Abstract
The redox kinetics involving the reaction of N, N′-phenylenebis(salicyalideneiminato)cobalt(III) ([CoSalophen]+) and l-cysteine (LSH) was studied using pseudo-first order approach under the following conditions, [H+] = 1.0 × 10−3 mol/dm3, μ = 0.1 C2 mol/dm3 (NaCl), λmax = 470 nm and T = 27 ± 1 °C in DMSO: H2O; 1:4 v: v medium. The redox reaction was 1st order in both [CoSalophen+] and [LSH], with the overall 2nd order. Hydrogen ion concentration effect revealed the activeness of both the protonated and deprotonated form of the reductant, positive Brønsted-Debye salt effect and was also ion catalyzed. There was no evidence suggesting an intermediate complex of significant stability in the reaction. Free radical was detected to take part and as such the reasonable mechanistic pathway for the reaction is suggested to be outer-sphere, hence proposed.
Keywords: Inorganic chemistry; Kinetics; Mechanisms; N,N′-phenylenebis(salicyalideneiminato)cobalt(III); l-Cysteine; Mixed aqueous medium
Inorganic chemistry; Kinetics; Mechanisms; N,N′-phenylenebis(salicyalideneiminato)cobalt(III); l-Cysteine; Mixed aqueous medium
1. Introduction
Schiff base complexes of cobalt(III) are utilized as impetuses for the oxygenation reactions of organic molecules (Niederhoffer et al., 1984), eminently in the preparative oxygenation of phenols (Matsuura, 1977), indols (Nishinaga et al., 1993) and amines (Nishinaga et al., 1988), a reversible process similar to Co(III)/Co(II) redox potential (Hirotsu et al., 1996). It likewise discovers applications in oxidative coupling catalytic reactions of phenols and oxidation of olefins (Zhang et al., 2003). The catalytic activity of Co(III) salen (bis(salicylidene)ethylenediamine) complexes demonstrates the catalytically dynamic species to be cobalt in the +3 oxidation state (Eichhorn et al., 1992, 1993; 1997; Eichhorn and Speiser, 1994; Nishinaga et al., 1983; Speiser and Stahl, 1995).
On the other hand, these complexes have been utilized extensively to imitate cobalamin (B12) coenzymes (Aly, 1998; Amirnasr et al., 2001; Cini et al., 1998; Polson et al., 1997), classified as an oxygen carrier (Niederhoffer et al., 1984) and oxygen activators (Bianchini and Zoellner, 1996; Henson et al., 1999; Yamada, 1999). Metal complexes of salophen (bis(salicylidene)phenylenediamine) and salen-type ligands also find applications in the intercalation of DNA base pairs (Kocak et al., 2016; Yilmaz et al., 2017) and for potentiometric discoveries of basic anions existing in biological and environmental systems (Kleij et al., 2006). Salophen-type ligands have additionally been utilized as nitrite sensors (Ganjali et al., 2004). In addition, Co(III) Schiff base complexes have been utilized as antimicrobial agents (Böttcher et al., 1997).
Furthermore, Co(III) salen complexes have shown an outstanding selectivity to poly(propylenecarbonate) development with 99% of carbonate linkages. This led to significant efforts been devoted to the synthesis of these complexes as well as mechanistic studies for a better understanding of their reaction mechanism (Cohen and Coates, 2006; Hošt’álek et al., 2015; Lu and Wang, 2004; Luinstra et al., 2005). Recently, Co(III) salen metal complexes have been used in the electro-reduction of CO2 to CO, acting as a multi-electron reducing agent (Solomon et al., 2018).
L-cysteine plays structural roles in proteins and can likewise partake in electron transfer reactions and its sulfhydryl group, thiolate (RS−) is stabilized in a particular environment. However, the sulfhydryl group of free l-cysteine is somewhat inert for redox reaction in physiological conditions. Thiolate is a relatively more potent nucleophilic agent than its protonated structure. As such, reactive l-cysteine is in its anionic structure mostly in proteins. In addition, l-cysteine is significant for numerous protein functions; the molecule is toxic to cells in both prokaryotes and eukaryotes even at low concentrations (El-Hag and Dahab, 2016; Özkan et al., 2002).
Thiol-containing compounds are easily susceptible to oxidation and hence a reason for most of their biological functions (Friedman, 1973; Jocelyn, 1972) and numerous kinetic investigations have been stated on the redox reactions with a view to understand the role of enzymes containing l-cysteine group in electron transfer reactions. Oxidation of thiols by metal ions (Amjad et al., 1977; Ayoko et al., 1993; Ayoko and Olatunji, 1983; Gangopadhyay et al., 1994; Ghosh et al., 1987; Grases et al., 1986; McAuley and Olatunji, 1977; Olatunji and Okechukwu, 1987; Ozawa et al., 1992; Sisley and Jordan, 1995) and non-metallic oxidants (Abedinzadeh et al., 1989; Elskens et al., 1988; Hupe and Wu, 1980; Konidari et al., 1992; Shaked et al., 1980; Weaver and Rabenstein, 1995; Zhao et al., 1994) have been stated to proceed through different mechanisms. An example is where dichromate was used, the overall reaction mechanism involved the formation a thioester as an intermediate, then intra- and inter-molecular redox reactions (Bose et al., 1992; Brauer and Wetterhahn, 1991). However, with Cr(VI) and l-cysteine, redox reaction occurs through an inner-sphere mechanism (Adari et al., 2006), but with Mo(VI) and 12-tungstocobaltate(III), the reaction occurs through an outer-sphere mechanistic pathway (Ayoko and Olatunji, 1983; Martin and Spence, 1970; Sami et al., 2009).
Reports have also been previously published involving oxidation of l-cysteine by Corey's reagents (Adari et al., 2006), trinuclear Mn(IV) species (Chakraborty et al., 2012) [Mn4O6]4+ core (Chakraborty et al., 2013), potassium ferrate (Read et al., 2000) and 11-tungstophosphovanate(V) (Sami et al., 2009).
Similarly, this research studies the kinetics of redox reaction of [CoSalophen]+, (Salophen = Bis(salicylidene)phenylenediamine) by l-cysteine (LSH) and reported herein for the mechanism of the reaction of this complex to be further understood.
2. Methodology
Chemicals used for this research work were of analytical quality and were used without further purification. The rates of reactions were studied by monitoring the reaction mixture as the absorbance decreases with increasing reaction time at 470 nm on a SHERWOOD colorimeter 254. The reducing agent used was L-cysteine (BDH) while sodium chloride (M&B) salt was used to stabilize the strength of ions in the reaction medium. Cobalt(II) chloride hexahydrate, salicyaldehyde, O-phenylenediamine, chloroform, ethanol, H2O2, and diethyl ether were obtained from Merck.
The Schiff base, bis(salicylidene)-1,2-phenylenediamine, (Salophen), the complex, N,N′-phenylenebis(salicylideneiminato)cobalt(III), ([CoSalophen]+) were synthesized and characterized according to the reported procedure (Pizzolato et al., 2013).
The Schiff base (ligand) was set up by refluxing ortho-substituted phenylenediamine (1.00 g, 9.25 mmol) with benzylaldehyde (1.97 mL, 18.50 mmol) in ethanol (30 mL) at 78 °C for 10 min and the crude cooled to 0 °C. Filtration was used to collect the precipitate, then washed and dried with ethanol and desiccator, respectively. Yield: 2.37 g (91%).
To prepare the Schiff base complex, a blend of the ligand (0.79 g, 2.50 mmol) together with hydrated cobalt(II) chloride (Aldrich) (0.63 g, 2.30 mmol) was stirred in methanol (60 mL) for 3 h at room temperature followed by oxidation with H2O2. Ethoxy ethane (20 mL) was added to induce precipitate formation. The precipitate was filtered, washed and recrystallized using filter paper, ethoxy ethane, and chloroform, respectively. Yield 0.59 g (78%).
The complex structure is depicted in Scheme 1.
Scheme 1.
Structure of N,N′-phenylenebis(salicylideneiminato)cobalt(III) complex.
Conductivity measurements were carried out at room temperature utilizing a HACH Sension5 conductivity meter. Molar conductivity of synthesized complex with the concentration of 1.0 × 10−4 mol/dm3 1:4, v: v of DMSO: H2O was obtained as 122 cm2/Ohm/mol with 12.2 × 10−6/Ohm/cm as the specific conductance, indicating an electrolytic ratio of 1:1.
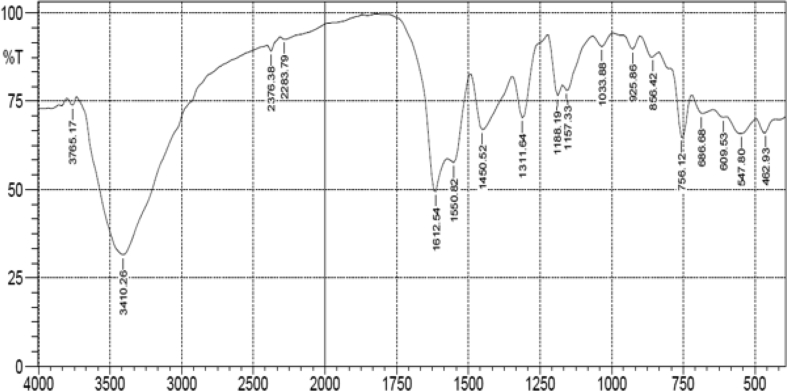
Fourier transform infrared (FT-IR) spectra were recorded on shimadzu varian 8400 spectrophotometer in KBr pellets. The FT-IR spectrum of the complex showed the complexation of the ligand with the complex by a slight shift of peak due to the imine (v(C=N)) bond from 1620cm−1 to 1612cm−1 (Salavati-niasari & Shaterian, 2007). Peaks around 547cm−1 and 462cm−1 was assigned to v(Co–O) and v(Co–N) respectively (Figures 1 and 2). Magnetic susceptibility (μeff, BM) measurement shown that the Cobalt(III) complex synthesized is of low spin inner orbital hybridized with a magnetic moment μeff of 0.2 BM. This value indicates the oxidation to Co(III) during the preparation of the complex(Abu-Surrah et al., 2008).
Figure 1.
FT-IR spectrum of the Salophen ligand.
Figure 2.
FT-IR spectrum of the Co(III)Salophen complex.
Spectrophotometric titration utilized for this study was the mole proportion method of stoichiometric determination. the stoichiometry of the reaction using the method of mole proportion. The concentration of [CoSalophen]+ was kept consistent while the proportion of [LSH]: [CoSalophen]+ was differed from (0.25–4.00) at μ = 0.1 C2 mol/dm3 (NaCl) and T = 27 ± 1 °C. A plot of absorbance against mole proportion gave the reaction stoichiometry. A point of inflexion on the graph indicated the mole proportion (Hamza et al., 2012).
Every kinetic measurement was done under condition of pseudo-first order with [LSH] in excess over [CoSalophen+] by at least 20 fold at 0.1 C2 mol/dm3 (NaCl), [H+] = 1.0 × 10−3 mol/dm3 (HCl) and T = 27 ± 1 °C. Pseudo-first order plots of log (At-A∞) versus time were made (where At and A∞ are the absorbance at time t and at the end of the reaction, respectively). Pseudo-first order rate constants, k1 values were obtained from the slopes of the plots. The second order constants (k2) were calculated as shown in Eq. (1):
| (1) |
The effect changing the concentration of H+ on the reaction rate was examined by changing the H+ concentration within the range of (1.0–18.0) × 10−3 mol/dm3, while keeping [CoSalophen+] and [LSH] of 2.0 × 10−4 mol/dm3 and 12.0 × 10−3 mol/dm3, respectively constant at T = 27 ± 1 °C and μ = 0.1 C2 mol/dm3. The second-order acid-dependent variation with [H+] was achieved from the plot of k2 against [H+] (Adetoro et al., 2011).
The effect of reaction rate as ionic strength of the medium of reaction is varied was examined within range of 0.04–0.18 C2 mol/dm3, with constant concentrations of the reactants at 27 ± 1 °C. A plot log k2 against √μ was also made.
The effect of added ions were examined for [Z] = 3.0–18.0 × 10−3 mol/dm3 ([Z] = Ca2+/SO42−) at constant [CoSalophen+], [LSH] and μ. The effect of temperature on the reaction rates was studied within 300–325 K. The data were then considered using Eyring plot of ln k2/T versus 1/T and thermodynamic parameters also were determined at constant [CoSalophen+], [LSH] and ionic strength. Free radical participation as the reaction is occurring was investigated by addition of 5 cm3 of 0.02 mol/dm3 solution of acrylamide to the mixture of reactions that has oxidized partially then addition of excess methanol.
During the reaction progress, comparison was made between the spectra of the mixture of reaction with that of the complex within the range of wavelength 400–700 nm. A graph of 1/k1 against 1/[LSH] of Michaelis-Menten type was also made.
3. Results and discussion
Stoichiometric results obtained for this study revealed a mole proportion to be 1 to 1 base on the reaction as depicted in Figure 3. The stoichiometric equation for the reaction is shown below:
| (2) |
Figure 3.
Absorbance versus mole ratio plot for the reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 × 10−4 mol/dm3, [H+] = 1.0 × 10−3 mol/dm3, μ = 0.1 C2 mol/dm3, [LSH] = (0.5–7.0) × 10−4 mol/dm3, T = 27 ± 1 °C and λmax = 470 nm.
A result of stoichiometry analogous to this research was reported previously in the reduction of heteropoly 11-tungstophosphovanadate(V) by l-cysteine (Komiyama et al., 1999).
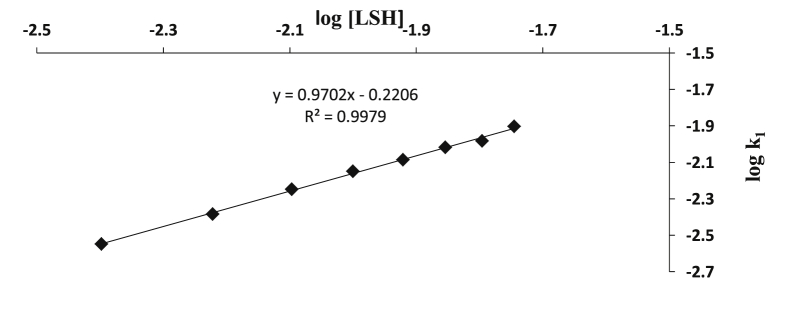
The plots of pseudo-first order, log (At − A∞) against time were linear to 80% completion of the reaction (Figure 4) suggesting a first order dependence in [CoSalophen+]. The order with respect to [LSH] was determined from a plot log k1 against log [LSH]. The slope of the plot was 0.97 (Figure 5). The values of the second order rate constants remained similar and fairly the same (Table 1). The rate law for the reaction is represented by Eq. (3).
| (3) |
where k2 = (6.9 ± 0.18) × 10−1 dm3/mol/s.
Figure 4.
Typical pseudo-first order plot for the reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 10−4 mol/dm3, LSH = 12.0 × 10−3 mol/dm3, [H+] = 1.0 × 10−3 mol/dm3, μ = 0.1 C2 mol/dm3, T = 27 ± 1 °C and λmax = 470 nm.
Figure 5.
Plot of log k1 versus log [LSH] for the redox reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 10−4 mol/dm3, [H+] = 1.0 × 10−3 mol/dm3, μ = 0.1 C2 mol/dm3, T = 27 ± 1 °C and λmax = 470 nm.
Table 1.
The pseudo-first order and second order rate constants for the reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 10−4 mol/dm3, T = 27 ± 1 °C and λmax = 470 nm.
| 103[LSH],(mol/dm3) | 103H+,(mol/dm3) | μ, (C2 mol/dm3) | 103 k1,(/s) | 101 k2,(dm3/mol/s) |
|---|---|---|---|---|
| 4.00 | 1.00 | 0.10 | 2.83 | 7.08 |
| 6.00 | 1.00 | 0.10 | 4.14 | 6.89 |
| 8.00 | 1.00 | 0.10 | 5.65 | 7.06 |
| 10.00 | 1.00 | 0.10 | 7.09 | 7.09 |
| 12.00 | 1.00 | 0.10 | 8.20 | 6.83 |
| 14.00 | 1.00 | 0.10 | 9.58 | 6.84 |
| 16.00 | 1.00 | 0.10 | 10.43 | 6.52 |
| 18.00 | 1.00 | 0.10 | 12.51 | 6.95 |
| 12.00 | 1.00 | 0.04 | 5.97 | 4.97 |
| 12.00 | 1.00 | 0.06 | 6.89 | 5.74 |
| 12.00 | 1.00 | 0.08 | 7.58 | 6.31 |
| 12.00 | 1.00 | 0.10 | 8.24 | 6.87 |
| 12.00 | 1.00 | 0.12 | 8.77 | 7.31 |
| 12.00 | 1.00 | 0.14 | 9.53 | 7.95 |
| 12.00 | 1.00 | 0.16 | 10.20 | 8.50 |
| 12.00 | 1.00 | 0.18 | 11.01 | 9.17 |
| 12.00 | 1.00 | 0.10 | 8.20 | 6.83 |
| 12.00 | 3.00 | 0.10 | 18.10 | 15.10 |
| 12.00 | 6.00 | 0.10 | 31.10 | 25.90 |
| 12.00 | 9.00 | 0.10 | 54.10 | 45.40 |
| 12.00 | 12.00 | 0.10 | 70.20 | 58.50 |
| 12.00 | 15.00 | 0.10 | 89.60 | 74.70 |
| 12.00 | 18.00 | 0.10 | 97.80 | 81.50 |
A second order dependence in [LSH] was previously reported in the reaction of LSH by trans-dichlorotetracyanoplatinate(IV) (Shi et al., 1996), heteropoly 11-tungstophosphovanadate (V) (Sami et al., 2009) and 12-tungstocobaltate(III), while a fractional order dependence in [LSH] in the oxidation of l-cysteine by Corey's reagent (Adari et al., 2006).
The reaction displayed acid independent and dependent pathways as obtained from a plot of k2 versus [H+] presented in Figure 6. Acid dependence effect of this kind is represented by Eq. (4)
| (4) |
a = 0.16 dm3/mol/s and b = 464.16 dm6/mol2/s.
Figure 6.
Plot of k2 versus [H+] for the reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 × 10−4 mol/dm3, [LSH] = 12.0 × 10−3 mol/dm3, [H+] = (1.0–18.0) × 10−3 mol/dm3, μ = 0.1 C2 mol/dm3, T = 27 ± 1, and λmax = 470 nm.
The final rate scheme for the reaction is presented in Eq. (5)
| (5) |
This nature of acid dependence is signifying that the un-protonated and protonated species of the reactant are reactive (Gupta and Gupta, 1984) as shown in Eq. (6).
| (6) |
Similar results of acid dependence were reported by earlier researchers in the reaction of l-cysteine (Ayoko et al., 1993; Ayoko and Olatunji, 1983; Chakraborty et al., 2012).
From Table 1, the results indicated the reaction rate to be enhanced as the strength of ions in the medium of the reaction is increased. A straight line graph was obtained from a graph of log k2 against √μ with positive slope (+1.1) (Figure 7), indicating a positive effect of salt based Brønsted – Debye model. This indicates the participation of the same charged species with a magnitude of 1 at the slow step (Onu et al., 2009).
Figure 7.
Plot of logk2 versus √μ for the reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 × 10−4 mol/dm3, [LSH] = 12.0 × 10−3 mol/dm3, [H+] = 1.0 × 10−3 mol/dm3, μ = (0.04–0.18) C2 mol/dm3, T = 27 ± 1, and λmax = 470 nm.
The reaction rate was found to be inhibited in the presence of added cations while anions catalyzed the reaction rate (Ca2+ and SO42-, Table 2). Catalysis of added ion suggests that the mechanism of the reaction is outer-sphere (Ibrahim et al., 2019). The formation of a gel was observed on adding acrylamide, which is a radical scavenger, to partially reacted mixture in excess methanol suggesting the presence of free radicals in the reaction (Alioke et al., 2012).
Table 2.
The Effect of added anion and cation on the rate of reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 10−4 mol/dm3, [LSH] = 12.0 × 10−3 mol/dm3, [H+] = 1.0 × 10−3 mol/dm3, μ = 0.1 C2 mol/dm3, T = 27 ± 1, and λmax = 470 nm.
| [Z] | 103 [Z], (mol/dm3) | 103 k1, (/s) | 101 k2, (dm3/mol/s) | |
|---|---|---|---|---|
| SO42- | 0.0 | 8.20 | 6.83 | |
| 3.0 | 8.39 | 6.99 | ||
| 6.0 | 8.59 | 7.16 | ||
| 9.0 | 8.67 | 7.22 | ||
| 12.0 | 8.78 | 7.31 | ||
| 15.0 | 8.95 | 7.45 | ||
| 18.0 | 9.10 | 7.58 | ||
| Ca2+ | 0.0 | 8.20 | 6.83 | |
| 3.0 | 7.14 | 5.95 | ||
| 6.0 | 5.56 | 4.63 | ||
| 9.0 | 4.14 | 3.45 | ||
| 12.0 | 3.48 | 2.90 | ||
| 15.0 | 2.55 | 2.13 | ||
| 18.0 | 0.94 | 0.79 | ||
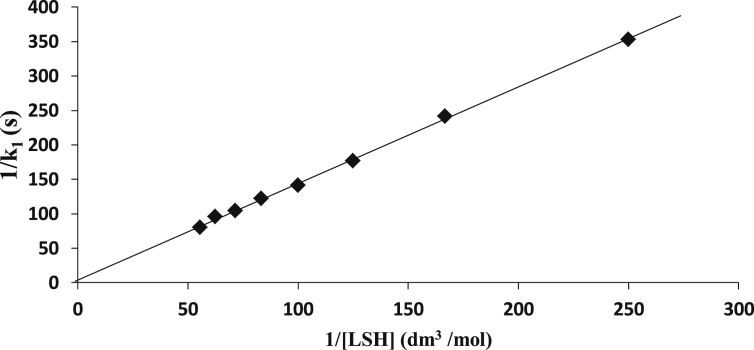
No shift in λmax (470 nm) was observed when the spectrum of the reaction mixture was matched with that of [CoSalophen]+ (Figure 8). Also, a Michaelis–Menten type plot of 1/k1 versus 1/[LSH] had negligible intercept suggesting the lack of evidence to support the presence of a stable and detectable intermediate in the reaction (Figure 9). This lack of evidence for the detectable intermediary complex is evidence for reactions occurring through outer-sphere mechanistic path.
Figure 8.
Plot for spectrophotometric test for the redox reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 10−4 mol/dm3, [LSH] = 12.0 × 10−3 mol/dm3, [H+] = 1.0 × 10−3 mol/dm3, μ = 0.1 C2 mol/dm3, T = 27 ± 1, and λmax = 470 nm.
Figure 9.
Michaelis-Menten plot for the redox reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 10−4 mol/dm3, [LSH] = 12.0 × 10−3 mol/dm3, [H+] = 1.0 × 10−3 mol/dm3, μ = 0.1 C2 mol/dm3, T = 27 ± 1, and λmax = 470 nm.
The results of temperature dependence on the rate constants and activation parameters for the reaction are depicted in Table 3. The relatively high negative value of ΔS∗ indicates that at the rate-determining step, the redox process is spontaneous (Watts, 1973).
Table 3.
Temperature dependent rate constants and activation parameters for the redox reaction of [CoSalophen]+ and LSH at [CoSalophen+] = 2.0 10−4 mol/dm3, [LSH] = 12.0 × 10−3 mol/dm3, [H+] = 1.0 × 10−3 mol/dm3, μ = 0.1C2 mol/dm3, T = 27 ± 1, and λmax = 470 nm.
| T (K) | 103 k1 (/s) | 101 k2 (dm3/mol/s) |
|---|---|---|
| 300 | 8.20 | 6.83 |
| 305 | 12.41 | 10.34 |
| 310 | 15.13 | 12.61 |
| 315 | 15.25 | 12.70 |
| 320 | 18.82 | 15.68 |
| 325 |
22.57 |
18.81 |
| Activation parameters | ||
| ΔHǂ = +28 kJ/mol | ΔSǂ = -158 J/mol/K | |
| ΔGǂ = +74 kJ/mol at 300 K | Ea = +29 kJ/mol | |
The quantitative test of Co2+ was established by adding a few drops of potassium thiocyanate in excess acetone to the reaction product. The blue solution formed in the reaction mixture showed the presence of Co2+ (Osunlaja et al., 2013). The presence of disulphide, LSSL was ascertained by comparing the reaction mixture's spectrum after the completion of the reaction with the spectrum obtained when LSH was treated the H2O2 which is known to oxidize thiols to disulphide (Amjad et al., 1977). A similarity in the spectra suggests oxidation of LSH to LSSL (Figure 10).
Figure 10.
UV- visible spectra of product of LSH oxidation by CoSalophen+ and hydrogen peroxide.
From the results obtained, Eqs. (7), (8), (9), and (10) is suggested to be the elementary steps involved for the reaction:
| (7) |
| (8) |
| (9) |
| (10) |
From the mechanism
| Rate = k3[CoSalophen+][HLSH+] + k5[CoSalophen+][LSH] | (11) |
| From Equation (7): [HLSH+] = K[LSH][H+] | (12) |
Substituting Eq. (12) in Eq. (11)
| Rate = k3K[CoSalophen+] [LSH][H+] + k5[CoSalophen+] [LSH] | (13) |
| = k5 + k3K[H+] ([CoSalophen+] [LSH]) | (14) |
Eq. (14) is equivalent to Eq. (5)where k5 = a = 0.16 dm3/mol/s and k3K = b = 464.16 dm6/mol2/s.
4. Conclusion
The reaction of [CoSalophen+] and LSH in DMSO: H2O; 1:4 media, displayed a stoichiometry of 1:1 based on the mole ratio. Second order overall was established for the reaction and the hydrogen ion concentration effect revealed the activeness of both the protonated and deprotonated form of the reductant. Positive salt effect based on the Brønsted-Debye was established during the course of the reaction. Free radical was detected to take part while intermediary complex with substantial stability was not detected as the reaction is progressing. From the outcomes, the reasonable mechanistic pathway for the reaction is suggested to be outer-sphere.
Declarations
Author contribution statement
S. Abdulsalam: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
S. O. Idris: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
G. A. Shallangwa, A. D. Onu: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Ahmadu Bello University Zaria for providing equipment for this research.
References
- Abedinzadeh Z., Gardes-Albert M., Ferradini C. Kinetic study of the oxidation mechanism of glutathione by hydrogen peroxide in neutral aqueous medium. Can. J. Chem. 1989;67(7):1247–1255. [Google Scholar]
- Abu-Surrah A.S., Abdel-Halim H.M., Feda’a M. Synthesis of cobalt (III), iron (III), and chromium (III) complexes with salicylaldiminato ligands: evaluation of the complexes as catalysts for oxidation of L-cysteine. Zeitschrift Für Naturforschung B. 2008;63(7):848–852. [Google Scholar]
- Adari K.K., Nowduri A., Vani P. Kinetics and mechanism of oxidation of l-Cysteine by Corey’s reagent. Transit. Met. Chem. 2006;31(6):745–748. [Google Scholar]
- Adetoro A., Iyun J.F., Idris S.O. Kinetic approach to the mechanism of redox reaction of pyrocatechol violet and nitrite ion in aqueous hydrochloric acid. Res. J. Appl. Sci. Eng. Technol. 2011;3(10):1159–1163. [Google Scholar]
- Alioke C.U., Ukoha P.O., Ukwueze N.N., Ujam O.T., Asegbeloyin J.N. 2012. Kinetics and Mechanism of the Reduction of N, N’-salicylideneiminationiron (III) Complex Ion by L-ascorbic Acid in Aqueous Acid Medium. [Google Scholar]
- Aly M.M. Recent developments in the metallosupramolecular and molecular structures of the cobalt, iron and vanadium complexes of the dianionic tetradentate Schiff base ligands of salicylideneimine and acetylacetoneimine. J. Coord. Chem. 1998;43(2–3):89–113. [Google Scholar]
- Amirnasr M., Schenk K.J., Gorji A., Vafazadeh R. Synthesis and spectroscopic characterization of [CoIII (salophen)(amine)2] ClO4 (amine= morpholine, pyrrolidine, and piperidine) complexes. The crystal structures of [CoIII (salophen)(morpholine)2] ClO4 and [CoIII (salophen)(pyrrolidine)2] ClO4. Polyhedron. 2001;20(7–8):695–702. [Google Scholar]
- Amjad Z., Chambers J.G., McAuley A. Metal-ion oxidations in solution. Part XX. The mechanism of the reaction of thallium (III) with 2-mercaptosuccinic acid in perchlorate media. Can. J. Chem. 1977;55(20):3575–3580. [Google Scholar]
- Ayoko G.A., Iyun J.F., Ekubo A.T. Oxidation of glutathione by diaquatetrakis (2, 2′-bipyridine)-μ-oxo diruthenium (III) ion in aqueous acidic solutions. Transit. Met. Chem. 1993;18(1):6–8. [Google Scholar]
- Ayoko G.A., Olatunji M.A. Oxidation of L-cysteine, mercaptoacetic acid and β-mercaptoethylamine by 12-tungstocobaltate (III) Polyhedron. 1983;2(7):577–582. [Google Scholar]
- Bianchini C., Zoellner R.W. Vol. 44. 1996. Activation of dioxygen by cobalt group metal complexes; pp. 263–339. (Advances in Inorganic Chemistry). [Google Scholar]
- Bose R.N., Moghaddas S., Gelerinter E. Long-lived chromium (IV) and chromium (V) metabolites in the chromium (VI)-glutathione reaction: NMR, ESR, HPLC, and kinetic characterization. Inorg. Chem. 1992;31(11):1987–1994. [Google Scholar]
- Böttcher A., Takeuchi T., Hardcastle K.I., Meade T.J., Gray H.B., Cwikel D., Kapon M., Dori Z. Spectroscopy and electrochemistry of cobalt (III) Schiff base complexes. Inorg. Chem. 1997;36(12):2498–2504. [Google Scholar]
- Brauer S.L., Wetterhahn K.E. Chromium (VI) forms a thiolate complex with glutathione. J. Am. Chem. Soc. 1991;113(8):3001–3007. [Google Scholar]
- Chakraborty M., Mandal P.C., Mukhopadhyay S. Mechanistic studies on the oxidation of thiols by a {Mn4O6}4+ core in aqueous acidic media. Polyhedron. 2012;45(1):213–220. [Google Scholar]
- Chakraborty M., Mandal P.C., Mukhopadhyay S. Kinetic studies on oxidation of L-cysteine and 2-mercaptoethanol by a trinuclear Mn (IV) species in aqueous acidic media. Inorg. Chim. Acta. 2013;398:77–82. [Google Scholar]
- Cini R., Moore S.J., Marzilli L.G. Strong trans influence methoxymethyl ligand in B12 cobaloxime and imine/oxime model complexes: structural, spectroscopic, and molecular mechanics investigations. Inorg. Chem. 1998;37(26):6890–6897. doi: 10.1021/ic980666i. [DOI] [PubMed] [Google Scholar]
- Cohen C.T., Coates G.W. Alternating copolymerization of propylene oxide and carbon dioxide with highly efficient and selective (salen) Co (III) catalysts: effect of ligand and cocatalyst variation. J. Polym. Sci. Part A Polym. Chem. 2006;44(17):5182–5191. [Google Scholar]
- Eichhorn E., Rieker A., Speiser B. The electrochemical oxidation of [CoII (salen)] in solvent mixtures—an example of a ladder scheme with coupled electron-transfer and solvent-exchange reactions. Angew Chem. Int. Ed. Engl. 1992;31(9):1215–1217. [Google Scholar]
- Eichhorn E., Rieker A., Speiser B., Sieglen J., Strähle J. Electrochemistry of oxygenation catalysts, Part 2 improved synthesis, crystal structure, and electrochemical properties of N, N′-Bis (salicylidene) ethylenediaminatocobalt (III) chloride. Zeitschrift Für Naturforschung B. 1993;48(4):418–424. [Google Scholar]
- Eichhorn E., Rieker A., Speiser B., Stahl H. Electrochemistry of oxygenation catalysts. 3.1 thermodynamic characterization of electron transfer and solvent exchange reactions of Co (salen)/[Co (salen)]+ in DMF, pyridine, and their mixtures. Inorg. Chem. 1997;36(15):3307–3317. doi: 10.1021/ic9703336. [DOI] [PubMed] [Google Scholar]
- Eichhorn E., Speiser B. Electrochemistry of oxygenation catalysts: Part 4. Solvent-composition-dependent isopotential points in cyclic voltammograms of Co (salen) J. Electroanal. Chem. 1994;365(1–2):207–212. [Google Scholar]
- El-Hag D.A., Dahab A.A. Identification and characterisation of disulphide bonds in therapeutic proteins by using Raman Spectroscopy. Adv. J Pharm. Life Sci. Res. 2016;4(3):50–59. [Google Scholar]
- Elskens M., Penninckx M., Vandeloise R., Vander Donckt E. Use of a simplex technique and contour diagrams for the determination of the reaction rate constants between glutathion and thiram in the presence of NADPH. Int. J. Chem. Kinet. 1988;20(11):837–848. [Google Scholar]
- Friedman M. Chemistry and biochemistry of the sulfhydryl group in amino acids, peptides and proteins. Biochem. Soc. Trans. 1973;64(1):54–59. [Google Scholar]
- Gangopadhyay S., Ali M., Dutta A., Banerjee P. Oxidation of thioglycolic acid and glutathione by (trans-cyclohexane-1, 2-diamine-N, N, N′, N′-tetraacetato) manganate (III) in aqueous media. J. Chem. Soc. Dalton Trans. 1994;6:841–845. [Google Scholar]
- Ganjali M.R., Shirvani-Arani S., Norouzi P., Rezapour M., Salavati-Niasari M. Novel nitrite membrane sensor based on cobalt (II) salophen for selective monitoring of nitrite ions in biological samples. Microchim. Acta. 2004;146(1):35–41. [Google Scholar]
- Ghosh S.K., Bose R.N., Gould E.S. Electron transfer. 89. Reductions of carboxylato-bound chromium (V) with mercapto acids. Inorg. Chem. 1987;26(22):3722–3727. [Google Scholar]
- Grases F., Palou J., Amat E. Kinetics and mechanism of the technetium (VII) oxidation of L-cysteine. Transit. Met. Chem. 1986;11(7):253–255. [Google Scholar]
- Gupta K.S., Gupta Y.K. Hydrogen-ion dependence of reaction rates and mechanism. J. Chem. Educ. 1984;61(11):972–977. American Chemical Society Publications. [Google Scholar]
- Hamza S.A., Iyun J.F., Idris S.O. Kinetics and mechanism of the redox reaction of toluidine blue and nitrite ions in aqueous acidic medium. Arch. Appl. Sci. Res. 2012;4(1):10–18. [Google Scholar]
- Henson N.J., Hay P.J., Redondo A. Density functional theory studies of the binding of molecular oxygen with Schiff’s base complexes of cobalt. Inorg. Chem. 1999;38(7):1618–1626. [Google Scholar]
- Hirotsu M., Kojima M., Nakajima K., Kashino S., Yoshikawa Y. Stereochemistry and electrochemistry of cobalt (II) and cobalt (III) complexes containing optically active tetradentate Schiff base ligands. Bull. Chem. Soc. Jpn. 1996;69(9):2549–2557. [Google Scholar]
- Hošt’álek Z., Mundil R., Císařová I., Trhlíková O., Grau E., Peruch F., Cramail H., Merna J. Salphen-Co (III) complexes catalyzed copolymerization of epoxides with CO2. Polymer. 2015;63:52–61. [Google Scholar]
- Hupe D.J., Wu D. Effect of charged substituents on rates of the thiol-disulfide interchange reaction. J. Org. Chem. 1980;45(15):3100–3103. [Google Scholar]
- Ibrahim I., Idris S.O., Abdulkadir I., Onu A.D. Kinetics and mechanism of the redox reaction of N, N′-phenylenebis-(salicylideneiminato) iron (III) with oxalic acid in mixed aqueous medium. Transit. Met. Chem. 2019;44(3):269–273. [Google Scholar]
- Jocelyn P.C. Biochemistry of the SH group. Biochem. Soc. Trans. 1972;1(3) 781-781. [Google Scholar]
- Kleij A.W., Kuil M., Lutz M., Tooke D.M., Spek A.L., Kamer P.C.J., van Leeuwen P.W.N.M., Reek J.N.H. Supramolecular zinc (II) salphen motifs: reversible dimerization and templated dimeric structures. Inorg. Chim. Acta. 2006;359(6):1807–1814. [Google Scholar]
- Kocak A., Yilmaz H., Faiz O., Andac O. Experimental and theoretical studies on Cu (II) complex of N, N′-disalicylidene-2, 3-diaminopyridine ligand reveal indirect evidence for DNA intercalation. Polyhedron. 2016;104:106–115. [Google Scholar]
- Komiyama M., Takeda N., Shigekawa H. Hydrolysis of DNA and RNA by lanthanide ions: mechanistic studies leading to new applications. Roy. Soc. Chem. 1999;16:1443–1451. [Google Scholar]
- Konidari C.N., Tzouwara-Karayanni S.M., Bowman L.E., Karayannis M.I. Kinetic and mechanistic study of the reaction of 2, 6-dichlorophenol-indophenol and cysteine. Talanta. 1992;39(7):863–868. doi: 10.1016/0039-9140(92)80107-o. [DOI] [PubMed] [Google Scholar]
- Lu X., Wang Y. Highly active, binary catalyst systems for the alternating copolymerization of CO2 and epoxides under mild conditions. Angew. Chem. Int. Ed. 2004;43(27):3574–3577. doi: 10.1002/anie.200453998. [DOI] [PubMed] [Google Scholar]
- Luinstra G.A., Haas G.R., Molnar F., Bernhart V., Eberhardt R., Rieger B. On the formation of aliphatic polycarbonates from epoxides with chromium (III) and aluminum (III) metal–salen complexes. Chem. A Eur. J. 2005;11(21):6298–6314. doi: 10.1002/chem.200500356. [DOI] [PubMed] [Google Scholar]
- Martin J.F., Spence J.T. Oxidation of thioglycolic acid by molybdenum (V) and molybdenum (VI) J. Phys. Chem. 1970;74(20):3589–3596. [Google Scholar]
- Matsuura T. Bio-mimetic oxygenation. Tetrahedron. 1977;33(22):2869–2905. [Google Scholar]
- McAuley A., Olatunji M.A. Metal-ion oxidations in solution. Part XIX. Redox pathways in the oxidation of penicillamine and glutathione by chromium (VI) Can. J. Chem. 1977;55(18):3335–3340. [Google Scholar]
- Niederhoffer E.C., Timmons J.H., Martell A.E. Thermodynamics of oxygen binding in natural and synthetic dioxygen complexes. Chem. Rev. 1984;84(2):137–203. [Google Scholar]
- Nishinaga, Ohara H., Tomita H., Matsuura T. Mechanism of Co (salen)-catalyzed oxygenolysis of indole ring as a model for the tryptophan-2, 3-dioxygenase reaction. Tetrahedron Lett. 1983;24(2):213–216. [Google Scholar]
- Nishinaga T., Moriyama H., Wazaki T., Mashino T., Fujii Y. Structural effect of cobalt Schiff base complex catalyst on its catalytic activity in dioxygenolysis of 3-methylindole. J. Mol. Catal. 1993;83(1–2):117–123. [Google Scholar]
- Nishinaga, Yamazaki S., Matsuura T. Catalytic dehydrogenation of secondary amines with cobalt schiff base complex-oxygen system. Tetrahedron Lett. 1988;29(33):4115–4118. [Google Scholar]
- Olatunji M.A., Okechukwu R.C. Rates and mechanisms of the reactions of octacyanomolybdate (V) anion with L-cysteine, penicillamine and thioglycolic acid in aqueous acidic solutions. Inorg. Chim. Acta. 1987;131(1):89–94. [Google Scholar]
- Onu A.D., Iyun J.F., Idris S.O. The kinetics of the reduction of tetraoxoiodate (VII) by N-(2-hydroxylethyl) ethylenediaminetriacetatocobaltate (II) ion in aqueous perchloric acid. J. Transit. Metal Chem. 2009;34(8):849–853. [Google Scholar]
- Osunlaja A.A., Idris S.O., Iyun J.F. Kinetics and mechanism of thiourea oxidation by oxygenated [Co2(O2)(NH3)10]5+ complex. J. Chem. Pharmaceut. Res. 2013;5(2):328–336. [Google Scholar]
- Ozawa T., Hanaki A., Onodera K. Spectroscopic studies on the production of hydroxyl radicals from the reactions of copper (II) polyamine-n-polycarboxylate complexes with hydrogen peroxide. Polyhedron. 1992;11(7):735–738. [Google Scholar]
- Özkan Y., Özkan E., Şimşek B. Plasma total homocysteine and cysteine levels as cardiovascular risk factors in coronary heart disease. Int. J. Cardiol. 2002;82(3):269–277. doi: 10.1016/s0167-5273(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Pizzolato E., Natali M., Posocco B., López A.M., Bazzan I., Di Valentin M., Galloni P., Conte V., Bonchio M., Scandola F. Light driven water oxidation by a single site cobalt salophen catalyst. Chem. Commun. 2013;49(85):9941–9943. doi: 10.1039/c3cc45457f. [DOI] [PubMed] [Google Scholar]
- Polson S.M., Cini R., Pifferi C., Marzilli L.G. Synthesis and x-ray structures of elusive imine/oxime-type organocobalt B12 complexes. NMR study suggesting steric strain within the axially ligated benzimidazole. Inorg. Chem. 1997;36(3):314–322. doi: 10.1021/ic9704492. [DOI] [PubMed] [Google Scholar]
- Read J.F., Bewick S.A., Graves C.R., MacPherson J.M., Salah J.C., Theriault A., Wyand A.E.H. The kinetics and mechanism of the oxidation of S-methyl-L-cysteine, L-cystine and L-cysteine by potassium ferrate. Inorg. Chem. Acta. 2000;303(2):244–255. [Google Scholar]
- Salavati-niasari M., Shaterian M. Oxidation of cyclohexene with tert -butylhydroperoxide catalysted by host ( nanocavity of zeolite-Y )/guest ( Mn ( II ), Co ( II ), Ni ( II ) and Cu ( II ) complexes of N , N -bis ( salicylidene ) phenylene-1 , 3-diamine ) nanocomposite materials. J. Mol. Catal. A Chem. 2007;261:147–155. [Google Scholar]
- Sami P., Venkateshwari K., Mariselvi N., Sarathi A., Rajasekaran K. Studies on electron transfer reactions: reduction of heteropoly 11-tungstophosphovanadate (V) by l-cysteine and thioglycolic acid in aqueous acid medium. Transit. Met. Chem. 2009;34(7):733–737. [Google Scholar]
- Shaked Z., Szajewski R.P., Whitesides G.M. Rates of thiol-disulfide interchange reactions involving proteins and kinetic measurements of thiol pKa values. Biochemistry. 1980;19(18):4156–4166. doi: 10.1021/bi00559a004. [DOI] [PubMed] [Google Scholar]
- Shi T., Berglund J., Elding L.I. Kinetics and mechanism for reduction of trans-dichlorotetracyanoplatinate (IV) by thioglycolic acid, L-cysteine, DL-penicillamine, and glutathione in aqueous solution. Inorg. Chem. 1996;35(12):3498–3503. [Google Scholar]
- Sisley M.J., Jordan R.B. Kinetic and equilibrium studies of the reactions of cysteine and penicillamine with aqueous iron (III) Inorg. Chem. 1995;34(24):6015–6023. [Google Scholar]
- Solomon M.B., Rawal A., Hook J.M., Cohen S.M., Kubiak C.P., Jolliffe K.A., D’Alessandro D.M. Electroactive Co (iii) salen metal complexes and the electrophoretic deposition of their porous organic polymers onto glassy carbon. RSC Adv. 2018;8(43):24128–24142. doi: 10.1039/c8ra04385j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser B., Stahl H. Complexation of [N, N′-Bis (salicylidene)-ethylenediiminato] cobalt (III) by anilines in dimethylformamide. Angew Chem. Int. Ed. Engl. 1995;34(10):1086–1089. [Google Scholar]
- Watts D.W. Reaction kinetics and mechanism. Phys. Chem. Org. Solvent Systems. 1973:681–731. [Google Scholar]
- Weaver K.H., Rabenstein D.L. Thiol/disulfide exchange reactions of ovothiol A with glutathione. J. Org. Chem. 1995;60(6):1904–1907. [Google Scholar]
- Yamada S. Advancement in stereochemical aspects of Schiff base metal complexes. Coord. Chem. Rev. 1999;190:537–555. [Google Scholar]
- Yilmaz H., Kocak A., Dilimulati M., Zorlu Y., Andac M. A new Co (III) complex of Schiff base derivative for electrochemical recognition of nitrite anion. J. Chem. Sci. 2017;129(10):1559–1569. [Google Scholar]
- Zhang Y.-L., Ruan W.-J., Zhao X.-J., Wang H.-G., Zhu Z.-A. Synthesis and characterization of axial coordination cobalt (III) complexes containing chiral Salen ligands. Polyhedron. 2003;22(12):1535–1545. [Google Scholar]
- Zhao R., Lind J., Merenyi G., Eriksen T.E. Kinetics of one-electron oxidation of thiols and hydrogen abstraction by thiyl radicals from. alpha.-amino CH bonds. J. Am. Chem. Soc. 1994;116(26):12010–12015. [Google Scholar]