Highlights
-
•
There is a high rate of visceral leishmaniasis (VL) relapse/recurrence among patients with concomitant HIV co-infection.
-
•
Fever, the most common symptom of VL is less frequent with relapse of VL. In contrast, there is high tissue parasite load.
-
•
Most of these patients need longer duration of treatment and combination regimens to achieve cure.
-
•
There is an urgent need to look for alternative better treatment options.
Abstract
Background
Human visceral leishmaniasis (VL) is a life-threatening protozoan disease caused by parasites belonging to the Leishmania donovani complex. Ethiopia has the highest VL-HIV co-infection rate in the world, with several of these patients presenting with repeated episodes of VL disease (ie, relapse). However, we lack data on how HIV patients with multiple VL relapse present clinically, and whether they continue to respond to currently available medicines.
Methods
The medical records of VL-HIV co-infected patients with multiple VL relapses at the Leishmaniasis Treatment and Research Center in Gondar, Ethiopia, between June 2012 and June 2016 were retrieved. Variables on their clinical and laboratory profiles were collected. Descriptive analysis was done to show the characteristics of the VL episodes.
Result
A total of 48 VL episodes in 12 patients were identified, the median number of episodes per patient was 5 (interquartile range, 4–8 episodes). The median time to relapse was 5 months (interquartile range, 3–5.5 months). Splenomegaly was present in 47 of the episodes (98%), fever or other accompanying symptoms were present in only 66% (32 out of 48). The median tissue parasite grade at VL diagnosis was 6+ (interquartile range, 5+– 6+). All patients were on antiretroviral therapy. The median duration of treatment per episode was 2 months (interquartile range, 2–2 months). All patients achieved parasitological cure at discharge at each episode.
Conclusions
Multiple recurrences of VL diseases were observed in HIV co-infected patients. With recurrent episodes, splenomegaly was found to be the main manifestation, whereas fever was less common. These patients came with recurrence of diseases in <6 months and required prolonged treatment to achieve cure.
Further research on prediction, prevention, and better management options for recurrent VL is needed. ORCID ID: https://orcid.org/0000-0002-1410-0454. (Curr Ther Res Clin Exp. 2020; 81:XXX–XXX)
Introduction
Human visceral leishmaniasis (VL) or kala-azar is a life-threatening protozoan disease caused by parasites belonging to the Leishmania donovani complex.1 More than 550 million people worldwide are at risk of developing the disease that is transmitted by the bite of a phlebotomine sand fly. The parasite targets tissue macrophages, leading to the typical manifestations of chronic fever, hepatosplenomegaly, and pancytopenia. VL is fatal if left untreated. In the Indian subcontinent and Eastern Africa, VL is caused by L donovani, and it is transmission is anthroponotic.1
Globally, the VL-HIV co-infection rate is estimated to be 2% to 9% in endemic areas but can reach more than 15% in some parts of northwest Ethiopia.2,3 More than 2500 VL cases are reported annually in Ethiopia, but underreporting seems likely.4 High treatment failure rates, a slow response to treatment, high mortality, multiple relapse, and atypical presentations are some of the common challenges in patients with VL-HIV co-infection.3,5, 7, 7 The main risk factors for relapse are a previous episode of VL and low CD4 count. The initiation of antiretroviral therapy (ART) and secondary prophylaxis can improve the relapse-free survival.3,8,9
Despite the increasing availability of VL drugs, ART and secondary prophylaxis, clinicians are confronted with HIV and other immunosuppressed patients developing multiple relapses, which present with substantial therapeutic challenges. Only a few case reports on multiple VL-HIV relapses have been published, mainly from Europe where L infantum is endemic.10,11 As to L donovani, we found 1 single case report from India12 and none from East Africa. Consequently, we lack data on how HIV patients with multiple VL relapse present clinically, and whether they continue to respond to currently available medicines. Given their repeated and prolonged exposure to various antileishmanial drugs, such cases could represent a source and reservoir of drug-resistant parasites. Here, we report 12 cases of HIV patients with frequent (>3 episodes) VL relapses of which at least 2 were treated at Leishmaniasis Research and Treatment Center (LRTC) in Gondar, Ethiopia.
Methods
Study setting
This study was carried out at LRTC of the University of Gondar. LRTC was established in collaboration to Drug for Neglected Diseases initiative in 2005. It is among the main VL treatment and clinical trial sites in northwest Ethiopia. A total of 300 to 400 VL patients are treated every year. The average HIV co-infection rate from monthly reports of the center ranges from 10% to 20%. It is run by Good Clinical Practice trained doctors, nurses, and other supportive staff. The laboratory technologists are also trained on Good Clinical Laboratory Practice. Standard operating procedures are in place. Monitoring and supervisions are conducted from time to time by sponsors and regulatory authorities. The paper medical records of treated patients are archived in the center. Anti-Leishmania medicines are available through supportive organizations such as Drug for Neglected Diseases initiative, Medecins san Frontieres, and the World Health Organization.
Study design and population
A retrospective, cohort, descriptive study was conducted entailing the medical records of HIV patients with frequent VL relapse (>3 episodes) and who have been treated at least 2 times at LRTC between June 2012 and June 2016.
VL diagnosis, treatment, and follow-up
At LRTC, VL is diagnosed by demonstration of the amastigote form of the parasites in spleen or bone marrow aspiration (70% and 30%, respectively).13 Splenic aspiration has a high diagnostic yield (93%–99%) and is the technique of choice. Bone marrow aspiration is used in case of contraindications for splenic aspiration.14 Parasite quantification is carried out according to grading of the World Health Organization technical report.15
The first-line regimen for VL was 17 days of sodium stibogluconate (SSG) and paromomycin (PM) injections. For VL patients with HIV co-infection, liposomal amphotericin B (L-AmpB) (30–40 mg/kg over 12 or 24 days) was preferably used for safety reasons.14,15 However, when there was scarcity of L-AmpB, SSG with or without PM combination might be used. Often, L-AmpB was reserved for the most severely sick patients. More recently, L-AmpB was used in combination with miltefosine PO for 28 days. In exceptional situations, unresponsive patients have been treated, on compassionate grounds, with pentamidine (4 mg/kg for 15 doses on alternative days), available through pentamidine secondary prophylaxis clinical trial.16,17 Pentamidine secondary prophylaxis trial was done to prevent VL relapse in HIV patients with low CD4 count while being on ART after initial cure of VL using monthly administration of pentamidine. Patients are appointed for regular follow-up every 3 months in the first year of treatment or earlier if they become symptomatic.
HIV diagnosis, treatment, and follow-up
According to the Ethiopian national guideline, HIV screening is done in all outpatient and inpatient departments of the hospital. All VL patients diagnosed with HIV will be started on ART. During VL treatment patient will be linked to the ART clinic in the hospital, where they will get adherence counseling, ART dispensing, CD4 and viral load measurement, social support, and referrals if needed.18 HIV viral load testing was not readily available and was done when resources allowed.
Data and statistical analysis
Medical chart number of HIV VL patients treated at LRTC were extracted from a paper leishmaniasis registration book. Using their chart number, paper medical charts were collected from the chart room, located at LRTC. From these medical charts, preset data variables on sociodemographic characteristics, clinical and laboratory profile, and treatment history of each patient was collected and entered into an Excel (Microsoft Corp, Redmond, Washington) sheet. The treating physicians (RM, HF, ED, and TM) interchangeably enter or double-check data entered in to Excel sheet for consistency and completeness compared with the paper medical records Descriptive analysis was done using frequency and percentages. The median and interquartile range (IQR) were calculated for numeric variables. Graphs were made using Excel version 15.
Ethical considerations
Ethical approval and waiver for consent was obtained from the institutional review board of University of Gondar to use this retrospective data from the medical charts of patients. The treating physicians, who are also the authors of this article, handled the patient data confidentially at all stages of the study.
Results
Over a 4-year period (from 2012 to 2016), completed records of 12 VL-HIV co-infected patients with more than 2 episodes of VL were found. These accounted for 48 treatment episodes. An illustrative case of typical frequent relapse is described in detail in the sections that follow, highlighting key features that are common to all the other patients.
Illustrative case
The first presentation of a 42-year-old male patient with HIV to the LRTC was in June 2012. He gave history of 2 VL treatments in the past, in 1996 and 1997. He was on ART (ie, tenofovir, lamivudine, and efavirenz) starting in 2008. Besides that, his medical history was unremarkable.
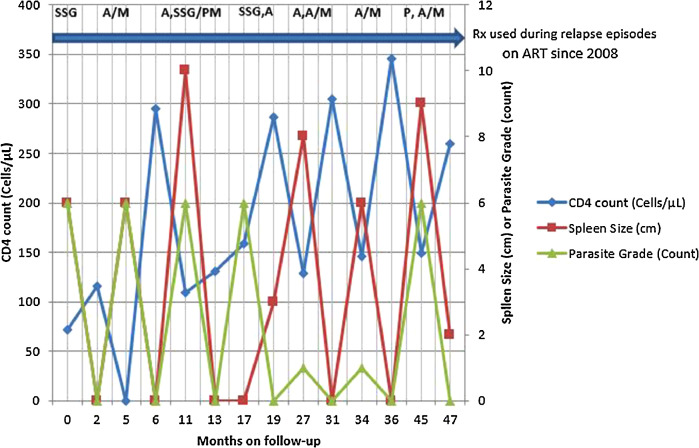
At presentation to LRTC, he had painless, nodular skin lesions involving the face and upper extremities, predominantly at the tip of the nose and on the elbows. On clinical examination, splenomegaly of 6 cm and multiple nodular lesions over the face and upper extremities was found.6 Microscopy from the skin lesions as well as a spleen aspirate showed L donovani bodies with a load of 6+. With this, second VL relapse (ie, the third VL episode) was diagnosed. At this diagnosis, his CD4 count was 72 cells/µL. After receiving 2 months of SSG treatment, he showed clinical improvement (eg, weight gain and spleen regression), the CD4 count increased to 116 cells/µL, there were no parasites in his bone marrow aspiration (spleen was not palpable) (see Figure 1).
Figure 1.
Evolution of CD4 count, spleen size, and Leishmania parasite grade during 7 visceral leishmaniasis (VL) relapses in an HIV co-infected patient. A = L-AmpB; A/M = A and M in combination therapy; A, SSG = first treatment course of L-AmpB followed by treatment with SSG; L-AmpB = liposomal amphotericin B; M = miltefosine; P = pentamidine; PM = paromomycin; SSG = sodium stiboglucinate. The arrow denotes use of antiretroviral treatment (ART).
Recruited into a clinical trial, the patient started to receive monthly pentamidine as a secondary prophylaxis after his initial cure for this episode of VL. On 3-month follow-up, enlarged spleen to a size of 6 cm below the coastal margin was detected. Otherwise, he did not have any subjective complaints and rather had increased hemoglobin (from 24.7 mg/dL to 38.0 mg/dL) and white blood cell count (from 1800/µL to 2900/µL) compared with the values at discharge. However, the splenic aspiration revealed 6+ grade LD bodies and the fourth VL episode was diagnosed. After treatment with L-AmpB and miltefosine combination therapy for 1 month, he was discharged cured (ie, repeat bone marrow aspirate showed no parasite), and nonpalpable spleen. The end of VL treatment, CD4 cell count was 295 cells/µL. The pentamidine prophylaxis was not continued.
After a relapse-free period of 5 months, during his routine follow-up visit, a splenomegaly of 10 cm was detected again. The spleen aspirate showed a grade +6 LD bodies when fifth VL episode of VL was diagnosed. At this time the CD4 count declined to 109 cells/µL. The patient was treated for 1 month with L-AmpB and showed only partial response with parasite grade decreased to 3+ on bone marrow aspiration. The treatment was continued with SSG/PM combination therapy for 17 days. Parasitological cure was achieved, the spleen was not palpable, and the CD4 increased to 131 cells/µL.
Four months after discharge, he presented with his sixth episode, again with a 6+ parasite grade, and CD4 count of 159 cells/µL. Treatment was started with SSG and changed to L-AmpB after 9 days due to increased creatinine levels. After 2 months of treatment with L-AmpB (2 courses of 40 mg/kg), the patient was cured and discharged with a CD4 count of 257 cells/µL. At this stage, HIV viral load test was done and it was undetectable.
After 8 relapse-free months, he presented again with no clinical symptoms but a splenomegaly of 8 cm below the costal margin. With parasite levels of 1+ in spleen aspirate at this time, he was treated for his seventh episode of VL. The CD4 count was 129 cells/µL before treatment for this episode. After 1 month of L-AmpB treatment, the parasite grade increased to 6+. The treatment with L-AmpB was prolonged for 1 more month. Although combination treatment with miltefonsine was planned, the latter medicine was not available at that time. Two months of treatment decreased the parasite grade to 1+. At this time, miltefosine was available and treatment continued in combination with L-AmpB to achieve parasitological cure. However, bone marrow parasite grade was 2+ after 1 month of the combination therapy requiring an additional month of treatment with the combination regimen before achieving parasitological cure. The CD4 count at end of this extended treatment episode was 305 cells/µL.
Three months after discharge, he presented again with splenomegaly of 6 cm, a 1+ parasite grade on spleen aspiration and a CD4 count of 146 cells/µL (eighth VL episode). He received 2 months of L-AmpB and miltefosine combination therapy and was discharged with non palpable spleen, no LD bodies on test of cure from bone marrow and a CD4 count of 346 cells/µL.
After 9 months, the patient presented again with splenomegaly of 9 cm and drop in his CD4 count to 149 cells/µL for his ninth episode of VL. During this episode he took 15 doses of 4 mg/kg pentamidine followed by L-AmpB and miltefosine treatment. The patient was discharged in May 2016 with nonpalpable spleen, improved hematogram, and CD4 count of 260 cells/µL.
Case series
In our cohort 12 patients had a total of 48 episodes, with the number of episodes ranging from 3 (n = 2) to 9 (n = 2) and with a median of 5 episodes (IQR, 4–8 episodes) per patient. The median age at first VL diagnosis was 31 years (IQR, 29.5–41 years) and all patients were men.
Relapse typically occurred in the first year after treatment of an episode of VL, with a median of 5 months (IQR, 3–5.5 months). Although splenomegaly was present in 98% (47 out of 48) of the episodes, spiking fever and other typical VL symptoms (such as weight loss) where absent in 33.3% (16 out 48) of the episodes. In 66.6% of the episodes, VL diagnosis was only suspected based on splenomegaly, altered hemogram, and/or declining CD4 counts. Treatment was typically associated with increases in the white blood cell count, the hematocrit, and the CD4 count. Tissue parasite counts at VL diagnosis were high, with a median of 6+ (IQR, 5+ to 6+).
Out of 48 episodes, baseline CD4 counts were available during the 41 episodes, with a value above 200 cells/µL in only 2 of the occasions. End of treatment CD4 count was available for the 39 episodes, with a value above 200 cells/µL in 15 occasions (Table 1). The median increase in CD4 count after treatment episode was 68 cells/µL (IQR, 27.5–129.5 cells/µL). Nine patients were on ART when diagnosed with VL for the first time at our center, and 3 patients started ART after they started VL treatment. In 2 cases, ART was changed to second line for virologically confirmed first-line treatment failure according to the national ART guidelines. Four other patients were found to have an undetectable viral load, 3 had high viral load (>1 million copies/mL) but were not changed to second-line ART (during the study period), and 3 were not tested (during the study period). In this cohort, only 1 patient received pentamidine as a secondary prophylaxis made available through a clinical trial after parasitological cure.
Table 1.
Overview of clinical and laboratory features, and treatment (Tx) details of patients with multiple visceral leishmaniasis protozoan disease relapses, Gondar, Ethiopia, 2012-2016.⁎
| Patient No. | No. of episodes | No. of episodes with fever | Pretreatment CD4 count (cells/µL) | CD4 change with Tx (cells/µL) | Median No. of WBC change with Tx (cells/µL) | HCT change with Tx (%) | Tx used at the site† | Median No. of courses of treatment | Duration between episodes (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 0 | 137 (114 to 148) | 120 (60 to 164) | 1100 | 1.7 (–3.5 to 4.6) | SSG; A/M; (A, SSG/PM); (SSG, A); (A, A/M); A/M; (P, A/M) | 2 | 5 (3 to 6.5) |
| 2 | 4 | 0 | 57 (57-57) | 23 (23- 23) | 400 | 11.9 (9.3-23.0) | (SSG, A/M); A/M; (A/M, A) | 2 | 8 (6.5 to 9.0) |
| 3 | 8 | 4 | 137 (105 to 180) | 79 (35 to 130) | 2450 | 2.25 (–1.9 to –6.5) | SSG; (A, SSG, A/M); (A, A/M); A/M; (A/M, SSG); A/M; P | 2 | 5 (3.2 to 9.7) |
| 4 | 9 | 3 | 48 (40 to 56) | Uk | –900 | –9.15 (–12 to –5) | (A, A/M, SSG/A); (A/M, SSG); (A/M/SSG); (A/M, P) | 3 | 8 (6.7 to 9.5) |
| 5 | 5 | 1 | Uk | Uk | 5500 | 7.3 (7.3 to 7.3) | (SSG, A); A/M | 1.6 | 7 (5.3 to 7.8) |
| 6 | 5 | 4 | 48 (36 to 55) | 11 (–32 to –31) | 1300 | 4.6 (–5.3 to –7.5) | (SSG, A/M); A; (A/M, A)(A, A/M); A/M | 2 | 3 (3.0) |
| 7 | 5 | 5 | 5 (4 to 7) | 22 (9 to 32) | 1300 | 3.4 (–8.6 to 9.7) | SSG/PM; A/M; A/M; A/M | 1 | 3 (3.0 to 4.0) |
| 8 | 4 | 4 | 69 (64 to 72) | 94 (82 to 180) | 1500 | 3.4 (2.5 to 4.0) | A/M; A/M; A/M; (A/M, SSG) | 2 | 1 (0.7 to 1.7) |
| 9 | 8 | 3 | 91 (46 to 96) | 79 (53 to 105) | 2700 | –10.4 (–24.3 to –4) | A/M; (A/M, SSG); SSG | 2 | 5 (4.5 to 6.0) |
| 10 | 3 | 3 | 123 (88 to 126) | 85(73 to 86) | 1200 | 1.4 (–4 to 1.7) | A/M; A/M; A/M | 2 | 3 (1.5 to 3.5) |
| 11 | 5 | 2 | 94 (81 to 94) | 67 (45 to 146) | 1500 | 0.1 (–1.1 to 0.5) | (A, A/M); A/M; SSG | 3 | 3 (1.5 to 3.5) |
| 12 | 3 | 3 | 115 (74 to 124) | 196 (139 to 230) | 1000 | 6.3 (5.7 to 8.3) | A; A/M; A/M | 1 | 7 (3.5 to 10.0) |
A = Liposomal amphotericin B; LRTC = Leishmaniasis Research and Treatment Center; M = miltefosine; P = pentamidine; PM = paromomycin; SSG = sodium stibogluconate; Uk = unknown.
Unless otherwise noted, values are presented as median (interquartile range).
Tx given for each episode is separated by a semi-colon and if more than 1 Tx regimen was given during an episode, these are grouped by parenthesis. For example, SSG; A/M; (A, SSG/PM); refers to a patient who received SSG during the first episode, A/miltefosine during the second episode, and was treated during the third episode with A monotherapy, followed by Tx with SSG/PM combination therapy.
In 30 of the episodes, a single course of treatment; that is, up to 1 month duration was used. The regimens were L-AmpB in 3, L-AmpB and miltefosine combination in 21, antimonials in 4, and pentamidine in 2 of the VL episodes. In the remaining 18 episodes, 2 or 3 courses of treatment (more than 1 month duration) were required to achieve cure. These included treatment with L-AmpB monotherapy followed by SSG. Among all 48 episodes, only 13 episodes attained an initial cure with the standard course of treatment (1 month duration). Most episodes (72.9% [35 out of 48]) required longer duration of treatment, with a median of 2 months (IQR, 2–2 months) (Table 1).
Discussion
In this case series we describe a subgroup of VL-HIV patients presenting with repeated relapses, despite achieving initial parasitological cure with antileishmanial treatments and initiation of ART. For patients in our cohort, the clinical presentation was not typical for patients who present with first episode of VL. Fever was only seen in 66.6% of patients, whereas most patients (95.7%) who present with first episode of VL have fever.19 Almost all patients (98%) presented with gradually increasing spleen size. Whether this indicates a form of primary immunological deficit of the host—leading to the multiple relapses—or a kind of tolerance toward the parasite, requires more research.
An interesting feature was that around 40% of patients were discharged with a CD4 count ≥200 cells/µL—a level considered to provide some protection against relapse—but nevertheless they returned with a relapse and decreased CD4 counts. Typically, a clear increase in CD4 counts was seen after VL treatment. This most likely relates to the fact that VL can induce lymphocytopenia, although it could also be related to immunological changes in VL that may lead to an increased HIV-related destruction of CD4 cells because VL and HIV have been known to mutually reinforce each other.2
Thirty-five out of 48 (72.9%) episodes were treated with combination and multiple courses of drugs. Although, L-AmpB and miltefosine were preferred for safety reasons, these have limited availability (at least during the study period) in our context and moreover, seem to be insufficiently effective in some situations. Treatment duration to achieve parasitological cure was generally long (on average 2 months) compared with the length of treatment duration in nonrelapsing patients, which is 1 month. Moreover, the effect of repeated exposure to multiple different drugs on the parasite is not well known. A major concern is that the repeated treatment with the same drugs could lead to emergence of drug resistance. As transmission is considered to be anthroponotic in East Africa, emerging resistance in HIV co-infected patients could potentially spread more widely to the general population. We did not observe a progressive decrease in treatment response across the different episodes, although patients require prolonged treatment to achieve cure. This requires detailed longitudinal studies on the genotypic and phenotypic characterization of parasites in HIV patients undergoing repeated and prolonged treatment for VL. Phenotypic studies have only been reported from L infantum endemic regions in Europe. Although 1 study reported decreased drug sensitivity toward amphotericin B after repeated treatment courses,20 this was not confirmed in other studies.21,22
Even after achieving fair CD4 count levels and HIV virological suppression, these patients do not keep the parasite under control, even months after initiation of ART. Therefore, immunological studies on why these patients continue to relapse are required and a better understanding of the immunological processes driving to escape parasite control need to be developed. Our results indicate the need for effective secondary prophylaxis in such patients. Pentamidine was found effective, safe, and feasible to reduce relapse rates in a study in Ethiopia.16,17 However, in this study, prophylaxis was discontinued once relapse—the main outcome—had occurred. Whether continuing pentamidine secondary prophylaxis, despite the development of relapse while on the prophylaxis, has any value in such patients requires further study. Alternatively, other anti-Leishmania drugs could be used for secondary prophylaxis for such patients. However, the currently available other anti-Leishmania medicines are used for first or second line treatment in East Africa. Therefore, this strategy could entail the risk of promoting the emergence of drug resistance against the key drugs, which could then spread more widely, compromising the efficacy of the currently used VL drugs in East Africa. This is why for East Africa, pentamidine was used for secondary prophylaxis in the above-mentioned studies because it is currently not used for first- or second-line VL treatment.
Combined with the fact that patients with frequent relapse had relatively few clinical symptoms, this raises the question whether these patients should be treated each time when presenting with splenomegaly and parasitologically confirmed relapse. To treat or not has been a recurring discussion amongst clinicians caring for patients with multiple relapses as described in this article. On one hand, it might feel useless to treat patients coming back every few months with a VL relapse until parasitological cure, knowing the patient to relapse again too soon. On the other hand, there are also a number of considerations that favor of treating patients with LV. Historically, most patients in whom treatment was withheld often showed deterioration of the clinical condition over time. In some cases, VL progressed over time. Others displayed persistently low CD4 counts and subsequently presented with other opportunistic infections such as tuberculosis, chronic diarrheal diseases, and cryptococcal meningitis. Thus, early detection of relapse and treatment might be preferable. We admit that these statements are based on our limited personal experience and discussions on this problematic issue with experts around the world would be useful.
Active chronic form of VL has been described using molecular diagnosis and culture for Leishmania in immune-compromised patients after demonstration of the persistent multiplication of the parasite both during asymptomatic period and clinical episodes of endemic disease.10 Thus, despite the parasitological cure demonstrated on microscopy in these patients, there is high possibility of ongoing multiplication of the parasite during what seemed to be disease-free period. Whether prolonged treatment after achieving parasitological cure—to achieve a deeper cure—combined with secondary prophylaxis has an additional value also remains unknown. Whereas randomized controlled trials might provide an answer here, the fact that such patients are relatively rare might require multisite or multicountry studies. Whether or not withholding treatment is ethical must certainly be discussed carefully when engaging in such a study.
The strengths of our study relate to the fact that because it was conducted in a clinical research center with Good Clinical Practice experience and the quality of the data collected was good. There are several important limitations to the study. First, it only provides information on patients deciding to re-present to our center, therefore we cannot claim that patients in our study are necessarily representative for all VL-HIV co-infected patients with multiple relapses in Ethiopia. Second, treatment was not standardized but also depended on the availability of drugs, and secondary prophylaxis was only provided to 1 patient who was enrolled in an ongoing trial by then. Third, drug resistance studies would have enriched our findings, as well as identifying the genotypic characterization of the parasites to assess the prevalence of reinfection versus relapse caused by the same parasite. Fourth, HIV viral load could not be monitored routinely due to the limited availability of the service. Fifth, there is no adherence check for ART after discharge, which may be responsible for the CD4 decline and the frequency of relapse.
Conclusions
With multiple relapses of VL in HIV co-infected patients, fever was not a prominent clinical feature. Most patients presented with gradually increasing spleen size and no other apparent symptom or sign. This may lead to miss diagnosis and/or delayed diagnosis. Whether to treat each relapse episode of VL in HIV patients presenting with multiple parasitologically confirmed VL remains unclear and requires further systematic studies. If left untreated, VL is universally fatal. However, when treated, such patients require combination drugs and prolonged VL treatment to achieve parasitological cure. Repeated drug exposure carries a risk of drug resistance.
Declaration of Competing Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
Acknowledgment
The authors thank all Leishmaniasis Treatment and Research Center staff members who are actively involved in the care of these patients. The authors also thank the Drugs for Neglected Disease Initiative and University of Gondar for supporting the Leishmaniasis Research and Treatment Center.
E. Diro, R. Mohammed, and J. van Griensven conceived and designed the work. R. Mohammed, E. Diro, T. Mekonnen, and H. Fikre performed the data collection. R. Mohammed, E. Diro, J. van Griensven, and A. Schuster analyzed the data. E. Diro, R. Mohammed, J. van Griensven, and A. Schuster wrote the manuscript. All authors read and approved the final manuscript.
Footnotes
These authors contributed equally to this work.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2020.100583.
Appendix A. Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- 1.van Griensven J, Diro E. Visceral leishmaniasis. Infect Dis Clin North Am. 2012 Jun;26(2):309–322. doi: 10.1016/j.idc.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet J-P. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008 Apr;21(2):334–359. doi: 10.1128/CMR.00061-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diro E, Lynen L, Ritmeijer K, Boelaert M, Hailu A, van Griensven J. Visceral Leishmaniasis and HIV coinfection in East Africa. PLoS Negl Trop Dis. 2014 Jun;8(6):e2869. doi: 10.1371/journal.pntd.0002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abongomera C, Diro E, Vogt F, Tsoumanis A, Mekonnen Z, Admassu H. The Risk and Predictors of Visceral Leishmaniasis Relapse in Human Immunodeficiency Virus-Coinfected Patients in Ethiopia: A Retrospective Cohort Study. Clin Infect Dis. 2017 Oct;65(10):1703–1710. doi: 10.1093/cid/cix607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diro E, van Griensven J, Mohammed R, Colebunders R, Asefa M, Hailu A. Atypical manifestations of visceral leishmaniasis in patients with HIV in north Ethiopia: a gap in guidelines for the management of opportunistic infections in resource poor settings. Lancet Infect Dis. 2015 Jan;15(1):122–129. doi: 10.1016/S1473-3099(14)70833-3. [DOI] [PubMed] [Google Scholar]
- 7.Diro E, Lynen L, Mohammed R, Boelaert M, Hailu A, van Griensven J. High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS Negl Trop Dis. 2014 May;8(5):e2875. doi: 10.1371/journal.pntd.0002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cota GF, de Sousa MR, Rabello A. Predictors of visceral leishmaniasis relapse in HIV-infected patients: a systematic review. PLoS Negl Trop Dis. 2011 Jun;5(6):e1153. doi: 10.1371/journal.pntd.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ter Horst R, Collin SM, Ritmeijer K, Bogale A, Davidson RN. Concordant HIV infection and visceral leishmaniasis in Ethiopia: the influence of antiretroviral treatment and other factors on outcome. Clin Infect Dis. 2008 Jun;46(11):1702–1709. doi: 10.1086/587899. [DOI] [PubMed] [Google Scholar]
- 10.Bourgeois N, Bastien P, Reynes J, Makinson A, Rouanet I, Lachaud L. “Active chronic visceral leishmaniasis” in HIV-1-infected patients demonstrated by biological and clinical long-term follow-up of 10 patients. HIV Med. 2010 Nov;11(10):670–673. doi: 10.1111/j.1468-1293.2010.00846.x. [DOI] [PubMed] [Google Scholar]
- 11.Bourgeois N, Lachaud L, Reynes J, Rouanet I, Mahamat A, Bastien P. Long-term monitoring of visceral leishmaniasis in patients with AIDS: relapse risk factors, value of polymerase chain reaction, and potential impact on secondary prophylaxis. J Acquir Immune Defic Syndr. 2008 May;48(1):13–19. doi: 10.1097/QAI.0b013e318166af5d. [DOI] [PubMed] [Google Scholar]
- 12.Patole S, Burza S, Varghese GM. Multiple relapses of visceral leishmaniasis in a patient with HIV in India: a treatment challenge. Int J Infect Dis. 2014 Aug;25:204–206. doi: 10.1016/j.ijid.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Diro E, Lynen L, Assefa M, Takele Y, Mengesha B, Adem E. Impact of the use of a rapid diagnostic test for visceral leishmaniasis on clinical practice in Ethiopia: a retrospective study. PLoS Negl Trop Dis. 2015 May;9(5):e0003738. doi: 10.1371/journal.pntd.0003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federal Ministry of Health E . Guidelines for diagnosis, treatment and prevention of leishmaniasis in Ethiopia. 2nd edition. Ethiopia; Addis Adaba: 2013. [Google Scholar]
- 15.WHO/Expert Committee on the Control of Leishmaniases, Marinkelle CJ. The control of leishmaniases. Bull World Health Organ [Internet] 1980;58(6):807–818. http://www.who.int/neglected_diseases/resources/who_trs_949/en/ Available from: [PMC free article] [PubMed] [Google Scholar]
- 16.Diro E, Ritmeijer K, Boelaert M, Alves F, Mohammed R, Abongomera C. Use of Pentamidine As Secondary Prophylaxis to Prevent Visceral Leishmaniasis Relapse in HIV Infected Patients, the First Twelve Months of a Prospective Cohort Study. PLoS Negl Trop Dis. 2015;9(10):e0004087. doi: 10.1371/journal.pntd.0004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diro E, Ritmeijer K, Boelaert M, Alves F, Mohammed R, Abongomera C. Long-term Clinical Outcomes in Visceral Leishmaniasis/Human Immunodeficiency Virus-Coinfected Patients During and After Pentamidine Secondary Prophylaxis in Ethiopia: A Single-Arm Clinical Trial. Clin Infect Dis. 2018 Jan;66(3):444–451. doi: 10.1093/cid/cix807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health of the Federal Democratic Republic of Ethiopia . National Consolidated Guidelines for Comprehensive HIV Prevention, Care and Treatment. Ministry of Health; Ethiopia: 2018. [Google Scholar]
- 19.Hurissa Z, Gebre-Silassie S, Hailu W, Tefera T, Lalloo DG, Cuevas LE. Clinical characteristics and treatment outcome of patients with visceral leishmaniasis and HIV co-infection in northwest Ethiopia. Trop Med Int Health. 2010 Jul;15(7):848–855. doi: 10.1111/j.1365-3156.2010.02550.x. [DOI] [PubMed] [Google Scholar]
- 20.Di Giorgio C, Faraut-Gambarelli F, Imbert A, Minodier P, Gasquet M, Dumon H. Flow cytometric assessment of amphotericin B susceptibility in Leishmania infantum isolates from patients with visceral leishmaniasis. J Antimicrob Chemother. 1999 Jul;44(1):71–76. doi: 10.1093/jac/44.1.71. [DOI] [PubMed] [Google Scholar]
- 21.Lachaud L, Bourgeois N, Plourde M, Leprohon P, Bastien P, Ouellette M. Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin Infect Dis. 2009 Jan;48(2):e16–e22. doi: 10.1086/595710. [DOI] [PubMed] [Google Scholar]
- 22.Durand R, Paul M, Pratlong F, Rivollet D, Dubreuil-Lemaire ML, Houin R. Leishmania infantum: lack of parasite resistance to amphotericin B in a clinically resistant visceral leishmaniasis. Antimicrob Agents Chemother. 1998 Aug;42(8):2141–2143. doi: 10.1128/aac.42.8.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/