Abstract
Background/Objectives
Abdominal pain is one of the known symptoms associated with coronavirus disease 2019. Little is known about the development of acute pancreatitis as a complication of severe acute respiratory syndrome coronavirus 2 infection. This case report describes the presentation of acute pancreatitis in two of three family members with severe COVID-19 infection.
Methods
Data were collected from three family members admitted with COVID-19 to the intensive care unit in March 2020. This study was reviewed and approved by the local data and ethics committee (31-1521-253).
Results
Two of the three family members were diagnosed with acute pancreatitis associated with SARS-CoV-2. Other causes of acute pancreatitis were excluded for both patients (including alcohol, biliary obstruction/gall stones, drugs, trauma, hypertriglyceridemia, hypercalcemia, and hypotension).
Conclusions
These cases highlight acute pancreatitis as a complication associated with COVID-19 and underlines the importance of measuring pancreas-specific plasma amylase in patients with COVID-19 and abdominal pain.
Keywords: Acute pancreatitis, COVID-19, SARS-CoV-2, Severe acute pancreatitis, Viral pancreatitis
1. Introduction
The ongoing pandemic with coronavirus disease-19 (COVID-19) has reached above 2.810.000 confirmed cases worldwide [1]. The severity of the disease ranges from subclinical infections to severe illness requiring hospital admission [2,3]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections primarily affect the respiratory tract [4], but gastrointestinal symptoms such as nausea, vomiting and diarrhea also occur [5,6]. Abdominal pain is most frequent in patients who are severely ill [7]. Viral RNA has been found in fecal specimens despite negative respiratory tests [8,9] and in gastrointestinal epithelium [5] suggesting a possible fecal-oral transmission route.
Acute pancreatitis is the most frequent gastrointestinal cause of hospital admission in the United States [10]. The development of acute pancreatitis is multifactorial requiring predisposition and relevant injury [11]. The most common causes are gallstones and alcohol abuse, but viral-induced acute pancreatitis has also been described [12]. This case report describes three first-degree relatives with severe COVID-19, two of which developed severe acute pancreatitis.
2. Methods
Data were collected from three family members admitted with COVID-19 to the Intensive Care Unit at Copenhagen University Hospital Hvidovre in the Capital Region of Denmark in March 2020. Acute pancreatitis was diagnosed according to the Atlanta classification.15 Throat swab samples and tracheal aspirates were collected from all patients and SARS-CoV-2 was detected using real time transcription–polymerase chain reaction assay. Blood samples and diagnostic imaging were performed according to the best clinical practice. This study was reviewed and approved by the local data and ethics committee (31-1521-253).
3. Results
Two of the three family members were diagnosed with acute pancreatitis associated with SARS-CoV-2. Other causes of acute pancreatitis were excluded for both patients (including alcohol, biliary obstruction/gall stones, drugs, trauma, hypertriglyceridemia, hypercalcemia, and hypotension).
Case 1
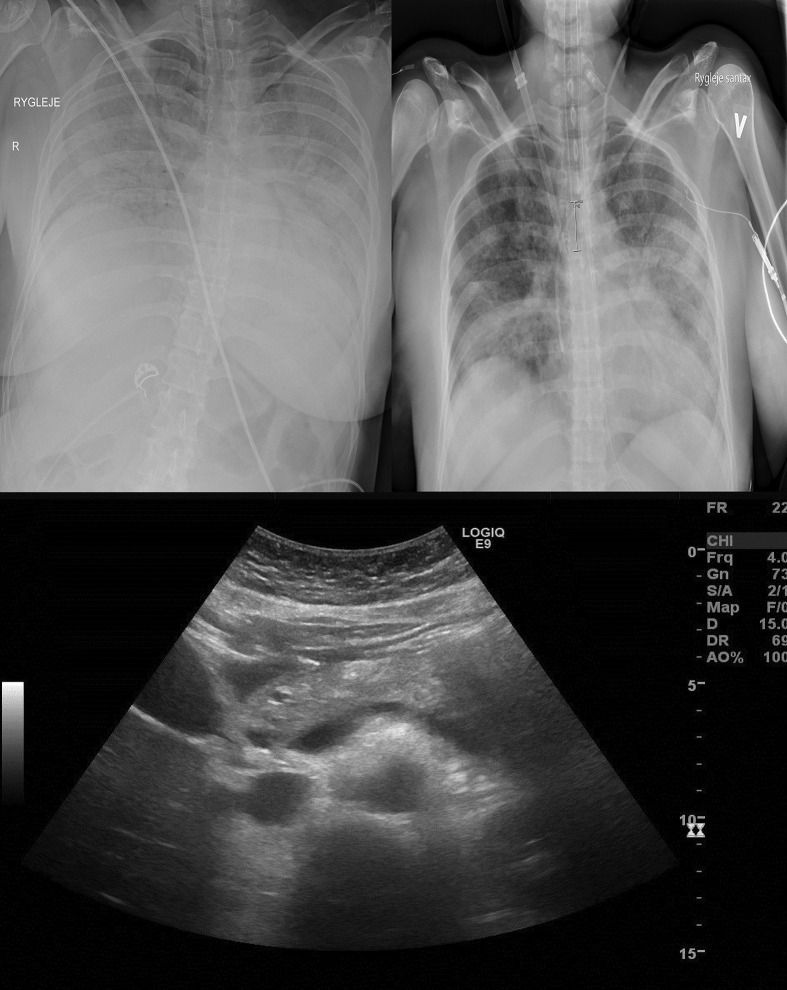
(daughter): A 47-year old previously healthy woman was admitted to the emergency department at our hospital with a fever, headache and neck pain for one week and anorexia, sore throat and dyspnea for a couple of days. The patient had a minimal alcohol intake and did not smoke. At admission, the patient was awake and hemodynamically stable with a mean arterial pressure of 93 mmHg. The patient showed signs of acute respiratory distress with a respiratory rate of 40/min, an oxygen-saturation (SaO2) of 80% on 15L of oxygen/min requiring intubation and mechanical ventilation. A chest X-ray showed bilateral massive opacities with air bronchograms (Fig. 1 ). The patient tested positive for SARS-CoV-2 RNA. The patient developed acute kidney failure and continuous veno-venous hemodialysis (CVVHD) was initiated at day two. The respiratory changes worsened over the next two weeks and the chest X-ray showed increasing perihilar involvement and consolidation. Fifteen days after admission, severe hypercapnia (pCO2> 8,5 kPa) was resolved with extracorporeal membrane oxygenation, which was maintained for three days. Tracheostomy was performed at day 25 as clinical progress was observed. Treatments included fluid resuscitation and intravenous antibiotics.
At admission, pancreas-specific plasma amylase was increased to 173 U/L. The value increased rapidly to >1500 U/L after 11 h (Fig. 2 ). Abdominal ultrasound showed evidence of acute pancreatitis with a diffusely voluminous pancreas without focal lesions or gallstones (Fig. 1). The Modified Glasgow Acute Pancreatitis Score was 5 points indicating severe acute pancreatitis. Plasma levels of triglycerides and calcium were normal. The amylase decreased from day four and reached the normal range at day 21. As of last update, the patient is still in intensive care.
Case 2
(mother): A 68-year old woman who was admitted to the Gastrounit at our hospital two days after Case 1. Comorbidities included hypertension, hypothyroidism and osteoporosis treated with losartan, levothyroxine, alendronate and cyanocobalamin. The patient was admitted with epigastric pain and fever as well as vomitus, diarrhea, fatigue and polydipsia. At admission, the patient had a temperature of 39.7 °C rectally, a C-reactive protein of 77 mg/L, tachypnea (30 breaths/min), hypoxia (8.5 kPa) and hypocapnia (3.8 kPa) on 4L of oxygen/min. Initial chest radiograph showed diffuse interstitial opacities. The respiratory insufficiency progressed during the first two days and the patient was intubated and admitted to the intensive care unit for mechanical ventilation. The patient tested positive for SARS-CoV-2 RNA. On day 3, the patient developed acute kidney failure and CVVHD treatment was initiated. Renal function did not recover, and the respiratory distress continued to worsen with bilateral progression of opacities peripherally and perihilar with consolidation. Treatment included fluid resuscitation and intravenous antibiotics.
At admission, the patient experienced abdominal pain and the clinical assessment showed direct epigastric and periumbilical tenderness. Slight abdominal distension was noted at day four and remitted afterwards. The amylase-level increased from 85 U/L on the day of admission to 934 U/L on day six (Fig. 2). The Modified Glasgow Acute Pancreatitis Score was 5 points indicating severe acute pancreatitis. Calcium-levels were normal and earlier measurements of triglycerides were also normal. A daily decline of amylase was measured afterwards until normal range was reached at day 22. As of last update, the patient is still in ICU.
Case 3
(father): One week after Case 1 was admitted, a 71-year old man was admitted to the emergency department. He was previously healthy, had a very low alcohol intake and did not smoke. Three days before admission the patient experienced gastrointestinal symptoms with anorexia and diarrhea as well as a fever (38.9 °C), dry cough, general malaise and fatigue. Initially, the patient was hypoxic, but reached a normal respiratory rate of 17 breaths/min and saturation (9.7 kPa) after treatment with oxygen (8L/min). A chest X-ray showed bilateral perihilar and interstitial opacities. The respiratory problems worsened and on day three, the patient developed acute respiratory distress. The patient was transferred to the intensive care unit and received invasive mechanical ventilation and intravenous antibiotics. Due to bradycardia and hypotension, noradrenaline and dopamine was added. After three weeks, the patient developed increasing creatinine and oliguria. The outcome was fatal. The patient did not show evidence of acute pancreatitis (Fig. 2).
Fig. 1.
Diagnostic imaging of Case 1. The top panel: chest x-ray at day 1 of admission (left) and at day 17 (right). Lower panel: ultrasound image of a voluminous pancreas and no signs of gallstones in the gallbladder and bile ducts.
Fig. 2.
Evolution of plasma amylase, C-reactive protein (CRP) and white blood cell count (WBC) during admission. ∗In Case 1, pancreas-specific plasma was measured until day 12. Afterwards, only total amylase was measured due to patient transfer and thus not plotted.
4. Discussion
Previous studies have reported acute inflammation in the pancreas due human immunodeficiency virus, mumps, Cytomegalovirus, Coxsackievirus B and Influenza A (H1N1). The incidence of viral acute pancreatitis is unknown [12].
We report two first-line relatives with acute pancreatitis associated with SARS-CoV-2 infection. According to the current guidelines [13], the diagnosis of acute pancreatitis requires at least two of the three following signs: 1) abdominal pain, 2) amylase or lipase >3 times the upper normal limit, and 3) characteristic findings on diagnostic imaging. The two cases described in this paper had severe acute pancreatitis, which itself may lead to multiorgan failure including adult respiratory distress and kidney failure as seen in both patients. On the other hand, COVID-19 may also lead to same organ failures and it is therefore not possible to evaluate if the acute pancreatitis contributed to the severe course of the disease. The third family member who was admitted to the intensive care unit with COVID-19 had respiratory distress and gastrointestinal symptoms, but not acute pancreatitis. Additional two family members (not described) tested positive for SARS-CoV-2, but only had mild symptoms and did not require hospitalization. We did not identify a likely explanation for why only two of the five cases developed acute pancreatitis.
Previous studies have verified that COVID-19 may be associated with gastrointestinal symptoms including abdominal pain [[5], [6], [7]] and have identified viral RNA in the gastrointestinal tract [5,8,9]. Wang et al. reported at admission 17% of 52 patients with COVID-19 had slightly abnormal amylase or lipase [14]. Several factors may contribute to the development of acute pancreatitis including pancreatic autodigestion, enzyme activation, complement system activation, microcirculation disturbance theory, leukocyte excessive activation, and pancreatic acinar cell apoptosis and necrosis. Theoretically viral pancreatitis develops due to direct destruction of pancreatic acinar cells by inflammation and edema. Alternatively, damage to the pancreatic acinar cells by the virus could lead to a leaking intracellular enzyme or precipitates a process of cell death. The entry receptor Angiotensin-converting enzyme 2 (ACE2) for SARS-CoV-2 has been identified in the gastrointestinal epithelium of infected cases. During the 2002–2004 outbreak, SARS-CoV used ACE2 for entry to host cells. Increased expression in pancreatic islets were observed, leading eventually to acute diabetes [15]. Genome sequences of SARS-CoV have shown that 79.6% are shared with SARS-CoV-2 [6]. The expression of ACE2 in the pancreas during SARS-CoV-2 infection could therefore lead to acute inflammation.
Nevertheless, the presented cases underline the importance of measuring lipase or amylase in patients with SARS-CoV-2 infections, especially if patients experience abdominal pain.
Author contributions
Dr Hadi, Amer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Hadi, Amer, Werge, Mikkel P., Gluud, Lise L., and Novovic, S contributed equally. Concept and design: Dr Gluud, Lise L. Acquisition, statistical analysis, or interpretation of data: Drs Hadi, Amer, Werge, Mikkel P., Gluud, Lise L, and Novovic S. Drafting of the manuscript: Dr Hadi, Amer. Critical revision of the manuscript for important intellectual content: all authors.
Funding/support
This work did not receive financial support.
Declaration of competing interest
None reported.
References
- 1.Johns Hopkins C.S.S.E. Coronavirus COVID-19 (2019-nCoV). Coronavirus COVID-19 glob cases by. Johns Hopkins CSSE. 2020:1. [Google Scholar]
- 2.Zhu J., Ji P., Pang J., Zhong Z., Li H., He C., et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nobel Y.R., Phipps M., Zucker J., Lebwohl B., Wang T.C., Sobieszczyk M.E., et al. Gastrointestinal symptoms and COVID-19: case-control study from the United States. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li L., Huang T., Wang Y., Wang Z., Liang Y., Huang T., et al. Novel coronavirus patients’ clinical characteristics, discharge rate and fatality rate of meta-analysis. J Med Virol. 2019 doi: 10.1002/jmv.25757. 2020:jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong S.H., Lui R.N., Sung J.J. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15047. 0–3. [DOI] [PubMed] [Google Scholar]
- 7.Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;1–9 doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amico F.D., Baumgart D.C., Danese S., Peyrin-biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peery A.F., Dellon E.S., Lund J., Crockett S.D., McGowan C.E., Bulsiewicz W.J., et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:313. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Testoni P.A. Acute recurrent pancreatitis: etiopathogenesis, diagnosis and treatment. World J Gastroenterol. 2014;20:16891–16901. doi: 10.3748/wjg.v20.i45.16891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lankisch P.G., Apte M., Banks P.A. Supplementary appendix: acute pancreatitis. Lancet. 2015;6736:1–32. doi: 10.1016/S0140-6736(14)60649-8. [DOI] [PubMed] [Google Scholar]
- 13.Banks P.A., Bollen T.L., Dervenis C., Gooszen H.G., Johnson C.D., Sarr M.G., et al. Classification of acute pancreatitis - 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 14.Wang F., Wang H., Fan J., Zhang Y., Wang H., Zhao Q. Pancreatic injury patterns in patients with COVID-19 pneumonia. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]