Abstract
Aims
To compare the insulin sensitivity and secretion indices of pregnant Bangladeshi women with GDM and normal glucose tolerance (NGT).
Methods
This cross sectional study was performed with 40 GDM and equal number of NGT pregnant women diagnosed on basis of WHO criterion-2013 during 24–40 weeks of gestation. Glucose was measured by glucose oxidase method and fasting insulin by chemiluminescent immunoassay. Equations of homeostatic model assessment (HOMA) were used to calculate indices of insulin resistance (HOMA-IR), β-cell function (HOMA-B) and insulin sensitivity (HOMA-%S).
Results
The GDM group had significantly higher insulin resistance as indicated by higher fasting insulin value [GDM vs. NGT; 10.23 (7.94–14.50) vs. 7.07 (5.28–11.07) µIU/ml] and HOMA-IR [GDM vs. NGT; 2.47 (1.75–3.43) vs. 1.50 (0.99–2.22)] and poor β-cell secretion [GDM vs. NGT; HOMA-B: 113.37 (90.30–191.35) vs. 150.98 (109.85–271.72), median (IQR); p < 0.001 for all]. HOMA-B was significantly lower in GDM than NGT with BMI < 23 kg/m2 [GDM vs. NGT; 63.37 (49.19–83.83) vs. 134.89 (93.50–193.17) ng/ml; p = 0.010] despite having statistically comparable difference in IR. BMI was found to be a significant predictor of HOMA-IR in GDM.
Conclusions
Though impairments of both insulin secretion and sensitivity are hallmarks in the pathogenesis of GDM, β-cell dysfunction contributes more to development of GDM in those with relatively lower BMI in our population.
Keywords: GDM, Insulin resistance, Insulin secretion, HOMA, HOMA-IR, HOMA-B
Introduction
Pregnancy induces composite changes in metabolism along with marked decrease in insulin sensitivity [1]. The latter half of pregnancy is a state of insulin resistance (IR) that poses a physiologic stress for the pancreatic β-cells. Maintenance of normal glucose homeostasis in pregnancy is dependent upon the capability of β-cells to increase the secretion of insulin and thereby compensate for this IR [2]. An insufficient compensatory response will result in maternal hyperglycemia, as occur in the setting of GDM [3].
GDM is a global public health concern. The most common identified risk factors for GDM are advanced age, high body mass index (BMI) and family history of DM [4]. Asian race has already been included as risk factor during routine screening as there is a high incidence of GDM among this population [5]. Insulin secretion and sensitivity capacities of Asian women might be different from those of women of Western countries. Since even non-obese Asians were found to exhibit onset of type 2 diabetes at younger age, insulin secretion appears to be a major factor in the development of type 2 diabetes in this population. In Asians, pancreatic β-cell mass is relatively smaller than the Westerners, and the insulin secretion capacity is also lower [6]. Though maternal overweight/ obesity is an established risk factor for GDM, recent review has found that the prevalence of GDM may be even higher among lean [7], As the pathophysiology of GDM is similar to that of type 2 diabetes, insulin secretion capacity might be a more important factor for inducing GDM in normal weight Asian women though interplay of decreased secretion and resistance of insulin cannot be overlooked.
These ethnic differences of IR and β-cell function in pregnancy have been described in some previous studies. Morkrid et al. concluded that pregnant women from East and South Asia are more insulin resistant and have poorer β-cell function than Western Europeans. In that longitudinal study, Asian women were more insulin resistant in early pregnancy. From early to 28 weeks of gestation, the women from South Asia were not able to increase their β-cell function mutual to the IR. The β-cell response relative to the pregnancy induced IR was therefore unbalanced compared with the Western Europeans [8].
Among various indices for measurement of insulin sensitivity/ resistance, the homeostasis model assessment (HOMA) model is widely used in research because of its simplicity. The HOMA of insulin resistance (HOMA-IR) index is regarded as an inexpensive and reliable measure of IR. On the other hand, HOMA of β-cell function (HOMA-B) index is a good measure of β-cell function [9]. HOMA-IR is considered as an accurate index of insulin sensitivity throughout pregnancy and it correlates well with the severity and pathophysiological heterogeneity of GDM [10]. Several previous studies found that HOMA-IR values in GDM group were significantly higher than in NGT women in various populations [2], [6], [8], [11], [12]. Researchers already revealed poorer HOMA-B index in GDM women of South Asia [6], [8], [13]. The present study was intended to examine the difference in the insulin sensitivity and secretion index between GDM and pregnant women with NGT using HOMA model in our population.
Materials and methods
Subjects
This study encompassed 40 women with GDM (age: 28.20 ± 5.00 years, BMI: 27.41 ± 3.37 kg/m2; mean ± SD) and equal number of women with NGT (age: 26.23 ± 4.64 years, BMI: 25.70 ± 3.71 kg/m2; mean ± SD) screened by 75-gm 3-samples OGTT following WHO 2013 criterion for GDM. Women after 24 weeks of gestation with singleton pregnancy attending the ‘GDM Clinic’ of department of Endocrinology BSMMU were screened and enrolled consecutively. Women with prior history of DM were excluded from this study.
Study design
It was a cross-sectional study carried out from April 2017 to September 2017. Prior to commencement of this study the research protocol was approved by the Institutional Review Board (IRB). After recording relevant clinical data, OGTT was performed following an overnight fast. Study subjects were enrolled as GDM or NGT on the basis of WHO 2013 diagnostic criteria. Thus 40 GDM patients and an equal number of NGT mothers were enrolled for the study. Fasting venous blood (4 ml) was collected for insulin and serum separated to be preserved at −80 °C until assay.
Analytic method
Plasma glucose was analyzed by glucose oxidase method by using Dimension EXL 200 Integrated Chemistry System (Siemens, Germany) on the same day of collection. Serum insulin levels was measured by chemiluminescent immunoassay method using Access Immunoassay System (REF- 33410), Beckman Coulter, Inc., USA. The coefficient variances (CV) for glucose were 2.02% for low level values and 2.07% for high level whereas intra-assay CV for insulin was 2.54%.
Assessment of insulin secretion and sensitivity index
Insulin resistance and secretion were evaluated using the equations of original HOMA model described by Matthews et al. [14].
Statistical analysis
All data were analysed using SPSS program (version 23.0) and expressed as frequencies or percentages for qualitative values and mean (±SD) for quantitative values. When quantitative values with skewed distribution were found, they were presented as median and interquartile range (25th −75th percentile). Comparison between subgroups was done by Chi-square test, unpaired t-test or Mann-Whitney U test as applicable. Correlation between BMI and various insulin indices were analyzed by Pearson’s Correlation tests. Multiple linear regression analysis was used to determine the predictors of IR. P value ≤ 0.05 was considered as statistically significant.
Results
As shown in Table 1, women with GDM were elder than those in the NGT group (p = 0.071) and they had significantly higher BMI values than their peers (p = 0.034). Furthermore, GDM subjects were more likely to have a family history of diabetes (p = 0.098). The GDM group had significantly higher insulin resistance as indicated by higher fasting insulin value [GDM vs. NGT; 10.23 (7.94–14.50) vs. 7.07 (5.28–11.07) µIU/ml] and HOMA-IR [GDM vs. NGT; 2.47 (1.75–3.43) vs. 1.50 (0.99–2.22)] and poor β-cell secretion [GDM vs. NGT; HOMA-B: 113.37 (90.30–191.35) vs. 150.98 (109.85–271.72), median (IQR); p < 0.001 for all].
Table 1.
Clinical characteristics and insulin indices in study subjects.
| Variables | GDM (n = 40) | NGT (n = 40) | p |
|---|---|---|---|
| Age (years, mean ± SD) | 28.20 ± 5.00 | 26.23 ± 4.64 | 0.071 |
| BMI (kg/m2, mean ± SD) | 27.41 ± 3.37 | 25.70 ± 3.71 | 0.034 |
| Gestational weeks at detection (mean ± SD) | 31.38 ± 4.21 | 31.40 ± 4.71 | 0.980 |
| Gravida | |||
| Primigravida | 16 (40.0) | 17 (42.5) | 0.820 |
| Multigravida | 24 (60.0) | 23 (57.5) | |
| History of previous macrosomia | 3 (7.5) | 1 (2.5) | 0.308* |
| History of abortion | 12 (30.0) | 13 (32.5) | 0.809 |
| Family history of DM in 1st degree relatives | 17 (42.5) | 10 (25.0) | 0.098 |
| Fasting insulin (µIU/ml) | 10.23 (7.94–14.50) | 7.07 (5.28–11.07) | 0.001† |
| HOMA-IR | 2.47 (1.75–3.43) | 1.50 (0.99–2.22) | <0.001† |
| HOMA-B | 113.37 (90.30–191.35) | 150.98 (109.85–271.72) | 0.014† |
| HOMA-%S | 40.62 (29.13–56.96) | 66.72 (45.03–100.81) | <0.001† |
(Within parenthesis are percentages over column total).
Significance values were calculated by Student’s t-test and χ2-test in data with normal distribution.
*by Fisher’s Exact test.
†Quantitative date with skewed distribution were expressed as median (interquartile range) and p-values were calculated using Mann-Whitney U test.
HOMA-IR: homeostasis model assessment of insulin resistance.
HOMA-B: homeostasis model assessment of β-cell function.
HOMA-%S: homeostasis model assessment of insulin sensitivity.
Comparison of clinical profile and insulin indices were done between GDM and NGT women who had BMI below 23 kg/m2 as well as equal to or above it (Table 2); and between the groups of GDM holding BMI cut off at 23 kg/m2 (Table 3). As depicted in Table 2, despite statistically similar age and gestational age, HOMA-B was significantly lower in GDM having BMI < 23 kg/m2 (p = 0.010) than that of the NGT group with similar BMI. IR indices did not differ statistically in between these two groups (fasting insulin, p = 0.571; HOMA-IR, p = 0.226; and HOMA-%S, p = 0.226). On the other hand, GDM women with BMI ≥ 23 kg/m2 and statistically similar age as well as gestational age exhibited greater IR (fasting insulin, p = 0.005; HOMA-IR, p < 0.001 and HOMA-%S, p < 0.001) along with decreased insulin secretion (HOMA-B, p = 0.020).
Table 2.
Comparison of clinical characters and insulin indices between GDM and NGT according to BMI cut off.
| Variables | BMI < 23 kg/m2 |
P | BMI ≥ 23 kg/m2 |
P | ||
|---|---|---|---|---|---|---|
| GDM (n = 4) | NGT (n = 11) | GDM (n = 36) | NGT (n = 29) | |||
| Age (years, mean ± SD) | 26.50 ± 2.88 | 24.27 ± 4.29 | 0.283 | 28.39 ± 5.17 | 26.97 ± 4.63 | 0.247 |
| Gestational age (weeks, mean ± SD) | 33.50 ± 3.10 | 31.00 ± 4.21 | 0.251 | 31.14 ± 4.28 | 31.55 ± 4.95 | 0.724 |
| *Fasting insulin (µIU/ml) | 6.11 (4.55–7.61) | 4.90 (4.12–6.50) | 0.571 | 11.55 (8.88–14.71) | 8.43 (5.79–11.67) | 0.005 |
| *HOMA-IR | 1.37 (1.03–2.14) | 0.88 (0.74–1.44) | 0.226 | 2.83 (1.92–3.53) | 1.62 (1.13–2.28) | <0.001 |
| *HOMA-B | 63.37 (49.19–83.83) | 134.89 (93.50–193.17) | 0.010 | 122.54 (101.13–199.29) | 190.86 (113.53–291.41) | 0.020 |
| *HOMA-%S | 77.63 (47.37–97.13) | 113.26 (69.23–134.47) | 0.226 | 35.39 (28.34–52.16) | 61.55 (43.83–88.32) | <0.001 |
Comparison between groups done by Student’s t test and Mann-Whitney U test as applicable.
Data were expressed as median (interquartile range).
Table 3.
Insulin indices in GDM according to BMI categories (23 kg/m2).
| Variables | Groups |
p | |
|---|---|---|---|
| BMI < 23 (kg/m2) (n = 4) | BMI ≥ 23 (kg/m2) (n = 36) | ||
| Fasting insulin (µIU/ml) | 6.11 (4.55–7.61) | 11.55 (8.88–14.71) | 0.001 |
| HOMA-IR | 1.37 (1.03–2.14) | 2.83 (1.92–3.53) | 0.011 |
| HOMA-B | 63.37 (49.19–83.83) | 122.54 (101.13–199.29) | 0.001 |
| HOMA-%S | 77.63 (47.37–97.13) | 35.39 (28.34–52.16) | 0.011 |
Data were expressed as median followed by interquartile range in parentheses.
Comparison between groups done by Mann-Whitney U test.
GDM group with BMI ≥ 23 kg/m2 had more insulin resistance (fasting insulin, p = 0.001; HOMA-IR, p = 0.011) and lower insulin sensitivity (HOMA-%S, p = 0.011) compared to GDM with BMI < 23 kg/m2 (Table 3). Similarly in Fig. 1, BMI showed positive correlation with HOMA-IR in both GDM and NGT groups.
Fig. 1.
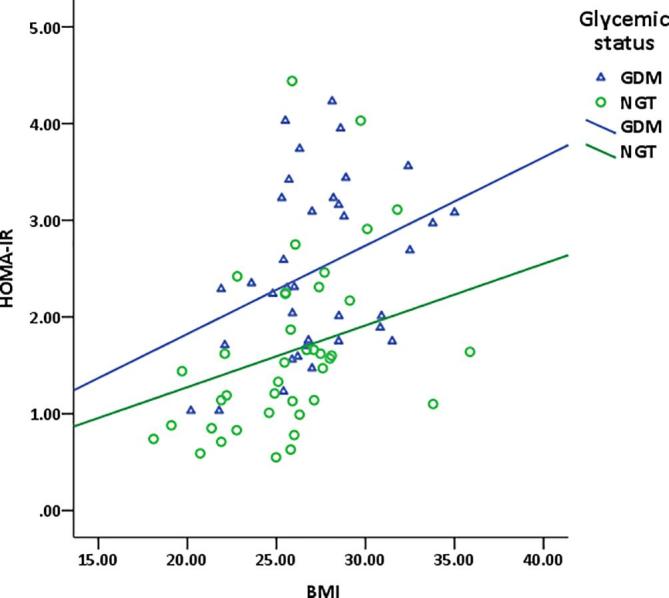
Relationship of BMI with HOMA-IR in women with or without GDM. GDM: gestational diabetes mellitus NGT: normal glucose tolerance HOMA-IR: homeostasis model assessment of insulin resistance BMI: body mass index *by Pearson’s correlation coefficient test.
Multiple linear regression analysis revealed that BMI was independent predictor of HOMA-IR in GDM (p = 0.008) as well as in NGT (p < 0.001). None of the risk factors (p = NS for all) like maternal age, family history of diabetes, gravida, previous history of abortion, previous history of macrosomia showed independent predictability over HOMA-IR in GDM (Table 4).
Table 4.
Multiple linear regression showing the predictive association of clinical and hormonal parameters with HOMA-IR.*
| Independent variable | GDM (n = 40) | NGT (n = 40) | ||
|---|---|---|---|---|
| R2 = 0.269 |
R2 = 0.428 |
|||
| β | p | β | p | |
| (Constant) | – | 0.512 | – | 0.030 |
| Age | −0.106 | 0.594 | −0.093 | 0.574 |
| BMI | 0.499 | 0.008 | 0.573 | <0.001 |
| Family history of DM | 0.164 | 0.306 | −0.423 | 0.004 |
| Gravida | −0.271 | 0.280 | 0.178 | 0.396 |
| Previous history of abortion | 0.129 | 0.482 | −0.208 | 0.236 |
| History of previous macrosomia | 0.051 | 0.752 | −0.175 | 0.235 |
Logarithmic transformation done for normal distribution of dependent variable.
Discussion
This study clearly demonstrated that women with GDM have lower insulin sensitivity and secretory capacity than their peers. Moreover, β-cell dysfunction and decreased insulin secretory capacity appears to be an important factor for development of GDM in our population especially in those with relatively lower BMI.
Observations resulting from this study dictate that significant differences occur in women in terms of the severity of IR and β-cell dysfunction at the time of diagnosis of GDM assessed by HOMA indices. BMI was found to have a strong correlation with IR in GDM. Consistent with previous study, GDM women with high BMI (≥23 kg/m2) had decreased insulin sensitivity and a significant enhancement in compensatory insulin secretion [15]. Interestingly, in women with lower BMI (<23 kg/m2), there was statistically significant decrease in HOMA-B index without any significant difference in IR. This indicates that GDM development in underweight women might be primarily related to poor insulin secretory capacity. As the pathophysiology of GDM is similar to that of type 2 diabetes and it has been postulated that lower insulin secretory capacity is a major risk factor for development of type 2 diabetes in non-obese Asians [6]. From this finding it appears that the role of β-cell dysfunction is important in the development of GDM in women with lean body weight. However, it should be mentioned that sample size was not sufficient enough for the lean group.
Of the many pathogenic mechanisms, IR and impaired β-cell function remain the hallmarks of GDM [2]. This study assessed IR with fasting insulin and HOMA-IR values and found those values to be significantly higher in GDM group than in NGT. Insulin secretory index HOMA-B was significantly lower in GDM than NGT mothers even with lesser insulin sensitivity as measured by HOMA-%S. Earlier studies also suggested that the pathogenesis of GDM is a defect of islet beta cell functions and compensatory increase in insulin secretion in response to increased IR during pregnancy [12]. Present findings are in line with other reports, that GDM is caused by both reduced insulin secretion and enhanced IR [12], [16]. Previous study reported HOMA-IR values of 2.3 (1.7–2.9) [median (IQR)] for Korean GDM women [6]; these values are not markedly different from those obtained in the present study (median HOMA-IR of 2.47 in GDM). In contrast, much higher value of HOMA-IR (6.59 ± 2.93, mean ± SD) were reported in a subset of Indian GDM mothers by Das et al. [17]; the discrepancy might be due to lower sample size (only 14 GDM mothers were included in that study).
Nevertheless, insulin secretory capacity measured by HOMA-B in this study was much lower than the findings by other investigators [10]. Pathophysiologic heterogeneity known between Asian and Caucasians have demonstrated lower pancreatic β-cell mass and insulin secretion capacity in the former [8]. This variance may also explain the onset of GDM despite lower IR even in non-obese younger mothers of our population.
Well recognized risk factors for GDM include overweight and obesity [11]. Moreover, pre-pregnancy BMI was found to have a far greater effect on IR in pregnancy in Asian women than Caucasians [18]. Present study could not assess the pre-pregnancy BMI, in contrast current BMI during pregnancy was used in various statistical calculation. In this study, BMI was found to have positive linear relationship with HOMA-IR which is in accordance with other reports [11], [15]. People with higher BMI usually have adipose tissue dysfunction which is known as the key determinant of obesity associated metabolic complications and IR [19]. So, in pregnancy with higher BMI a significant insulin response enhancement is needed to overcome both pregnancy associated IR and decreased insulin sensitivity due to adipose tissue dysfunction. Mismatching of these events culminates into dysglycemia to cause GDM. Current findings also suggest that BMI might be causally related to IR and thereby perpetuate GDM. Our analyses revealed significant positive linear relationship between HOMA-IR and BMI even in NGT women. As we know that pathophysiology of GDM is multifactorial, there might be other genetic and humeral determinants for development of GDM in our population.
In this study, variables were analyzed between BMI groups holding BMI cut-off at 23 kg/m2. Indices of IR, fasting insulin level and HOMA-IR, were higher in GDM than non-GDM but the increase was minimal in the normal weight GDM mothers. In contrast, there was significant difference of insulin resistance/sensitivity indices in overweight group. On the other hand, HOMA-B, an index of insulin secretion capacity, was decreased in all GDM women regardless of body weight. The decline was more important in normal weight GDM women considering the minimal change in IR. Thus GDM development in normal weight women was mostly related to insulin secretory dysfunction. In agreement with this, other studies demonstrated that GDM development in lean GDM women is primarily related to poor insulin secretory capacity, whereas in overweight GDM women it is associated with both IR and to inadequate insulin secretion [11], [20]. Willer et al. also found that impaired insulin secretion is the predominant defect in lean GDM and this inadequate β-ccll secretory capacity does not abate after delivery [21]. Recently it was found that leaner women with low IR and impaired insulin secretion account for about 40% of GDM in Japan [22]. Significant numbers of GDM turns to type 2 DM in future [22]. Other than being obese and having high IR, impaired insulin secretion in lean mothers might be a key determinant of onset of GDM and type 2 DM in Asian population. In this context, it is worth mentioning that, we have observed that genetic background may have potential impact over defect in β-cell function in lean GDM women. Investigators of our GDM study group had observed significantly higher frequency of GDM in relatively lean individuals with risk variants of TCF7L2 polymorphism [23]. TCF7L2 genetic variant is known to be associated with reduced insulin secretion in response to both glucose and incretin hormones [24]. Though it is too early to acclaim but cannot be overlooked that intrinsic defect in insulin secretory capacity seems to be more important factor for inducing GDM in normal weight pregnant women.
The present study has certain limitations. First, apart from HOMA model, other indices (like insulin secretion-sensitivity index-2, Matsuda index, insulinogenic index etc.) for estimation of insulin resistance and β-cell dysfunction could not be calculated. Second, we could not use pre-pregnancy BMI for comparison between the study subjects. Third, we were unable to analyse postpartum glucose tolerance status and other perinatal outcomes.
In summary, this study clearly demonstrates that there is decreased insulin sensitivity and poor β-cell function in GDM compared to those without glucose aberration. In overweight women, GDM was found to be associated with both IR and inadequate insulin secretion. On the other hand, in women with low body mass index, decreased insulin secretory capacity seems to be the primary defect causing GDM in our population. Further studies are warranted to elucidate the impact of β-cell dysfunction in development of GDM in young and normal weight mothers.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank the department of Microbiology, BSMMU and DNA Solution Laboratory, Dhaka for their technical support.
Funding
This work was supported by BSMMU as research grant.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcte.2020.100226.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Shalayel MHF, Noaemi MC, Ahmed SAM. Insulin resistance in the third trimester of pregnancy suffering from gestational diabetes mellitus or impaired glucose tolerance. In: Radenkovic M, editor. Gestational Diabetes, Croatia: In Tech; http://www.intechopen.com/books/gestational-diabetes/insulin-resistance-in-the-third-trimester-of-pregnancy-suffering-from-gestational-diabetes-mellitus.net/; 2011 [accessed 4 April 2017].
- 2.Retnakaran R., Ye C., Kramer C.K., Connelly P.W., Hanley A.J.G., Sermer M. Evaluation of circulating determinants of Beta-cell function in women with and without gestational diabetes. J Clin Endocrinol Metab. 2016;101(7):2683–2691. doi: 10.1210/jc.2016-1402. https://doi:10.1210fjc.2016-1402 [DOI] [PubMed] [Google Scholar]
- 3.Ferrara A., Ehrlich S.F. Strategies for diabetes prevention before and after pregnancy in women with GDM. Curr Diabetes Rev. 2011;7:75–83. doi: 10.2174/157339911794940738. https://doi:10.2174/157339911794940738 [DOI] [PubMed] [Google Scholar]
- 4.Liu L., Zhang Y., Li L. Risk factor of gestational diabetes among healthy Chinese women: an observational study. Biomed Res. 2017;28(5):2126–2130. https://www.alliedacademics.org [Google Scholar]
- 5.Cheung N.W., Wasmer G., Al-Ali J. Risk factors for gestational diabetes among Asian women. Diabetes Care. 2001;24(5):955–956. doi: 10.2337/diacare.24.5.955. [DOI] [PubMed] [Google Scholar]
- 6.Yang S.J., Kim T.N., Baik S.H., Kim T.S., Lee K.W., Nam M. Insulin secretion and insulin resistance in Korean women with gestational diabetes mellitus and impaired glucose tolerance. Korean J Intern Med. 2013;28:306–313. doi: 10.3904/kjim.2013.28.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen C.L., Pham N.M., Binns C.W., Duong D.V., Lee A.H. Prevalence of gestational diabetes mellitus in Eastern and South eastern Asia: a systemic review and meta-analysis. J Diabetes Res. 2018 doi: 10.1155/2018/6536974. 6536974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morkrid K., Jenum A.K., Sletner L., Vandal M.H., Waage C.W., Nakstad B. Failure to increase insulin secretory capacity during pregnancy induced insulin resistance is associated with ethnicity and gestational diabetes. Eur J Endocrinol. 2012;167:579–588. doi: 10.1530/EJE-12-0452. [DOI] [PubMed] [Google Scholar]
- 9.Song Y., Manson J.E., Tinker L., Howard B.V., Kaller L.H., Nathan L. Insulin sensitivity and insulin secretion determined by homeostasis model assessment (HOMA) and risk of diabetes in a multiethnic cohort of women: the Women’s health initiative observational study. Diabetes Care. 2007;30(7):1747–1752. doi: 10.2337/dc07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokup A., Ciastek B.R., Goralczyk K., Walentowicz M., Szymariski M., Rosc D. Insulin resistance as estimated by the homeostatic method at diagnosis of gestational diabetes: estimation of disease severity and therapeutic needs in a population-based study. BMC Endocr Disord. 2013;13:21–30. doi: 10.1186/1472-6823-13-21. https://doi:10.1186/1472-6823-13-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S., Kim M.Y., Baik S.H., Woo J.T., Kwon Y.J., Daily J.W. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of pre-pregnancy BMI. Eur J Clin Med. 2013;67:196–201. doi: 10.1038/ejcn.2012.207. https://doi:10.1038/ejcn.2012.207 [DOI] [PubMed] [Google Scholar]
- 12.Wei J., Jianbo G., Cheng J. Gestational diabetes and impaired glucose tolerance in pregnant women. Pak J Med Sci. 2014;30(6):1203–1208. doi: 10.12669/pjms.306.5755. https://doi:10.12669/pjms.306.5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekelund M., Shaat N., Almgren P., Berntorp K. Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia. 2009;53(3):452–457. doi: 10.1007/s00125-009-1621-3. [DOI] [PubMed] [Google Scholar]
- 14.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. PMID: 3899825. [DOI] [PubMed] [Google Scholar]
- 15.Genova M., Ananieva T.A., Tzatchev T. Impact of body mass index on insulin sensitivity/Resistance in pregnant women with and without gestational diabetes mellitus. Acta Med Bulgarica. 2013;11(2):60–67. http://hdl.handle.net/10861/716 [Google Scholar]
- 16.Saisho Y., Miyakoshi K., Ikenoue S., Kasuga Y., Matsumoto T., Minegishi K. Marked decline in beta cell function during pregnancy leads to development of glucose intolerance in Japanese women. Endocor J. 2013;60(4):533–539. doi: 10.1507/endocrj.EJ12-0356. [DOI] [PubMed] [Google Scholar]
- 17.Das S., Behera M.K., Misra S., Baliarsihna A.K. Beta-cell function and insulin resistance in pregnancy and their relation to fetal development. Metab Syndr Relat Disord. 2010;8(1):25–32. doi: 10.1089/met.2009.0017. https://doi:10.1089/met.2009.0017 [DOI] [PubMed] [Google Scholar]
- 18.Retnakaran R., Hanley A.J.G., Connelly P.W., Sermer M., Zinman B. Ethnicity modifies the effect of obesity on insulin resistance in pregnancy: a comparison of Asians, South Asian and Caucasian women. J Clin Endocrinol Metab. 2006;91:93–97. doi: 10.1210/jc.2005-1253. [DOI] [PubMed] [Google Scholar]
- 19.Longo M., Zatterale F., Naderi J., Parrillo L., Formisano P., Raciti G.A. Adipose tissue dysfunction as determinant of obesity associated metabolic complications. Int J Mol Sci. 2019;20:2358. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endo S., Maeda K., Suto M., Kaji T., Morina M., Kinoshita T. Differences in insulin sensitivity in pregnant women with overweight and gestational diabetes mellitus. Gynecol Endocrinol. 2006;22(6):343–348. doi: 10.1080/09513590600724836. [DOI] [PubMed] [Google Scholar]
- 21.Willer A.K., Prager R., Waldhausl W., Pacini G., Thomaseth K., Wagner O.F. Pronounced insulin resistance and inadequate β-cell secretion characterize lean gestational diabetes during and after pregnancy. Diabetes Care. 1997;20(18):1717–1723. doi: 10.2337/diacare.20.11.1717. PMID:9353615. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa S., Kobayashi Y. Leaner women with impaired insulin secretion accounts for about 40% of gestational diabetes mellitus in Japan. J Pregn. 2019 doi: 10.1155/2019/7578403. 7578403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mashfiqul-Hasan Nusrat-Sultana, Sharmin-Jahan Sandesh-Panthi, Yasmin-Aktar Atiqur-Rahman M, Fariduddin M., Hasanat M.A. TCF7L2 gene rs7903146 polymorphism may confer expression of gestational diabetes mellitus in relatively young and lean mothers. Diabet Obesity Int J. 2016;6:1–7. [Google Scholar]
- 24.Prato SD, Bianchi C, Daniele, G. Abnormalities of insulin secretion and β-cell function in type 2 diabetes. In: RIG Holt, Cs Cockram, A Flyvbjerg, BJ Goldstein, editors. Textbook of Diabetes, West Sussex: Wiley-Blackwell; 2017, p. 161-172.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


