Abstract
The main route of transmission of the human coronaviruses (HCoVs), and presumably also of the new pandemic SARS-CoV-2, is via droplets and close contacts, however their fecal elimination also suggests the possible spread via water. A scientific literature search was thus carried out to highlight the current state of the art and knowledge gaps regarding coronavirus in water. Since 1978 only 22 studies have met the inclusion criteria, and considered heterogeneous purposes, detection methods and types of water. In vitro experiments have addressed the recovery efficiency of analytical methods, survival in different types of water and the removal efficiency of water treatments. Field studies have monitored coronaviruses in surface waters, sewage, slurry, and biosolids. Overall, at the lab scale, HCoVs or surrogates can survive for several days at 4 °C, however their persistence is lower compared with non-enveloped viruses and is strongly influenced by temperature and organic or microbial pollution. HCoVs have rarely been detected in field investigations, however may be due to the low recovery efficiency of the analytical methods. The scarcity of information on HCoV in the environment suggests that research is needed to understand the fate of these viruses in the water cycle.
Keywords: SARS-CoV-2, Coronavirus, Water, Wastewater, Survival, Recovery efficiency
Graphical abstract
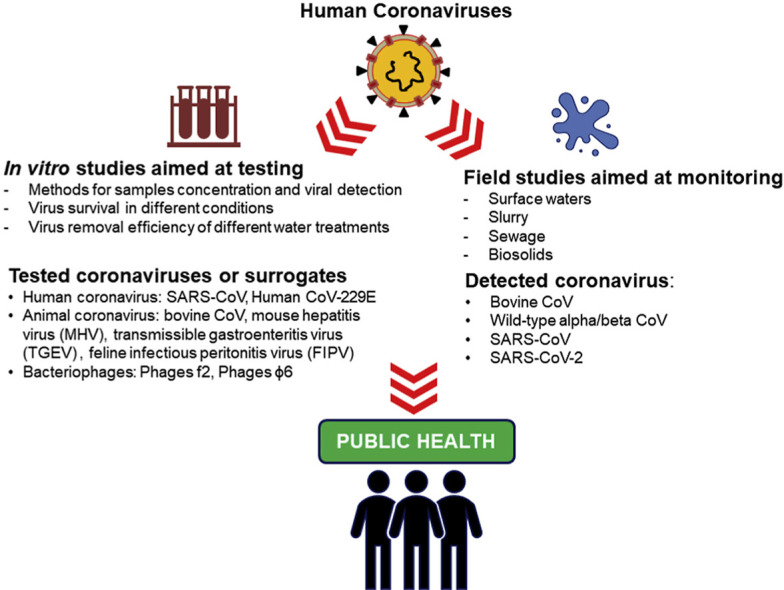
Highlights
-
•
Only 22 heterogeneous studies addressed Human CoV or surrogates in waters.
-
•
Without better knowledge, waterborne circulation of SARS-CoV-2 cannot be excluded.
-
•
Urgent research on coronavirus presence and persistence in the environment is needed.
1. Introduction
The appearance of new viruses with a high epidemic potential is often the result of complex dynamics involving animals, humans and the environment (Coker et al., 2011). Coronavirus can be considered as a paradigm of this phenomenon, in the last 18 years it has caused three new alarming diseases: severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and the current coronavirus disease 2019 (COVID-19) (WHO, 2020a). The family of Coronaviridae includes strains that infect humans with a wide range of clinical symptoms, from those associated with the common cold to potentially lethal respiratory syndromes. Other Coronaviridae strains infect birds and mammals (ICTV, 2012). The high variability of this virus makes cross-infections among species possible, potentially leading to spillover (Chan et al., 2015). In fact before the outbreak of the current virus, a number of authors addressing the environmental circulation of viruses had already highlighted the potential new pandemic threat posed by coronavirus (Wigginton and Ellenberg, 2015 ; Morse et al., 2012 ; Santos and Monteiro, 2013).
Although the main route of transmission of these viruses is via droplets and close contacts, the possible environmental spread via water, bioaerosols, and food should not be neglected. In fact, the fecal elimination of coronavirus is well-known and has been confirmed for SARS-CoV (Yam et al., 2003; Poon et al., 2004; Wang et al., 2005a; Petrich et al., 2006; He et al., 2007), MERS CoV (Drosten et al., 2013; Zhou et al., 2017) and SARS-CoV-2 (Holshue et al., 2020; Zhang et al., 2020). The potential fecal-oral transmission was recently highlighted by Yeo et al. (2020). Moreover, possible transmission through bioaerosols from toilet flushing was demonstrated in Hong Kong for the SARS epidemic cluster in Amoy Gardens (Watts, 2003; Yu et al., 2004) and was recently suggested for SARS-CoV-2 (Ong et al., 2020). Finally, the involvement of fecally-contaminated food in coronavirus transmission is generally not considered but cannot be ruled out, given the survival of the human or animal coronavirus on vegetables (Mullis et al., 2013; Yépiz-Gómez et al., 2013).
At present, COVID-19 is responsible for a rapidly expanding global epidemic with tens of thousands of cases and thousands of deaths (Heymann and Shindo, 2020; Di Marco et al., 2020). As a result, a pandemic was declared by WHO on March 11, 2020 (WHO, 2020b). It is therefore possible that the virus may be released with wastewater and from there contaminate other water bodies (surface, sea, groundwater), generating aerosols. In fact, sewage from hospitals, especially infectious disease units, may contain the epidemic virus, thus requiring efficient disinfection before discharge into natural waters.
Owing to concerns about SARS-CoV-2 water pollution, North Korea has recently started to monitor surface waters used as sources of drinking water, as recently announced in newspapers by the Korean government-funded news agency (Byung-joon, 2020 on Yonhap News Agency), even though other countries are reassuring the public about the safety of drinking water (La Rosa et al., 2020). The problem of water pollution was also addressed in a technical brief of the WHO on water, sanitation, hygiene and waste management for COVID-19 (March 3, 2020). This focused on enteric viruses, including coronaviruses, and indicates that measures used for the non-enveloped enteric virus abatement should be even more efficient for coronavirus (WHO, 2020c). Nevertheless, the brief confirms the lack of knowledge regarding coronavirus in water.
Knowledge of the presence of the coronavirus (and more specifically of SARS-CoV-2) in wastewater, along with its survival and removal by different treatment approaches could be very useful for risk assessment and management.
2. Literature search strategy
In order to define the state of the art regarding coronavirus (generally and for epidemic strains) in the water environment, a literature search was conducted on April 20, 2020 using three bibliographic databases (PubMed, Scopus, and Web of Science) without time limitations to include even the oldest papers. The search was performed with the following keywords: coronavirus, SARS-CoV-2, SARS, MERS, COVID-19 associated with water, wastewater, sewage, slurry, sludge, and biosolids. To give a comprehensive view of the topic, the literature search was extended to preprints using the medRxiv server (https://www.medrxiv.org/), with the same criteria cited above. Papers were screened in relation to the title and abstract in order to eliminate duplicates and to check whether they complied with the aim of the survey. The selected papers were read entirely to collect data on: coronavirus type and strain, type of water sample, type of study (experimental or field), detection methods, monitoring data, survival data, effect of disinfection, and treatments.
The time span covered by the papers identified by the search extended from 1978 to 2020, however the number of specific papers corresponding to the keywords used was very small: only 22, of which 4 papers were preprints from MedRxiv. These papers are reported and summarized in Table 1 .
Table 1.
Description of the reviewed studies with attention focused on coronavirus and/or surrogates (papers are listed in chronological order of publication).
| Author and date of publication | Type of study | Type of CoV or surrogate a | Aim of the study | Study design |
|||
|---|---|---|---|---|---|---|---|
| d | Type of water sample (n° of samples) | Virus concentration method | Virus detection method b | ||||
| Derbyshire and Brown (1978) | Field | Coronavirus growing on primary cell cultures | Virological characterization of environmental matrices impacted by livestock | 2-L | Cattle and pig slurry (n° 56) | Different virus concentration methods according to matrix type | Primary cell cultures of PK and EBK cells |
| 20-L | Runoff, surface waters and groundwaters (n° 102) | ||||||
| Duan et al. (2003) | In vitro | SARS-CoV strain P9 | Survival at room temperature in water and different surfaces (9 samplings over 120 h period assay) | 300-μL | sterilized water spiked with SARS-CoV to a final quantity of 106 TCID50 | NA | Infective assay on cell line Vero-E6 |
| Wang et al. (2005b) | In vitro | SARS-CoV | 1. Survival assay in various water matrices at 4 °C and 20 °C (9 samplings over 14 days period assay) | 100-ml | Different water samples spiked with SARS-CoV to a final quantity of 105 TCID50/ml:
|
NA | Infectivity assay onto Vero E6 cell line and RT-PCR |
|
2. Disinfection assay in wastewaters using sodium hypochlorite and chlorine dioxide | 100-ml | Domestic sewages spiked with SARS-CoV and phage f2 to a final quantity 101.75 TCID50/ml and 1.1 × 105 PFU/L, respectively | NA | Infectivity assay onto Vero E6 cell line | ||
| Wang et al. (2005c) | In vitro |
|
Recovery efficiency of virus concentration methods based on electropositive filter media particle | 100-ml | Hospital sewage samples spiked with SARS-CoV and phage f2 to a final quantity of 102–103 TCID50/ml for both | NA | Infectivity assay onto Vero E6 cell line |
| Field |
|
To investigate potential fecal-oral transmission of SARS-CoV | 2.5-L | Hospital sewage before disinfection (n° 5) | Electropositive filters | Both infectivity assay onto Vero E6 cell line and RT-PCR | |
| 25-L | Hospital sewage after disinfection (n° 5) | ||||||
| Casanova et al. (2009) | In vitro |
|
Survival assay in various water matrices at 4 °C and 23–25 °C (6 samplings over 49 days period assay) | 45-ml | Different water samples spiked with TGEV and MHV at a final quantity ∼105 MPN/ml and ∼107 MPN/ml, respectively:
|
NA | Infective assay on ST cell cultures for TGEV and DBT cell cultures for MHV. |
| Gundy et al. (2009) | In vitro |
|
Survival assay in various matrices at 23 °C and only for filtered tap water the assay was carried out also at 4 °C (6 samplings over 21 days period assay) | 30-ml | Different water samples spiked with HCoV and FIPV at a final quantity of 105 TCID50/ml for both:
|
NA | Infectivity assay on MRC-5 cell line for HCoV and CRFK cell line for FIPV |
| Fan et al. (2010) | In vitro | MHV strain A59 | Detection efficiency of a methodology based on spectroscopy | 1-ml | Deionized water samples spiked with MHV at a final quantity of 106-107 PFU/ml | NA | Surface-enhanced Raman spectroscopy (SERS) followed by multivariate statistical analyses for the interpretation of SERS spectral data (specific for each virus strain) |
| Schwarte et al. (2011) | Field | Bovine CoV | To evaluate the effects of grazing management on sediment, phosphorus and pathogen loading | Not specified | Simulated runoff (n° 360) and cow feces (n° 90) | Not specified | RT-qPCR |
| Bibby et al. (2011) | Field | Human CoV | To develop an approach for describing the diversity of human pathogenic viruses in an environmentally isolated viral metagenome | 1-L | Treated sewage sludge (Class B biosolid) | Sample concentration according to standardized US procedure for virus concentration in sludge | Shotgun sequencing techniques |
| Bibby and Peccia (2013) | Field | Human CoV | To describe the human virus diversity in wastewater sample, and to understand infectious risks associated with land application | 250-ml | Untreated sewage sludge (n° 5) and treated sewage sludge (n° 5) | Sample concentration according to procedure described in literature | Shotgun sequencing techniques |
| Abd-Elmaksoud et al., 2014 | In vitro | Bovine CoV | Recovery efficiency using glass wool filter as technique for water samples concentration. The different turbidity is used to simulate agricultural runoff | 20-L | Tap water spiked with bovine CoV at a final concentration of 250 GC/L, and added with different quantity of dried agricultural soil to produce three different turbidity level | Glass wool filtration | RT-qPCR |
| Corsi et al. (2014) | Field | Bovine CoV | To examine the occurrence, hydrologic variability, and seasonal variability of human and bovine viruses in surface water | 20-L | River waters impacted by rural or urban runoff (n° 63) | Automatic sampling procedure and concentration based on prefiltration and glass wool filtration. | RT-qPCR |
| Casanova and Weaver (2015) | In vitro | Phage φ6 | Survival assay at 22 and 30 °C (10 samplings over 10 days period assay) | 45-ml | Pasteurized raw sewage spiked with φ6 at a final concentration of ∼107 PFU/ml | NA | Plaque assay |
| Ye et al. (2016) | In vitro |
|
|
30-ml | Pasteurized and unpasteurized raw sewage spiked with MHV and φ6 at a final concentration of 3 × 104 PFU/ml and 5 × 105 PFU/ml, respectively | NA |
|
| Christensen and Myrmel (2018) | In vitro | Bovine CoV | Removal efficiency of coagulation-filtration system at bench scale and using three different coagulant (zirconium, chitosan and polyaluminium chloride). | 400-ml | Wastewaters spiked with bovine CoV at a final concentration of 104 PCRU/ml | Centrifugation and filtration steps | Both infectivity assay based on HRT-18G and RT-qPCR |
| Blanco et al. (2019) | In vitro | TGEV strain PUR46-MAD | Recovery efficiency of an optimized methodology for virus concentration, based on glass wool filtration | 50-L | Surface waters spiked with TGEV at a final concentration of 5.7 × 106 TCID50/L | Glass wool filtration | Infectivity assay based on swine testis (ST) cell line |
| Field | Wild-type alpha/beta CoV | To verify the efficiency of the optimized procedure in detecting viruses (HAV and coronavirus) in natural environment | 20-L | Surface waters (n° 21) | Glass wool concentration with an optimization set up in the in vitro study using TEGV | Semi-nested RT-PCR for wild-type alpha/beta CoV and sequencing | |
| Ahmed et al. (2020) | Filed | SARS-CoV-2 | - To monitor SARS-CoV-2 in a pumping station and WWTPs, after first COVID-19 cases in Australia - To estimate COVID-19 prevalence in the study area from SARS-CoV-2 data in wastewaters (Wastewater-based epidemiology) |
100-200-ml | Raw sewages (n° 9). | Automatic 24h sampling procedure and concentration based on different methods: - Electronegative membranes; - Ultrafiltration (cut-off 10 kDa) |
RT-qPCR and sequencing |
| Wang et al. (2020) | Field | SARS-CoV-2 | To monitor SARS-CoV-2 in a hospital setting for COVID-19 patients (surface, sewage, personal protective equipment) | Not specified | Wastewater at different step of the treatment in a disinfection pool: untreated (n° 3), partially treated (n° 1), treated (n° 1). | Not specified | RT-qPCR and infectivity assay onto Vero E6 cell line |
| Medema et al. (2020) (pre-print on medRxiv ∗) | Field | SARS-CoV 2 | To monitor SARS-CoV-2 in WWTP from cities and Schiphol Airport, before and after first COVID-19 cases in The Netherlands | 250-ml | Raw sewages (n° 24) | Automatic 24h sampling procedure and concentration by ultrafiltration (cut-off 100 kDa) | RT-PCR |
| Nemudryi et al. (2020) (pre-print on medRxiv ∗) | Field | SARS-CoV-2 | To monitor SARS-CoV-2 in municipal wastewaters, after first COVID-19 cases in USA (Montana) To determine the phylogenetic origin of SARS-CoV-2 |
500-ml | Raw sewages (n° 7 in triplicate) | Two different sampling strategy (manual and automatic 24h samplings) and concentration by ultrafiltration (cut-off 10 kDa) | RT-qPCR and sequencing |
| Wu et al. (2020) (pre-print on medRxiv ∗) | Field | SARS-CoV 2 |
|
Not specified | Raw sewages (n° 14) | Automatic 24h sampling procedure, filtration on 0.2 μm membrane and centrifugation with polyethylene glycol 8000 | RT-qPCR and sequencing |
| Wurtzer et al. (2020) (pre-print on medRxiv ∗) | Field | SARS-CoV-2 | To monitor SARS-CoV-2 in urban WWTP after first COVID-19 cases in France | 11-ml | Wastewater samples both raw (n° 23) and treated (n° 8) | Ultracentrifugation (details not provided) | RT-qPCR |
∗ Pre-prints means preliminary reports that have not been peer-reviewed and retrieved from medRxiv database.
FIPV = Feline Infectious Peritonitis Virus; HCoV = Human coronavirus; MHV = Murine hepatitis virus; NA = not applicable; TGEV = Transmissible gastroenteritis virus.
CRFK = Crandell Reese feline kidney; DBT = delayed brain tumor; EBK = embryonic bovine kidney; HRT = human rectal tumor; MRC-5 are fetal human lung fibroblast; PK = pig kidney; ST = swine testicular.
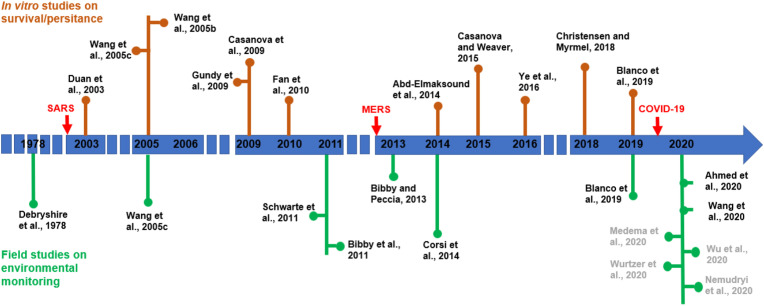
With the exception of one study published in 1978, the other papers were published after the SARS (late 2002/early 2003) and MERS (late 2012) emergencies. Fig. 1 reports the timeline of the reviewed studies, divided on the basis of topic: nine papers consisted of in vitro studies, eleven papers were monitoring studies, and two papers included both research aspects. The papers were not comparable, because they had different purposes and used different methodologies.
Fig. 1.
Timeline of the reviewed papers divided according to the topic which they addressed. Arrows indicate the emergence of pandemic infections due to coronaviruses. Authors in dark grey refer to pre-prints preliminary report retrieved from medRxiv.
3. In vitro experiments on spiked samples
In total there were 11 in vitro studies, seven of which used surrogates, one human coronavirus, and three used both. Seven papers investigated animal coronaviruses, in particular bovine CoV (BCoV), the mouse hepatitis virus (MHV) responsible for respiratory and enteric symptoms in laboratory mice, the transmissible gastroenteritis virus (TGEV), responsible for diarrheal disease in swine and the feline infectious peritonitis virus (FIPV) (Abd-Elmaksoud et al., 2014 ; Christensen and Myrmel, 2018 ; Casanova et al., 2009 ; Fan et al., 2010 ; Ye et al., 2016 ; Blanco et al., 2019 ; Gundy et al., 2009). In addition, bacteriophages (f2, φ6) were employed as model viruses, because they share similar features with coronavirus, such as an envelope and their small size (up to 120 nm). They also have the advantages of being harmless to humans and can be easily detected with simple cultural methods (Wang et al., 2005b , c ; Casanova and Weaver, 2015 ; Ye et al., 2016). In addition, phage Φ6 is considered as a conservative surrogate of coronavirus because it has a double-stranded RNA genome that confers greater stability than the single-stranded RNA genomes of coronavirus (Casanova and Weaver, 2015). Only four studies experimented with the human coronavirus, in particular SARS-CoV and Human CoV-229E (Duan et al., 2003 ; Wang et al., 2005b , c ; Gundy et al., 2009).
The aims of in vitro experiments can be divided into two groups: to test the recovery efficiency of different methodologies for sample concentration and viral detection (5 papers), and to assess the virus survival in different types of water and/or at different temperatures (6 papers), or to evaluate the virus removal efficiency of different water treatments (2 papers). The studies carried out by Wang et al. (2005b) and Ye et al. (2016) had more than one aim (see Table 1).
3.1. Recovery efficiency of analytical methods
Current methods are set up for non-enveloped viruses (i.e. adenovirus, norovirus, hepatitis A virus), which are normally linked to waterborne transmission (Bosch et al., 2008; La Rosa et al., 2012). Coronavirus, as well as other enveloped viruses, exhibit structural and biochemical properties, which suggest that the same methods would not have the same recovery efficiency. Different methods have therefore been tested on different water samples spiked with different viruses.
Two papers focused on glass wool filtration methods widely used for non-enveloped viruses, which consist in an initial phase of adsorption and elution to positively charged glass wool, followed by a second phase of precipitation with polyethylene glycol 6000 (PEG) (Abd-Elmaksoud et al., 2014; Blanco et al., 2019). Abd-Elmaksoud et al. (2014) analysed 20 L of dechlorinated tap water with different turbidities, spiked with BCoV (detected with PCR). They found highly variable virus recovery rates (18.1 ± 20.1%). Results from the samples with low turbidity reported the highest recovery efficiency (25.8 ± 21.3%). Blanco et al. (2019) optimized the glass wool filtration method using 50 L of surface waters spiked with TGEV (detected with cell cultures) by raising the pH of the buffer eluent to 11.0 (at 9.5 for non-enveloped viruses), by increasing the contact time from 10 to 20 min, and using a 20% concentration of PEG instead of 10%. The virus recovery thus improved from 0.40% to 5.1 ± 1.4%. Although both studies used the same method, the results are not directly comparable because the studies differed in terms of coronavirus strain, type and volume of water samples, quantity of virus seeded and detection method.
Wang et al. (2005c) evaluated a virus concentration method based on the adsorption on electropositive filter media particle columns, elution with broth at pH 7.2, and precipitation by 10% PEG. They analysed 100-ml of sewage spiked with SARS-CoV and phage f2 (detected with cultural methods) and obtained a much higher recovery efficiency for phage f2 (on average 127.1%, ranging from 33.6 to 260.0%) than for SARS-CoV (1.02% ranging from 0 to 21.4%).
Ye et al. (2016) applied a two-step method to wastewater samples based on a preliminary centrifugation, aimed at removing solid particles, and then ultrafiltration on the liquid fraction. Both solid and liquid parts were analysed with cultural methods. Small wastewater samples (30-ml) were spiked with MHV and phage φ6 (both enveloped) and with phages MS2 and T3 (non-enveloped), and the method was optimized for both fractions to increase the recovery rates of the enveloped viruses. They achieved mean recovery rates of 25.1% for MHV, 18.2% for ϕ6, 55.6% for MS2 and 85.5% for T3, thus confirming the lower efficiency of the concentration methods for enveloped viruses. In addition, although up to 26% of the two enveloped viruses adsorbed to the solid fraction (compared to 6% of the two non-enveloped ones), only 3.7% of MHV and 2% of MS2 were recovered from it.
A completely different approach was followed by Fan et al. (2010) who tested the efficiency of a methodology for virus detection based on surface-enhanced Raman spectroscopy (SERS) coupled with statistical analysis methods to interpret the SERS spectral pattern. They used deionized water spiked with MHV, with a very high final concentration (106-107 PFU/ml). The results demonstrated that this methodology was able to differentiate viruses at the strain level, however its real usefulness for rapid detection and identification of viruses in environmental water samples was questionable owing to the low sensitivity.
The small numbers of studies on the detection methods for coronavirus and surrogates in water is likely motivated by the assumption that they are not considered to be waterborne Although the results of these studies are not directly comparable, they indicate that methods normally used for non-enveloped viruses need to be improved for enveloped viruses, and that in any case they have a lower efficiency.
3.2. Survival in different types of water and removal efficiency of treatments
In the survival studies, a variable persistence of viruses was identified, depending on the type of virus, type of water sample, and temperature. Duan et al. (2003) found a reduction in SARS-CoV infectivity up to an undetectable level after 3–4 days at room temperature in 300-μL of sterilized water, initially containing a viral titer of 106 TCID50. The persistence of infective SARS-CoV was reduced in 2 day at 20 °C in all the water types tested by Wang et al. (2005b) (namely hospital wastewater, domestic sewage and tap water) but at 4 °C it increased to 14 days, which was the duration of the entire study period. Over a 21-day study period, Gundy et al. (2009) compared the survival of HCoV-229E and FIVP with that of poliovirus 1 in filtered, unfiltered tap water and wastewater. In tap water, coronavirus showed a 3 Log10 decrease in 10 days at 23 °C and over a longer period (estimated >100 days) at 4 °C. In addition, FIVP inactivation at 4 °C was faster compared to HCoV-229E. In wastewater (only at 23 °C) both coronaviruses survived for a shorter period: 2–4 days. Poliovirus survived longer than coronaviruses in all tested water samples at 23 °C, similar to 4 °C in tap water.
Other studies showed a prolonged survival of animal coronavirus and surrogates in different water matrices. Casanova et al. (2009) reported a 2 Log10 decline in TGEV and MHV infectivity after more than 15 days in reagent-grade water at 25 °C. The survival was strongly prolonged at 4 °C: over 49 days, no reduction was observed both in reagent-grade and lake water for MHV, whilst a 1 Log10 reduction after 14 days was observed in lake water for TGEV. This study also demonstrated a faster decline in infectivity in wastewater: in particular a 2 Log10 reduction was observed after nine and seven days for TGEV and MHV, respectively, in pasteurized sewage at 23–25 °C (room temperature) and 2 Log10 and 1 Log10 reductions after 35 days at 4 °C. In a later work, Casanova and Weaver (2015) found a 2 log10 reduction of phage φ6 after five days in pasteurized raw sewage at 22 °C, but with nonlinear inactivation kinetics. Studying MHV and phage φ6 in raw sewage, Ye et al. (2016) compared the virus survival in pasteurized and unpasteurized wastewater. While in pasteurized sewage, they found a 2 Log10 reduction at 25 °C after 30–40 h for MHV and >50 h for phage φ6, in the unpasteurized samples, the inactivation time was reduced to 13 and 7 h, respectively, indicating the possible role of other microorganisms (bacteria and protozoa) in the viral inactivation. The reduction in the enveloped viruses was significantly slower in wastewater at 10 °C compared to 25 °C. In the same study, phages MS2 and T3 (non-enveloped) survived longer in all conditions and matrices.
Although these studies are fragmentary and not directly comparable, they indicate that human coronavirus and surrogates are less resistant than non-enveloped viruses in water environments, that their survival is generally reduced in waters with organic and microbial pollution, and that viral inactivation increases with increasing temperatures.
Several studies have considered the efficacy of disinfection treatments against SARS-CoV and surrogates on surfaces (Kampf et al., 2020). However, water disinfection was only addressed by Wang et al. (2005b) who analysed the resistance to different chlorine solutions of SARS-CoV and phage f2 seeded into 100-ml domestic sewage. During a 30 min disinfection assay, SARS-CoV was completely inactivated with 10 mg/L chlorine or 20 mg/L chlorine dioxide, while phage f2 needed a higher chlorine concentration (40 mg/L) and was not completely inactivated even by 40 mg/L chlorine dioxide.
Christensen and Myrmel (2018) tested the virus removal efficiency at the bench scale of a coagulation-filtration system previously optimized using three different coagulants (zirconium, chitosan and polyaluminium chloride) in reducing BCoV and other viruses (Hepatis A virus, bovine norovirus and MS2). Diluted and undiluted water samples (400-ml) from water treatment plants were spiked with viral mixtures. After the addition of coagulants, centrifugation and filtration steps, the supernatant and filtrate were analysed for quantification. A combination of flocculation and filtration led to a decline in viral presence from 10 to 70% depending on the type of virus, coagulant and presence of natural organic matter: BCoV was reduced more (mean 4 Log10) in undiluted water by all the three coagulants than in diluted water. In this last example, chitosan performed the best.
4. Environmental monitoring studies
As of 2019, a total of seven studies had monitored the presence of coronavirus (SARS and animal) in water, sewage, slurry or biosolids, generally as part of a wider project. Overall, their findings are heterogeneous in terms of the goals of the study, matrices and detection methods.
Derbyshire and Brown (1978) analysed different environmental matrices impacted by breeding activities, for a total of 158 samples collected from surface runoff, surface waters and groundwaters (20-L each) as well as slurry from pig and cattle sources (2-L each). The study used different virus concentration methods based on the type of environmental matrices, and seeded the eluates on primary cell cultures. From slurry samples (56), the study found 25 porcine enteroviruses, three adenovirus and one coronavirus. The analysis of water samples (102) revealed the presence of only three porcine enteroviruses, and one bovine enterovirus, however no coronavirus was found.
Wang et al. (2005c) analysed a total of 20 sewage samples before (2.5-L) and after (25-L) disinfection by chlorine, from hospitals receiving SARS patients. Samples were concentrated by electropositive filter media particle, the virus recovery was also assessed in the same study (see Section 3.2), and the eluates were then analysed for SARS-CoV using both cell culture and RT-PCR. All samples were negative for infectious SARS-CoV, however the genome was detected in all samples before disinfection (100%), and in three samples after disinfection (15%).
Schwarte et al. (2011) studied the impact of grazing animals on the microbial and chemical-physical water quality of pasture streams, using different simulated scenarios in a rural area. BCoV, together with bovine enterovirus and bovine rotavirus, were measured from simulated runoff (360 samples) and cow feces (90 samples) as viruses are commonly shed by grazing animals. The pathogen load was analysed using multiplex RT-qPCR however the procedure for sample concentration was not described. Bovine enterovirus was detected both in cow feces (24.3%) and in the runoff samples (ranging from 8.3% to 16.7%), while bovine coronavirus was only present in one feces sample (1.1%). Bovine rotavirus was not detected in any of the samples.
Bibby et al. (2011, 2013) characterized viral pathogens in sewage sludge through metagenomic analysis (the entire genome extracted was sequenced by shotgun pyrosequencing). In their two studies, they followed different procedures for virus recovery from sludge but in both cases, they found that coronaviruses were the most abundant human viruses, occurring in over 80% of samples (Bibby and Peccia, 2013). The human coronavirus strains 229E and HKU1 were identified. Both studies produced a list of the most abundant viruses, which could be extremely useful in quantitative pathogen monitoring.
Corsi et al. (2014) collected samples from three streams in the USA running through an area with rural and urban land use. They used an automated large-volume sample collection and virus concentration system based on pre-filtration and glass-wool filter. The virus eluates were then analysed using RT-qPCR for BCoV and various other viruses. Around 20-L composite samples were obtained mixing 5-L subsamples collected over time by the system in two different conditions (low-flow period and during runoff events) for a total of 63 samples. The eluates were analysed using RT-qPCR to measure both human (adenovirus, enterovirus, norovirus genogroups I and II, hepatitis A virus, and rotavirus) and bovine (adenovirus, enterovirus, rotavirus group A, polyomavirus, coronavirus, and bovine viral diarrhoea virus types 1 and 2) enteric viruses. During the study period, all the human viruses were detected in the samples, as well as numerous animal and bovine viruses except BCoV.
In the most recent study, Blanco et al. (2019) concentrated 10-L samples of surface waters by an optimized glass wool filtration method (see Section 3.1) and used a semi-nested RT-PCR for wild-type alpha/beta CoV followed by sequencing. They analysed a total of 21 samples and found only one positive result for alphacoronavirus, which was related to a novel rodent/shrew-specific clade by the sequence analysis.
In the studies conducted until 2019, field investigations also confirmed the scarcity of data on coronavirus in water. Coronaviruses were rarely searched for and more rarely found, although the lack of positive results could derive from the low recovery efficiency of the methods used as shown above. However, in the early months of 2020, six field studies focused on a SARS-CoV-2 search in wastewater samples owing to the increasing focus on the environmental circulation of the new coronavirus.
In Australia, Ahmed et al. (2020) collected 100–200 ml of sewage (nine samples), concentrated them with two different methods (filtration by electronegative membranes and ultrafiltration) and analysed the concentrates with RT-qPCR using with two different primer-probe sets for nucleocapsid protein gene. The authors obtained one positivity for each concentration method (not the same sample) but with only one set of primers and at very low titers: 1.2 and 1.9 genomic copies/100 ml. In China, Wang et al. (2020) sampled the wastewater of a hospital at various stages of a multi-stage disinfection with sodium hypochlorite. Five samples (three before disinfection and one for each of the disinfection stages) were analysed with RT-qPCR and a culture assay. The samples from the inlet and after the first disinfection stage were positive, the sample after disinfection was negative. All were non infective.
The four other monitoring studies on this topic were retrieved from medRxiv as preliminary reports, which had not yet been peer-reviewed. In the Netherlands, Medema et al. (2020) monitored sewage samples and obtained positive signals only in 14 (77.8%) of the 18 samples collected after the occurrence of the first cases of COVID-19. In the USA (Massachusetts), Wu et al. (2020) collected 10 samples from WWTPs after the first known cases of COVID-19 and detected SARS-CoV-2 in all of them with approximately 100 genomic copies/ml. The same result was obtained after the storage of samples at 4 °C for 24 h and for a week. Again in the USA (Montana), Nemudryi et al. (2020) collected seven samples of raw sewage, which tested positive for SARS-CoV-2, with a viral load from 100 to 2000 genomic copies/L. In France, Wurtzer et al. (2020) collected both raw and treated wastewater from urban WWTP (31 samples), obtaining 100% and 75% positivity, respectively, with a 2 Log10 reduction after treatment.
Overall, these recent studies confirm the lack of standardized concentration methods for enveloped viruses (see Table 1) and the need to use the same method in order to be able to compare results from different studies.
5. Conclusions
Concerns about possible secondary transmission of the novel SARS-CoV-2 via water are growing with the evidence of its fecal elimination. Moreover, the results of in vitro experiments of prolonged virus survival with declining temperatures suggest that coronavirus excreted in feces could reach wastewater treatment plants in an infective state, especially in cool climates. However, current knowledge is very scarce and fragmentary. Prior to COVID-19, interest in this topic was very low owing to the common belief that enveloped viruses cannot survive for extended periods in water. However, the assumption that SARS-CoV-2 is not involved in environmental circulation cannot be accepted without better knowledge, as highlighted by the detection of SARS-CoV-2 RNA in wastewater by six different global research groups.
The ongoing SARS-CoV-2 emergency and its rapid spread demands new attention on its detection in water. The scarcity of information on the presence and persistence of coronavirus in the environment merits urgent research.
In the meantime, we should respond to the ongoing pandemic by taking precautions and assume that there is a potential for secondary transmission. In particular, we believe that research should address the following:
-
•
Set up efficient methods to concentrate and detect enveloped viruses (and coronavirus in particular) from water matrices;
-
•
Evaluate the survival of these viruses in natural conditions, at different temperatures and in different types of water;
-
•
Assess the efficiency of water treatments and disinfection to avoid contamination from urban and hospital wastewater;
-
•
Evaluate the implications for water reuse for agriculture including the possibility of food (raw vegetables) contamination;
-
•
Establish a surveillance system through sewage monitoring of the potential virus circulation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to thank English for Academics (e4ac.com) for editing and proofreading the manuscript.
References
- Abd-Elmaksoud S., Spencer S.K., Gerba C.P., Tamimi A.H., Jokela W.E., Borchardt M.A. Simultaneous concentration of bovine viruses and agricultural zoonotic bacteria from water using sodocalcic glass wool filters. Food Environ. Virol. 2014;6(4):253–259. doi: 10.1007/s12560-014-9159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020:138764. doi: 10.1016/j.scitotenv.2020.138764. (in press). Available online 18 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Viau E., Peccia J. Viral metagenome analysis to guide human pathogen monitoring in environmental samples. Lett. Appl. Microbiol. 2011;52(4):386–392. doi: 10.1111/j.1472-765X.2011.03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013;47(4):1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco A., Abid I., Al-Otaibi N., Pérez-Rodríguez F.J., Fuentes C., Guix S., Pintó R.M., Bosch A. Glass wool concentration optimization for the detection of enveloped and non-enveloped waterborne viruses. Food Environ. Virol. 2019;11(2):184–192. doi: 10.1007/s12560-019-09378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A., Guix S., Sano D., Pinto R.M. New tools for the study and direct surveillance of viral pathogens in water. Curr. Opin. Biotechnol. 2008;19(3):295–301. doi: 10.1016/j.copbio.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byung-joon K. Yonhap News Agency; 2020. N. Korea to Examine Drinking Water Sources to Ward off Coronavirus.https://en.yna.co.kr/view/AEN20200206001551325 6 Feb 2020. [Google Scholar]
- Casanova L.M., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.M., Weaver S.R. Inactivation of an enveloped surrogate virus in human sewage. Environ. Sci. Technol. Lett. 2015;2(3):76–78. doi: 10.1021/acs.estlett.5b00029. [DOI] [Google Scholar]
- Chan J.F., Lau S.K., To K.K., Cheng V.C., Woo P.C., Yuen K.Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen E., Myrmel M. Coagulant residues’ influence on virus enumeration as shown in a study on virus removal using aluminium, zirconium and chitosan. J. Water Health. 2018;6(4):600–613. doi: 10.2166/wh.2018.028. [DOI] [PubMed] [Google Scholar]
- Coker R., Rushton J., Mounier-Jack S., Karimuribo E., Lutumba P., Kambarage D., Pfeiffer D.U., Stärk K., Rweyemamu M. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect. Dis. 2011;11(4):326–331. doi: 10.1016/S1473-3099(10)70312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi S.R., Borchardt M.A., Spencer S.K., Hughes P.E., Baldwin A.K. Human and bovine viruses in the Milwaukee River watershed: hydrologically relevant representation and relations with environmental variables. Sci. Total Environ. 2014;490:849–860. doi: 10.1016/j.scitotenv.2014.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire J.B., Brown E.G. Isolation of animal viruses from farm livestock waste, soil and water. J. Hyg. 1978;81(2):295–302. doi: 10.1017/s0022172400025134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco M., Baker M.L., Daszak P., De Barro P., Eskew E.A., Godde C.M., Harwood T.D., Herrero M., Hoskins A.J., Johnson E., Karesh W.B., Machalaba C., Garcia J.N., Paini D., Pirzl R., Smith M.S., Zambrana-Torrelio C., Ferrier S. Opinion: sustainable development must account for pandemic risk. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117(8):3888–3892. doi: 10.1073/pnas.2001655117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Seilmaier M., Corman V.M., Hartmann W., Scheible G., Sack S., Guggemos W., Kallies R., Muth D., Junglen S., Müller M.A., Haas W., Guberina H., Röhnisch T., Schmid-Wendtner M., Aldabbagh S., Dittmer U., Gold H., Graf P., Bonin F., Rambaut A., Wendtner C.M. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect. Dis. 2013;13(9):745–751. doi: 10.1016/S1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.M., Zhao X.S., Wen R.F., Huang J.J., Pi G.H., Zhang S.X., Han J., Bi S.L., Ruan L., Dong X.P. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003;16:246–255. [PubMed] [Google Scholar]
- Fan C., Hu Z., Riley L.K., Purdy G.A., Mustapha A., Lin M. Detecting food- and waterborne viruses by surface-enhanced Raman spectroscopy. J. Food Sci. 2010;75(5):M302–M307. doi: 10.1111/j.1750-3841.2010.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1(1):10–14. [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J. Washington state 2019-nCoV case investigation team. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Zhuang H., Zhao C., Dong Q., Peng G., Dwyer D.E. Using patient collected clinical samples and sera to detect and quantify the severe acute respiratory syndrome coronavirus (SARS-cov) Virol. J. 2007;4:32. doi: 10.1186/1743-422X-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D.L., Shindo N. COVID-19: what is next for public health? Lancet. 2020. [DOI] [PMC free article] [PubMed]
- Ictv . In: Virus Taxonomy. Classification and Nomenclature of Viruses. 9th Report of the International Committee on Taxonomy of Viruses (ICTV) King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. International Union of Microbiological Societies Virology Division; 2012. 2012. [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Fratini M., della Libera S., Iaconelli M., Muscillo M. Emerging and potentially emerging viruses in water environments. Ann. Ist. Super Sanita. 2012;48(4):397–406. doi: 10.4415/ANN_12_04_07. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Rossi P. Coronavirus. Acqua di rubinetto sicura. Nessun rischio neanche dai sistemi fognari. 2020. https://www.quotidianosanita.it/scienza-e-farmaci/articolo.php?articolo_id=82163 Quotidiano Sanità online. 5 March 2020. Available at: article in Italian)
- Medema G., Heijnen L., Elsinga G., Italiaander R. Presence of SARS-Coronavirus-2 in sewage. 2020. medRxiv. [DOI] [PubMed]
- Morse S.S., Mazet J.A.K., Woolhouse M., Parrish C.R., Carroll D., Karesh W.B., Zambrana-Torrelio C., Lipkin W.I., Daszak P. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis L., Saif L.J., Zhang Y., Zhang X., Azevedo M.S. Stability of bovine coronavirus on lettuce surfaces under household refrigeration conditions. Food Microbiol. 2013;30(1):180–186. doi: 10.1016/j.fm.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Surya K., Wiegand T., Buyukyoruk M., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. 2020. medRxiv. [DOI] [PMC free article] [PubMed]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. J. Am. Med. Assoc. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrich A., Mahony J., Chong S., Broukhanski G., Gharabaghi F., Johnson G., Louie L., Luinstra K., Willey B., Akhaven P., Chui L., Jamieson F. Multicenter comparison of nucleic acid extraction methods for detection of severe acute respiratory syndrome coronavirus RNA in stool specimens. J. Clin. Microbiol. 2006;44:2681–2688. doi: 10.1128/JCM.02460-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon L.L., Chan K.H., Wong O.K., Cheung T.K., Ng I., Zheng B., Seto W.H., Yuen K.Y., Guan Y., Peiris J.S. Detection of SARS coronavirus in patients with severe acute respiratory syndrome by conventional and real-time quantitative reverse transcription-PCR assays. Clin. Chem. 2004;50(1):67–72. doi: 10.1373/clinchem.2003.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R., Monteiro S. Epidemiology, control, and prevention of emerging zoonotic viruses. In: Cook Nigel., editor. Viruses in Food and Water. Risk Surveillance and Control. Woodhead Publishing Limited; 2013. [DOI] [Google Scholar]
- Schwarte K.A., Russell J.R., Kovar J.L., Morrical D.G., Ensley S.M., Yoon K.J., Cornick N.A., Cho Y.I. Grazing management effects on sediment, phosphorus, and pathogen loading of streams in cool-season grass pastures. J. Environ. Qual. 2011;40(4):1303–1313. doi: 10.2134/jeq2010.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Wu X.M., Xiao W.J., Zhu X.M., Gu C.Q., Yin J., Wei W., Yao W., Liu C., Li J.F., Ou G.R., Wang M.N., Fang T.Y., Wang G.J., Qiu Y.H., Wu H.H., Chao F.H., Li J.W. Excretion and detection of SARS coronavirus and its nucleic acid from digestive system. World J. Gastroenterol. 2005;11(28):4390–4395. doi: 10.3748/wjg.v11.i28.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Guo T.K., Zhen B., Kong Q.X., Yi B., Li Z., Song N., Jin M., Xiao W.J. Concentration and detection of SARS coronavirus in sewage from Xiao Tang Shan hospital and the 309th hospital. J. Virol. Methods. 2005;128:156–161. doi: 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y., Guo B.Z., Liu C., Ou G.R., Wang M.N., Fang T.Y., Chao F.H., Li J.W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.04.024. (in press). Available online 17 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. Report details lessons from SARS outbreak. Lancet. 2003;362(9391):1207. doi: 10.1016/S0140-6736(03)14561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci.: Water Res. Technol. 2015;1:735. [Google Scholar]
- Who Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it Available at: (last accessed 15 March 2020)
- Who WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available at: (last accessed 15 March 2020)
- Who . 2020. WHO Technical Brief: Water, Sanitation, Hygiene and Waste Management for COVID-19. WHO reference number: WHO/2019-NcOV/IPC_WASH/2020.1. [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Moniz K., Erickson T., Chai P., Thompson J., Alm E. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. 2020. medRxiv. [DOI] [PMC free article] [PubMed]
- Wurtzer S., Marechal V., Mouchel J.M., Moulin L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. 2020. medRxiv. [DOI]
- Yam W.C., Chan K.H., Poon L.L., Guan Y., Yuen K.Y., Seto W.H., Peiris J.S. Evaluation of reverse transcription-PCR assays for rapid diagnosis of severe acute respiratory syndrome associated with a novel coronavirus. J. Clin. Microbiol. 2003;41(10):4521–4524. doi: 10.1128/JCM.41.10.4521-4524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yépiz-Gómez M.S., Gerba C.P., Bright K.R. Survival of respiratory viruses on fresh produce. Food Environ Virol. 2013;5:150–156. doi: 10.1007/s12560-013-9114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T.S., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H.W., Leung D.Y.C., Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen C., Zhu S., Shu C., Wang D., Song J. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2(8):123–124. [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li C., Zhao G. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017:3. doi: 10.1126/sciadv.aao4966. eaao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]