Abstract
Background
Chelation therapy is promoted and practiced around the world as a form of alternative medicine in the treatment of atherosclerotic cardiovascular disease. It has been suggested as a safe, relatively inexpensive, non‐surgical method of restoring blood flow in atherosclerotic vessels. However, there is currently limited high‐quality, adequately‐powered research informing evidence‐based medicine on the topic, specifically regarding clinical outcomes. Due to this limited evidence, the benefit of chelation therapy remains controversial at present. This is an update of a review first published in 2002.
Objectives
To assess the effects of ethylene diamine tetra‐acetic acid (EDTA) chelation therapy versus placebo or no treatment on clinical outcomes among people with atherosclerotic cardiovascular disease.
Search methods
For this update, the Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases, the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials register to 6 August 2019. We searched the bibliographies of the studies retrieved by the literature searches for further trials.
Selection criteria
We included studies if they were randomised controlled trials of EDTA chelation therapy versus placebo or no treatment in participants with atherosclerotic cardiovascular disease. The main outcome measures we considered include all‐cause or cause‐specific mortality, non‐fatal cardiovascular events, direct or indirect measurement of disease severity, and subjective measures of improvement or adverse events.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality using standard Cochrane procedures. A third author considered any unresolved issues, and we discussed any discrepancies until a consensus was reached. We contacted study authors for additional information.
Main results
We included five studies with a total of 1993 randomised participants. Three studies enrolled participants with peripheral vascular disease and two studies included participants with coronary artery disease, one of which specifically recruited people who had had a myocardial infarction. The number of participants in each study varied widely (from 10 to 1708 participants), but all studies compared EDTA chelation to a placebo. Risk of bias for the included studies was generally moderate to low, but one study had high risk of bias because the study investigators broke their randomisation code halfway through the study and rolled the placebo participants over to active treatment. Certainty of the evidence, as assessed by GRADE, was generally low to very low, which was mostly due to a paucity of data in each outcome's meta‐analysis. This limited our ability to draw any strong conclusions. We also had concerns about one study's risk of bias regarding blinding and outcome assessment that may have biased the results.
Two studies with coronary artery disease participants reported no evidence of a difference in all‐cause mortality between chelation therapy and placebo (risk ratio (RR) 0.97, 95% CI 0.73 to 1.28; 1792 participants; low‐certainty). One study with coronary artery disease participants reported no evidence of a difference in coronary heart disease deaths between chelation therapy and placebo (RR 1.02, 95% CI 0.70 to 1.48; 1708 participants; very low‐certainty). Two studies with coronary artery disease participants reported no evidence of a difference in myocardial infarction (RR 0.81, 95% CI 0.57 to 1.14; 1792 participants; moderate‐certainty), angina (RR 0.95, 95% CI 0.55 to 1.67; 1792 participants; very low‐certainty), and coronary revascularisation (RR 0.46, 95% CI 0.07 to 3.25; 1792 participants). Two studies (one with coronary artery disease participants and one with peripheral vascular disease participants) reported no evidence of a difference in stroke (RR 0.88, 95% CI 0.40 to 1.92; 1867 participants; low‐certainty). Ankle‐brachial pressure index (ABPI; also known as ankle brachial index) was measured in three studies, all including participants with peripheral vascular disease; two studies found no evidence of a difference in the treatment groups after three months after treatment (mean difference (MD) 0.02, 95% CI ‐0.03 to 0.06; 181 participants; low‐certainty). A third study reported an improvement in ABPI in the EDTA chelation group, but this study was at high risk of bias. Meta‐analysis of maximum and pain‐free walking distances three months after treatment included participants with peripheral vascular disease and showed no evidence of a difference between the treatment groups (MD ‐31.46, 95% CI ‐87.63 to 24.71; 165 participants; 2 studies; low‐certainty). Quality of life outcomes were reported by two studies that included participants with coronary artery disease, but we were unable to pool the data due to different methods of reporting and varied criteria. However, there did not appear to be any major differences between the treatment groups. None of the included studies reported on vascular deaths. Overall, there was no evidence of major or minor adverse events associated with EDTA chelation treatment.
Authors' conclusions
There is currently insufficient evidence to determine the effectiveness or ineffectiveness of chelation therapy in improving clinical outcomes of people with atherosclerotic cardiovascular disease. More high‐quality, randomised controlled trials are needed that assess the effects of chelation therapy on longevity and quality of life among people with atherosclerotic cardiovascular disease.
Plain language summary
Chelation therapy for atherosclerotic cardiovascular (heart and circulation) disease
Background
Atherosclerosis is caused by fatty deposits that cause a narrowing of people's arteries and restrict blood flow. People with blocked arteries are more likely to have strokes, heart attacks, and narrow blood vessels in their feet. Chelation therapy involves infusions into the bloodstream of substances believed to remove metals from the blood. This treatment is offered to people with atherosclerotic cardiovascular disease as a way of breaking down the blockages in their blood vessels. Chelation therapy is practiced in several places around the world as an alternative form of medicine, but there is currently a lack of knowledge surrounding this treatment. More information is needed to understand if this treatment should be more widely recommended.
Key results
This review included evidence from five studies with a total of 1993 participants (current until August 2019). Three studies enrolled participants with peripheral vascular disease, and two of the studies included participants with coronary artery disease, one of which specifically recruited people who had had a heart attack. All five studies compared chelation therapy with no treatment or placebo. Only two of the studies (both of which included participants with coronary artery disease) reported death from any cause, and these reported no difference in overall deaths between those that received chelation therapy and those who did not. Ony one study (in people with coronary artery disease) reported cardiovascular death, and this study found no difference between in risk between those who had chelation therapy and those who did not. Two studies of people with coronary artery disease reported rates of heart attack and angina, and found no difference in the risk of these between participants who had chelation treatment and those who did not. Similarly, two studies (one in people with coronary artery disease and one in people with peripheral vascular disease) reported the chance of having a stroke, and found no clear difference in the chance of this between people who did or did not received chelation treatment. Two studies in people with peripheral vascular disease used an indirect measure of blood flow known as the ankle‐brachial pressure index (ABPI), or ankle brachial index. These studies did not show any differences in this measure between people who received chelation therapy for three or six months and those who did not get the treatment. There was also no clear differences in the distance participants could walk.
We could not combine specific measures of quality of life in a single analysis. Looking at the two studies in people with coronary artery disease that reported this outcome, there was no difference in the quality of life reported by people who received chelation therapy and those who did not get the treatment. Two studies reported information about adverse events, but we could not combine this in a single analysis because they reported them in different ways and the events were different. However, the people who had chelation therapy did not appear to have any increase in either minor or major adverse events, compared with people who did not have the therapy.
Certainty of the evidence
We considered most of the data we found to be of low certainty, mostly because there were very few studies that provided data. Even though we included five studies, not all of them reported on each outcome. There is currently not enough evidence about the effects of chelation therapy on blockages in the blood vessels of people with atherosclerotic cardiovascular (heart and circulation) disease.
Conclusions
Overall, this review did not find any clear differences between people treated with chelation and people given the control, for the outcomes we evaluated. None of the outcomes included more than two studies, therefore it is difficult at this time to determine if these are true findings or just because there is not enough data. Further high‐quality trials that focus on clinical outcomes are necessary.
Summary of findings
Summary of findings 1. EDTA compared to placebo for atherosclerotic cardiovascular disease.
| EDTA compared to placebo for atherosclerotic cardiovascular disease | ||||||
| Patient or population: atherosclerotic cardiovascular disease Setting: outpatient clinics Intervention: EDTA Comparison: placebo | ||||||
| Outcomes | No. of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Comments | |
| Risk with placebo | Risk difference with EDTA | |||||
| All‐cause mortality follow up: range 1 years to 5 years | 1792 (2 RCTs) | ⊕⊕⊝⊝ LOW a,b | RR 0.97 (0.73 to 1.28) | Study population | Two studies with coronary artery disease participants | |
| 102 per 1000 | 3 fewer per 1000 (28 fewer to 29 more) | |||||
| Coronary heart disease death follow up: mean 5 years | 1708 (1 RCT) | ⊕⊝⊝⊝ VERY LOW c,d | RR 1.02 (0.70 to 1.48) | Study population | One study with coronary artery disease participants | |
| 59 per 1000 | 1 more per 1000 (18 fewer to 28 more) | |||||
| Myocardial infarction follow up: range 1 years to 5 years | 1792 (2 RCTs) | ⊕⊕⊕⊝ MODERATE e | RR 0.81 (0.57 to 1.14) | Study population | Two studies with coronary artery disease participants | |
| 75 per 1000 | 14 fewer per 1000 (32 fewer to 10 more) | |||||
| Angina follow up: range 1 years to 5 years | 1792 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW e,f,g | RR 0.95 (0.55 to 1.67) | Study population | Two studies with coronary artery disease participants | |
| 26 per 1000 | 1 fewer per 1000 (12 fewer to 18 more) | |||||
| Stroke follow up: range 6 months to 5 years | 1867 (2 RCTs) | ⊕⊕⊝⊝ LOW e,h | RR 0.88 (0.40 to 1.92) | Study population | One study with coronary artery disease participants and one study with peripheral vascular disease participants | |
| 14 per 1000 | 2 fewer per 1000 (9 fewer to 12 more) | |||||
| Ankle‐brachial pressure index at 3 months post‐treatment | 181 (2 RCTs) | ⊕⊕⊝⊝ LOW e,h | ‐ | The mean ankle‐brachial pressure index at 3 months post‐treatment was 0.56. | MD 0.02 higher (0.03 lower to 0.06 higher) | Two studies with peripheral vascular disease participants |
| Maximum walking distance (m) at 3 months post‐treatment | 165 (2 RCTs) | ⊕⊕⊝⊝ LOW e,h | ‐ | The mean maximum walking distance (m) at 3 months post‐treatment was 112.5. | MD 31.46 m lower (87.63 lower to 24.71 higher) | Two studies with peripheral vascular disease participants |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EDTA: ethylene diamine tetra‐acetic acid; RCT: randomised controlled trials; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngrade 1 level: death rates very different between two studies reporting. bDowngrade 1 level: only one of two studies in the meta‐analysis provided events for this outcome, making the estimate imprecise. cDowngrade 1 level: only one study reporting on this outcome so cannot evaluate inconsistency. dDowngrade 2 levels: only one study included in this analysis and the confidence intervals are very wide. eDowngrade 1 level: very wide confidence interval around point estimate makes it difficult to interpret true association. fDowngrade 1 level: two studies included in analysis are seemingly consistent but variability is 35%, indicating moderate heterogeneity. gDowngrade 1 level: two studies reporting on this outcome use different definitions and methods to determine angina. hDowngrade 1 level: one included study had a high risk of bias that might have affected the outcome assessment.
Background
Description of the condition
Atherosclerotic cardiovascular disease is characterised by an accumulation of plaques or lesions that result from an accumulation of lipids, followed by chronic inflammation at susceptible sites in the artery wall. This leads to thickening of the artery walls, and can affect the entire artery tree (Aziz 2016). Disease progression usually includes microcalcifications, extracellular matrix breakdown, interplaque haemorrhage, degradation of the fibrous cap, plaque erosion and rupture (Bakic 2007). A person with atherosclerosis may remain asymptomatic for a long time. However, in combination with other risk factors, such as age and an unhealthy lifestyle (including consumption of a high fat, high sugar diet), it can eventually contribute to the narrowing of blood vessels. The resulting restriction of blood flow causes ischaemia, with its accompanying symptoms (Aziz 2016; INTERHEART 2004). An atherosclerotic lesion may rupture, leading to either a stroke or heart attack.
Description of the intervention
Treatment with ethylene diamine tetra‐acetic acid (EDTA) for metal poisoning has been established for decades, but less well‐established is its usefulness for cardiovascular disease, which has been claimed since the 1950s (Clarke 1955; Clarke 1956). This recommendation is based on a physiologic premise: since metastatic calcium deposits can be removed by chelation, the calcium involved in atheroma could be evacuated in a similar way. This theoretical mechanism could potentially lead to an improvement in the outcomes of people with coronary disease. Initial claims about the effect of EDTA on lowering serum calcium levels were based on animal experiments (Clarke 1956). However, it was the result of active administration of EDTA to people at that time that provided encouragement for the use of this form of therapy. The continued use and promotion of chelation therapy is hampered by the limited availability of randomised controlled trials showing benefit.
Chelation therapy is promoted as a form of alternative medicine to treat atherosclerosis and relieve symptoms of cardiovascular disease (Hiatt 1997). It has been suggested to be a safe, relatively inexpensive, non‐surgical method of restoring blood flow in atherosclerotic vessels, thus preventing chronic symptoms such as angina pectoris and claudication, or acute symptoms such as myocardial infarction or stroke. Several clinics have been set up worldwide offering it to people with atherosclerotic cardiovascular disease (Ernst 1997; van Rij 1994).
How the intervention might work
Chelation therapy consists of a series of intravenous infusions containing EDTA, in combination with other substances. EDTA, a water soluble compound, has been found to be effective in chelating and removing some toxic metals from the blood (Green 1993). It is capable of combining with polyvalent cations, such as calcium ions, to form a soluble non‐ionic complex that can be excreted (Wilder 1962).
Proponents of chelation believe that a mechanism taking place at the arterial wall can lead to regression of atherosclerotic plaques (Clarke 1960; Green 1993; Meltzer 1960). There have been case reports suggesting that EDTA chelation therapy in people with angina led to alleviation of symptoms (Clarke 1956; Meltzer 1960). On the other hand, a review of chelation therapy for peripheral arterial occlusive disease carried out in 1997 failed to show that chelation therapy was superior to placebo in any well conducted, controlled trial (Ernst 1997). This review reported that some of the uncontrolled studies showed the treatment was associated with considerable risks, such as hypocalcaemia and kidney damage (Ernst 1997). Proponents, however, claim these effects occur only if people are given overdose levels of EDTA and in the presence of already existing kidney damage (Ernst 1997).
Why it is important to do this review
In a book by Drs Walker and Shah on chelation therapy, the authors noted that "all possible mechanisms of action of chelation therapy for producing the observed beneficial effects are still incompletely documented. And this incomplete understanding of why and how it works becomes a useful argument employed by medical opponents of the method. There has, in fact, been no full‐scale study of the technique." (Walker 1997). Since the publication of this book, several small and medium‐scale clinical trials have been conducted on the use of chelation therapy in cardiovascular diseases, especially peripheral vascular diseases. The National Heart, Lung and Blood Institute (NHLBI) and the National Center for Complementary and Alternative Medicine collaborated to conduct the National Institutes of Health (NIH) Trial to Assess Chelation Therapy (TACT). The TACT trial enrolled around 1708 participants and aimed to determine whether chelation therapy has an effect on clinical endpoints, such as mortality, myocardial infarction, stroke and hospitalisation (TACT 2013).
This Cochrane Review is an update of a review first published in 2002 (Dans 2002), and aims to incorporate the latest available randomised controlled trial evidence to assess the effects of EDTA chelation therapy versus placebo or no treatment on clinical outcomes among people with atherosclerotic cardiovascular disease.
Objectives
To assess the effects of ethylene diamine tetra‐acetic acid (EDTA) chelation therapy versus placebo or no treatment on clinical outcomes among people with atherosclerotic cardiovascular disease.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) of chelation therapy compared to placebo or no treatment.
Types of participants
We included trials in people with atherosclerotic cardiovascular disease. This could be cerebrovascular disease, coronary artery disease, or peripheral vascular disease.
Types of interventions
We included trials evaluating intravenous infusions containing EDTA compared to placebo infusions or no treatment.
Types of outcome measures
We considered the following outcome measures:
Primary outcomes
all‐cause mortality;
coronary heart disease (CHD) deaths;
vascular deaths
Secondary outcomes
non‐fatal events, including acute coronary syndromes (e.g. myocardial infarction and unstable angina pectoris);
cerebrovascular events, such as stroke;
direct test of disease severity (e.g. digital subtraction angiograms for peripheral arterial disease);
indirect tests of disease severity (e.g. ankle‐brachial pressure index (ABPI; also known as ankle brachial index));
participant symptoms, such as walking distance for claudicants and quality of life;
adverse events.
Search methods for identification of studies
There were no restrictions on language.
Electronic searches
For this update, the Cochrane Vascular Information Specialist first searched the following databases for relevant trials on 5 August 2015:
the Cochrane Vascular Specialised Register (5 August 2015);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 7) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy we used to search CENTRAL.
The Cochrane Vascular Information Specialist subsequently conducted systematic top‐up searches of the following databases for RCTs and controlled clinical trials without language, publication year or publication status restrictions on 6 August 2019:
the Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched to 6 August 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (CRSO 2019, Issue 7);
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (searched from 1 January 2017 to 6 August 2019);
Embase Ovid (searched from 1 January 2017 to 6 August 2019);
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Ebsco (searched from 1 January 2017 to 6 August 2019);
AMED Ovid (searched from 1 January 2017 to 6 August 2019).
The Information Specialist modelled search strategies for the listed databases on the search strategy designed for CENTRAL. Where appropriate, the Information Specialist combined these with adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6, Lefebvre 2011). Search strategies for major databases are provided in Appendix 2.
The Information Specialist also performed top‐up searches of the following trials registries on 6 August 2019:
ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
Searching other resources
We searched the bibliographies of the studies retrieved by the literature searches for further trials.
Data collection and analysis
Selection of studies
For this update, two review authors (MVS, RF) checked all identified trials to determine potentially relevant articles for full text retrieval. The same two review authors then assessed retrieved studies for eligibility, according to the specified inclusion criteria. We passed issues that were unresolved in the assessment and data extraction process on to a third review author for resolution. We then discussed any unresolved issues until we reached a consensus.
Data extraction and management
Three review authors (MVS, FT, ALD) independently extracted data from the included studies. They collected information from each trial on study design, participant characteristics, interventions, comparators, and outcomes. In instances where there were inconsistencies and discrepancies in data extraction, the reviewers discussed these until they established a consensus.
Assessment of risk of bias in included studies
For this update, the review authors assessed the risk of bias for each trial as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool assesses the presence of the risk of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases that do not fit into these categories. Two review authors (MVS, ALD) carried out the 'Risk of bias' assessment independently for each trial, and asked a third author (DVS) to resolve disagreements when needed.
Measures of treatment effect
For dichotomous outcomes, we used the risk ratio (RR) with its 95% confidence interval (CI) as a measure of treatment effect. We analysed continuous scales of measurements as mean difference (MD) with a 95% CI.
Unit of analysis issues
The individual participant was the unit of analysis. There were no studies that included a cross‐over or cluster‐randomised design. If there are such studies in future updates, we will include them and analyse as appropriate.
Dealing with missing data
We extracted the data that we needed for this review from the primary studies. We used the sample size that investigators indicated as randomised and extracted the number of events reported in the results. Where appropriate, we used the number of participants randomised in the meta‐analyses, on an intention‐to‐treat basis. We planned to contact investigators where data were missing.
Assessment of heterogeneity
We tested heterogeneity and variability between trials using the Chi2 test for heterogeneity and the I2 statistic. We considered I2 values above 50% to indicate the possibility of substantial heterogeneity (Higgins 2011). We evaluated meta‐analyses that exceeded this threshold and inspected the individual studies within them for possible sources of heterogeneity. If we did not identify any sources of heterogeneity that lead us to believe that meta‐analysis would not be appropriate, we used a random‐effects model for that outcome.
Assessment of reporting biases
We intended to assess asymmetry in funnel plots to evaluate reporting, or publication, bias if there were sufficient numbers of studies within the analyses for a meaningful interpretation, i.e. at least 10 (Higgins 2011). However, we included too few studies to make this feasible.
Data synthesis
We used a fixed‐effect analysis to pool current data under the assumption that all studies measured the same factor and that variation of effect size was due to sampling error. If there was evidence of statistical heterogeneity, as indicated by an I2 value greater than 50%, we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We used subgroup analysis to present the results by participants with peripheral vascular disease versus those with coronary artery disease. In the event of identifying substantial statistical heterogeneity, we had planned to investigate potential causes and perform subgroup analysis. We only undertook this type of subgroup analysis for outcomes where there were sufficient studies and a large enough sample size to make subgroup analysis meaningful.
Sensitivity analysis
We had planned to undertake sensitivity analysis for meta‐analyses that included studies that were at higher risk of bias, or included studies that exerted greater than 50% weight on the overall analyses. As with subgroup analyses, we planned to undertake these types of sensitivity analyses for meta‐analyses that included sufficient studies and sample sizes for the sensitivity analyses to be meaningful.
Summary of findings and the assessment of the certainty of the evidence
We included a 'Summary of findings' table in this review to provide a quick reference of the most important findings. This includes an assessment of the certainty of evidence for all relevant outcomes. We used the GRADE approach to determine the certainty of the evidence, which considers the overall risk of bias of the included studies, the directness of the evidence, inconsistency within the results, precision of the estimate and risk of publication bias (Balshem 2011). The seven outcomes that we included in the 'Summary of findings' table were all‐cause mortality, coronary heart disease deaths, myocardial infarction, angina, stroke, ankle‐brachial pressure index and maximum walking distance. We created the 'Summary of findings' table using GRADEpro GDT software.
Results
Description of studies
Results of the search
We included two new studies in this update (Knudston 2002; TACT 2013), with 19 references between them. We identified two ongoing studies (TACT2; TACT3a), and excluded one study (TACT‐PAD). See Figure 1 for further details.
1.
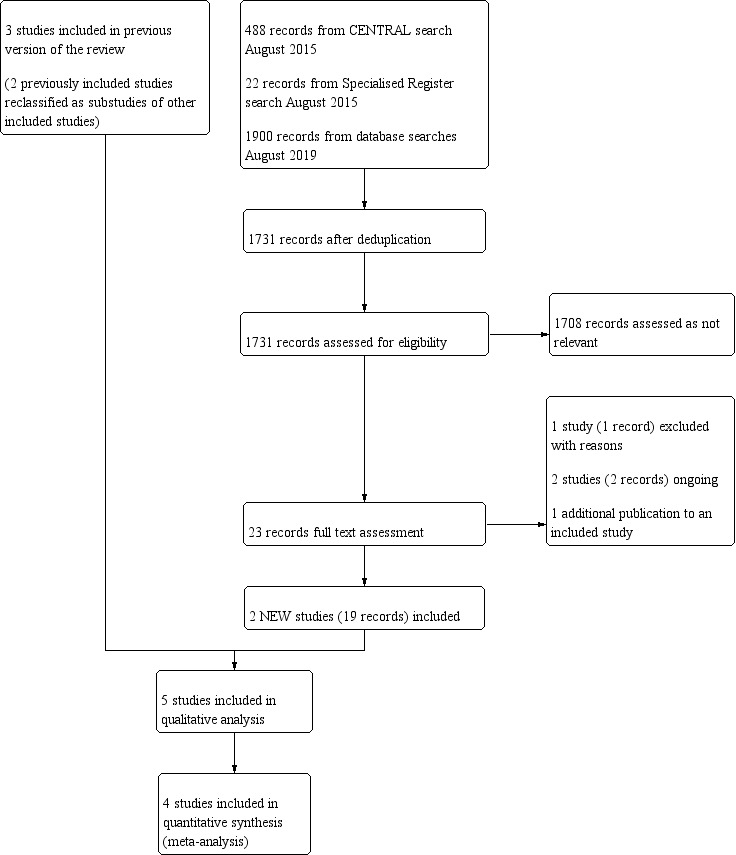
Study flow diagram.
We reclassified two previously included studies as additional publications of included studies (Guldager 1993; Sloth‐Nielsen 1991). In addition, we reclassified two previously excluded studies as additional publications of included studies (Anderson 2003; Guldager 1996).
Included studies
See Characteristics of included studies for full details of included studies.
Five RCTs with a total of 1993 participants satisfied the inclusion criteria for this review, and we included these for 'Risk of bias' assessment and data extraction (Guldager 1992; Knudston 2002; Olszewer 1990; TACT 2013; van Rij 1994). The TACT 2013 trial was the largest RCT, with 1708 adults enrolled, while the smallest trial randomised only 10 people (Olszewer 1990). All studies compared disodium EDTA to placebo. The placebo was either an isotonic solution (Guldager 1992; van Rij 1994), distilled water (Olszewer 1990), or a dextrose solution (Knudston 2002; TACT 2013).
Three studies enrolled participants with peripheral vascular disease (Guldager 1992; Olszewer 1990; van Rij 1994), and two of the studies included participants with coronary artery disease (Knudston 2002; TACT 2013), one of which specifically recruited post‐myocardial infarction participants (TACT 2013).
The studies by Guldager 1992, Olszewer 1990 and van Rij 1994 used 20 infusions for the duration of treatment, compared to 33 infusions for Knudston 2002 and 40 infusions for TACT 2013. The treatment times for these infusions varied greatly: Guldager 1992 described their treatment as five to nine weeks, van Rij 1994 reported 10 weeks of treatment, and Knudston 2002 reported 27 weeks. Olszewer 1990 did not describe how long the 20‐infusion treatment took. The TACT 2013 trial reported 30 weeks' treatment for the first 30 infusions, after which the participants received the final 10 infusions between two and eight weeks apart. Follow‐up time also varied, with Guldager 1992 following up for six months, Knudston 2002 and van Rij 1994 for one year, and TACT 2013 for five years or until the end of the study, which ever came first. Olszewer 1990 did not report their follow‐up time.
Excluded studies
We excluded the TACT‐PAD trial because it was not an RCT and only included a single treatment arm. This study was undertaken by the researchers who performed the TACT 2013 trial, but evaluated only people with diabetes and peripheral arterial disease. See Characteristics of excluded studies for further details.
Ongoing studies
We identified two ongoing studies (TACT2; TACT3a), and will include the results from these in the review when the trials have completed and further information is available. The studies are set to complete in 2021 and 2022, respectively.
See Characteristics of ongoing studies for further details.
Risk of bias in included studies
The methodological quality of the included studies was assessed using Cochrane's 'Risk of bias' tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the risk of bias as high, unclear or low, based on seven domains that may affect study results: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
See Figure 2 and Figure 3 and Characteristics of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four of five studies were clearly randomised, and we deemed these to be at low risk of bias for random sequence generation (Guldager 1992; Knudston 2002; TACT 2013; van Rij 1994). We graded Olszewer 1990 as unclear risk, because the study gave insufficient information about how the random sequence was generated.
Three studies had low risk of bias for allocation concealment because we deemed these to have appropriately concealed allocation (Knudston 2002; TACT 2013; van Rij 1994). We found two studies to be of unclear risk, primarily because they did not report explicit details about how the allocation sequence was protected (Guldager 1992; Olszewer 1990).
Blinding
Double‐blinding was stated or implied in all of the studies. Four studies had low risk of performance bias with regard to blinding of participants and personnel, as they used appropriate and sufficient measures to maintain blinding throughout the study period (Guldager 1992; Knudston 2002; TACT 2013; van Rij 1994). The studies did not explicitly state whether assessments done during the study (such as laboratory tests) led to unblinding of treatment, but there was an effort to maintain blinding. Olszewer 1990 had a high risk of performance bias as they broke their blinding halfway through the treatment period, and gave EDTA to participants assigned to the placebo group. Single‐blinding of participants was reported, and study personnel were aware of the treatment allocation.
Three of the five studies had low risk of detection bias, as they used appropriate measures to maintain blinding through follow‐up and analysis of the findings and blinding of outcome assessors (Knudston 2002; TACT 2013; van Rij 1994). Guldager 1992 had a high risk of detection bias as they only maintained blinding through the treatment period and broke blinding during the follow‐up assessment and analysis period. Olszewer 1990 also had a high risk of detection bias, for the same reason that they had a high risk of performance bias; they broke their blinding after the participants had received 10 of 20 infusions, and rolled all placebo participants over to active treatment.
Incomplete outcome data
In the assessment of incomplete outcome data, three of the five studies showed a low risk of bias (Knudston 2002; TACT 2013; van Rij 1994). These three studies explicitly described an intention‐to‐treat analysis, which helps in the assessment of attrition bias in the studies' reported analyses. The TACT 2013 trial also reported that they performed a sensitivity analysis to check the robustness of the results. The study by Guldager 1992 showed unclear risk of bias, as they did not include some of the participants in some outcomes. Consequently, we cannot discount a possible risk of bias, especially for subjective outcomes. We deemed the study by Olszewer 1990 to be at high risk, because it prematurely stopped one group and reassigned the participants in the placebo group to active treatment. This makes it impossible to conduct a proper comparison of the active treatment group with the control group.
Selective reporting
Four of the five studies had low risk of reporting bias (Guldager 1992; Knudston 2002; Olszewer 1990; TACT 2013). All of those studies clearly specified their outcomes and reported what was indicated. We considered van Rij 1994 to be at unclear risk of reporting bias because they stated that they would report findings after six and 12 months of follow‐up, but did not report their outcomes beyond three months' follow‐up.
Other potential sources of bias
We noted a high risk of bias for the Knudston 2002 study, in terms of differences in the baseline risk of the included participants. At baseline, more people in the placebo group had multivessel disease, nitrate use, or triple therapy use. There were more post‐myocardial infarction participants in the chelation group than in the placebo group. This baseline difference would most likely affect the direction of the outcome.
We also rated the Olszewer 1990 study to be at high risk of bias. The trial's decision to break the code and move the control group into the intervention group because of a seemingly positive response after 10 infusions was premature, and not part of the described protocol. This casts doubt on the true effect of treatment, as accurate assessments of the control group during the prespecified period were not possible.
We deemed the remaining three studies to be at low risk of other bias (Guldager 1992; TACT 2013; van Rij 1994).
Effects of interventions
See: Table 1
All‐cause mortality
Two studies with coronary artery disease participants reported specifically on all‐cause mortality (Knudston 2002; TACT 2013), although Knudston 2002 reported no deaths in either group. We found no evidence of a difference between the EDTA chelation and placebo arms (RR 0.97, 95% CI 0.73 to 1.28; 1792 participants; 2 studies; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: EDTA versus placebo, Outcome 1: All‐cause mortality
Coronary heart disease (CHD) deaths
Only TACT 2013 reported data on CHD deaths. We can draw no overall conclusions since the analysis included only one study with coronary artery disease participants, but there was no evidence of a difference between the EDTA and placebo arms (RR 1.02, 95% CI 0.70 to 1.48; 1708 participants; 1 study; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: EDTA versus placebo, Outcome 2: Coronary heart disease death
Vascular deaths
None of the included studies reported specifically on vascular deaths as an outcome.
Non‐fatal events including acute coronary syndromes such as myocardial infarction and unstable angina pectoris
Non‐fatal events, including acute coronary syndromes such as myocardial infarction and unstable angina pectoris, were reported in two studies with coronary artery disease participants (Knudston 2002; TACT 2013).
Myocardial infarction events were reported in two studies (Knudston 2002; TACT 2013). There was no evidence of a difference in this event between the treatment arms (RR 0.81, 95% CI 0.57 to 1.14; 1792 participants; 2 studies; moderate‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: EDTA versus placebo, Outcome 3: Myocardial Infarction
Both Knudston 2002 and TACT 2013 reported angina as an outcome. In the Knudston 2002 study, the researchers reported on cases of worsening angina. The TACT 2013 study reported on hospitalisations for angina. When combined in a single meta‐analysis, there was no evidence of a difference between the treatment groups for angina (RR 0.95, 95% CI 0.55 to 1.67; 1792 participants; 2 studies; very low‐certainty evidence; Analysis 1.4), although it should be noted that the point estimates for the two studies fall in opposite directions.
1.4. Analysis.

Comparison 1: EDTA versus placebo, Outcome 4: Angina
Knudston 2002 and TACT 2013 both reported coronary revascularisation. Since there was evidence of substantial heterogeneity, we used a random‐effects model to evaluate this outcome. There was no clear difference between the treatment groups for the outcome of coronary revascularisation (RR 0.46, 95% CI 0.07 to 3.25; 1792 participants; 2 studies; I2 = 56% Analysis 1.5), although the confidence interval is very wide, making it difficult to draw any overall conclusions.
1.5. Analysis.

Comparison 1: EDTA versus placebo, Outcome 5: Coronary revascularisation
Cerebrovascular events
Two studies reported stroke events (Guldager 1992; TACT 2013). Guldager 1992 included participants with peripheral vascular disease and TACT 2013 included participants with coronary artery disease. There was no evidence of a difference between those receiving EDTA and those receiving the placebo (RR 0.88, 95% CI 0.40 to 1.92; 1867 participants; 2 studies; low‐certainty evidence; Analysis 1.6). There was no clear difference between the subgroups (P = 0.43).
1.6. Analysis.

Comparison 1: EDTA versus placebo, Outcome 6: Stroke
Direct test of disease severity
A subgroup analysis of the Guldager 1992 study stated that they performed arteriograms on 30 participants before and after treatment. The authors reported that only two participants showed improvement, but did not state which treatment group they were in (Sloth‐Nielsen 1991). The study authors reported that there was no evidence of a difference between the groups based on the arteriograms. None of the other included studies reported on arteriograms to evaluate disease severity.
Indirect tests of disease severity
Three studies, all including participants with peripheral vascular disease, reported on ABPI as an outcome (Guldager 1992; Olszewer 1990; van Rij 1994). Guldager 1992 and van Rij 1994 both reported ABPI figures three months after treatment (as the absolute value, not the change in ABPI). When included in meta‐analysis, there was no evidence of a difference between the treatment groups (MD 0.02, 95% CI ‐0.03 to 0.06; 181 participants; 2 studies; low‐certainty evidence; Analysis 1.7). Guldager 1992 also reported on ABPI six months after treatment, and while we cannot draw any overall conclusions from a single study, we found no evidence of a difference between the treatment groups (MD 0.03, 95% CI ‐0.02 to 0.08; 123 participants; 1 study; Analysis 1.8).
1.7. Analysis.

Comparison 1: EDTA versus placebo, Outcome 7: Ankle‐brachial pressure index at 3 months post‐treatment
1.8. Analysis.

Comparison 1: EDTA versus placebo, Outcome 8: Ankle‐brachial pressure index at 6 months post‐treatment
Olszewer 1990 did not report any effect size measure, so we could not include it in the meta‐analysis, but after 10 treatments the study authors reported a clear improvement in the EDTA treatment group. This led them to break the blinding and give EDTA to the placebo group as well as to the original EDTA group.
Participant symptoms
Walking distances
Four studies reported walking ability (Guldager 1992; Knudston 2002; Olszewer 1990; van Rij 1994), but we only included Guldager 1992 and van Rij 1994 in the meta‐analysis. Both Guldager 1992 and van Rij 1994 included participants with peripheral vascular disease. Maximum walking distance, measured at three months post‐treatment, showed no evidence of a difference between the treatment groups (MD ‐31.46, 95% CI ‐87.63 to 24.71; 165 participants; 2 studies; low‐certainty evidence; Analysis 1.9). Guldager 1992 also evaluated maximum walking distance six months after treatment, and found no difference between the groups (MD ‐14.00, 95% CI ‐66.93 to 38.93; 107 participants; 1 study; Analysis 1.10). Guldager 1992 and van Rij 1994 also evaluated pain‐free walking distance at three months, but again, there was no evidence of a difference between the treatment groups (MD ‐7.73, 95% CI ‐22.59 to 7.13; 165 participants; 2 studies; Analysis 1.11). Guldager 1992 also showed no difference in pain‐free walking distance six months after treatment (MD ‐22.00, 95% CI ‐49.56 to 5.56; 107 participants; 1 study; Analysis 1.12).
1.9. Analysis.

Comparison 1: EDTA versus placebo, Outcome 9: Maximum walking distance (m) at 3 months post‐treatment
1.10. Analysis.

Comparison 1: EDTA versus placebo, Outcome 10: Maximum walking distance (m) at 6 months post‐treatment
1.11. Analysis.

Comparison 1: EDTA versus placebo, Outcome 11: Pain‐free walking distance (m) at 3 months post‐treatment
1.12. Analysis.

Comparison 1: EDTA versus placebo, Outcome 12: Pain‐free walking distance (m) at 6 months post‐treatment
Olszewer 1990, which assessed participants with peripheral vascular disease, also reported on walking distance. Since the methods were unclear and the study did not include an effect size, we could not add this to the meta‐analysis. The study authors noticed an improvement in some of the participants and chose to break the blinding. The study authors determined that participants receiving the EDTA chelation treatment had made significant improvements in walking distance compared with the placebo group, and rolled all the placebo participants to active treatment.
Knudston 2002, who included participants with coronary artery disease, reported on change in walking time to onset of ischaemia. They measured this from baseline to the end of treatment, and reported that there were no differences between the treatment groups.
Quality of Life
Two studies of people with coronary artery disease reported on Quality of Life (QoL) (Knudston 2002; TACT 2013).
Knudston 2002 reported QoL at the end of the treatment period (27 weeks), using several established QoL tools.
The Duke Activity Status Index (DASI) can reach a maximum score of 58.2, with a higher score indicating better physiologic reserve (Hlatky 1989).
The Seattle Angina Questionnaire (SAQ) scores range from 1 to 100, with a higher score indicating better levels of functioning (Spertus 1995).
Short‐Form 36 (SF‐36): mental and physical component summary scores range from 0 to 100, with a higher score indicating better health‐related quality of life (Ware 1994).
Knudston 2002 reported mean baseline and follow‐up values as well as the change score for each QoL assessment. However, the change score only reported a range and not the standard deviation, making inclusion in meta‐analysis inappropriate. When we evaluated follow‐up values for the SF‐36 mental health component, these showed a difference between the treatment and control groups. However, after evaluating baseline values, this was most likely due to the fact that there was already a difference present at baseline. Because of these inconsistencies, we chose not to include the QoL measures in a meta‐analysis at this time and instead described the results of the study for this outcome. After the treatment period, there was no change in the DASI scores for either treatment group. The EDTA chelation group had a mean change of ‐0.2 (95% CI ‐3.2 to 2.7) points and the placebo group had a mean change of 1.9 (95% CI ‐0.6 to 4.5) points; there was no clear difference between the groups. For the SAQ exertion component, both groups saw an improvement: EDTA MD 7.3 (95% CI 2.2 to 12.5) and placebo MD 8.3 (95% CI 3.9 to 12.8), but there was no clear difference between the EDTA and placebo groups. For the SF‐36 mental component summary score, there was no difference from baseline for either treatment group: EDTA MD 2.1 (‐0.4 to 4.6) and placebo MD 2.1 (‐0.4 to 4.5). Finally, for the SF‐36 physical component summary score, the placebo group had a mean increase (that is improvement) from baseline (MD 5.0, 95% CI 2.7 to 7.3) and the EDTA group found no evidence of an improvement (MD 2.2, 95% CI ‐0.5 to 4.9), but there were no overall differences between the two groups.
TACT 2013 stated that they would collect QoL measures using DASI and the SF‐36 mental health component. However, in the brief abstract that reported on these outcomes, authors only stated that both groups saw an improvement in DASI at six months, but that there was no difference between the treatment groups (MD 0.9, 95% CI ‐0.7 to 2.6). The same was true for the SF‐36 mental health component (MD 1.0, 95% CI ‐1.0 to 2.0).
van Rij 1994, who included participants with peripheral vascular disease, reported subjective measures that they described as 'lifestyle measures'. We did not include these findings in the meta‐analysis, as the measures are not commonly used today and are therefore unlikely be reflected in other similar studies. The instruments used were the Life in New Zealand National Survey (Russell 1991), which evaluates physical activity, perceived fitness, smoking, alcohol consumption and dietary practices for the four weeks prior to the questionnaire. They used the General Health Questionnaire (Goldberg 1972), evaluating anxiety and depression as well as the Profile of Mood States (McNair 1971), measuring tension, anger, depression, confusion, fatigue and vigour. Finally, van Rij 1994 used a visual analogue scale to evaluate participants' global feeling of well‐being and the effect of poor circulation on their social and private activities. At the end of treatment, and three months after treatment, there was little or no difference between the treatment groups regarding the measures of lifestyle.
Adverse events
Two studies reported on adverse events experienced by the participants in the different treatment arms (Guldager 1992; TACT 2013). Guldager 1992 included participants with peripheral vascular disease and TACT 2013 included participants with coronary artery disease. Guldager 1992 reported many different types of events separately and included: hypocalcaemic symptoms, fatigue, faintness, gastrointestinal symptoms, serum creatinine increase, proteinuria, phlebitis at infusion site, pain at infusion site, headache, Raynaud's phenomenon, metallic taste and dermatitis. These events were reported separately for the treatment and placebo groups. TACT 2013 simply reported serious adverse events as a single outcome, which included: death, heart failure, tachycardia, infusion site discomfort, abdominal cramping and reductions in calcium. It is unclear if a participant could have multiple adverse events and would therefore be included multiple times, making meta‐analysis inappropriate. While some of the event types overlap with those in the Guldager 1992 study, the TACT 2013 did not report the figures for the individual event types, and only reported the data as a single group of serious adverse events. We could not extract these or combine them with the other study.
Therefore, due to the large number of different events reported by Guldager 1992 and the fact that TACT 2013 only reported adverse events as a single group, meta‐analysis was not appropriate. Instead, we chose to create a table describing the different events reported by the studies (Table 2).
1. Adverse events.
| Study | Events in EDTA arm | n in EDTA arm | Events in placebo arm | n in placebo arm |
|
Guldager 1992 Combined events |
111 | 80 | 74 | 79 |
|
Guldager 1992 Hypocalcaemic symptoms |
6 | 80 | 2 | 79 |
|
Guldager 1992 Fatigue |
12 | 80 | 11 | 79 |
|
Guldager 1992 Faintness |
11 | 80 | 1 | 79 |
|
Guldager 1992 Gastrointestinal symptoms |
11 | 80 | 7 | 79 |
|
Guldager 1992 Serum‐creatinine increase |
7 | 80 | 9 | 79 |
|
Guldager 1992 Proteinuria |
10 | 80 | 4 | 79 |
|
Guldager 1992 Phlebitis at infusion site |
35 | 80 | 28 | 79 |
|
Guldager 1992 Pain at infusion site |
9 | 80 | 5 | 79 |
|
Guldager 1992 Headache |
7 | 80 | 7 | 79 |
|
Guldager 1992 Raynaud's Phenomenon |
1 | 80 | 0 | 79 |
|
Guldager 1992 Metallic taste |
1 | 80 | 0 | 79 |
|
Guldager 1992 Dermatitis |
1 | 80 | 0 | 79 |
|
TACT 2013 Combined events |
100 | 839 | 127 | 869 |
EDTA: ethylene diamine tetra‐acetic acid
Subgroup and sensitivity analyses
We have reported subgroup analyses grouped by study participants with peripheral vascular disease and coronary artery disease in the sections above. As all of the meta‐analyses included very few studies and participants, we did not undertake further subgroup or sensitivity analyses.
Discussion
Summary of main results
We included five randomised controlled trials (RCTs) with a total of 1993 participants in this review. All of the studies compared ethylene diamine tetra‐acetic acid (EDTA) chelation treatment with a placebo control. Three studies enrolled participants with peripheral vascular disease while two of the studies included participants with coronary artery disease. Not all studies reported all our outcomes of interest, and we only found one outcome (stroke) which had data from both a study in participants with peripheral vascular disease and a study in participants with coronary artery disease. We did not detect any subgroup differences.
We found no evidence of a difference between EDTA chelation treatment and placebo for the primary outcomes of all‐cause mortality (low‐certainty evidence) and coronary heart disease deaths (very low‐certainty evidence). None of the included studies reported specifically on vascular death as an outcome. We also found no evidence of a difference between the treatment and control groups for non‐fatal events, such as myocardial infarction (moderate‐certainty evidence), angina (very low‐certainty evidence) and stroke (low‐certainty evidence). A few of the included studies reported on indirect measures of disease, specifically ankle‐brachial pressure index (ABPI), but we found no evidence of differences after three or six months of follow‐up (low‐certainty evidence). We also found no evidence of differences for measures of walking distances (low‐certainty evidence). We could not evaluate quality of life measures with meta‐analysis, but evidence from the few studies that reported on these outcomes was not sufficient to show a difference between the treatment and control groups. Few studies reported on adverse events, and the types of events varied greatly, but there did not appear to be any increase of adverse events in the treatment compared with the control group.
Overall completeness and applicability of evidence
Although we included five studies in this review, the meta‐analyses that we performed generally only included data from one or two studies. For several of our outcomes of interest, the studies either did not report them at all, or they did not report them in a way that allowed us to pool them in a meta‐analysis. These limitations reduce the completeness and applicability of the evidence. Also, several outcomes that we considered focused on various direct and indirect measurements of disease severity, such as ABPI and walking distances, and the included studies evaluated and reported these in different ways, thus limiting the applicability of any conclusions that we could draw about these outcomes.
The Trial to Assess Chelation Therapy (TACT) trial was the only included study that was designed and conducted in a manner to attempt to answer the question of whether chelation therapy for people with atherosclerosis can reduce clinically relevant endpoints, such as mortality and cardiovascular events (TACT 2013). Despite the larger sample size compared with the other included studies, this trial only reported a trend to benefit on the composite endpoint of death, reinfarction, stroke, coronary revascularization, or hospitalisation for angina, and not on the outcomes individually. The borderline effect warrants further study, because the strength of evidence is currently insufficient.
The TACT trial highlighted the possible importance of different subgroups, such as type of primary myocardial infarction, or people with diabetes, and their response to chelation treatment (TACT 2013). A post‐hoc analysis of TACT participants with diabetes and peripheral artery disease (162/1708), reported that chelation therapy reduced the primary endpoint (myocardial infarction, stroke, coronary revascularisation, and hospitalisation for angina) compared to placebo infusions (hazard ratio 0.52; 95% CI 0.30 to 0.92) (Ujueta 2019). This finding is being investigated further in ongoing trials (TACT2; TACT3a).
Only one study reported a clinical improvement with chelation therapy (Olszewer 1990). This study found improvements in ABPI and walking distances halfway through the treatment and chose to break the randomisation code. The investigators found the improvements to be concentrated in the EDTA treatment group, so they decided to give the active treatment to those previously receiving placebo. These changes to the protocol made it impossible to compare directly the effects of EDTA chelation with a placebo after the prescribed treatment and follow‐up. This study, which only included 10 participants, is the only RCT that provided evidence in support of EDTA chelation treatment in those with atherosclerotic cardiovascular disease, and the evidence is highly questionable.
Quality of the evidence
The overall risk of bias of the included studies ranged from low risk to high risk, but the majority of included studies were either at low or moderate risk of bias. Olszewer 1990 was the only study with a consistent high risk of bias, due to their deviation from the protocol and provision of active treatment to the placebo control group for the latter half of the treatment period.
The Table 1 provides details of the certainty of evidence as determined by GRADE for the outcomes: all‐cause mortality, coronary heart disease death, myocardial infarction, angina, stroke, ABPI and maximum walking distance. Overall, the GRADE rating of certainty of evidence was low to very low. This was mainly due to imprecision of the evidence (as a result of including so few studies in each analysis), as well as issues with heterogeneity of the findings and possible concerns with risk of bias that stem from a single study that did not adhere to blinding during the follow‐up outcome measurement phase.
Potential biases in the review process
The process used in this systematic review was based on the Cochrane guidelines for review development and followed the procedures outlined per stage of the review, thus limiting biases that may surface at any phase in the review process (Higgins 2011).
There were very few outcomes that we could combine in this systematic review, so there was no need to make assumptions or additional calculations that may have created a potential bias. Although the reported findings from the TACT 2013 trial showed fewer cardiovascular events (as a composite outcome) with chelation than with placebo, these findings were not supported within our review's meta‐analysis. We did not use the same composite outcome as the TACT 2013 trial, and we collapsed the four treatment groups down to two, in order to compare those who received EDTA chelation treatment and those who received the placebo infusions, regardless of whether or not they also received high‐dose vitamins and minerals.
Agreements and disagreements with other studies or reviews
A meta‐analysis of unpublished data reported a high correlation between improvement in cardiovascular function and treatment with EDTA (Chappel 1994). However, the review included studies that were not randomised controlled trials. Another systematic review, which included randomised trials similar to our systematic review, concluded that the best available evidence does not support the use of EDTA chelation therapy (Seely 2005).
The TACT 2013 study was the first large trial on chelation and atherosclerotic cardiovascular disease that reported a trend to benefit in composite endpoints of total mortality, recurrent MI, stroke, coronary revascularization, or hospitalisation for angina. Yet a reappraisal by Sidhu 2013 and a review by Avila 2014 supported the need for further replicative clinical studies before chelation can be considered or accepted as one of the therapies for post‐myocardial infarction participants.
Authors' conclusions
Implications for practice.
At the present time, there is insufficient evidence to determine the effectiveness of chelation therapy to improve clinical outcomes among people with atherosclerotic cardiovascular disease. Wider acceptance of this treatment in clinical practice must be preceded by conducting well‐designed randomised clinical trials with adequate sample size. Such trials should focus on clinical outcomes, quality of life outcomes and adverse events, especially among people at risk for coronary or cerebrovascular disease.
Implications for research.
To date, trials on chelation therapy have centred on peripheral vascular disease, more specifically on treatment of intermittent claudication. Only two trials have been completed that included people with coronary artery disease. Therefore, it is important that larger, methodologically sound, randomised controlled trials continue to be conducted in people for whom the treatment is intended, e.g. people with coronary and cerebrovascular disease. It is also important that future trials of chelation therapy include endpoints that show its effects on longevity and quality of life, rather than on mechanistic outcomes. In addition, the proper recording and reporting of safety issues or adverse events should always be part of research involving novel treatments such as this. In as much as benefits need to be reported, there is always a need to balance it with any risk present.
What's new
| Date | Event | Description |
|---|---|---|
| 13 September 2019 | New citation required but conclusions have not changed | Searches rerun. Two new studies included, one new study excluded, two new ongoing studies identified. Review text amended to reflect current Cochrane policies. No change to conclusions. |
| 13 September 2019 | New search has been performed | Searches rerun. Two new studies included, one new study excluded, two new ongoing studies identified. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 29 May 2008 | Amended | Converted to new review format. |
| 30 October 2006 | Amended | Minor edit |
| 25 May 2005 | New search has been performed | Review updated with the addition of one ongoing study and minor style guide changes |
Acknowledgements
The review authors wish to thank their family, friends and colleagues in the profession for all the support. In addition, the review authors would also like to thank and commend Marlene Stewart and Cathryn Broderick of Cochrane Vascular for their never‐ending patience and full assistance in making sure this systematic review was updated.
The review authors are also very appreciative of the support provided by the Philippine College of Physicians for the completion of this update.
The review authors, and the Cochrane Vascular Editorial base, are grateful to the following external reviewers for their time and comments: Jeffrey J Siracuse, MD, Associate Professor of Surgery and Radiology, Boston University, USA, and Marcial Fallas‐Camacho, Hospital Clinica Biblica, San Jose, Costa Rica. We are also grateful to the peer reviewer who opted to remain anonymous.
Appendices
Appendix 1. CENTRAL search strategy 5 August 2015
| Search run on 5 August 2015 | ||
| #1 | MESH DESCRIPTOR Cardiovascular Diseases EXPLODE ALL TREES | 66195 |
| #2 | cardiovasc*:TI,AB,KY | 29252 |
| #3 | coronary:TI,AB,KY | 29987 |
| #4 | heart:TI,AB,KY | 69447 |
| #5 | myocardial:TI,AB,KY | 22684 |
| #6 | circulat*:TI,AB,KY | 16321 |
| #7 | CAD:TI,AB,KY | 1867 |
| #8 | MESH DESCRIPTOR Ischemia | 719 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD):TI,AB,KY | 7815 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 6604 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 2835 |
| #12 | (claudic* or IC):TI,AB,KY | 2587 |
| #13 | (isch* or CLI):TI,AB,KY | 19917 |
| #14 | arteriopathic:TI,AB,KY | 7 |
| #15 | dysvascular*:TI,AB,KY | 9 |
| #16 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 78 |
| #17 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 116 |
| #18 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 71 |
| #19 | ((iliac or femoral or popliteal or femoro* or fempop* or crural) near3(occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 788 |
| #20 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1062 |
| #21 | (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY | 917 |
| #22 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 | 152382 |
| #23 | MESH DESCRIPTOR Chelation Therapy EXPLODE ALL TREES | 61 |
| #24 | MESH DESCRIPTOR Chelating Agents EXPLODE ALL TREES | 1973 |
| #25 | ethylenediamine*:TI,AB,KY | 205 |
| #26 | (ethylen* near2 diamine*):TI,AB,KY | 40 |
| #27 | chelat*:TI,AB,KY | 884 |
| #28 | EDTA:TI,AB,KY | 816 |
| #29 | #23 OR #24 OR #25 OR #26 OR #27 OR #28 | 3030 |
| #30 | #22 AND #29 | 488 |
Appendix 2. Literature searches August 2018 and 2019
| Source | Search strategy | Hits retrieved |
| CENTRAL | #1 MESH DESCRIPTOR Cardiovascular Diseases EXPLODE ALL TREES 92399 #2 cardiovasc*:TI,AB,KY 49961 #3 coronary:TI,AB,KY 43789 #4 heart:TI,AB,KY 104388 #5 myocardial:TI,AB,KY 32708 #6 circulat*:TI,AB,KY 23247 #7 CAD:TI,AB,KY 3397 #8 MESH DESCRIPTOR Ischemia 1542 #9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY 12285 #10 ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 10672 #11 (peripheral near3 dis*):TI,AB,KY 4875 #12 (claudic* or IC):TI,AB,KY 4130 #13 (isch* or CLI):TI,AB,KY 32430 #14 arteriopathic:TI,AB,KY 7 #15 dysvascular*:TI,AB,KY 23 #16 (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 130 #17 (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 227 #18 ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 101 #19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) near3(occlus* or reocclus* or re‐occlus* or steno*or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 1486 #20 MESH DESCRIPTOR Leg EXPLODE ALL TREES 2801 #21 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY 1731 #22 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 230870 #23 MESH DESCRIPTOR Chelation Therapy EXPLODE ALL TREES 74 #24 MESH DESCRIPTOR Chelating Agents EXPLODE ALL TREES 2865 #25 ethylenediamine*:TI,AB,KY 245 #26 (ethylen* near2 diamine*):TI,AB,KY 50 #27 chelat*:TI,AB,KY 1291 #28 EDTA:TI,AB,KY 1033 #29 #23 OR #24 OR #25 OR #26 OR #27 OR #28 4412 #30 #22 AND #29 759 #31 01/01/2015 TO 14/08/2018:CD 465323 #32 #30 AND #31 283 |
15 August 2018: 283 6 August 2019: 113 |
| Clinicaltrials.gov | Cardiovascular or coronary or heart or myocardial or Ischemia or atherosclerosis or arteriosclerosis | Chelation or Chelating or ethylenediamine or EDTA | 15 August 2018: 0 6 August 2019: 0 |
| ICTRP Search Portal | Cardiovascular or coronary or heart or myocardial or Ischemia or atherosclerosis or arteriosclerosis | Chelation or Chelating or ethylenediamine or EDTA | 15 August 2018: 5 6 August 2019: 1 |
| MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) 1946 to present (2017, 2018 AND 2019 ONLY) | 1 exp Cardiovascular Diseases/ 2209343 2 cardiovasc*.ti,ab. 381507 3 coronary.ti,ab. 363097 4 heart.ti,ab. 732184 5 myocardial.ti,ab. 309715 6 circulat*.ti,ab. 366774 7 CAD.ti,ab. 33317 8 ISCHEMIA/ 47686 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 172168 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 143710 11 (peripheral adj3 dis*).ti,ab. 38010 12 (claudic* or IC).ti,ab. 62275 13 (isch* or CLI).ti,ab. 347926 14 arteriopathic.ti,ab. 162 15 dysvascular*.ti,ab. 217 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 710 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1826 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1484 19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno*or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 7457 20 exp LEG/bs [Blood Supply] 25049 21 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 9734 22 or/1‐21 3277858 23 exp Chelation Therapy/ 1351 24 exp Chelating Agents/ 134882 25 ethylenediamine*.ti,ab. 11668 26 (ethylen* adj2 diamine*).ti,ab. 1458 27 chelat*.ti,ab. 60560 28 EDTA.ti,ab. 33715 29 or/23‐28 199079 30 22 and 29 13731 31 randomized controlled trial.pt. 466776 32 controlled clinical trial.pt. 92580 33 randomized.ab. 419286 34 placebo.ab. 191058 35 drug therapy.fs. 2039779 36 randomly.ab. 295554 37 trial.ab. 436605 38 groups.ab. 1824095 39 or/31‐38 4261870 40 exp animals/ not humans.sh. 4486684 41 39 not 40 3684348 42 30 and 41 2507 43 (2017* or 2018*).ed. 1591726 44 42 and 43 182 45 from 44 keep 1‐182 182 |
15 August 2018: 182 6 August 2019: 179 |
| Embase 1974 to present (2017, 2018 AND 2019 ONLY) | 1 exp cardiovascular disease/ 3430790 2 cardiovasc*.ti,ab. 523409 3 coronary.ti,ab. 483622 4 heart.ti,ab. 955801 5 myocardial.ti,ab. 408418 6 circulat*.ti,ab. 440655 7 CAD.ti,ab. 54144 8 ischemia/ 70891 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 221615 10 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 185745 11 (peripheral adj3 dis*).ti,ab. 51387 12 (claudic* or IC).ti,ab. 60628 13 (isch* or CLI).ti,ab. 478891 14 arteriopathic.ti,ab. 179 15 dysvascular*.ti,ab. 229 16 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 938 17 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 2541 18 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 1937 19 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno*or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 10054 20 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 13112 21 or/1‐20 4409661 22 exp chelation therapy/ 3721 23 exp chelating agent/ 296819 24 ethylenediamine*.ti,ab. 11008 25 (ethylen* adj2 diamine*).ti,ab. 1655 26 chelat*.ti,ab. 65391 27 EDTA.ti,ab. 42205 28 or/22‐27 356023 29 21 and 28 44546 30 randomized controlled trial/ 485046 31 controlled clinical trial/ 453436 32 random$.ti,ab. 1254892 33 randomization/ 78352 34 intermethod comparison/ 224167 35 placebo.ti,ab. 263094 36 (compare or compared or comparison).ti. 439852 37 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1682909 38 (open adj label).ti,ab. 61736 39 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 201044 40 double blind procedure/ 144948 41 parallel group$1.ti,ab. 20897 42 (crossover or cross over).ti,ab. 89779 43 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 271853 44 (assigned or allocated).ti,ab. 320163 45 (controlled adj7 (study or design or trial)).ti,ab. 281055 46 (volunteer or volunteers).ti,ab. 217627 47 trial.ti. 234687 48 or/30‐47 3865784 49 29 and 48 8652 50 (2017* or 2018*).em. 2792765 51 49 and 50 779 52 from 51 keep 1‐779 779 |
15 August 2018: 779 6 August 2019: 628 |
| CINAHL (2017, 2018 AND 2019 ONLY) | S46 S44 AND S45 25 S45 EM 2017 OR EM 2018 410,156 S44 S29 AND S43 418 S43 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 344,690 S42 MH "Random Assignment" 39,170 S41 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" 32,863 S40 MH "Crossover Design" 11,254 S39 MH "Factorial Design" 921 S38 MH "Placebos" 8,370 S37 MH "Clinical Trials" 93,027 S36 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,539 S35 TX crossover OR "cross‐over" 14,645 S34 AB placebo* 28,548 S33 TX random* 220,802 S32 TX trial* 252,333 S31 TX "latin square" 143 S30 S22 AND S29 466 S29 S23 OR S24 OR S25 OR S26 OR S27 OR S28 2,165 S28 TX EDTA 477 S27 TX chelat* 1,526 S26 TX ethylen* n2 diamine* 33 S25 TX ethylenediamine* 140 S24 (MH "Chelating Agents+") 1,104 S23 (MH "Chelation Therapy") 360 S22 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 463,910 S21 (femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 1,094 S20 (MH "Leg/BS") 450 S19 TX (iliac or femoral or popliteal or femoro* or fempop* or crural) n3(occlus* or reocclus* or re‐occlus* or steno*or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 878 S18 TX (lower n3 extrem*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter* 119 S17 TX limb n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 277 S16 TX leg n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 126 S15 TX dysvascular* 172 S14 TX arteriopathic 10 S13 TX isch* or CLI 39,688 S12 TX claudic* or IC 5,894 S11 TX peripheral n3 dis* 9,264 S10 TX (arter* or vascular or vein* or veno* or peripher*) n3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) 12,678 S9 TX atherosclero* or arteriosclero* or PVD or PAOD or PAD 26,494 S8 TX Ischemia 25,852 S7 TX CAD 4,214 S6 TX circulat* 47,409 S5 TX myocardial 51,509 S4 TX heart 177,885 S3 TX coronary 63,781 S2 TX cardiovasc* 126,171 S1 (MH "Cardiovascular Diseases+") 306,442 |
15 August 2018: 25 6 August 2019: 59 |
| AMED (Allied and Complementary Medicine) 1985 to July 2019 (2017, 2018 AND 2019 ONLY) | 1 cardiovasc*.ti,ab. 2619 2 coronary.ti,ab. 1294 3 heart.ti,ab. 5122 4 myocardial.ti,ab. 845 5 circulat*.ti,ab. 980 6 CAD.ti,ab. 155 7 ISCHEMIA/ 266 8 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 810 9 ((arter* or vascular or vein* or veno* or peripher*) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 461 10 (peripheral adj3 dis*).ti,ab. 439 11 (claudic* or IC).ti,ab. 1031 12 (isch* or CLI).ti,ab. 1687 13 arteriopathic.ti,ab. 1 14 dysvascular*.ti,ab. 58 15 (leg adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 21 16 (limb adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 32 17 (lower adj3 extrem* adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 25 18 ((iliac or femoral or popliteal or femoro* or fempop* or crural) adj3 (occlus* or reocclus* or re‐occlus* or steno*or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 52 19 ((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) adj3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*)).ti,ab. 110 20 or/1‐19 12379 21 exp Chelation therapy/ 40 22 exp Chelating agents/ 163 23 ethylenediamine*.ti,ab. 8 24 (ethylen* adj2 diamine*).ti,ab. 11 25 chelat*.ti,ab. 300 26 EDTA.ti,ab. 117 27 or/21‐26 364 28 20 and 27 70 29 exp CLINICAL TRIALS/ 3788 30 RANDOM ALLOCATION/ 314 31 DOUBLE BLIND METHOD/ 667 32 Clinical trial.pt. 1212 33 (clinic* adj trial*).tw. 5438 34 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 2866 35 PLACEBOS/ 591 36 placebo*.tw. 3132 37 random*.tw. 17749 38 PROSPECTIVE STUDIES/ 1119 39 or/29‐38 22789 40 28 and 39 7 41 ("2017" or "2018").yr. 3424 42 40 and 41 0 |
15 August 2018: 0 6 August 2019: 0 |
Data and analyses
Comparison 1. EDTA versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 2 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.28] |
| 1.1.1 Coronary artery disease participants | 2 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.73, 1.28] |
| 1.2 Coronary heart disease death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Coronary artery disease participants | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Myocardial Infarction | 2 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.14] |
| 1.3.1 Coronary artery disease participants | 2 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.14] |
| 1.4 Angina | 2 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.67] |
| 1.4.1 Coronary artery disease participants | 2 | 1792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.55, 1.67] |
| 1.5 Coronary revascularisation | 2 | 1792 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.07, 3.25] |
| 1.5.1 Coronary artery disease participants | 2 | 1792 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.07, 3.25] |
| 1.6 Stroke | 2 | 1867 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.40, 1.92] |
| 1.6.1 Coronary artery disease participants | 1 | 1708 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.35, 1.81] |
| 1.6.2 Peripheral vascular disease participants | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 71.65] |
| 1.7 Ankle‐brachial pressure index at 3 months post‐treatment | 2 | 181 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.06] |
| 1.7.1 Peripheral vascular disease participants | 2 | 181 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.06] |
| 1.8 Ankle‐brachial pressure index at 6 months post‐treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.8.1 Peripheral vascular disease participants | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9 Maximum walking distance (m) at 3 months post‐treatment | 2 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐31.46 [‐87.63, 24.71] |
| 1.9.1 Peripheral vascular disease participants | 2 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐31.46 [‐87.63, 24.71] |
| 1.10 Maximum walking distance (m) at 6 months post‐treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10.1 Peripheral vascular disease participants | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11 Pain‐free walking distance (m) at 3 months post‐treatment | 2 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐7.73 [‐22.59, 7.13] |
| 1.11.1 Peripheral vascular disease participants | 2 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐7.73 [‐22.59, 7.13] |
| 1.12 Pain‐free walking distance (m) at 6 months post‐treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.12.1 Peripheral vascular disease participants | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Guldager 1992.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind trial Intention‐to‐treat: not stated Country: Denmark Setting: outpatient clinic |
|
| Participants |
Number randomised: N = 159 (EDTA n = 80; placebo n = 79) Exclusions post‐randomisation: N = 6 (EDTA n = 5; placebo n = 1) Losses to follow‐up: (more likely study withdrawal than dropout) N = 4 (2 deteriorations leading to vascular surgery, 1 death, 1 participant started chelation therapy at a private clinic) Age (mean years ± SD): EDTA 64 ± 7; placebo 66 ± 9 Gender (M n (%)): EDTA n = 48 (60%); placebo n = 55 (70%) Inclusion criteria: stable intermittent claudication for at least 12 months; pain‐free walking distance range of 50 m to 200 m, measured on treadmill at a speed of 3.6 km/hr with 10° inclination; ABPI of worse leg < 0.8 Exclusion criteria: vascular surgery within last 12 months, ischaemic rest pain or gangrene, moderate or severe venous insufficiency, renal insufficiency, diabetes mellitus, thyroid or parathyroid disorders, hepatic dysfunction, significant cardio‐pulmonary failure, e.g. acute myocardial infarction within last 12 months, tuberculosis, pregnancy, other conditions which could limit the person's walking distance or reliable interpretation of the study, people receiving anticoagulants, nitroglycerine or lithium, EDTA chelation therapy within last 24 months. |
|
| Interventions |
Treatment: EDTA 3 g + NaCl 8.4 g in 1 litre normal saline solution x 20 infusions Control: 1 litre normal saline solution x 20 infusions Duration of treatment: 5 to 9 weeks Follow up: 3 and 6 months |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done in blocks of 10. |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | As this was a double‐blind study, blinding of participants and personnel was assured, at least until the end of the treatment period. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessments done during the double‐blind study period had low risk of bias. As the code was broken after the last participant completed the treatment period, the 3‐month and 6‐month post‐treatment measurements were done with outcome assessors aware of treatment assignments and therefore had high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six participants who did not complete treatment were not included in the subjective evaluation. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the plan were reported. |
| Other bias | Low risk | Free from other sources of bias. |
Knudston 2002.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled clinical trial. Intention‐to‐treat: yes Country: Alberta, Canada Setting: outpatient clinic |
|
| Participants |
Number randomised: N = 84 (EDTA n = 41; placebo n = 43) Exclusions post‐randomisation: none Losses to follow‐up: no dropouts were reported, however there were 4 people unable to complete protocol in the control group and 2 unable to complete protocol in the treatment group. Age (mean years ± SD): EDTA 66 ± 9.1; placebo 65 ± 8.5 Gender (M n (%)): EDTA 33 (85.4%); placebo 32 (83.7) Inclusion criteria: aged 21 years or older with proven coronary artery disease or documented myocardial infarction and stable angina while receiving optimal therapy; 1 mm of horizontal or down sloping ST‐segment depression from the isoelectric line 80 milliseconds after the J point on treadmill test 2 and 14 minutes from the onset of exercise. Exclusion criteria: planned revascularisation, previous chelation therapy, heart failure, inability to walk on the treadmill, resting ECG changes that would interfere with ischaemic assessment, abnormal renal or liver function, untreated lipid abnormality at time of randomisation. |
|
| Interventions |
Treatment: EDTA weight‐adjusted with a maximum total dose for each treatment of 3 g in 500 mL of 5% dextrose in water x 33 infusions Control: 500 mL of 5% dextrose in water with 20 mL 0.9% sodium chloride x 33 infusions Each 5% dextrose in water solution also contained 750 mg of magnesium sulphate, 5 g of ascorbic acid and 5 g of sodium bicarbonate (titrated to physiologic pH). All participants also received oral multivitamin therapy, 2 tablets 3 times daily except on treatment days. Duration of treatment: 27 weeks total; twice weekly for 15 weeks and once monthly for an additional 3 months. Follow up: 1 year from randomisation |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacist assigned the randomised therapy and prepared 'indistinguishable' solutions. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | As this was a double‐blind study, blinding of participants and personnel to treatment was assured. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment was assured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six people were unable to complete protocol; four in the placebo group and two in the treatment group; clinical events were presented on an intent‐to‐treat basis. There were apparently no dropouts. |
| Selective reporting (reporting bias) | Low risk | Only 39 participants per group were analysed with regards to outcomes involving subjective and treadmill data for between‐group comparisons; for clinical events, all participants were included in the between‐group analysis |
| Other bias | High risk | Despite utilising a randomisation process, there was more multivessel disease, nitrate use and triple therapy at baseline in the placebo group and more past myocardial infarction in the chelation group. These differences may tend to favour chelation and therefore the conclusion of 'no effect' is strengthened. |
Olszewer 1990.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind for 10 treatments, then completed in an open single treatment fashion. Intention‐to‐treat: not stated Country: Brazil Setting: outpatient clinic |
|
| Participants |
Number randomised: N = 10 (EDTA n = 5; placebo n = 5) Exclusions post‐randomisation: not stated Losses to follow up: not stated Age (mean years (range)): 47 (41 to 53) Gender: All male Inclusion criteria: peripheral vascular disease (Fontaine Stage II) ‐ intermittent claudication; walking test ‐ claudication between 100 m and 300 m; master exercise test ‐ claudication with < 40 steps; bicycle stress test ‐ claudication before 3 minutes at 50 km/hr Exclusion criteria: pain at rest or at night, gangrene |
|
| Interventions |
Treatment: EDTA 10 mL (1.5 g) x 20 Control: distilled water 10 mL x 20 (although halfway through all control participants rolled‐over to active treatment for remaining 10 infusions) Duration of treatment: not stated Follow up: not stated |
|
| Outcomes |
(observations made after 10 infusions and after 20 infusions) |
|
| Notes | Adequate allocation concealment initially, but the code was broken before end of study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study states that the 2 groups were "randomly" and equally divided |
| Allocation concealment (selection bias) | Unclear risk | The identification of the contents of each vial was in a sealed envelope by the manufacturer; it was not explicitly stated how the dispensing of the treatment was done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | For the first 10 treatments, the blinding of participants and personnel was maintained; in the remaining 10 sessions, only the participants were blinded to treatment assignment and the placebo group was shifted to active treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was blinded for the first 10 infusions, however in the remaining 10 infusions the investigators broke the code and the placebo group was shifted to active treatment: Outcome assessment was therefore not blinded in this case. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The incompleteness in outcome data is due to the premature stoppage of the placebo arm and then the shifting of this arm to active treatment; the proposed number of sessions for both groups was not followed. |
| Selective reporting (reporting bias) | Low risk | All outcomes indicated were reported in tables with group differences specified. |
| Other bias | High risk | The breaking of the code and shifting of the control group into the intervention group because of a seemingly positive response after 10 infusions was premature and perhaps not part of the planned protocol; this casts doubt on the true effect of treatment as accurate assessments with a control group on the prespecified period could not be derived. |
TACT 2013.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind trial Intention‐to‐treat: yes Country: USA & Canada Setting: outpatient clinics |
|
| Participants |
Number randomised: N = 1708 (EDTA n = 839; placebo n = 869) Exclusions post‐randomisation: none Losses to follow up: N = 22 (EDTA n = 13; placebo n = 9) Age (mean years (range)): EDTA 65 (58.8 to 71.6); placebo 65.5 (58.7 to 72.2) Gender (M n (%)): EDTA 687 (82%); placebo 83%) Inclusion criteria: history of myocardial infarction 6 weeks or more prior to enrolment; willing to participate Exclusion criteria: women of childbearing potential, serum creatinine level > 2.0 mg/dL, platelet count < 100,000/µL, abnormal liver function studies, blood pressure > 160/100 mmHg, intolerance to chelation or vitamin components, history of chelation treatment within 5 years, planned coronary or carotid revascularisation or history of revascularisation within last 6 months, history of cigarette smoking within 3 months, active heart failure or heart failure hospitalisation within 6 months, or inability to tolerate 500 mL infusions weekly |
|
| Interventions | Participants were randomised to one of four groups:
For the purposes of this review, we combined groups 1 and 2 that were both receiving active EDTA chelation treatment. Treatment: standard multi component disodium EDTA chelation solution x 40 infusions Control: 500 mL of normal saline and 1.2% dextrose x 40 infusions During infusion phase, all participants received a daily low‐dose of vitamin B6 25 mg, zinc 25 mg, copper 2 mg, manganese 15 mg and chromium 50 µg to reduce depletion by chelation treatment. Duration of treatment: first 30 infusions occurred weekly and final 10 infusions could "occur between 2 weeks and up to 8 weeks apart". Follow‐up: total of 5 years, with quarterly telephone contact and annual clinic visits |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using permuted blocks stratified by clinical site. |
| Allocation concealment (selection bias) | Low risk | Secure web‐based randomisation was performed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study with the blinded active chelation solution prepared by a central pharmacy. Placebo infusions were shipped with identical packaging and 2 separate placebo syringes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded independent clinical events committee at Brigham Women's Hospital adjudicated all nonprocedural components of the primary endpoint. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 13 in the chelation group and 9 in the placebo group who were lost to follow‐up. Looking at the results, this may decrease confidence minimally. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes were reported using between group analysis. |
| Other bias | Low risk | No other sources of bias were identified. |
van Rij 1994.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind trial. Intention‐to‐treat: yes Country: New Zealand Setting: outpatient clinic |
|
| Participants |
Number randomised: N = 32 (EDTA n = 15; placebo n = 17) Exclusions post‐randomisation: none up to 3‐month assessment Losses to follow up: none up to 3‐month assessment Age (mean years ± SD): EDTA 67.7 ± 7.0; placebo 66.9 ± 6.7 Gender (M n (%)): EDTA 13 (87%); placebo 15 (88%) Inclusion criteria: intermittent claudication < 20% variation in measured walking distance over 3 consecutive assessments performed on different days, older than 45 years Exclusion criteria: other debilitating disease that affected walking, significant renal disease, diabetes mellitus |
|
| Interventions |
Treatment: EDTA 3 g + MgCl 0.76 g + NaHCO3 0.84 g in 500 mL normal saline x 20 infusions Control: 500 mL normal saline x 20 infusions Both groups' infusions contained Parentrovite (thiamine hydrochloride 250 mg, riboflavine phosphate 5; 5 mg, pyridoxine hydrochloride 50 mg, ascorbic acid 500 mg, nicotinamide 160 mg, sodium pantothenate 5 mg, glucose anhydrous 1000 mg, sodium metabisulphite 4 mg); Both groups also received oral daily vitamin supplements. Duration of treatment: 10 weeks Follow up: after 10 infusions, 3, 6 and 12 months |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Preparation of assigned infusions were conducted independently by the hospital pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | As this was a double‐blind study with the infusions indistinguishable by container, labelling or colour, participant and personnel blinding was assured. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments of participants and data were done by different staff groups who worked independently and were blind to treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no complications or withdrawals from the study in either group up to the 3‐month assessment, but no indication of outcomes reported after 3 months of follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All initially stated outcomes were reported with between‐group analysis, but there was no evidence of outcomes reported at 6 and 12 months. |
| Other bias | Low risk | No other sources of bias were identified. |
ABPI: ankle‐brachial pressure index (also known as ankle brachial index) ECG: electrocardiogram EDTA: ethylene diamine tetra‐acetic acid eGFR: estimated glomerular filtration rate MgCl: magnesium chloride mmHg: millimetres of mercury NaCl: sodium chloride NaHCO3: sodium hydrogen carbonate SD: standard deviation TET: treadmill exercise test VO2: maximal oxygen uptake
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| TACT‐PAD | Non‐RCT: single‐arm treatment study evaluating EDTA for people with diabetes and critical limb ischaemia |
EDTA: ethylene diamine tetra‐acetic acid RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
TACT2.
| Study name | Trial to Assess Chelation Therapy 2 (TACT2) |
| Methods | Phase 3, double‐blind, RCT, 2 x 2 factorial design |
| Participants | 1200 participants with diabetes and prior myocardial infarction |
| Interventions | Active disodium EDTA (chelation) + active oral multi vitamins/minerals (OMVM) Active disodium EDTA (chelation) + placebo OMVM Placebo disodium EDTA (chelation) + active OMVM Placebo disodium EDTA (chelation) + placebo OMVM |
| Outcomes | Composite of all‐cause mortality, myocardial infarction, stroke, coronary revascularisation or hospitalisation for unstable angina over 5 years |
| Starting date | October 2016 |
| Contact information | Gervasio Lamas, MD, TACT2 Study Chair, Mt. Sinai Medical Center, Miami, Florida, United States |
| Notes | Expected completion in 2021 |
TACT3a.
| Study name | Trial to Assess Chelation Therapy in Critical Limb Ischemia (TACT3a) |
| Methods | Phase 3, double‐blind, RCT |
| Participants | 50 participants with diabetes and critical limb ischaemia |
| Interventions | Drug: edetate disodium: (chelation) solution contains up to 3 g of edetate disodium adjusted based on creatinine clearance, 2 g of magnesium chloride, 100 mg of procaine hydrochloride, 2500 U of heparin, 7 g of ascorbate, 2 milliequivalent (mEq) potassium chloride (KCl), 840 mg sodium bicarbonate, 250 mg pantothenic acid, 100 mg of thiamine, 100 mg of pyridoxine, and sterile water to complete 500 mL. Placebo infusions consist of 500 mL normal saline. Treatment will consist of 40 active or placebo infusions over 30 weeks. |
| Outcomes | Coronary revascularization, stroke, MI, death (all‐cause), or major amputation during an average 1.25 years of follow‐up |
| Starting date | 19 March 2019 |
| Contact information | Francisco Ujueta, MD, Mount Sinai Medical Center, Miami, Florida, United States |
| Notes | Expected completion in 2022 |
EDTA: ethylene diamine tetra‐acetic acid OMVM; oral multi vitamins/minerals RCT: randomised controlled trial
Differences between protocol and review
We made a very minor modification to the objectives of the original protocol, to limit direct test of disease severity to digital subtraction angiogram only.
For sensitivity analysis plans, we added that we would evaluate studies with higher risk of bias and meta‐analyses where one study has a large influence on the results.
The original protocol and review used a quality assessment instrument developed by the Philippine Cardiovascular Research Group. For this update we used the Cochrane 'Risk of bias' tool (Higgins 2011),
Contributions of authors
MVVS: joint primary author, contact reviewer, appraised studies and extracted data RF: joint primary author, appraised studies using GRADE ALD: author, reviewer, resolved discrepancies FT: author, reviewer, appraised studies and extracted data DVS: author, reviewer, resolved discrepancies
Sources of support
Internal sources
No sources of support supplied
External sources
-
Philippine College of Physicians, Philippines
Provided funds for the completion of this review update. The grant included payment for utilities used ‐ a faster local internet service, and meeting and communication expenses.
-
The Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
MVVS: the host institution received a minimal grant from the Philippine College of Physicians to assist the review authors to complete the review. The grant included payment for utilities used ‐ a faster local internet service, and meeting and communication expenses. RF: none known ALD: is an investigator in the Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS) trial which is funded by Bayer. FNT: none known DVS: is an investigator in the Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (COMPASS) trial which is funded by Bayer.
These authors contributed equally to this work
These authors contributed equally to this work
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Guldager 1992 {published data only}
- Guldager B, Faergeman O, Jorgensen SJ, Nexo E, Jelnes R. Disodium-ethylene diamine tetraacetic acid (EDTA) has no effect on blood lipids in atherosclerotic patients. A randomized, placebo-controlled study. Danish Medical Bulletin 1993;40(5):625-7. [PubMed] [Google Scholar]
- Guldager B, Jelnes R, Jorgensen SJ, Nielsen JS, Klaerke A, Mogensen K, et al. EDTA treatment of intermittent claudication - a double-blind, placebo-controlled study. Journal of Internal Medicine 1992;231(3):261-7. [DOI] [PubMed] [Google Scholar]
- Guldager B, Jelnes R, Jorgensen SJ, Sloth-Nielsen J, Klaerke A, Mogensen K, et al. EDTA versus placebo treatment in intermittent claudication. A double-blind randomized trial [EDTA versus placebobehandling af claudicatio intermittens]. Ugeskrift for Laeger 1992;154(23):1618-21. [PubMed] [Google Scholar]
- Guldager B, Jorgensen PJ, Grandjean P. Metal excretion and magnesium retention in patients with intermittent claudication treated with intravenous disodium EDTA. Clinical Chemistry 1996;42(12):1938-42. [PubMed] [Google Scholar]
- Sloth-Nielsen J, Guldager B, Mouritzen C, Lund EB, Egebland M, Norregaard O, et al. Arteriographic findings in EDTA chelation therapy on peripheral arteriosclerosis. American Journal of Surgery 1991;162(2):122-5. [DOI] [PubMed] [Google Scholar]
Knudston 2002 {published data only}
- Anderson TJ, Hubacek J, Wyse DG, Knudtson ML. Effect of chelation therapy on endothelial function in patients with coronary artery disease: PATCH substudy. Journal of the American College of Cardiology 2003;41(3):420-5. [DOI] [PubMed] [Google Scholar]
- Knudston ML, Wyse DG, Galbraith PD, Brant R, Hildebrand K, Paterson D, et al. Chelation therapy for ischemic heart disease: a randomized trial. Journal of the American Medical Association 2002;287:481-6. [DOI] [PubMed] [Google Scholar]
Olszewer 1990 {published data only}
- Olszewer E, Sabbag FC, Carter JP. A pilot double-blind study of sodium magnesium EDTA in peripheral vascular disease. Journal of the National Medical Association 1990;82(3):173-7. [PMC free article] [PubMed] [Google Scholar]
TACT 2013 {published data only}
- Drisko J, Alexander KP, Roberts RS, Chappell LT, Lee KL, Boineau R, et al. Post-myocardial infarction treatment with edetate disodium was safe in the trial to assess chelation therapy. Circulation 2015;132 (Suppl 3):A10466. [Google Scholar]
- Escolar E, Lamas GA, Mark DB, Boineau R, Goertz C, Rosenberg Y, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circulation: Cardiovascular Quality and Outcomes 2014;7(1):15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolar E, Lamas GA. Clinical benefit of EDTA chelation therapy in patients with diabetes in the trial to assess chelation therapy (TACT). Circulation 2013;128(22 Suppl 1):A11546. [Google Scholar]
- Issa OM, Roberts R, Mark DB, Boineau R, Goertz C, Rosenberg Y, et al. Effect of high-dose oral multivitamins and minerals in participants not treated with statins in the randomized Trial to Assess Chelation Therapy (TACT). American Heart Journal 2018;195:70-7. [DOI] [PubMed] [Google Scholar]
- Lamas GA, Boineau R, Goertz C, Mark DB, Rosenberg Y, Stylianou M, et al. EDTA chelation therapy alone and in combination with oral high-dose multivitamins and minerals for coronary disease: the factorial group results of the Trial to Assess Chelation Therapy. American Heart Journal 2014;168(1):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas GA, Boineau R, Goertz C, Mark DB, Rosenberg Y, Stylianou M, et al. Oral high-dose multivitamins and minerals alone and in combination with chelation therapy for coronary disease: a randomized clinical trial. Journal of Alternative and Complementary Medicine 2014;20(5):A2-3. [Google Scholar]
- Lamas GA, Boineau R, Goertz C, Mark DB, Rozema TC, Nahin RL, et al. Results of the trial to assess chelation therapy. Circulation 2012;126(23):2778-9. [Google Scholar]
- Lamas GA, Escolar E, Boineau R, Nahin R, Goertz C, Mark D, et al. Clinical benefit of chelation therapy in post-MI patients with diabetes and peripheral artery disease. Circulation 2014;130:A18819. [Google Scholar]
- Lamas GA, Escolar E, Boineau R, Nahin R, Goertz C, Mark D, et al. Graded benefit of EDTA chelation in post-MI patients with diabetes treated with different hypoglycemic strategies. Circulation 2014;130:A16630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Design of the Trial to Assess Chelation Therapy (TACT). American Heart Journal 2012;163:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Disodium EDTA chelation for post myocardial infarction patients. Journal of Alternative and Complementary Medicine 2014;20(5):A2. [Google Scholar]
- Lamas GA, Goertz C, Boineau R, Mark DB, Rozema T, Nahin RL, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. Journal of the American Medical Association 2013;309(12):1241-50. [DOI] [PMC free article] [PubMed]
- Lamas GA, Guarneri E, Mark DB, Boineau R, Bradley R, Goertz C, et al. Effect of high-dose oral multi-vitamins and minerals in patients not treated with statins in the trial to assess chelation therapy. Circulation 2015;132 (Suppl 3):A10476. [Google Scholar]
- Lamas GA, Nahin RL, Lindblad L, Goertz C, Boineau R, Chappell T, et al. Clinical benefit of EDTA-based chelation therapy and high-dose oral multivitamins and multiminerals in TACT-an expanded comparison of 2 factorial groups. Circulation 2013;128 (Suppl 22):A11054. [Google Scholar]
- Lewis EF, Lamas G, Mark D, Rosenberg Y, Boineau R, Roberts RS, et al. Differential outcomes with chelation therapy among patients with anterior versus non-anterior myocardial infarction: a TACT substudy. Journal of the American College of Cardiology 2014;63(12 Suppl 1):A1565. [Google Scholar]
- Mark DB, Anstrom KJ, Boineau R, Goertz C, Rozema TC, Knight JD, et al. Quality of life outcomes in the trial to assess chelation therapy (TACT). Circulation 2012;126(23):2779-80. [Google Scholar]
- Ujueta F, Arenas IA, Escolar E, Diaz D, Boineau R, Mark DB, et al. The effect of EDTA-based chelation on patients with diabetes and peripheral artery disease in the Trial to Assess Chelation Therapy (TACT). Journal of Diabetes and its Complications 2019;33(7):490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Rij 1994 {published data only}
- Rij A, Soloman C, Packer S, Hopkins W. Chelation therapy for intermittent claudication: a double-blind randomised controlled trial. New Zealand Medical Journal 1998;111:17. [DOI] [PubMed]
- Rij AM, Solomon C, Packer SG, Hopkins WG. Chelation therapy for intermittent claudication: a double-blind, randomized, controlled trial. Circulation 1994;90(3):1194-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
TACT‐PAD {published data only}
- NCT03424746. Chelation therapy in diabetic patients with critical limb ischemia (TACT-PAD). clinicaltrials.gov/ct2/show/NCT03424746 (first received 7 February 2018).
References to ongoing studies
TACT2 {published data only}
- NCT02733185. Trial to assess chelation therapy 2 (TACT2). clinicaltrials.gov/ct2/show/NCT02733185 (first received 11 April 2016).
TACT3a {published data only}
- NCT03982693. Trial to assess chelation therapy in critical limb ischemia (TACT3a). clinicaltrials.gov/ct2/show/NCT03982693 (first received 11 June 2019).
Additional references
Anderson 2003
- Anderson TJ, Hubacek J, Wyse DG, Knudtson ML. Effect of chelation therapy on endothelial function in patients with coronary artery disease: PATCH substudy. Journal of the American College of Cardiology 2003;41(3):420-5. [DOI] [PubMed] [Google Scholar]
Avila 2014
- Avila MD, Escolar E, Lamas GA. Chelation therapy after the trial to assess chelation therapy: results of a unique trial. Current Opinion in Cardiology 2014;29:481-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aziz 2016
- Aziz M, Yadav KS. Pathogenesis of atherosclerosis. A review. Medical & Clinical Reviews 2016;2(3):22. [Google Scholar]
Bakic 2007
- Bakic M. Pathogenetic aspects of atherosclerosis. Acta Medica Medianae 2007;46(1):25-9. [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Chappel 1994
- Chappel LT, Stahl JP, Evans R. EDTA chelation treatment for vascular disease: a meta-analysis using unpublished data. Journal of Advancement in Medicine 1994;7(3):131-42. [Google Scholar]
Clarke 1955
- Clarke NE, Clarke CN, Mosher RE. The "in vivo" dissolution of metastatic calcium. An approach to atherosclerosis. American Journal of Medical Sciences 1955;229(2):142-9. [DOI] [PubMed] [Google Scholar]
Clarke 1956
- Clarke NE, Clarke CN, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. American Journal of Medical Sciences 1956;232(6):654-66. [DOI] [PubMed] [Google Scholar]
Clarke 1960
- Clarke N. Atherosclerosis, occlusive vascular disease and EDTA. American Journal of Cardiology 1960;6(2):1-3. [DOI] [PubMed] [Google Scholar]
Ernst 1997
- Ernst E. Chelation therapy for peripheral arterial occlusive disease: a systematic review. Circulation 1997;96(3):1031-3. [DOI] [PubMed] [Google Scholar]
Goldberg 1972
- Goldberg D. The detection of psychiatric illness by questionnaire. London, England: Oxford University Press, Inc, 1972. [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 27 December 2018. Hamilton (ON): McMaster University (developed by Evidence Prime).Available at gradepro.org.
Green 1993
- Green S. Chelation Therapy: unproven claims and unsound theories. Nutrition Forum 1993.
Guldager 1993
- Guldager B, Faergeman O, Jorgensen SJ, Nexo E, Jelnes R. Disodium-ethylene diamine tetraacetic acid (EDTA) has no effect on blood lipids in atherosclerotic patients. A randomized, placebo-controlled study. Danish Medical Bulletin 1993;40(5):625-7. [PubMed] [Google Scholar]
Guldager 1996
- Guldager B, Jorgensen PJ, Grandjean P. Metal excretion and magnesium retention in patients with intermittent claudication treated with intravenous disodium EDTA. Clinical Chemistry 1996;42(12):1938-42. [PubMed] [Google Scholar]
Hiatt 1997
- Hiatt WR. Current and future drug therapies for claudication. Vascular Medicine 1997;2(3):257-62. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hlatky 1989
- Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Amercian Journal of Cardiology 1989;64(10):651-4. [DOI] [PubMed] [Google Scholar]
INTERHEART 2004
- Yusuf S, Hawken S, Ounpuu S on behalf of the INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937-52. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
McNair 1971
- McNair D, Lorr M, Droppleman LF. Manual for the profile of mood states. San Diego, USA: Educational and Industrial Testing Service, 1971. [Google Scholar]
Meltzer 1960
- Meltzer LE, Ural ME, Kitchell JR. The treatment of coronary artery disease with disodium EDTA. In: Metal-Binding in Medicine, edited by Seven MJ. Philadelphia: Lippincott, 1960. [Google Scholar]
Russell 1991
- Russell DG, Wilson NC. Life in New Zealand Commission Report I: Executive Overview. Dunedin, New Zealand: University of Otago, 1991. [Google Scholar]
Seely 2005
- Seely DM, Wu P, Mills EJ. EDTA chelation therapy for cardiovascular disease: a systematic review. BMC Cardiovascular Disorders 2005;5:doi:10.1.1186/1471-2261-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sidhu 2013
- Sidhu MS, Saour BM, Boden WE. A TACTful reappraisal of chelation therapy in cardiovascular disease. Nature Reviews. Cardiology 2014;11(3):180-3. [DOI] [PubMed] [Google Scholar]
Sloth‐Nielsen 1991
- Sloth-Nielsen J, Guldager B, Mouritzen C, Lund EB, Egebland M, Norregaard O, et al. Arteriographic findings in EDTA chelation therapy on peripheral arteriosclerosis. American Journal of Surgery 1991;162(2):122-5. [DOI] [PubMed] [Google Scholar]
Spertus 1995
- Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. Journal of the American College of Cardiology 1995;25(2):334-41. [DOI] [PubMed] [Google Scholar]
Ujueta 2019
- Ujueta F, Arenas IA, Escolar E, Diaz D, Boineau R, Mark DB, et al. The effect of EDTA-based chelation on patients with diabetes and peripheral artery disease in the Trial to Assess Chelation Therapy (TACT). Journal of Diabetes and its Complications 2019;33(7):490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1997
- Walker M, Shah H. Everything you should know about chelation therapy. New Canaan, Connecticut: Keats Publishing, 1997. [Google Scholar]
Ware 1994
- Ware Jr JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: a user's manual.. Boston, USA: Health Assessment Lab, New England Medical Center, 1994. [Google Scholar]
Wilder 1962
- Wilder LW, De Jode LR, Milstein SW, Howard JM. Mobilization of atherosclerotic plaque calcium with EDTA utilizing the isolation-perfusion principle. Surgery 1962;52(5):793-5. [PubMed] [Google Scholar]
References to other published versions of this review
Dans 2002
- Dans AL, Tan FN, Villarruz-Sulit EC. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD002785] [DOI] [PMC free article] [PubMed] [Google Scholar]


