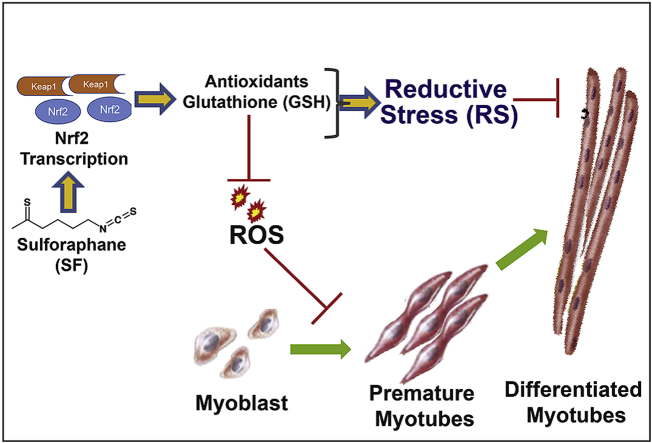
Abstract
Myo-satellite cells regenerate and differentiate into skeletal muscle (SM) after acute or chronic injury. Changes in the redox milieu towards the oxidative arm at the wound site are known to compromise SM regeneration. Recently, we reported that abrogation of Nrf2/antioxidant signaling promotes oxidative stress and impairs SM regeneration in C57/Bl6 mice. Here, we investigated whether the activation of intracellular Nrf2 signaling favors antioxidant transcription and promotes myoblast differentiation. Satellite cell-like C2C12 myoblasts were treated with sulforaphane (SF; 1.0 & 5.0 μM) to activate Nrf2/antioxidant signaling during proliferation and differentiation (i.e. formation of myotubes/myofibers). SF-mediated Nrf2 activation resulted in increased expression of Nrf2-antioxidants (e.g. GCLC and G6PD) and augmented the production of reduced glutathione (GSH) leading to a reductive redox state. Surprisingly, this resulted in significant inhibition of myoblast differentiation, as observed from morphological changes and reduced expression of MyoD, Pax7, and Myh2, due to reductive stress (RS). Furthermore, supplementation of N-acetyl-cysteine (NAC) or GSH-ester or genetic knock-down of Keap1 (using siRNA) also resulted in RS-driven inhibition of differentiation. Interestingly, withdrawing Nrf2 activation rescued differentiation potential and formation of myotubes/myofibers from C2C12 myoblasts. Thus, abrogation of physiological ROS signaling through over-activation of Nrf2 (i.e. RS) and developing RS hampers differentiation of muscle satellite cells.
Keywords: Reductive stress, Pro-oxidative setting, Satellite cells, Skeletal muscle regeneration, Nrf2-signaling, Differentiation markers, Reactive oxygen species (ROS)
Graphical abstract
Highlights
-
•
Sulforaphane activates Nrf2 and establishes reductive stress (RS) in C2C12 myoblasts.
-
•
RS abolishes basal ROS and significantly impede the differentiation of myoblasts.
-
•
Augmentation of glutathione using pharmacological agents (NAC and GSH-ester) promotes RS and impairs differentiation.
-
•
Precluding RS restores the myoblast differentiation process.
1. Introduction
Skeletal muscle (SM) contributes to about 50% of the total body mass. SM generates force through the contractile movement of myofibers during physical activity, which are tightly regulated through bioenergetics, stress response, antioxidant defense systems, and redox metabolism [[1], [2], [3], [4]]. Adult SM tissue regains regenerative capacity upon injury or damage [[5], [6], [7]]. Satellite cells undergo regeneration to form myofibers and maintain muscle mass through their activation following injury [6,8,9]. Myogenic differentiation is a multistep process consisting of the expression of various transcription factors such as paired-box (Pax3 and Pax7) [[10], [11], [12], [13]] and canonic myogenic regulatory factors (MRFs; Myf-5, MyoD, myogenin and Mrf4) for the development [14,15]. The adult SM requires Pax7 and MyoD in the satellite stem cells to regulate cell lineage during SM regeneration [[16], [17], [18]].
SMs including muscle precursor cells are constantly exposed to a pro-oxidizing environment due to their high rate of oxygen consumption and metabolic activity [19,20]. During aging or chronic conditions such as diabetes mellitus and AIDS, loss of skeletal muscle mass and activity are coupled with decreased glutathione and increased oxidative stress (OS) [[21], [22], [23]]. In addition, sarcopenia, an age-related condition, characterized by a reduction in the size and number of muscle fibers is strongly associated with increased OS [[24], [25], [26]]. While OS impairs muscular strength and health, it is likely that the use of antioxidants would be beneficial. However, a pro-oxidative signaling is crucial for satellite cell activation at the wound site of a regenerating skeletal muscle [20,[27], [28], [29], [30]]. Nonetheless, response to extreme changes in the redox conditions (i.e. oxidative vs. reductive stresses) during SM regeneration remains unexplored.
Here, we hypothesized that an excess reductive capacity (i.e. abundant intracellular antioxidants) may abrogate the obligatory pro-oxidant signals required for the activation of satellite cells (i.e. proliferation and differentiation), thereby delaying or dampening the process of muscle regeneration. In this study, we investigated whether the activation of Nrf2 induces RS and impairs proliferation and differentiation of muscle stem cells, which are crucial for SM regeneration.
2. Material and methods
2.1. Cell culture, treatments and experiment design
C2C12 myoblasts were cultured in proliferation medium (DMEM containing 10% FBS). The cultured myoblasts (~80% confluency) were induced to myogenic differentiation by replacing proliferation medium with differentiation medium [31] (DMEM containing 2% horse serum). Myoblasts were subjected to pro-reductive environment or RS by dose-dependent treatment of sulforaphane (SF), N-acetyl cysteine (NAC) and glutathione reduced ethyl ester (GEE) or Keap1-knockdown using shRNA during proliferation (24 h) and differentiation phases (up to 5 days of differentiation). Following these treatments, the proliferating and differentiating myoblasts were analyzed for rate of differentiation (morphological, gene and protein markers) by qPCR [Primers for qPCR: Catalase (F-GGAGGCGGGAACCCAATAG; R-GTGTGCCATCTCGTCAGTGAA), Gclc (F-GGACAAACCCCAACCATCC; R-GTTGAACTCAGACATCGTTCCT), Gclm (F-CTTCGCCTCCGATTGAAGATG; R-AAAGGCAGTCAAATCTGGTGG), G6pd (F-TCAGACAGGCTTTAACCGCAT; R-CCATTCCAGATAGGGCCAAAGA), Nqo1 (F-AGGATGGGAGGTACTCGAATC; R-TGCTAGAGATGACTCGGAAGG), Gapdh (F-TGACCTCAACTACATGGTCTACA; R-CTTCCCATTCTCGGCCTTG), Myod1 (F-CCACTCCGGGACATAGACTTG; R-AAAAGCGCAGGTCTGGTGAG), Myogenin (F-GAGACATCCCCCTATTTCTACCA; R-GCTCAGTCCGCTCATAGCC)] and immunoblotting [Antibodies details: NQO1 (Abcam, AB34173); GSR (AB16801); GCLC (AB41463); GCLM (AB81445); G6PD (NB100-236); HO-1 (AB13248); SOD1 (AB13498); SOD2 (AB13534); MYOGENIN (AB124800); MYH2 (AB124937); CATALASE (EMD Millipore, 219010); GAPDH (Cell Signaling, D16H11)], respectively [31], glutathione redox state by enzyme kinetics (glutathione reductase recycling assay) [32], reactive oxygen species measurements (using Fluorescent probe) [33], and apoptosis using Annexin-V/propidium iodide [32,34] (FACS). Detailed methods are provided in the supplemental section.
2.2. Statistical analysis
Data are expressed as mean ± SEM. One-way ANOVA analysis was used to determine significant differences between control and SF/NAC/GEE treated groups, and Student T-test was used to compare the control and Keap1 knockdown cells. All the statistical comparisons were made between controls/undifferentiated cells to differentiated cells vs respective drug treated groups in each figure. p values smaller than 0.05 were considered statistically significant.
3. Results
3.1. Sulforaphane (SF) induces reductive stress (RS) and inhibits myoblast differentiation
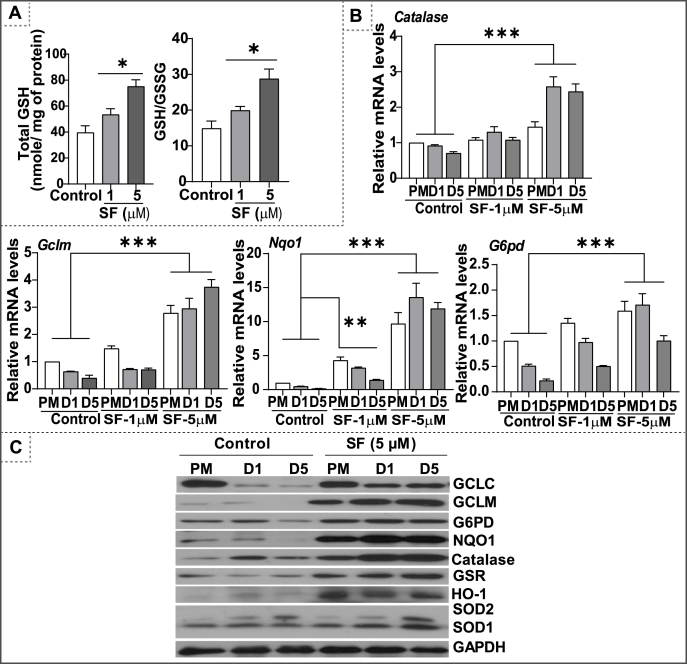
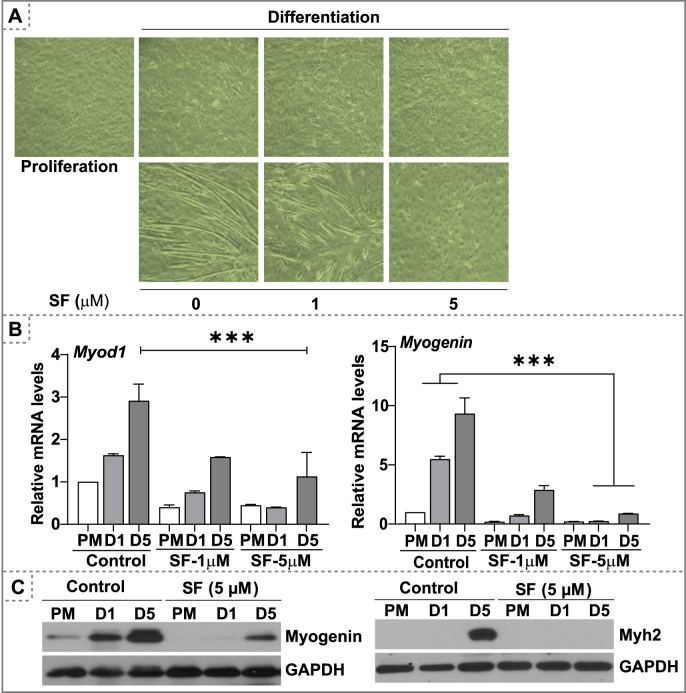
Glutathione (GSH) is an essential non-protein antioxidant thiol involved in maintaining redox homeostasis by neutralizing reactive oxygen species and scavenging oxidized proteins [35,36]. Previous studies demonstrated SF could enhance GSH levels and result in an intracellular reductive state [37,38]. Here, we tested the effect of SF on the status of Nrf2-regulated antioxidant genes during the proliferation and differentiation phases of myoblast growth. Exposing the cells with SF resulted in a dose dependent increase in glutathione (GSH) levels and redox ratio (GSH/GSSG; Fig. 1A) along with an upregulation of antioxidant genes, suggestive of an intensification of reductive environment (Fig. 1B). Of note, key antioxidant proteins involved in glutathione biosynthesis, GCLC, GCLM and G6PD were downregulated during the differentiation phase of myoblasts, indicating that a pro-oxidative setting is crucial for the differentiation process (Fig. 1C and Fig. S1). In contrast, SF treatment upregulated GCLM levels by 15–20 fold and NQO1 and catalase levels by 6–8 fold resulting in reductive stress (RS) during the differentiation phase of myoblasts (Fig. 1C and Fig. S1). Previously, we reported that Nrf2 loss-associated OS results in delayed regeneration of skeletal muscle. In this study, we hypothesized that enhanced antioxidant levels might accelerate myogenic differentiation. C2C12 myoblasts were pre-treated with a non-toxic dose of SF (1–5 μM) during proliferation (24 h) and differentiation (until day 5). Paradoxically, SF (1 and 5 μM) treatment showed diminished C2C12 myoblast differentiation (Fig. 2A). These findings were accompanied by a decrease in the gene expression of myogenic differentiation markers, during SF treatment (Fig. 2B). Early differentiation markers, Myod1 and myogenin levels were significantly decreased under 5.0 μM SF treatment (Fig. 2C). Myh2, a terminal differentiation marker was also downregulated by SF on day-1 and day-5 of differentiation (Fig. 2C). Overall, the overabundant intracellular glutathione and antioxidative stress resulted in a poor formation of myotubes, suggesting an impaired differentiation due to reductive stress.
Fig. 1.
Sulforaphane treatment establishes reductive stress in C2C12 myoblasts. (A) Glutathione levels and its redox (GSH/GSSG) ratio in the myoblasts following sulforaphane (SF) treatment was measured using enzymatic recycling assay. (B) qPCR based quantification of gene expression for some of the classical Nrf2-driven antioxidants (Gclc, Gclm, Nqo1, G6pd & Catalase) during proliferation (PM) and differentiation (day 1 & 5) phases. (C) Immunoblot analyses of antioxidant enzymes (i.e. GSR, GCLM, GCLC, NQO1, G6PD, Catalase, HO1 and SOD1/2) in proliferating and differentiating myoblasts on different time points (Differentiation on day 1 & 5). *p < 0.05, **p < 0.01 and ***p < 0.001, n = 3–4.
Fig. 2.
Reductive stress impairs myoblast differentiation. C2C12 myoblasts were cultured in proliferation medium to 70–80% confluence, subjected to differentiation with or without SF (1.0 & 5.0 μM). (A) Bright field microscopy images of C2C12 myoblast during proliferation and differentiation phase under SF treatment (day 1 & 5). (B) Q-PCR based relative gene expression of myoblast differentiation markers (Myod1 and Myogenin) during myoblast proliferation and differentiation (day 1 & 5). (E) Immunoblots for differentiation markers (Myogenin and Myh2) during myoblast proliferation and differentiation (day1 & 5) phases. ***p < 0.001, n = 3–4.
3.2. A pro-oxidative milieu is a pre-requisite for myogenic differentiation
Since we observed diminished levels of antioxidants during basal differentiation of myoblasts, we next addressed whether SF-mediated increase in antioxidants resulted in decreased cellular ROS (than the basal/physiological settings), which may lead to an impaired differentiation. To this end, we determined the ROS levels by flow cytometric analysis of proliferating & differentiating myoblasts incubated with DCFDA, a ROS sensitive probe that fluoresce upon oxidation. During normal differentiation, increased fluorescence was observed as an indication of elevated ROS levels in C2C12 myoblast (Fig. S2). On day-1 of differentiation, ROS levels were increased significantly from basal levels and further increased on days 3 & 5 (Figs. S1A–B). This finding is consistent with our observation that antioxidant proteins are decreased during myoblast differentiation (Fig. 1C). While the levels of antioxidants were augmented by SF treatment (1, 3 and 5 μM), basal ROS levels (observed during proliferation) and elevated ROS levels (observed during normal differentiation) were significantly decreased (Fig. S1). Though treatment with a lower dose of SF (1.0 μM) showed no significant change in ROS levels, 5.0 μM of SF dramatically decreased the ROS levels, which was associated with poor differentiation (Fig. 2). These results suggest that a moderate generation of ROS is necessary to facilitate the myoblast differentiation. In addition, shifting the redox milieu towards the reductive arm by SF treatment appears to prevent the differentiation of myoblasts into myotubes (Fig. 2A–C). Furthermore, FACS using Annexin V/Propidium Iodide (PI) and immunoblotting analyses indicated no evidence for apoptosis in the SF-treated myoblasts that experienced poor to no differentiation (Fig. S2).
3.3. Direct augmentation of intracellular glutathione also hampers myoblast differentiation
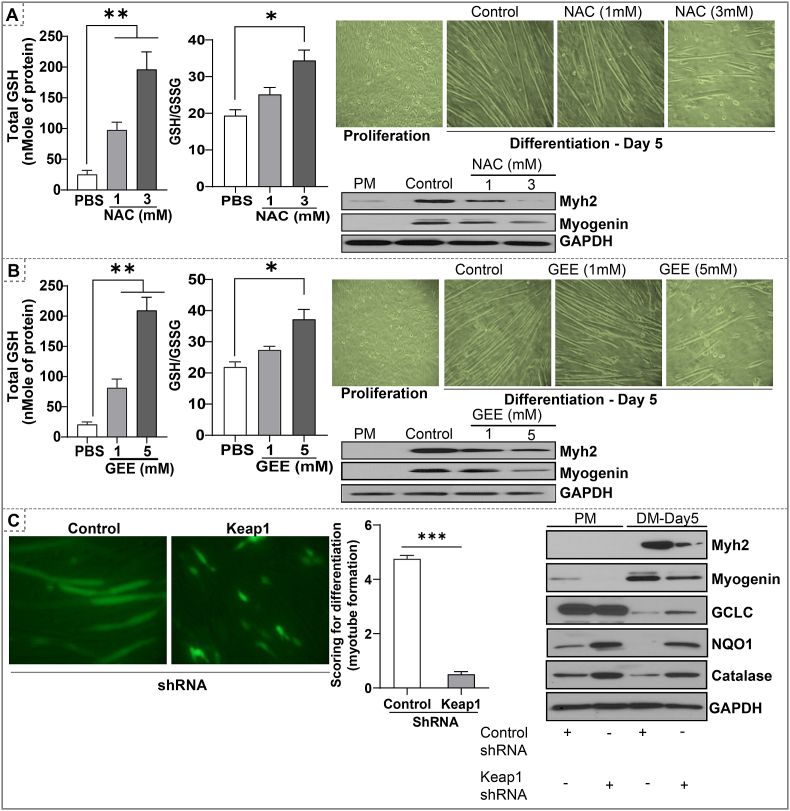
We next determined whether direct augmentation of intracellular glutathione could result in RS and inhibit myoblast differentiation. To address this possibility, we governed the effects of N-acetyl cysteine (NAC), a precursor of glutathione synthesis [39], and glutathione ethyl ester (GEE), a cell-permeable derivative of glutathione [40]. Both NAC and GEE supplementations significantly increased total GSH levels and the GSH/GSSG ratio (Fig. 3A–B). Either NAC or GEE at the concentration of 1.0 mM increases the total GSH levels by ~3 fold and the GSH/GSSG ratio by 1.5 fold (P < 0.05; Fig. 3A–B). Under these conditions, both NAC and GEE moderately inhibited the myoblast differentiation. The higher doses of NAC (3 mM) or GEE (5.0 mM) significantly increased total GSH levels by 7–8 fold and GSH/GSSG ratio by 2–3 fold (P < 0.01) which resulted in substantial inhibition of myotube formation (Fig. 3A–B). Immunoblot analysis of early and terminal differentiation markers, myogenin and Myh2, respectively, further corroborated with the inhibition of myoblast differentiation (Fig. 3A–B). These results suggest that reductive conditions established by NAC or GEE are sufficient to inhibit the myogenic differentiation process.
Fig. 3.
Direct augmentation of glutathione (i.e. NAC, GEE) or Keap1-silencing induce RS and inhibits myoblast differentiation. (A) NAC (1 and 3 mM) or (B) GEE treatment (1 and 5 mM) was performed in proliferating myoblast for 24 h and differentiating myoblasts until day-5. Total GSH and GSH/GSSG ratio in proliferating C2C12 cells treated with NAC/GEE, Bright field images of proliferating and differentiating myoblast (Magnification = 20X), immunoblot analysis of Myh2, and Myogenin in proliferating (PM) and differentiating (day 5) myoblast treated with NAC or GEE. *p < 0.05 and **p < 0.01, n = 3–4. (C) Immunofluorescence images of differentiating (day 5) myoblast after shRNA-mediated knockdown of Keap1 during proliferation phase. (B) GFP expressing myotubes were scored between 0-5 and the average of scoring were presented in bar graphs. (C) Western blots of markers of myoblast differentiation and antioxidants proteins in day 5 of differentiating myoblast after shRNA-mediated knockdown of Keap1. *p < 0.05, **p < 0.01 and ***p < 0.001, n = 3–4.
3.4. Silencing Keap1 activates the Nrf2/antioxidant signaling and retards myoblast differentiation
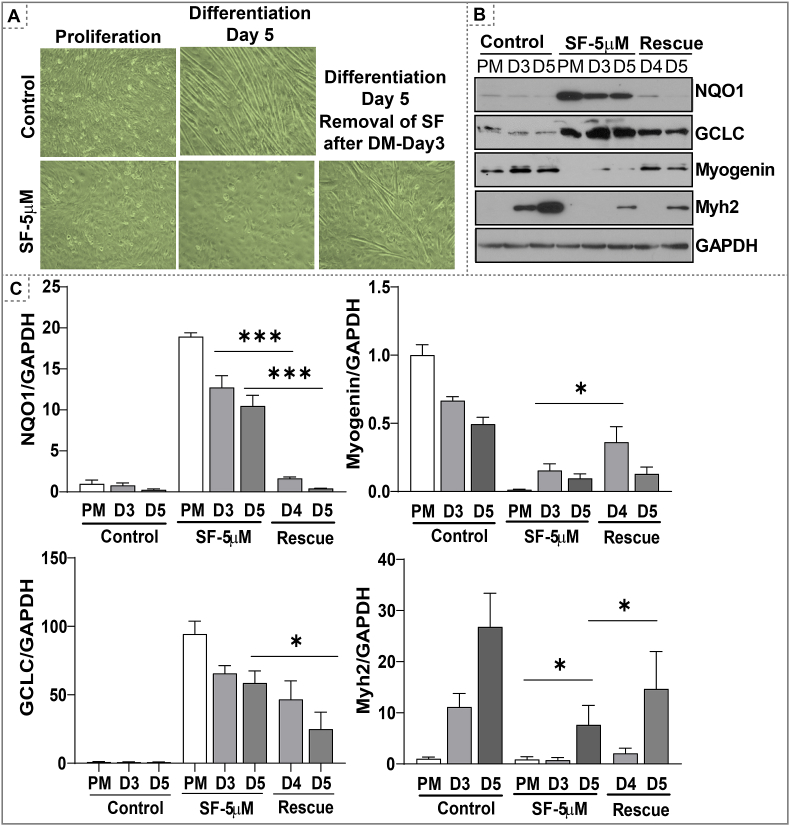
Keap1 is a negative regulator of Nrf2 activation [[41], [42], [43]]. Under basal conditions, Keap1 binds to Nrf2 and facilitates its proteasomal degradation [44]. Here, we silenced Keap1 by shRNA to inhibit proteasomal degradation of Nrf2 and induce Nrf2-dependent antioxidant signaling (Fig. 3C). The proliferating myoblasts were transfected with four different Keap1 shRNAs (1 to 4) and the protein levels of NQO1, a classical target of Nrf2, were measured to determine the type of shRNA that efficiently silences Keap1. Myoblasts expressing Keap1 shRNA-4 with highest Keap1 silencing showed impaired differentiation when compared to myoblasts transfected with mock shRNA (Fig. 3C). Rate of differentiation was semi-quantitatively analyzed by scoring on a scale of 1–10 (1 – Proliferating cells vs. 10 – fully differentiated myotubes) (Fig. 3C). Both early and late differentiation markers, myogenin and Myh2 protein levels were decreased in differentiating myoblasts expressing Keap1 shRNA-4 in relation to control shRNA (Fig. 3C). In addition, antioxidant proteins (GCLC, NQO1, and catalase) were also significantly increased in proliferating and differentiating myoblasts expressing Keap1 shRNA-4 (Fig. 3C). These results indicate that a genetic silencing of Keap1 and resultant activation of the Nrf2 antioxidant pathway impairs myoblast differentiation due to an enhanced reductive condition (i.e. RS). Of note, preventing RS through SF washout on day 3, efficiently rescued the differentiation of myoblasts (Fig. 4A-C).
Fig. 4.
Preventing RS (by withdrawing SF) rescues myoblast differentiation. Myoblasts were treated with SF (5 μM) during proliferation and differentiation phases until day 3. Removal of SF (washout) on day 3 by replenishing cells with fresh differentiation medium and allowed to differentiate until day 5. (A) Bright field images of proliferating and differentiating myoblast until day 5 under SF or rescued SF treatment. (B) Western blots of markers of myoblast differentiation and antioxidant proteins in proliferating and differentiating myoblasts. (C) Densitometry analysis of NQO1, myogenin, GCLC and MYH2 normalized with GAPDH was presented in bar graphs. *p < 0.05 and ***p < 0.001, n = 3–4.
4. Discussion
Nrf2/antioxidant signaling constitutes cellular defense systems and protects them during stress conditions. We previously demonstrated that an age-related loss of Nrf2 function results in oxidative stress (OS) and delays satellite cell activation and skeletal muscle (SM) regeneration [45]. In the present investigation, we attempted to attenuate OS-mediated impaired SM regeneration by pharmacologically/genetically activating Nrf2-antioxidant signaling. Unexpectedly, a pharmacological activation of Nrf2 by sulforaphane (SF) resulted in a gradual inhibition of myoblast differentiation. Importantly, the genetic silencing of Keap1, a suppressor of Nrf2, promoted the Nrf2-dependent induction of antioxidants, which also resulted in significantly impaired myoblast differentiation. These results indicate that shifting the redox milieu towards the reductive arm (i.e. RS) could negatively influence the process of myogenic differentiation.
Our data demonstrate a marked decrease in expression of major antioxidant enzymes (GCLC, GLCM, G6PD, etc.) at both protein (Fig. 1C) and transcript levels (Fig. 1B) during the normal transition of myoblasts from proliferation to differentiation. These observations indicate a prerequisite of restrained Nrf2-antioxidant signaling in cells that undergo differentiation. Downregulation of antioxidant enzymes (i.e. GCLC and GCLM) resulted in the depletion of GSH and increased GSSG to maintain a pro-oxidative condition during myoblast differentiation. Notably, under SF treatment, antioxidant levels were uplifted and subsequently led to an increase in intracellular GSH levels (Fig. 1A), causing RS that inhibited the myoblast differentiation. These findings suggest that while a pro-oxidative setting favors myocyte differentiation, RS seem to prevent this process. Therefore, the future studies warranted to define an optimal intracellular redox environment that could facilitate the activation of muscle stem cells and promote differentiation of myoblasts into myotubes, thereby triggering the regeneration process in response to injury or damage.
During RS, inhibition of myoblast differentiation was accompanied with a decrease of early (myogenin and Myod1) and terminal (Myh2) myogenic differentiation markers (Fig. 2). In contrast, age-related OS has been reported to impair satellite cell activation causing inhibition of myogenic differentiation [45]. Age-related OS has been demonstrated to promote apoptosis of muscle and progenitor satellite cells leading to muscle loss [46,47]. Of note, RS partially induced C2C12 myoblast proliferation and did not induce apoptosis during either the proliferation or differentiation phase (Fig. S2). To our surprise, the myoblasts under RS were neither proliferating nor differentiating, suggesting that the RS engages a quiescent state (G1 phase of the cell cycle) in the myoblasts. Interestingly, preventing the RS by withdrawing SF restored the myogenic differentiation process. Although a G1 arrest is typically coupled to the differentiation process, an inhibition of the differentiation process was observed without apoptosis. Of note, besides G1 arrest, several factors and events such as activation of p38 MAPK kinase and histone deacetylase are essential to drive the differentiation process [48,49]. One or more of these events may be hindered by an extensive reductive redox (i.e. RS) condition in the myoblasts receiving chronic SF. These interesting questions are to be addressed in future studies. Consistent with this notion, the SF wash-out experiments (Fig. 4) partially rescued the differentiation, indicating that the cells have been maintained in a functionally active and conditionally reversible state. Thus, the RS-induced cell cycle arrest could be related to cell senescence-associated cytostasis [50].
In summary, this study demonstrates that suppression of Nrf2-antioxidant signaling occurs during physiological myogenic differentiation that creates a pro-oxidative environment, which is essential for myoblast differentiation. Nonetheless, activation of Nrf2/antioxidant signaling establishes a reductive condition that causes RS, which impairs myogenic differentiation.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Acknowledgments
This study was supported by funding from NHLBI (1HL118067), NIA (AG042860), the AHA (BGIA 0865015F), University of Utah Center for Aging Pilot grant (2009), the start-up funds from the Division of Cardiovascular Medicine/Department of Medicine, University of Utah and by Department of Pathology, the University of Alabama at Birmingham, AL (for NSR) and UAB-AMC21 grant by the University of Alabama at Birmingham, AL.
Authors thank Dr. Madhusudhanan Narasimhan (TTUHSC) for informal discussion on this manuscript. Authors also thank Dr. Gobinath Shanmugam, Dr. Kishore Kumar SN, Dr. Sini Sunny and Dr. Christopher Davidson for their editorial assistance. Author appreciate Miss. Snekha N. Rajasekaran for grammar check on the revision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101492.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bo H. Mitochondrial redox metabolism in aging: effect of exercise interventions. J. Sport Health Sci. 2013;2(2):67–74. [Google Scholar]
- 2.Agerholm M. Perturbations of NAD(+) salvage systems impact mitochondrial function and energy homeostasis in mouse myoblasts and intact skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2018;314(4):E377–e395. doi: 10.1152/ajpendo.00213.2017. [DOI] [PubMed] [Google Scholar]
- 3.Elkalaf M., Andel M., Trnka J. Low glucose but not galactose enhances oxidative mitochondrial metabolism in C2C12 myoblasts and myotubes. PloS One. 2013;8(8) doi: 10.1371/journal.pone.0070772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He F. Redox mechanism of reactive oxygen species in exercise. Front. Physiol. 2016;7:486. doi: 10.3389/fphys.2016.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrhardt J., Morgan J. Regenerative capacity of skeletal muscle. Curr. Opin. Neurol. 2005;18(5):548–553. doi: 10.1097/01.wco.0000177382.62156.82. [DOI] [PubMed] [Google Scholar]
- 6.Tedesco F.S. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J. Clin. Invest. 2010;120(1):11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musar A. The basis of muscle regeneration. Adv. Biol. 2014;2014:16. [Google Scholar]
- 8.Almeida C.F. Muscle satellite cells: exploring the basic biology to rule them. Stem Cell. Int. 2016;2016:14. doi: 10.1155/2016/1078686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Relaix F., Zammit P.S. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139(16):2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- 10.Relaix F. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435(7044):948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 11.Buckingham M. Skeletal muscle progenitor cells and the role of Pax genes. C R Biol. 2007;330(6–7):530–533. doi: 10.1016/j.crvi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Kassar-Duchossoy L. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19(12):1426–1431. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young A.P., Wagers A.J. Pax3 induces differentiation of juvenile skeletal muscle stem cells without transcriptional upregulation of canonical myogenic regulatory factors. J. Cell Sci. 2010;123(Pt 15):2632–2639. doi: 10.1242/jcs.061606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zammit P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Lepper C., Fan C.M. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48(7):424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu X., Wang H., Hu P. Stem cell activation in skeletal muscle regeneration. Cell. Mol. Life Sci. 2015;72(9):1663–1677. doi: 10.1007/s00018-014-1819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang J.S., Krauss R.S. Muscle stem cells in developmental and regenerative myogenesis. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13(3):243–248. doi: 10.1097/MCO.0b013e328336ea98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepper C., Conway S.J., Fan C.M. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460(7255):627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzel F.R. Formation of reactive oxygen species at increased contraction frequency in rat cardiomyocytes. Cardiovasc. Res. 2006;71(2):374–382. doi: 10.1016/j.cardiores.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Kozakowska M. The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015;36(6):377–393. doi: 10.1007/s10974-015-9438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44(2):532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 22.Morley J.E., Anker S.D., von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014;5(4):253–259. doi: 10.1007/s13539-014-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pace G.W., Leaf C.D. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 1995;19(4):523–528. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 24.Niess A.M., Simon P. Response and adaptation of skeletal muscle to exercise--the role of reactive oxygen species. Front. Biosci. 2007;12:4826–4838. doi: 10.2741/2431. [DOI] [PubMed] [Google Scholar]
- 25.Powers S.K., Talbert E.E., Adhihetty P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011;589(Pt 9):2129–2138. doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng S.J., Yu L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010;11(4):1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langen R.C. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. Faseb. J. 2004;18(2):227–237. doi: 10.1096/fj.03-0251com. [DOI] [PubMed] [Google Scholar]
- 28.Langen R.C. Tumor necrosis factor-alpha inhibits myogenesis through redox-dependent and -independent pathways. Am. J. Physiol. Cell Physiol. 2002;283(3):C714–C721. doi: 10.1152/ajpcell.00418.2001. [DOI] [PubMed] [Google Scholar]
- 29.Fedorova M., Kuleva N., Hoffmann R. Reversible and irreversible modifications of skeletal muscle proteins in a rat model of acute oxidative stress. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2009;1792(12):1185–1193. doi: 10.1016/j.bbadis.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Le Moal E. Redox control of skeletal muscle regeneration. Antioxidants Redox Signal. 2017;27(5):276–310. doi: 10.1089/ars.2016.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolus D.J. Recurrent heat shock impairs the proliferation and differentiation of C2C12 myoblasts. Cell Stress & Chaperones. 2018;23(3):399–410. doi: 10.1007/s12192-017-0851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanmugam G. A biphasic effect of TNF-α in regulation of the Keap1/Nrf2 pathway in cardiomyocytes. Redox biology. 2016;9:77–89. doi: 10.1016/j.redox.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eruslanov E., Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 34.Li H. PFN2a suppresses C2C12 myogenic development by inhibiting proliferation and promoting apoptosis via the p53 pathway. Cells. 2019;8(9):959. doi: 10.3390/cells8090959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patlevic P. Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integr Med Res. 2016;5(4):250–258. doi: 10.1016/j.imr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espinosa-Diez C. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dias I.H. Sulforaphane restores cellular glutathione levels and reduces chronic periodontitis neutrophil hyperactivity in vitro. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forman H.J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspect. Med. 2009;30(1–2):1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014;141(2):150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Mårtensson J., Jain A., Meister A. Glutathione is required for intestinal function. Proc. Natl. Acad. Sci. U.S.A. 1990;87(5):1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanmugam G. Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice. Redox Biol. 2019;27:101212. doi: 10.1016/j.redox.2019.101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuadrado A. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18(4):295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98(3):1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itoh K., Mimura J., Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxidants Redox Signal. 2010;13(11):1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 45.Shelar S.B. Disruption of nuclear factor (erythroid-derived-2)-like 2 antioxidant signaling: a mechanism for impaired activation of stem cells and delayed regeneration of skeletal muscle. Faseb. J. 2016;30(5):1865–1879. doi: 10.1096/fj.201500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narasimhan M. Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radic. Biol. Med. 2014;71:402–414. doi: 10.1016/j.freeradbiomed.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomes M.J. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8(12):20428–20440. doi: 10.18632/oncotarget.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mal A., Harter M.L. MyoD is functionally linked to the silencing of a muscle-specific regulatory gene prior to skeletal myogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100(4):1735–1739. doi: 10.1073/pnas.0437843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puri P.L., Sartorelli V. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell. Physiol. 2000;185(2):155–173. doi: 10.1002/1097-4652(200011)185:2<155::AID-JCP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 50.Kornienko J.S. High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci. Rep. 2019;9(1):1296. doi: 10.1038/s41598-018-37972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.