Abstract
Inappropriate complementary feeding contributes to growth faltering, anaemia, and cognitive impairments. Limited programmatic evidence exists on the effectiveness of low‐iron micronutrient powders (MNPs) on anaemia and motor development when provided every other day in the first year of life. This study used an existing demonstration project to evaluate associations between exposure of low‐iron MNPs, anaemia, and motor development of infants in Southern Ethiopia. Using a retrospective cohort design, 200 infants aged 9 to 12 months (98 MNP exposed; 102 unexposed) were recruited, and data on socio‐economic characteristics, anthropometric measures, dietary diversity scores (DDS), haemoglobin concentrations, and motor development acquisition were collected, and MNP exposed and unexposed groups were compared. Logistic regressions were run to identify predictors of acquisition of motor development milestones. Sociodemographic characteristics and dietary diversity scores were similar between MNP exposed and unexposed groups. Provision of low‐iron (6 mg) MNP every other day, for 3 months, was associated with reduced risk of anaemia and stunting and increased achievement of motor development. After adjusting for age, infants exposed to MNPs had a higher likelihood of standing alone (AOR = 3.1; 95% CI [1.53, 6.46]) and walking alone (4.9; 95% CI [2.12, 11.37]) than unexposed ones. Exposure to MNPs, DDS, stunting, and mothers education were associated with acquisition of motor development milestones. Provision of low‐iron dose MNPs on alternate days is associated with lower prevalence of anaemia, stunting, and increased motor development achievements. Integrating routine monitoring of motor development milestones with growth monitoring and anaemia screening can inform nutrition interventions to support optimal brain development.
Keywords: anaemia, home fortification, iron, motor development
Key messages.
Low‐iron micronutrient powders provided to infants on alternate days are associated with reduced anaemia and stunting.
Low‐iron micronutrient powders provided to infants on alternate days are associated with increased motor development.
Monitoring gross motor development in early life should be integrated into the health system to promote optimal brain development
1. INTRODUCTION
Worldwide, it is estimated that more than two billion people are deficient in key vitamins and minerals (Tulchinsky, 2010). Infants and children in low‐ and middle‐income countries (LMIC) are disproportionately affected by micronutrient deficiencies (Black et al., 2013). The high energy and nutrient requirements needed to support the rapid growth that occurs during the complementary feeding period (6–23 months of age) makes infants and young children more vulnerable to micronutrient deficiencies (Dewey & Brown, 2003).
Unfortunately, inappropriate complementary feeding practices, characterized by late introduction and inadequate intake of nutrient‐dense complementary foods, are widespread in LMIC and contribute significantly to growth faltering (Krasevec, An, Kumapley, Bégin, & Frongillo, 2017). Growth faltering in young children is associated with serious short‐ and long‐term consequences including impaired physical and cognitive development (Dewey & Begum, 2011). Growth faltering and nutrient deficiencies are often experienced during the first years of life, posing a particular concern as this is when the basis for lifetime brain function is established (Victora, de Onis, Hallal, Blössner, & Shrimpton, 2010).
Several randomized controlled trials have shown that home fortification is effective in preventing or treating anaemia and key micronutrients deficiencies such as iron deficiency, but results on growth and development remain inconsistent (De‐Regil, Suchdev, Vist, Walleser, & Peña‐Rosas, 2013; Dewey, Yang, & Boy, 2009). The mixed results on the effectiveness of home fortification on growth and development may partly be related to the timing, dosage, and combination of nutrients of the home‐fortification interventions. Indeed, interventions started in the first year of life may be more effective than those started in the second year of life. For example, the earlier the brain is protected from suboptimal iron status, the better it is for cognitive function (Cusick & Georgieff, 2012). However, interventions with iron among iron‐replete infants have also been shown to be associated with long‐term negative growth and cognitive outcomes (Lönnerdal, 2017; Lozoff, Castillo, Clark, & Smith, 2012). Consequently, more effective and safer intervention modalities maximizing benefits while minimizing adverse outcomes are needed (Bruins et al., 2015).
Alternative home‐fortification regimens with the lowest efficacious dose of iron or the provision of micronutrient powders (MNPs) on alternate days has been suggested (Dewey & Baldiviez, 2012; Paganini & Zimmermann, 2017), but whether these alternative regimens are associated with reduced risk of anaemia, stunting, and developmental impairments remain unknown. Filling this evidence gap is particularly important for countries such as Ethiopia where not only growth faltering (38%) and anaemia (56%) prevalence is high, but also dietary iron intake is also high (Abebe, Haki, & Baye, 2018; CSA & ICF, 2017; EPHI, 2013).
Therefore, the present retrospective cohort study used the opportunity of an existing demonstration project in Southern Ethiopia to evaluate whether exposure to low‐iron (6 mg) MNPs provided every other day in the first year of life is associated with anaemia, growth, and acquisition of motor development millstones in infants aged 9 to 12 months.
2. METHODS
2.1. Study site
The study was conducted in Aleta Chuko district, Sidama Zone, Southern Nations, Nationalities, and People's region (SNNPR) of Ethiopia. The prevalence of stunting (aged 6–59 months) in SNNPR is equal to the national average (CSA & ICF, 2017). The population heavily depends on staples such as “Enset” (Ensete ventricosum)—a tuber crop also known as false banana—and maize (Zea mays L.).
2.2. Programme description
Home fortification with multiple MNPs was implemented by Terebeza Development Association with the support of Micronutrient Initiatives, now known as Nutrition International. The project was implemented in five districts, namely, East Badewacho, Shebedino, Aleta Chuko, Geta, and Damot Pulasa. From these five districts, three (Korke, Chuko lemela, and Rufo waeno) were purposively selected for this study. The data collection was done stepwise, with each district serving as a central study site. The whole survey was finished in 2 weeks time. So the data were collected within 1–2 weeks of the termination of the MNP exposure. The children that participated in this study are those enrolled in the programme when they were 6–9 months. These children were 9 to 12 months when the assessment was done. Those exposed to MNPs were therefore exposed for 3–6 months.
2.3. Sampling of study participants
Totally, 1,729 infant age 6–23 months were identified in the three kebele of which 456 were 9 to 12 months of age. A list of “exposed” and “unexposed” children was generated. Then children were selected randomly using computer generated random numbers. From these 456 infants, 149 were exposed to intermittent (every other day) low‐iron (6 mg) MNPs in the 3 months preceding the survey. A subsample (n = 216) of eligible infants, 108 from each exposed and unexposed infants, were randomly selected from the three districts. The age of the infants was confirmed by checking the health post records and through maternal recall. The inclusion criteria for this study were to be apparently healthy with no signs of severe malnutrition and with no disabilities that prevent accurate measurement of motor development. A total of 98 exposed and 102 unexposed completed the study. Among those that did not complete the study, n = 12 had parents that refused the finger prick for haemoglobin testing and n = 4 were sick during the data collection. The study being part of a larger study that has several outcomes of interest, the sample size was estimated to detect a medium effect size (Cohen's d = 0.5) difference between any two outcomes assuming α = 0.05, and a power of 90%. This was calculated using G power software.
2.4. Ethics
The protocol was approved by the Institutional Review Board of the College of Natural and Computational Sciences, Addis Ababa University. Permission and support letters were also obtained from the Sidama Zone Health Bureau, SNNPR, Ethiopia. Because of illiteracy, informed consent was obtained orally in the presence of the health extension workers and the MNP project coordinator. All the questionnaires were translated to the local language, and data collection was interview based.
2.5. Sociodemographic and anthropometric characteristics
Information about the household sociodemographic characteristics including livelihood activities and education level of primary caregivers, ownership of land and livestock were collected using a pretested questionnaire administered by trained and experienced data collectors. The interviewees were mothers except in two cases where the primary caregivers were close relatives. A primary caregiver is defined as any person taking care of the child.
All anthropometric measurements were made by the same persons to avoid interexaminer errors; z‐scores length‐for‐age (LAZ), weight‐for‐age (WAZ), and weight‐for‐length (WLZ) were calculated using World Health Organization (WHO) multicentre growth reference data (WHO, 2006) using the software ENA 2007. Stunting, underweight, and wasting were defined as LAZ, WAZ, or WLZ < −2, respectively.
2.6. Infant feeding practices
Infant feeding practices was assessed using the WHO infant and young child feeding indicators (WHO, 2010). Information about food groups consumed in the 24 hr prior the survey was collected using multiple pass open recall technique with questions and probes about the food consumed over the prior 24 hr with additional information about the ingredients of mixed dishes. The foods were then categorized into the following seven food groups to calculate the dietary diversity score: (a) grains, roots, and tubers; (b) legumes and nuts; (c) dairy products (milk, yogurt, and cheese); (d) eggs; (e) flesh foods (meat, fish, poultry, and liver/organ meats); (f) vitamin A‐rich fruits and vegetables; and (g) other fruits and vegetables. All children were still breastfed at the time of the survey. The proportion of breastfed infants meeting the minimum meal frequency (≥3) and the minimum dietary diversity score (≥4) was calculated.
2.7. Haemoglobin measurements
Haemoglobin was measured using a portable photometer (Hemocue HB 301). After cleaning the infants' middle finger with a disinfectant wipe, the side of the finger was pricked using a lancet. After wiping the first two drops, light pressure was applied and the third drop was collected using a cuvette and haemoglobin concentrations were recorded. Measurements were adjusted for altitude and anaemia was defined as haemoglobin concentrations <11.0 g/dl (WHO, 2011). Infants' with severe anaemia (≤7 g/dl) were referred to the healthy facility for follow‐up.
2.8. Motor development
Motor development was assessed by the first author using the WHO Multicentre Growth Reference Study guidelines (Wijnhoven et al., 2004). Six distinct gross motor milestones were assessed for this study: (a) sitting without support, (b) hands‐knees crawling, (c) standing with assistance, (d) walking with assistance, (e) standing alone, and (f) walking alone. These are simple measurements to take and are considered to be universal and fundamental to the acquisition of self‐sufficient erect locomotion.
2.9. Quality control
All data collectors were experienced nurses and health extension workers. Data collectors have received 3 days training on anthropometric, motor milestone development, and haemoglobin measurements. The training was followed by a pretest on subjects not included in the actual study. The field supervision was supported by three experienced supervisors (one/site), and the first author checked the completeness and quality of the data on a daily basis. Data collectors were blinded to whether the infant was exposed and unexposed. All anthropometric measurements and motor development assessments were taken by the first author using calibrated measuring board and weighting scales. Triplicate measures of weight, length, middle upper arm circumference and head circumference were taken using standardized techniques. Measurements were taken at the health post with infants wearing light clothing and no shoes (WHO, 2006) using height boards and weighing scales (SECA).
2.10. Statistical analysis
The data were double entered in Epi‐data statistical software (version 20), was cleaned, and exported to SPPS (IBM SPSS Statistics 20.0, Chicago, IL, USA) for statistical analyses. All continuous variables were checked for normality using the Shapiro–Wilk test. Continuous variables are presented as mean ± SD and categorical data as frequencies. If data were normally distributed, independent Student's t‐test was used to compare means of exposed and unexposed groups; otherwise, Mann–Whitney U test was used. Chi‐square test was used for categorical variables to test differences between MNP exposed and unexposed groups. In all comparisons, differences were considered statistically significant at P < 0.05. The association between motor milestone development (standing alone and walking alone) with MNP exposure, feeding practice, nutritional status, haemoglobin, and some socio‐economic and demographic characteristics were investigated using bivariate and multivariate logistic regression analyses. Variables associated with motor development milestones at 𝑃 values ≤ 0.2 were entered in multivariate logistic regression that controlled for confounding variables. The odds ratio and 95% confidence interval (CI) are presented. A mediation analysis was then conducted to estimate the direct and indirect effect of MNP exposure on motor development using PROCESS for SPSS version 3 (https://www.processmacro.org/index.html). Regressions were run with and without haemoglobin concentrations as a mediator. For all statistical comparisons, P values less than 0.05 were considered to be statistically significant.
3. RESULTS
From the 216 infant (108 per arm) meeting the eligibility criteria, a total of 200 (98 MNP exposed/102 unexposed) completed the study. The average caregiver or mother was in her twenties and was married (Table 1). About a third of the mothers (29.6% MNP exposed/39.2% unexposed) did not have any formal education. The average household family size was higher in the MNP exposed (5.1) than in the unexposed (4.6) group (P < 0.05). The average age of the children in this study was similar between the two groups (~11 months). Most of the study participants were from male‐headed farming households, with over half of owning less than 1 ha of land. Most of the socio‐economic variables were similar between the MNP exposed and unexposed groups, except for chicken ownership that was significantly higher in the unexposed group. More than 90% of the study participants had access to potable water (public tap or stand pipe) and improved toilet facilities (pit latrines).
Table 1.
Selected sociodemographic characteristics by exposure to micronutrient powder (MNP)
| All | MNP exposed | MNP unexposed | P values | |
|---|---|---|---|---|
| N = 200 | n = 98 | n = 102 | ||
| Child's age | 10.7 ± 1.1 | 10.9 ± 1.0 | 10.5 ± 1.1 | 0.08 |
| Child's sex (male:female) | 1.0 | 1.0 | 1.0 | 0.89 |
| Mothers' age (years) | 0.22 | |||
| <20 | 3 (1.5) | 3 (3.2) | — | |
| 20–24 | 54 (27.0) | 25 (25.5) | 29 (28.4) | |
| 25–30 | 123 (61.5) | 58 (59.2) | 65 (63.7) | |
| >30 | 20 (10.0) | 12 (12.2) | 8 (7.8) | |
| Mother's education | 0.52 | |||
| No formal | 69 (34.5) | 29 (29.6) | 40 (39.2) | |
| Primary | 95 (47.5) | 49 (50.0) | 46 (45.1) | |
| Secondary | 36 (18.0) | 20 (20.4) | 16 (15.7) | |
| Married | 200 (100) | 98 (100) | 102 (100) | |
| Household size | 4.9 ± 1.6 | 5.1 ± 1.7 | 4.6 ± 1.5 | 0.03* |
| Livelihood activity | 0.74 | |||
| Farming | 188 (94.0) | 91(92.9) | 97 (95.1) | |
| Trading | 8 (4.0) | 5 (5.1) | 3 (2.9) | |
| Other | 4 (2.0) | 2 (2.0) | 2 (2.0) | |
| Monthly income (ETB) | — | 0.00 (0.00, 500.00)a | 275.00 (0.00, 500.00)a | — |
| Land size (≤1 ha) | 123 (61.5) | 62 (63.3) | 61(59.8) | 0.62 |
| Owns cow or oxen | 122 (61.0) | 65 (66.3) | 57 (55.9) | 0.13 |
| Owns chicken | 98 (49.0) | 41 (41.8) | 57 (55.9) | 0.05* |
| Access to potable drinking water | 196 (98) | 96 (98) | 100 (98) | 0.97 |
| Improved toilet facility (pit latrines) | 74 (37) | 43 (43.9) | 31 (30.4) | 0.10 |
Note. Values are mean ± SD or n (%). ETB: Ethiopian birr.
Values are median and first and third quartiles.
Significant differences in mean or proportion between MNP exposed and unexposed groups at P < 0.05 levels using independent t‐test (two‐tailed) or chi‐square test.
All children were still breastfed at the time of the survey, and about 73% were fed the minimum number of times (≥3) per day, but small number of children met the minimum (≥4) dietary diversity score (Table 2). There was no statistically significant difference in meal frequency and dietary diversity between MNP exposed and unexposed groups. The proportion of children that consumed animal source foods in the 24 hr preceding the survey was very low (<5%). All the children had received vitamin A supplements in the last 6 months.
Table 2.
Infant (9 to 12 months) feeding practices, anthropometry, haemoglobin, and morbidity measures by micronutrient powder (MNP) exposure
| All | MNP exposed | MNP unexposed | P values | |
|---|---|---|---|---|
| N = 200 | n = 98 | n = 102 | ||
| Feeding practices | ||||
| Currently breastfed | 200 (100) | 98 (100) | 102 (100) | — |
| Fed minimum number of meals/day (≥3) | 147 (73.5) | 72 (73.5) | 75 (73.5) | 0.99 |
| Dietary diversity score (DDS) | 2.3 ± 0.8 | 2.2 ± 0.8 | 2.4 ± 0.8 | 0.23 |
| Met minimum DDS (≥4 food groups) | 7 (3.5) | 3 (3.1) | 4(3.9) | 0.74 |
| Vitamin A supplement within the last 6 months | 200 (100) | 98 (100) | 102 (100) | — |
| Anthropometric characteristics | ||||
| Stunting (LAZ < −2) | 60 (30.0) | 22 (22.4) | 38 (37.3) | 0.02* |
| Underweight (WAZ < −2) | 8 (4.0) | 3 (3.1) | 5 (4.9) | 0.51 |
| Wasting (WLZ < −2) | 6 (3.0) | 3 (3.1) | 3 (2.9) | 0.96 |
| MUAC (cm)b | 13.2 ± 1.0 | 13.3 ± 1.1 | 13.0 ± 0.8 | 0.02* |
| Head circumference (cm)b | 46.9 ± 2.1 | 47.4 ± 1.8 | 46.4 ± 2.3 | <0.001* |
| Haemoglobin and morbidity | ||||
| Haemoglobin level (g/dl)a | 11.8 ± 3.7 | 12.4 ± 3.6 | 11.3 ± 3.8 | 0.03* |
| Anaemic status | 82(41.0) | 30(30.6) | 52(51.0) | 0.003* |
| Child sick in the past 1 week | 23 (11.5) | 15 (15.3) | 8 (7.8) | 0.10 |
Note. Values are mean ± SD or n (%). LAZ: z‐scores length‐for‐age; WLZ: z‐scores weight‐for‐length; MUAC: middle upper arm circumference; WAZ: z‐scores weight‐for‐age.
Haemoglobin values were adjusted for altitude.
Mann–Whitney U test was used for comparing exposed and unexposed groups.
Significant mean differences or proportion between unexposed and exposed groups at P < 0.05 levels using two‐tailed independent t‐test (continuous variables) or chi‐square (frequencies) test.
The stunting prevalence was unacceptably high (30%) and was significantly lower in the MNP exposed than in the unexposed group (P < 0.05). Middle upper arm circumference, head circumference, and haemoglobin concentrations were significantly higher in the MNP exposed than in the unexposed ones. Anaemia prevalence in the MNP exposed group was 20 percentage points lower than in the unexposed group. Although a higher proportion of children in the MNP exposed group were reported to be sick in the 1 week prior the survey, the difference between the exposed and unexposed groups was not statistically significant (P > 0.05).
Differences in the acquisition of motor development milestones were observed between the MNP exposed and unexposed groups (Table 3). The proportion of infants standing and walking alone was higher in the MNP exposed than in the unexposed group (P < 0.05). These associations between MNP exposure and standing and walking alone remained significant in the multivariate logistic regression that adjusted for age and other covariates (Table 4). Infants exposed to MNP had a threefold (adjusted odds ratio [AOR] = 3.1; 95% CI [1.53, 6.46]) likelihood of standing alone and a fourfold likelihood (4.9; CI [2.12, 11.37]) of walking alone. Dietary diversity score (2.0; CI [1.01, 3.91]) and child sickness (0.2; CI [0.04, 0.88]) in the past week were significant predictors of standing alone, whereas stunting (0.4; CI [0.14, 0.92]) and mothers “education level” (0.3; CI [0.13, 0.92]) were significant predictors of walking alone (P < 0.05).
Table 3.
Motor development milestones of infants (9 to 12 months) by MNP exposure
| All | MNP exposed | MNP unexposed | P values | |
|---|---|---|---|---|
| N = 200 | n = 98 | n = 102 | ||
| Motor development milestones | ||||
| Sitting without support | 200 (100) | 98 (100) | 102 (100) | |
| Hand and knee crawling | 200 (100) | 98 (100) | 102 (100) | |
| Stand with assistance | 189 (94.5) | 94 (95.9) | 93 (91.2) | 0.174 |
| Walking with assistance | 130 (65.0) | 67 (68.4) | 71 (69.6) | 0.850 |
| Standing alone | 60 (30.0) | 38 (38.8) | 22 (21.6) | 0.008* |
| Walking alone | 48 (24.0) | 35 (35.7) | 13 (12.7) | <0.001* |
Note. Values are mean ± SD or n (%). MNP: micronutrient powder.
Significant differences between unexposed and exposed groups at P < 0.05 levels using chi‐square test (two‐tailed).
Table 4.
Factors predicting standing and walking alone
| Standing alone | Walking alone | |||||||
|---|---|---|---|---|---|---|---|---|
| Able | Unable | COR | AOR | Able | Unable | COR (95% CI) | AOR (95% CI) | |
| N (%) | N (%) | (95% CI) | (95% CI) | |||||
| MNP exposed | ||||||||
| No | 22 (36.7) | 80 (57.1) | 1 | 1 | 13 (27.1) | 89 (58.6) | 1 | 1 |
| Yes | 38 (63.3) | 60 (42.9) | 2.3 * (1.24, 4.30) | 3.1 * (1.53, 6.46) | 35 (72.9) | 63 (41.4) | 3.8 * (1.86, 7.77) | 4.9 * (2.12, 11.37) |
| Dietary diversity score | ||||||||
| Not adequate (≤3) | 28 (46,7) | 88 (62.9) | 1 | 1 | 23 (47.9) | 93 (61.2) | 1 | 1 |
| Adequate | 32 (53.3) | 52 (37.1) | 1.9 * (1.05, 3.57) | 2.0 * (1.01, 3.91) | 25 (52.1) | 59 (38.8) | 1.7 (0.89, 3.29) | 1.8 (0.81, 3.82) |
| Fed min. number of meals/day | ||||||||
| Not adequate | 10 (16.7) | 43 (30.7) | 1 | 1 | 7 (14.6) | 46 (30.3) | 1 | 1 |
| Adequate (≥ 3) | 50 (83.3) | 97 (69.3) | 2.2 * (1.03, 4.78) | 2.1 (0.903,4.66) | 41 (85.4) | 106 (69.7) | 2.5 * (1.06, 6.09) | 2.2 (0.86, 5.72) |
| Haemoglobin level | ||||||||
| <11(g/dl) | 31 (51.7) | 52 (37.1) | 1 | 1 | 23 (47.9) | 58 (38.2) | 1 | 1 |
| ≥11(g/dl) | 29 (48.3) | 88 (62.9) | 0.7 (0.35, 1.20) | 0.6 (0.29, 1.19) | 25 (52.1) | 94 (61.8) | 0.7 (0.36, 1.33) | 0.6 (0.25, 1.27) |
| Stunting | ||||||||
| LAZ ≤ −2 | 11 (18.3) | 61 (43.6) | 1 | 1 | 7 (14.6) | 65 (42.8) | 1 | 1 |
| LAZ > −2 | 49 (81.7) | 79 (56.4) | 0.7 (0.36, 1.40) | 0.8 (0.37, 1.65) | 41(85.4) | 87 (57.2) | 0.3 * (0.13, 0.76) | 0.4 * (0.14, 0.91) |
| Child sickness | ||||||||
| Yes | 3 (5.0) | 20 (14.3) | 1 | 1 | 3 (6.2) | 20 (13.2) | 1 | 1 |
| No | 57 (95.0) | 120 (85.7) | 0.3 (0.09, 1.11) | 0.2 * (0.04, 0.88) | 45 (93.8) | 132 (86.8) | 0.4 (0.13, 1.55) | 0.3 (0.06, 1.30) |
| Mother education | ||||||||
| No education | 26 (43.3) | 46 (32.9) | 1 | 1 | 19 (39.6) | 53 (34.9) | 1 | 1 |
| Primary education | 22 (36.7) | 76 (54.3) | 0.4 (0.18, 1.04) | 2.1 (0.96, 4.42) | 16 (33.3) | 82 (53.9) | 0.5 (0.19, 1.14) | 2.1 (0.87, 5.12) |
| Secondary education | 12 (20.0) | 18 (12.9) | 0.9 (0.35, 2.03) | 0.7(0.28, 1.74) | 13 (27.1) | 17 (11.2) | 0.3 * (0.10, 0.63) | 0.3 * (0.13, 0.93) |
Note. MNP: micronutrient powder; COR: crude odds ratio; AOR, adjusted odds ratio; CI: confidence interval; multivariate logistic regression adjusted for age.
Associations are significant at P < 0.05.
Bold values are statistically significant variables.
Our exploratory mediation analyses suggested that improved motor development milestone is directly explained by MNP exposure, but a smaller but significant share of the associations is mediated by an increase in haemoglobin concentration (Figure 1).
Figure 1.
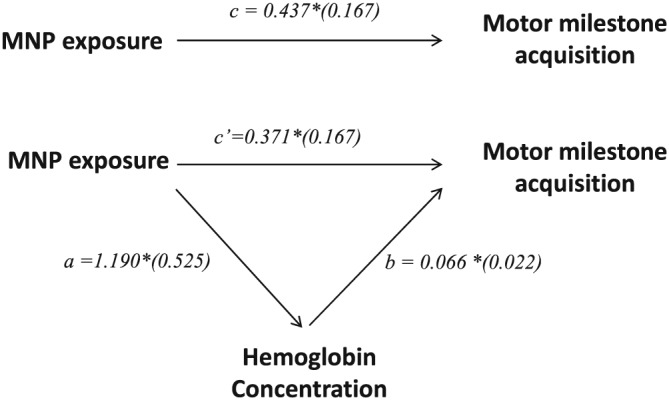
Path diagram presenting mediation effect of micronutrient powder (MNP) exposure on motor milestone acquisition through increased haemoglobin concentration; Note: a and b coefficients define the indirect effect of MNP exposure on motor milestone acquisition through haemoglobin concentrations; c′ coefficient denotes the direct effect of MNP exposure on motor milestone acquisition
4. DISCUSSION
The present retrospective cohort study used the opportunity of an existing demonstration project to show that a low‐iron (6 mg) MNP provided every other day for infants in first year of life is associated with increased achievement of motor milestone development and a lower risk of anaemia. MNP exposure in the first year of life is directly and indirectly (through increased haemoglobin concentration) associated with improved motor development outcome. Dietary diversity predicted ability to stand alone, whereas stunting and mothers' education predicted ability to walk alone.
Inadequate complementary feeding is widespread as illustrated by the very low proportion of infants meeting the minimum dietary diversity score. This finding is in line with findings from previous studies in LMIC including Ethiopia (Baye, Guyot, Icard‐Verniere, & Mouquet‐Rivier, 2013; Mayen, Marques‐Vidal, Paccaud, Bovet, & Stringhini, 2014; Mengistu, Moges, Samuel, & Baye, 2017). Diets of infants in this and other Ethiopian settings are predominantly plant‐based with little or no consumption of nutritious food groups such as animal source foods (Baye et al., 2013). Such a low dietary diversity score has consistently been found associated with higher risk of anaemia and stunting among infants and young children (Gashu et al., 2016; Krasevec et al., 2017). The finding that 30% of our study participants were stunted and 40% were anaemic suggests that these infants are already at an increased risk of cognitive deficit that, without timely intervention, may be irreversible. Given that achieved motor development milestones are consistently found to mediate cognitive function (Marrus et al., 2017; Oudgenoeg‐Paz, Leseman, & Volman, 2015), the significant association between dietary diversity score, stunting, and motor development milestones (standing and walking alone) further supports the need for urgent interventions that save infants' developing brains from nutritional insults.
Among such interventions, home fortification of complementary foods with MNPs has been promoted in various LMICs (De‐Regil et al., 2013). Although found highly effective in preventing or treating anaemia, recent studies have shown concern over the use of MNPs with standard iron concentrations of 12.5 mg (Bruins et al., 2016; Paganini & Zimmermann, 2017). This concern arises from the increased risk of diarrhoea and gut inflammation, particularly when MNPs are provided to iron‐replete children (Lönnerdal, 2017; Paganini & Zimmermann, 2017). The provision of iron to iron‐replete infants was also associated with increased stunting and lower long‐term cognitive function (Lönnerdal, 2017; Lozoff et al., 2012). These findings pose serious challenge to interventions that aim to prevent anaemia or micronutrient deficiencies and the associated adverse effects.
In an effort to increase benefit and minimize adverse effects of MNPs containing iron, the present demonstration project used a low‐iron (6 mg) dose provided on alternative days. This was informed by consumption and epidemiologic surveys that showed mild to moderate prevalence of anaemia despite a high (excessive) iron intake, even among young children (Abebe et al., 2018; CSA & ICF, 2017). In spite of this modified regimen, MNP exposure was associated with lower rates of anaemia and stunting (P < 0.05). This finding, if confirmed through community‐based clinical trials, can present an alternative for programmes in countries such as Ethiopia, where both the risk of inadequate and excessive iron exists.
Although MNP interventions are often designed as a strategy to prevent anaemia, they may also confer additional advantages in supporting cognitive and motor development (Adu‐Afarwuah et al., 2007; Larson & Yousafzai, 2017; Matias et al., 2017). Indeed, the present study showed that MNP exposure was significantly associated with increased motor milestone achievement such as standing and walking alone. Our mediation analyses revealed that the effect of MNP exposure on motor development milestone achievements was in part explained by increased haemoglobin concentration. However, a far more substantial share of the association was not related to an increase in haemoglobin concentrations. This finding is of interest and may have important programmatic implications. First, in an effort to minimize possible adverse effects, screening and targeting only anaemic infants for MNP interventions may not be an option as some of the positive effects on motor development are not related to haemoglobin concentrations (anaemia). Second, micronutrient deficiencies without anaemia can affect motor milestone achievements and possibly have implication on cognitive development (Krebs, Lozoff, & Georgieff, 2017).
The present study has several limitations that need to be considered when interpreting our findings. First, this is an opportunistic study that was tied to an existing project and as a result, we had no control over the enrolment of infants or on the implementation of intervention. Consequently, the comparisons between the exposed and the unexposed may be affected by possible preintervention differences. However, the comparability in sociodemographic characteristics between the exposed and the unexposed is reassuring and suggests minimal bias, if any. Given that this is a retrospective cohort study, causal inferences cannot be made and differences between the exposed and unexposed group should be interpreted as associations. Although we have tried to capture morbidity, our sample size might have not been adequate enough to detect differences between the two groups (exposed or unexposed).
Notwithstanding the above limitations, the present study has shown that provision of a low‐iron dose MNP on alternate days, in the first year of life, is associated with lower prevalence of anaemia, stunting, and increased motor development achievements. Given that a share of the associations between MNP and motor development milestones is independent of haemoglobin concentrations, targeting only anaemic children for MNP interventions may not be effective. Interventions that improve complementary diets in the first year of life are needed not only to prevent anaemia or micronutrient deficiencies but also to support the developing brain. A community‐based clinical trial is needed to establish the effectiveness of this modified regimen. Nevertheless, integrating simple, but useful monitoring tools such as acquisition of motor development milestones (proxy for cognitive development) into the health system, along with anaemia screening and growth monitoring can inform intervention that aim to improve child nutrition and promote optimal brain development.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
CONTRIBUTIONS
All authors were involved in developing the study design. AG and KB designed the study; AG coordinated and supervised the fieldwork. AL provided critical information about the demonstration project. AG and KB analysed and interpreted the data. AG wrote the first draft of the manuscript. All the authors contributed to manuscript preparation.
ACKNOWLEDGMENTS
The authors thank all of the children and families who took part in this survey. Nutrition international (formerly Micronutrient initiative) is acknowledged for allowing us to use their demonstrating project for this study. Dr. Tadesse Alemu's in‐field and statistical support is acknowledged.
Geletu A, Lelisa A, Baye K. Provision of low‐iron micronutrient powders on alternate days is associated with lower prevalence of anaemia, stunting, and improved motor milestone acquisition in the first year of life: A retrospective cohort study in rural Ethiopia. Matern Child Nutr. 2019;15:e12785 10.1111/mcn.12785
REFERENCES
- Abebe, Z. , Haki, G. D. , & Baye, K. (2018). Simulated effects of home fortification of complementary foods with micronutrient powders on risk of inadequate and excessive intakes in West Gojjam, Ethiopia. Maternal & Child Nutrition, 14(1), e12443 10.1111/mcn.12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah, S. , Lartey, A. , Brown, K. H. , Zlotkin, S. , Briend, A. , & Dewey, K. G. (2007). Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: Effects on growth and motor development. The American Journal of Clinical Nutrition, 86(2), 412–420. 10.1093/ajcn/86.2.412 [DOI] [PubMed] [Google Scholar]
- Baye, K. , Guyot, J.‐P. , Icard‐Verniere, C. , & Mouquet‐Rivier, C. (2013). Nutrient intakes from complementary foods consumed by young children (aged 12–23 months) from North Wollo, northern Ethiopia: The need for agro‐ecologically adapted interventions. Public Health Nutrition, 16(10), 1741–1750. 10.1017/S1368980012005277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , De Onis, M. , … Uauy, R. (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet, 382(9890), 427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- Bruins, M. J. , Kupka, R. , Zimmermann, M. B. , Lietz, G. , Engle‐Stone, R. , & Kraemer, K. (2016). Maximizing the benefits and minimizing the risks of intervention programs to address micronutrient malnutrition: Symposium report. Maternal & Child Nutrition, 12(4), 940–948. 10.1111/mcn.12334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins, M. J. , Mugambi, G. , Verkaik‐Kloosterman, J. , Hoekstra, J. , Kraemer, K. , Osendarp, S. , … Verhagen, H. (2015). Addressing the risk of inadequate and excessive micronutrient intakes: Traditional versus new approaches to setting adequate and safe micronutrient levels in foods. Food & Nutrition Research, 59(1), 26020 10.3402/fnr.v58.26020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Statistical Agency—CSA/Ethiopia, & ICF (2017). Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia: CSA and ICF; Retrieved from http://dhsprogram.com/pubs/pdf/FR328/FR328.pdf [Google Scholar]
- Cusick, S. E. , & Georgieff, M. K. (2012). Nutrient supplementation and neurodevelopment: Timing is the key. Archives of Pediatrics & Adolescent Medicine, 166(5), 481–482. 10.1001/archpediatrics.2012.199 [DOI] [PubMed] [Google Scholar]
- De‐Regil, L. M. , Suchdev, P. S. , Vist, G. E. , Walleser, S. , & Peña‐Rosas, J. P. (2013). Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Evidence‐Based Child Health: A Cochrane Review Journal, 8(1), 112–201. 10.1002/ebch.1895 [DOI] [PubMed] [Google Scholar]
- Dewey, K. G. , & Baldiviez, L. M. (2012). Safety of universal provision of iron through home fortification of complementary foods in malaria‐endemic areas. Advances in Nutrition, 3(4), 555–559. 10.3945/an.111.001131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, K. G. , & Begum, K. (2011). Long‐term consequences of stunting in early life. Maternal & Child Nutrition, 7, 5–18. 10.1111/j.1740-8709.2011.00349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, K. G. , & Brown, K. H. (2003). Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food and Nutrition Bulletin, 24(1), 5–28. 10.1177/156482650302400102 [DOI] [PubMed] [Google Scholar]
- Dewey, K. G. , Yang, Z. , & Boy, E. (2009). Systematic review and meta‐analysis of home fortification of complementary foods. Maternal & Child Nutrition, 5(4), 283–321. 10.1111/j.1740-8709.2009.00190.x [DOI] [Google Scholar]
- EPHI (Ethiopian Public Health Institute) . (2013). Ethiopian National Food Consumption Survey. Addis Ababa, Ethiopia.
- Gashu, D. , Stoecker, B. J. , Adish, A. , Haki, G. D. , Bougma, K. , & Marquis, G. S. (2016). Ethiopian pre‐school children consuming a predominantly unrefined plant‐based diet have low prevalence of iron‐deficiency anaemia. Public Health Nutrition, 19(10), 1834–1841. 10.1017/S1368980015003626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasevec, J. , An, X. , Kumapley, R. , Bégin, F. , & Frongillo, E. A. (2017). Diet quality and risk of stunting among infants and young children in low‐and middle‐income countries. Maternal & Child Nutrition, 13, e12430 10.1111/mcn.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs, N. F. , Lozoff, B. , & Georgieff, M. K. (2017). Neurodevelopment: The impact of nutrition and inflammation during infancy in low‐resource settings. Pediatrics, 139(Supplement 1), S50–S58. 10.1542/peds.2016-2828G [DOI] [PubMed] [Google Scholar]
- Larson, L. M. , & Yousafzai, A. K. (2017). A meta‐analysis of nutrition interventions on mental development of children under‐two in low‐and middle‐income countries. Maternal & Child Nutrition, 13(1), e12229 10.1111/mcn.12229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnerdal, B. (2017). Excess iron intake as a factor in growth, infections, and development of infants and young children. The American Journal of Clinical Nutrition, 106(Supplement 6), 1681S–1687S. 10.3945/ajcn.117.156042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff, B. , Castillo, M. , Clark, K. M. , & Smith, J. B. (2012). Iron‐fortified vs low‐iron infant formula: Developmental outcome at 10 years. Archives of Pediatrics & Adolescent Medicine, 166(3), 208–215. 10.1001/archpediatrics.2011.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus, N. , Eggebrecht, A. T. , Todorov, A. , Elison, J. T. , Wolff, J. J. , Cole, L. , … Swanson, M. R. (2017). Walking, gross motor development, and brain functional connectivity in infants and toddlers. Cerebral Cortex, 28(2), 750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias, S. L. , Mridha, M. K. , Tofail, F. , Arnold, C. D. , Khan, M. S. A. , Siddiqui, Z. , … Dewey, K. G. (2017). Home fortification during the first 1000 d improves child development in Bangladesh: A cluster‐randomized effectiveness trial–3. The American Journal of Clinical Nutrition, 105(4), 958–969. 10.3945/ajcn.116.150318 [DOI] [PubMed] [Google Scholar]
- Mayen, A.‐L. , Marques‐Vidal, P. , Paccaud, F. , Bovet, P. , & Stringhini, S. (2014). Socioeconomic determinants of dietary patterns in low‐and middle‐income countries: A systematic review. The American Journal of Clinical Nutrition, 100(6), 1520–1531. 10.3945/ajcn.114.089029 [DOI] [PubMed] [Google Scholar]
- Mengistu, G. , Moges, T. , Samuel, A. , & Baye, K. (2017). Energy and nutrient intake of infants and young children in pastoralist communities of Ethiopia. Nutrition, 41, 1–6. 10.1016/j.nut.2017.02.012 [DOI] [PubMed] [Google Scholar]
- Oudgenoeg‐Paz, O. , Leseman, P. P. , & Volman, M. (2015). Exploration as a mediator of the relation between the attainment of motor milestones and the development of spatial cognition and spatial language. Developmental Psychology, 51(9), 1241–1253. 10.1037/a0039572 [DOI] [PubMed] [Google Scholar]
- Paganini, D. , & Zimmermann, M. B. (2017). The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. The American Journal of Clinical Nutrition, 106(Supplement 6), 1688S–1693S. 10.3945/ajcn.117.156067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulchinsky, T. H. (2010). Micronutrient deficiency conditions: Global health issues. Public Health Reviews, 32(1), 243–255. 10.1007/BF03391600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora, C. G. , de Onis, M. , Hallal, P. C. , Blössner, M. , & Shrimpton, R. (2010). Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics, peds‐2009, 125, e473–e480. 10.1542/peds.2009-1519 [DOI] [PubMed] [Google Scholar]
- WHO (2006). WHO child growth standards: Length/height for age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age, methods and development. Geneva: World Health Organization. [Google Scholar]
- WHO (2010). Indicators for assessing infant and young child feeding practices: Part 2: Measurement. Geneva: World Health Organization. [Google Scholar]
- WHO . (2011). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Download from: http://www.who.int/vmnis/indicators/haemoglobin.pdf.
- Wijnhoven, T. M. , de Onis, M. , Onyango, A. W. , Wang, T. , Bjoerneboe, G.‐E. A. , Bhandari, N. , … Al Rashidi, B. (2004). Assessment of gross motor development in the WHO Multicentre Growth Reference Study. Food and Nutrition Bulletin, 25(1_suppl1), S37–S45. 10.1177/15648265040251S106 [DOI] [PubMed] [Google Scholar]


