Abstract
Meningiomas are common intracranial tumors that rarely metastasize. We present a highly unusual case of a 42-year-old man with direct seeding of meningioma to the abdominal wall. The patient had a history of multiple operations for a recurrent intracranial meningioma with decompressive craniectomy and preservation of the calvarial bone flap by implantation into the subcutaneous layer of the anterior abdominal wall. Following removal of the bone flap, a new abdominal wall mass was identified, consistent with iatrogenic implantation of anaplastic meningioma.
Keywords: Anaplastic meningioma, Metastatic meningioma, Calvarial bone flap preservation, Central nervous system neoplasms
Introduction
Meningiomas are common intracranial tumors that arise from the arachnoid cap cells of the meninges [1]. Most meningiomas are benign, slowly growing tumors that can be cured by neurosurgical resection. Metastases occur in less than 1 in 1000 cases [2] and can arise via several mechanisms: dissemination via the cerebrospinal fluid, blood, or lymph, or iatrogenic seeding of surgical tracts [2].
The World Health Organization (WHO) stratifies meningiomas into three major groups, reflected by the WHO grades I (benign), II (atypical), and III (malignant). The WHO classification is based on morphologic criteria and is an important predictor of survival.
We present a case of grade II/III meningioma presenting in the abdominal wall following resection of recurrent intracranial meningioma with decompressive craniectomy and subsequent cranioplasty. In the interval between the craniectomy and cranioplasty, the calvarial bone flap had been preserved by implantation in the subcutaneous space of the abdominal wall.
Case report
In 2016, a 39-year-old male presented initially to an outside institution with right hand weakness and was found to have a large extra-axial mass along the left parietal convexity. He underwent gross total resection of the tumor, which was determined upon pathological examination to be an atypical meningioma, WHO grade II.
Follow-up MRI performed approximately 9 months following the resection revealed 2 recurrent enhancing extra-axial masses with broad dural attachment underlying and at the margins of the prior craniotomy. The larger mass measured up to 4.7 cm, produced mass effect upon and reactive edema within the underlying right parietal lobe parenchyma, and extended superficially into the craniotomy kerf, elevating the bone flap (Fig. 1). Repeat resection was performed 4 months following this imaging. Due to significant brain swelling at the time of surgery, a decompressive craniectomy was performed, and the calvarial bone flap was implanted into the subcutaneous tissues of the right anterior abdominal wall for preservation while the parenchymal edema subsided. Pathological examination of the recurrent tumor revealed anaplastic meningioma, WHO grade III, which indicated histologic progression from the original tumor.
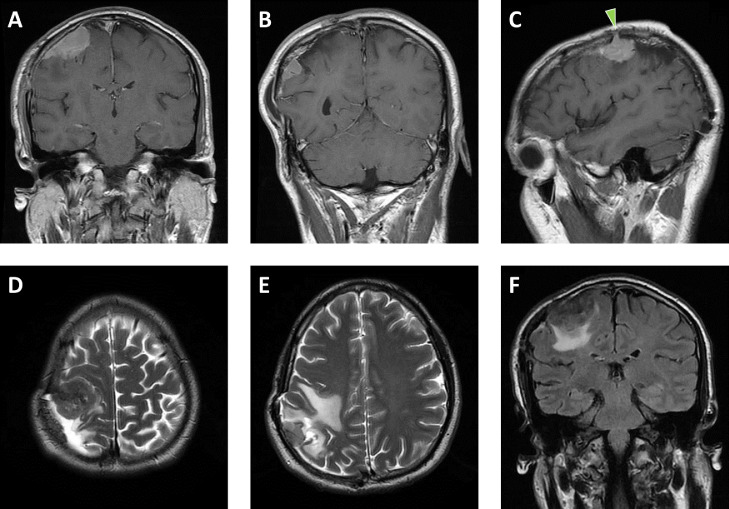
Fig. 1.
Initial meningioma recurrence. Coronal postcontrast T1-weighted MR images of the brain (A, B) depict 2 dural-based, enhancing masses underlying a right parietal craniotomy flap. Sagittal postcontrast T1-weighted image (C) illustrates one of these masses extending into the craniotomy kerf (green arrowhead). Axial T2-weighted images (D, E) depict areas of hypointensity within the masses and reactive edema in the underlying parietal lobe, which is also highlighted on the coronal fluid-attenuated inversion recovery image (F). The edema results in moderate elevation of the craniotomy flap (B, E). (Color version of figure is available online.)
Three months later, after the brain edema had decreased, the bone flap was retrieved from the abdominal wall and cranioplasty was performed. The bone flap was left unanchored due to concern for future recurrent swelling and increased pressure. The patient then underwent a course of radiation therapy targeting the site of resected anaplastic meningioma.
He was thereafter lost to neurosurgical follow-up for nearly a year, at which time repeat MRI revealed re-recurrence characterized by multiple enhancing extra-axial masses underlying the calvarial bone flap and significant elevation of the flap secondary to marked parietoocciptal edema (Fig. 2). A third craniotomy and resection were performed, and the histologic diagnosis was recurrent anaplastic meningioma, WHO grade III. The patient subsequently received a course of chemotherapy with temozolomide.
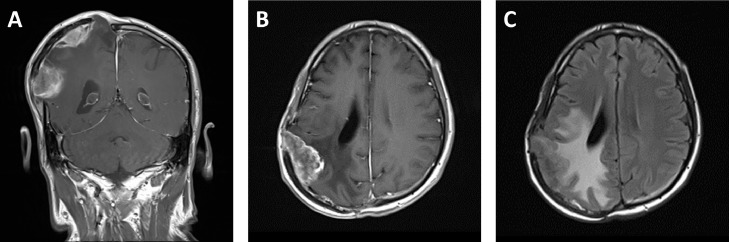
Fig. 2.
Second meningioma recurrence. Coronal (A) and axial (B) postcontrast T1-weighted MR images of the brain depict multiple dural-based enhancing masses underlying the right parietal craniotomy flap. The axial fluid-attenuated inversion recovery image (C) shows significant reactive edema in the underlaying parietal lobe. Associated brain swelling markedly elevates the craniotomy flap (A).
Three months later, the patient presented to an outside emergency department with abdominal complaints and back pain. A contrast-enhanced abdominal CT was performed, revealing a multilobulated, enhancing, 7.6 × 10.7 × 8.2 cm mass in the right anterior abdominal wall, coincident with the site of prior calvarial bone flap implantation. The mass extended from the skin surface, through the abdominal wall musculature, and into the pre-peritoneal fat, focally displacing the parietal peritoneum posteriorly (Fig. 3A). The CT also revealed multiple lucent osseous lesions as well as a pathologic fracture of the L1 vertebra with an associated soft tissue mass extending into the right ventrolateral spinal canal and invading the right psoas muscle (Fig. 3B and C).
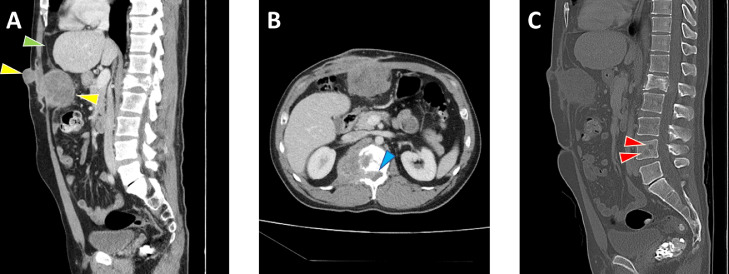
Fig. 3.
Abdominal wall implantation and lumbar spine meningioma metastases. Sagittal (A) and axial (B) contrast-enhanced CT images of the abdomen and pelvis on soft-tissue windows reveal a lobulated mass (yellow arrowheads) in the anterior abdominal wall at the site of prior craniotomy flap storage extending from the skin surface to the preperitoneal fat and deflecting the parietal peritoneum (green arrowhead). Also seen is a pathologic fracture of the L1 vertebra with associated enhancing soft tissue mass extending into the spinal canal (blue arrowhead) and right psoas muscle. Sagittal CT image on bone window (C) reveals additional lytic lesions in the L4 vertebra (red arrowheads) in addition to better delineating the L1 pathologic fracture. (Color version of figure is available online.)
Osseous metastatic disease was confirmed by subsequent contrast-enhanced MRI of the lumbar spine which revealed STIR hyperintensity and enhancement corresponding to the lucencies seen on CT (Fig. 4A-D). At the L1 level, there was ventral epidural extension of the soft tissue component producing flattening of the distal conus medullaris, but with preservation of a small amount of cerebrospinal fluid surrounding the conus and cauda equina nerve roots (ESCC grade 2).
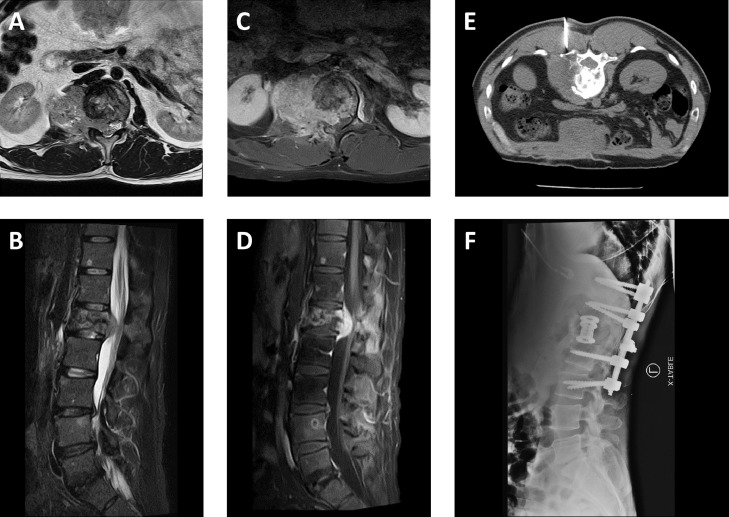
Fig. 4.
Spinal cord compression due to metastatic meningioma. Axial T2-weighted (A), sagittal short tau inversion recovery (B), and axial (C) and sagittal (D) postcontrast T1-weighted fat suppressed MR images of the lumbar spine reveal an enhancing mass associated with a pathologic fracture at the L1 level extending into the right ventrolateral epidural space and deforming the contour of the conus medullaris. Additional enhancing metastatic lesions are also seen in the L4 vertebral body. The mass at L1 underwent CT-guided biopsy (E) demonstrating anaplastic meningioma, following by decompression, corpectomy, and T11-L3 spinal fixation with associated hardware seen on lateral radiograph (F).
Thereafter, the patient had a prolonged and complex course including the development of multiple additional metastases to the thoracolumbar spine and bony pelvis. Biopsy of the L1 lesion followed by decompression and corpectomy were eventually performed given worsening neurologic symptoms and pain secondary to compression fracture (Fig. 4E and F). Pathological assessment of the spinal metastases also indicated anaplastic meningioma, WHO grade III, consistent with the histopathology of the patient's prior intracranial lesions.
Discussion
Iatrogenic implantation of meningioma is extremely rare, and distant iatrogenic implants are even rarer. Several cases of implantation seeding of meningioma to the scalp have been reported as a complication of surgical resection, [3], [4], [5], [6], [7], [8], [9]. However, only 2 other cases of distant extracranial iatrogenically implanted lesions have been reported in the literature, 1 occurring in the abdominal wall [10] and the other in the skin of the thigh [11]; both cases were presumed secondary to surgical site seeding. The authors of these cases stressed the importance of avoiding possible tumor implantation of operative fields by replacing surgical instruments when another operative site is accessed.
Decompressive craniectomy is used to treat elevated intracranial pressure caused by cerebral edema, intracranial hemorrhage, or mass lesion [12]. One method of storing the bone flap for subsequent cranioplasty is to embed it through an incision in the abdominal subcutaneous layer. In the present case, such placement of the bone flap may have resulted in microscopic tumor cell seeding of the abdominal wall. A rich blood supply in the subcutaneous space and a less restrictive anatomic compartment may have also contributed to the growth of the abdominal tumor. Potential strategies for controlling the risk of iatrogenic seeding in cases of malignant meningioma requiring decompressive craniectomy include cryopreservation of the bone flap or use of synthetic allograft material for subsequent cranioplasty.
Assessing risk factors for iatrogenic meningioma implantation is difficult due to the rarity of the event. Nevertheless, evidence suggests that iatrogenic seeding may be associated with higher grade meningiomas [9]. In a study of scalp incisions seeded by meningiomas, 3 out of 4 cases displayed histologic progression at the time of implantation [9]. The tumor in the present case displayed histologic progression from atypical to anaplastic meningioma prior to discovery of the abdominal tumor. The tendency of higher-grade tumors to produce greater levels of vascular endothelial growth factor, thus favoring angiogenesis and vascular invasion [13], may also contribute to seeding propensity at surgical sites.
The differential diagnosis for the abdominal wall meningioma includes metastasis via hematogenous or lymphatic routes. Unlike the aforementioned cases of iatrogenic meningioma implantation, the patient in this report was found to have multiple metastases to the spine in addition to the subcutaneous abdominal tumor, raising the possibility that the abdominal tumor was seeded by systemic dissemination rather than by direct inoculation. However, meningioma metastases are most commonly seen in the lungs and pleura, liver, lymph nodes, and bone, rather than in the abdominal wall [14,15]. Thus, the abdominal wall mass in the present case is favored to be secondary to the subcutaneous implantation of the calvarial bone flap.
Conclusion
Implantation of meningioma cells via abdominal wall bone flap preservation following craniectomy is a possible mechanism for iatrogenic spread of malignant meningiomas. Alternative methods of bone flap preservation or cranioplasty may be warranted in patients with known high grade meningiomas to mitigate the risk of implantation seeding.
References
- 1.Thomas R.Z., Dalal I. Extracranial metastases of anaplastic meningioma. BJR Case Rep. 2017 7;3(2) doi: 10.1259/bjrcr.20150092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enam S.A., Abdulrauf S., Mehta B., Malik G.M., Mahmood A. Metastasis in meningioma. Acta Neurochir (Wien) 1996;138(10):1172–1177. doi: 10.1007/bf01809747. discussion 1177-8. [DOI] [PubMed] [Google Scholar]
- 3.Kok D.L., Hendry S., Alvarez B. Iatrogenic subcutaneous metastasis from WHO Grade I intracranial meningioma. J Clin Neurosci. 2018;58:224–225. doi: 10.1016/j.jocn.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Singh R.V., Yeh J.S., Campbell D.A. Implantation meningioma in temporalis muscle: case report. Br J Neurosurg. 1994;8(1):93–95. doi: 10.3109/02688699409002400. [DOI] [PubMed] [Google Scholar]
- 5.Lüdemann W.O., Obler R., Tatagiba M., Samii M. Seeding of malignant meningioma along a surgical trajectory on the scalp. Case report and review of the literature. J Neurosurg. 2002;97(3):683–686. doi: 10.3171/jns.2002.97.3.0683. [DOI] [PubMed] [Google Scholar]
- 6.Akai T., Shiraga S., Iizuka H., Kishibe M., Kawakami S., Ueda Y. Recurrent meningioma with metastasis to the skin incision–case report. Neurol Med Chir (Tokyo) 2004;44(11):600–602. doi: 10.2176/nmc.44.600. [DOI] [PubMed] [Google Scholar]
- 7.Ozer E., Kalemci O., Acar U.D., Canda S. Pin site metastasis of meningioma. Br J Neurosurg. 2007;21(5):524–527. doi: 10.1080/02688690701485255. [DOI] [PubMed] [Google Scholar]
- 8.Tahir M.Z., Shamim M.S., Chishti K.N. Recurrent atypical meningioma seeding to surgical scar. Neurol India. 2009;57(2):222–224. doi: 10.4103/0028-3886.51307. [DOI] [PubMed] [Google Scholar]
- 9.Avecillas-Chasin J.M., Saceda-Gutierrez J., Alonso-Lera P., Garcia-Pumarino R., Issa S., López E. Scalp metastases of recurrent meningiomas: aggressive behavior or surgical seeding? World Neurosurg. 2015;84(1):121–131. doi: 10.1016/j.wneu.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Sadahira Y., Sugihara K., Manabe T. Iatrogenic implantation of malignant meningioma to the abdominal wall. Virchows Arch. 2001;438(3):316–318. doi: 10.1007/s004280000347. [DOI] [PubMed] [Google Scholar]
- 11.Maddah G., Shabahang H., Zehi V., Sharifi Sistani N., Mashhadi Nejad H. Iatrogenic seeding of tumor cells in thigh soft tissue upon surgical removal of intracranial meningioma. Basic Clin Neurosci. 2016;7(2):159–164. doi: 10.15412/J.BCN.03070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schirmer C.M., Ackil A.A, Jr., Malek A.M. Decompressive craniectomy. Neurocrit Care. 2008;8(3):456–470. doi: 10.1007/s12028-008-9082-y. [DOI] [PubMed] [Google Scholar]
- 13.Guevara P., Escobar-Arriaga E., Saavedra-Perez D., Martinez-Rumayor A., Flores-Estrada D., Rembao D. Angiogenesis and expression of estrogen and progesterone receptors as predictive factors for recurrence of meningioma. J Neurooncol. 2010;98(3):379–384. doi: 10.1007/s11060-009-0086-z. [DOI] [PubMed] [Google Scholar]
- 14.Abboud M., Haddad G., Kattar M., Aburiziq I., Geara F.B. Extraneural metastases from cranial meningioma: a case report. Radiat Oncol. 2009;4:20. doi: 10.1186/1748-717X-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beutler B.D., Nguyen E.T., Parker R.A., Tran C., Acharya J., Torres F.A. Metastatic meningioma: Case report of a WHO grade I meningioma with liver metastases and review of the literature. Radiol Case Rep. 2019;15(2):110–116. doi: 10.1016/j.radcr.2019.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]