Abstract
Advanced lung cancer can have numerous extra-pulmonary complications. Due to the proximity to the heart, cardiac invasion and the subsequent seeding of the tumor into the vascular system present numerous challenges in physician team care coordination. Here we have a 55-year-old male who presented with stroke symptoms in the setting of undiagnosed advanced lung cancer complicated by direct tumor invasion into the left atria and mixed embolic showering phenomenon and thrombotic hypercoagulability. Review of both the embolic showering phenomenon and hypercoagulability will be discussed as both can result in embolic occlusion or metastatic seeding at distant sites. Fewer than ten cases of spontaneous tumor embolization resulting from lung cancer invasion of the pulmonary vein have been reported. Poor prognosis of advanced lung cancer requires delicate, yet pragmatic conversations between care teams and the patient.
Keywords: Lung cancer, Hypercoagulability, Showering, Embolus, Thrombus, Stroke
Highlights
-
•
Lung cancer has potential sequelae that extend well beyond confines of pulmonary pleura.
-
•
Caution is needed when differentiating between tumor extension and metastatic seeding for atrial masses in the setting of lung cancer.
-
•
The showering phenomenon of tumor emboli should be considered when presented with stroke symptoms that cannot be attributed to a unilateral deficit or when multiple areas of the brain are affected.
1. Introduction
Primary lung cancer is a global health problem as it is the second most common cancer in men and women and is the leading cause of death attributed to cancer [1]. Differentiated into small cell and non-small cell varieties, older age and smoking have been the strongest risk factors associated with lung cancer development [2]. Treatment and survival rates are largely dependent on the stage of cancer found at diagnosis, with end stage and metastatic disease traditionally having poorer prognosis and decreased quality of life [3]. Extrapulmonary manifestations and paraneoplastic syndromes may occur in conjunction with lung cancer and are particularly pronounced in late stages of the disease, with symptoms often matching the site of metastasis [4]. Due to the proximity to the heart, cardiac invasion and the subsequent seeding of the tumor into the vascular system present numerous challenges in physician team care coordination [4,5].
Here we present a case of a 55-year-old male who presented with stroke symptoms in the setting of undiagnosed advanced lung cancer complicated by direct tumor invasion into the left atria and mixed embolic showering phenomenon and thrombotic hypercoagulability.
2. Case report
55-year-old Caucasian male presented to the emergency room with 6-h history of acute left facial palsy, bilateral upper extremity weakness, and right sided vision loss. These symptoms suddenly occurred when the patient was standing in line at a bank and denied any previous episodes. The patient admitted that he has had poor medical follow-up with physicians, with anemia and hemoptysis laboratory orders pending at his primary care physician's office for the last three months. The patient's son mentioned a potential left lower lung mass seen on outpatient x-ray done several months ago. Social history was significant for 35 pack year smoking history and family history was positive for pancreatic cancer in father and metastatic breast cancer in mother. Review of systems revealed unintentional weight loss of 15 pounds over the last two months, shortness of breath, and fatigue.
Physical exam revealed left upper and lower extremity weakness (3/5 on all range of motion), left facial palsy, right gaze deviation, and mild to moderate dysarthria with slurring but grossly understandable speech, with an initial National Institute of Health Stroke Score (NIHSS) of 6, resulting in calling a code stroke [6]. CBC, CMP, troponins, and PT/PTT were within standard ranges for the entirety of the patient's hospital course.
Computerized tomography (CT) of the head revealed a 3.2 cm hypodense lesion in the left frontal lobe with surrounding calcification versus hemorrhage (Fig. 1). Subsequent CT perfusion further revealed a small matched defect in right frontal lobe suggestive of core infarct, right lung mass consistent with malignant neoplasm, mediastinal lymphadenopathy, multiple filling defects of the right pulmonary artery and branches, and compression of the superior vena cava. No overt vessel occlusion was found at that time and tissue plasminogen activator was not administered. The patient was admitted to the stroke unit for further workup and observation.
Fig. 1.

CT head without contrast done at admission. Red circle highlights 3.2cm hypodense lesion. L for left, P for posterior. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
During this time, the patient was started on oral aspirin 168mg and atorvastatin 80mg, intravenous dexamethasone 4mg, and subcutaneous enoxaparin 40mg. Upon admission to the stroke floor, the patient reported resolution of left upper and lower extremity weakness, with physical and occupational therapy evaluation noting new balance and gait instability compared to family reported baseline.
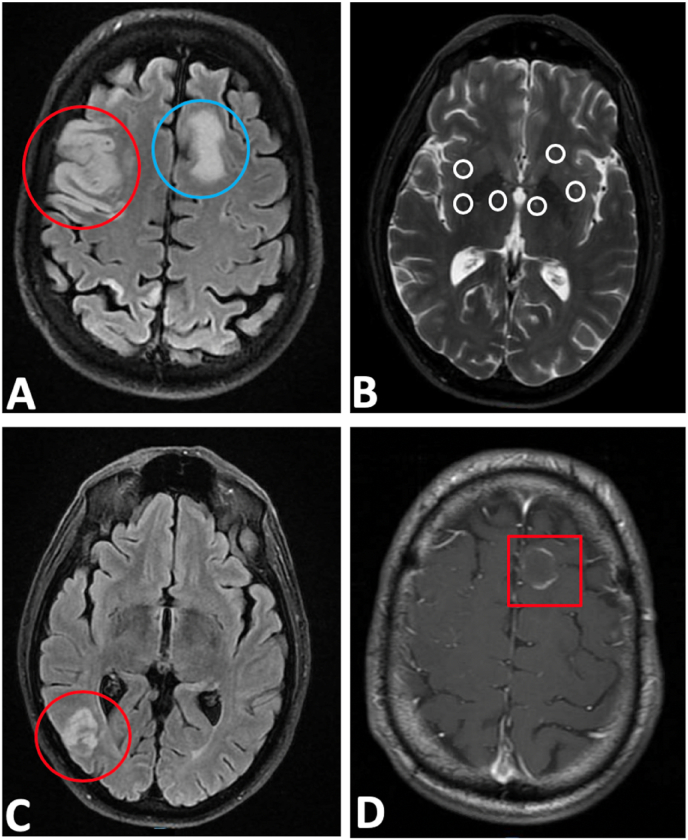
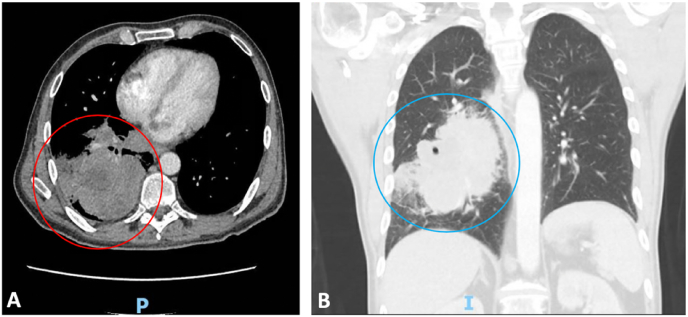
Magnetic resonance imaging (MRI) brain showed a moderate size acute infarction in the right frontoparietal lobe with small amount of internal petechial hemorrhage, numerous scattered infarctions of the right cerebral hemisphere, cerebellar hemisphere, and left frontoparietal deep white matter suggestive of embolic etiology, and a rim-enhancing lesion in the left frontal lobe with surrounding edema suggestive of metastatic disease (Fig. 2A–D). Hematology/oncology and pulmonology consultation resulted in CT chest and abdomen, showing large 8.9cm necrotic right infrahillar and lower lung mass, pulmonary embolism in distal right main pulmonary artery with extension into the right upper and lower lobe segmental artery branches, and scattered hypodensities measuring 6 mm throughout the right hepatic lobe likely representing metastasis in the setting of malignancy (Fig. 3A–B). Transthoracic echocardiogram revealed normal left ventricular ejection fraction of 55% with a 3.7 × 1.4 cm mass in the left atrium with a patent foramen ovale.
Fig. 2.
A-D: MRI brain showed: A. A moderate size acute infarction in the right frontoparietal lobe (red circle) and left frontoparietal deep white matter suggestive of embolic etiology (blue circle).
B. Small amount of internal petechial hemorrhage (white circles).
C. Numerous scattered infarctions of the right cerebral (red circle) and cerebellar hemisphere.
D. Rim-enhancing lesion (red square) in the left frontal lobe with surrounding edema suggestive of metastatic disease. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
A-B:
A. 8.9 cm necrotic right infrahillar lower lung mass (red circle). P for posterior.
B. Coronal view of mass (blue circle) with associated pulmonary embolism in distal right main pulmonary artery with extension into the right upper and lower lobe segmental artery branches. I for inferior. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Despite consultation with the cardiothoracic and interventional radiologic team and extensive discussion regarding the extremely poor prognosis regarding the clinical picture of advanced lung cancer with metastasis, the patient and family elected to proceed with CT-guided needle biopsy of the lung. Pathology confirmed primary lung adenocarcinoma with cytology showing infiltrating malignant glandular epithelium containing moderate cellularity with single-lying pleomorphic cells with enlarged nuclei, anisonucleosis, and prominent nucleoli. Immunohistochemistry studies supported the diagnosis with the further delineation of a TP53 splice site mutation and cells positive for CK7, TTF-1, Napsin-A, and p63.
Upon palliative and goals of care discussions, the patient and family elected to proceed with comfort care, however were unable to wait for insurance authorization for hospice placement. The patient left against medical advice and was subsequently lost to follow-up.
3. Discussion
Advanced lung cancer or stage IV/metastatic lung cancer is defined as when the disease has spread beyond the original lung to the opposite lung, pericardium, chest, or other bodily sites [1]. According to the National Cancer Institute, greater than 55% of patients are found to have stage IV lung cancer at the time of initial diagnosis; however, this rate has been steadily dropping and may be attributed to a confluence of factors such as increased knowledge regarding the health effects of smoking as well as new guidelines and better techniques for lung cancer detection [7,8]. In many of these cases, however, patients often present to care due to acute symptoms or complications arising from non-pulmonary causes [9]. Hypercoagulability in malignancy is common as the coagulation cascade is activated by tumor cells, resulting in venous thrombosis in distant sites [10,11]. Malignancy-associated thrombosis tends to follow an anatomic pattern of involvement and includes large artery bifurcations, the cerebral circulation, or organs with high blood flow, such as the liver in this case [12,13].
Atrial extension of primary lung cancer occurs when the tumor grows beyond the confines of the lung pleura and tissue and pushes through the pulmonary veins to gain access to the heart [14]. In comparison to the more common hematologic metastasis to the heart, direct cardiac invasion of lung cancer is rare as it requires contiguous connection between the primary tumor and secondary site [15]. Recent studies have demonstrated that only 4.2% of advanced lung cancer have involvement of the pulmonary veins and just 0.9% of those cases have extension into the left atrium via the pulmonary vein, of which the latter presented in the aforementioned case [16,17]. Atrial extensions of tumors have been more commonly discovered in sarcomas than in bronchogenic or pulmonary sources, in which its rarity can result in confusion with other cardiac pathologies [18]. Transthoracic and transesophageal echocardiograms are often used to assess the effect of tumor invasion on cardiac function, while continuity between the lung primary and cardiac mass is confirmed via CT [1,19]. As seen with the case patient, direct tissue biopsy is still required to diagnose the type of cancer and determine the appropriate therapy.
The showering phenomenon occurs when tumor fragments are sheared and enter the body's circulation, resulting in either embolic occlusion or metastatic seeding at distant sites [20,21]. Fewer than ten cases of spontaneous tumor embolization resulting from lung cancer invasion of the pulmonary vein have been reported [21]. The incidence of lung cancer with atrial extensions that progress to showering emboli is unclear, with sporadic case reports in the past decade [22]. From the limited research, it appears that the majority tend to cause partial occlusion at large vessels (50%), followed by stroke or stroke-like symptoms (30%) and other areas (20%) such as the contralateral lobe of the lung, lower and upper extremities, kidneys, and spleen [23]. Due to poor prognosis and limited life expectancy, minimal research and guidelines exist regarding care and treatment options [24]. Therefore, a multidisciplinary approach is critical to address the physical, emotional, and spiritual needs of the patient and family in preparation for discussions regarding end of life and goals of care [25].
4. Conclusion
Lung cancer has potential sequelae that extend well beyond confines of pulmonary pleura. The case presented highlights advanced lung cancer with the rare findings of direct invasion of the left atrium resulting in stroke due to hypercoagulable thrombi versus showering tumor emboli. Caution is needed when differentiating between tumor extension and metastatic seeding for atrial masses in the setting of lung cancer. The showering phenomenon of tumor emboli should be considered when presented with stroke symptoms that cannot be attributed to a unilateral deficit or when multiple areas of the brain are affected. Poor prognosis of advanced lung cancer requires delicate, yet pragmatic conversations between care teams and the patient.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Matthew Migliozzi: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. George Nguyen: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. Bina Kviatkovsky: Investigation, Resources, Writing - original draft, Writing - review & editing, Supervision. Christine Lomiguen: Conceptualization, Writing - original draft, Writing - review & editing, Project administration.
Declaration of competing interest
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmcr.2020.101064.
Appendix A. Supplementary data
References
- 1.Nasim F., Sabath B.F., Eapen G.A., Lung Cancer Med. Clin. North Am. 2019;103:463–473. doi: 10.1016/j.mcna.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Mao Y., Yang D., He J., Krasna M.J. Epidemiology of lung cancer. Surg. Oncol. Clin. N. Am. 2016;25:439–445. doi: 10.1016/j.soc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch F.R., Scagliotti G.V., Mulshine J.L., Kwon R., Curran W.J., Jr., Wu Y.L., Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 4.Riihimaki M., Hemminki A., Fallah M., Thomsen H., Sundquist K., Sundquist J., Hemminki K. Metastatic sites and survival in lung cancer. Lung Canc. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Bussani R., De-Giorgio F., Abbate A., Silvestri F. Cardiac metastases. J. Clin. Pathol. 2007;60:27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwah L.K., Diong J. National institutes of health stroke scale (NIHSS) J. Physiother. 2014;60:61. doi: 10.1016/j.jphys.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Gohagan J.K., Prorok P.C., Hayes R.B., Kramer B.S., Prostate L.C. Ovarian cancer screening trial Project T: the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial of the national cancer Institute: history, organization, and status. Contr. Clin. Trials. 2000;21:251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 8.Tanoue L.T., Tanner N.T., Gould M.K., Silvestri G.A. Lung cancer screening. Am. J. Respir. Crit. Care Med. 2015;191:19–33. doi: 10.1164/rccm.201410-1777CI. [DOI] [PubMed] [Google Scholar]
- 9.Godoy M.C.B., White C.S., Erasmus J.J., Wu C.C., Truong M.T., Munden R.F., Chiles C. Extrapulmonary neoplasms in lung cancer screening. Transl. Lung Cancer Res. 2018;7:368–375. doi: 10.21037/tlcr.2018.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammouda A., Souilah S., Ferhat-Hamida M.Y., Amir Z.C., Aouichat-Bouguerra S., Hariti G. Activation of coagulation in patients with lung cancer. Ann. Biol. Clin. (Paris) 2019;77:272–280. doi: 10.1684/abc.2019.1445. [DOI] [PubMed] [Google Scholar]
- 11.Syrigos K., Grapsa D., Sangare R., Evmorfiadis I., Larsen A.K., Van Dreden P., Boura P., Charpidou A., Kotteas E., Sergentanis T.N., Elalamy I., Falanga A., Gerotziafas G.T. Prospective assessment of clinical risk factors and biomarkers of hypercoagulability for the identification of patients with lung adenocarcinoma at risk for cancer-associated thrombosis: the observational ROADMAP-CAT study. Oncol. 2018;23:1372–1381. doi: 10.1634/theoncologist.2017-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdol Razak N.B., Jones G., Bhandari M., Berndt M.C., Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers (Basel) 2018;10 doi: 10.3390/cancers10100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble S., Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br. J. Canc. 2010;102(Suppl 1):S2–S9. doi: 10.1038/sj.bjc.6605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin M.T., Ku S.C., Wu M.Z., Yu C.J. Intracardiac extension of lung cancer via the pulmonary vein. Thorax. 2008;63:1122. doi: 10.1136/thx.2007.090373. [DOI] [PubMed] [Google Scholar]
- 15.Freitas C., Lopes J.P., Araujo D. Myxoma or lung cancer? Arch. Bronconeumol. 2018;54:42. doi: 10.1016/j.arbres.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Park J.H., Seo H.S., Park S.K., Suh J., Kim D.H., Cho Y.H., Lee N.H. Spontaneous systemic tumor embolism caused by tumor invasion of pulmonary vein in a patient with advanced lung cancer. J. Cardiovasc. Ultrasound. 2010;18:148–150. doi: 10.4250/jcu.2010.18.4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballo P., Laureano R., Briganti M., Passaleva M.T., Piani F., Piga C., Tatini S., Santoro G.M. Left atrial mass invasion from pulmonary neoplasm extension via the right upper pulmonary vein presenting as ipsilateral stroke. Case Rep. Med. 2016;2016:7084234. doi: 10.1155/2016/7084234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehan V.K., Deshpande J., Dalvi B.V., Kale P.A. Direct extension of bronchogenic carcinoma through pulmonary veins into the left atrium mimicking left atrial myxoma. Chest. 1992;101:1722–1723. doi: 10.1378/chest.101.6.1722. [DOI] [PubMed] [Google Scholar]
- 19.Cipriano F., Lu Dessoti, Rodrigues A.J., Vicente W.V.A., Chahud F., Evora P.R.B. Report of a lung carcinoma extended to the left atrium through pulmonary vein. J. Thorac. Dis. 2018;10:E46–E51. doi: 10.21037/jtd.2017.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morasch M.D., Shanik G.D. Tumor embolus: a case report and review of the literature. Ann. Vasc. Surg. 2003;17:210–213. doi: 10.1007/s10016-001-0251-0. [DOI] [PubMed] [Google Scholar]
- 21.Schreffler S.M., Paolo W.F., Kloss B.T. Spontaneous showering of tumor emboli in a patient with advanced primary lung cancer: a case report. Int. J. Emerg. Med. 2012;5:27. doi: 10.1186/1865-1380-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadeghpour A., Alizadehasl A. Showering emboli of an atrial mass: a fatal phenomenon. Res. Cardiovasc. Med. 2013;2:77–78. doi: 10.5812/cardiovascmed.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi A.K., Pearson A.C., Orsinelli D.A. Tumor invasion of the pulmonary veins: a unique source of systemic embolism detected by transesophageal echocardiography. J. Am. Soc. Echocardiogr. 1995;8:97–99. doi: 10.1016/s0894-7317(05)80364-5. [DOI] [PubMed] [Google Scholar]
- 24.Nestle U., De Ruysscher D., Ricardi U., Geets X., Belderbos J., Pottgen C., Dziadiuszko R., Peeters S., Lievens Y., Hurkmans C., Slotman B., Ramella S., Faivre-Finn C., McDonald F., Manapov F., Putora P.M., LePechoux C., Van Houtte P. ESTRO ACROP guidelines for target volume definition in the treatment of locally advanced non-small cell lung cancer. Radiother. Oncol. 2018;127:1–5. doi: 10.1016/j.radonc.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Ferrell B., Sun V., Hurria A., Cristea M., Raz D.J., Kim J.Y., Reckamp K., Williams A.C., Borneman T., Uman G., Koczywas M. Interdisciplinary palliative care for patients with lung cancer. J. Pain Symptom Manag. 2015;50:758–767. doi: 10.1016/j.jpainsymman.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.