Abstract
Alpha-terpineol is a monoterpenoid found in many essential oils, being widely used in food and household products. In vitro antioxidant and anti-inflammatory activities have already been associated with this alcohol; therefore, this study aimed to check if these properties were also present in vivo, counteracting the oxidant and inflammatory effects of a high-fat diet, as well as if there were differences in the biological activities among the two α-terpineol enantiomers. Thus, this work evaluated the effect of supplementation of α-terpineol enantiomers (at 25, 50 and 100 mg/kg of diet) on biological parameters of diet-induced obese Sprague-Dawley rats. In general, α-terpineol improved the nutritional parameters of rats fed a high-fat diet. The intake of α-terpineol at concentrations ≥50 mg/kg was able to reestablish the insulin sensibility and reduced (p < 0.05) serum levels of pro-inflammatory cytokines TNF-α and IL-1β, when compared with the control group. The intake of R-(+)- and (–)-α-terpineol decreased the TNF-α level by approximately 1.5 and 3.4 times, respectively, when compared with the high-fat group, regardless of the concentration. Moreover, both enantiomers at 50 mg/kg decreased the levels of serum thiobarbituric acid reactive substances (TBARS) by 2.6–4.2 times, while hepatic TBARS were reduced in approximately 1.6 times, regardless of the compound and concentration tested. Further experiments are suggested to confirm the mechanisms and the security of α-terpineol in different experimental models and more extended exposure experiments.
Keywords: Food science, Antioxidant, Bioaroma, Biological activity, Interleukin, Inflammation
Food science; Antioxidant; Bioaroma; Biological activity; Interleukin; Inflammation
1. Introduction
Obesity is currently recognized as one of the most important public health problems worldwide due to its contribution to the significant increase in premature death and early disability. This condition is closely related to a higher risk for the development of a series of chronic non-communicable diseases such as insulin resistance, type 2 diabetes, hypertension, dyslipidemias, liver and cardiovascular diseases, and certain types of cancer [1, 2]. Excessive consumption of products with a high energy density and high fat and/or sugar content is among the leading causes of obesity, as well as the reduction of physical activity associated with lifestyle changes generated by urbanization [3]. Thus, molecules targeting this problem have attracted the interest of many scientists, and the use of terpenes in this sense has already yielded relevant results [4], encouraging other studies with chemically related molecules.
Limonene is the most abundant monoterpene in nature. Its S-(–)- enantiomer is found in low concentrations in Mentha and conifers oils, while R-(+)-limonene is the main constituent of orange peel oil [5]. In 2017, Brazil alone exported more than 50 thousand tons of R-(+)-limonene-containing by-products (0.8.106 kg D-limonene, 22.4.106 kg citrus terpene and 27.1.106 kg orange essential oil) at prices ranging from US$6.5 to US$9/kg [6]. The large availability and low price of limonene have attracted the interest of researchers aiming more noble uses of this underutilized product [7, 8].
The biotechnological oxyfuncionalization of limonene, which has been widely reported over the past 50 years [9, 10], is one of the strategies to add value to this by-product, since oxidized counterparts of limonene have shown interesting biological properties both in vitro and in vivo. One of the most notable examples is perillyl alcohol, which has shown the potential for treating malignant glioma in humans [11]. Carvone, in turn, besides inhibiting chemically induced lung and stomach cancers, could also act as antimicrobial agent, insect repellent, potato sprouting inhibitor, also inducing glutathione S-transferase [12].
The biotransformation of limonene into α-terpineol, for instance, has been described using a wide variety of biocatalysts, but the bioprocess employing Shingobium sp. is particularly interesting, since α-terpineol production in the order of hundreds of grams per liter has been reported [13, 14]. Moreover, this microorganism could biotransform both R-(+)- and S-(–)-limonene, yielding R-(+)- and S-(–)-α-terpineol, respectively [14]. In terms of bioactivity, several biological properties have been attributed to α-terpineol [15]. However, as far as we know, no studies are dealing with the effect of α-terpineol in high-fat obesity models, although it presents anti-inflammatory and antioxidant activities, which are closely related, for example, to protection against the pathophysiological mechanisms involved in the genesis of obesity [16, 17, 18]. Moreover, only scarce information regarding the differences in the bioactivity between α-terpineol enantiomers is found, although it is widely known that stereochemistry might be determinant to some physiological properties [19]. In this way, it was hypothesized that the consumption of α-terpineol could counteract the oxidant and inflammatory effects associated with a high-fat diet and that this biological property could also be dependent on the spatial configuration of this molecule.
Based on the above, this preliminary study employed an experimental model of obesity to investigate if a diet containing α-terpineol could attenuate metabolic disorders caused by a hyperlipidic diet and also identify possible differences in the physiological responses for α-terpineol enantiomers.
2. Materials and methods
2.1. Chemicals
All the reagents used in the experiments presented analytical grade, except α-terpineol, which was obtained as described in the sequence.
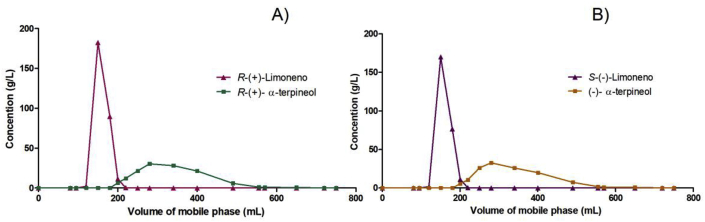
Considering that the α-terpineol standards commercially available lack good enantiomeric excess and considering that Sphingobium sp. can specifically convert R-(+)- and S-(–)-limonene to R-(+)- and S-(–)-α-terpineol, respectively [13], we produced our own test materials, following the procedure optimized by Molina et al., 2019 [14]. Briefly, the biotransformation system consisted of a 250 mL Erlenmeyer flask filled with 37.5 mL of an aqueous phase containing 3.5 g/L (OD600 = 10) frozen biomass and 12.5 mL of soybean oil, which presented 350 g/L substrate (limonene). After incubation for 72 h (for R-(+)-limonene biotransformation) or 120 h (for S-(–)-limonene biotransformation) at 200 rpm and 28 °C, the organic layer was recovered and extracted with the same volume of ethanol. This alcoholic fraction was concentrated on rotary evaporator (40 °C under vacuum) and the resulting oily substance (5 mL) was fractionated in an open column (37 mm diameter × 15 cm stationary phase) containing activated (105 °C/3h) silica gel 60 (Merck Millipore, Darmstadt, Germany) eluted with hexane:ethyl acetate (9:1) (adapted from Madyastha and Renganathan [20]). The eluate between 210 mL and 560 mL, containing the α-terpineol fraction (Figure 1), was concentrated on a rotary evaporator, analyzed in a gas chromatograph (Section 2.2) and kept in amber flasks at −18 °C until being used to prepare the feed for the animal model (Section 2.3).
Figure 1.
Separation curves of limonene (▲) and α-terpineol (■) in the oily fraction resulting from the biotransformation of (A) R-(+)- and (B) S-(–)-limonene. This procedure was carried out in an open column (37 mm diameter × 15 cm stationary phase) containing activated (105 °C/3h) silica gel 60 eluted with hexane:ethyl acetate (9:1).
2.2. Gas chromatography analysis
The volatile compounds present in the ethanol extract were analyzed in HP-7890A gas chromatography coupled with a Flame Ionization Detector (GC-FID). A HP-5 capillary column (30 m length × 0.25 mm internal diameter × 0.25 μm film width) was used for routine analysis and for quantifying the compounds, while a β-DEX 120 chiral column (60 m length × 0.25 mm internal diameter 0.25 μm film width) was used to identify the enantiomers present in the sample. Helium was used as carrier gas at a constant flow of 1.0 mL/min, and the split ratio was 1:10. For the analysis with HP-5 column, the oven temperature was preserved at 80 °C for the initial 3 min, increased at 20 °C/min until the final temperature of 200 °C, which was maintained constant for additional 4 min. As for the analysis with the chiral column, the oven temperature was maintained constant at 120 °C. The enantiomeric excess (ee) was calculated based on the peak areas of each enantiomer.
2.3. Biological assay
2.3.1. Diets
The experimental diets were elaborated according to the American Institute of Nutrition (AIN-93G), for growing rodents. The lean control group (AIN - pair-fed) consumed a normolipidic diet in the same amount of diet consumed by HF group (AIN-93G diet modified to hyperlipidic with 35% fat as 31% lard and 4% vegetable oil [21]) (Tables 1 and 2). In the experimental diets, R-(+)- and (–)-α-terpineol were added in the soybean oil at three different concentrations, 25, 50, and 100 mg/kg of diet, and these diets were offered ad libitum. These concentrations were c.a. one order of magnitude lower than NOAEL (No Observed Adverse Effect Level - 250 mg/kg bw) of α-terpineol [22].
Table 1.
Table of contents (g/kg) for each diet.
| Ingredients (g) | Groups∗ |
|||||||
|---|---|---|---|---|---|---|---|---|
| AIN | HF | S25 | S50 | S100 | R25 | R50 | R100 | |
| Casein | 145 | 145 | 145 | 145 | 145 | 145 | 145 | 145 |
| Corn starch | 453 | 269 | 269 | 269 | 269 | 269 | 269 | 269 |
| Maltodextrin | 132 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 |
| Sucrose | 100 | 58.5 | 58.5 | 58.5 | 58.5 | 58.5 | 58.5 | 58.5 |
| Cellulose | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Mineral mix | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 | 35.0 |
| Vitamin mix | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| L-cystine | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Choline bitartrate | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| TBHQ | 0.0140 | 0.0140 | 0.0140 | 0.0140 | 0.0140 | 0.0140 | 0.0140 | 0.0140 |
| Soybean oil | 70.0 | 40.0 | 40.0 | 40.0 | 39.9 | 40.0 | 40.0 | 39.9 |
| Lard | - | 310 | 310 | 310 | 310 | 310 | 310 | 310 |
| α-Terpineol | - | - | 0.0250 | 0.0500 | 0.100 | 0.0250 | 0.0500 | 0.100 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
Supplemented groups - S25: hyperlipidic diet plus 25 mg/kg of (−)-α-terpineol; S50: hyperlipidic diet plus 50 mg/kg of (−)-α-terpineol; S100: diet hyperlipidic diet plus 100 mg/kg of (−)-α-terpineol; R25: hyperlipidic diet plus 25 mg/kg of R-(+)-α-terpineol; R50: hyperlipidic diet plus 50 mg/kg of R-(+)-α-terpineol; R100: hyperlipidic diet plus 100 mg/kg of R-(+)-α-terpineol (Table 2).
Table 2.
Proximate composition of the diets (g/100g).
| Parameter | Groups |
|||||||
|---|---|---|---|---|---|---|---|---|
| AIN | HF | S25 | S50 | S100 | R25 | R50 | R100 | |
| Moisture | 8.10 ± 0.06a | 5.20 ± 0.39b | 5.10 ± 0.08b | 4.70 ± 0.06c | 4.50 ± 0.02d | 4.50 ± 0.12d | 4.40 ± 0.47de | 4.30 ± 0.14e |
| Proteinns | 13.1 ± 0.8 | 12.6 ± 0.3 | 12.7 ± 0.3 | 12.6 ± 0.2 | 12.9 ± 0.6 | 12.8 ± 0.8 | 12.5 ± 0.3 | 12.4 ± 0.2 |
| Ashns | 2.90 ± 1.4 | 2.30 ± 0.35 | 2.90 ± 0.04 | 3.00 ± 0.13 | 3.00 ± 0.07 | 3.00 ± 0.15 | 2.80 ± 0.02 | 2.90 ± 0.09 |
| Lipids | 15.1 ± 0.9b | 38.0 ± 0.9a | 36.3 ± 0.4a | 37.8 ± 0.2a | 36.7 ± 0.2a | 37.1 ± 0.1a | 37.0 ± 0.1a | 37.2 ± 0.4a |
| Carbohydrates | 60.8 ± 3.2a | 41.9 ± 1.9b | 43.0 ± 1.6b | 41.9 ± 1.2b | 42.9 ± 1.7b | 42.6 ± 2.3b | 43.3 ± 1.8b | 42.9 ± 1.7b |
| Caloric value (kJ/100g) | 1806 ± 20b | 2344 ± 15a | 2321±9a | 2331 ± 12.9a | 2324 ± 14a | 2325 ± 12a | 2327 ± 10a | 2324.±13a |
Results presented as the mean ± standard deviation (n = 3). Averages in the lines followed by same letters do not differ from each other at the 5% level of significance by the Tukey Test. Hyperlipidic (HF) and hyperlipidic groups supplemented with 25 mg/kg of R - (+) - α-terpineol (R25) and (−) - α-terpineol (S25), 50 mg/kg of R - (+) - α-terpineol (R 50) and (−) - α-terpineol (S 50) and 100 mg/kg of R-(+)-α-terpineol (R 100) and (−) - (S100).
nsNot significant by the Tukey test at the 5% probability level.
The proximate composition of the diets was done by the following analyses: moisture (105 °C), protein and ash, according to the methodologies of the Association of Official Analytical Chemists [23]; lipids by Bligh and Dyer [24]; total carbohydrate content (including total dietary fiber) was calculated by the difference between 100 and the sum of moisture, protein, lipids, and ashes; energy value was estimated based on the protein, lipid, and carbohydrate levels, according to the Atwater system, using the specific coefficients (carbohydrates and proteins 17 kJ/g and lipids 38 kJ/g) [25].
2.3.2. Experimental design
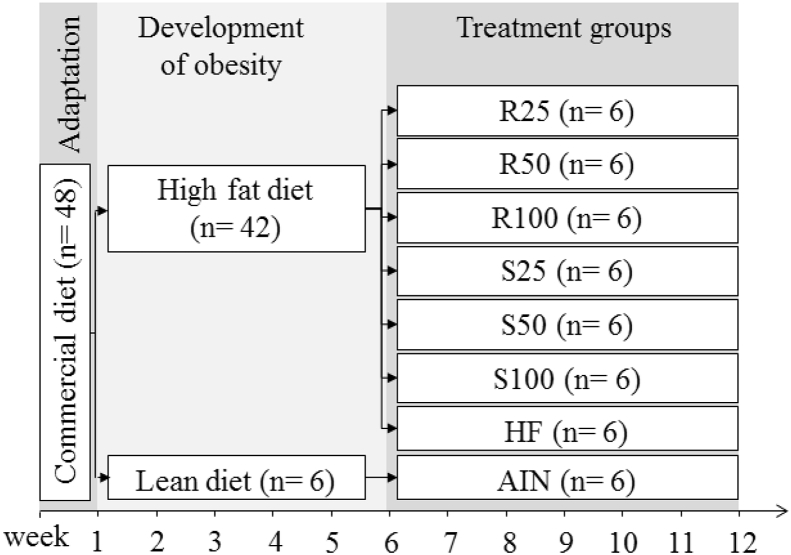
All experiments were performed following the Brazilian National Council for Control of Animal Experimentation (CONCEA), and the protocol was approved by the institutional Ethics Committee on the Use of Animals - UNICAMP (#3843-1/2015). Forty-eight male Sprague-Dawley rats (21 days old) weighing approximately 150 g were randomized into eight groups (n = 6). The animals were kept in individual growth cages and submitted to a period of adaptation and acclimatization, consuming a commercial diet (Labina, Purina, Paulínia, SP, Brazil). The environmental temperature was controlled (21 ± 3 °C) under a 12-hour light-dark cycle, with free access to water and food. After seven days of adaptation, the lean group, identified as AIN, kept receiving AIN-93G diet, while the obese animals were fed AIN-93G diet modified to high-fat and hypercaloric (HF) diet for the next five weeks for the development of obesity. After this period, the animals were randomly divided into seven groups, namely HF (only HF diet); R25; R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with25, 50 or 100 mg/kg (–)-α-terpineol, respectively). This treatment lasted six weeks, and during this period the lean control group (AIN) received the same amount of diet consumed by HF group (pair-fed) to match the food intake of both groups (Figure 2). Body weight was measured once a week.
Figure 2.
Design of the animal experiment. The diet composition of each treatment group is described in Figures 1 and 4. AIN group fed AIN-93G diet; HF – AIN-93G diet modified to hyperlipidic content (35% fat); R25, R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg of R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with 25, 50 or 100 mg/kg of (–)-α-terpineol, respectively).
2.3.3. Analytical assays
Glucose tolerance test (GTT) and insulin sensitivity test (ITT) [26] were performed after 12 h of fasting in the 10th and 11th experimental weeks, respectively. For GTT, glucose (2.0 g/kg bw) was administered intraperitoneally in fasted animal, and a small cut at the tip was done for blood collection. The blood glucose level was measured on strips inserted into a digital glucose meter (FreeStyle Lite, Abbott, Alameda, CA, USA) at 0, 30, 60, 90 and 120 min after glucose administration. For ITT, insulin was injected intraperitoneally (5.0 U/kg bw), and blood glucose concentration was measured at 0, 15, 30, 45, 60, 90 and 120 min, as previously described. The ratio of the decay constant (KITT) was calculated using the formula 0.693/T1/2, where T1/2 is the time required to reduce baseline glycaemia by half. Serum glucose T1/2 was calculated from the slope of the glucose decay curve during its linear phase [27].
At the end of the 12th experimental week, the animals fasted for 12 h were euthanized by exsanguination through the cardiac puncture. Serum and plasma were separated and frozen at −80 °C until the analyses. Heart, liver, kidneys, spleen, cecum, brown and epididymal adipose tissue were removed, cleaned with saline solution (0.9% NaCl), and weighed. Liver samples from each animal were collected and stored in formalin to perform the histological evaluation after being stained with hematoxylin and eosin. The image of the slides was obtained using Nikon Eclipse E-400 photomicroscope (Nikon, Tokyo, Japan) with an increase of 40X. Hepatic tissue structures and their microscopic changes were observed, and a descriptive analysis of the morphological characteristics was performed. The remaining liver tissue was frozen in liquid nitrogen and stored at −80 °C for future analysis.
The levels of the enzymes alanine aminotransferase - ALT (Wiener lab 1761302, Rosario, Argentina) and aspartate aminotransferase - AST (Wiener lab 1751302) were evaluated by the enzymatic-colorimetric method to investigate possible hepatic damage caused by the α-terpineol intake. Inflammatory cytokines (TNFα and IL-1β) were evaluated in serum using ELISA kit (Peprotech®, Rocky Hill, CT, USA).
Thiobarbituric acid reactive substances (TBARS) in serum and hepatic tissue were quantified according to Cazarin et al. [28]. A standard curve was obtained using 1.1.3.3-tetraethoxypropane, and the results were expressed as nmol MDA (malondialdehyde)/mg of tissue or nmol MDA/mL of serum. Protein concentration in tissue homogenates was determined by the Bradford method [29] using bovine serum albumin as standard to normalize the results.
2.4. Statistical analyses
The results obtained were presented as a mean ± standard deviation. For the data with normal distribution, a parametric statistical test (ANOVA and Tukey test) with a significance level of 5% (p < 0.05) was applied. For data that do not present a normal distribution, nonparametric test was done (Kruskal-Wallis followed by Dunn). Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA. USA).
3. Results
3.1. Production of α-terpineol enantiomers
The biotransformation of R-(+)-limonene by Sphingobium sp. yielded, after extraction and purification, an oily solution containing (as a percentage in GC-FID) 94.5% R-(+)-α-terpineol, 2.8% S-(–)-α-terpineol and 2.7% residual R-(+)-limonene. This product, here called R-(+)-α-terpineol, presented 97.3% purity of α-terpineol with an enantiomeric excess (ee) of 94.2% of the R form. The biotransformation of S-(–)-limonene, on the other hand, resulted in 64.6% S-(–)-α-terpineol, 33.9% R-(+)-α-terpineol and 1.5% residual S-(–)-limonene. In this case, the so-called (–)-α-terpineol presented 98.5% purity of α-terpineol with an enantiomeric excess of 31.5% of the S- isomer.
3.2. Weight gain and serum lipids
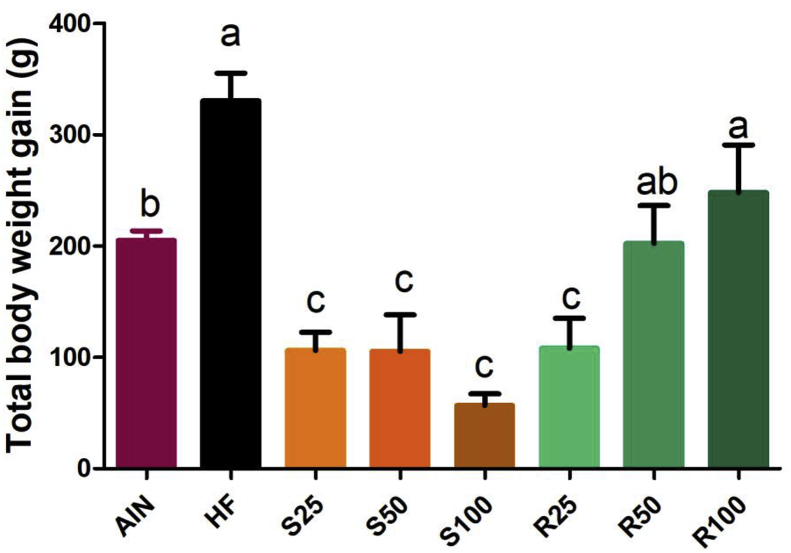
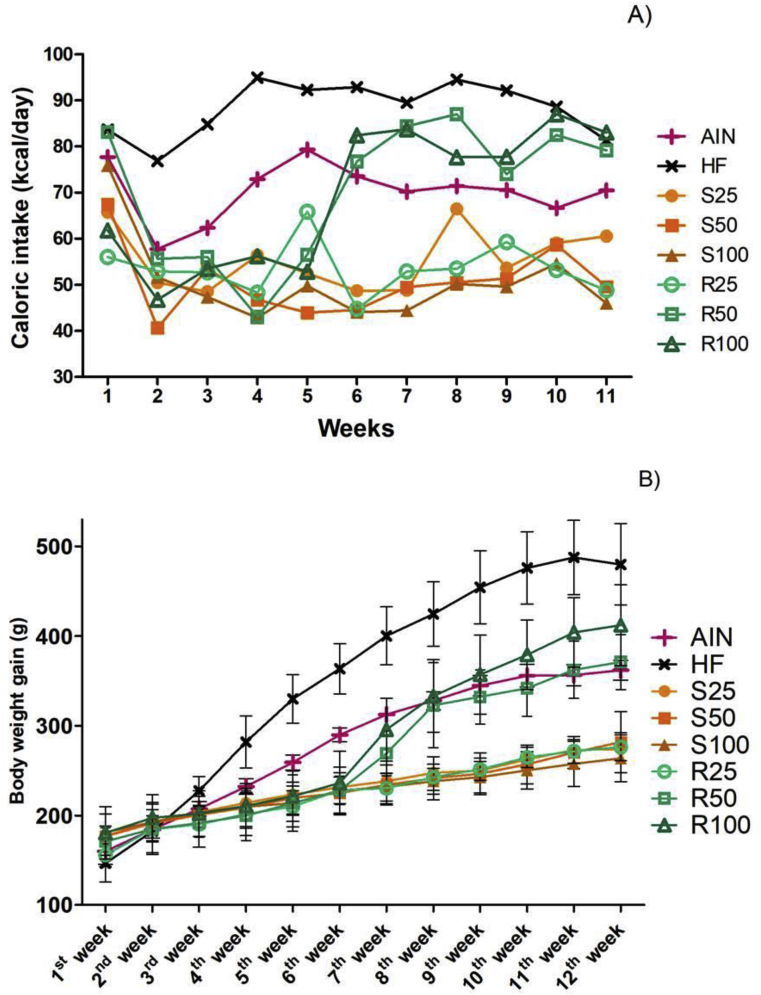
The mean total weight gain of each group is shown in Figure 3. More detailed data, i.e., weekly caloric intake, may be found in Figure 4. HF group presented higher weight gain (and higher weekly caloric intake), while almost all α-terpineol fed groups had the lowest weight gain (and lowest weekly caloric intake). No statistically significant differences were observed for total weight gain between HF, R50 and R100.
Figure 3.
Total weight gain of Sprague Dawley rats submitted to different treatments for 12 experimental weeks. Results expressed as a mean ± standard deviation. AIN group fed AIN-93G diet; HF – AIN-93G diet modified to hyperlipidic content (35% fat); R25, R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg of R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with 25, 50 or 100 mg/kg of (–)-α-terpineol, respectively). Different letters indicate the significant statistical difference (p < 0.05). One way ANOVA, with Tukey post-test (n = 6 per group).
Figure 4.
Weekly caloric intake (A) and weekly evolution of body weight gain (B) of Sprague Dawley rats submitted to different treatments for 12 experimental weeks. Results expressed as the mean ± standard deviation (n = 6 per group). AIN group fed AIN-93G diet; HF – AIN-93G diet modified to hyperlipidic content (35% fat); R25, R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with 25, 50 or 100 mg/kg (–)-α-terpineol, respectively).
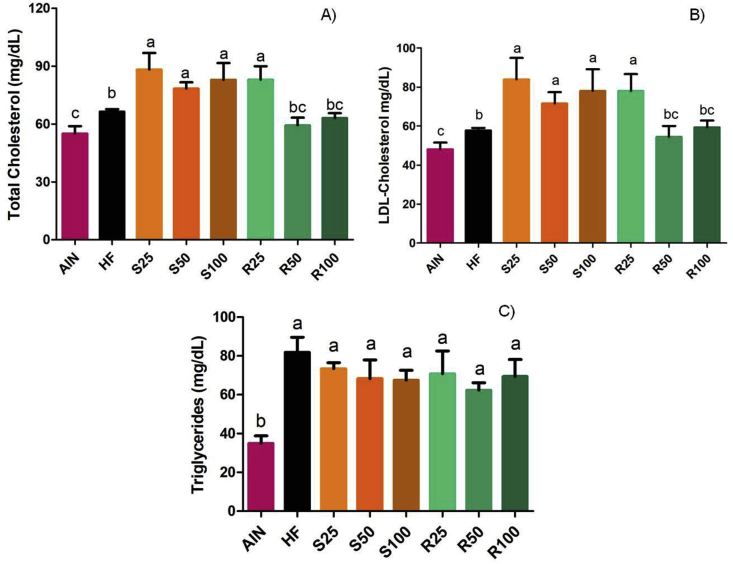
In terms of serum lipids, Figure 5 indicates that the intake of high-fat diet increased total cholesterol, LDL-cholesterol, and triglycerides when compared with the lean group, confirming that the intake of a high-fat diet promotes dyslipidemia associated with obesity. Additionally, the consumption of the diet supplemented with (–)-α-terpineol (all doses) and the diet supplemented with 25 mg/kg of R-(+)-α-terpineol resulted in a further increase in the level of total and LDL-cholesterol, compared with HF control group. The consumption of R50 and R100 diets did not modify the serum lipid profile.
Figure 5.
Serum total cholesterol (A), LDL-cholesterol (B) and triglycerides (C) from Sprague-Dawley rats. Results expressed as a mean ± standard deviation. AIN group fed AIN-93G diet; HF – AIN-93G diet modified to hyperlipidic content (35% fat); R25, R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with 25, 50 or 100 mg/kg (–)-α-terpineol, respectively). Different letters indicate the significant statistical difference (p < 0.05). One way ANOVA with Tukey post-test (n = 6 per group).
3.3. Hepatic lipid accumulation and function
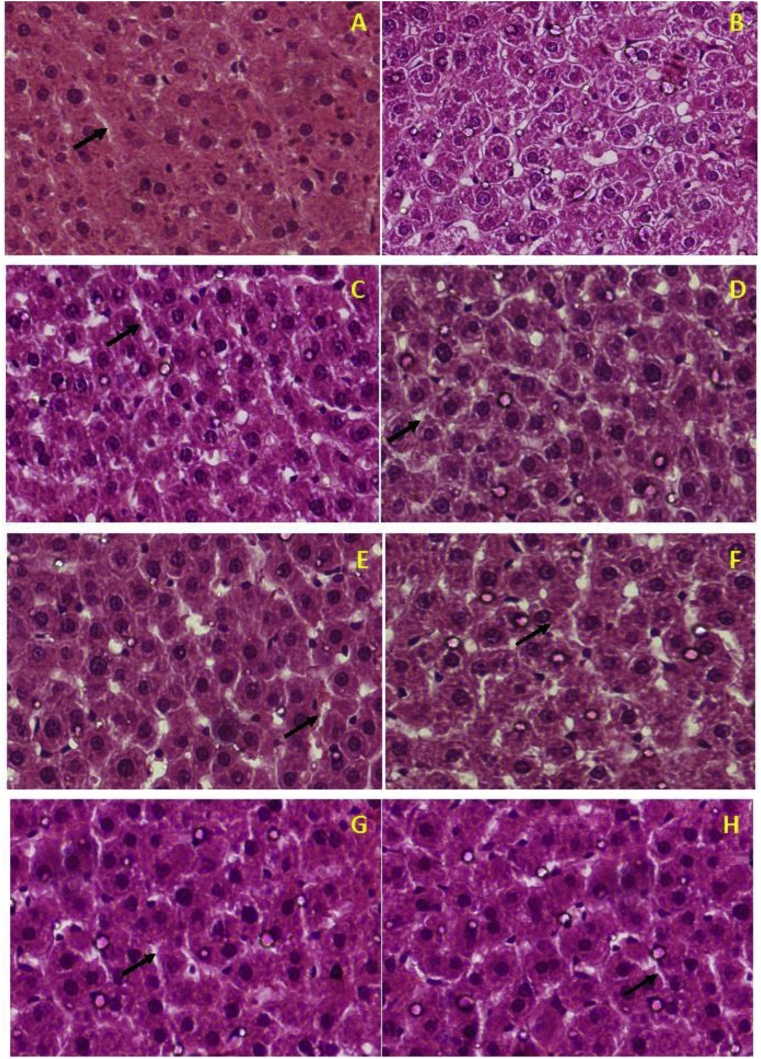
Liver fatty accumulation or Non-Alcoholic Fatty Liver Disease (NAFLD) is a condition prevalent in obese people [30]. The accumulation of lipid droplets in hepatocytes was evaluated by histological analysis in the liver tissue. Rats fed a normolipidic diet showed normal tissue morphology, with hepatocytes of hexagonal structure with centrally arranged nuclei and visible sinusoids (black arrows), without fat deposition (Figure 6A). On the other hand, animals fed an HF diet (Figure 6B) showed significant accumulation of lipid droplets in the hepatocyte cytoplasm, besides the loss of the identification of hepatic sinusoids compared to AIN group. The ingestion of α-terpineol seemed to counteract this effect, since fewer lipid droplets were found in hepatocytes, in comparison with HF group. Although there is a small accumulation of fat in some regions, the tissues showed hepatocytes with normal morphology and visible sinusoids (Figure 6C–H), similar to the normolipidic group (AIN).
Figure 6.
Histological analysis of the liver: AIN (A); HF (B); S25 (C); S50 (D); S100 (E); R25 (F); R 50 (G); R100 (H). AIN group fed AIN-93G diet; HF – AIN-93G diet modified to hyperlipidic content (35% fat); R25, R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with 25, 50 or 100 mg/kg (–)-α-terpineol, respectively). Histological sections were stained with hematoxylin and eosin. Black arrows: sinusoids. An increase of 40X.
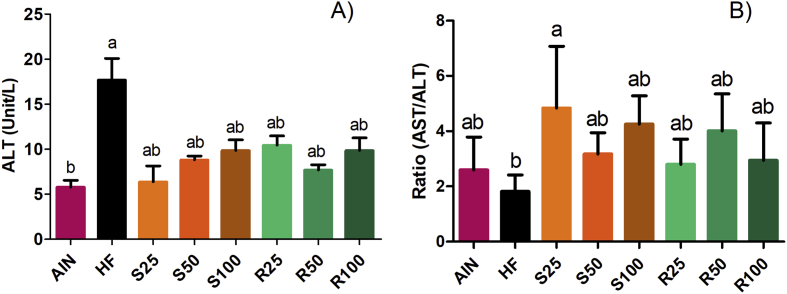
The fatty acid accumulation in the hepatocytes can also change the level of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes, which are used as indicators of onset or progression of liver injury [30]. Also, the AST/ALT ratio has been used as a marker of insulin resistance [31]. Corroborating the histological analysis, the level of ALT and the AST/ALT ratio results indicate that α-terpineol enantiomers intake did not promote change in the liver function (Figure 7). In fact, the serum levels of ALT in the hyperlipidic control group (HF) were significantly higher (p < 0.05) than the other experimental groups. However, no statistically significant differences were observed for ALT levels between the lean group (AIN) and the α-terpineol-treated animals.
Figure 7.
Serum levels of alanine aminotransferase (ALT) (A) and aspartate aminotransferase/alanine aminotransferase ratio (AST/ALT) (B) in Sprague Dawley rats. Results expressed as a mean ± standard deviation. AIN group fed AIN-93G diet; HF – AIN-93G diet modified to hyperlipidic content (35% fat); R25, R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with 25, 50 or 100 mg/kg (–)-α-terpineol, respectively). Different letters indicate the significant statistical difference (p < 0.05). One way ANOVA with Tukey post-test (n = 6 per group).
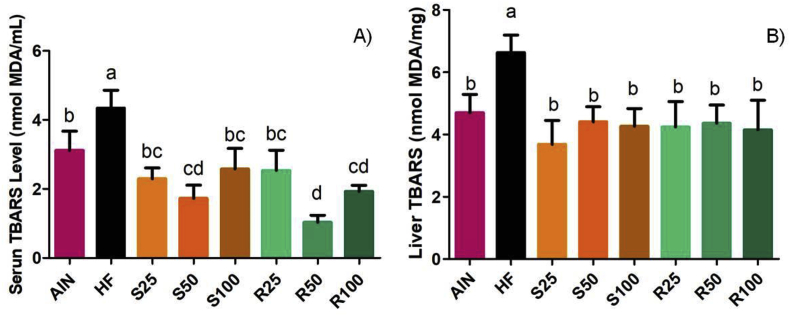
The level of thiobarbituric acid reactive substances (TBARS) is widely used as indicators of lipid peroxidation and to estimate the degree of oxidative stress. As shown in Figure 8A, the serum levels of TBARS of animals fed a high-fat diet (HF group) were significantly higher compared with the lean control group (AIN). However, the supplementation of α-terpineol in a high-fat diet promoted a significant reduction (40–70%) in these substances compared with HF group, restoring the levels found for a normolipidic diet (groups S25, S100, R25), or even lower levels (groups S50, R50 and R100). Similar behavior was observed in the liver TBARS level, which was higher for HF group, and no statistical differences were evidenced for the other groups (Figure 8B).
Figure 8.
Levels of thiobarbituric acid reactive substances (TBARS) in serum (A) and liver (B) of Sprague-Dawley rats supplemented with R-(+) and (–)-α-terpineol. Results expressed as a mean ± standard deviation. AIN group fed AIN-93G diet; HF – AIN-93G diet modified to hyperlipidic content (35% fat); R25, R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg of R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with 25, 50 or 100 mg/kg of (–)-α-terpineol, respectively). Different letters indicate the significant statistical difference (p < 0.05). One way ANOVA with Tukey post-test (n = 6 per group).
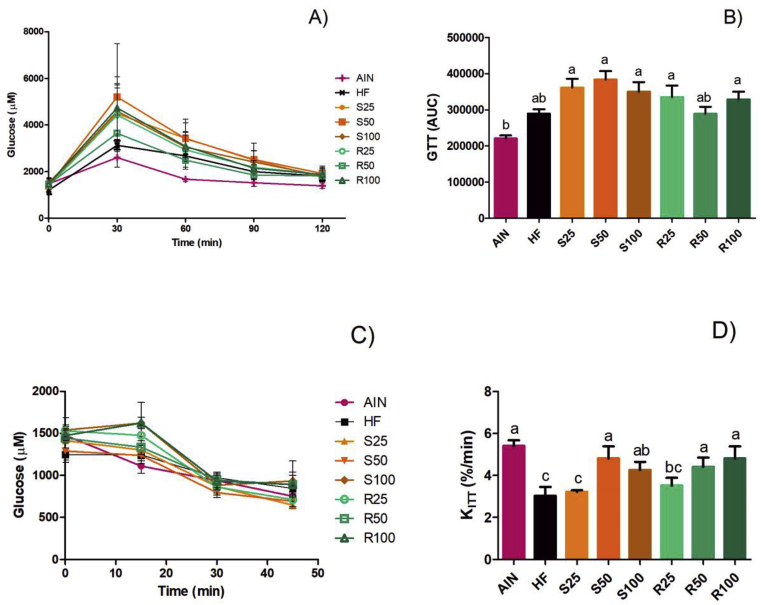
3.4. Glucose tolerance test (GTT) and insulin tolerance test (ITT)
The glucose tolerance test (GTT) was performed to assess the effect of α-terpineol enantiomers on the glycemic response against a glucose overload. There was no significant difference in the basal glycemia (t = 0 min) between treatments (p < 0.05), and in all experimental groups the blood glucose peak occurred 30 min after the intraperitoneal injection of the glucose solution. The hyperlipidic diets increased GTT levels, in comparison with the normolipidic group (AIN). This parameter was even higher for groups receiving α-terpineol, although not statistically different from HF group, except for S50 (Figure 9).
Figure 9.
Glycemic curve during 120 min after intraperitoneal injection of glucose solution (A), area under the curve during GTT (B) and glucose response (C) and glucose decay rate (D) in insulin tolerance test. KITT calculated from the glycemia collected in the insulin tolerance test. Results expressed as a mean ± standard deviation. AIN group fed AIN-93G diet; HF – AIN-93G diet modified to hyperlipidic content (35% fat); R25, R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg of R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with 25, 50 or 100 mg/kg of (–)-α-terpineol, respectively). Different letters indicate the significant statistical difference (p < 0.05). One way ANOVA with Tukey post-test (n = 6 per group).
On the other hand, α-terpineol intake could improve insulin resistance in rats fed a high-fat diet. As shown in Figure 9, the supplementation of more than 50 mg/kg of α-terpineol in a high-fat diet could induce an increase in the insulin sensitivity of the animals, reestablishing the KITT values found for rats fed normolipidic diet.
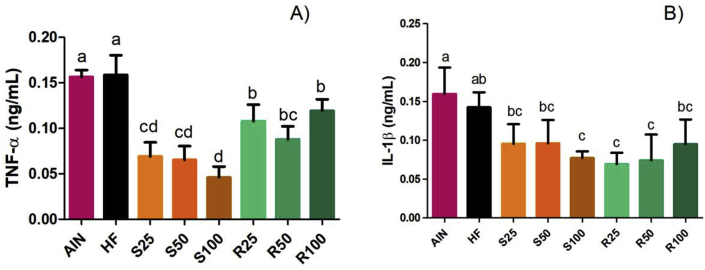
3.5. Levels of pro-inflammatory interleukins
The levels of TNF-α and IL-1β pro-inflammatory cytokines were equivalent in both fat and lean controls (groups HF and AIN) and significantly lower (p < 0.05) in the groups treated with α-terpineol, although the levels of IL-1β in groups S25, S50 and R100 did not significantly differ from HF. Apparently, the effect of α-terpineol on the reduction of these interleukins was not dose-dependent. In the case of TNF-α, a significant difference was observed in the interleukin reduction effect of each α-terpineol enantiomers: while the treatment with (–)-α-terpineol provided 60–70% reduction of TNF-α, R-(+)-α-terpineol caused only 30–40% reduction (Figure 10).
Figure 10.
Effect of α-terpineol enantiomers intake on the level of pro-inflammatory cytokines TNF-α (A) and IL-1β (B). Results expressed as a mean ± standard deviation. AIN group fed AIN-93G diet; HF – AIN-93G diet modified to hyperlipidic content (35% fat); R25, R50, and R100 (HF diet supplemented with 25, 50 or 100 mg/kg of R-(+)-α-terpineol, respectively); and S25, S50 and S100 (HF diet supplemented with 25, 50 or 100 mg/kg of (–)-α-terpineol, respectively). Different letters indicate the significant statistical difference (p < 0.05). One way ANOVA with Tukey post-test (n = 6 per group).
4. Discussion
Obesity is a worldwide health problem which can lead to various complications related to glucose metabolism, such as the development of hyperlipidemia, insulin resistance, type 2 diabetes, cardiovascular diseases, culminating in the metabolic syndrome [32, 33]. The intake of a hypercaloric diet, especially the high-fat (HF) diet contributes to energetic imbalance and weight gain. This weight gain is related to the accumulation of abnormal or excessive fat in the visceral or subcutaneous tissue, which can be infiltrated by lymphocytes and macrophages, contributing to production and release of pro-inflammatory cytokines and installation of the subclinical or chronic inflammation observed in obesity [34, 35]. Also, liver damage is one of the effects of HF diet intake, evidenced by increase in transaminases level [31]. It is well documented that obesity is a clinical state of chronic low-grade inflammation characterized by the production of a wide variety of biochemical mediators, including pro-inflammatory cytokines [36]. The increase in the release of tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) is directly related to the physiological response observed in the inflammatory processes [34, 37], as well as to the increase of adipose tissue characteristic of obesity. The experimental model used in this study was not able to produce all the effects associated with obesity, such as the increase in pro-inflammatory cytokines serum levels (TNF-α and IL-1β) compared with the lean control group (AIN). However, the effect was observed on weight gain, accumulation of lipid droplets in hepatocytes, increase in serum ALT level and lipid profile, as well as TBARS level (serum and liver), and insulin sensitivity.
The different weight gains for animals fed a high-fat diet supplemented with α-terpineol (Figure 3) were correlated to the lower weekly caloric intake among these groups (Figure 4), which, in turn, could be associated with diet palatability as well as with a possible modulation of appetite signaling in the regions of hypothalamus responsible for food intake via the gut-brain axis [38, 39, 40]. However, this hypothesis could not be confirmed with the data obtained in this experiment because neither this molecular pathway nor the microbiota composition were evaluated. The decreased weight gain was more pronounced in S groups, and it was associated with a lower caloric intake, which resulted in lower body weight over the experiment (Figure 4). The diets supplemented with 50 and 100 mg/kg R-(+)-α-terpineol led the animals to increase the food intake, resulting in more weight gain compared with the other treatments. Because this molecular pathway was not evaluated, the weight gain observed in R-(+)-α-terpineol group could be useful in some conditions, such as in possible prevention of cachexia. This is a condition developed by patients with cancer and other chronic diseases, and it is characterized by anorexia and loss of adipose tissue and muscle mass [41]. Thus, cachexia significantly decreases the life quality of these patients, besides increasing mortality rates [42].
Another relevant data refer to the serum lipid profile. Despite the alerting effect of S-(–)-α-terpineol intake on total and LDL cholesterol, the diet supplementation with 50 and 100 mg/kg R-(+)-α-terpineol did not modify the levels of total and LDL-cholesterol. Imaizumi et al. [43] and Choi et al. [44] demonstrated a similar hypercholesterolemic effect in Wistar rats and C57BL/6mice fed with α-terpineol-supplemented diet. However, in this study, steatosis was not observed in the animals, while increased lipogenesis and lipid accumulation in hepatocytes were evidenced in mice receiving 500 mg α-terpineol/kg bw/day for two weeks [44]. The mechanism involved in these responses is still unclear and needs to be elucidated with more specific molecular studies.
The liver is composed by hepatocytes arranged in plaques and in the structural unit, called hepatic lobe. The accumulation of fat inside the hepatocytes is a natural mechanism used to store energy. However, in overload situations, fat accumulation contributes to the development of hepatic dysfunction [45]. In general, liver parameters for rats fed a high-fat diet were also better when α-terpineol supplementation occurred, indicating that this monoterpenoid could have some role in protecting the hepatic tissue. Bioactive compounds are known do exert direct and indirect effects on obesity and inflammation [46]. In this particular case, terpenes could also have worked on the decrease in inflammation biomarkers. Lower lipid droplet accumulation reestablished normal hepatocyte morphology, and a decrease in ALT and TBARS levels were noticed in the treated groups. The results on TBARS, specifically, are in agreement with previous studies that show the in vitro antioxidant action of α-terpineol in different assays [16, 17]. In this study, we showed that this antioxidant activity was also valid for in vivo systems.
In terms of insulin resistance, we evidenced that 50 mg of α-terpineol per kg of diet, or more, would restore the “normal” (AIN group) values of KITT, counteracting the effect of the high-fat diet. This result could be related to the decrease in fatty acid deposits and lipotoxicity (data not shown) and the reduction of proinflammatory cytokines (Figure 10) in the groups treated with α-terpineol, variables that contribute to the disruption of insulin signaling, causing insulin resistance in muscle, liver and adipose tissue [47].
The cytokine TNF-α is related to acute and chronic inflammatory responses and contributes to the development of a variety of chronic inflammatory diseases, such as type 2 diabetes. This molecule is produced by various cell types, especially macrophages present in the adipose tissue characteristic in obesity. The cytokine IL-1β is also a potent proinflammatory cytokine, and it is involved in a variety of cellular activities, including cell proliferation, differentiation, and apoptosis, in addition to promoting the progression of a series of autoimmune diseases [16, 48, 49]. The consumption of a high-fat diet has been associated with systematic low-grade inflammation due to modulation of the microbiota and increased translocation of LPS through the intestinal mucosa [50]. The ingestion of certain levels of α-terpineol also seemed to counteract some variables changed by the intake of a high-fat diet, such as ALT secretion, the levels of TBARS levels, pro-inflammatory cytokines (TNF-α and IL-1β), and the development of insulin resistance. Although there was no difference between the lean (AIN) and fat (HF) groups regarding the levels of TNF-alpha and IL-B, α-terpineol intake decreased the level of these two pro-inflammatory cytokines, suggesting that the presence of these compounds in the diet could contribute to decreasing the inflammatory status in the body, counteracting inflammation processes. In fact, there is some evidence in the literature that α-terpineol has anti-inflammatory activity in vitro [51, 52] and this study suggests that this activity can be also evidenced in vivo. Our results showed that the effect of α-terpineol on the levels of TNF-α varied according to the special configuration of this molecule: the intake of a diet supplemented with (–)-α-terpineol (S groups) resulted in a more pronounced effect on the levels of TNF-α than the diet supplemented with R-(+)-α-terpineol (R groups). This particularity requires more molecular studies to elucidate how each different enantiomer could modulate this pathway. We hypothesize that this modulation may be related to the inhibition of the NF-kB cell signaling pathway, which controls the transcription of genes for most inflammatory cytokines [53, 54]. These results are important and quite promising because they reinforce previous studies indicating that not only α-terpineol but also other terpene compounds [55, 56, 57] could act in the inhibition of pro-inflammatory cytokines. This property is particularly important for studies on obesity, since it is well documented that this condition results in a low-grade inflammation [36].
In general, the effects of α-terpineol were apparently independent of the spatial configuration of its molecule. No significant differences were found for the effects of R-(+)- and (–)-α-terpineol on ALT, TBARS, GTT, KITT (at 50ppm and 100ppm) and TNF-α levels. However, significant differences were evidenced for body weight gain, IL-1β level, total cholesterol and LDL-cholesterol. These differences in the enantiomers responses need to be better elucidated at a molecular level and in different experimental models. In fact, the differences in biological activities among enantiomers have been widely reported in the literature. In terms of monoterpenes, the R-(–) and S-(+)- enantiomers of carvone, for example, have presented different biological effect in vivo (see a brief discussion in Souza et al. [58]). In case of α-terpineol, as far as we know, the only difference so far reported for the biological activities between its enantiomers is related to aroma character: while R-(+)-α-terpineol has a lilac odor, the S-(–)- isomer has coniferous odor character [59]. In this sense, the biotechnological production of α-terpineol becomes an attractive strategy, particularly for biomedical applications of this compound, where enantiomeric purity is a relevant parameter. Thus, although not as good as described earlier [13], the ee of the products obtained in this study (94.2% for R form in R-(+)-α-terpineol and 31.5% for the S form in (–)-α-terpineol) are much better than the values found for commercial α-terpineol standards. Alpha-terpineol from Aldrich (90%, technical grade, CAS 98-55-5, Cat. number 432628), α-terpineol from SAFC (≥96%, CAS 10482-56-1, Cat. number W304506) and α-terpineol from BTC (96%, CAS 98-55-5, Cat. number #144440), for instance, have an ee for the S-(–)- isomer of 16%, 8.9% and 18.5%, respectively, while the ee for Fluka's (+)-α-terpineol (≥97%, CAS 7785-53-7, Cat. number 83073) is only 30% for R-(+)-α-terpineol (data not shown). In nature, reasonably high ee (78%) was found in litchis flavor for S-(–)-α-terpineol, while 37.2% ee for R-(+)-enantiomer was detected in mango flavor and a racemic mixture was found in yellow passion fruit.
In conclusion, this study showed preliminary data about the effects of α-terpineol enantiomers in a high-fat diet model, as well as its potentiality to treat different health conditions. Thus, we recommend more studies to elucidate the molecular basis of the observed effects (especially on inflammation) as well as the response of these compounds in long term studies.
Declarations
Author contribution statement
Gardênia Martins de Sousa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Cinthia B. B. Cazarin, Mário R. Maróstica Junior, Juliano L. Bicas: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Celina de Almeida Lamas, Valéria H. A. C. Quitete, Glaucia M. Pastore: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the National Counsel of Technological and Scientific Development (CNPq) (301108/2016-1, 403328/2016-0, 473981/2012-2 and 400411/2016-4). This work was also supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. The authors thank Espaço da Escrita – Pró-Reitoria de Pesquisa - UNICAMP - for the language services provided.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Halliwell B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 2.Galic S., Oakhill J.S., Steinberg G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Mattes R. Energy intake and obesity: ingestive frequency outweighs portion size. Physiol. Behav. 2014;134:110–118. doi: 10.1016/j.physbeh.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Jing L., Zhang Y., Fan S., Gu M., Guan Y., Lu X., Huang C., Zhou Z. Preventive and ameliorating effects of citrus d-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2013;715:46–55. doi: 10.1016/j.ejphar.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Surburg H., Panten J. sixth ed. Wiley-VCH; Germany: 2016. Common Fragrance and Flavor Materials: Preparation, Properties and Uses. [Google Scholar]
- 6.Brazil, Comex Stat, http://comexstat.mdic.gov.br/pt/geral (accessed December 10, 2018).
- 7.de Carvalho C.C.C.R., da Fonseca M.M.R. Biotransformation of terpenes. Biotechnol. Adv. 2006;24:134–142. doi: 10.1016/j.biotechadv.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Lange B.M. Biosynthesis and biotechnology of high-value p-menthane monoterpenes, including menthol, carvone, and limonene. Adv. Biochem. Eng. Biotechnol. 2015;148:319–353. doi: 10.1007/10_2014_289. [DOI] [PubMed] [Google Scholar]
- 9.Bicas J.L., Dionisio A.P., Pastore G.M. Bio-oxidation of terpenes: an approach for the flavor industry. Chem. Rev. 2009;109:4518–4531. doi: 10.1021/cr800190y. [DOI] [PubMed] [Google Scholar]
- 10.Felipe L.d.O., Oliveira A.M.d., Bicas J.L. Bioaromas – perspectives for sustainable development. Trends Food Sci. Technol. 2017;62:141–153. [Google Scholar]
- 11.da Fonseca C.O., Teixeira R.M., Silva J.C.T., de Saldanha da Gama Fischer J., Meirelles O.C., Landeiro J.A., Quirico-Santos T. Long-term outcome in patients with recurrent malignant glioma treated with perillyl alcohol inhalation. Anticancer Res. 2013;33:5625–5631. [PubMed] [Google Scholar]
- 12.de Carvalho C.C.C.R., da Fonseca M.M.R. Carvone: why and how should one bother to produce this terpene. Food Chem. 2006;95:413–422. [Google Scholar]
- 13.Bicas J.L., Fontanille P., Pastore G.M., Larroche C. A bioprocess for the production of high concentrations of R-(+)-α-terpineol from R-(+)-limonene. Process Biochem. 2010;45:481–486. [Google Scholar]
- 14.Molina G., Pessôa M.G., Bicas J.L., Fontanille P., Larroche C., Pastore G.M. Optimization of limonene biotransformation for the production of bulk amounts of α-terpineol. Bioresour. Technol. 2019;294:122180. doi: 10.1016/j.biortech.2019.122180. [DOI] [PubMed] [Google Scholar]
- 15.Khaleel C., Tabanca N., Buchbauer G. α-Terpineol, a natural monoterpene: a review of its biological properties. Open Chem. 2018;16:349. [Google Scholar]
- 16.Marostica M.R., Silva T.A.A.R.E., Franchi G.C., Nowill A., Pastore G.M., Hyslop S. Antioxidant potential of aroma compounds obtained by limonene biotransformation of orange essential oil. Food Chem. 2009;116:8–12. [Google Scholar]
- 17.Bicas J.L., Neri-Numa I.A., Ruiz A.L.T.G., De Carvalho J.E., Pastore G.M. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem. Toxicol. 2011;49:1610–1615. doi: 10.1016/j.fct.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 18.França B.K., Melo Alves M.R., Silveira Souto F.M., Tiziane L., Freire Boaventura R., Guimarães A., Alves A. Peroxidação lipídica e obesidade: Métodos para aferição do estresse oxidativo em obesos. GE J. Port. Gastrenterol. 2013;20:199–206. [Google Scholar]
- 19.Nguyen L.A., He H., Pham-Huy C. Chiral drugs: an overview. Int. J. Biomed. Sci. 2006;2:85–100. [PMC free article] [PubMed] [Google Scholar]
- 20.Madyastha K.M., Renganathan V. Metabolism of alpha-terpineol by Pseudomonas incognita. Can. J. Microbiol. 1984;30:1429–1436. doi: 10.1139/m84-228. [DOI] [PubMed] [Google Scholar]
- 21.Reeves P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 22.European Food Safety Authority Scientific Opinion on the safety and efficacy of aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols and esters with esters containing tertiary alcohols ethers (chemical group 6) when used as flavourings for all animal species. EFSA J. 2012;10:2966. [Google Scholar]
- 23.AOAC . eighteenth ed. 2006. Official Methods of Analysis, Association of Official Analytical Chemists, Gaithersburgs, MD. [Google Scholar]
- 24.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 25.FAO, Food Energy - Methods of Analysis and Conversion Factors, ftp://ftp.fao.org/docrep/fao/006/y5022e/y5022e00.pdf (accessed May 3, 2017).
- 26.Vinue A., Gonzalez-Navarro H. Glucose and insulin tolerance tests in the mouse. Methods Mol. Biol. 2015;1339:247–254. doi: 10.1007/978-1-4939-2929-0_17. [DOI] [PubMed] [Google Scholar]
- 27.Bonora E., Moghetti P., Zancanaro C., Cigolini M., Querena M., Cacciatori V., Corgnati A., Muggeo M. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J. Clin. Endocrinol. Metab. 1989;68:374–378. doi: 10.1210/jcem-68-2-374. [DOI] [PubMed] [Google Scholar]
- 28.Cazarin C.B.B., da Silva J.K., Colomeu T.C., Batista Â.G., Meletti L.M.M., Paschoal J.A.R., Bogusz Junior S., Braga P.A.d.C., Reyes F.G.R., Augusto F., de Meirelles L.R., Zollner R.d.L., Maróstica Júnior M.R. Intake of Passiflora edulis leaf extract improves antioxidant and anti-inflammatory status in rats with 2,4,6-trinitrobenzenesulphonic acid induced colitis. J. Func. Foods. 2015;17:575–586. [Google Scholar]
- 29.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Marchesini G., Moscatiello S., Di Domizio S., Forlani G. Obesity-associated liver disease. J. Clin. Endocrinol. Metabol. 2008;93:s74–s80. doi: 10.1210/jc.2008-1399. [DOI] [PubMed] [Google Scholar]
- 31.Kawamoto R., Kohara K., Kusunoki T., Tabara Y., Abe M., Miki T. Alanine aminotransferase/aspartate aminotransferase ratio is the best surrogate marker for insulin resistance in non-obese Japanese adults. Cardiovasc. Diabetol. 2012;11:117. doi: 10.1186/1475-2840-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastien M., Poirier P., Lemieux I., Despres J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014;56:369–381. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Jaramillo P., Gomez-Arbelaez D., Lopez-Lopez J., Lopez-Lopez C., Martinez-Ortega J., Gomez-Rodriguez A., Triana-Cubillos S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Invest. 2014;18:37–45. doi: 10.1515/hmbci-2013-0053. [DOI] [PubMed] [Google Scholar]
- 34.Lee H., Lee I.S., Choue R. Obesity, inflammation and diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013;16:143–152. doi: 10.5223/pghn.2013.16.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellulu M.S., Patimah I., Khaza'ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro A.M., Macedo-de la Concha L.E., Pantoja-Meléndez C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev. Med. Hosp. Gen. 2017;80:101–105. [Google Scholar]
- 37.Esser N., Legrand-Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Simpson K.A., Martin N.M., Bloom S.R. Hypothalamic regulation of food intake and clinical therapeutic applications. Arq. Bras. Endocrinol. Metabol. 2009;53:120–128. doi: 10.1590/s0004-27302009000200002. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K., Jayasena C.N., Bloom S.R. Obesity and appetite control. Exp. Diabetes Res. 2012;2012:824305. doi: 10.1155/2012/824305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneeberger M., Gomis R., Claret M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 2014;220:T25–46. doi: 10.1530/JOE-13-0398. [DOI] [PubMed] [Google Scholar]
- 41.Aoyagi T., Terracina K.P., Raza A., Matsubara H., Takabe K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015;7:17–29. doi: 10.4251/wjgo.v7.i4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Haehling S., Anker S.D. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 2014. J. Cachexia Sarcopenia Muscle. 2014;5:261–263. doi: 10.1007/s13539-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imaizumi K., Hanada K., Mawatari K., Sugano M. Effect of essential oils on the concentration of serum lipids and apolipoproteins in rats. Agric. Biol. Chem. 1985;49:2795–2796. [Google Scholar]
- 44.Choi Y.-J., Sim W.-C., Choi H.K., Lee S.-H., Lee B.-H. α-Terpineol induces fatty liver in mice mediated by the AMP-activated kinase and sterol response element binding protein pathway. Food Chem. Toxicol. 2013;55:129–136. doi: 10.1016/j.fct.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 45.Bertram H.C., Larsen L.B., Chen X., Jeppesen P.B. Impact of high-fat and high-carbohydrate diets on liver metabolism studied in a rat model with a systems biology approach. J. Agric. Food Chem. 2012;60:676–684. doi: 10.1021/jf203994k. [DOI] [PubMed] [Google Scholar]
- 46.Batista A.G., Soares E.S., Mendonca M.C.P., da Silva J.K., Dionisio A.P., Sartori C.R., da Cruz-Hofling M.A., Marostica M.R. Jaboticaba berry peel intake prevents insulin-resistance-induced tau phosphorylation in mice. Mol. Nutr. Food Res. 2017:61. doi: 10.1002/mnfr.201600952. [DOI] [PubMed] [Google Scholar]
- 47.Boucher J., Kleinridders A., Kahn C.R. Insulin receptor signaling in normal and insulin-resistant states. CSH Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao R., Zhou H., Su S.B. A critical role for interleukin-1beta in the progression of autoimmune diseases. Int. Immunopharm. 2013;17:658–669. doi: 10.1016/j.intimp.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Kawaguchi K., Matsumoto T., Kumazawa Y. Effects of antioxidant polyphenols on TNF-alpha-related diseases. Curr. Top. Med. Chem. 2011;11:1767–1779. doi: 10.2174/156802611796235152. [DOI] [PubMed] [Google Scholar]
- 50.Duan Y., Zeng L., Zheng C., Song B., Li F., Kong X., Xu K. Inflammatory links between high fat diets and diseases. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Oliveira M.G., Marques R.B., de Santana M.F., Santos A.B., Brito F.A., Barreto E.O., De Sousa D.P., Almeida F.R., Badaue-Passos D., Jr., Antoniolli A.R., Quintans-Junior L.J. alpha-terpineol reduces mechanical hypernociception and inflammatory response. Basic Clin. Pharmacol. Toxicol. 2012;111:120–125. doi: 10.1111/j.1742-7843.2012.00875.x. [DOI] [PubMed] [Google Scholar]
- 52.Held S., Schieberle P., Somoza V. Characterization of alpha-terpineol as an anti-inflammatory component of orange juice by in vitro studies using oral buccal cells. J. Agric. Food Chem. 2007;55:8040–8046. doi: 10.1021/jf071691m. [DOI] [PubMed] [Google Scholar]
- 53.Hassan S.B., Gali-Muhtasib H., Goransson H., Larsson R. Alpha terpineol: a potential anticancer agent which acts through suppressing NF-kappaB signalling. Anticancer Res. 2010;30:1911–1919. [PubMed] [Google Scholar]
- 54.Nogueira M.N., Aquino S.G., Rossa Junior C., Spolidorio D.M. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1beta, IL-6 and IL-10 on human macrophages. Inflamm. Res. 2014;63:769–778. doi: 10.1007/s00011-014-0749-x. [DOI] [PubMed] [Google Scholar]
- 55.Ramalho T.R., Oliveira M.T., Lima A.L., Bezerra-Santos C.R., Piuvezam M.R. Gamma-terpinene modulates acute inflammatory response in mice. Planta Med. 2015;81:1248–1254. doi: 10.1055/s-0035-1546169. [DOI] [PubMed] [Google Scholar]
- 56.Kim D.S., Lee H.J., Jeon Y.D., Han Y.H., Kee J.Y., Kim H.J., Shin H.J., Kang J., Lee B.S., Kim S.H., Kim S.J., Park S.H., Choi B.M., Park S.J., Um J.Y., Hong S.H. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-kappaB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015;43:731–742. doi: 10.1142/S0192415X15500457. [DOI] [PubMed] [Google Scholar]
- 57.Chen L., Zhao L., Zhang C., Lan Z. Protective effect of p-cymene on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2014;37:358–364. doi: 10.1007/s10753-013-9747-3. [DOI] [PubMed] [Google Scholar]
- 58.Souza F.V.M., da Rocha M.B., de Souza D.P., Marçal R.M. (−)-Carvone: antispasmodic effect and mode of action. Fitoterapia. 2013;85:20–24. doi: 10.1016/j.fitote.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Boelens M.H., Boelens H., van Gemert L.J. Sensory properties of optical isomers. Perfum. Flavor. 1993;18 [Google Scholar]