Abstract
There is evidence in the general population that adhering to a high protein and low carbohydrate diet may help in losing weight. However, there is little evidence among postpartum women. The aim of this study is to evaluate the effect of a high protein diet on weight loss among postpartum women. A parallel‐randomized controlled trial with 94 postpartum women was conducted in a maternity ward in Mesquita county (recruitment from February 2009 to December 2010) and in a polyclinic in Rio de Janeiro city (recruitment from December 2010 to December 2011). Women were randomized to the intervention group (IG) or control group (CG), and both groups received an isocaloric diet (1,800 kcal). Additionally, the IG received approximately 25 g of protein obtained from 125 g per week of sardine to increase daily dietary protein content and was advised to restrict carbohydrate intake. The CG received nutritional counselling to follow the national nutrition guidelines (15% protein, 60% carbohydrates, and 25% lipids). A linear mixed‐effects model was used to test the effect of high protein intake and macronutrient intake on weight loss during the postpartum period. Body weight decreased in the IG compared with the CG (ß = −0.325; p = 0.049) among overweight and obese postpartum women. The percentage of energy intake from lipid (ß = −0.023; p = 0.050) was negatively associated with body weight, and carbohydrate intake (ß = 0.020; p = 0.026) was positively associated with body weight over time among all women. Protein intake and lower carbohydrate intake may be used as a dietary strategy to improve body weight loss during the postpartum period.
Keywords: low‐income countries, macronutrients, maternal obesity, maternal postpartum weight loss, protein intake, randomized controlled trial
Key messages.
There is limited available evidence regarding the relationship between high protein intake and weight retention during the postpartum period.
Body weight decreased in the intervention group when compared with the control group among overweight and obese postpartum women.
High protein and low CH intake may be used as a dietary strategy to improve body weight maintenance during the postpartum period.
Dietary counselling during the postpartum period improved weight loss and prevented weight retention.
More research should be conducted to test the safety of a high protein diet during the postpartum period.
1. INTRODUCTION
Failure to return to prepregnancy weight after childbirth may contribute to weight retention, which can ultimately lead to long‐term maternal obesity (Adegboye & Linne, 2013; Endres et al., 2015) and other chronic diseases later in life (Fraser et al., 2011; Rasmussen & Abrams, 2011; Shao et al., 2017). Socio‐demographics factors, such as low education, high parity, and Black race (Endres et al., 2015), as well as potentially modifiable behaviours, such as reduced breastfeeding duration and lack of physical activity, can increase the risk of weight retention (Hollis et al., 2017; Lovelady, 2011; Martin, MacDonald‐Wicks, Hure, Smith, & Collins, 2015). According to Hollis et al. (2017), higher postpartum weight retention is associated with a greater number of modifiable risk factors, such as excessive gestational weight gain and breastfeeding for less than 6 months. Postpartum women retained more than two additional kilograms of body weight for each modifiable risk factor (Hollis et al., 2017).
Previous studies have shown evidence that energy restriction and aerobic exercise among postpartum women promote weight loss and prevent excessive weight retention (Adegboye & Linne, 2013; Nascimento, Pudwell, Surita, Adamo, & Smith, 2014). In generally, diets were based on energy restriction, energy goals, healthy eating, or nutritional counselling (Choi, Fukuoka, & Lee, 2013). According to Wiltheiss et al. (2013), energy restriction should be the focus of dietary interventions aimed at improving weight loss among obese and overweight postpartum women.
In addition, some studies focused on the role of the macronutrient ratio, such as low fat and CH intake, low glycaemic load, or high protein intake for weight loss, but these studies were not conducted among postpartum women (Campos‐Nonato, Hernandez, & Barquera, 2017; Ebbeling et al., 2012; Soenen et al., 2012). Although the exact mechanism by which protein intake promotes weight loss during the postpartum period is still unclear, there is compelling evidence regarding the effects of high protein diets on satiety and thermogenesis among adults, which could improve body weight maintenance for longer periods (Astrup, Raben, & Geiker, 2015).
To the best of our knowledge, no other clinical trial based on high protein dietary intake has been performed among postpartum women. The present study was conducted based on a previous observational study that demonstrated greater postpartum weight loss among participants consuming a high protein diet (Castro, Kac, de Leon, & Sichieri, 2009). Furthermore, maternal nutritional requirements are increased in the postpartum period; therefore, in addition to caloric intake, protein intake should be enhanced to support exclusive breastfeeding (Marangoni et al., 2016). Thus, the aim of this study was to evaluate the effect of high protein intake on weight loss during the first six postpartum months.
2. METHODS
2.1. Study population and design
This is a parallel‐randomized controlled trial (RCT) with 94 postpartum women who gave birth between February 2009 and February 2011. In total, 106 postpartum women were recruited from the public maternity ward of the Municipal Hospital Leonel de Moura Brizola in Mesquita County from February 2009 to December 2010 and from the Piquet Carneiro Policlinic in Vila Isabel district from December 2010 to December 2011. However, 12 women were excluded because they were enrolled after more than two postpartum months (Figure 1). The recruitment of women in the polyclinic occurred after recruitment in the maternity ward hospital was finished, as the main objective was to fill the total targeted sample size. Both study sites are located in Rio de Janeiro state in Brazil. Figure 1 shows the study design from recruitment to follow‐up.
Figure 1.
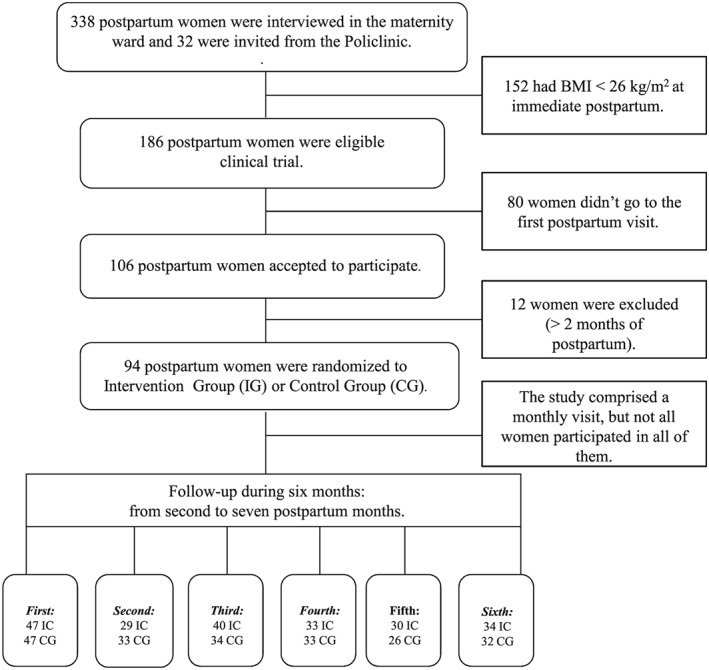
Flow of postpartum women participants
The eligibility criteria to participate in the clinical trial were age between 18 and 45 years, body mass index (BMI) ≥ 26 kg/m2 immediately postpartum (cut‐off based on the Institute of Medicine criteria to classify overweight prepregnancy BMI; IOM, 1992), no pre‐existing chronic diseases, a singleton pregnancy, and between 4 and 8 weeks after childbirth. A weight loss of 5% is expected to have an impact on the metabolic profile from the immediate postpartum period to the baseline of the study; therefore, we only considered women with BMI ≥ 26 kg/m2.
2.2. Sample size and randomization
The targeted sample size of 148 postpartum women was projected to provide 80% or more power to detect a 1.2 kg/m2 difference in BMI between groups over 6 months of postpartum follow‐up, with a standard deviation of ± 2.5 kg/m2 and using a 2‐sided t test with a significance level of 5%. This difference represents a 5% change in the weight of a woman with a BMI of 26 kg/m2 and a height of 1.60 m. After adding 20% for possible losses, a total of 180 women were intended to be recruited.
A list of random numbers was generated using the Statistical Analysis System (SAS) software (SAS version 9.3, Institute, Cary, NC, USA). The enrolled postpartum women were allocated to the intervention group (IG) or control group (CG) according to a random list. Women were randomized after baseline data collection at the first postnatal visit, which occurred approximately 4–8 weeks after birth. All participants provided informed written consent. This research was approved by the Ethics Committee (protocols number CAAE–0014.0.259.000–08) of the Social Medicine Institute of State University of Rio de Janeiro and was registered on the site http://www.ClinicalTrials.gov as identifier NCT00969488.
2.3. Measurements
The study follow‐up occurred over 6 months (starting at least 30 days after delivery) and women were invited to come to the clinic each month for a total of six visits: first visit in the second month; second visit in the third month; third visit in the fourth month, fourth visit in the fifth month, and sixth visit in the seventh month). Not all women participated in all six interviews (Figure 1). Certified nutritionists conducted the interviews, collected all the data (dietary, socio‐demographic, and anthropometric) and conducted nutritional counselling. During the first visit, a structured questionnaire was administered to acquire socio‐demographic data: age (years), education (years of schooling), parity (≥ 1 or ≤ 2), total family income (dollars); self‐reported race (Black/Brown or White), and breastfeeding [yes (exclusive or partial) or not]. Maternal height and weight were collected at this time by trained interviewers according to standardized procedures (Lohman, Roche, & Martorell, 1988). Height (cm) was measured with a portable stadiometer (AlturaExata®, Brazil). Weight (kg) and body fat percentage (BF%) were estimated by bioelectrical impedance using a Tanita® scale (Tanita Inner Scan, Illinois, USA). Women were measured without shoes and wearing light clothes.
Early pregnancy body mass index (EPBMI, kg/m2) was calculated using early pregnancy weight, which was obtained from the prenatal records, when weight was measured before the 13th gestational week. In the absence of this information, self‐reported prepregnancy weight (PPG) in kg was used. Gestational weight gain (GWG) was calculated using the difference between the last measured weight before delivery (after 38 weeks) and the early pregnancy weight.
2.4. Dietary variables
A validated semiquantitative food frequency questionnaire (FFQ) (Sichieri & Everhart, 1998) consisting of 81 food items was administered at the 1st postpartum follow‐up visit (during the second postpartum month), and the participants were asked to respond based on their diet during the 1 month after to childbirth (referred to the first 30 days after delivery). A programme developed in SAS was used to convert food frequencies and number of portions into estimated meanFs of daily intake (Sichieri, 1998). The Brazilian Food Composition Table (NEPA, 2006) was used to assess the nutritional composition of foods. The Department of Agriculture Food Composition Table (USDA, 2010) was adopted when a certain food item was not available on the Brazilian table.
2.5. Interventions
All women were advised (counselled) to adopt an isocaloric diet to lose weight. The advised diet contained approximately 1,800 kcal in six daily meals (breakfast, morning snack, lunch, afternoon snack, dinner, and supper) and was based on a fixed menu (Chart 1). The menu included the exact number of portions of each of the following eight recommended food groups (Philippi, Latterza, Cruz, & Ribeiro, 1999): (a) breads, cereals, roots, and tubers;(b) vegetables;(c) fruits; (d) lean meats, eggs and fish; (e) milk and dairy products; (f) beans; (g) oils and fats; and (h) sugars and sweets.
Chart 1.
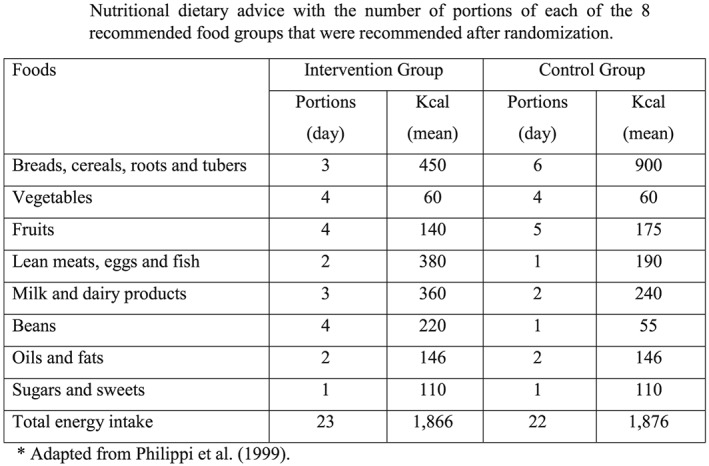
Nutritional dietary advice with the number of portions of each of the eight recommended food groups that were recommended after randomization
Women in the IG also received instructions to follow a high protein diet. Women were encouraged to eat foods with high protein content [beans (four portions per day), milk and dairy products (three portions per day), and lean meats, eggs, and fish (two portions per day)], to avoid carbohydrate intake at dinner, and to consume a maximum of three portions per day of bread, pasta, rice, potatoes, or roots. This group also received six cans per month of sardines soaked in tomato sauce (125 g) to ensure they consumed at least 25 g of protein per week.
The CG received standard nutritional counselling to follow an isocaloric diet that included six portions of breads, cereals, roots, or tubers; one portion of lean meats, eggs, or fish; two portions of milk and dairy products; and one portion of beans distributed in six daily meals. To avoid losses to follow‐up and to promote dietary adherence, women in the CG received 2 kg of pasta per month.
For each group, we provided printed diet information and food recipes and individual counselling during monthly meetings. Women received a list of food content by food group that could be used as equivalent intake.
2.6. Statistical analysis
Weight loss (kg) over the six postpartum months (from the second to seventh postpartum month) was defined as the primary outcome. The baseline characteristics of postpartum women were described using means, standard deviations (SD) and frequencies (%) according to the randomized group. Chi‐square and Student's t‐tests were applied to verify anthropometric and socio‐demographic baseline differences between groups.
Linear mixed‐effects regression models (LME) were used to evaluate the effect of the intervention on weight (as continuous variable) over 6 months postpartum (from second to seventh postpartum months), using an unstructured covariance matrix. The LME model included time (months), intervention (IG and CG) and interaction of time, and intervention variables. The interactions of the quadratic postpartum months (months*months) variable was tested to verify the linearity of the data. However, this measure was not statistically significant and, therefore, not included in the final model. Analyses were conducted on an intention‐to‐treat (ITT) basis, with participants analysed in the group, they were allocated to regardless of treatment compliance. Model 1 included all 94 postpartum women. Then, Model 2 included only those 65 overweight and obese postpartum women (BMI ≥ 25 kg/m2).
Predicted models of body weight according to dietary IGs and CGs included the time variable, the intervention variable (IG vs. CG) and the interaction of the IG and CG with time (month). Additionally, three independent models were performed, one for each macronutrient percentage (% of lipid, % of protein, and % of carbohydrate intake) of total energy intake variable reported by postpartum women in the FFQ at baseline, with continuous weight loss as the outcome in all cases. These predicted models included the macronutrient intake percentage, time variable, and the interaction of the macronutrient intake percentage with time variable. In addition, the values were adjusted by the IG and CG variables and by the interaction of IG and CG variables with time (month) and with the macronutrient percentage. Statistical significance was set at p < 0.05. Statistical analyses were performed using SAS.
3. RESULTS
There were no significant differences between the IG and CG groups at baseline for weight, height, gestational weight gain, age, family income, schooling, and age in the complete sample of 94 women and in the overweight and obese subset group of 65 postpartum women. In the group of all women, the IG participants had a higher protein intake percentage in the 1st postpartum follow‐up visit (p < 0.01) (Table 1).
Table 1.
Anthropometric and socio‐demographic characteristics at baseline (first follow‐up visit) of postpartum women according to dietary intervention groups. Mesquita, Rio de Janeiro, 2011
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variables | IG* (n = 47) | CG**(n = 47) | IG (n = 30) | CG (n = 35) | ||
| Mean (SD) | p valuea | Mean (SD) | p valuea | |||
| Dietary intake | ||||||
| Energy (kcal) | 2,062 (992) | 2,035 (1,007) | 0.798 | 2,109 (1,045) | 2,092 (745) | 0.939 |
| Protein (%) | 19.5 (3.23) | 18.4 (4.26) | 0.007 | 18.6 (3.08) | 18.3 (4.28) | 0.716 |
| Lipid (%) | 11.1 (2.89) | 10.9 (2.46) | 0.511 | 25.3 (6.35) | 24.4 (5.76) | 0.548 |
| Carbohydrate (%) | 56.3 (9.02) | 57.6 (7.62) | 0.124 | 56.9 (8.56) | 58.2 (7.58) | 0.517 |
| Anthropometrics | ||||||
| Weight (kg) | 75.9 (16.0) | 75.7 (13.8) | 0.954 | 78.6 (12.4) | 75.1 (10.4) | 0.215 |
| Height (m) | 1.61 (0.07) | 1.61 (0.06) | 0.941 | 1.61 (5.73) | 1.61 (6.73) | 0.918 |
| BMIc (kg/m2 ) | 28.9 (4.89) | 29.1 (5.40) | 0.929 | 30.4 (3.69) | 29.1 (3.33) | 0.156 |
| Body fat (%) | 35.5 (7.12) | 36.2 (7.03) | 0.667 | 35.1 (6.83) | 35.9 (5.13) | 0.638 |
| PGWe (kg) | 68.6 (13.3) | 71.0 (15.8) | 0.456 | 74.3 (3.04) | 69.3 (12.8) | 0.184 |
| PGBMIf (kg/m2 ) | 26.5 (4.32) | 27.5 (5.43) | 0.362 | 28.7 (5.28) | 26.9 (4.06) | 0.152 |
| GWGg (kg) | 12.7 (6.48) | 14.7 (10.1) | 0.293 | 13.8 (11.3) | 12.4 (6.59) | 0.584 |
| Socio‐demographic | ||||||
| Age (y) | 25.7 (4.98) | 26.2 (5.51) | 0.645 | 26.7 (6.07) | 25.9 (5.09) | 0.542 |
| Education (y) | 8.9 (3.41) | 8.5 (3.48) | 0.638 | 8.6 (3.48) | 9.0 (3.42) | 0.753 |
| Incomed (dollar) | 356 (232) | 327 (201) | 0.551 | 368 (203) | 368 (244) | 0.999 |
| Parity (n) | 2.25 (1.22) | 2.19 (1.26) | 0.686 | 2.55 (1.44) | 2.42 (1.56) | 0.773 |
| N (%) | p valueb | N (%) | p valueb | |||
| Skin colour | 0.472 | 0.566 | ||||
| Black/Brown | 37 (78.7) | 34 (72.3) | 105 (80.1) | 116 (82.9) | ||
| White | 10 (21.3) | 13 (27.7) | 26 (19.9) | 24 (17.1) | ||
| Breastfeeding | 0.606 | 0.648 | ||||
| Exclusive/partial | 26 (70.3) | 25 (75.8) | 29 (96.7) | 33 (94.3) | ||
| No | 11 (29.7) | 08 (24.2) | 01 (3.33) | 02 (5.71) | ||
IG = Intervention group.
CG = Control group.
Student.
Chi‐square.
Body mass index.
Total family income.
Pregestational weight.
Pregestational body mass index.
Gestational weight gain. Model 1 included all 94 postpartum women and Model 2 included only the subset with 65 overweight and obese postpartum women.
Overweight and obese postpartum women in the IG had higher weight loss over time when compared with the CG (ß = −0.325; SD = 0.16; p = 0.049; Table 2 ). Postpartum women in the IG when compared with women in the CG lost more weight until the fourth month of follow‐up (Figure 2). The data show a significant negative effect of lipid intake on body weight change (ß = −0.023; SD = 0.012; p = 0.050), whereas the percentage of carbohydrate intake was positively associated with body weight over time (ß = 0.020; SD = 0.009; p = 0.026) in the model including all postpartum women (Table 3).
Table 2.
Regression coefficients modelsa estimates predicting body weight according to dietary intervention. Mesquita, Rio de Janeiro, 2011
| Model 1 (n = 94)a | Model 2a (n = 65) | |||
|---|---|---|---|---|
| Parameters | ß (SD) | p value | ß (SD) | p value |
| Intercept | ||||
| Weight (kg) | 76.1 (2.26) | < 0.001 | 78.5 (2.07) | < 0.001 |
| IG*/CG** | 3.09 (3.20) | 0.337 | 3.90 (2.85) | 0.176 |
| Rate of body weight loss | ||||
| Month | −0.133 (0.09) | 0.122 | −0.070 (0.12) | 0.553 |
| IG*/CG**month | −0.210 (0.12) | 0.088 | −0.325 (0.16) | 0.049 |
IG = Intervention group.
CG = Control group.
Models were fitted using a random intercept. Model 1 included all 94 postpartum women and Model 2 included only the subset with 65 overweight and obese postpartum women.
Figure 2.
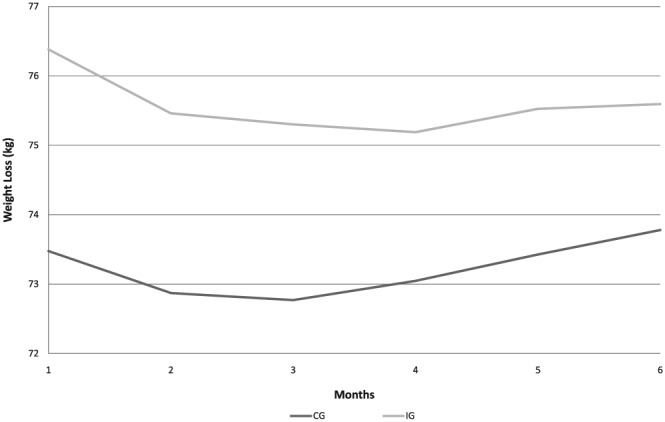
Predicted means of body weight loss according to dietary intervention group (IG) and control group (CG)
Table 3.
Regression coefficients from models estimates for the percent of energy from protein, carbohydrate and lipid dietary intake according to postpartum predicting body weight loss. Mesquita, Rio de Janeiro, 2011
| Model 1 (n = 94)a | Model 2 (n = 65)a | |||
|---|---|---|---|---|
| ß (SD) | p value | ß (SD) | p value | |
| Rate of body weight loss | ||||
| Percent of Protein × Month | −0.031 (0.018) | 0.088 | −0.027 (0.023) | 0.238 |
| Percent of Carbohydrate × Month | 0.020 (0.009) | 0.026 | 0.020 (0.011) | 0.078 |
| Percent of Lipids × Month | −0.023 (0.012) | 0.050 | −0.024 (0.014) | 0.088 |
Models were fitted using a random intercept. Model 1 included all 94 postpartum women and Model 2 included only the subset with 65 overweight and obese postpartum women.
4. DISCUSSION
This RCT compared the effect of a high protein diet to an isocaloric energy diet on weight loss during a period of 6 months postpartum (from the second to the seventh postpartum month) and showed a differential weight loss between the subgroup of overweight and obese women participants in the IG. It was observed that higher weight loss occurred in the second postpartum months and weight loss then persisted until the 4th visit at the fifth postpartum month. A second interesting result was the negative effect of the percentage of lipid derived from the baseline reported dietary intake with weight loss over time, whereas the percentage of carbohydrate intake from the total reported energy intake was associated with weight increase.
To the best of our knowledge, this is the first RCT conducted during the postpartum period that used a high protein diet as an intervention. Generally, caloric diet restriction, a healthy diet, dietary counselling, increased breastfeeding duration, and increased physical activity have been identified as strategies to prevent weight retention among normal and overweight postpartum women (Adegboye & Linne, 2013; Berger, Peragallo‐Urrutia, & Nicholson, 2014; Choi et al., 2013; Hollis et al., 2017; Lovelady, 2011; Østbye, Peterson, Krause, Swamy, & Lovelady, 2012).
Various hypotheses pertaining to protein metabolism have been raised to explain weight loss increase among subjects that adhered to a high protein diet (Pesta & Samuel, 2014). Furthermore, the literature has shown the benefits of a high protein diet on weight change (Castro et al., 2009; Soenen et al., 2012; Wycherley, Moran, Clifton, Noakes, & Brinkworth, 2012), diet‐induced thermogenesis (Westerterp, 2004), and a satiating effect, which could be important factors for weight loss and weight management during the postpartum period (Cuenca‐Sánchez, Navas‐Carrillo, & Orenes‐Piñero, 2015; Pesta & Samuel, 2014). Moreover, a high protein diet has a lower glycaemic index that seems to be more effective for body weight loss (Horan, McGowan, Gibney, Donnelly, & McAuliffe, 2014; Radulian, Rusu, Dragomir, & Posea, 2009); however, this finding is still controversial (Louie, Markovic, Ross, Foote, & Brand‐Miller, 2015). According to a study conducted by Thomas, Elliott, Fau, Baur, and Baur (2007), lowering the glycaemic load in the diet appears to be a more effective method of promoting weight loss and improving lipid profiles and can be more easily incorporated into a person's lifestyle when compared with conventional and highly restricted energy and low‐fat diets. Clifton's meta‐analysis showed that higher protein, low‐carbohydrate diets may offer a slight advantage in terms of weight loss and loss of fat mass compared with a normal protein diet among adults (Clifton, Condo, & Keogh, 2014). In addition, there is evidence that very low‐carbohydrate diets may lead to greater short‐term weight loss than do low‐fat diets (Fields, Ruddy, Wallace, Shah, & Millstine, 2016; Hall et al., 2015).
In our study, although we did not have the objective of evaluating the effect of the glycaemic index on weight variation, we observed that the percentage of carbohydrates was positively associated with weight variation in all women. However, this result was not found in the subgroup of women with overweight and obesity. More recently, an RCT conducted with 460 postpartum women in Dublin, Ireland, revealed greater weight loss from preconception to 3 months postpartum among the IG with a higher glycaemic index diet (Horan et al., 2014).
Additionally, in our RCT, we observed an additional effect of the high protein diet on weight loss among overweight and obese women. In addition to the high energy expenditure among overweight and obese women and the metabolic effect of a protein diet among postpartum women with excessive weight (Prentice, Black, Coward, & Cole, 1996), behaviour, life style and socio‐demographic issues may also explain greater weight loss in a subgroup formed by women with excessive weight in our study. Overweight and obese women are more motivated to lose weight after their third pregnancy according to Bastian et al. (2010). Health, motivation, and family support could be factors, and obese women could be more concerned about their weight‐related outcomes than primiparous women and pregestational normal weight women. A pilot‐study conducted by Haby, Glantz, Hanas, and Premberg (2015) with 100 obese pregnant women showed that an intervention based on nutrition support and advice, that prescribed physical activity resulted in lower weight gain and weight retention among the IG when compared with the CG.
Nevertheless, long‐term intervention studies are necessary to confirm that weight loss is maintained in the late postpartum period (Herring et al., 2017). In many studies, no effect was observed between counselling and weight retention (Althuizen, van der Wijden, van Mechelen, Seidell, & van Poppel, 2013; Aşcı & Rathfisch, 2016; van der Pligt et al., 2016). An RCT conducted among 102 pregnant women in Istanbul, Turkey, tested the effect of lifestyle interventions on gestational weight gain and weight retention. The results showed that the IG increased protein and vegetable intake as well as the percentage of protein from the diet compared with the CG from preconception to the postpartum period. However, the intervention was not effective in preventing weight retention, only in avoiding excessive gestational weight gain (Aşcı & Rathfisch, 2016).
However, there is still no consensus, as other studies have found satisfactory results from counselling. The intensity of the nutritional and behavioural advice has been discussed (Phelan et al., 2014). Individualized and structured diets (O'Toole, Sawicki, & Artal, 2003) and reduced energy intake (Wiltheiss et al., 2013) were found to be more predictive of lower body weight retention among overweight and obese postpartum women. According to Jackson, Wardle, Johnson, Finer, and Beeken (2013), advice from health professionals increased motivation among 810 overweight and obese adults in a survey across Great Britain. The authors concluded that increasing health professional capacity building is important for weight counselling.
This study has some limitations that should be considered when interpreting the results. First, the calculated sample size was not achieved, and the statistical power to detect changes between groups varied from 5.1% to 23.5% when only overweight and obese postpartum women were considered. Despite this limitation, we observed a borderline and a significant difference in postpartum weight loss among the participants in the IG versus CG, respectively, among all postpartum women and overweight and obese postpartum women.
The present study addresses an import factor that ensures the validity of our results. Although the follow‐up time was not long, it was sufficient to detect the effect of high protein content in reducing postpartum weight. This finding is particularly important because few studies have a greater than 6 months, long‐term postpartum follow‐up (Althuizen et al., 2013; Phelan et al., 2014; Vesco et al., 2016). Additionally, studies have tried to understand factors related to pregnancy outcomes (Poston et al., 2017), such as maternal weight gain, weight retention, and dietary barriers, which are related to more difficulties with losing weight (Carter‐Edwards et al., 2009; Christenson, Johansson, Reynisdottir, Torgerson, & Hemmingsson, 2016; Davis, Shearrer, Tao, Hurston, & Gunderson, 2017; Opie, Neff, & Tierney, 2016). According to Vesco et al. (2016), a postpartum weight loss maintenance programme could be added to prevent high postpartum weight regain. These authors followed 114 obese pregnant women and showed that at 1‐year postpartum, over half of the women between both the intervention and CGs were at or below their baseline weight.
Good participant compliance to their allocated group adds to the strength of our study. The weight loss in the IG was significant and observed with or without adjustment for energy intake. Dietary compliance was obtained via the intensive dietetic and professional support provided throughout the study. This strategy supports the evidence that achieving weight goals is more successful when instructions are provided by trained and qualified dieticians (Opie et al., 2016). One important issue to consider is the fact that the reproductive period is a phase of life when women are very motivated to adopt healthy behaviours, contributing to long‐term dietary changes. Additionally, most people are able to lose weight on diet plans that impose calorie restrictions over a short‐term period.
Therefore, monthly individual visits, prescriptive diet plans, and regular weight monitoring are initiatives that might favour the adoption of healthy behaviours (Opie et al., 2016; Phelan et al., 2014). However, these practices may limit the potential translation of current findings into clinical and public health practice where such intensive dietetic support may not be realistic or affordable. To ensure that the study findings would be of relevance to health professionals, the proposed dietary plans were designed to incorporate all core food groups with a high protein diet and a moderate to low carbohydrate intake. Although the dietary plans were relatively prescriptive in our study, participants had choices within food groups. This approach was also used in an RCT in an antenatal health group in Gothenburg, Sweden. In this study, obese women could choose their food intake from a list of equivalent foods (Haby et al., 2015).
This result indicates that protein intake may be an important dietary tool to increase weight loss during the postpartum period. Future studies should be performed to evaluate whether this difference persists over a longer postpartum period (Louie et al., 2015). With our results, we were able to verify a higher body weight loss during the postpartum period. This finding could be very important considering that this phase of life is associated with weight retention and maintenance of excess weight or obesity development (Gilmore, Klempel‐Donchenko, & Redman, 2015). Moreover, the greater gestational weight gain among the CG could have important implications for overweight and obesity later in life as well as with offspring (Catov, Abatemarco, Althouse, Davis, & Hubel, 2015; Widen et al., 2015).
5. CONCLUSIONS
In summary, a high protein diet seems to be more effective in promoting weight loss in overweight and obese postpartum women. A diet with higher carbohydrate content was associated with weight increase. The use of a structured eating dietary plan, which allows flexibility but limits choices, can assist in weight change. Protein intake may be used as a dietary strategy to improve body weight maintenance during the postpartum period.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
MBTC conceptualized the study and collected the data, performed the statistical analysis and the interpretation of the data, draft de manuscript, and contributed to the discussion of the results. DBC and RS performed the statistical analysis and the interpretation of the data and reviewed the manuscript. MCA, INB, and ARAA drafted the manuscript and contributed to the discussion of the manuscript. GK reviewed and contributed to the discussion of the manuscript.
ACKNOWLEDGEMENTS
The authors pleasantly thank all the postpartum women and their babies, the study interviewers, the maternity ward staff, and the nutrition students for their commitment, effort, and contributions to the study.
Castro MBTd, Cunha DB, Araujo MC, et al. High protein diet promotes body weight loss among Brazilian postpartum women. Matern Child Nutr. 2019;15:e12746 10.1111/mcn.12746
REFERENCES
- Adegboye, A. R. A. , & Linne, Y. M. (2013). Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database of Systematic Reviews, 7 10.1002/14651858.CD005627.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althuizen, E. , van der Wijden, C. L. , van Mechelen, W. , Seidell, J. C. , & van Poppel, M. N. (2013). The effect of a counselling intervention on weight changes during and after pregnancy: A randomised trial. BJOG, 120(1), 92–99. 10.1111/1471-0528.12014 [DOI] [PubMed] [Google Scholar]
- Aşcı, Ö. , & Rathfisch, G. (2016). Effect of lifestyle interventions of pregnant women on their dietary habits, lifestyle behaviors, and weight gain: A randomized controlled trial. Journal of Health, Population, and Nutrition, 35, 7 10.1186/s41043-016-0044-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup, A. , Raben, A. , & Geiker, N. (2015). The role of higher protein diets in weight control and obesity‐related comorbidities. International Journal of Obesity, 39(5), 721–726. 10.1038/ijo.2014.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian, L. A. , Pathiraja, V. C. , Krause, K. , Namenek Brouwer, R. J. , Swamy, G. K. , Lovelady, C. A. , & Østbye, T. (2010). Multiparity is associated with high motivation to change diet among overweight and obese postpartum women. Womens Health Issues, 20(2), 133–138. 10.1016/j.whi.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, A. A. , Peragallo‐Urrutia, R. , & Nicholson, W. K. (2014). Systematic review of the effect of individual and combined nutrition and exercise interventions on weight, adiposity and metabolic outcomes after delivery: Evidence for developing behavioral guidelines for post‐partum weight control. BMC Pregnancy and Childbirth, 14, 319 10.1186/1471-2393-14-319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos‐Nonato, I. , Hernandez, L. , & Barquera, S. (2017). Effect of a high‐protein diet versus standard‐protein diet on weight loss and biomarkers of metabolic syndrome: A randomized clinical trial. Obesity Facts, 10(3), 238–251. 10.1159/000471485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter‐Edwards, L. , Østbye, T. , Bastian, L. A. , Yarnall, K. S. , Krause, K. M. , & Simmons, T. J. (2009). Barriers to adopting a healthy lifestyle: Insight from postpartum women. BMC Research Notes, 2, 161 10.1186/1756-0500-2-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, M. B. , Kac, G. , de Leon, A. P. , & Sichieri, R. (2009). High‐protein diet promotes a moderate postpartum weight loss in a prospective cohort of Brazilian women. Nutrition, 25(11–12), 1120–1128. 10.1016/j.nut.2009.02.006 [DOI] [PubMed] [Google Scholar]
- Catov, J. M. , Abatemarco, D. , Althouse, A. , Davis, E. M. , & Hubel, C. (2015). Patterns of gestational weight gain related to fetal growth among women with overweight and obesity. Obesity (Silver Spring), 23(5), 1071–1078. 10.1002/oby.21006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. , Fukuoka, Y. , & Lee, J. H. (2013). The effects of physical activity and physical activity plus diet interventions on body weight in overweight or obese women who are pregnant or in postpartum:A systematic review and meta‐analysis of randomized controlled trials. Preventive Medicine, 56(6), 351–364. 10.1016/j.ypmed.2013.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson, A. , Johansson, E. , Reynisdottir, S. , Torgerson, J. , & Hemmingsson, E. (2016). Women's perceived reasons for their excessive postpartumweight retention: A qualitative interview study. PLoS One, 11(12), e0167731 10.1371/journal.pone.0167731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton, P. M. , Condo, D. , & Keogh, J. B. (2014). Long term weight maintenance after advice to consume low carbohydrate, higher protein diets–a systematic review and meta analysis. Nutrition, Metabolism, and Cardiovascular Diseases, 24(3), 224–235. 10.1016/j.numecd.2013.11.006 [DOI] [PubMed] [Google Scholar]
- Cuenca‐Sánchez, M. , Navas‐Carrillo, D. , & Orenes‐Piñero, E. (2015). Controversies surrounding high‐protein diet intake: Satiating effect and kidney and bone health. Advances in Nutrition, 6(3), 260–266. 10.3945/an.114.007716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. N. , Shearrer, G. E. , Tao, W. , Hurston, S. R. , & Gunderson, E. P. (2017). Dietary variables associated with substantial postpartum weight retention at 1‐year among women with GDM pregnancy. BMC Obes, 4, 31 10.1186/s40608-017-0166-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Agriculture (2010). USDA . National Nutrient Database for Standard Reference. Release 23.. Retrieved July 2010. http://www.nal.usda.gov/fnic/foodcomp
- Ebbeling, C. B. , Swain, J. F. , Feldman, H. A. , Wong, W. W. , Hachey, D. L. , Garcia‐Lago, E. , & Ludwig, D. S. (2012). Effects of dietary composition on energy expenditure during weight‐loss maintenance. JAMA, 307(24), 2627–2634. 10.1001/jama.2012.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres, L. K. , Straub, H. , McKinney, C. , Plunkett, B. , Minkovitz, C. S. , Schetter, C. D. , … Community Child Health Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development . (2015). Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstetrics and Gynecology, 125(1), 144–152. 10.1097/AOG.0000000000000565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, H. , Ruddy, B. , Wallace, M. R. , Shah, A. , & Millstine, D. (2016). Are low‐carbohydrate diets safe and effective? The Journal of the American Osteopathic Association, 116(12), 788–793. 10.7556/jaoa.2016.154 [DOI] [PubMed] [Google Scholar]
- Fraser, A. , Tilling, K. , Macdonald‐Wallis, C. , Hughes, R. , Sattar, N. , Nelson, S. M. , & Lawlor, D. A. (2011). Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: The Avon Longitudinal Study of Parents and Children (ALSPAC). The American Journal of Clinical Nutrition, 93(6), 1285–1292. 10.3945/ajcn.110.008326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore, L. A. , Klempel‐Donchenko, M. , & Redman, L. M. (2015). Pregnancy as a window to future health: Excessive gestational weight gain and obesity. Seminars in Perinatology, 39(4), 296–303. 10.1053/j.semperi.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haby, K. , Glantz, A. , Hanas, R. , & Premberg, Å. (2015). Mighty Mums–An antenatal health care intervention can reduce gestational weight gain in women with obesity. Midwifery, 31(7), 685–692. 10.1016/j.midw.2015.03.014 [DOI] [PubMed] [Google Scholar]
- Hall, K. D. , Bemis, T. , Brychta, R. , Chen, K. Y. , Courville, A. , Crayner, E. J. , … Yannai, L. (2015). Calorie for calorie, dietary fat restriction esults in more body fat loss than carbohydrate restriction in people with obesity. Cell Metabolism, 22(3), 427–436. 10.1016/j.cmet.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring, S. J. , Cruice, J. F. , Bennett, G. G. , Darden, N. , Wallen, J. J. , Rose, M. Z. , … Foster, G. D. (2017). Intervening during and after pregnancy to prevent weight retention among African American women. Preventive Medical Reports, 7, 119–123. 10.1016/j.pmedr.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis, J. L. , Crozier, S. R. , Inskip, H. M. , Cooper, C. , Godfrey, K. M. , Harvey, N. C. , … Robinson, S. M. (2017). Modifiable risk factors of maternal postpartum weight retention: An analysis of their combined impact and potential opportunities for prevention. International Journal of Obesity, 41(7), 1091–1098. 10.1038/ijo.2017.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan, M. K. , McGowan, C. A. , Gibney, E. R. , Donnelly, J. M. , & McAuliffe, F. M. (2014). Maternal diet and weight at 3 months postpartum following a pregnancy intervention with a low glycaemic index diet: Results from the ROLO randomised control trial. Nutrients, 6(7), 2946–2955. 10.3390/nu6072946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (1992). Nutrition during pregnancy. An implementation guide. Washington, DC: The National Academy Press. [Google Scholar]
- Jackson, S. E. , Wardle, J. , Johnson, F. , Finer, N. , & Beeken, R. J. (2013). The impact of a health professional recommendation on weight loss attempts in overweight and obese British adults: A cross‐sectional analysis. BMJ Open, 3(11), e003693 10.1136/bmjopen-2013-003693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman, T. G. , Roche, A. F. , & Martorell, R. (1988). Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books. [Google Scholar]
- Louie, J. C. , Markovic, T. P. , Ross, G. P. , Foote, D. , & Brand‐Miller, J. C. (2015). Effect of a low glycaemic index diet in gestational diabetes mellitus on post‐natal outcomes after 3 months of birth: A pilot follow‐up study. Maternal & Child Nutrition, 11(3), 409–414. 10.1111/mcn.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelady, C. (2011). Balancing exercise and food intake with lactation to promote post‐partum weight loss. The Proceedings of the Nutrition Society, 70(2), 181–184. 10.1017/S002966511100005X [DOI] [PubMed] [Google Scholar]
- Marangoni, F. , Cetin, I. , Verduci, E. , Canzone, G. , Giovannini, M. , Scollo, P. , … Poli, A. (2016). Maternal diet and nutrient requirements in pregnancy and breastfeeding. An Italian consensus document. Nutrients, 8(10). 10.3390/nu8100629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, J. , MacDonald‐Wicks, L. , Hure, A. , Smith, R. , & Collins, C. E. (2015). Reducing postpartum weight retention and improving breastfeeding outcomes in overweight women: A pilot randomised controlled trial. Nutrients, 7(3), 1464–1479. 10.3390/nu7031464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento, S. L. , Pudwell, J. , Surita, F. G. , Adamo, K. B. , & Smith, G. N. (2014). The effect of physical exercise strategies on weight loss in postpartum women: A systematic review and meta‐analysis. International Journal of Obesity, 38(5), 626–635. 10.1038/ijo.2013.183 [DOI] [PubMed] [Google Scholar]
- NEPA (2006). Tabela brasileira de composição de alimentos [Brazilian Food Composition Table] (2nd ed.). Campinas: Avaiable from http://www.unicamp.br/nepa/taco/contar/taco_versao2.pdf: NEPA‐UNICAMP. accessed July 2010 [Google Scholar]
- Opie, R. S. , Neff, M. , & Tierney, A. C. (2016). A behavioural nutrition intervention for obese pregnant women: Effects on diet quality, weight gain and the incidence of gestational diabetes. The Australian & New Zealand Journal of Obstetrics & Gynaecology, 56(4), 364–373. 10.1111/ajo.12474 [DOI] [PubMed] [Google Scholar]
- Østbye, T. , Peterson, B. L. , Krause, K. M. , Swamy, G. K. , & Lovelady, C. A. (2012). Predictors of postpartum weight change among overweight and obese women: Results from the active mothers postpartum study. Journal of Women's Health (2002), 21(2), 215–222. 10.1089/jwh.2011.2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole, M. L. , Sawicki, M. A. , & Artal, R. (2003). Structured diet and physical activity prevent postpartum weight retention. Journal of Women's Health (2002), 12(10), 991–998. 10.1089/154099903322643910 [DOI] [PubMed] [Google Scholar]
- Pesta, D. H. , & Samuel, V. T. (2014). A high‐protein diet for reducing body fat: Mechanisms and possible caveats. Nutrition & Metabolism (London), 11(1), 53 10.1186/1743-7075-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan, S. , Phipps, M. G. , Abrams, B. , Darroch, F. , Grantham, K. , Schaffner, A. , & Wing, R. R. (2014). Does behavioral intervention in pregnancy reduce postpartum weight retention? Twelve‐month outcomes of the fit for delivery randomized trial. The American Journal of Clinical Nutrition, 99(2), 302–311. 10.3945/ajcn.113.070151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi, S. T. , Latterza, A. R. , Cruz, A. T. R. , & Ribeiro, L. C. (1999). Pirâmide alimentar adaptada: Guia para escolha dos alimentos. Revista de Nutrição, 12(1). 10.1590/S1415-52731999000100006), 65–80. [DOI] [Google Scholar]
- Poston, L. , Bell, R. , Briley, A. L. , Godfrey, K. M. , Nelson, S. M. , Oteng‐Ntim, E. , … Wardle, J. (2017). Programme Grants for Applied Research In Improving pregnancy outcome in obese women: The UK pregnancies better eating and activity randomised controlled Trial. Southampton (UK): NIHR Journals Library. [PubMed] [Google Scholar]
- Prentice, A. M. , Black, A. E. , Coward, W. A. , & Cole, T. J. (1996). Energy expenditure in overweight and obese adults in affluent societies: An analysis of 319 doubly‐labelled water measurements. European Journal of Clinical Nutrition, 50(2), 93–97. [PubMed] [Google Scholar]
- Radulian, G. , Rusu, E. , Dragomir, A. , & Posea, M. (2009). Metabolic effects of low glycaemic index diets. Nutrition Journal, 8, 5 10.1186/1475-2891-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, K. M. , & Abrams, B. (2011). Gestational weight gain and later maternal health: Are they related? The American Journal of Clinical Nutrition, 93(6), 1186–1187. 10.3945/ajcn.111.016758 [DOI] [PubMed] [Google Scholar]
- Shao, Y. , Qiu, J. , Huang, H. , Mao, B. , Dai, W. , He, X. , … Zhang, Y. (2017). Pre‐pregnancy BMI, gestational weight gain and risk of preeclampsia: A birth cohort study in Lanzhou, China. BMC Pregnancy and Childbirth, 17(1), 400 10.1186/s12884-017-1567-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichieri, R. (1998). Epidemiologia da Obesidade Rio de Janeiro: EdUERJ.
- Sichieri, R. , & Everhart, J. E. (1998). Validity of Brazilian food frequency questionnaire against dietary recalls and estimated energy intake. Nutrition Research, 18(10), 1649–1659. 10.1016/S0271-5317(98)00151-1 [DOI] [Google Scholar]
- Soenen, S. , Bonomi, A. G. , Lemmens, S. G. , Scholte, J. , Thijssen, M. A. , van Berkum, F. , & Westerterp‐Plantenga, M. S. (2012). Relatively high‐protein or 'low‐carb' energy‐restricted diets for body weight loss and body weight maintenance? Physiology & Behavior, 107(3), 374–380. 10.1016/j.physbeh.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Thomas, D. E. , Elliott, E.J. Fau ‐ Baur, L. , & Baur, L . (2007). Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database of Systematic Reviews, 18(3), CD005105 10.1002/14651858.CD005105.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pligt, P. , Olander, E. K. , Ball, K. , Crawford, D. , Hesketh, K. D. , Teychenne, M. , & Campbell, K. (2016). Maternal dietary intake and physical activity habits during the postpartum period: Associations with clinician advice in a sample of Australian first time mothers. BMC Pregnancy and Childbirth, 16, 27 10.1186/s12884-016-0812-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesco, K. K. , Leo, M. C. , Karanja, N. , Gillman, M. W. , McEvoy, C. T. , King, J. C. , … Stevens, V. J. (2016). One‐year postpartum outcomes following a weight management intervention in pregnant women with obesity. Obesity (Silver Spring), 24(10), 2042–2049. 10.1002/oby.21597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerterp, K. R. (2004). Diet induced thermogenesis. Nutrition & Metabolism (London), 1(1), 5 10.1186/1743-7075-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widen, E. M. , Factor‐Litvak, P. R. , Gallagher, D. , Paxton, A. , Pierson, R. N. , Heymsfield, S. B. , & Lederman, S. A. (2015). The pattern of gestational weight gain is associated with changes in maternal body composition and neonatal size. Maternal and Child Health Journal, 19(10), 2286–2294. 10.1007/s10995-015-1747-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltheiss, G. A. , Lovelady, C. A. , West, D. G. , Brouwer, R. J. , Krause, K. M. , & Østbye, T. (2013). Diet quality and weight change among overweight and obese postpartum women enrolled in a behavioral intervention program. Journal of the Academy of Nutrition and Dietetics, 113(1), 54–62. 10.1016/j.jand.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wycherley, T. P. , Moran, L. J. , Clifton, P. M. , Noakes, M. , & Brinkworth, G. D. (2012). Effects of energy‐restricted high‐protein, low‐fat compared with standard‐protein, low‐fat diets: A meta‐analysis of randomized controlled trials. The American Journal of Clinical Nutrition, 96(6), 1281–1298. 10.3945/ajcn.112.044321 [DOI] [PubMed] [Google Scholar]


