all possible measures should be implemented to reduce the risk of exposure (90). Health care workers are at elevated risk for contracting this virus, as demonstrated by Wu et al. (16), noting 1,716 of the 44,672 infected individuals (3.8%) were health care workers. This fact emphasizes the need for self-protection with PPE before caring for potentially exposed COVID-19 patients and provides further rationale for delaying elective procedures. In teaching hospitals, it is imperative to minimize exposure among trainees and nonessential staff (e.g., medical students) not only for their own safety and that of their patients, but also for conservation of PPE and for avoiding the unnecessary increase in the number of asymptomatic vectors. Finally, transmission between health care workers is also a major concern, especially in the setting of emergency or suboptimal logistics, or when devices for PPE have become scarce.
Figure 3.
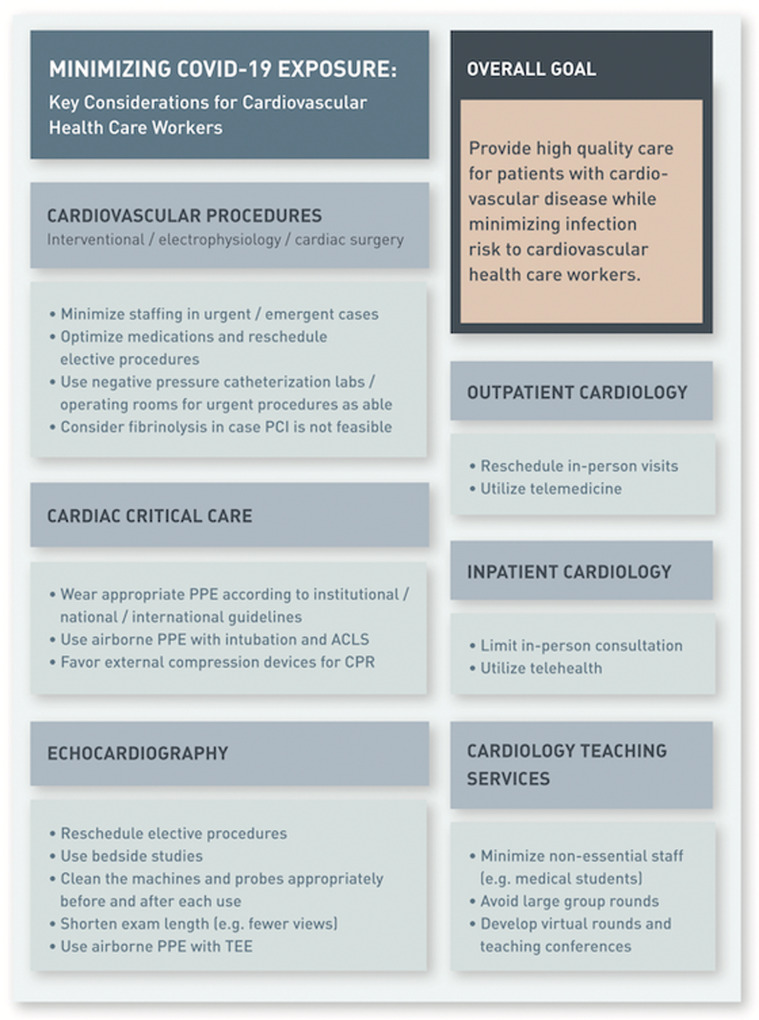
Considerations Regarding COVID-19 for Cardiovascular Health Care Workers by Specialty
Infographic with important considerations regarding coronavirus disease 2019 (COVID-19) for cardiovascular disease health care workers by specialty. ACLS = advanced cardiac life support; CPR = cardiopulmonary resuscitation; PCI = percutaneous coronary intervention; PPE = personal protective equipment; TEE = transesophageal echocardiography.
Triaging CV patients and visits
There are numerous considerations specific to the care of CV patients that should be taken into account to minimize risk for COVID-19 transmission to patients and health care workers; these considerations are outlined in Table 6 . One important mechanism to help prevent transmission is the use of telemedicine. This technology, already used by numerous large health care systems around the world, is ideal in public health crises as it allows for patients to be triaged while minimizing exposure of patients and health care workers to potential infection. Additionally, telemedicine provides an opportunity for specialists that might not otherwise be available to evaluate patients. Although there are currently barriers to the widespread implementation of telemedicine, such as coordination of testing in patients triaged as high risk, this is a technology that will likely prove important to promote viral containment (91). Other essential principles are to minimize nonessential/nonurgent in-person health care worker-patient interactions as much as possible (i.e., social distancing) and limit elective cardiac catheterization, operating room, and echocardiographic procedures. If such procedures are necessary, the number of required personnel should be kept to a minimum.
Table 6.
Considerations for Cardiovascular Health Care Workers and Health Systems Regarding COVID-19 and CVD
| Health Care Worker | Health Systems |
|---|---|
| E-visits/telehealth for triage and patient management, when feasible | Providing and expanding the knowledge and infrastructure for e-visits/telehealth |
| Adherence to guidelines for optimal use of PPE | Preparing sufficient PPE for patient families and healthcare personnel |
| Self-reporting symptoms, if present, and halting the role as health care worker in case symptoms arise | Improving patient and public education regarding indications for quarantine versus hospital presentation |
| Limit elective procedures (i.e. echocardiography, cardiac catheterization) if not urgent/emergent | Improve testing availability so appropriate containment can be achieved |
PPE = personal protective equipment; other abbreviations as in Table 1.
Table 7.
CV Society Guideline Key Considerations With Regard to CVD and COVID-19
| Society/Guideline (Ref. #) | Key Recommendations |
|---|---|
| ACC Clinical Guidance (93) | Establish protocols for diagnosis, triage, isolation of COVID-19 patients with CVD or CV complications Develop acute myocardial infarction–specific protocols (i.e., PCI and CABG) for COVID-19 outbreak |
| ESC Council on Hypertension Statement on COVID-19 (94) | There is insufficient evidence regarding the concerns surrounding safety of ACE inhibitor or ARB treatment in patients with COVID-19 Current recommendations are to continue ACE inhibitor or ARB therapy given no sufficient evidence to discontinue therapy because of this infection |
| European Society of Hypertension (95) | Patients with hypertension should receive treatment with ACE inhibitors and ARBs according to 2018 ESC/ESH guidelines despite COVID-19 infection status (102) In, the case of shock, health care workers should continue or discontinue ACE inhibitor and ARB therapy on case-by-case basis |
| Hypertension Canada (96) | Patients with hypertension should continue their home blood pressure medical regimen |
| Canadian Cardiovascular Society (97) | Continuation of ACE inhibitor, ARB, and ARNI therapy is strongly recommended in COVID-19 patients |
| Internal Society of Hypertension (98) | Endorse the ESC Hypertension Statement |
ACC = American College of Cardiology; ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor neprilysin inhibitor; CABG = coronary artery bypass graft; ESC = European Society of Cardiology; PCI = percutaneous coronary intervention; other abbreviations as in Table 1.
Considerations for Health Systems and Management of Noninfected CV Patients
CV societal leadership
Recently, due to potential health concerns for the CV health care workers and investigators, and to avert deterioration of the COVID-19 outbreak, the American College of Cardiology made the unprecedented but appropriate decision to cancel the 2020 Scientific Sessions meeting. Similarly, a number of medical conferences around the world are either being canceled or postponed (92). Additionally, given the clear implications of this pandemic on CV care, numerous societies have already weighed in with guidance statements, which are summarized in Table 7 . The American College of Cardiology Clinical Bulletin provides a practical clinical summary about key implications and recommendations for CV care of COVID-19 patients (93). The European Society of Cardiology Council on Hypertension and European Society of Hypertension statements acknowledge the questions regarding ACE inhibitor and ARB therapy in the setting of COVID-19 patients (94,95). These societies as well as a number of others agree that further data would be vital to inform decisions on adjusting regimens of these agents in the setting of this outbreak (96, 97, 98). Moving forward, these important CV societies among other large physician groups and health systems will be critical allies to advance the knowledge generation and CV care in patients infected with this virus.
Preparing for hospital surges and prioritizing care for the critically ill
A comprehensive package of measures is required for hospital systems to fully prepare for COVID-19 (Table 5). A significant increase in COVID-19 patients should be anticipated. At the same time, provisions for general health services for acute and severe chronic illnesses must be maintained. Specifically, regarding CV care, as the pandemic surges, hospitals may prioritize the treatment of severe and high-risk patients and enact policy to prevent overwhelming of the health care system by the “worried well.” Given concerns of hospitals exceeding capacity, specific protocols will need to be developed for the care of CV patients while preserving limited in-patient resources and minimizing health care worker and patient exposures. There are reports of individual centers developing alternate ST-segment elevation MI pathways in the setting of the COVID-19 crisis, such as using fibrinolytic therapy if delays to primary percutaneous coronary intervention are anticipated when hospitals are at capacity or staffing for the catheterization lab is inadequate (51). A recent report from China also suggests substantial delays in door-to-device time in patients presenting with STEMI during the COVID-19 outbreak (99). Additionally, repurposing cardiac ICUs as medical ICUs for the care of patients with COVID-19 will likely become necessary, but this may limit the quality of specialty care for CV patients. Given the need for ICU beds after cardiac surgery, medical management or percutaneous interventional approaches may need to be preferentially considered for urgent scenarios that cannot wait (e.g., percutaneous coronary intervention rather than coronary artery bypass graft surgery or transcatheter valve solutions rather than surgery) to minimize ICU bed utilization. Furthermore, as mentioned, appropriate use and careful selection of ECMO-appropriate patients as well as having established ECMO protocols for COVID-19 patients are important strategies to consider (60).
Need for education
Information on the most up-to-date evidence surrounding management and treatment of patients with COVID-19 should be widely disseminated and freely available and should be provided in illustrative formats (e.g., infographics) that improve public knowledge and understanding. The free flow of communication between health care workers and hospitals is paramount to effectively combat the pandemic. The care of patients with COVID-19 will require the expertise of many specialty services including pulmonology/critical care, infectious diseases, cardiology, surgery, pharmacy, and hospital administration among others. Optimal infection control and treatment strategies for COVID-19 should be shared with the entire health care community. Accordingly, every effort must be made to provide clear and unambiguous information to patients and decision makers, countering myths and false news that may generate panic or false optimism. As the evidence base surrounding COVID-19 and its management is evolving on a daily basis, the dissemination of accurate information must occur in real time.
Ethical challenges
The unprecedented challenge represented by COVID-19 has brought novel and dramatic ethical dilemmas, ranging from policy issues (e.g., focusing on containment and mitigation vs. herd immunity), as well as clinical dilemmas (e.g., considering all patients alike vs. triaging patients according to age, comorbidities, and expected prognosis, similar to other catastrophic circumstances). Close interaction among patient advocates, government officials, and regulators, as well as physician groups, hospital administrators, and other societal leaders will be essential to navigate these ethical challenges.
Conclusions and Future Directions
The COVID-19 pandemic has affected hundreds of thousands of patients and poses a major health threat on an international scale. The CV community will play a key role in the management and treatment of patients affected by this disease in addition to providing continuity of care to noninfected patients with underlying CVD. In the coming months, efforts toward evaluating new therapies will be crucial to the treatment of this virus, and as this process develops, further appreciation of the intricate interplay among COVID-19, CVD, and the various stakeholders involved including patients, health care workers, and health care systems will be crucial to improving outcomes in at-risk and infected patients. Prospective randomized clinical trials and cohort studies are ongoing and will be important to helping treat patients affected by this virus.
A number of theories exist regarding the elevated risk for adverse events for patients with CVD who develop COVID-19. In particular, better understanding of the relationships involving the ACE2 protein, antihypertensive agent use, and COVID-19 prognosis will have important implications for patients with both COVID-19 and CVD. Outside of the scope of individual trials, concerted efforts by all health care workers and incisive leadership are required to help mitigate the health risk to the population at large, as well as to CV health care workers, as demonstrated by the difficult decision to cancel the 2020 American College of Cardiology Scientific Sessions. Efficient use of resources, including leveraging of the telehealth capabilities, and optimal adherence to preventative population-wide and health care worker-level measures will enable the transition from this critical period until the disease outbreak is contained.
Acknowledgment
The authors credit Julie Der Nigoghossian for assistance with graphic design.
Footnotes
Dr. Madhavan has received support from an institutional grant by the National Institutes of Health/National Heart, Lung, and Blood Institute to Columbia University Irving Medical Center (T32 HL007854). Dr. Bikdeli has served as a consulting expert, on behalf of the plaintiff, for litigation related to a specific type of inferior vena cava filters. Dr. Brodie has received research support from ALung Technologies; and has served on the Medical Advisory Boards of ALung Technologies, Baxter, BREETHE, Xenios, and Hemovent. Dr. Kirtane has received support from institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, Philips, and ReCor Medical. Dr. Stone has received speaking or other honoraria from Cook, Terumo, Qool Therapeutics, and Orchestra Biomed; has served as a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, and Matrizyme; and has received equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, and Valfix. Dr. Krumholz has worked under contract with the Centers for Medicare and Medicaid Services to support quality measurement programs; has received a research grant, through Yale, from Medtronic and the U.S. Food and Drug Administration to develop methods for post-market surveillance of medical devices; has received research grants from Medtronic; and has received a research grant from Johnson & Johnson, through Yale University, to support clinical trial data sharing; has received a research agreement, through Yale University, from the Shenzhen Center for Health Information for work to advance intelligent disease prevention and health promotion; has collaborated with the National Center for Cardiovascular Diseases in Beijing; has received payment from the Arnold & Porter Law Firm for work related to the Sanofi clopidogrel litigation; has received payment from the Ben C. Martin Law Firm for work related to the Cook CELECT inferior vena cava filter litigation; has received payment from the Siegfried and Jensen Law Firm for work related to Vioxx litigation; has chaired the Cardiac Scientific Advisory Board for UnitedHealth; was a participant/participant representative of the IBM Watson Health Life Sciences Board; has served on the Advisory Boards of Element Science and Facebook; has served on the Physician Advisory Board for Aetna; and cofounded HugoHealth, a personal health information platform, and Refactor Health, an enterprise health care artificial intelligence–augmented data enterprise. Dr. Parikh has received institutional grants/research support from Abbott Vascular, Shockwave Medical, TriReme Medical, Sumodics, Silk Road Medical, and the National Institutes of Health; has received consulting fees from Terumo and Abiomed; and has served on the Advisory Boards of Abbott, Medtronic, Boston Scientific, CSI, and Philips. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available at:
- 3.Biondi-Zoccai G., Landoni G., Carnevale R., Cavarretta E., Sciarretta S., Frati G. SARS-CoV-2 and COVID-19: facing the pandemic together as citizens and cardiovascular practitioners. Minerva Cardioangiol. 2020 Mar 9 doi: 10.23736/S0026-4725.20.05250-0. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 Mar 11 doi: 10.1007/s00392-020-01626-9. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 Mar 5 doi: 10.1038/s41569-020-0360-5. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 11 doi: 10.1016/S0140-6736(20)30566-3. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su S., Wong G., Shi W. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge X.Y., Li J.L., Yang X.L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H., Yang P., Liu K. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 Mar 3 doi: 10.1007/s00134-020-05985-9. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 Feb 19 doi: 10.1016/S1473-3099(20)30120-1. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Coronavirus Disease 2019 (COVID-19) Situation report—46. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-covid-19.pdf?sfvrsn=96b04adf_2 Available at:
- 14.Johns Hopkins University Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available at:
- 15.Zhang S., Diao M., Yu W., Pei L., Lin Z., Chen D. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: A data-driven analysis. Int J Infect Dis. 2020;93:201–204. doi: 10.1016/j.ijid.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 doi: 10.1001/jama.2020.2648. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 18.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L.Q., Huang T., Wang Y.Q. 2019 novel coronavirus patients' clinical characteristics, discharge rate and fatality rate of meta-analysis. J Med Virol. 2020 Mar 12 doi: 10.1002/jmv.25757. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 Feb 7 doi: 10.1001/jama.2020.1585. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. JAMA. 2020 Mar 11 doi: 10.1001/jama.2020.3633. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 Mar 3 doi: 10.1007/s00134-020-05991-x. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee P.I., Hu Y.L., Chen P.Y., Huang Y.C., Hsueh P.R. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020 Feb 25 doi: 10.1016/j.jmii.2020.02.011. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W., Zhang Q., Chen J. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12 doi: 10.1056/NEJMc2003717. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W.-H., Strych U., Hotez P.J., Bottazzi M.E. The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. 2020 Mar 3 doi: 10.1007/s40475-020-00201-6. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 Feb 7 doi: 10.1097/CM9.0000000000000744. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13 doi: 10.1001/jamainternmed.2020.0994. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan W.J., Ni Z.Y., Hu Y., for the China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 Feb 28 doi: 10.1056/NEJMoa2002032. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porcheddu R., Serra C., Kelvin D., Kelvin N., Rubino S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J Infect Dev Ctries. 2020;14:125–128. doi: 10.3855/jidc.12600. [DOI] [PubMed] [Google Scholar]
- 31.Govaert T.M., Thijs C.T., Masurel N., Sprenger M.J., Dinant G.J., Knottnerus J.A. The efficacy of influenza vaccination in elderly individuals: a randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–1665. [PubMed] [Google Scholar]
- 32.Liu W.M., van der Zeijst B.A., Boog C.J., Soethout E.C. Aging and impaired immunity to influenza viruses: implications for vaccine development. Hum Vaccin. 2011;7 Suppl:94–98. doi: 10.4161/hv.7.0.14568. [DOI] [PubMed] [Google Scholar]
- 33.Zidar D.A., Al-Kindi S.G., Liu Y. Association of lymphopenia with risk of mortality among adults in the US general population. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby P., Ridker P.M., Hansson G.K., Leducq Transatlantic Network on Atherothrombosis Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tall A.R., Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 38.Davis M.M., Taubert K., Benin A.L. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. J Am Coll Cardiol. 2006;48:1498–1502. doi: 10.1016/j.jacc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Liu R., Ming X., Xu O. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aslam S., Mehra M.R. COVID-19: yet another coronavirus challenge in transplantation. J Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:893–896. [PubMed] [Google Scholar]
- 42.Danziger-Isakov L.A., Husain S., Mooney M.L., Hannan M.M., ISHLT Infectious Disease Council The novel 2009 H1N1 influenza virus pandemic: unique considerations for programs in cardiothoracic transplantation. J Heart Lung Transplant. 2009;28:1341–1347. doi: 10.1016/j.healun.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Sarkisian L., Saaby L., Poulsen T.S. Clinical characteristics and outcomes of patients with myocardial infarction, myocardial injury, and nonelevated troponins. Am J Med. 2016;129:446. doi: 10.1016/j.amjmed.2015.11.006. e5–21. [DOI] [PubMed] [Google Scholar]
- 44.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 45.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 Feb 24 doi: 10.1016/S2213-2600(20)30079-5. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020 Mar 10 doi: 10.1016/j.pcad.2020.03.001. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36:78–80. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Feb 18 doi: 10.1016/S2213-2600(20)30076-X. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16 doi: 10.1093/eurheartj/ehaa190. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng J., Huang J., Pan L. How to balance acute myocardial infarction and COVID-19: the protocols from Sichuan Provincial People’s Hospital. Intensive Care Med. 2020 Mar 11 doi: 10.1007/s00134-020-05993-9. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood S. COVID-19 and the Heart: Insights from the Front Lines. TCTMD. March 12, 2020. https://www.tctmd.com/news/covid-19-and-heart-insights-front-lines Available at:
- 53.Welt F.G.P., Shah P.B., Aronow H.D. Catheterization laboratory considerations during the coronavirus (COVID 19) pandemic: from tACC’s Interventional Council and SCAI. J Am Coll Cardiol. 2020 Mar 16 doi: 10.1016/j.jacc.2020.03.021. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen C., Zhou Y., Wang D.W. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020 Mar 5 doi: 10.1007/s00059-020-04909-z. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buzon J., Roignot O., Lemoine S. Takotsubo cardiomyopathy triggered by influenza A virus. Intern Med. 2015;54:2017–2019. doi: 10.2169/internalmedicine.54.3606. [DOI] [PubMed] [Google Scholar]
- 56.Zompatori M., Ciccarese F., Fasano L. Overview of current lung imaging in acute respiratory distress syndrome. Eur Respir Rev. 2014;23:519–530. doi: 10.1183/09059180.00001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson N.D., Fan E., Camporota L. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 58.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 59.Karmpaliotis D., Kirtane A.J., Ruisi C.P. Diagnostic and prognostic utility of brain natriuretic peptide in subjects admitted to the ICU with hypoxic respiratory failure due to noncardiogenic and cardiogenic pulmonary edema. Chest. 2007;131:964–971. doi: 10.1378/chest.06-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacLaren G., Fisher D., Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020 Feb 19 doi: 10.1001/jama.2020.2342. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research. 2020 Apr 5 doi: 10.1016/j.thromres.2020.04.013. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Feb 19 doi: 10.1111/jth.14768. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan B.E., Chong V.C.L., Chan S.S.W. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020 Mar 4 doi: 10.1002/ajh.25774. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 64.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020 Mar 27 doi: 10.1111/jth.14817. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witt D.M., Nieuwlaat R., Clark N.P. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2:3257–3291. doi: 10.1182/bloodadvances.2018024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu C.M., Cheng V.C., Hung I.F., for the HKU/UCH SARS Study Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Byetta. [Package insert] Amylin Pharmaceuticals Inc.; San Diego, CA: 2007. [Google Scholar]
- 69.KALETRA(R) Oral Film Coated Tablets, Oral Solution, Lopinavir Ritonavir Oral Film Coated Tablets, Oral Solution. [Package insert] AbbVie Inc.; North Chicago, IL: 2013. [Google Scholar]
- 70.DeCarolis D.D., Westanmo A.D., Chen Y.C., Boese A.L., Walquist M.A., Rector T.S. Evaluation of a potential interaction between new regimens to treat hepatitis C and warfarin. Ann Pharmacother. 2016;50:909–917. doi: 10.1177/1060028016660325. [DOI] [PubMed] [Google Scholar]
- 71.Frost C.E., Byon W., Song Y. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br J Clin Pharmacol. 2015;79:838–846. doi: 10.1111/bcp.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mueck W., Kubitza D., Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76:455–466. doi: 10.1111/bcp.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itkonen M.K., Tornio A., Lapatto-Reiniluoto O. Clopidogrel increases dasabuvir exposure with or without ritonavir, and ritonavir inhibits the bioactivation of clopidogrel. Clin Pharmacol Ther. 2019;105:219–228. doi: 10.1002/cpt.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marsousi N., Daali Y., Fontana P. Impact of boosted antiretroviral therapy on the pharmacokinetics and efficacy of clopidogrel and prasugrel active metabolites. Clin Pharmacokinet. 2018;57:1347–1354. doi: 10.1007/s40262-018-0637-6. [DOI] [PubMed] [Google Scholar]
- 75.Brilinta (Ticagrelor). [Prescribing information] AstraZeneca LP; Wilmington, DE: July 2011. [Google Scholar]
- 76.Brilinta (Ticagrelor). [Product monograph] AstraZeneca Canada Inc.; Mississauga, Ontario: May 2011. [Google Scholar]
- 77.Angiolillo D.J., Rollini F., Storey R.F. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation. 2017;136:1955–1975. doi: 10.1161/CIRCULATIONAHA.117.031164. [DOI] [PubMed] [Google Scholar]
- 78.Kengreal. [Package insert] Chiesi USA, Inc.; Cary, NC: 2015. [Google Scholar]
- 79.Mulangu S., Dodd L.E., Davey R.T., Jr. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 82.Page R.L., 2nd, O'Bryant C.L., Cheng D. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e32–e69. doi: 10.1161/CIR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 83.Tonnesmann E., Kandolf R., Lewalter T. Chloroquine cardiomyopathy—a review of the literature. Immunopharmacol Immunotoxicol. 2013;35:434–442. doi: 10.3109/08923973.2013.780078. [DOI] [PubMed] [Google Scholar]
- 84.Day M. COVID-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 85.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4 doi: 10.1002/ddr.21656. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferrario C.M., Jessup J., Chappell M.C. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 88.van Doremalen N., Bushmaker T., Morris D. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Mar 17 doi: 10.1056/NEJMc2004973. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chow T.T., Kwan A., Lin Z., Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006;64:371–378. doi: 10.1016/j.jhin.2006.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adams J.G., Walls R.M. Supporting the health care workforce during the COVID-19 global epidemic. JAMA. 2020 Mar 12 doi: 10.1001/jama.2020.3972. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 91.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020 Mar 11 doi: 10.1056/NEJMp2003539. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 92.Rimmer A. Covid-19: medical conferences around the world are cancelled after US cases are linked to Massachusetts meeting. BMJ. 2020;368:m1054. doi: 10.1136/bmj.m1054. [DOI] [PubMed] [Google Scholar]
- 93.American College of Cardiology COVID-19 Clinical Guidance for the Cardiovascular Care Team. https://www.acc.org//∼/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/2020/02/S20028-ACC-Clinical-Bulletin-Coronavirus.pdf Available at:
- 94.European Society of Cardiology Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. March 13, 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang Available at:
- 95.European Society of Hypertension. Statement of the European Society of Hypertension (ESH) on hypertension. Renin Angiotensin System blockers and COVID-10. Available at: https://www.eshonline.org/spotlights/esh-statement-on-covid-19/. Accessed on March 12, 2020.
- 96.Hypertension Canada’s Statement on: Hypertension, ACE-Inhibitors and Angiotensin Receptor Blockers and COVID-19. March 13, 2020. https://hypertension.ca/wp-content/uploads/2020/03/2020-30-15-Hypertension-Canada-Statement-on-COVID-19-ACEi-ARB.pdf Available at:
- 97.Canadian Cardiovascular Society COVID-19 and concerns regarding use of ACEi/ARB/ARNi medications for heart failure or hypertension. https://www.conferenceharvester.com/Uploads/Documents/9006/CCSCHFSstatementregardingCOVID.pdf Available at:
- 98.International Society of Hypertension A Statement From the International Society of Hypertension on COVID-19. https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/ Available at:
- 99.Tam C.F., Cheung K.S., Lam S. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020 doi: 10.1161/CIRCOUTCOMES.120.006631. CIRCOUTCOMES120006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang Z., Yi F., Wu K. Clinical characteristics of coronavirus pneumonia 2019 (COVID-19): an updated systematic review. March 12, 2020. Available at: [DOI]
- 101.Lu Y., Wang P., Zhou T. Comparison of prevalence, awareness, treatment, and control of cardiovascular risk factors in China and the United States. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williams B., Mancia G., Spiering W. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]