Abstract
Food packaging materials are commonly derived from petroleum that increases global contamination; this raises the interest to evaluate raw material from renewable sources such as whey protein for the development of packaging materials, especially to produce active films. This research aimed to evaluate whey protein-based film properties when natamycin, nanoemulsioned α-tocopherol, or both were added. An oil-in-water (O/W) nanoemulsion of antioxidant (α-tocopherol) was prepared by microfluidization technique. Four films were prepared with different levels of natamycin and nanoemulsified α-tocopherol and were characterized in terms of physicochemical, mechanical, optical-properties, water vapor barrier, FTIR, microstructure, antioxidant and antimicrobial activity. The natamycin, nanoemulsified α-tocopherol, or both did not modify the moisture content of the films. Moreover lead to a significant reduction of tensile strength and elastic modulus, while presenting growth in the elongation at break. Film opacity, the total color difference, the UV-Vis light barrier, and the water vapor permeability values increased when compounds were incorporated into the film. The microstructure studies showed uniformly distributed porosity throughout the films. The addition of nanoemulsioned α-tocopherol into whey protein-based films provoked antioxidant activity and the addition of natamycin produced films with effectivity against C. albicans, P. chrysogenum, and S. cerevisiae, allowing develop a material appropriate for use as active food packaging.
Keywords: Food science, Food technology, Materials science, Nanotechnology, Nanoemulsion, Whey protein-based films, Active packaging, α-tocopherol, Natamycin
Food Science, Food Technology, Materials Science, Nanotechnology, Nanoemulsion, Whey protein-based films, Active Packaging, α-tocopherol, Natamycin.
1. Introduction
Up to 2015, around 6300 million tons of plastic waste has been produced, 79% are discarded, 12% are burned, and only 9% are recycled (Zhao et al., 2018). Landfill and incineration are traditional methods to handle plastic waste leading to a negative environmental impact (Khalil et al., 2016). This context has led to the implementation of laws on the reasonable use of plastic materials, as in the case of Europe regarding the reduction of usage of light plastic bags with the Directive (EU) 2015/720. The growing worldwide problem of the contamination of petroleum-based plastics and the need to generate new materials as alternatives for food packaging have opened a field of research for the development of biodegradable containers from renewable sources (Dammak et al., 2017).
An alternative to petroleum-based plastic could be materials based on biopolymers derived from animal sources such as collagen (Cazón et al., 2017), gelatin (Dammak et al., 2017), chitosan (Ahmed and Ikram., 2016; Cazón et al., 2017), chitosan and gelatin (Bonilla and Sobral., 2016; Pérez-Córdoba et al., 2018), whey proteins isolated and concentrated whey (Ghadetaj et al., 2018; Schmid et al., 2017); or from vegetal sources, such as Zein (Oymaci and Altinkaya., 2016), cellulose (Lavoine et al., 2016), starch (Ollé-Resa, Gerschenson, and Jagus., 2014a, 2016), and gluten (Fajardo et al., 2010), among others.
The whey proteins have been of great interest because, at present, the world manufacture of cheese whey annually is around 1.8–1.9 × 108 tons (Zhou et al., 2019). The predominant proteins in whey are α-lactalbumin and β-lactoglobulin, with a high nutritional value representing about 50% and 20% of the total protein content, these proteins can form emulsions, foams, gels, and a high potential to form biopolymer-based materials for food applications (Ghadetaj et al., 2018; Ozer et al., 2016; Schmid et al., 2018; Shokri and Ehsani, 2017). The packaging materials made with these proteins are also edible and biodegradable, presenting an excellent barrier to gases compared with conventional plastics (Oymaci and Altinkaya., 2016). Moreover, these materials can serve as vehicles of bioactive compounds allowing the development of active food packaging (Atares and Chiralt., 2016).
Antimicrobial substances have been studied as fungicides, natural extracts, enzymes, bacteriocins, and organic acids for their application in biodegradable films (Altenhofen et al., 2012; Pintado et al., 2010). A cause of food spoiling corresponds to the growth of microorganisms, such as molds and yeasts, which compromise food stability and threaten consumer safety because they can produce mycotoxins (Moatsou et al., 2015). Natamycin is a natural additive obtained by the fermentation of the bacterium Streptomyces natalensis; it is a natural antifungal agent and is widely used to prevent contamination by yeasts and molds in different food products. Natamycin has advantages as an additive because it does not have color, odor, and taste; also, the Food and Drug Administration has considered natamycin as a recognized product as safe and the European Union as a natural preservative (E235). This fungicide of the polyene macrolide group acts on the ergosterol of the cell membrane and can be used for cheese surface treatment (Ramos et al., 2012; Ollé-Resa, Gerschenson, and Jagus., 2014a; Moatsou et al., 2015).
The lipid oxidation is another cause of food deterioration and has effects on bad odors, color loss, harmful compounds, and the development of off-flavors. The α-tocopherol is generally accepted as a soluble lipid antioxidant it is recommended for use in foods containing fats, oils and has been used in edible films by Martins et al. (2012) in chitosan, Martelli et al. (2017) in carboxymethyl cellulose, Noronha et al. (2014) in methylcellulose, Shokri and Ehsani (2017) in whey protein concentrate and Bonilla and Sobral (2016); Pérez-Córdoba et al. (2017, 2018) in gelatin-chitosan.
Tocopherols were cataloged as substances generally GRAS for food products by the Code of Federal Regulations; hence the use of α-tocopherol as an antioxidant natural has increased the interest in protecting and extending the shelf life in food (Granda-Restrepo et al., 2009; Shokri and Ehsani, 2017). Although α-tocopherol is poorly soluble in aqueous systems and very hard to integrate within film-forming, which are aqueous or hydrophilic systems, incorporation of α-tocopherol in the biopolymer matrix is facilitated by emulsions or nanoemulsions (Pérez-Córdoba et al., 2018). For this reason, there is an opportunity to incorporate the active compounds of natural origin and recognized as GRAS into the packaging material (FDA, 2001), so that from the packaging, they exert their function as antioxidants and antifungals.
The primary objective was to evaluate the effect of the inclusion of natamycin, nanoemulsioned α-tocopherol, and a mixture of them on chemical, physical, mechanical properties, antioxidant, and antimicrobial activity of whey protein-based films.
2. Materials and methods
2.1. Raw materials and reagents
The following materials were used: Whey protein concentrate (WPC, 80% protein) obtained from Ingredientes y Productos Functionales (IPF, Colombia), Natamycin (NAT), containing 50% lactose and 50% natamycin (ZSEB-E Co. Ltd, China), α-tocopherol (α-TOC) (97% Sigma-Aldrich), glycerol (Gly, J.T.Baker, Germany) used for film flexibility as a plasticizer, polysorbate 80 (Tween® 80, Sigma-Aldrich), sorbitan monoester (Span® 60, Sigma-Aldrich) as surfactants. Potassium persulfate, ABTS•+: 2.2′-Azino-bis (3-ethylbenzenothiazoline6- sulfonic acid), Trolox: 6-hydroxy-2,5,7,8- tetramethylchroman-2-carboxylic acid, chloride acid, ethanol, methanol, iron trichloride, acetate buffer, TPTZ: 2,4,6-Tri(2-pyridyl)-s-triazine and DPPH: 2,2-diphenyl-1-picrylhydrazyl of Sigma-Aldrich. The microorganisms used for testing were C. albicans ATCC 10231, Saccharomyces cerevisiae ATCC 2601, and Penicillium chrysogenum ATCC 10106 provided by Scorpionism Ofidism research group of University of Antioquia, the culture media used for fungi and yeasts were Sabouraud agar and Sabouraud broth (Merk, Germany).
2.2. Preparation of nanoemulsion of tocopherol
An oil-in-water (O/W) nanoemulsion was prepared at 1.29% (w/w) α-TOC, by spontaneous emulsification followed by microfluidization, according to Bouchemal et al. (2004), Kaur et al. (2016), and Pérez-Córdoba et al. (2018), respectively. Two phases were prepared separately; an aqueous phase composed of 97.6% weight/weight (w/w) of distilled water and 0.30% (w/w) Tween 80 mixed at 25 °C/10 min with a magnetic stirrer (RH basic2, IKA, Germany); and an organic phase composed of 1.29% (w/w) α-TOC, 0.19% (w/w) of Span 60 and 0.61% (w/w) of ethanol were mixed at 35 °C/10 min. Thereupon these phases were mixed and homogenized at 7000 rpm/5 min using an UltraTurrax (Ultra-Turrax® IKA T25, Labotechnik, Bodensee, Germany), achieving a hydrophilic-lipophilic balance (HLB = 11) to obtain a coarse emulsion. Finally, the nanoemulsion was obtained using a microfluidizer (M-110S, Microfluidics, USA) at 70 MPa and 3 processing cycles (Pérez-Córdoba et al., 2018).
2.3. Droplet size and stability of nanoemulsion of α-TOC
The stability and mean droplet size were measured using a multi-sample analytical centrifuge LUMiSizer® LS 610 (LUM GmbH, Berlin, Germany) at 20 °C and 865 nm. Nanoemulsion was centrifuged at 4000 rpm (2325.4 × g) for approximately 2 h (Dammak et al., 2017).
2.4. Films preparation
Four film-forming solutions (FFS) were formulated, as shown in Table 1. The FFS was prepared under continuous stirring dissolving WPC powder in distilled water, natamycin, nanoemulsioned α-TOC, or both and then glycerol added as a plasticizer, the pH of FFS was adjusted to 7 with 0.1 M NaOH (Ramos et al., 2013). Then, FFS was heated to 90 °C/20 min in a water bath (Marconi®, MA-184, SP, Brazil) and homogenized at 7000 rpm (Bonilla and Sobral., 2016).
Table 1.
Formulation of FFS for WPC films loaded with active compounds. F0: Film without active compounds (Control film); F1: 2% α-TOC – 0 ppm NAT; F2: 0% α-TOC – 300 ppm NAT; F3: 2% α-TOC – 300 ppm NAT.
| Film | Component | F0 | F1 | F2 | F3 |
|---|---|---|---|---|---|
| Composition | α-TOC (%) | 0 | 2 | 0 | 2 |
| NAT (ppm) | 0 | 0 | 300 | 300 | |
| Formulation of FFS (% w/w) | WPC | 10.0 | 10.0 | 10.0 | 10.0 |
| Distilled water | 85.0 | 52.5 | 85.0 | 52.5 | |
| Glycerol | 5.0 | 5.0 | 5.0 | 5.0 | |
| Nanoemulsion | 0 | 32.5 | 0 | 32.5 | |
| NAT | 0 | 0 | 0.0123 | 0.0123 |
Films were made by the solvent casting method in polystyrene Petri dishes (14 cm), dried at 30 °C/12 h in an oven (Marconis MA035/5, SP, Brazil). The films were conditioned in chambers carrying saturated solutions of NaBr (RH ≈ 58% and water activity, aw ≈ 0.575) at 25 °C/7 days before all analyses. All characterizations were carried out in triplicates, at least.
2.5. Films characterization
2.5.1. Thickness
Film were measured using a digital micrometer (Mitutoyo IP65 Quantumike, Japan), at 10 random positions.
2.5.2. Moisture content
Moisture content (MC) (% w/w) was determined gravimetrically, following the methodology described by Ghadetaj et al. (2018), and Pérez-Córdoba et al. (2018).
2.5.3. Solubility in water
The solubility in water (S) (% w/w) was measured in discs of 20 mm diameter, which were weighed and shaken (60 rpm) with 50 mL of distilled water at 24 °C/24 h (Shaker SOLAB-SL223, Sao Paulo). The samples were removed from the distilled water and dried at 105 °C/24 h to know the final weight of dry matter (Pérez-Córdoba et al., 2018; Schmid et al., 2017).
2.5.4. Mechanical properties
The mechanical properties of films (ten samples sheets from each film) were measured determining EB: elongation at break, TS: tensile strength, and EM: elastic modulus by the ASTM D882/12 (ASTM, 2001) standard method using a texture analyzer (TA.XT2i, Stable Micro System, UK).
2.5.5. Optical properties (Color, opacity and UV-Vis light barrier)
The color values of films were determined using a colorimeter MiniScan MSEZ 1049 (HunterlLab, Reston, VA, USA) with a measuring cell with an opening of 30 cm, D65 (daylight) lamp, angle of 10° (Bonilla and Sobral., 2016). The coordinates CIELab, L∗ (lightness), a∗(red-green), and b∗(yellow-blue) were obtained, allowing the calculation of the total color difference (ΔE∗) using Eq. (1) (Dammak et al., 2017; Ghadetaj2018).
| (1) |
Where
Moreover, film opacity was measured following the Hunterlab method in the reflectance mode, using the same equipment for the color analysis (Bonilla and Sobral., 2016).
The UV-Vis light barrier was measured as transmittance (%) using a UV-Vis spectrophotometer LAMBDA 35 (Perkin Elmer, Waltham, MA, USA) in wavelength range from 200 to 900 nm (Dammak et al., 2017; Ramos et al., 2012).
2.5.6. Water vapor permeability
Water vapor permeability (WVP) (g.m−1.s−1.Pa−1) was obtained gravimetrically at 25 °C, according to the method reported by Pérez-Córdoba et al. (2018). The aluminum cells had a permeation area of 0.0032 m2, and they contained silica gel (~0% RH). Films were disposed at the top of the cell and placed in a desiccator containing distilled water with 100% RH and were weighed for 7 days. The WVP was calculated as follow (Equation 2):
| (2) |
Where w/t (g.s−1): coefficient of the linear regression, ΔP (Pa): partial vapor pressure difference between water and the dry atmosphere (3169.1 Pa), x (mm): film thickness, and A (m2): permeation area. (Ahmed and Ikram., 2016; Bonilla and Sobral., 2016; Dammak et al., 2017; Marra et al., 2016) Three replicates per film were analyzed.
2.5.7. Microstructure: Scanning electron microscopy (SEM)
The microstructure of the cross-sections and the upper surface of the films samples were pre-conditioned using silica gel for 10 days at room temperature and were visualized by SEM, using a HITACHI Tabletop Microscope TM3000 (Hitachi Ltd, Tokyo, Japan), with a wide magnification range of 15–15,000X. The images were captured using an accelerating voltage of 15 kV.
2.5.8. Fourier-transform infrared spectroscopy (FTIR)
FTIR spectroscopy was used to analyze the chain interactions into films, the spectra of the films were obtained in transmittance mode from 650 to 4000 cm−1 at a resolution of 1 cm−1, using a spectrometer (FTIR-ATR Spectrum One, Perkin Elmer) equipped with the Spectrum One software (Bonilla and Sobral., 2016; Lara et al., 2019).
2.5.9. Antioxidant properties
2.5.9.1. Sample preparation
The solutions containing α-tocopherol were prepared by the extraction method described by Contreras-Calderón et al. (2011) with slight modifications; 0.1 g from each film were placed with 6 g of methanol-water (50:50, v/v), and were mixed in a vortex (BOECO, Germany) for 1 min, after that, the samples were shaken 1 h at 25 °C in a shaker (Heidolph Unimax 1010, Germany). The samples were centrifuged at 6000 rpm/10 min (BOECO C-28A, Germany), and the supernatant was recovery. The process was repeated but adding 6 g of acetone-water (60:40, v/v) to the residue, finally, it was adjusted up to 25 g with deionized water.
2.5.9.2. ABTS•+ method
The ABTS free radical scavenging method was done according to previous works (Bonilla and Sobral., 2016; Contreras-Calderón et al., 2011; Dammak et al., 2017; López de Dicastillo et al., 2016; Pérez-Córdoba et al., 2018; Re et al., 1999). A solution of ABTS•+ was prepared (1:1) with potassium persulfate (2.45 mM) and ABTS (7 mM), and kept for 16 h in the dark. Later, to prepare the ABTS•+ working solution, an aliquot of ABTS•+ solution was diluted with ethanol to reach an absorbance of 0.70 ± 0.02, using a UV-Vis spectrophotometer at 730 nm. Aqueous solutions of Trolox from o to 500 μM were used for calibration. 100 μL of film extraction, diluted with Trolox standard or water was added to 1 mL of ABTS•+ working solution and hatced 30 min at 30 °C.
2.5.9.3. DPPH• method
Scavenging of DPPH• radical was measured using a modified Alam et al. (2013) method. 0.2 mL of film extraction and 2 mL of methanol was mixed, after that, 2 mL of DPPH• radical solution (120 μM) was added, and kept in the dark 30 min. Subsequently, the absorbance was determined at 515 nm using a UV-Vis spectrophotometer (Mapada-UV330 Shanghai, China).
2.5.9.4. FRAP assay
The FRAP assay was made following Contreras-Calderon et al. (2011). FRAP reagent was made with 2.5 mL of FeCl3 (20 mM), 25 mL acetate buffer 0.3 M at pH 3.6, and 2.5 mL of TPTZ (prepared in 40 mM HCl). After that, 30 μL of film extract or Trolox was mixed with 90 μL distilled water, 900 μL of FRAP, and incubated at 35 °C/30 min. Absorbance values at 595 nm were measured using a UV-Vis spectrophotometer (Mapada-UV330 Shanghai, China), and for calibration curve was used solutions from 0 to 500 μM of Trolox. The results for all antioxidant properties were expressed as Trolox Equivalent Antioxidant Capacity TEAC (μmol/g films).
2.5.10. Antimicrobial activity
2.5.10.1. Growth conditions
Candida albicans and Saccharomyces cerevisiae were grown in 100 mL Sabouraud broth at 37 °C/12 h in a continuously agitated shaker until its exponential growth phase. Penicillium chrysogenum was grown in inclined agar tubes using 50 mL Sabouraud agar at 37 °C until sporulation was reached. On the day of the antimicrobial test, the spores were scraped off with sterile water (Ciro2014; Ollé Resa, Gerschenson and Jagus., 2014a).
2.5.10.2. Agar diffusion method
The antimicrobial effect of films on the test microorganisms was evaluated by the agar diffusion method. 100 μL of inoculum with turbidity equivalent to a McFarland 0.5 (using a spectrophotometer at 625nm) containing 1.5 × 108 CFU/mL of each microorganism, C. albicans, S. cerevisiae, and P. chrysogenum were prepared and spread on the surface of Petri dishes carrying Sabouraud agar (Bonilla and Sobral., 2016; Ciro2014).
Discs of 7 mm diameter were cut from treatments F0 (negative control), F2, F3 and positive control (discs from filter paper soaked with 300 ppm NAT) were placed on each plate previously inoculated, after that all plates are incubated at 25 °C/24 h (Ollé Resa, Gerschenson and Jagus., 2014a). The zone of inhibition (mm2) was calculated from the area of the whole zone subtracted from the disc area.
2.5.11. Statistical analysis
Statistical analyses of data were performed through a variance analysis (ANOVA) employment the Statgraphics® centurion XV software. The differences were tested using Duncan's multiple range test at p < 0.05.
3. Results and discussion
3.1. Characterization of nanoemulsion
It is necessary to ensure the quality of the nanoemulsion of α-TOC production before applying it to WPC film to guarantee that it will be stable during film processing. In this sense, the evaluation of the mean droplet size and nanoemulsion stability is indispensable. Then, the nanoemulsion presented a monomodal distribution, with an average diameter of 113.50 ± 1.6 nm. Pérez-Córdoba et al. (2018) reported similar results about the mean droplet size for nanoemulsions of α-tocopherol/cinnamaldehyde, and α-tocopherol/garlic oil, 123.1 ± 1.5 nm, 111.0 ± 2.0 nm respectively. The experimental results of the stability showed that stability values for nanoemulsion correspond to 0.588 for the first month, and 0.481 a second month. Values less than 0.5 indicate a phase separation and instability of nanoemulsions (Dammak2019).
3.2. Film characterization
All WPC films were transparent, with no apparent defects by naked eye observation such as bubbles, scratches, phase separation, or cracks. The thickness was maintained in all films (Table 2), and no statistical difference was observed between data (p = 0.30), allowing to calculate an average thickness of 0.126 ± 0.021 mm, comparable to those reported by Ramos et al. (2013) with a thickness of 0.13 ± 0.04 mm for films produced with film-forming solution with the same protein concentration. Therefore, the addition of active compounds did not modify this property.
Table 2.
Physicochemical and mechanical properties of WPC films incorporated with NAT or nanoemulsions of α-TOC, or both. F0: Film without active compounds (Control film); F1: 2% α-TOC – 0 ppm NAT; F2: 0% α-TOC – 300 ppm NAT; F3: 2% α-TOC – 300 ppm NAT.
| Film |
Thickness |
Moisture |
Solubility in |
TS |
EB |
EM |
|---|---|---|---|---|---|---|
| (mm) |
content (%) |
water (%) |
(MPa) |
(%) |
(MPa) |
|
| n | 10 | 3 | 3 | 10 | 10 | 10 |
| F0 | 0.122 ± 0.017a | 28.3 ± 2.3a | 58.9 ± 0.7a | 10.8 ± 0.6a | 29.4 ± 10.2a | 2.4 ± 0.4a |
| F1 | 0.119 ± 0.037a | 28.3 ± 2.0a | 55.4 ± 1.1b | 3.3 ± 0.6b | 48.6 ± 14.9b | 0.8 ± 0.2b |
| F2 | 0.124 ± 0.010a | 31.3 ± 5.7b | 58.8 ± 0.8a | 6.4 ± 0.9c | 45.6 ± 9.2b | 1.5 ± 0.3c |
| F3 | 0.139 ± 0.020a | 29.5 ± 3.2a | 55.3 ± 1.5b | 5.9 ± 0.7c | 46.4 ± 11.5b | 1.3 ± 0.2c |
Mean values ± standard deviation. Different letters in the same column indicate significant differences (p < 0.05) among the average values obtained by application of Duncans test. TS: Tensile strength; EB: Elongation at break; EM: Elastic modulus.
3.2.1. Moisture content (MC) and solubility (S) in water
Table 2 presented the values obtained for S and MC for WPC films. No significant difference (p = 0.72) was observed for MC of all films, allowing calculation of an average value of 28.6 g of water/100 g of dry matter. Neither NAT, nanoemulsion of α-TOC, or both did not modify the hygroscopicity, as Pérez-Córdoba et al. (2018) showed in chitosan-gelatin films. These results are interesting because eventual differences in MC could affect the results of some physical properties owing to the plasticizing effect of water.
Regarding film solubility in water, it was observed that F1 and F3 showed slightly lower S (p < 0.05) than the F0 and F2, whereas no significant difference (p > 0.05) was observed between these last two films. According to these results, the presence of the nanoemulsioned α-TOC contributed to reducing the solubility of the film, and all results of S showed that WPC films maintain their integrity after 24 h in water, due to the establishment of disulfide bonds between protein molecules generated after the heat treatment (Ramos et al., 2013).
3.2.2. Mechanical properties
The incorporation of active compounds produced films (F1, F2, F3) with lower TS and EM, which means, less resistant and stiff films, and higher EB, or more stretchable one, than F0 (p < 0.05) (Table 2). Nevertheless, NAT was the active component that provoked the less considerable changes in TS and EM of films (F2, F3). Thus, the film (F1) with only nanoemulsioned α-tocopherol presented the lower values of TS (p < 0.05) and EM. Interestingly, this behavior was not observed for EB, where all films (F1, F2, F3) presented similar values (p > 0.05). Apparently, the active compounds seem to present a plasticizer effect on films.
TS values significantly decreased from 10.8 ± 0.6 (F0) to 3.3 ± 0.6 MPa (F1) (p < 0.05) for films loaded with nanoemulsioned α-tocopherol (Table 2). These results of weaker films agree with those presented by Ramos et al. (2012) with antimicrobial agents including natamycin on WPI films, alginate/pectin films loaded with NAT Krause et al. (2012) and Noronha et al. (2014) on methylcellulose films loaded with α-tocopherol nanocapsules. Also, Martins et al. (2012) found on chitosan films that α-tocopherol incorporation reduced TS (p < 0.05), and Bahram et al. (2014) noted that the presence of Cinnamon Essential Oil inside to WPC films caused a decrease in TS (p < 0.05). Pérez-Córdoba et al. (2018) also have similar behavior for TS on gelatin-chitosan films incorporated with α-tocopherol, garlic oil, and cinnamaldehyde. The addition of α-TOC inside WPC film produces a film structure with less chain mobility, flexibility, and resistance to fracture Martins et al. (2012).
The EM results behave like those of TS. The addition of nanoemulsion of α-TOC, NAT, or both into the WPC-films reduced the stiffness (p < 0.05) because these compounds modified the intermolecular linkage in the protein chains (Ghadetaj et al., 2018). In general, a high EM could be expected since a higher degree of denaturation generates more disulfide bonds. Nevertheless, the addition of nanoemulsions of α-TOC with hydrophobic ingredients and surfactants modified their interactions with film materials. Furthermore, it might perform a plasticizer action and increase polymer mobility.
Analogous effects have been shown on WPI films with the addition of Grammosciadium ptrocarpum Bioss essential oils (Ghadetaj et al., 2018) and Natamycin (Ramos et al., 2012). The EB in control (F0) film was 29.4% (Table 2), the inclusion of nanoemulsioned α-TOC, NAT, or both (F1, F2, and F3) did not change EB significantly between these last ones (p > 0.05). Bahram et al. (2014) revealed a growth in EB by the addition of cinnamon essential oil or lipids in protein films. The films loaded of compounds are higher than the control; these results reflect the grade of chemical and structural compatibility of compounds on WPC films.
3.2.3. Optical properties (Color, opacity and UV-Vis light barrier)
Color measurements to WPC films are shown in Table 3. These attributes can affect product appearance and consumer acceptability. According to these results (Table 3), compounds loaded led to an increase in the L∗, a∗, b∗, and opacity values of the produced films.
Table 3.
Color (L∗: Luminosity, a∗: Chroma range green-red, b∗: Chroma range blue-yellow, ΔE∗: total color difference), opacity, and water vapor permeability of WPC films. F0: Film without active compounds (Control film); F1: 2% α-TOC – 0 ppm NAT; F2: 0% α-TOC – 300 ppm NAT; F3: 2% α-TOC – 300 ppm NAT.
| Film | L∗ | a∗ | b∗ | ΔE∗ | Opacity | WVP (×10−10gm−1s−1Pa−1) |
|---|---|---|---|---|---|---|
| F0 | 89.7 ± 0.4a | −1.9 ± 0.1a | 9.8 ± 1.3a | 9.0 ± 1.4a | 4.2 ± 0.6a | 1.4 ± 0.0a |
| F1 | 90.9 ± 0.6b | −1.5 ± 0.1b | 10.7 ± 1.6b,c | 9.5 ± 1.7a,b | 5.9 ± 0.7b | 1.9 ± 0.1b |
| F2 | 90.5 ± 0.3c | −1.3 ± 0.1c | 10.2 ± 0.8a,b | 9.1 ± 0.8a | 6.5 ± 1.0c | 1.3 ± 0.1c |
| F3 | 90.9 ± 0.3b | −1.6 ± 0.1d | 11.4 ± 0.9c | 10.0 ± 0.9b | 6.1 ± 0.5b,c | 1.6 ± 0.0d |
Mean values ± standard deviation, n = 3. Different letters in the same column indicate significant differences (p < 0.05) among the average values obtained by application of Duncans test.
Although L∗ and a∗ show some significant difference (p < 0.05), this value was around 90 and -1.5; hence these films presented a slightly yellowish color and translucid. These values were higher than WPI films with nanoemulsions of Grammosciadium ptrocarpum Bioss this value the L∗, a∗ was 82 and -5.5, respectively (Ghadetaj et al., 2018). Also, Ghadetaj et al. (2018) showed that the incorporation of nanoemulsions had no significant effect on the L∗, a∗, and b∗ values of WPI films. Regarding the results of ΔE∗, it was observed that WPC-film had a yellow aspect, and the ΔE∗ value of the control film (F0) was 9.0 ± 1.4 (Table 3). The addition of additives increased ΔE∗value (p < 0.05), with the highest ΔE∗ (10.0 ± 0.9) observed for F3, as a consequence of the yellow color of α-TOC. This result was consistent with Bahram et al. (2014), which found that the incorporation of Cinnamon Essential Oil into WPC films increases the ΔE∗value of WPC film.
The films were virtually transparent (opacity ~0). Films were less transparent when the active compounds are added in its structure (F1, F2, and F3) presenting values of 5.9, 6.5, and 6.1, respectively (Table 3). The films presented a rise in opacity caused by natamycin, and this may be associated to the low solubility of natamycin in water whose substance was 50% pure in lactose; also, the natamycin molecule has a hydrophobic and hydrophilic component Krause et al. (2012). Also, it can be affected by the procedure steps, the size, and concentration of the loaded compounds.
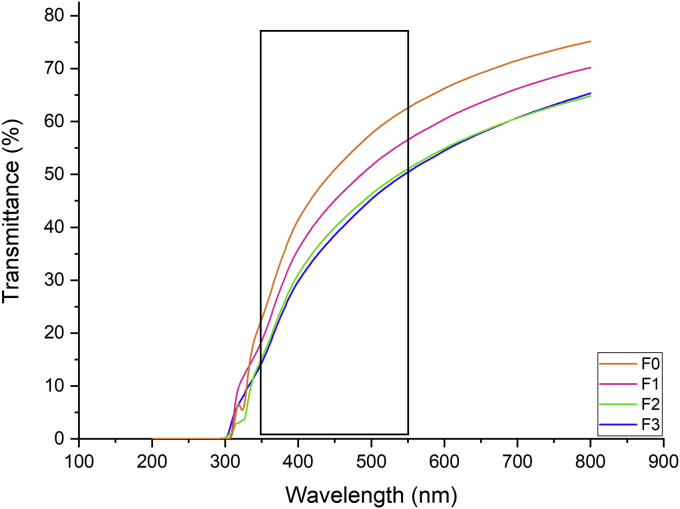
The light transmission of all films is shown in Figure 1. All WPC films have high barrier properties in the UV range (200–300 nm), which may be due to the high content of aromatic amino acids in the proteins into films, that can absorb UV (Ramos et al., 2012), independently of the presence of the active components. Regarding the visible light, the domain comprising wavelengths of 350–550 nm are the most damaging (rectangle in Figure 1), the maximum damage occurring at about 450 nm (Robertson., 2013), provoking chemical reactions induced by visible light (e.g., Vitamin B2). Then, in this domain, the F3 and F2 presented the best barrier to light (Figure 1).
Figure 1.
Light transmission spectra (UV-Vis) of WPC film loaded with nanoemulsioned α-TOC, NAT, and its mixture. F0: Film without active compounds (Control film); F1: 2% α-TOC – 0 ppm NAT; F2: 0% α-TOC – 300 ppm NAT; F3: 2% α-TOC – 300 ppm NAT.
3.2.4. Water vapor permeability (WVP)
WVP is an important property for biodegradable films since it is essential to determine the feasibility of films to avoid deterioration reactions in foods (Martins et al., 2012).
The presence of nanoemulsioned α-TOC, NAT, or both changed WVP values in films and their values are presented in Table 3. Despite of the significant differences between all films, the values are very close, and with the same order of magnitude. The F0 film showed a value for WVP of 1.4 × 10−10 g.m−1.s−1.Pa−1 and it increases to 1.9 × 10−10 g.m−1.s−1.Pa−1 (F1) and 1.6 × 10−10 g.m−1.s−1.Pa−1 (F3) (Table 3). Although α-TOC is hydrophobic, WVP values increased when nanoemulsioned α-TOC was incorporated into WPC films. Maybe the effect of nanoemulsioned α-TOC over the cohesion forces of the protein network lets the movement of water vapor throughout the WPC matrix (Ghadetaj et al., 2018; Ramos et al., 2013) and in chitosan-based films containing α-tocopherol (Martins et al., 2012). Besides, nanoemulsioned α-TOC incorporated increases the molecular spaces between the protein network and reduces the intermolecular interaction which is why increasing the permeability of WPC films.
However, natamycin exhibited a decrease of WVP with a value of 1.3 × 10−10 g.m−1.s−1.Pa−1 (F2) and this value are much lower than the control film, whose value was 1.4 × 10−10 g.m−1.s−1.Pa−1. Ramos et al. (2012) and Ollé Resa et al. (2014b) noted a similar result with a significant difference for tapioca starch films in which the use the natamycin reduced the WVP value. Thus, all films, independently of additives, were high permeable to the water vapor.
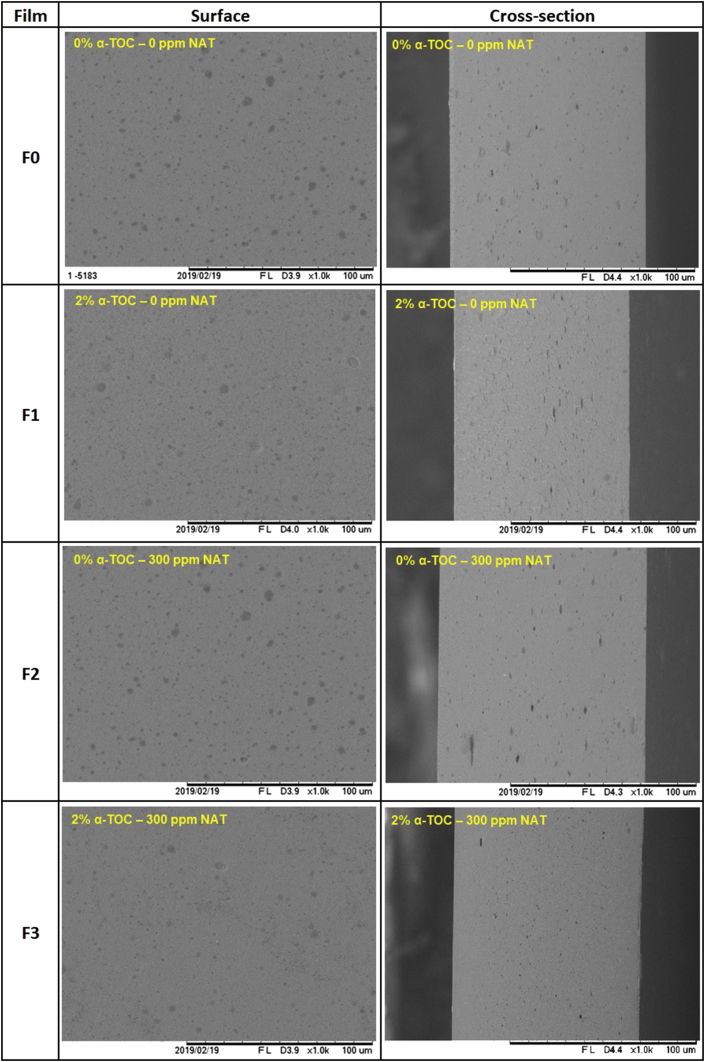
3.2.5. Microstructure
Figure 2 shows SEM micrographs of cross-sections and surface morphology. Surfaces with some heterogeneous surface can be observed in all films, with circular structures seems having be occupied by glycerol droplets, distributed in the cross-section. On another side, eventually separation between the active compounds and the WPC was not observed, indicating a high compatibility of the nanoemulsioned α-TOC, NAT and its mixture with the film matrix, producing a relatively smooth and compact morphology. Moreover, some open porous was observed in the cryo-fractured surface of films (Figure 2). Similar results were obtained by Noronha et al. (2014) using α-TOC nanocapsules loaded in methylcellulose film, which observed a smooth and compact structure probably due to the strong chemical bond of each component loaded.
Figure 2.
SEM micrographs of the surface and cross-section of whey protein concentrate films incorporated with nanoemulsioned α-TOC, NAT, and its mixture. F0: Film without active compounds (Control film); F1: 2% α-TOC – 0 ppm NAT; F2: 0% α-TOC – 300 ppm NAT; F3: 2% α-TOC – 300 ppm NAT.
The incorporation of nanoemulsion can decrease the miscibility of WPC-films, whereas producing a less compact and more porous structure (Oymaci and Altinkaya., 2016). The little orifices in all films were the marks of droplets that remained in the film during fracturing with nitrogen. Film F2 showed a surface free of crystals, in comparison to those presented by Krause et al. (2012) with alginate/pectin films that indicate that the added of natamycin showed the presence of crystals and a surface with non-uniform.
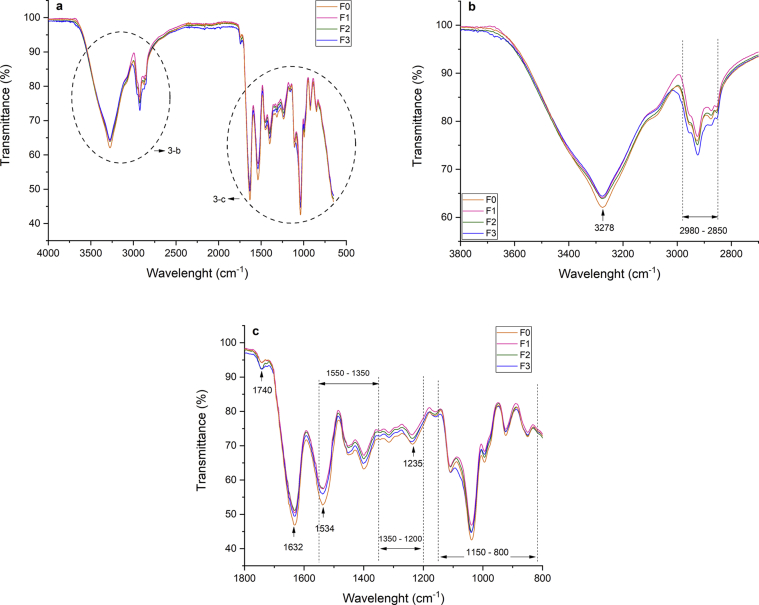
3.2.6. Fourier-transform infrared spectroscopy
FTIR spectroscopy was used to determine α-TOC and NAT presence and know structural changes on WPC films (Figure 3). All WPC films spectra showed bands ranging from 3150 to 3600 cm−1 attributed to free OH and N–H groups on protein strands, respectively; the bands from 2850 to 2980 cm−1 associated to C–H stretching bond, the peaks from 1600 to 1700 cm−1 are attributed to Amide-I related to C=O stretch and C–N vibrations; the peaks from 1400 to 1550 cm−1 assigned to Amide-II (groups N–H); the peaks on 1200-1350 cm−1 related to Amide III with C–N stretching and N–H bonds, peaks from 800 to 1150 cm−1 associated to absorption bonds of glycerol (Ghadetaj et al., 2018; Ramos et al., 2013).
Figure 3.
FT-IR spectra of WPC film loaded with O/W nanoemulsioned α-TOC, NAT, and its mixture: (a) range at 4000 - 600 cm−1; (b) range at 3800 - 2700 cm−1 and (c) range at 800 - 800 cm−1. F0: Film without active compounds (Control film); F1: 2% α-TOC – 0 ppm NAT; F2: 0% α-TOC – 300 ppm NAT; F3: 2% α-TOC – 300 ppm NAT.
By the addition of NAT (F2), the peak value increase in the range of 2920 cm−1 associated with C–H stretching vibration, it is also represented in the F3 film. The peaks ranging from 3500 cm−1 to 3350 cm−1 are also characteristic of N–H stretch of natamycin and possibly were overlaid by O–H peaks of Gly, α-TOC, and WPC; similar changes are shown in alginate active films with natamycin (Bierhalz et al., 2013) and polyethylene with natamycin (Grafia et al., 2018). For F1, the intensity of the band in 2920 cm−1 decreased, Martins et al. (2012) showed the possibly the creation of new connections on film by the presence of α-TOC and surfactant. For F3, the band intensity in 3278 cm−1 decreased, which is associated to N–H free O–H protein.
Generally, active compounds were stayed in the network of the WPC film by the establishment of chemical bonds and by physical entrapment too. Bahram et al. (2014) with adding cinnamon essential oil and Ghadetaj et al. (2018) with the inclusion of Grammosciadium ptrocarpum Bioss essential oils have shown similar results on the structural properties of the films.
3.2.7. Antioxidant activity
The antioxidant activity of the WPC films, determined by ABTS•+, DPPH•, and FRAP, and was expressed as μmol TE/g film, and are presented in Table 4. As predictable, F0 and F2 did not present any radical scavenging activity in tested methods because neither WPC nor NAT have this property. Only films containing α-TOC (F1 and F3) showed antioxidant activity. The α-TOC can donate a hydrogen atom to quench free radicals by producing α-tocopheroxyl radicals until the decolorization of ABTS and DPPH radical, respectively (Re et al., 1999; Contreras-Calderón et al., 2011).
Table 4.
Trolox Equivalent Antioxidant Capacity (TEAC) and Inhibition halos against Candida albicans, Penicillium chrysogenum and Saccharomyces cerevisiae of WPC films. F0: Film without active compounds (Control film); F1: 2% α-TOC – 0 ppm NAT; F2: 0% α-TOC – 300 ppm NAT; F3: 2% α-TOC – 300 ppm NAT.
| Film | TEAC (μmol TE/g dried film) |
Zone of inhibition (mm2) |
||||
|---|---|---|---|---|---|---|
| ABTS∗+ | FRAP | DPPH∗ | C. albicans | P. chrysogenum | S. cerevisiae | |
| F0 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a |
| F1 | 19.5 ± 1.1b | 9.5 ± 0.9b | 28.8 ± 0.5b | - | - | - |
| F2 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 170.1 ± 22.0b | 315.3 ± 35.5b | 334.9 ± 40.5b |
| F3 | 14.6 ± 1.2c | 4.4 ± 1.0c | 26.5 ± 0.7c | 150.9 ± 29.3b | 288.2 ± 39.6b | 303.5 ± 14.5b |
Mean values ± standard deviation. Different letters in the same column indicate significant differences (p < 0.05) among the average values obtained by application of Duncans test.
The film F1 presented an antioxidant activity higher (p < 0.05) than F3, suggesting that NAT reduced the action of α-TOC, or, in other words, they have antagonist effect. F1 presented an antioxidant activity of 19.5 ± 1.1, 9.5 ± 0.9, and 28.8 ± 0.5 μmol TE/g film for ABTS•+, FRAP and DPPH•, respectively. F3 presented values of 14.6 ± 1.2, 4.4 ± 1.0, and 26.5 ± 0.7 μmol TE/g film in the same order (Table 4). Although F3 has natamycin together with α-TOC its antioxidant capacity (ABTS•+ and DPPH•) is even higher than shown in gelatin films loaded with α-tocopherol which published values of 2.6 ± 0.1 and 0.2 ± 0.0 μmol TE/g film for ABTS•+ and DPPH• respectively (Pérez et al., 2018).
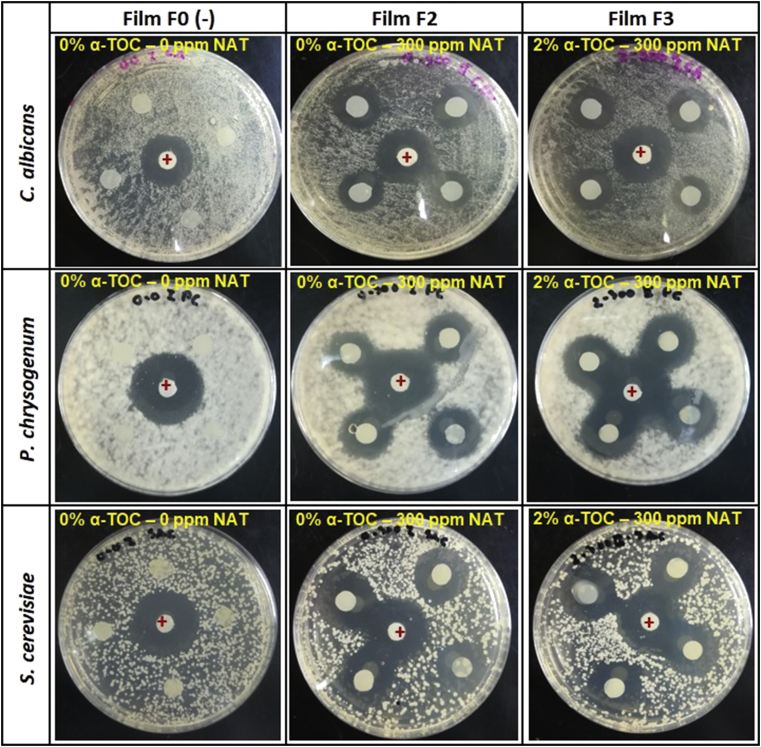
3.2.8. Antimicrobial activity
As a could be expected, the WPC films with natamycin (F2 and F3) were efficacious against C. albicans, P. chrysogenum, and S. cerevisiae when evaluated an agar diffusion method (Figure 4, Table 4). Among additives, only natamycin has antimicrobial activity. Thus, films F0 and F1 (without natamycin) did not show antimicrobial activity, whereas films F2 and F3 presented an area of inhibition more extensive than control films, owing to the diffusion of antimicrobial across the WPC films, and the presence of α-TOC (F3) only leads to a reduction in inhibition area nevertheless did not modify the antimicrobial activity of the films (p > 0.05). Ollé Resa et al. (2014b) published analogous results about the antimicrobial activity against S. cerevisiae on starch edible films, Ramos et al. (2012) reported that whey protein isolate films loaded with natamycin present inhibition against one yeast, Y. lipolytica. Also, Fajardo et al. (2010) evaluated that chitosan coating loaded with natamycin against Penicillium crustosum, P. roqueforti, P. commune, and Aspergillus niger. These studies showed that natamycin obstacle fungal growth by attachment particularly to ergosterol, which is only present in moulds and yeast plasma membranes. The natamycin impedes vacuolar by interaction with ergosterol (Ollé Resa, Gerschenson, and Jagus., 2014a). The diffusion of NAT into agar gel was similar for the yeast and moulds test due to the same agar was used for their growth, nevertheless the different diameters obtained were related with sensitivity to natamycin and different growth rate for the microorganisms tested (Figure 4 and Table 4).
Figure 4.
Inhibition of Candida albicans, Penicillium chrysogenum and Saccharomyces cerevisiae following disk diffusion assay of WPC films. F0: Film without active compounds (Control film); F1: 2% α-TOC – 0 ppm NAT; F2: 0% α-TOC – 300 ppm NAT; F3: 2% α-TOC – 300 ppm NAT.
4. Conclusion
WPC films loaded with α-tocopherol and natamycin were successfully prepared by the casting method. The characterization of the WPC films showed that active compounds are well distributed. The incorporation of α-tocopherol and natamycin into WPC film did not affect the moisture content, nevertheless decreased the TS, EM and increased EB, showing that the nanoemulsioned α-TOC had a plasticizer effect on films. Water vapor permeability of the film increased despite the hydrophobic nature of α-tocopherol but not increased for film with NAT. All films have an excellent barrier property in the UV region (200–550 nm). The FTIR spectra indicated that nanoemulsioned α-tocopherol, and natamycin remained stable during the process of making the films, which shown antioxidant capacity and antimicrobial activity against C. albicans, P. chrysogenum, and S. cerevisiae respectively.
The WPC films added with nanoemulsioned α-TOC, and natamycin have great potential for application in food packaging as a vehicle for active compounds and becoming as an alternative to typical materials for possibly enhancing safety, improving quality and increasing the shelf life of food packaged with them.
Practical applications
This research develops a food active packaging from renewable sources such as whey protein, with nanoemulsioned α-tocopherol and natamycin as antioxidant and antimicrobial compounds for better food protection. Physicochemical, mechanical, optical-properties, water vapor barrier, FTIR microstructure, antioxidant and antimicrobial activity were characterized to evaluate the potential for practical application as active packaging for the food industry.
Declarations
Author contribution statement
Camilo Agudelo-Cuartas: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Diana Granda-Restrepo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Paulo J.A. Sobral: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hugo Hernandez, Wilson Castro: Analyzed and interpreted the data; Wrote the paper.
Funding statement
Camilo Agudelo Cuartas was supported by the Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación "Francisco José de Caldas" a Colombian doctoral scholarship COLCIENCIAS (Convocatoria 727 of 2015).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Agudelo Cuartas gratefully acknowledge Laboratorio de Tecnología de Alimentos (Universidad de Sao Paulo, Brasil), Universidad Nacional de Frontera, Sullana - Perú, Proyecto: Creación del Laboratorio de Investigación en Inocuidad (CUI N° 2439545), and Ingredientes y Productos Functionales (IPF, Colombia).
References
- Ahmed S., Ikram S. Chitosan and gelatin based biodegradable packaging films with UV-light protection. J. Photochem. Photobiol. B Biol. 2016;163:115–124. doi: 10.1016/j.jphotobiol.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Alam Md. Nur., Jahan N., Rafiquzzaman Md. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceut. J. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenhofen M., Krause A., Kieckbusch T. Modelling natamycin release from alginate/chitosan active films. Int. J. Food Sci. Technol. 2012;47(4):740–746. [Google Scholar]
- ASTM . Standards Designations: D882. Annual Book of ASTM Standards. American Society for Testing and Materials; Philadelphia, PA, USA: 2001. Standard test method for tensile properties of thin plastic sheeting; pp. 162–170. [Google Scholar]
- Atares L., Chiralt A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016;48:51–62. [Google Scholar]
- Bahram S., Rezaei M., Soltani M., Kamali A. Whey protein concentrate edible film activated with cinnamon essential oil. J. Food Process. Preserv. 2014;38(3):1251–1258. [Google Scholar]
- Bierhalz A., Sa Silva M., de Sousa H., Braga M. Influence of natamycin loading methods on the physical characteristics of alginate active films. J. Supercrit. Fluids. 2013;76:74–82. [Google Scholar]
- Bonilla J., Sobral P.J.A. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016;16:17–25. [Google Scholar]
- Bouchemal K., Briançon S., Perrier E., Fessi H. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int. J. Pharm. 2004;280(1–2):241–251. doi: 10.1016/j.ijpharm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Cazón P., Velázquez G., Ramírez J., Vázquez M. Polysaccharide-based films and coatings for food packaging: a review. Food Hydrocolloids. 2017;68:136–148. [Google Scholar]
- Ciro G., Zapata J.E., López J. In vitro evaluation of bixa orellana L. (Annatto) seeds as potential natural food preservative. J. Med. Plants Res. 2014;8(21):772–779. [Google Scholar]
- Contreras-Calderón J., Calderón-Jaimes L., Guerra-Hernández E., García-Villanova B. Antioxidant capacity, phenolic content and Vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011;44(7):2047–2053. [Google Scholar]
- Dammak I., de Carvalho R., Fávaro C., Laurenço R. Properties of active gelatin films incorporated with rutin-loaded nanoemulsions. Int. J. Biol. Macromol. 2017;98:39–49. doi: 10.1016/j.ijbiomac.2017.01.094. [DOI] [PubMed] [Google Scholar]
- Dammak I., Lourenço R., Sobral P.J.A. Active gelatin films incorporated with pickering emulsions encapsulating hesperidin: preparation and physicochemical characterization. J. Food Eng. 2019;240:9–20. [Google Scholar]
- Fajardo P., Martins J.T., Fuciños C., Pastrana Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of saloio cheese. J. Food Eng. 2010;101(4):349–356. [Google Scholar]
- FDA CFR - Code of federal Regulations title 21. Part 182 substances generally recognized as safe. Food Drug Admin. 2001 [Google Scholar]
- Ghadetaj A., Almasi H., Mehryar L. Development and characterization of whey protein isolate active films containing nanoemulsions of Grammosciadium ptrocarpum Bioss. Essential oil. Food Packag. Shelf Life. 2018;16:31–40. [Google Scholar]
- Grafia A., Vázquez M., Bianchinotti M., Barbosa S. Development of an antifungal film by polyethylene surface modification with natamycin. Food Packag. Shelf Life. 2018;18:191–200. [Google Scholar]
- Granda-Restrepo D., Soto-Valdez H., Peralta E., Troncoso-Rojas R. Migration of α-tocopherol from an active multilayer film into whole milk powder. Food Res. Int. 2009;42(10):1396–1402. [Google Scholar]
- Kaur K., Kaur J., Kumar R., Mehta S.K. Formulation and physiochemical study of A-tocopherol based oil in water nanoemulsion stabilized with non toxic, biodegradable surfactant: sodium stearoyl lactate. Ultrasonics Sonochem. 2016 doi: 10.1016/j.ultsonch.2016.08.026. [DOI] [PubMed] [Google Scholar]
- Khalil H.P.S., Davoudpour Y., Saurabh C., Hossain M.D. A review on nanocellulosic fibres as new material for sustainable packaging: process and applications. Renew. Sustain. Energy Rev. 2016;64:823–836. [Google Scholar]
- Krause A., Altenhofen M., Kieckbusch T. Natamycin release from alginate/pectin films for food packaging applications. J. Food Eng. 2012;110(1):18–25. [Google Scholar]
- Lara B., Araújo A., Dias M., Guimarães M. Morphological, mechanical and physical properties of new whey protein isolate/polyvinyl alcohol blends for food flexible packaging. Food Packag. Shelf Life. 2019;19:16–23. [Google Scholar]
- Lavoine N., Guillard V., Desloges I., Gontard N., Bras J. Active bio-based food-packaging: diffusion and release of active substances through and from cellulose nanofiber coating toward food-packaging design. Carbohydr. Polym. 2016;149:40–50. doi: 10.1016/j.carbpol.2016.04.048. [DOI] [PubMed] [Google Scholar]
- López de Dicastillo C., Rodríguez F., Guarda A., Galotto M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016;136:1052–1060. doi: 10.1016/j.carbpol.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Marra A., Silvestre C., Duraccio C., Cimmino S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016;88:254–262. doi: 10.1016/j.ijbiomac.2016.03.039. [DOI] [PubMed] [Google Scholar]
- Martelli S., Motta C., Caon T., Alberton J. Edible carboxymethyl cellulose films containing natural antioxidant and surfactants: $α$-Tocopherol stability, in vitro release and film properties. LWT-Food Sci. Technol. 2017;77:21–29. [Google Scholar]
- Martins J., Cerqueira M., Vicente A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocolloids. 2012;27(1):220–227. [Google Scholar]
- Moatsou G., Moschopoulou E., Beka A., Tsermoula P. Effect of natamycin-containing coating on the evolution of biochemical and microbiological parameters during the ripening and storage of ovine hard-gruyere-type cheese. Int. Dairy J. 2015;50:1–8. [Google Scholar]
- Noronha C., Matos S., Calegari R., Manique P. Characterization of antioxidant methylcellulose film incorporated with α-tocopherol nanocapsules. Food Chem. 2014;159:529–535. doi: 10.1016/j.foodchem.2014.02.159. [DOI] [PubMed] [Google Scholar]
- Ollé Resa C., Gerschenson L., Jagus R. Natamycin and nisin supported on starch edible films for controlling mixed culture growth on model systems and port salut cheese. Food Contr. 2014;44:146–151. [Google Scholar]
- Ollé Resa C., Jagus R., Gerschenson L. Natamycin efficiency for controlling yeast growth in models systems and on cheese surfaces. Food Contr. 2014;35(1):101–108. [Google Scholar]
- Ollé Resa C., Gerschenson L., Jagus R. Starch edible film supporting natamycin and nisin for improving microbiological stability of refrigerated argentinian port salut cheese. Food Contr. 2016;59:737–742. [Google Scholar]
- Oymaci P., Altinkaya S. Improvement of barrier and mechanical properties of whey protein isolate based food packaging films by incorporation of Zein nanoparticles as a novel bionanocomposite. Food Hydrocolloids. 2016;54:1–9. [Google Scholar]
- Ozer B., Uz M., Oymaci P., Altinkaya S. Development of a novel strategy for controlled release of lysozyme from whey protein isolate based active food packaging films. Food Hydrocolloids. 2016;61:877–886. [Google Scholar]
- Pérez-Córdoba L., Sobral P.J.A. Physical and antioxidant properties of films based on gelatin, gelatin-chitosan or gelatin-sodium caseinate blends loaded with nanoemulsified active compounds. J. Food Eng. 2017;213:47–53. [Google Scholar]
- Pérez-Córdoba L., Norton I., Batchelor H., Gkatzionis K. Physico-chemical, antimicrobial and antioxidant properties of gelatin-chitosan based films loaded with nanoemulsions encapsulating active compounds. Food Hydrocolloids. 2018;79:544–559. [Google Scholar]
- Pintado C., Ferreira M., Sousa I. Control of pathogenic and spoilage microorganisms from cheese surface by whey protein films containing malic acid, nisin, and natamycin. Food Contr. 2010;21(3):240–246. [Google Scholar]
- Ramos Ó., Santos A., Leão M., Pereira J. Antimicrobial activity of edible coatings prepared from whey protein isolate and formulated with various antimicrobial agents. Int. Dairy J. 2012;25(2):132–141. [Google Scholar]
- Ramos Ó., Reinas I., Silva S., Fernandes J. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocolloids. 2013;30(1):110–122. [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Robertson G. third ed. Taylor & Francis Group; New York: 2013. Food Packaging: Principles and Practice. [Google Scholar]
- Schmid M., Pröls S., Kainz D., Hammann F., Grupa U. Effect of thermally induced denaturation on molecular interaction-response relationships of whey protein isolate based films and coatings. Prog. Org. Coating. 2017;104:161–172. [Google Scholar]
- Schmid M., Merzbacher S., Müller K. Time-dependent crosslinking of whey protein based films during storage. Mater. Lett. 2018;215:8–10. [Google Scholar]
- Shokri S., Ehsani A. Efficacy of whey protein coating incorporated with lactoperoxidase and α-tocopherol in shelf life extension of pike-perch fillets during refrigeration. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;85:225–231. [Google Scholar]
- Zhao Y., Lv X., Ni H. Solvent-based separation and recycling of waste plastics: a review. Chemosphere. 2018;209:707–720. doi: 10.1016/j.chemosphere.2018.06.095. [DOI] [PubMed] [Google Scholar]
- Zhou X., Hua X., Huang L., Xu Y. Bio-utilization of cheese manufacturing wastes (cheese whey powder) for bioethanol and specific product (galactonic acid) production via a two-step bioprocess. Bioresour. Technol. 2019;272:70–76. doi: 10.1016/j.biortech.2018.10.001. [DOI] [PubMed] [Google Scholar]