Abstract
The superior ophthalmic vein (SOV) approach through the facial vein is usually preferred for transvenous embolization of a cavernous sinus dural arteriovenous fistula (CS DAVF) when the ipsilateral inferior petrosal sinus is angiographically occluded. However, navigating the microcatheter can sometimes be difficult because of stenosis or tortuous angulation at the junction between the angular vein and SOV. We present a novel transvenous access route to treat a CS DAVF using the ipsilateral deep facial vein through the SOV to reach the cavernous sinus. A 66-year-old woman presented with left-sided chemosis, exophthalmos, and external ophthalmoplegia. Angiography showed a left CS DAVF associated with a dilated SOV and retrograde cortical venous reflux. A dilated drainage vein, which branched from the SOV, ran through the lateral aspect of the orbit and exited the orbit through the inferior orbital fissure. This vein connected with the ipsilateral deep facial vein draining into the facial and internal jugular veins. We performed transvenous embolization via the SOV approach through the deep facial vein and achieved complete obliteration, by placing 3 platinum coils, without complications. Ophthalmic veins may connect with the cavernous sinus and pterygoid plexus, passing through the superior and inferior orbital fissures, respectively. Our case suggests that the deep facial vein may provide access to the SOV through the inferior orbital fissure without passing the difficult tortuous angle between the angular vein and SOV.
Keywords: Cavernous sinus dural arteriovenous fistula, Deep facial vein, Inferior orbital fissure, Transvenous embolization, Superior ophthalmic vein
Introduction
Cavernous sinus (CS) dural arteriovenous fistulas (DAVFs) are recognized as a common subtype of intracranial DAVF, which is defined as an abnormal arteriovenous connection involving the dura mater within or near the wall of the CS [1]. Although the natural history of CS DAVFs is reported to be relatively benign, with the potential to spontaneously resolve through thrombosis, aggressive lesions involving retrograde cortical venous reflux require prompt treatment [2]. Transvenous embolization (TVE) via the ipsilateral inferior petrosal sinus (IPS) is preferred as the primary approach route when treating a CS DAVF, regardless of the opacification of the IPS. However, an angiographically occluded IPS sometimes makes catheterization difficult, with a reported success rate of approximately 50%-80% [3]. An ipsilateral superior ophthalmic vein (SOV) approach should be considered as another option [4] in cases of good contrast opacification of the SOV. The most commonly used approach to reach the CS through the SOV is the ipsilateral facial vein (FV), however, navigating the microcatheter from the FV to the SOV can sometimes be difficult because of severe stenosis or tortuosity at the junction of the angular vein and SOV [5].
We describe a case of CS DAVF successfully treated via the SOV approach through the ipsilateral deep facial vein (DFV). The DFV may provide a relatively straight and short access course to the SOV through the inferior orbital fissure, without passing the difficult tortuous angle between the angular vein and SOV. To our knowledge, there are no published reports regarding the usefulness of the DFV in reaching the SOV for the treatment of CS DAVF.
Case presentation
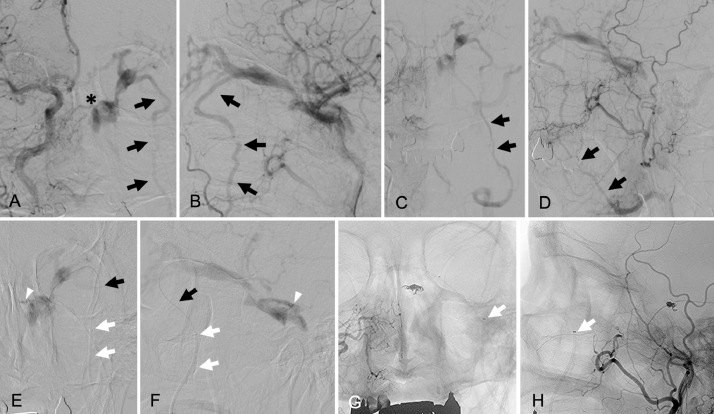
A 66-year-old woman who presented with left-sided chemosis, exophthalmos, and diplopia was referred to our hospital. On ophthalmological examination, a Hess chart showed a unilateral external ophthalmoplegia. Her intraocular pressure was 24 mmHg in the left eye and 14 mmHg in the right eye. Three-dimensional computed tomographic angiography images showed a left CS DAVF associated with a dilated SOV. A dilated vein (Fig. 1A, B, white arrows) ran along the lateral aspect of the orbit and drained into the DFV. Bone-subtracted computed tomographic angiography clearly showed that the dilated drainage vein (Fig. 1C, white arrows) was connected with the SOV near the junction of the CS. Right common carotid angiograms showed that a dilated draining vein branched from the SOV connected with the ipsilateral DFV (Fig. 2A, B, black arrows) and no opacification was recognized in the ipsilateral IPS. A shunting point in the superior-medial wall of the CS was also clearly visible (Fig. 2A, asterisk). The late venous phase of the right common carotid angiograms (Fig. 2C, D, black arrows) showed that the DFV was draining into the internal jugular vein through the ipsilateral FV.
Fig. 1.
Three-dimensional computed tomographic angiography (3D-CTA) images. The images (A: anteroposterior view, B: lateral view, C: subtracted CTA of the lateral view) show the left cavernous sinus dural arteriovenous fistula (CS DAVF) associated with a dilated superior ophthalmic vein (SOV) and retrograde cortical venous reflux into the superficial middle cerebral vein. The dilated drainage vein (white arrows) branching from the SOV runs along the lateral aspect of the left orbit and drains into the deep facial vein (DFV) through the inferior orbital fissure.
Fig. 2.
Right common carotid angiograms (A: anteroposterior view, B: lateral view) show the left cavernous sinus dural arteriovenous fistula (CS DAVF) and dilated superior ophthalmic vein (SOV) associated with the drainage vein (black arrows) running through the lateral aspect of the left orbit. The asterisk in panel A indicates the shunting point on the superior-medial wall of the CS. The narrowing of the drainage vein is visible where it passes through the inferior orbital fissure. The late arterial phase of the right common carotid angiograms (C: anteroposterior view, D: lateral view) show the dilated deep facial vein draining into the ipsilateral facial vein (black arrows). Super-selective angiograms (E: anteroposterior view, F: lateral view), after advancing the microcatheter to the shunting point, show the venous angioarchitecture in the CS. White arrowheads mark the tip of the microcatheter placed in the shunting point. Note the microcatheter (black arrows) supported by the 4-French distal access catheter (white arrows) running through the deep facial vein. Unsubtracted, final right common angiograms (G: anteroposterior view, H: lateral view) show the complete obliteration of the CS DAVF and the coil configuration placed at the shunting point. Note the tip of the 4-French support catheter (arrows) placed just proximal to the inferior orbital fissure.
Endovascular treatment was performed under local anesthesia. Consistent with reports in the literature, we usually select ipsilateral IPS cannulation as the primary TVE approach, regardless of IPS opacification. However, as catheterization of the occluded IPS was unsuccessful after several attempts, we changed our strategy. We planned to perform TVE via the SOV approach through the DFV. A 4-French distal access catheter (Cerulean G; Medikit, Tokyo, Japan), supported by a 7-French guiding catheter (Roadmaster; Goodman, Aichi, Japan), was advanced into the ipsilateral DFV just below the inferior orbital fissure (Fig. 2E-H, white arrows). A microcatheter (Excelsior 1018; Stryker Neurovascular, Fremont, MN, USA) was advanced (Fig. 2E, F, black arrows) over a 0.016 inch microguidewire (GT wire 16; Terumo, Tokyo, Japan) into the SOV through the dilated vein running along the lateral aspect of the orbit (Fig. 2E, F, black arrows). When the microcatheter reached in the SOV, we carefully advance the tip of the microcatheter into the shunting point (Fig. 2E, F, white arrowheads). Three detachable platinum coils were delivered into the shunt and complete obliteration of the CS DAVF was achieved (Fig. 2G, H). During microcatheter positioning and coil delivery, the 4-French distal access catheter placed in the DFV offered substantial support (Fig. 2G, H, white arrows). Total procedure time from groin puncture to removal of sheaths was 185 minutes, including the time of initial attempt for the catheterization into the occluded IPS. No procedure-related complications were observed during the perioperative period. The patient's postoperative course was uneventful, and her ocular symptoms resolved completely within 1 week. Her diplopia had completely disappeared by her 6-month visit to the outpatient clinic, and no recurrent findings were observed on her 12-month-follow-up angiograms.
Discussion
The aim of the treatment for CS DAVF is to completely obliterate both the fistula and cortical venous reflux, while avoiding introducing new cranial nerve palsy. Endovascular treatment has been widely used to achieve this outcome; TVE is the preferred method and is performed by accessing the CS via the ipsilateral IPS or SOV. The ipsilateral IPS is frequently selected as the primary treatment route, regardless of its occlusion, because it provides a relatively straight and short course to the CS [6]. Because the ipsilateral IPS is occluded in more than half of cases, access through the sinus is problematic and, in these situations, access to the CS must be achieved through other venous approaches. Multiple alternative approach routes have been reported in the literature, including the contralateral IPS through the circular sinus, pterygoid plexus, superior petrosal sinus, clival venous plexus and facial vein-SOV [7], [8], [9]. In situations when the ipsilateral IPS is not an accessible route, the facial vein-SOV approach should be considered a reasonable alternative [10]. However, severe tortuosity, abrupt angle and/or stenosis at the junction of the angular vein and the SOV sometimes prevent the navigation of the microcatheter into the SOV. To overcome this, various approaches have been reported, such as direct puncture of a surgically exposed SOV [11] or percutaneous preseptal puncture [12]. In this report, we present a novel transvenous access route to reach the CS from the ipsilateral DFV directly connecting with the SOV. To our knowledge, there are no reported cases of CS DAVF treated by TVE through the ipsilateral DFV. The advantage of this access route is that it can provide an easy access to the SOV through the inferior orbital fissure, without passing the difficult angle between the angular vein and SOV. Our total procedure time was 185 minutes, most of it was spent as an initial attempt for the catheterization into the occluded IPS. After deciding to change the approach route, we could reach the CS in a short time without passing the severe tortuosity at the junction of the angular vein and SOV. Thus, the procedure time for our novel access route was considered to be shorter than standard approach [4]. There have been reports in the literature regarding the potential risk of procedural complications, such as venous injury or rupture, when passing the tortuous route between the angular vein and the SOV. The facial vein-SOV route is surrounded by soft tissue and not supported by bony and dural structures, which is an important difference from the IPS route. Thus, improper manipulation of devices carries a greater risk of procedural complications, such as preseptal hematoma formation during the procedure [4]. The disadvantage of our method is that a 4-French distal access catheter cannot be passed through the inferior orbital fissure (Fig. 2E-H, white arrows), then the catheter may not provide enough support to deliver the microcatheter more distally. However, the DFV in our case was relatively straight and directly connected with the SOV near the CS. Therefore, we were able to reach the CS with the microcatheter and manipulate it into the shunting point, resulting in complete obliteration of the fistula using a minimum number of coils. We should also discuss the potential complications associated with our approach compared with the standard procedure. Because the most of the catheterization procedure in our approach is extracranial, we believe that the introduction of our novel approach does not increase unusual risks. However, careful manipulation of the microguidewire or microcatheter is mandatory to prevent the intraorbital hemorrhage, which can be caused by penetration of a fragile intraorbital vein.
Anatomically, the ophthalmic veins communicate with the pterygoid plexus through the inferior orbital fissure, and the pterygoid plexus also communicates with the FV via the various branches of the DVF [13,14]. This venous angioarchitecture makes the SOV approach through the DFV possible when an occluded IPS makes access difficult. To date, there have been few detailed descriptions of the connection between the CS and the pterygoid plexus. Jahan et al. [15] reported the case of a CS DAVF treated with TVE via the contralateral pterygoid plexus. In their report, retrograde catheterization to the pterygoid plexus was achieved through the maxillary vein supported by the guiding catheter placed in the retromandibular vein. Communication between the CS and pterygoid plexus consists of the vein passing the foramen of Vesalius and the emissary vein of the foramen lacerum. These veins were used in their report to reach the CS, however, they concluded that the technical difficulties of the catheterization was the major disadvantage. In our case, the direct connection between the CS and DFV made it easy to navigate the catheter without passing the pterygoid plexus. Although a venous connection in our case is rare, our novel approach route, a relatively straight and short course though the DFV to the SOV, should be considered when this route is clearly recognizable on the angiograms of patients with a CS DAVF.
Conclusion
Although TVE via ipsilateral IPS, regardless of its occlusion, is a first-line treatment approach that should be considered when treating a CS DAVF, an understanding of variable venous anatomy is essential for various alternative approaches to the CS. We present a rare case of CS DAVF treated by TVE via the ipsilateral DVF; this novel approach allowed easy access to the SOV through the inferior orbital fissure without passing the difficult tortuous angle between the angular vein and the SOV.
Acknowledgments
Acknowledgements
None.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interests.
Disclosure of funding and financial support: The authors have no personal, financial, or institutional interest in any of the drugs, materials, and devices described in this article.
Ethical considerations: This report is in accordance with the Declaration of Helsinki and International Council for Harmonisation/Good Clinical Practice guidelines. Institutional Review Board approval was obtained for this study. An informed written consent was obtained from a patient and patient information was anonymized and deidentified prior to the analysis.
References
- 1.Berenstein A., Lasjaunias P., ter Brugge K.G. Dural arteriovenous shunts. In: Berenstein A, Lasjaunias P, ter Brugge KG, editors. Surgical Neuroangiography. Springer; New York: 2004. pp. 565–607. [Google Scholar]
- 2.Viñuela F., Fox A.J., Debrun G.M., Peerless S.J., Drake C.G. Spontaneous carotid-cavernous fistulas: clinical, radiological, and therapeutic considerations. Experience with 20 cases. J Neurosurg. 1984;60(5):976–984. doi: 10.3171/jns.1984.60.5.0976. [DOI] [PubMed] [Google Scholar]
- 3.Fang B., Qian C., Yu J., Xu L., Jiang D., Xu J. Transarterial embolization of cavernous sinus dural arteriovenous fistulas with ipsilateral inferior petrosal sinus occlusion via the ascending pharyngeal artery. World Neurosurg. 2018;117:e603–e611. doi: 10.1016/j.wneu.2018.06.098. [DOI] [PubMed] [Google Scholar]
- 4.Fujita A., Kohta M., Sasayama T., Kohmura E. Impact of transvenous embolization via superior ophthalmic vein on reducing the total number of coils used for patients with cavernous sinus dural arteriovenous fistula. Neurosurg Rev. 2019 doi: 10.1007/s10143-019-01227-9. [DOI] [PubMed] [Google Scholar]
- 5.Choi J.H., Shin Y.S., Kim B.S. Making microguidewire loop facilitates navigation through tortuous or abruptly angulated head and neck veins to access cavernous sinus dural arteriovenous fistulas. World Neurosurg. 2019;129:e561–e565. doi: 10.1016/j.wneu.2019.05.216. [DOI] [PubMed] [Google Scholar]
- 6.Rhim J.K., Cho Y.D., Park J.J., Jeon J.P., Kang H.S., Kim J.E. Endovascular treatment of cavernous sinus dural arteriovenous fistula with ipsilateral inferior petrosal sinus occlusion: a single-center experience. Neurosurgery. 2015;77(7):192–199. doi: 10.1227/NEU.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 7.Yu S.C., Cheng H.K., Wong G.K., Chan C.M., Cheung J.Y., Poon W.S. Transvenous embolization of dural carotid-cavernous fistulae with transfacial catheterization through the superior ophthalmic vein. Neurosurgery. 2007;60(6):1032–1038. doi: 10.1227/01.NEU.0000255455.05355.31. [DOI] [PubMed] [Google Scholar]
- 8.Jahan R., Gobin Y.P., Glenn B., Duckwiler G.R., Viñuela F. Transvenous embolization of a dural arteriovenous fistula of the cavernous sinus through the contralateral pterygoid plexus. Neuroradiology. 1998;40(3):189–193. doi: 10.1007/s002340050566. [DOI] [PubMed] [Google Scholar]
- 9.Mounayer C., Piotin M., Spelle L., Moret J. Superior petrosal sinus catheterization for transvenous embolization of a dural carotid cavernous sinus fistula. AJNR Am J Neuroradiol. 2002;23(7):1153–1155. [PMC free article] [PubMed] [Google Scholar]
- 10.Biondi A., Milea D., Cognard C., Ricciardi G.K., Bonneville F., van Effenterre R. Cavernous sinus dural fistulae treated by transvenous approach through the facial vein: report of seven cases and review of the literature. AJNR Am J Neuroradiol. 2003;24(6):1240–1246. [PMC free article] [PubMed] [Google Scholar]
- 11.Miller N.R. Diagnosis and management of dural carotid-cavernous sinus fistulas. Neurosurg Focus. 2007;23(5):E13. doi: 10.3171/FOC-07/11/E13. [DOI] [PubMed] [Google Scholar]
- 12.Heran M.K.S., Volders D., Haw C., Shewchuk J.R. imaging-guided superior ophthalmic vein access for embolization of dural carotid cavernous fistulas: report of 20 cases and review of the literature. AJNR Am J Neuroradiol. 2019;40(4):699–702. doi: 10.3174/ajnr.A5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborn A.G. Craniofacial venous plexuses: angiographic study. AJR Am J Roentgenol. 1981;136(1):139–143. doi: 10.2214/ajr.136.1.139. [DOI] [PubMed] [Google Scholar]
- 14.Remington L.A. Orbital blood supply. In: Remington LA, editor. Clinical anatomy and physiology of the visual system. 3rd edition. Butterworth-Heinemann; Oxford: 2012. pp. 202–217. [Google Scholar]
- 15.Jahan R., Gobin Y.P., Glenn B., Duckwiler G.R., Viñuela F. Transvenous embolization of a dural arteriovenous fistula of the cavernous sinus through the contralateral pterygoid plexus. Neuroradiology. 1998;40(3):189–193. doi: 10.1007/s002340050566. [DOI] [PubMed] [Google Scholar]