Highlights
-
•
Human connectome project examining adolescent anxiety and depression.
-
•
N = 225 planned, clinical and control adolescents, ages 14–17.
-
•
Description of clinical and functional imaging measures from first 140 participants.
-
•
Data publicly available via national institute of health data archive.
Abstract
We present a Human Connectome Project study tailored toward adolescent anxiety and depression. This study is one of the first studies of the Connectomes Related to Human Diseases initiative and is collecting structural, functional, and diffusion-weighted brain imaging data from up to 225 adolescents (ages 14–17 years), 150 of whom are expected to have a current diagnosis of an anxiety and/or depressive disorder. Comprehensive clinical and neuropsychological evaluations and longitudinal clinical data are also being collected. This article provides an overview of task functional magnetic resonance imaging (fMRI) protocols and preliminary findings (N = 140), as well as clinical and neuropsychological characterization of adolescents. Data collection is ongoing for an additional 85 adolescents, most of whom are expected to have a diagnosis of an anxiety and/or depressive disorder. Data from the first 140 adolescents are projected for public release through the National Institutes of Health Data Archive (NDA) with the timing of this manuscript. All other data will be made publicly-available through the NDA at regularly scheduled intervals. This article is intended to serve as an introduction to this project as well as a reference for those seeking to clinical, neurocognitive, and task fMRI data from this public resource.
1. Introduction
Adolescence is a critical time for understanding brain changes associated with psychiatric disorders, as nearly half of lifetime diagnoses begin by age 14 (Kessler et al., 2005). Focusing on brain bases of adolescent psychiatric disorders is critical because of increasing annual rates of major depressive episodes in adolescents (+52% from 2005–2017 [Twenge et al., 2019]) and a doubling of adolescent emergency room visits due to suicidal attempts/ideation from 2007–2015 (Burstein et al., 2019; see also Miron et al., 2019). The brain's cognitive control-, emotion-, and reward-related circuitries are undergoing substantial development during adolescence (Gogtay et al., 2004; Hare et al., 2008; Insel et al., 2017; Monk et al., 2003; Ofen et al., 2007; Olson et al., 2015; Pehlivanova et al., 2018; Somerville et al., 2011; see also Ahmed et al., 2015; Casey, 2015; Somerville et al., 2010). These circuits have also been implicated in the development and persistence of two of the most common types of adolescent psychiatric disorders: anxiety and depression. We detail the Human Connectome Project (HCP) study of adolescent anxiety and depression and discuss National Institute of Mental Health (NIMH) research domain criteria (RDoC)-consistent neuroimaging tasks selected to elicit activation in cognitive control-, emotion-, and reward-related brain regions. We also provide preliminary task-functional magnetic imaging (fMRI) results and detail adolescent clinical and neuropsychological characterization.
The HCP provides resources for acquiring, processing, analyzing, and sharing standardized, open-access neuroimaging data (hereafter, HCP infrastructure; e.g., Van Essen et al., 2012a; Barch et al., 2013; Glasser et al., 2013). This infrastructure has been adopted by Connectomes Related to Human Diseases (CRHD) projects aimed at better understanding brain changes associated with human pathologies. We present a CRHD project, undertaken by the Boston Adolescent Neuroimaging of Depression and Anxiety consortium (BANDA). The BANDA study is utilizing the HCP infrastructure to collect and disseminate multimodal MRI data, along with clinical and neuropsychological data from 225 adolescents (ages 14–17). One-hundred-and-fifty adolescents are planned to have a diagnosis of an anxiety and/or depressive disorder.
There are three primary goals of the BANDA study. First, it aims to collect a rich dataset of brain imaging, clinical, and cognitive measures from depressed and anxious, as well as healthy adolescents, and to make these data openly-available to the biomedical research community. Second, because most imaging tools have been developed using adult samples, this project, along with the Human Connectome Project Development (HCP-D) study, aims to offer the resources for researchers to create adolescent-specific tools (e.g., white matter and functional connectivity atlases) to enhance the analysis of structural and functional brain development during this critical period (e.g., Tahmasebi et al., 2012). Finally, this project collects longitudinal clinical data and will allow researchers to use these data to test predictive markers of the development, progression, and remission of depressive and anxious symptoms in adolescence.
The present article serves as an introduction to the BANDA study and as a methodological reference for those seeking to use this study's task functional magnetic resonance imaging (fMRI), clinical, or neuropsychological data. We present descriptive analyses from the first 140 participants. Similar to the descriptions of other HCP projects, these analyses involve an overview of sample characteristics and brain activation patterns during functional imaging tasks (Barch et al., 2013; Somerville et al., 2018). In a companion paper, Siless and colleagues (Siless et al., 2020) thoroughly detail image acquisition protocols, image quality assessments, and image harmonization with other HCP studies. Both reports were timed with the projected public release of data from the first 140 participants to the National Institutes of Health Data Archive (NDA).
2. Overview of the BANDA study
2.1. Study sample
This project will collect multimodal MRI data, along with clinical and neuropsychological data from 225 adolescents, scanned at ages 14–17. Adolescence is often broadly defined (e.g., pubertal onset through early adulthood). However, we selected this age range for two primary reasons. First, in this age range, we can be reasonably certain that the majority of participants will have entered puberty (Parent et al., 2003; Patten and Viner, 2007). Second, relevant brain responses (i.e., emotional and reward system neural activations) peak, and individual differences in these responses also are at their apex within this age range (Somerville et al., 2010). Thus, this age range may allow researchers to examine whether this variation relates to adolescent mood and anxious symptomology, which often emerges during adolescence (Somerville et al., 2010).
Participants are recruited from a wide range of sources including: psychological clinics, bus and train advertisements, newsletters to special interest groups, and social media. Interested parents and adolescents undergo a preliminary screen via phone for inclusion/exclusion criteria. Inclusion/exclusion criteria are verified during the initial study visit (Table 1; Supplementary Table 1). In terms of participant matriculation and ongoing participant tracking, prospective patients matriculate into the imaging portion of the study (see Section 2) if they meet the general inclusion/exclusion criteria and have a diagnosis of an anxious and/or depressive disorder (see Section 3.1.2 for definitions).
Table 1.
Summary of inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age 14–17 years at time of scanning | Premature birth (37 weeks, or 34 for twins) or less than 5 lbs. at birth |
| Parent and child fluent in English | Serious medical conditions |
| Safe to enter MRI | History of serious head injury |
| Parent and child meet intellectual abilities as measured by a score of 85 or higher on the Wechsler Abbreviated Scale of Intelligence, 2nd Edition (WASI-II) | Hospitalization > 2 days for neurological or cardiovascular disease |
| Diagnosis of autism spectrum disorder | |
| Use of daily preventive migraine medication; migraine within 72 h of scan |
Note: See Supplementary Table 1 for detailed list of exclusion criteria.
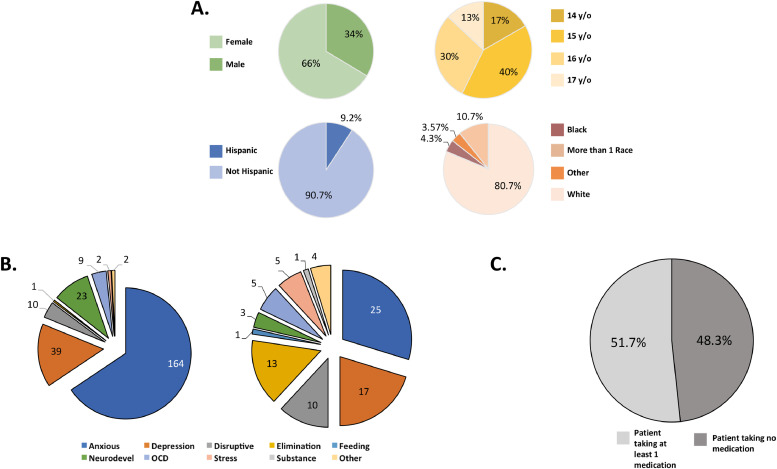
The BANDA consortium is a partnership formed in the Greater Boston area, allowing for us to sample from a racially- and ethnically-diverse community. This project strives to approximate the racial and ethnic diversity of the broader U.S. population (Falk et al., 2013; LeWinn et al., 2017). The BANDA study oversamples female participants to approximate annual and lifetime prevalence sex-disparities in both adolescent anxiety and depression trends—where females show prevalence rates at least 12% greater than males (see https://nimh.nih.gov/health/statistics; Fig. 1; Supplementary Table 2).
Fig. 1.
Brief characteristics of the first 140 participants. A. Sex, Age, Ethnicity, and Race. Full demographic characteristics may be found in Supplementary Table 1. B. Count of patient current (left) and historical (right) DSM-5 diagnosis types. Full diagnostic characteristics may be found Supplementary Tables 3 and 4. OCD= obsessive-compulsive disorder. C. Patient psychiatric medication status. Detailed medication use descriptions may be found in Supplementary Table 5.
2.2. Relationship to other imaging projects
Harmonization of imaging sequences and behavioral measures across studies offer opportunities to evaluate results on different samples and to jointly test hypotheses across projects. The BANDA study has worked in parallel with the HCP-D study—an ongoing effort collecting imaging and behavioral data from 1300+ healthy children, adolescents, and young adults (Somerville et al., 2018; Harms et al., 2018)—to harmonize certain image acquisition parameters and cognitive measures. BANDA has also harmonized imaging sequences, behavioral measures, and fMRI tasks with three other CRHD projects examining adult anxiety, depression, and disordered emotional states. These partner CRHD projects include the Dimensional Connectomics of Anxious Misery (PI: Sheline; University of Pennsylvania), the Perturbation of the Treatment of Resistant Depression Connectomes by Fast-Acting Therapies (PIs: Espinoza, Narr, Wang; University of California-Los Angeles), and the Mapping Connectomes for Disordered Mental States (PI: Williams; Stanford University). Together with our partner CRHD projects, we will collect 900+ participants, most of whom suffer from anxious and/or depressive symptoms. Comprehensive details of imaging hardware and parameter harmonization with other HCP studies may be found in a companion manuscript (Siless et al., 2020 ). Finally, this project has also harmonized specific cognitive and neuropsychological measures with other, non-HCP large-scale, developmental neuroimaging projects (Adolescent Brain Cognitive Development study [ABCD; Volkow et al., 2018], Philadelphia Neurodevelopmental Cohort [Gur et al., 2014; Satterthwaite et al., 2016]).
This project also has many unique features. We selected a rigorous clinical battery with diagnostic interviews and clinical dimensional measures assessing depressive and anxious constructs (e.g., state anxiety and anhedonia). Additionally, dimensional measures investigate constructs that are relevant to general adolescent maladaptive behaviors (e.g., problem behaviors, risky and impulsive decision making). Given that one aim of this project is clinical prediction, clinical data are also collected every six months for at least one year post-imaging. Finally, fMRI tasks were selected to evoke brain activations in regions relevant to general adolescent development, as well as anxiety and depression.
3. Participant schedule
Those passing preliminary screening for inclusion/exclusion criteria undergo clinical and neuropsychological testing (Session 1). Parents provide informed consent and adolescents assent to participate at the beginning of Session 1. Session 1 data are acquired from one of three clinical sites: the Center for Anxiety and Related Disorders at Boston University; the Center for Depression, Anxiety, and Stress Research at McLean Hospital/Harvard Medical School; and the Child Cognitive Behavioral Therapy Program at Massachusetts General Hospital/Harvard Medical School. In Session 2, participants undergo brain imaging and an eye-tracking session (outside of scanner) at the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital. Attempts are made to minimize the time between Session 1 evaluations and Session 2 imaging. 75.7% of participants were imaged within two weeks of Session 1, 92.1% of participants were imaged within three weeks, 98.6% were imaged within 5 weeks. Sessions 3 and 4 are clinical longitudinal follow-up sessions occurring at 6 and 12 months after Session 2, respectively. These sessions use similar clinical assessments as Session 1 (except Session 3 is online, whereas Session 4 is in-person). At the time of writing this report, data are still being collected on the participants detailed here; thus, Sessions 3 and 4 are not discussed further. A Consolidated Standard of Report Trials (CONSORT) flowchart is available in Supplementary Figure 1 to illustrate group sample sizes and attrition during Sessions 1 and 2.
4. P articipant characterization
4.1. Diagnostic interviews and clinical classification
Diagnoses of present and historical psychiatric disorders are given by trained researchers who are, or are under the supervision of, licensed clinical psychologists. Diagnoses are approved by a clinical psychologist and given according to the Diagnostic and Statistical Manual of Mental Health Disorders, 5th Edition (DSM-5; American Psychiatric Association, 2013). Adolescents receive the Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (K-SADS; Kaufman et al., 1997) based upon DSM-4 structures. At study onset, the K-SADS had not yet adopted DSM-5 criteria. Study clinicians (authors Henin, Hirshfeld-Becker, and Auerbach), thus systematically reviewed each K-SADS module to ensure that prompts could probe DSM-5 diagnostic criteria. Where required, prompts were added to address potential changes to disorder classifications (e.g., DSM-5 persistent depressive disorder). Thus, the K-SADS used here was adapted to also provide DSM-5 diagnoses. The K-SADS is a semi-structured diagnostic interview assessing lifetime and current mental disorders, which yields reliable and valid psychiatric diagnoses within age ranges 7–18 (Kaufman et al., 1997). Participating parents also receive the K-SADS Parent Report module to gather additional data. Parent and adolescent reports are used in the diagnostic characterization of the adolescent. Suicide risk is assessed using the Columbia Suicide Severity Rating Scale (Posner et al., 2011) and if the participant is deemed to be at imminent clinical risk (e.g., has endorsed suicidal intent) by the interviewing researcher/supervising clinical psychologist, s/he is unenrolled from the study and appropriate measures are taken. The K-SADS and the Columbia Suicide Severity Rating Scale combined took on average approximately 1–3 h, depending upon patient status, to complete. Family history of psychiatric disorders from an adolescents’ first- and second-degree relatives are assessed by a standardized interview of the participating parent (Weissman et al., 2000), which took approximately 20 min to complete.
4.1.1. Inter-rater agreement on clinical classifications
Diagnostic reliability of primary DSM-5 clinical classifications (depression and/or anxiety disorders) for the first 140 participants was estimated. This was achieved by comparing original clinical diagnoses to those given by a blinded, licensed psychologist (author Rosso) who: (1) Was not involved in any of the original clinical classifications, (2) had no prior knowledge of the original clinical classifications, and (3) had no prior knowledge of the random stratification procedures (below). Audio recordings of parent and adolescent K-SADS were selected from 15 participants (~10% of sample) whom had complete audio data files. Stratification ensured an equal probability of representation from each clinical site (i.e., 5 per site), and an equal representation of control participants, and participants with depressive and anxiety disorders. Cohen's Kappa statistic was used to assess inter-rater agreement (Cohen, 1960). BANDA inter-rater agreement for anxiety (κ = 0.55) and depression (κ = 0.66) diagnoses fall within the moderate to substantial range (Landis and Koch, 1977). BANDA depression inter-rater agreement also exceeded that of the initial child and pediatric DSM-5 field trials (κBANDA = 0.66 vs. κDSMtrials = 0.28; Freedman et al., 2013). There was no basis for direct comparison between BANDA anxiety disorders and child and pediatric DSM-5 field trials. However, BANDA anxiety agreement scores were greater than adult generalized anxiety disorder inter-rater agreement scores assessed during DSM-5 field trials (κBANDA = 0.55 vs. κDSMtrials = 0.20; Freedman et al., 2013).
4.2. Putative study groupings
For group-level descriptive analyses, participants were placed into one of three study categories using DSM-5 diagnoses to illustrate broad trends associated with anxiety and depression in the present data. Users of these data are not constrained to the study groupings described here, as K-SADS characterization yields an abundance of diagnostic information (Fig. 1; see also Supplementary Tables 3–4). The three study groups discussed are: the Control Adolescent Group (CA), the Depressed Adolescent Group (DA), and the Anxious Adolescent Group (AA). CA participants have no current DSM-5 disorder, nor a historical diagnosis of anxiety or depression. DA participants meet the DSM-5 criteria for a current depressive disorder. Thus, DA participants have clinically significant depressive symptoms present within 2-weeks at the time of the K-SADS interview. This grouping encompasses participants whom met the criteria for a current diagnosis of: major depressive disorder, dysthymia, an unspecified depressive disorder (i.e., depression not otherwise specified), or adjustment disorder with depressed mood. DA participants may also have a current or historical anxiety disorder. AA participants meet the DSM-5 criteria for at least one of the following anxiety disorders: generalized anxiety, social anxiety, specific phobia, agoraphobia, panic disorder, separation anxiety, or single-incident posttraumatic stress disorder. Participants included in the AA group do not meet the criteria for a current diagnosis of any of the depressive disorders described above. Participants in the AA group may have a historical diagnosis of a depressive disorders. However, depressive symptomology is currently subthreshold or, where diagnostic criteria permit (e.g., major depressive disorder), in “full remission.” Approximately 85% of the AA group meet the current diagnostic criteria for generalized anxiety disorder (58.50%), social phobia (46.80%), and/or separation anxiety disorder (8.51%; see Supplementary Tables 3–4).
4.3. Clinically-relevant dimensional measures
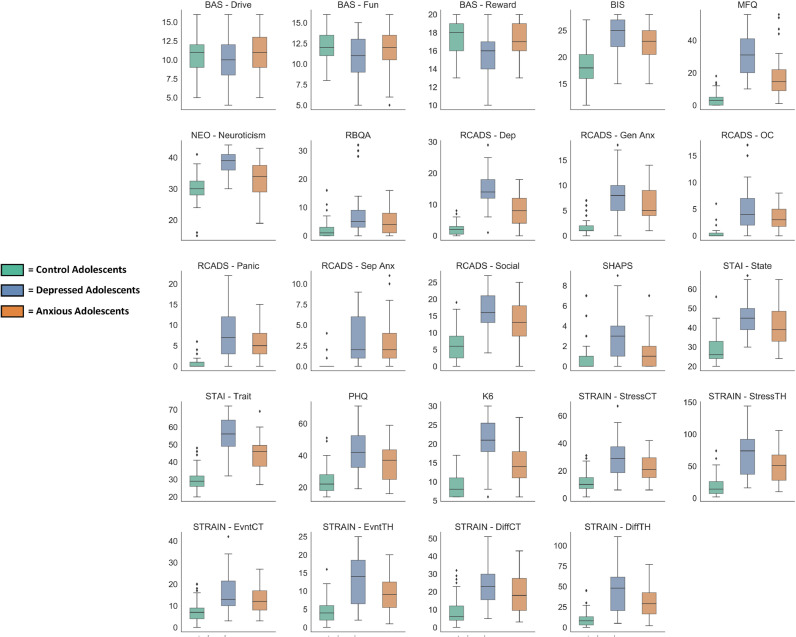
Dimensional measures were selected in consultation with study clinicians and by surveying research to probe constructs that cut across traditional diagnostic categories of anxiety and depression. Seven self-report measures were selected to characterize dimensions of adolescents’ moods, personality traits, thoughts, and behaviors (Fig. 2; Table 2). Administration time for these seven self-report measures took on average about 25 min to complete. An additional measure was selected to assess adolescents’ histories of stressful life experiences (Slavich et al., 2019), which took on average around 20 min to complete. Detailed information on adolescent dimensional measures may be found in Supplementary Appendix I. Dimensional data are collected from select measures by parent report on the adolescent (e.g., behavioral inhibition, negative and positive affect, problem behaviors; Supplementary Table 6).
Fig. 2.
Mean clinical dimension measure scores displayed by study groupings on select subscales. Measures and abbreviations are listed from left to right and top to bottom. Behavioral Inhibition and Behavioral Activation Questionnaire (BIS-BAS; Carver and White, 1994) –Drive (BAS-Drive) subscale score, Fun Seeking (BAS-Fun) subscale score, Reward Responsiveness (BAS-Reward) subscale score, and Behavioral Inhibition Scale (BIS) subscale score. Mood and Feelings Questionnaire total score (MFQ; Angold et al., 1995). NEO Five Factor Personality Inventory - Neuroticism subscale score (NEO—Neuroticism; McCrae and Costa, 2004). Risky Behavior Questionnaire for Adolescents total score (RBQA; Auerbach and Gardiner, 2012). Revised Child Anxiety and Depression Scale (RCADS; de Ross et al., 2000) Major Depressive Disorder subscale score (RCADS-Dep), Generalized Anxiety Disorder subscale score (RCADS-GenAx), Obsessive Compulsive Disorder subscale score (RCADS-OC), Panic Disorder subscale score (RCADS-Panic), Separation Anxiety Disorder subscale score (RCADS-SepAnx), and Social Phobia subscale score (RCADS-Social). Snaith-Hamilton Pleasure Scale total score (SHAPS; Snaith et al., 1995). State-Trait Anxiety Inventory (STAI; Spielberger et al., 1970), State subscale score (STAI-State) and Trait subscale score (STAI-Trait). Adolescent Stress and Adversity Inventory (STRAIN; Slavich et al., 2019). STRAIN Physical Health Complaints/Symptoms score (PHQ), STRAIN Mental Health Complaints/Symptoms score (K6), Total Count of Stressors (STRAIN-StressCT), Total Severity of Stressors (STRAIN-StressTH), Total Count of Acute Life Events (STRAIN-EvntCT), Total Severity of Acute Life Events (STRAIN-EvntTH), Total Count of Chronic Difficulties (STRAIN-DiffCT), and Total Severity of Chronic Difficulties (STRAIN-DiffTH). Detailed information on these clinical measures may be found in Supplementary Appendix I.
Table 2.
Adolescent Self-report Clinical Measures on Select Subscales.
| Domain | Measure | Subscale | Cronbach's α |
|---|---|---|---|
| Motivational systems | Behavioral Inhibition and Activation Questionnaire (BIS-BAS) | BIS | 0.85 |
| BAS-Drive | 0.75 | ||
| BAS-Fun | 0.62 | ||
| BAS-Reward | 0.77 | ||
| Depressive feelings and behaviors | Mood and Feelings Questionnaire (MFQ) | 0.96 | |
| Anxiety, depression, and vulnerability to stress | Neuroticism Subscale (NEO—Neuroticism) | 0.90 | |
| Anxious and depressive symptoms | Revised Child Anxiety and Depression Scale (RCADS) | RCADS-Dep | 0.93 |
| RCADS-GenAnx | 0.89 | ||
| RCADS-OC | 0.80 | ||
| RCADS-Panic | 0.90 | ||
| RCADS-SepAnx | 0.78 | ||
| RCADS-Social | 0.91 | ||
| Risky behavior | Risky Behavior Questionnaire for Adolescents (RBQA) | 0.82 | |
| Hedonic capacity | Snaith-Hamilton Pleasure Scale (SHAPS) | 0.72 | |
| Anxious tendencies and anxious state | State-Trait Anxiety Inventory (STAI) | STAI-State | 0.93 |
| STAI-Trait | 0.95 |
Note: Behavioral Inhibition and Behavioral Activation Questionnaire (BIS-BAS; Carver and White, 1994) – Behavioral Inhibition Scale (BIS) subscale, Drive (BAS-Drive) subscale, Fun Seeking (BAS-Fun) subscale, and Reward Responsiveness (BAS-Reward) subscale. Mood and Feelings Questionnaire (MFQ; Angold et al., 1995). NEO Five Factor Personality Inventory - Neuroticism subscale (NEO—Neuroticism; McCrae and Costa, 2004). Revised Child Anxiety and Depression Scale (RCADS; de Ross et al., 2000) Major Depressive Disorder subscale (RCADS-Dep), Generalized Anxiety Disorder subscale (RCADS-GenAx), Obsessive Compulsive Disorder subscale (RCADS-OC), Panic Disorder subscale (RCADS-Panic), Separation Anxiety Disorder subscale (RCADS-SepAnx), and Social Phobia subscale (RCADS-Social). Risky Behavior Questionnaire for Adolescents (RBQA; Auerbach and Gardiner, 2012). Snaith-Hamilton Pleasure Scale total score (SHAPS; Snaith et al., 1995). State-Trait Anxiety Inventory (STAI; Spielberger et al., 1970), State subscale (STAI-State) and Trait subscale (STAI-Trait). Detailed information on these clinical measures may be found in Supplementary Appendix I.
4.4. Cognitive and neuropsychological measures
Data characterizing adolescents’ cognitive/neuropsychological function are collected to assess cognitive constructs that could be related to general adolescent development, while also taking participant burden into consideration. Many of the BANDA measures are consistent with the original HCP and HCP-D studies (Barch et al., 2013), ABCD Study (Luciana et al., 2018), the Philadelphia Neurodevelopmental Cohort study (Satterthwaite et al., 2016), and several ongoing CRHD studies (Table 3; Supplementary Figure 2). Measures were selected from the National Institutes of Health (NIH) Toolbox (Gershon et al., 2013; Heaton et al., 2014) and the University of Pennsylvania Computerized Neuropsychological Test Battery (Penn Test Battery; R.C. Gur et al., 2010). Adolescent participants complete a total of 9 computerized measures from these standardized batteries; five of which are from the NIH toolbox. Adolescents and their participating parent also complete a two-subtest Wechsler Abbreviated Scale of Intelligence, 2nd Edition (WASI-II) which allows for the estimation of a normative Full-scale IQ, along with Verbal Comprehension and Perceptual Reasoning abilities. Administration time for adolescent completion of cognitive and neuropsychological measures was approximately 75 min.
Table 3.
Cognitive and Neuropsychological Measures.
| Task | Domain | Battery | Reporting | Harmonization |
|---|---|---|---|---|
| Delay Discounting | Impulsivity/self-regulation | Penn | Child | 2, 4 |
| Dimensional Card Sort | Cognitive flexibility/attention | NIH | Child | 1, 2, 3, 4, 5, 7 |
| Emotion Recognition | Facial emotion recognition | Penn | Child | 1, 2, 3, 4, 6 |
| Flanker | Inhibition/attention | NIH | Child | 1, 2, 3, 4, 5, 7 |
| List Sorting | Working memory | NIH | Child | 1, 2, 3, 4, 5, 7 |
| Matrix Reasoning | Nonverbal reasoning | Penn | Child | 1, 3, 4, 6 |
| Oral Reading | Reading decoding | NIH | Child | 1, 2, 3, 5 |
| Pattern Comparison | Processing speed | NIH | Child | 1, 2, 3, 4, 5, 7 |
| WASI-II | Full-scale intelligence | WASI | Parent/Child | |
| Word Memory | Verbal episodic memory | Penn | Child | 1, 3, 4, 6 |
Note: Task, Domains, and Harmonization with other “Big Data” Imaging Studies. 1 = original HCP, 2= HCP-Development, 3= Connectomes Related to Human Disease Project-Perturbation of the Treatment of Resistant Depression Connectome by Fast-Acting Therapies, 4= Dimensional Connectomics of Anxious Misery, 5=the ABCD study, 6= Philadelphia Neurodevelopmental Cohort, 7= Connectomes Related to Human Disease Project-Mapping Connectomes of Disordered Mental States. WASI-II = Wechsler Abbreviated Scale of Intelligence, 2nd Edition. NIH= task selected from National Institutes of Health Toolbox. Penn = University of Pennsylvania Computerized Neuropsychological Test Battery.
4.5. Additional characterization
Adolescent-report data on his/her relative physical development, primary- and secondary-sex characteristics are acquired (Taylor et al., 2001). Data from the Chapman Handedness Inventory are acquired to assess lateral-hand dominance of the adolescent (Chapman and Chapman, 1987). Demographic data (e.g., race, ethnicity, parental education, household income) are obtained via parent report, along with data on adolescents’ current psychiatric medication use (Fig. 1; Supplementary Table 5).
5. Functional imaging tasks in the BANDA study
We selected three tasks which probe psychological constructs of interest and that have been shown to elicit activation in neurocircuitry relevant to general adolescent development, as well as anxiety and depression (Fig. 3). BANDA targets three RDoC constructs: Responsiveness to Reward (Positive Valence Systems Domain), Acute Threat (Negative Valence Systems Domain), and Cognitive Control (Cognitive Systems Domain; see nimh.nih.gov/research-priorities/rdoc/constructs). Initial piloting of fMRI tasks in adult participants yielded activation patterns in brain regions thought to be related to these constructs and also activation patterns generally consistent with previous reports. Here, we discuss these tasks and demonstrate preliminary brain activation patterns from the first 140 adolescents participating in the BANDA study. fMRI tasks were adapted from extant reports, but were re-programmed from proprietary stimulus presentation software to an open-source stimulus presentation software (Pierce, 2007). Task code and stimuli are retrievable from https://github.com/BANDA-connect. Abbreviated versions of the fMRI tasks are used to train each participant on task procedures, before she/he begins scanning. Participant performance is monitored during practice to ensure that the participant is performing each task correctly, and s/he is given multiple attempts to practice the task if needed. Instructions for each task are repeated before the presentation of the task in the scanner. Training on tasks and presentation follows a standardized administration protocol to ensure all participants receive the same instructions and a similar study experience (Siless et al., 2020).
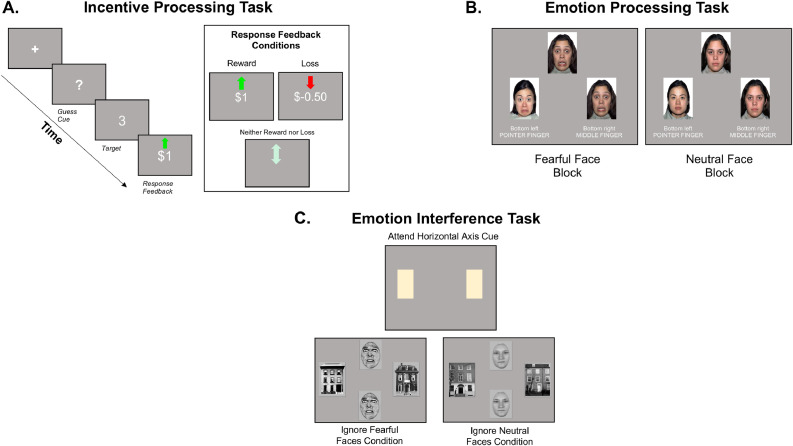
Fig. 3.
Example trials of BANDA fMRI tasks. A. Incentive Processing Task (IPT). B. Emotion Processing Task (EPT). C. Emotion Interference Task (EIT).
5.1. MRI acquisition
Comprehensive information on hardware, sequences, and acquisition harmonization with other HCP imaging projects is described in our companion article (Siless et al., 2020). Briefly, images are collected on a Siemens 3T Prisma MRI using a 64-channel head coil. The study used 52 head elements. Stimuli are back-projected onto a screen and observed through a mirror mounted on the task responses.
Only T1-weighted (T1w), T2-weighted (T2w), and task echo-planar image (EPI) acquisition parameters are described here. Task-fMRI images are acquired using 2-D multiband, gradient-recalled echo-planar imaging via publicly-available sequences identical to those used in other HCP studies (cmrr.umn.edu/multiband). Sequences offer a 2.0 mm isotropic voxel with whole-brain coverage from 72 oblique, axial slices, with multiband acceleration factor = 8, repetition time (TR) = 800 ms, echo time (TE) = 37 ms, flip angle = 52° Tasks are acquired using an even number of EPI runs, with two different phase encoding directions (i.e., anterior-posterior, posterior-anterior). One multi-echo magnetization-prepared rapid gradient echo (MPRAGE) T1w and one T2w image is acquired with acquisition parameters matching those of the HCP along with an additional volume navigators (vNavs) setter for prospective motion correction (Tisdall et al., 2012). The vNavs-enabled sequences estimate motion throughout the T1w and T2w scans and reacquires/replaces k-space data unduly affected by motion. T1w and T2w scans feature a 0.8 mm isotropic voxel size with 208 slices, field-of-view = 256 × 240 × 167 mm, acquired in the sagittal orientation. T1w: TR= 2400 ms, TE= 2.18 ms, T2w: TR = 3200 ms, TE= 564 ms.
5.2. Image processing and analysis
Task fMRI and structural images were processed in accordance to HCP minimally-preprocessed guidelines (Glasser et al., 2013) using processing workflows for Version 3.26.1 of the HCP minimally preprocessed pipeline (retrieved from: github.com/Washington-University/Pipelines/releases/tag/v3.26.1).
Anatomical Processing Workflows. PreFreeSurfer, FreeSurfer, PostFreeSurfer workflows were used to process T1w and T2w images. Processing procedures are described in detail elsewhere (see Glasser et al., 2013). Briefly, PreFreeSurfer workflow performed: gradient nonlinearity distortion corrections, co-registered T1w and T2w images, applied a bias-field correction using individual participant's spin-echo field maps, and spatially-normalized participants’ T1w image to the Montreal Neurological Institute (MNI) template (i.e., MNI152). The FreeSurfer and PostFreeSurfer workflows focus upon the corrected and spatially-normalized T1w outputs from the PreFreeSurfer workflow. Here, participants’ anatomical data are segmented into cortical and subcortical structures, cortical structures are reconstructed into pial and white matter surfaces, the cortical surface is smoothed, and cortical surface data are registered (via convexity of sulci, curvature of gyral and sulcal folds, and T1w/T2w myelin maps [i.e., multimodal surface matching; Robinson et al., 2104]) to a standard surface template (i.e., 32k_fs_LR mesh; Van Essen et al., 2012b). The final output of these workflows is the participants’ data in a single, down-sampled (~2 mm) space of 91, 282 Gy matter coordinates (i.e., “grayordinates”); MNI (volume) and a standardized surface template (32k_fs_LR mesh). This “grayordinate” space reflects an efficient amalgam of volume (i.e., voxels) and surface (i.e., vertices) data in a common geometrical and informatics framework.
Functional Processing and Analysis. fMRIVolume and fMRISurface workflows also correct for distortion and spatially-normalize participants’ functional data to the standard grayordinate space created by the anatomical workflows (above). These too are described in detail elsewhere (see Glasser et al., 2013). Briefly, these functional workflows remove spatial distortions via gradient nonlinearity corrections, realign volumes to compensate for participant motion, adjust for bias fields via dual-phase encoded spin-echo corrections (i.e., topup; Andersson et al., 2003), register fMRI data to participants’ MNI-based anatomical data, normalize each timeseries by the (whole-brain) global mean, and remove extra-parenchymal voxels. Cortical data are then placed in the standard surface-space (i.e., 32k_fs_LR mesh) using transformations obtained during the anatomical workflows and a spatial filter is applied so that higher-noise voxels (greater than 0.5 standard deviations above mean coefficient of variation within a 5 mm Gaussian neighborhood) are excluded from surface maps. Functional data are spatially smoothed (2 mm full width at half maximum [FWHM]) on the mesh surface and within the subcortical values, and subjected to a high-pass filter of 0.005 Hz.
To obtain activations within the task fMRI data, task-versus-rest, and condition-versus-condition contrasts are modeled in FMRIB Software Library (FSL; Smith et al., 2004) using a generalized linear modeling (GLM) convolution approach with a double-gamma hemodynamic response function, while also removing variance accounted for by translational and rotational head motion estimates. In the HCP workflows, an additional smoothing kernel is applied prior to GLM, resulting in a final applied spatial smoothing factor of FWHM ≈ 3.5 mm. Group-level z-scores of task activation were derived using FSL's Permutation Analysis of Linear Models (PALM; Winkler et al., 2014). Brain activation maps for each functional imaging task, for selected contrasts of interest are displayed in Figs. 4–6 and Supplementary Figure 3.
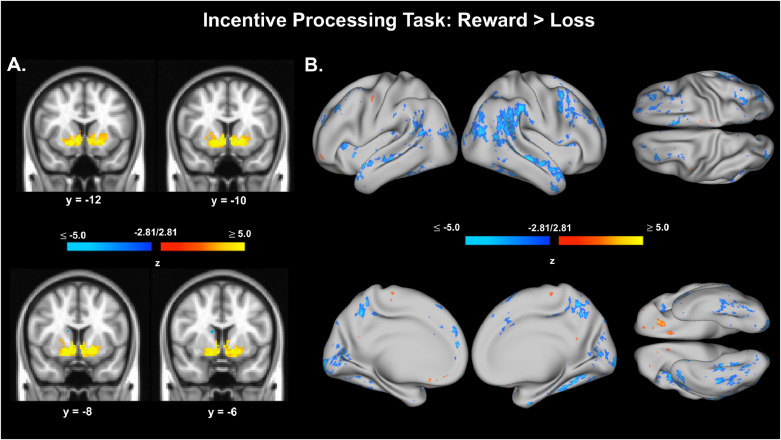
Fig. 4.
Preliminary activation results for the Incentive Processing Task, Reward > Loss contrast. A. Voxel data from Incentive Processing Task, Reward > Loss contrast. Voxels shown are thresholded at z = ± 2.81, p < .005, uncorrected. Voxel data were also thresholded with an additional volume correction of (k) requiring greater than 9 contiguous voxels (k > 9). B. Surface (vertex) data from Incentive Processing Task, Reward > Loss contrast. Vertices shown are thresholded at z = ± 2.81, p < .005, uncorrected.
Fig. 6.
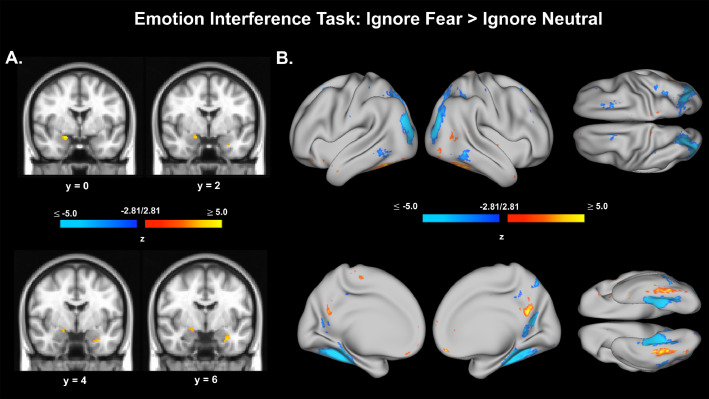
Voxel and vertex preliminary activation results for the Emotion Interference Task, Ignore Fear > Ignore Neutral contrast. A. Voxel data from Emotion Interference Task, Ignore Fear > Ignore Neutral contrast. Voxel data were also thresholded with an additional volume correction of (k) requiring greater than 9 contiguous voxels (k > 9). B. Surface (vertex) data from Emotion Interference Task, Ignore Fear > Ignore Neutral contrast. Vertices shown are thresholded at z = ± 2.81, p < .005, uncorrected.
5.3. Responsiveness to reward and the incentive processing task (IPT)
The responsiveness-to-reward construct is reflected in physiological signatures and behavioral markers associated with the anticipation or receipt of rewards. Striatal structures comprise key nodes within the brain's reward and motivation system (RMS; Bartra et al., 2013; Haber and Knutson, 2009; Shultz, 2000, 2002). Adolescence is thought to be characterized by greater sensitivity of the RMS to incentives, as well as a greater tendency for reward-seeking behaviors (Casey, 2015; Somerville et al., 2010; see also Buckholtz and Faigman, 2014; Cohen and Casey, 2014). Evidence robustly links alterations to the brain's RMS and depression (Dillon et al., 2014; Forbes et al., 2006; Luking et al., 2016a; Pizzagalli et al., 2009; Sharp et al., 2014; Stringaris et al., 2015; see also Bress et al., 2013; Kerestes et al., 2014; Luking et al., 2016b; Nelson et al., 2015; Pizzagalli, 2014). There are also findings demonstrating anxiety-related activation changes in RMS relative to sample controls (Benson et al., 2015; Lahat et al., 2016).
The IPT, adapted from Delgado and colleagues (2000), presents the prospect of monetary rewards to evoke activation in the RMS (from Barch et al., 2013). This task was chosen for the BANDA study for several reasons. First, IPT-type tasks consistently elicit suprathreshold RMS activation in healthy youth and adults (Delgado et al., 2000; Forbes et al., 2009; Hubbard et al., in press; May et al., 2004; Speer et al., 2015; Tricomi et al., 2004). Second, IPTs are also used by the original HCP and HCP-D studies (Barch et al., 2013; Somerville et al., 2018) and two CRHD projects (one on adult anxiety, one on adult disordered emotional states). Third, youth with depression or risk for depression show blunted RMS responses given the prospect of incentives relative to non-MDD youth (Forbes et al., 2006; Stringaris et al., 2015).
Participants complete two runs (2 min 52 s per run; 5 min 44 s total) of a block-design, IPT adapted from Delgado et al., 2000 (see also Barch et al., 2013; Speer et al., 2015). Before the task begins, participants are reminded that their responses will result in winning or losing actual money. Participants guess via index- or middle-finger button press whether a to-be-revealed number (between 1–9) is greater than or less than 5. Participants then receive an image of the actual number and visual feedback regarding whether they had guessed correctly. Guessing correctly results in a green, upward-facing arrow and “+$1″, indicating that participants will have $1 added to their task winnings. Guessing incorrectly results in a red, downward-facing arrow and “-$0.50″, indicating that the participants will have $0.50 taken from their task winnings. Punishment trials are half of the magnitude of reward trials to account for greater sensitivity of participants to loss compared to reward (Tversky and Kahneman, 1991; from Barch et al., 2013). Feedback is also given if the number that was revealed was 5; by a gray, double-headed, horizontal arrow. The gray arrow indicates that the participant neither wins nor loses money on that trial. If the participant fails to respond in the time allotted by the trial (1.5 s), “no response” is presented on screen and the participant is shown that s/he will not win or lose money for that trial. The number of reward and loss trials is controlled so that each block features primarily reward or primarily loss trials. Response times (RTs) are acquired for each button press within the 1.5 s allotted by the trial. The number of button presses during the trial period is also recorded.
The question mark cue is presented for 1.5 s, followed by feedback for 1.0 s, then by a 1.0 s inter-trial interval. There are 8 trials per block. There are two types of experimental blocks (28 s/block) and one fixation (baseline; 15 s/block) block. Block conditions are balanced and pseudo-randomized across each run. Each block type (Reward, Loss, Fixation) is presented 8 times (4 times/run). In the Reward blocks, participants receive 6 pseudo-randomly interspersed reward trials and either 1 neutral and 1 loss trial, 2 neutral trials, or 2 loss trials. In the Loss blocks, participants receive 6 pseudo-randomly interspersed loss trials and either 1 neutral and 1 reward trial, 2 neutral trials, or 2 reward trials.
Questions are placed on the screen after the second run of the IPT. These questions will allow the assessment of whether groups or individuals differ in their perceived winnings and whether these perceptions are related to RMS activation (Schultz, 2017). Up to three prompts inquire about participants’ perceived winnings. The participant responds to these prompts via finger pad. First, s/he is asked, “Do you think you won money, lost money, or broke even?” This serves as a stem question to set up the following questions. However, if the participant responds that they perceived they “broke even,” no follow-up questions are presented. Next, s/he is asked, “How much do you think you [won/lost]?” This question has three available responses to qualify the magnitude of participants’ perceived winnings or losses: “A little”, “Average”, or “A Lot”. Finally, the screen prompts participants to: “Guess how much you [won/lost]”. There are four available responses aimed at quantifying the perceived magnitude of winning or losing in US dollars: “0–5″, “5–10″, “10–15″, “15+”.
5.3.1. IPT preliminary data analysis
IPT functional imaging data were available and analyzed for 133 adolescents (AA=43, CA=51, DA=38). Reward, Loss, and Reward > Loss conditions were modeled, along with translational and rotational head motion parameter estimates, using standard GLM approaches in FSL. Consistent with the HCP young adult and developmental cohorts, brain activation in response to reward compared to loss (Reward > Loss conditions) are presented here (Barch et al., 2013; Somerville, 2018). This contrast captured differential activation patterns associated with the prospect of rewards, while distinguishing activations related to the general task experience (e.g., visual/perceptual load, motor responses) and the prospect of losses. We expected positive activations for this contrast within ventral striatum (Barch et al., 2013; Somerville, 2018). Voxel and vertex data were thresholded using cutoff values z= ± 2.81, p < .005, uncorrected. Voxel data were also thresholded with an additional volume correction of (k) requiring greater than 9 contiguous voxels (k > 9) in an attempt to ensure clusters of activation had reasonable mass, without omitting activation clusters from smaller brain regions. This approach using a relatively liberal lower-limit threshold is consistent with the approach of the original HCP study to describe general activation patterns in a preliminary dataset (Barch et al., 2012). The p < .005 cutoff value was chosen consistent with recent analyses demonstrating that beginning with a voxel-wise p-value of this magnitude can identify regions of interest with strong to moderate strength of evidence for a target effect (Chen et al., 2019; see also Chen et al., 2020). The IPT and Reward > Loss contrast produced expected, positive activations in ventral striatum (Fig. 4), lending confidence that this task can evoke canonical reward-related neural signatures in this sample.
5.4. Acute threat and the emotion processing task (EPT)
The acute-threat construct is reflected in physiological signatures and behavioral markers associated with aversive (e.g., threatening or fear-inducing) stimuli or cues. A primary node within fear- or threat-based brain circuitry is the amygdala (Barrett and Wager, 2006; LeDoux, 2000; J. 2007; LeDoux and Brown, 2016; LeDoux and Pine, 2016). Amygdalar activation is overexpressed in adolescents relative to children and adults, particularly in threatening contexts (Ahmed et al., 2015; Casey, 2015; Somerville et al., 2010). Fear-related brain circuitry is consistently implicated in anxiety and depression or risk factors for these disorders (Bishop et al., 2004a; Chai et al., 2015; Christensen et al., 2015; Kilgore and Yurgelun-Todd, 2005; McClure et al., 2007; Phan et al., 2006; Thomas et al., 2001; Yang et al., 2010; see also Dillon et al., 2014; Etkin and Wager, 2007; Hofmann et al., 2011; Kerestes et al., 2014; Stuhrmann et al., 2011).
The EPT (Chai et al., 2015; Hariri et al., 2002; see also Barch et al., 2013) was chosen to target the acute threat construct and evoke fear-related brain activity for several reasons. First, EPTs have been shown to evoke group-level, suprathreshold activation in the amygdalae in adults and youth (Barch et al., 2013; Somerville, 2018). Second, an EPT version is used by the original HCP and HCP-D studies, and two current CRHD projects in adult anxiety and depression. Finally, viewing threatening facial expressions within this task is associated with increases in amygdala BOLD activation for anxious and depressed participants, as well as for those at risk for developing anxiety or depression, relative to study controls (Chai et al., 2015; Kilgore and Yurgelun-Todd, 2005; McClure et al., 2007; Phan et al., 2006; Thomas et al., 2001; Yang et al., 2010; see also Etkin and Wager, 2007; Kerestes et al., 2014; Stuhrmann et al., 2011).
Participants complete two runs (5 min 24 s per run, 10 min 48 s total) of a block-design, EPT adapted from Hariri et al., 2002 (from Barch et al., 2013). Participants indicate via index- or middle-finger button press which of two pictures at the bottom of the screen match one picture presented above (target). When the picture to the left of the screen matches the target picture, participants are instructed to respond with their index finger. When the picture to the right of the screen matches the target picture, participants are instructed to respond with their middle finger. RTs and accuracy of responses are acquired within the 3 s allotted for each trial. Each trial consists of three pictures from a single condition (one at the top, two at the bottom). Each block consists of six trials and lasts 18 s. There are four experimental conditions, one control condition (Object stimuli), and one baseline condition (fixation cross). Six blocks of each condition are presented across two runs.
Four experimental conditions consist of Fearful, Happy, Neutral, and Sad face stimuli taken from the Radboud and NimStim databases (Langner et al., 2010; Tottenham et al., 2009) and Object stimuli consisting of fruits and vegetables scaled to approximately the same size as the face stimuli (Chai et al., 2015). Face stimuli are from 72 actors (50% female). Each actor is presented as a target once for each facial expression (Fearful, Happy, Neutral, Sad). The Sad condition was not part of the original task adapted from Hariri et al. (2002), nor is it part of other HCP studies. We did not include the Sad condition for the first 17 (AA = 1, CA =14, DA = 2) participants. However, because activation in amygdalae for depressed persons tends to be reliably sensitive to this condition (Stuhrmann et al., 2011), we decided to include this once we began recruiting more depressed adolescents. All actors are facing forward and the images are cropped to contain only the actors’ head, neck, and a portion of their shoulders. Object stimuli consist of 72 unique pictures of fruits and vegetables. Each Object stimulus is presented in only a single trial. Conditions are interspersed pseudo-randomly across two runs. Target responses (index or middle finger) are balanced across conditions and appear pseudo-randomly across runs.
5.4.1. EPT preliminary data analysis
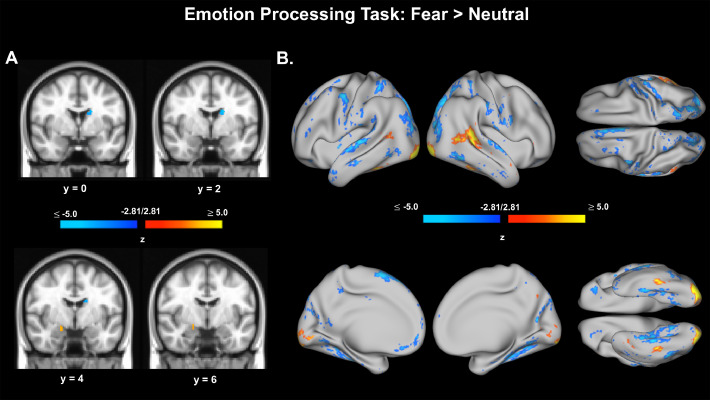
EPT functional data were available and analyzed for 134 adolescents (AA=44, CA=51, DA=39). Fearful, Happy, Neutral, Sad, Object, Face > Object, Fear > Neutral conditions were modeled, along with translational and rotational head motion parameter estimates, using standard GLM approaches in FSL. We present brain activation in response to fearful faces compared to neutral faces (Fear > Neutral). This contrast elucidates differential brain activation patterns associated with fearful face processing while distinguishing activations related to the general task experience (e.g., visual/perceptual load, face features, motor responses). We expected positive activations for this contrast within amygdalae. Voxel and vertex data were thresholded at z= ± 2.81, p < .005, uncorrected. Voxel data were also thresholded with an additional volume correction of (k) requiring greater than 9 contiguous voxels (k > 9) in an attempt to ensure clusters of activation had reasonable mass, without omitting activation clusters from smaller brain regions. Fear > Neutral contrast produced slight positive activations in the amygdala (Fig. 5), lending some confidence that this task can evoke canonical threat-related neural signatures in this sample. Results from the canonical, Face > Object contrast are also presented in Supplementary Material (Supplementary Figure 3).
Fig. 5.
Voxel and vertex preliminary activation results for the Emotion Processing Task, Fear > Neutral contrast. A. Voxel data from Emotion Processing Task, Fear > Neutral contrast. Voxels shown are thresholded at z = ± 2.81, p < .005, uncorrected. Voxel data were also thresholded with an additional volume correction of (k) requiring greater than 9 contiguous voxels (k > 9). B. Surface (vertex) data from Emotion Processing Task, Fear > Neutral contrast. Vertices shown are thresholded at z = ± 2.81, p < .005, uncorrected.
5.5. Cognitive control and the emotion interference task (EIT)
The cognitive-control construct is reflected in physiological signatures and behavioral markers during task conditions requiring goal-directed performance. One's capacity to bias cognitive and neural systems in service of goal-directed performance is thought to reflect his/her cognitive control ability. Adolescents have difficulty exerting cognitive control during emotional distraction relative to other forms of distraction, and relative to adults in these same conditions (Cohen et al., 2016; Cohen-Gilbert and Thomas, 2013; Dreyfus et al., 2014; Grose-Fifer et al., 2013; see Ahmed et al., 2015). Cognitive control impairments during emotional distraction are thought to be an essential feature of anxiety and depression (Eysenck et al., 2007; Gotlib and Joormann, 2010; Hubbard et al., 2016, b; Joormann, 2011; Koster et al., 2011; Nolen-Hoeksema et al., 2008; Schweizer et al., 2019; Shi and Liu, 2016; Siegle et al., 2002; Whitmer and Gotlib, 2013).
Prefrontal cortex (PFC) is critical for goal-directed performance (Botvinick et al., 2001; Miller and Cohen, 2001; Miller and Buschman, 2013). PFC regions are active during emotional-distraction scenarios (Ochsner et al., 2008). This activation is thought to reflect the implementation of cognitive control processes (Banich et al., 2009; Bishop, 2007; Ochsner and Gross, 2005). PFC gray matter and structural/functional connections are also underdeveloped in adolescents relative to adults (Gogtay et al., 2004; Insel et al., 2017; Ofen et al., 2007; Pehlivanova et al., 2018; see also Casey, 2015). Depressed and anxious persons also show alterations in PFC functioning during (or proximal to) exposure to emotional material (Bishop et al., 2004b; Fales et al., 2008, 2009; Hooley et al., 2009; Mayberg et al., 1999; Siegle et al., 2002, G.J. 2007; Telzer et al., 2008; Wang et al., 2008; see also Auerbach et al., 2013; Bishop, 2007b; Drevets, 1999; Davidson et al., 2002; Foland-Ross and Gotlib, 2012; Hofmann et al., 2011; Kerestes et al., 2014; Mayberg, 2009).
The EIT (Fales et al., 2008a; Vuilleumier et al., 2001; Wojciulik et al., 1998) was chosen for the BANDA study for several reasons. First, the EIT has been shown in adults to elicit activation in cognitive-control-related brain regions (e.g., anterior cingulate cortex and lateral PFC; Fales et al., 2008a; Vuilleumier et al., 2001). Second, this task is currently being used in an adult CRHD project on anxiety, potentially allowing for adult and adolescent comparisons. Finally, adult anxiety- and depression-related activation changes in PFC have been demonstrated on variants of the EIT. Specifically, depressed and more anxious participants tend to show decreases in PFC BOLD activation when instructed to ignore fearful-face pictures on the EIT, relative to non-depressed or less anxious participants (e.g., Bishop et al., 2004b; Fales et al., 2008a, Fales et al., 2008b). Depression-related increases in amygdalar activation have also been observed during this condition (Fales et al., 2008a, Fales et al., 2008b).
Participants complete four runs (3 min 54 s per run, 15 min 36 s total) of an event-related design, EIT. Responses are given via index- or middle-finger button press. Participants indicate whether two pictures in either a horizontal or vertical axis are identical or different. Each trial presents two pairs of pictures (1 pair in the horizontal axis, 1 pair in the vertical axis). Each run features 24 trials wherein participants need to attend to the pairs of pictures presented in a specific axis. Before each run participants are cued signaling whether they need to attend to the pictures in the horizontal or vertical axis, and thus, ignore pictures in the orthogonal axis. RTs and accuracy of responses are acquired within 2.2 s allotted for each response.
Trials begin with a 1 s fixation cross, 0.25 s presentation of the picture stimuli, and allow 2.2 s for a response. There are two different classes of pictures (faces and houses), with the former class also featuring two different types of facial expressions (neutral and fearful). There are four conditions: attention to fearful faces, attention to neutral faces, ignoring fearful faces, ignoring neutral faces (Fales et al., 2008a, Fales et al., 2008b; Vuilleumier et al., 2001). Each condition is presented 24 times across the 4 runs, and an equal number of times in each axis orientation. Inter-trial intervals of 2150, 4660, 9680, and 12,190 ms are randomly (but equally) distributed throughout each run.
5.5.1. EIT preliminary data analysis
EIT functional data were available and analyzed for 133 adolescents (AA=44, CA=51, DA=38). Attention to Fearful Faces, Attention to Neutral Faces, Ignoring Fearful Faces, Ignoring Neutral Faces, Attending Faces > Attending Houses, and Ignoring Fearful Faces > Ignoring Neutral Faces conditions were modeled, along with translational and rotational head motion parameter estimates, using standard GLM approaches in FSL. Consistent with adult anxiety and depression research, we present brain activation in response to adolescents’ attempts at ignoring fearful faces compared to ignoring neutral faces (Ignore Fear > Ignore Neutral; Bishop et al., 2004b; Fales et al., 2008a, Fales et al., 2008b). This contrast captures differential brain activation patterns associated with attempts to ignore emotionally-evocative information (i.e., fearful faces) while distinguishing activations related to the general task experience (e.g., visual/perceptual load, face features, motor responses) and may correspond to the cognitive control construct. We assumed that we would find prefrontal activations associated with this contrast. However, we could not predict the direction of these activations a priori. An a priori directional prediction at the sample level is complicated due to sample heterogeneity (i.e., control, anxious, and depressed participants) and because these participants are adolescents–a group who more generally show cognitive control deficits in the presence of emotional stimuli (Cohen et al., 2016; Cohen-Gilbert and Thomas, 2013; Dreyfus et al., 2014; Grose-Fifer et al., 2013; see also Ahmed et al., 2015). Voxel and vertex data were thresholded at z= ± 2.81, p < .005, uncorrected. Voxel data were also thresholded with an additional volume correction of (k) requiring greater than 9 contiguous voxels (k > 9) in an attempt to ensure clusters of activation had reasonable mass, without omitting activation clusters from smaller brain regions. We observed negative activation in superior PFC and other PFC regions. This activation was greater when participants were trying to ignore neutral faces, relative to fearful faces (as indicated by negative values; Fig. 6). This finding is consistent with reports of adolescents, as well as anxious and depressed adults, having difficulty controlling attention away from emotionally-evocative information. Increased activations observed for the Ignore Fear > Ignore Neutral contrast in amygdalae and fusiform gyri, areas involved in emotion and face processing, also lend preliminary support for this interpretation.
6. Considerations for using BANDA data
6.1. Exploration of the neural correlates of clinical diversity
This project features a wide range of psychiatric symptoms and comorbidities. For categorical analyses described here, we parse this sample in terms of three study categories (i.e., AA, CA, and DA participants). However, prospective users of BANDA data are not constrained to these labels. The large sample of adolescent patients and the breadth of clinical information collected will allow for many clinically- and scientifically-meaningful comparisons. For example, interested researchers may examine brain or behavioral differences between lifetime and present episodes of depression. The addition of the Family History Screen will also allow for the assessment of transgenerational, neural effects of psychopathology (Chai et al., 2015, 2016; Huang et al., 2011; Hung et al., 2017; Luking et al., 2016a; Morgan et al., 2014; Peterson et al., 2009; Sharp et al., 2014; Stringaris et al., 2015). Moderate to substantial inter-rater agreement on DSM-5 categories for depression and anxious classifications also suggests that researchers may also explore the relationships of these traditional diagnostic labels with measures of adolescent brain and behaviors with a reasonable degree of confidence in these diagnostic classifications.
In this preliminary sample, control and clinical adolescents demonstrated considerable variability in their responses to the clinically-relevant dimensional measures. Importantly, this variability was accompanied by mostly desirable levels of internal consistency, with 93% of subscales falling in the Acceptable (α ≥ 0.7) to Excellent (α ≥ 0.9) range (Median Cronbach's α = 0.89; Table 2). The breadth of, and variability within, the measured clinical dimensions should provide a wealth of opportunities for investigators to account for a wide range of individual differences using the structural, functional, or diffusion imaging data. For instance, a dimensional approach could elucidate common factors across diagnoses that are the product of, or give rise to, specific neural findings (e.g., decreased functional connectivity between frontal regions and amygdalae during the viewing of fearful stimuli).
6.2. Substance use and BOLD signal
Medication is a general challenge for clinical imaging research (Iannetti and Wise, 2007). The exact effects of psychiatric medications on BOLD signal are not known. However, psychiatric medications do affect the physiological processes underlying BOLD signal activations (Dukart et al., 2018; Harris and Reynell, 2017). It was not feasible for this project to solely recruit unmedicated or treatment-naïve participants, nor would such a select group offer a representative sample of adolescents with depression or anxiety. Moreover, due to concerns for participants’ well-being, we did not require a washout period for medications before brain imaging.
One consideration for using this project's BOLD imaging data are the effects that medication status or type might have on functional imaging findings. About half of patients in the present sample were taking at least one psychotropic medication. If this trend continues in recruitment, reasonably large sample sizes of medicated and unmedicated adolescent patients will be obtained and may permit assessments of patient medication status on BOLD imaging data. Records of medication types and status of all participants are also collected which may be used as covariates in analyses (Supplementary Table 5).
Participants meeting criteria for current substance-use disorders are not included in this study. However, those with past substance-use disorders may be included. Researchers using these data may consider excluding those with historical substance-use disorders (Supplementary Table 4). In the present sample, one participant within the anxious adolescent group (2.6% of AA group) met the criteria for a previous substance-use disorder. Data from self-report substance abuse items will be available pertaining to alcohol, caffeine, nicotine, and illicit substance use. Group-level descriptive statistics of these items are featured in Supplementary Table 7.
6.3. Cross-study harmonization and comparisons
Harmonization of behavioral and imaging measures between the BANDA study and extant or future projects may allow for direct comparisons across a wide array of populations. The choice to use the computerized NIH Toolbox and Penn Test Battery measures affords cognitive and neuropsychological comparisons with at least 7 other “big-data” imaging projects. Moreover, because diverse samples are continuing to be collected on these measures and these data are being curated and shared, comprehensive norms may one day be available which will provide an invaluable resource for evaluating a participant's ability in light of a population-representative distribution (Beaumont et al., 2013). Similarly, the use of harmonized imaging acquisition parameters, hardware, and functional tasks may allow for comparisons of brain imaging findings or joint analyses across multiple imaging projects. Such comparisons, however, are not as straightforward as comparing behavioral measures (Vollmar et al., 2010). Thus, additional analytic approaches and additional care in interpretation of comparisons between harmonized imaging data from different projects may be necessary (Adhikari et al., 2018; Fortin et al., 2018; Karyumak et al., 2019; Yu et al., 2018; see also Harms et al., 2018; Siless et al., 2020).
6.4. Recommendations for future analyses
The present task-fMRI analyses used the HCP minimal preprocessing workflow (Glasser et al., 2013). To remain as consistent as possible with extant HCP methods presentations (Barch et al., 2013; Somerville et al., 2018) and to provide a general illustration of our minimally processed data, comprehensive denoising of imaging data was not part of the present analysis workflow. Thus, only minimal attempts to account for non-neural variance in these data were employed (i.e., covarying for translational and rotational head motion). Future analyses must consider employing additional practices to account for non-neural influences on these data (e.g., frame censoring [Power et al., 2012], component-based denoising [Behzadi et al., 2007; Salimi-Khorshidi et al., 2015]).
In keeping with other HCP reports to provide a general overview of all of the data, we did not exclude participants’ data from our analyses based upon task fMRI performance (Barch et al., 2013; Somerville et al., 2018). Task fMRI performance data are made publicly available, along with study images. Users of task fMRI data should consider excluding participants based upon performance criteria. For convenience, we suggest here minimum guidelines for participant exclusion based upon performance data. At minimum, users of the ECT and EIT measures should consider excluding participants who responded to fewer than 70% of trials and had less than 60% accuracy in their responding. Applying these criteria to the present sample would exclude 0.75% (n = 1) and 11.28% (n = 15), for the ECT and EIT; respectively. For the IPT, there is no measure of response accuracy. However, response rate and response bias (i.e., percent of index- or middle-finger button presses) are recorded. For this task, we also suggest excluding participants who responded to fewer than 70% of trials. Additionally, we suggest excluding participants who showed greater than 90% response bias for a single-button response. Because the probability of a correct or incorrect response was fixed at 50%, >90% suggests that participants were not thoughtfully performing the task. Applying these criteria to the IPT would exclude 0.75% (n = 1) participants.
Voxel and surface maps were displayed here using a lower-limit threshold of z = ± 2.81, corresponding to an uncorrected p-value of 0.005. This approach of using a relatively liberal lower-limit threshold was consistent with the approach used in the original HCP study (Barch et al., 2012) and it was undertaken to merely describe sample-wide trends in functional imaging data for specific contrasts of interest. This allowed us to illustrate activations that are likely sufficient for ROI-based analyses, as well demonstrate activations which may survive more rigorous (e.g., Bonferroni), grayordinate-wise correction methods (i.e., z ≈ ± 5). We suggest that prospective users of these data with the goal of grayordinate-wise or voxel-wise inferential testing (i.e., making population-level claims based upon sample-level evidence) consider rigorous family-wise error and/or false-discovery rate corrections to ensure the generalizability of their findings.
6.5. Pre-registration and transparent science
There are no contingencies placed upon accessing de-identified data from the BANDA study for research purposes. However, we encourage prospective users to pre-register hypothesis-driven research before analyzing these data (e.g., Open Science Framework). In attempting to circumvent entering bias into future random-effects investigations on these data, we chose not to publish group-level averages or differences (or individual difference relationships) on task-fMRI analyses here (Kerr, 1998). In terms of necessary statistical effect sizes for prediction of clinical dimensions, assuming a 10–20% loss of data for any given measure (e.g., attrition, poor image quality) from the proposed final sample will require a correlation effect size between r = 0.194 - 0.205, to reject the null hypothesis (α = 0.05) and achieve 80% power. If data-driven, predictive modeling is used, preregistration of discovery-set models before applying to validation data is also encouraged. Finally, we encourage users to deposit their code and results generated from analyses of BANDA brain data to one of many neuroscience repositories (e.g., NeuroVault, OpenNeuro).
7. Conclusion
The BANDA study reflects a novel effort seeking to understanding neural and behavioral aspects of adolescent anxiety and depression. This project capitalizes on the methods and philosophy of the HCP to provide the largest, open-access brain-imaging dataset of anxious and depressed adolescents to date. One goal in collecting and making data available is to help further developmental, neuropsychopathological approaches to understanding anxiety and depression. We hope that these data provide a useful resource for the neuroimaging and clinical communities in gaining insight into brain and behavior relationships in adolescent anxiety and depression.
Disclosures
Over the past three years, DAP received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, and Takeda Pharmaceuticals and an honorarium from Alkermes. SGH receives financial support from the Alexander von Humboldt Foundation and compensation for his work as editor from SpringerNature and the Association for Psychological Science. SGH also receives compensation for this role as an advisor from the Palo Alto Health Sciences and for his work as a Subject Matter Expert from John Wiley & Sons, Inc. and SilverCloud Health, Inc. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
Author statements
NA Hubbard: writing, visualization, validation, supervision, formal analysis, project administration; V Siless: writing, data curation, project administration, data collection, supervision, methodology; IR Frosch: writing, visualization, data collection, supervision, project administration; M Goncalves: formal analysis, software, writing, data curation, visualization; N Lo: formal analysis, software, data curation, visualization; J Wang: data collection, data curation; CCC Bauer: methodology; K Conroy: data collection; E Cosby: data collection; A Hay: data collection, supervision; R Jones: data collection, data curation; M Pinaire: data collection; F Vaz De Souza: data collection; G Vergara: data collection; S Ghosh: conceptualization, methodology, validation, data curation, writing, supervision, funding acquisition, project administration; A Henin: conceptualization, writing, data collection, supervision, funding acquisition, project administration; Hirshfeld-Becker: conceptualization, writing, data collection, supervision, funding acquisition; SG Hofmann: conceptualization, writing, supervision, funding acquisition, project administration; IM Rosso: conceptualization, writing, validation, funding acquisition; RP Auerbach: conceptualization, writing, validation, funding acquisition, project administration; DA Pizzagalli: conceptualization, writing, supervision, funding acquisition, project administration; A Yendiki: conceptualization, writing, project administration, data collection, supervision, methodology, funding acquisition; JDE Gabrieli: conceptualization, writing, project administration, supervision, funding acquisition; S Whitfield-Gabrieli: conceptualization, writing, project administration, supervision, funding acquisition.
Acknowledgments
This project was supported by the National Institute of Mental Health, U01MH108168 (JDEG, SWG), and (F32MH114525 to NAH). NAH was partially supported by the Brain and Behavior Research Foundation. AY was partially supported by R01EB021265 and U01EB026996. DAP was partially supported by R37MH068376 and R01MH101521. SSG was partially supported by R01EB020740 and P41EB019936. SGH was partly supported by R01AT007257, R01MH099021 and the James S. McDonnell Foundation. IMR was partially supported by R01MH096987 and the Brain and Behavior Research Foundation. This project was made possible by the resources provided by Shared Instrumentation Grants 1S10RR023401, 1S10RR019307, and 1S10RR023043. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of any other sponsor.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102240.
Appendix. Supplementary materials
References
- Adhikari B.M., Jahanshad N., Shukla D., Glahn D.C., Blangero J., Reynolds R.C., Kochunov P. Heritability estimates on resting state fMRI data using enigma analysis pipeline. Pacific Symp. Biocomput. 2018;212669:308–318. doi: 10.1142/9789813235533_0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.P., Bittencourt-Hewitt A., Sebastian C.L. Neurocognitive bases of emotion regulation development in adolescence. Dev. Cogn. Neurosci. 2015;15:11–25. doi: 10.1016/j.dcn.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Skare S., Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar image: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Angold A., Costello E.J., Messer S.C., Pickles A., Winder F., Silver D. Development of a short questionnaire for use in epidemiological studies of depression of children and adolescents. Int. J. Methods Psych. Res. 1995;5:237–249. [Google Scholar]
- Auerbach R.P., Gardiner C.K. Moving beyond the trait conceptualization of self-esteem: the prospective effect of impulsiveness, coping, and risky behavior engagement. Behav. Res. Thearpy. 2012;50:596–603. doi: 10.1016/j.brat.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Auerbach R.P., Webb C.A., Gardiner C.K., Pechtel P. Behavioral and neural mechanisms underlying cognitive vulnerability models of depression. J. Psychother. Integr. 2013;23(3):222–235. doi: 10.1037/a0031417. [DOI] [Google Scholar]
- Banich M.T., Mackiewicz K.L., Depue B.E., Whitmer A., Miller G.A., Heller W. Cognitive control mechanisms, emotion & memory: a neural perspective with implications for psychopathology. Neurosci. Behav. Rev. 2009;33(5):613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Burgess G.C., Harms M.P., Peterson S.E., Schlagger B.L., Corbetta M., Snyder A.Z. Function in the human connectome: task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F., Wager T.D. The structure of emotion: evidence from neuroimaging studies. Curr. Dir. Psychol. Sci. 2006;15(2):79–83. doi: 10.1111/j.0963-7214.2006.00411.x. [DOI] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. The valuation system: a coordinate-based meta-analysis of bold fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont J.L., Havlik R., Cook K.F., Hays R.D., Wallner-Allen K., Korper S.P., Gershon R. Norming plans for the nih toolbox. Neurology. 2013;80:87–92. doi: 10.1212/WNL.0b013e3182872e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for bold and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson B.E., Guyer A.E., Nelson E.E., Pine D.S., Ernst M. Role of contingency in striatal response to incentive in adolescents with anxiety. Cognit. Affect. Behav. Neurosci. 2015;15(1):155–168. doi: 10.3758/s13415-014-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S., Duncan J., Brett M., Lawrence A.D. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat. Neurosci. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bishop S.J., Duncan J., Lawrence A.D. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J. Neurosci. 2004;24(46):10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S.J. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn. Sci. (Regul. Ed.) 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108(3):624–652. doi: 10.1037/0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bress J.N., Foti D., Kotov R., Klein D.N., Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50(1):74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Buckholtz J.W., Faigman D.L. Promises, promises for neuroscience and law. Curr. Biol. 2014;24(18):R861–R867. doi: 10.1016/j.cub.2014.07.057. [DOI] [PubMed] [Google Scholar]
- Burstein B., Agostino H., Greenfield B. Suicidal attempts and ideation among children and adolescents in US emergency departments, 2007-2015. JAMA Pediatr. 2019;173(6):598–600. doi: 10.1001/jamapediatrics.2019.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C.S., White T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. JPSP. 1994;67:319–333. [Google Scholar]
- Casey B.J. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu. Rev. Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Hirshfeld-Becker D., Biederman J., Uchida M., Doehrmann O., Leonard J.A., Gabrieli J.D.E. Functional and structural brain correlates of risk for major depression in children with familial depression. NeuroImage: Clini. 2015;8:398–407. doi: 10.1016/j.nicl.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Hirshfeld-Becker D., Biederman J., Uchida M., Doehrmann O., Leonard J.A., Whitfield-Gabrieli S. Altered intrinsic functional brain architecture in children at familial risk of major depression. Biol. Psychiatry. 2016;80(11):849–858. doi: 10.1016/j.biopsych.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman L.J., Chapman J.P. The measurement of handedness. Brain Cogn. 1987;6(2):175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Chen G., Xiao Y., Taylor P.A., Rajendra J.K., Riggins T., Geng F., Redcay E., Cox R.W. Handling multiplicity in neuroimaging through bayesian lenses with multilevel modeling. Neuroinformatics. 2019;17(4):515–545. doi: 10.1007/s12021-018-9409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Taylor P.A., Cox R.W., Pessoa L. Fighting or embracing multiplicity in neuroimaging? neighborhood leverage versus global calibration. NeuroImage. 2020;2019(206) doi: 10.1016/j.neuroimage.2019.116320. doi:j.neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R., Ameringen M.V., Hall G. Increased activity of frontal and limbic regions to emotional stimuli in children at-risk for anxiety disorders. Psychiatry Res.: Neuroimag. 2015;233(1):9–17. doi: 10.1016/j.pscychresns.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreements for nominal scales. Educ. Psychol. Meas. 1960;1(1):37–46. [Google Scholar]
- Cohen-Gilbert J.E., Thomas K.M. Inhibitory control during emotional distraction across adolescence and early adulthood. Child Dev. 2013;84(6):1–22. doi: 10.1111/cdev.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.O., Casey B.J. Rewiring juvenile justice: the intersection of developmental neuroscience and legal policy. Trends Cogn. Sci. (Regul. Ed.) 2014;18(2):63–65. doi: 10.1016/j.tics.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Cohen A.O., Breiner K., Steinberg L., Bonnie R.J., Scott E.S., Taylor-Thompson K.A., Casey B.J. When is an adolescent an adult? assessing cognitive control in emotional and nonemotional contexts. Psychol. Sci. 2016;27(4):549–562. doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Pizzagalli D., Nitschke J.B. Depression: perspectives from affective neuroscience. Annu. Rev. Psychol. 2002;53(1):545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Nystrom L.E., Fissell C., Noll D.C., Fiez J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- de Ross R.L., Gullone E., Chorpita B.F. The revised child anxiety and depression scale: a psychometric investigation with Australian youth. Behav. Change. 2000;19(2):90–101. [Google Scholar]
- Dillon D.G., Rosso I.M., Pechtel P., Killgore W.D.S., Rauch S.L., Pizzagalli D.A. Peril and pleasure: an RDoC-inspired examination of threat responses and reward processing in anxiety and depression. Depress. Anxiety. 2014;31(3):233–249. doi: 10.1002/da.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C. Prefrontal cortical-amygdalar metabolism in major depression. Annu. New York Acad. Sci. 1999;877(1):614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Dreyfuss M., Caudle K., Drysdale A.T., Johnston N.E., Cohen A.O., Somerville L.H., Casey B.J. Teens impulsively react rather than retreat from threat. Dev. Neurosci. 2014;36(3–4):220–227. doi: 10.1159/000357755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart, J., Holiga, S., Chatham, C., Hawkins, P., Forsyth, A., McMillan, R., & Sambataro, F. (2018). Cerebral blood flow predicts differential neurotransmitter activity. Scientific Reports, 8, 1–11. doi: 10.1038/s41598-018-22444-0. [DOI] [PMC free article] [PubMed]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck M.W., Derakshan N., Santos R., Calvo M.G. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fales C.L., Barch D.M., Rundle M.M., Mintun M.A., Snyder A.Z., Cohen J.D., Sheline Y.I. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol. Psychiatry. 2008;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales C.L., Barch D.M., Rundle M.M., Mintun M.A., Mathews J., Snyder A.Z., Sheline Y.I. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J. Affect. Disord. 2008;112(1–3):206–211. doi: 10.1016/j.jad.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E.B., Hyde L.W., Mitchell C., Faul J., Gonzalez R., Heitzeg M.M., Schulenberg J. Proceedings of the National Academy of Sciences. Vol. 110. 2013. What is a representative brain? neuroscience meets population science; pp. 17615–17622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross L.C., Gotlib I.H. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Front. Psychol. 2012;3:1–17. doi: 10.3389/fpsyg.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., May J.C., Siegle G.J., Ladoucer C.D., Ryan N.D., Carter C.S., Dahl R.E. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J. Child Psychol. Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Brown S.M., Kimak M., Ferrell R.E., Manuck S.B., Hariri A.R. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol. Psychiatry. 2009;14(1):60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin J.P., Parker D., Tunc B., Watanabe T., Elliot M.A., Ruparel K., Shinohara R.T. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2018;161 doi: 10.1016/j.neuroimage.2017.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R., Lewis D.A., Michels R., Pine D.S., Schultz S.K., Tamminga C.A., Yager J. The initial field trials of DSM-5: new blooms and old thorns. JAMA Psychiatry. 2013;170(1):1–5. doi: 10.1176/appi.ajp.2012.12091189. [DOI] [PubMed] [Google Scholar]
- Gershon R.C., Wagster M.V., Hendrie H.C., Fox N.A., Cook K.F., Nowinski C.J. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80:S2–S6. doi: 10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiruopoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Jenkinson M. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Thompson P.M. Proceedings of the National Academy of Sciences. Vol. 101. 2004. Dynamic mapping of human cortical development during childhood through early adulthood; pp. 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Joormann J. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose-Fifer J., Rodrigues A., Hoover S., Zottoli T. Attentional capture by emotional faces in adolescence. Adv. Cognit. Psychol. 2013;9(2):81–91. doi: 10.2478/v10053-008-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.C., Richard J., Hughett P., Calkins M.E., Macy L., Bilker W.B., Gur R.E. A cognitive neuroscience based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J. Neurosci. Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.C., Calkins M.E., Satterthwaite T.D., Ruparel K., Bilker W.B., Moore T.M., Gur R.E. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014;71(4):366–374. doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2009;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Tessitore A., Mattay V.S., Fera F., Weinberger D.R. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Harms M.P., Somerville L.H., Ances B.M., Andersson J., Barch D.M., Bastiani M., Yacoub E. Imaging in the human connectome projects in development and aging: connectomics across the lifespan. Neuroimage. 2018;183:972–984. doi: 10.1016/j.neuroimage.2018.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J.J., Reynell C. How do antidepressants influence the BOLD signal in the developing brain? Dev. Cogn. Neurosci. 2017;25:45–57. doi: 10.1016/j.dcn.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Akschoomoff N., Tulsky D., Mungas D., Weintraub S., Dikmen S., Gershon R. Reliability and validity of composite scores from the nih toolbox cognition battery in adults. J. Int. Neuropsychol. Soc. 2014;20(6):588–598. doi: 10.1017/S1355617714000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S.G., Ellard K.K., Siegle G.J. Neurobiological correlates of cognitions in fear and anxiety: a cognitive-neurobiological information processing model. Cognit. Emotion. 2011;26(2):282–299. doi: 10.1080/02699931.2011.579414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley J.M., Gruber S.A., Parker H.A., Guillaumot J., Rogowska J., Yurgelun-Todd D.A. Cortico-limbic response to personally challenging emotional stimuli after complete recovery from depression. Psychiatry Res.: Neuroimaging. 2009;171(2):106–119. doi: 10.1016/j.psycychresns.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Huang H., Fan X., Williamson D.E., Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36(3):684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard N.A., Hutchinson J.L., Turner M.P., Montroy J., Bowles R.P., Rypma B. Depressive thoughts limit working memory capacity in dysphoria. Cognit. Emotion. 2016;30(2):193–209. doi: 10.1080/02699931.2014.991694. [DOI] [PubMed] [Google Scholar]
- Hubbard, N.A., Romeo, R.R., Grotzinger, H., Giebler, M., Imhof, A., Bauer, C.C.C., and Gabrieli, J.D.E. Reward-sensitive basal ganglia stabilize the maintenance of goal-relevant neural patterns in adolescents. Journal of Cognitive Neuroscience, in press. [DOI] [PMC free article] [PubMed]
- Hung Y., Saygin Z.M., Biederman J., Hirshfeld-Becker D., Uchia M., Doehermann O., Gabrieli J.D.E. Impaired frontal-limbic white matter maturation in children at risk for major depression. Cerebral. Cortex. 2017;27(9):4478–4491. doi: 10.1093/cercor/bhw250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianetti G.D., Wise R.G. BOLD functional mri in disease and pharmacological studies: room for improvement? Magn. Reson. Imaging. 2007;25(6):978–988. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Insel C., Kastman E.K., Glenn C.R., Somerville L.H. Development of corticostriatal connectivity constrains goal-directed behavior during adolescence. Nat. Commun. 2017;8(1):1605–1610. doi: 10.1038/s41467-017-01369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayumak S., Bouix S., Ning L., James A., Crow T., Shenton M., Rathi Y. Retrospective harmonization of multi-site diffusion mri data acquired with different acquisition parameters. Neuroimage. 2019;184:180–200. doi: 10.1016/j.neuroimage.2018.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kerestes R., Davey C.G., Stephanou K., Whittle S., Harrison B.J. Functional brain imaging studies of youth depression: a systematic review. NeuroImage: Clin. 2014;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr N.L. HARKing: hypothesizing after the results are known. Personal. Social Psychol. Rev. 1998;2(3):196–217. doi: 10.1207/s15327957pspr0203_4. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Demler O., Frank R.G., Olfson M., Pincus H.A., Walters E.E., Zaslavsky A.M. Prevalence and treatment of mental disorders, 1990 to 2003. New Engl. J. Med. 2005;352(24):2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D.S., Yurgelun-Todd D.A. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Koster E.H.W., Lissnyder E.D., Derakshan N., Raedt R.D. Understanding depressive rumination from a cognitive science perspective: the impaired disengagement hypothesis. Clin. Psychol. Rev. 2011;31(1):138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Lahat A., Benson B., Pine D.S., Fox N.A., Ernst M. Neural responses to reward in childhood: relations to early behavioral inhibition and social anxiety. Soc. Cogn. Affect. Neurosci. 2016;13(3):281–289. doi: 10.1093/scan/nsw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- Langner O., Dotsch R., Bijlstra G., Wigboldus D.H.J., Hawk S.T., van Knippenberg A. Presentation and validation of the Radboud faces database. Cognit. Emotion. 2010;24(8):1377–1388. [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr. Biol. 2007;17(20):R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E., Pine D.S. Using neuroscience to help understand fear and anxiety: a two-system framework. Am. J. Psychiatry. 2016;173(11):1083–1093. doi: 10.1176/appi.ajp.2016.16030353. [DOI] [PubMed] [Google Scholar]
- LeWinn K.Z., Sheridan M.A., Keyes K.M., Hamilton A., McLaughlin K.A. Sample composition alters associations between age and brain structure. Nat. Commun. 2017;8(1):1–14. doi: 10.1038/s41467-017-00908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M., Bjork J.M., Nagel B.J., Barch D.M., Gonzalez R., Nixon S.J., Banich M.T. Adolescent neurocognitive development impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev. Cogn. Neurosci. 2018;32:67–79. doi: 10.1016/j.dcn.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking K.R., Pagliaccio D., Luby J.L., Barch D.M. Depression risk predicts blunted neural responses to gains and enhanced responses to losses in healthy children. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(4):328–337. doi: 10.1016/j.jaac.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking K.R., Pagliaccio D., Luby J.L., Barch D.M. Reward processing and risk for depression across development. Trends Cogn. Sci. (Regul. Ed.) 2016;20(6):456–468. doi: 10.1016/j.tics.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J.C., Delgado M.R., Dahl R.E., Stenger V.A., Ryan N.D., Fiez J.A., Carter C.S. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol. Psychiatry. 2004;55(4):359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Liotti M., Brannan S.K., McGinnis S., Mahurin R.K., Jerabek P.A., Fox P.T. Reciprocal limbic-cortical function and negative mood: converging pet findings in depression and normal sadness. Am. J. Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S. Targeted electrode-based modulation of neural circuits for depression. J. Clin. Investig. 2009;119(4):717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E.B., Monk C.S., Nelson E.E., Parrish J.M., Adler A., Blair R.J.R., Pine D.S. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch. Gen. Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Buschman T.J. Cortical circuits for the control of attention. Curr. Opin. Neurobiol. 2013;23(2):216–222. doi: 10.1016/j.conb.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miron O., Yu K., Wilf-Miron R. Suicide rates among adolescents and young adults in the United States, 2000-2017. JAMA. 2019;321(23):2362–2364. doi: 10.1001/jama.2019.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., Zarahn E., Bilder R.M., Leibenlift E., Pine D.S. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/S1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Morgan J.K., Shaw D.S., Forbes E.E. Maternal depression and warmth during childhood predict age 20 neural response to reward. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(1):108–117. doi: 10.1016/j.jaac.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B.D., Perlman G., Klein D.N., Kotov R., Hajcak G. Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am. J. Psychiatry. 2015;173(12):1223–1230. doi: 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Wisco B.E., Lyubomirsky S. Rethinking rumination. Perspect. Psychol. Sci. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. (Regul. Ed.) 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Hughes B., Robertson E.R., Cooper J.C., Gabrieli J.D.E. Neural systems supporting the control of affective and cognitive conflicts. J. Cogn. Neurosci. 2008;21(9):1841–1854. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N., Kao Y., Sokol-Hessner P., Kim H., Whitfield-Gabrieli S., Gabrieli J.D.E. Development of the declarative memory system in the human brain. Nat. Neurosci. 2007;10(9):1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Olson I.R., Von Der Heide R.J., Alm K.H., Vyas G. Development of the uncinated fasciculus: implications for theory and developmental disorders. Dev. Cogn. Neurosci. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A., Teilmann G., Juul A., Skakkebaek N.E., Toppari J., Bourguignon J. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr. Rev. 2003;24(5):668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Patton G.C., Viner R. Pubertal transitions in health. The Lancet. 2007;369(9567):1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Pehlivanova M., Wolf D.H., Sotiras A., Kaczkurkin A.N., Moore T.M., Ciric R., Satterthwaite T.D. Diminished cortical thickness is associated with impulsive choice in adolescence. J. Neurosci. 2018;38(10):2471–2481. doi: 10.1523/JNEUROSCI.2200-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B.S., Warner V., Bansal R., Zhu H., Hao X., Liu J., Weissman M.M. Cortical thinning in persons at increased familial risk for major depression. PNAS. 2009;106(15):6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Tancer M.E. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol. Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Peirce J.W. PsychoPy-Psychophysics in software in python. J. Neurosci. Methods. 2007;162(1–2):8–13. doi: 10.1016/j.neumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A., Holmes A.J., Dillon D.G., Goetz E.L., Birk J.L., Bogdan R., Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A. Depression, stress and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014;10(1):393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K., Brown G.K., Stanley B., Brent D.A., Yershova K.V., Oquendo M.A., Mann J.J. The Colombia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry. 2011;168(12):1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlagger B.L., Peterson S.E. Spurious but systematic correlations in functional connectivity mri networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functioning mri data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2015;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Connolly J.J., Ruparel K., Calkins M.E., Jackson C., Elliott M.A., Gur R.E. The Philadelphia neurodevelopmental cohort: a publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2016;124(Part B):1115–1119. doi: 10.1016/j.neuroimage.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Reward prediction error. Current Biol. 2017;27:R365–R377. doi: 10.1016/j.cub.2017.02.064. [DOI] [PubMed] [Google Scholar]
- Schweizer S., Satpute A.B., Atzil S., Field A.P., Hitchcock C., Black M., Dalgleish T. The impact of affective information on working memory: a pair of meta- analytic reviews of behavioral and neuroimaging evidence. Psychol. Bull. 2019;145(6):566–609. doi: 10.1037/bul0000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C., Kim S., Herman L., Pane H., Reuter T., Strathearn L. Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. J. Abnorm. Psychol. 2014;123(2):298–309. doi: 10.1037/a0036191. [DOI] [PubMed] [Google Scholar]
- Shi Z., Liu P. Worrying thoughts limit working memory capacity in math anxiety. PLoS ONE. 2016;11(10) doi: 10.1371/journal.pone.0165644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G.J., Steinhauer S.R., Thase M.E., Stenger V.A., Carter C.S. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol. Psychiatry. 2002;51(9):693–707. doi: 10.1016/S0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle G.J., Thompson W., Carter C.S., Steinhauer S.R., Thase M.E. Increased amygdala and decreased dorsolateral prefrontal bold responses in unipolar depression: related and independent features. Biol. Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Siless V., Hubbard N.A., Wang J., Jones R., Lo N., Bauer C.C.C., Yendiki A. Image acquisition and quality assurance in the Boston Adolescent Neuroimaging of Depression and Anxiety study. NeuroImage: Clinical. 2020;26:102242. doi: 10.1016/J.NICL.2020.102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Stewart J.G., Esposito E.C., Shields G.S., Auerbach R.P. The stress and adversity inventory for adolescents (adolescent STRAIN): associations with mental and physical health, risky behaviors and psychiatric diagnoses in youth seeking treatment. J. Child Psychol. Psychiatry. 2019;60(9):998–1009. doi: 10.1111/jcpp.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith R.P., Hamilton M., Morley S., Humayan A., Hargreaves D., Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton pleasure scale. Br. J. Psych. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Bookheimer S.Y., Bucker R.L., Burgess G.C., Curtiss S.W., Dapretto M.…Barch D.M. The lifespan human connectome project in development: A large-scale study of brain connectivity development in 5-21 year olds. Neuroimage. 2018;183:456–468. doi: 10.1016/j.neuroimage.2018.08.050. 10.1016/j.neuroimage. 2018.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer M.E., Bhanji J.P., Delgado M.R. Savoring the past: positive memories evoke value representations in the striatum. Neuron. 2015;84(4):847–856. doi: 10.1016/j.neuron.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Luschene E. Consulting Psychologists Press; Palo Alto, CA: 1970. Manual For the State-Trait Anxiety Interview (Self-Evaluation Questionnaire) [Google Scholar]
- Stringaris A., Belil P.V.R., Artiges E., Lemaitre H., Gollier-Briant F., Wolke S., Paillère-Martinot M.L. The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. Am. J. Psychiatry. 2015;172(12):1215–1223. doi: 10.1176/appi.ajp.2015.14101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A., Suslow T., Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol. Mood Anxiety Disord. 2011;1(1):10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasebi A.M., Artiges E., Banaschewski T., Barker G.J., Bruehl R., Büchel, Paus T. Creating probabilistic maps of the face network in the adolescent brain: a multicenter functional MRI study. Hum. Brain Mapp. 2012;33(4):938–957. doi: 10.1002/hbm.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.J.C., Whincup P.H., Hindmarsh P.C., Lampe F., Odoki K., Cook D.G. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr. Perinat. Epidemiol. 2001;15(1):88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Mogg K., Bradley B.P., Mai X., Ernst M., Pine D.S., Monk C.S. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol. Psychol. 2008;79(2):216–222. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K.M., Drevets W.C., Dahl R.E., Ryan N.D., Birhmaher B., Eccard C.H., Casey B.J. Amygdala response to fearful faces in anxious and depressed children. Arch. Gen. Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tisdall M.D., Hess A.T., Reuter M., Meintjes E.M., Fischl B., Van Der Kouwe A.J.W. Volumetric navigators (vNavs) for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn. Reson. Med. 2012;68(2):389–399. doi: 10.1002/mrm.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Nelson C. The NIMSTIM set of facial expressions: judgements from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E.M., Delgado M.R., Fiez J.A. Modulation of caudate activity by action contingency. Neuron. 2004;41(2):281–292. doi: 10.1016/S0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Tversky A., Kahneman D. Loss aversion in riskless choice: a reference-dependent model. Q. J. Econ. 1991;106(4):1039–1061. doi: 10.2307/2937956. [DOI] [Google Scholar]
- Twenge J.M., Cooper A.B., Joiner T.E., Duffy M.E., Binau S.G. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005-2017. J. Abnormal Psychol. 2019;128(3):185–199. doi: 10.1037/abn0000410. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C., Ugurbil K., Auerbach E., Barch D., Behrens T.E.J., Bucholz R., Yacoub E. The human connectome project: a data acquisition perspective. Neuroimage. 2012;62(4):2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Glasser M.F., Dierker D.L., Harwell J., Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb. Cortex. 2012;22:2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Koob G.F., Croyle R.T., Bianchi D.W., Gordon J.A., Koroshetz W.J., Weiss S.R.B. The conception of the ABCD study: from substance use to a broad nih collaboration. Dev. Cogn. Neurosci. 2018;32:4–7. doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar C., O’Muircheartaigh J., Barker G.J., Symms M.R., Thomson P., Kumari V., Koepp M.J. Identical, but not the same: intra-site and inter-site reproducibility of fractional anisotropy measures on two 3.0T scanners. Neuroimage. 2010;51:1384–1394. doi: 10.1016/j.neuroimage.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. Effects of attention and emotion on face processing in the human brain: an event related fMRI study. Neuron. 2001;30(3):829–841. doi: 10.1016/S0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Wang L., LaBar K.S., Smoski M., Rosenthal M.Z., Dolcos F., Lynch T.R., McCarthy G. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163(2):143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M.M., Wickramaratne P., Adams P., Wolk S., Verdeli H., Olfson M. Brief screening for family psychiatric history. Arch. Gen. Psychiatry. 2000;57(7):675–682. doi: 10.1001/archpsyc.57.7.675. 10-1001/pubs.Arch Gen Psychiatry-ISSN-003-990x-57-7-yoa8214. [DOI] [PubMed] [Google Scholar]
- Whitmer A.J., Gotlib I.H. An attentional scope model of rumination. Psychol. Bull. 2013;139(5):1036–1061. doi: 10.1037/a0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciulik E., Kanwisher N., Driver J. Covert visual attention modulates face- specific activity in the human fusiform gyrus: fMRI study. J. Neurophysiol. 1998;79(3):1574–1578. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Yang T.T., Simmons A.N., Matthews S.C., Tapert S.F., Frank G.K., Max J.E., Paulus M.P. Adolescents with major depression demonstrate increased amygdala activation. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(1):42–51. doi: 10.1016/j.jaac.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., McCall D.M., Homayouni R., Tang L., Chen Z., Schoff D., Ofen N. Age-associated increase in mnemonic strategy use is linked to prefrontal cortex development. Neuroimage. 2018;181:162–169. doi: 10.1016/j.neuroimage.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.