Abstract
Food insecurity and poor infant and young child feeding (IYCF) practices contribute to undernutrition. The Kanyakla Nutrition Program was developed in rural Kenya to provide knowledge alongside social support for recommended IYCF practices. Utilizing a social network approach, the Kanyakla Nutrition Program trained community health workers (CHWs) to engage mothers, fathers, and grandparents in nutrition education and discussions about strategies to provide instrumental, emotional, and information support within their community. The 12‐week programme included six sessions and was implemented on Mfangano Island, Kenya, in 2014–2015. We analysed intervention effects on (a) nutrition knowledge among community members or CHWs and (2) IYCF practices among children 1–3 years. Nutrition knowledge was assessed using a postintervention comparison among intervention (community, n = 43; CHW, n = 22) and comparison groups (community, n = 149; CHW, n = 64). We used a quasi‐experimental design and difference‐in‐difference to assess IYCF indicators using dietary recall data from an ongoing cohort study among intervention participants (n = 48) with individuals living on Mfangano Island where the intervention was not implemented (n = 178) before the intervention, within 1 month postintervention, and 6 months postintervention. Findings showed no effect of the intervention on IYCF indicators (e.g., dietary diversity and meal frequency), and less than 15% of children met minimum acceptable diet criteria at any time point. However, knowledge and confidence among community members and CHWs were significantly higher 2 years postintervention. Thus, a social network approach had an enduring effect on nutrition knowledge, but no effects on improved IYCF practices.
Keywords: complementary feeding, food security, Kenya, Lake Victoria, nutritional status, social network
Key messages.
We evaluated the effect of a social network nutrition intervention that included various community members such as fathers and grandmothers on nutrition knowledge and infant and young child feeding (IYCF) practices in Kenya.
Few children met the IYCF recommendations for complementary feeding, with less than 15% meeting minimum acceptable diet criteria.
The intervention improved nutrition knowledge among caregivers and volunteer community health workers (CHWs) but had no impact on IYCF practices.
Social network groups may provide a platform for addressing structural barriers, including seasonality of local food production, to better support participants in applying their nutrition knowledge.
1. INTRODUCTION
Over 155 million children under 5 years old are stunted, defined as two standard deviations below the World Health Organization (WHO) reference standards for height for age (Food and Agriculture Organization [FAO], International Fund for Agricultural Development, UNICEF, World Food Programme, & WHO, 2017). Stunting can be irreversible (Dewey & Adu‐afarwuah, 2008; Victora, de Onis, Hallal, Blossner, & Shrimpton, 2010) and is associated with more illnesses, lower survival from infectious diseases, poorer learning, and poorer quality of life (Black et al., 2013; Liu et al., 2012). The large majority of stunted children live in low‐ and middle‐income countries where appropriate infant and young child feeding (IYCF) practices, including appropriate breastfeeding (i.e., exclusive breastfeeding until 6 months and breastfeeding until 2 years) and complementary feeding (i.e., minimum dietary diversity [MDD] and minimum meal frequency [MMF]; WHO, 2008), are critical to preventing stunting and enabling children to meet their growth and developmental potential (Black et al., 2013; Britto et al., 2017).
A range of barriers constrain the adoption of recommended IYCF practices. Limited nutrition knowledge, taboos and cultural beliefs (e.g., mothers have insufficient milk), and family members who negatively influence feeding practices can all affect whether people understand and follow IYCF recommendations (Thuita, Martin, Ndegwa, Bingham, & Mukuria, 2015). In addition, poverty and food insecurity, maternal time burden and work load, poor maternal mental health and care, and more can further diminish households' ability to follow these recommendations, even when they know of and understand them (Campisi, Cherian, & Bhutta, 2017; WHO, 2008). Although nutrition education interventions alone are often insufficient to improve IYCF practices (Dewey & Adu‐afarwuah, 2008; Imdad, Yakoob, & Bhutta, 2011), interventions that provide food or ongoing support to carry out recommendations are more likely to be successful (Graziose, Downs, O'Brien, & Fanzo, 2014). Interventions that engage social networks to address gaps in nutrition knowledge while fostering community‐level support for meeting recommendations (e.g., instrumental and emotional) thus have the potential to provide a cost‐effective and sustainable intervention and merit investigation.
Social networks may play a role in providing psychosocial, emotional, and material resources for maintaining IYCF recommendations (i.e., meeting breastfeeding and complementary feeding guidelines; Thuita et al., 2015). Through altruistic and transactional exchanges, such as meal sharing, money lending, or provision of child care, social support may directly strengthen IYCF practices (De Weerdt & Dercon, 2006; Kaschula, 2011; Mukuria, Martin, Egondi, Bingham, & Thuita, 2016). Social support is composed of multiple functional elements: informational support provides assistance or feedback to help solve a problem or maintain best practices; companionship involves spending time alongside others in recreational activities; emotional support involves care and empathy; and instrumental support provides material support such as loans, food sharing, or labour in‐kind (Sherbourne & Stewart, 1991). Instrumental social support is thought to be the element that most effectively fosters improved food security (Cohen & Wills, 1985; Tsai et al., 2011) and may also support improved IYCF practices.
Social networks also engage diverse community members in nutrition, a domain traditionally ascribed to women. Although mothers are often the primary caregivers and focus of nutrition interventions, other family members, including fathers and grandparents, play an important and increasingly acknowledged role in maternal and child health (Aboud & Singla, 2012; Aubel, 2012; Bezner‐Kerr, Dakishoni, Shumba, Msachi, & Chirwa, 2008; Mitchell‐Box & Braun, 2013; Tomlinson, Rahman, Sanders, Maselko, & Rotheram‐Borus, 2014). Recent research shows that when mothers experience more social support actions, they are more likely to feed their infants the recommended number of meals and a diverse diet (Mukuria et al., 2016). Further, social networks contribute to social capital, meaning participation in social interactions as well as norms, values, and beliefs (Agampodi, Agampodi, Glozier, & Siribaddana, 2015). Social capital is a determinant of health and a predictor of better health (Agampodi et al., 2015).
The Kanyakla Nutrition Program, which we study here, was designed to specifically address the social dimensions of nutrition behaviours and galvanize support to improve nutrition behaviours by engaging social networks. Evidence from Kenya demonstrates a benefit of engaging broader members of social networks on child nutrition outcomes (Mukuria et al., 2016), lending support for this approach. The traditional role of fathers and grandmothers further underscores the utility of a social network approach. In Kenya, fathers are often social gatekeepers, mediators of women's economic access, and enforcers of cultural practices (Mukuria et al., 2016; Thuita et al., 2015). Grandmothers are also central community figures in Kenya who hold and propagate health and child rearing beliefs, many of which may be contrary to current health science (Mukuria et al., 2016; Thuita et al., 2015). Thus, the engagement of fathers, grandparents, and wider social networks has the potential to improve the enabling environment for meeting recommended IYCF practices.
The Kanyakla Nutrition Program was implemented by Organic Health Response on Mfangano Island, Kenya, to address high rates of child malnutrition. In Kenya in 2014, 26% of children under age 5 years were stunted (length‐for‐age/height‐for‐age z‐score < −2) and 4% were wasted (weight‐for‐height z‐score < −2; Kenya Demographic and Health Survey, 2014). At our study site, Mfangano Island, 33% of children less than age 2 years were stunted, 5% were wasted, and 13% of infants were born with low birthweight in 2012 (Fiorella, 2015). Further, in 2010, 96% of Mfangano residents faced some level of food insecurity (Fiorella et al., 2014), which has been associated with poor early childhood development outcomes (Milner, Fiorella, Mattah, Bukusi, & Fernald, 2017). Despite the vast natural resources of the Lake Victoria fisheries, fish access is mediated by income (Fiorella et al., 2014) and has declined due to overfishing, destructive practices, and environmental changes that have reduced incomes and altered fish access (Abila, 2000; Abila, 2003; Njiru, Kazungu, Ngugi, Gichuki, & Muhoozi, 2008; Omwoma et al., 2014). Further, a high prevalence of HIV/AIDS in these communities reduces the labour force and can push fishers to use illegal and unsustainable methods (Fiorella et al., 2017), which may further exhaust fish stocks and negatively affect local incomes and food access.
In this study, we report on an evaluation of the Kanyakla Nutrition Program. We tested the hypotheses that (a) community members and volunteer community health workers (CHWs), known as community health volunteers in Kenya, who participated in the intervention demonstrated higher nutrition knowledge postintervention than those in the comparison group, and (b) children whose household members participated in the intervention have better feeding practices (measured through WHO IYCF indicators) compared with comparison children.
2. METHODS
2.1. Study overview
Our study included two components: (a) a cross‐sectional postintervention assessment of nutrition knowledge among CHWs and community members who participated in the programme (CHWs, n = 22; community members, n = 43) and those who did not participate in the programme (CHWs, n = 64; community members, n = 149) and (b) a difference‐in‐difference evaluation of IYCF practices comparing participants in the Kanyakla Nutrition Program and comparison participants before, immediately after, and 6 months postintervention (Figure 1).
Figure 1.
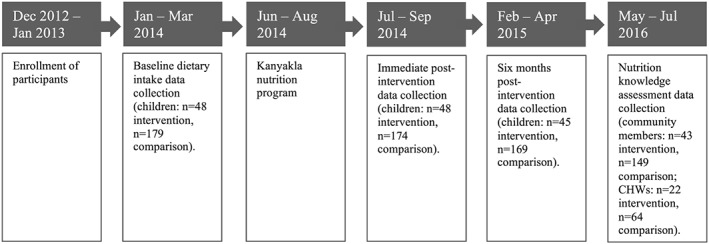
Timeline of participant engagement, intervention, and evaluation
2.2. Kanyakla Nutrition Program intervention
Social network groups called kanyaklas (which means “together” in Luo, the local language) participated in six curriculum sessions over 12 weeks facilitated by CHWs who were trained on each topic the week before leading the related session. The curriculum emphasized both knowledge and activating social support for enacting IYCF practices using hands‐on activities, dramas, and demonstrations about family planning, safe pregnancy, nutrition, and IYCF practices. In addition to addressing knowledge gaps and questions, Kanyakla groups generated strategies to address future challenges to nutrition and food security, such as support for breastfeeding mothers and recognition of childhood malnutrition. The programme aimed to engage social support networks, including fathers, grandparents, and other community members, and strengthen relationships with CHWs to drive sustainable behaviour changes. The objectives of the intervention were to improve IYCF practices and establish social network support for access to nutritious foods (DeLorme et al., 2018).
The most intensive elements of the Kanyakla Nutrition Program were offered from June 2014 to August 2014. Following the intensive training sessions, the Kanyakla groups were encouraged to continue meeting. From August 2014 through August 2015, CHWs were convened in bimonthly meetings to refresh their training, discuss issues that arose with their groups, and consider next steps. Although meetings were more sporadic following the conclusion of the curriculum sessions, approximately 50% of groups continued to meet.
2.3. Study population
2.3.1. Research on Environmental and Community Health parent study
An ongoing parent study, Research on Environmental and Community Health (RECH; meaning “fish” in Luo, the local language), aimed to analyse the interactions between declining fish catch and food security, livelihoods, fish consumption, and child growth and development on Mfangano Island (Fiorella et al., 2015). All households on the island meeting the eligibility criteria of having a child under the age of 2 years and resident of Mfangano Island at the time of enrolment were randomly selected using an enumerated sampling list and random number generation (Fiorella, 2015). The RECH study focuses on the dietary intake of a target child, who was under the age of two at the start of the study, and that child's primary caregiver living in the same household. For the nutrition knowledge assessment among community members and the study of IYCF practices, households were selected from the RECH cohort.
Participants were enrolled for the RECH study from December 2012 to March 2013. Data were collected using surveys, 24‐hr dietary recalls, and anthropometric measurements administered every 3 months for 2 years. Data collection tools were developed by piloting and adapting validated standardized instruments. Methods have been described previously (Fiorella et al., 2015; Fiorella et al., 2017; Milner et al., 2017).
2.3.2. Nutrition knowledge sampling
In June to July 2016, 2 years postintervention, nutrition knowledge questionnaires were administered to CHWs and community members who participated in the Kanyakla Nutrition Program (CHWs, n = 22; community members, n = 43) and a comparison group (CHWs, n = 64; community, n = 149). All CHWs who participated in the Kanyakla Nutrition Program were sampled. The community member intervention group was composed of individuals who had been enrolled in the parent RECH study and participated in the Kanyakla Nutrition Program. Community members and CHWs in the comparison group were opportunistically sampled based on attendance at meetings about the expansion of the Kanyakla Nutrition Program into regions where the Kanyakla Nutrition Program was not offered. All community members who were enrolled in the Kanyakla Nutrition Program and in the RECH study were found by enumerators and agreed to participate in the nutrition knowledge survey. Structured in‐person interviews were conducted by trained enumerators in the local language, Luo, and lasted approximately 15 minutes Verbal consent was obtained from study participants prior to questionnaire administration.
2.3.3. IYCF practice sampling
For the purposes of selecting the intervention and comparison groups and to reduce unintended cross‐over of the intervention, the island's four recognized geographic regions were used: East, West, North, and South. The nutrition programme was offered to all households in Mfangano East. The intervention group is therefore composed of those households who self‐selected to participate in the Kanyakla Nutrition Program: 47 adults and 48 children under the adults' care (one case of twins). The comparison group is composed of the RECH cohort members who did not receive the intervention (hereafter, “comparison participants”) and did not live in areas where the intervention was offered: 176 primary caregivers and 179 children (three cases of twins). Participants who lived in villages where the intervention was offered, but did not choose to participate, are excluded from the analysis as there is a high probability of intervention cross‐over and they are likely different from participants who would self‐select to participate in the intervention. Consent was obtained from all male and female heads of household upon enrolment into the RECH cohort. Where there was only a female head of household, her consent was obtained. At the time of data collection for this evaluation, participant children were between the ages of 1 and 3 years.
Participants with complete data for IYCF indicators, intervention participation, and household food insecurity at baseline and either of the postintervention time points were included. As expected in a study within a migratory community, some participants were lost to follow‐up or did not complete all surveys at all time points, resulting in fluctuations in sample size over time. Immediately postintervention, 48 children remained in the intervention group and 174 remained in the comparison group; 6 months postintervention, the intervention group numbered 45 children and the comparison group numbered 169 children. We compared baseline and follow‐up data for children who were missing at each time point. At baseline, caregivers who were missing data 6 months postintervention were significantly younger, and their households had a significantly lower asset index. However, there was no significant difference in caregiver age and asset index between the intervention and comparison groups 6 months postintervention time point, and their omission therefore is expected to introduce negligible bias to the analysis.
We used three time periods of the larger study to evaluate the nutrition intervention. The first data collection time point, “baseline,” was immediately before the intervention started. The second time point, “immediately postintervention,” was the month following the intensive phase of the intervention was completed. The third time point, “6 months postintervention,” was 6 months after the conclusion of the intervention and the longest possible follow‐up period within the context of the ongoing study (Figure 1).
2.4. Evaluation indicators
2.4.1. Indicators of nutrition knowledge
Nutrition knowledge was characterized using a 22‐item questionnaire. The scale included 16 true–false questions and six open‐ended questions, to which responses were characterized as correct or incorrect. In addition to scoring overall nutrition knowledge, three subscales were created to focus on specialized topics: breastfeeding practices (seven items), complementary feeding practices (eight), and caregiver confidence and behaviour (five). The breastfeeding practices subscale included questions on breastfeeding benefits, positions, and duration (Guyon, Quinn, Nielsen, & Stone‐Jimenez, 2015). The complementary feeding practices subscale included questions on appropriate feeding practices and the daily number of food groups to be fed to a young child (Guyon et al., 2015). The caregiver confidence and behaviour subscale included questions on recognizing signs of malnutrition, hunger cues, and confidence about nutrition knowledge (Guyon et al., 2015; Menon, Ruel, & Arimond, 2005; USAID Infant & Young Child Nutrition Project, 2011). Questions were selected based on the knowledge taught in the curriculum. Questions were translated and back‐translated and were piloted to ensure understanding of meaning and that open‐ended questions generated relevant answers.
2.4.2. IYCF practices
Dietary data
Dietary data for participating children were collected via 24‐hr open‐response dietary recalls with the primary caregiver (Gibson, 2005). The dietary recalls provided the total number of meals consumed and a list of all foods and amounts consumed in the last 24 hr. These foods were coded based on food group definitions from FAO and WHO indicator guidelines (FAO and FHI 360, 2016; WHO, 2008). The seven food groups include grains/roots/tubers, legumes/nuts, dairy, flesh foods, eggs, vitamin A‐rich fruits and vegetables, and other fruits and vegetables (WHO, 2008). Dietary recalls are generally a better reflection of average intake than food frequency questionnaires and are therefore the recommended form of dietary assessment in epidemiological studies (Kelada, Shelton, Kaufmann, Khoury, & Genotype, 2001; Prentice et al., 2011). Raw counts of the number of meals and the number of food groups the child consumed in 24 hr are presented.
MDD is a proxy indicator of diet quality in children age 6 months to 2 years (WHO, 2008). To meet the MDD indicator criteria, a child must consume foods in at least four out of seven designated food groups in 24 hr (WHO, 2008). A food consumed in any amount more than a condiment (e.g., a small amount of tomato for flavour) is counted (WHO, 2008). Meeting MDD criteria means the child is more likely to have consumed at least one animal‐source food, one fruit or vegetable, and one grain (WHO, 2008). Meeting the MDD criteria is associated with decreased risk of underweight and stunting (Marriott, White, Hadden, Davies, & Wallingford, 2012). This indicator is separately calculated for children who are breastfeeding.
MMF is a proxy indicator of energy intake from food other than breast milk in children age 6 months to 2 years (WHO, 2008). Children meet the MMF criteria by consuming a minimum of three meals in 24 hr if they are concurrently breastfeeding or four meals in 24 hr if they are not breastfeeding (WHO, 2008); all meals and snacks are also included. Meeting MMF criteria is associated with a reduced risk of being underweight (Bhutta et al., 2013).
Minimum acceptable diet (MAD) is a summary indicator of dietary quality. For children who are currently breastfeeding, both MMF and MDD criteria must be met for the MAD to be met; for children who are not currently breastfeeding, the MMF criteria must be met and the child must receive two dairy servings and consume four out of the six non‐dairy food groups described above for the MAD to be met (WHO, 2008).
Iron‐rich foods include flesh foods, iron supplements, iron‐fortified foods such as baby formula, and micronutrient packets with iron (WHO, 2008). Consumption of iron‐rich foods is an indicator of micronutrient adequacy in children but assesses only iron and does not reflect general micronutrient intake (WHO, 2008). Consumption of iron‐rich foods is associated with lower risk of being anaemic in a review of low‐ and middle‐income countries (Pasricha, Drakesmith, Black, Hipgrave, & Biggs, 2013).
2.5. Additional variables
Descriptive variables for primary caregivers such as age, sex, marital status, relationship to the child, and educational status were compared between the intervention and comparison groups to determine their similarity at baseline (Appendix S1). These characteristics were selected as they are expected to affect IYCF practices. We created an asset index that included household ownership of a bed, wall unit or cupboard, sofa without cushions, and sofa with permanent cushions. The asset index ranged from 0 to 4, with a higher number indicating a higher socio‐economic status. Other measures such as income, expenditures, and occupation tend to fluctuate more and reflect short‐term socio‐economic status (Falkingham & Namazie, 2002; Sahn & Stifel, 2003).
Descriptive variables for children included age and sex. Birthweight data were collected; however, this variable was excluded due to the high number of missing values, many values outside acceptable ranges, and an apparent lack of scales at facilities recording birthweights. Additional descriptive statistics including IYCF indicators were used to inform the dietary patterns of the children at baseline. Since at the time of the nutrition intervention, most children were older than 1 year, we are unable to assess the intervention's effect on breastfeeding behaviours. Therefore, breastfeeding practices were not an outcome of interest.
Household food security was assessed using the Food and Nutrition Technical Assistance Project Household Food Insecurity Access Scale (HFIAS). The HFIAS consists of nine questions pertaining to domains of the household food insecurity experience: anxiety and uncertainty about the household food supply, insufficient quality, and insufficient quantity of food intake. The primary caregiver was asked how frequently specific experiences of household food insecurity occurred during the previous 30 days: never, rarely (1–2 times), sometimes (3–10 times), or often (10+ times). The HFIAS score is the sum of the nine responses coded as never = 0, rarely = 1, sometimes = 2, and often = 3. The HFIAS score is continuous ranging from 0 (food secure) to 27 (severely food insecure; Coates, Swindale, & Bilinsky, 2007).
2.6. Data analysis
The data were merged and analysed in Stata IC version 14 (Stata, 2017).
2.6.1. Nutrition knowledge
We compared scores among the intervention and comparison groups in overall nutrition knowledge and knowledge subscales using one‐sided t tests to assess the null hypothesis that nutrition knowledge scores were not significantly different between the groups or that they were lower among the intervention group. We were interested specifically in whether the intervention was associated with improved scores. Due to the concerns around one‐sided t tests (Bland & Altman, 1994), we also ran two‐sided t tests that had similar results and patterns of significance.
2.6.2. IYCF practices
Using unadjusted regression models, we analysed differences in IYCF indicators between the intervention and comparison groups at each of the three time points. These initial analyses supported the use of difference‐in‐difference analyses to assess trends in IYCF indicators among the intervention and comparison groups. Though the IYCF indicators used in this analysis were measured at baseline, they were not measured at additional preintervention time points so we assessed the assumption of parallel trends using food security through HFIAS score from 12 months preintervention. HFIAS score was correlated with the IYCF indicators in our study population and has been found to be a robust predictor in other studies (Macharia, Ochola, Mutua, & Kimani‐Murage, 2018; Hanselman et al., 2018). Visual comparison and regression analysis confirmed parallel trends. Regression models including an interaction term between time point and intervention participation were used to conduct the difference‐in‐difference analysis. Random effects at the individual level were included to account for the propensity for an individual's IYCF practice patterns at T n to match their patterns at T n − 1. Due to the differences in household food insecurity scores between the intervention and comparison groups at baseline and the possibility that the intervention influenced the observed trend in household food insecurity, HFIAS scores were included in the final adjusted difference‐in‐difference regression model. Only children with complete data available for the two comparison time points were included in the respective difference‐in‐difference analyses.
We conducted subsequent multivariable analyses controlling for variables that differed between the intervention and comparison groups at baseline (caregiver's age and household food insecurity score), and an additional full model including variables indicated through F value comparison and Akaike information criterion/Bayesian information criterion analysis was built to provide an expanded view of associations of the intervention with IYCF practices (Appendix S1).
2.7. Ethical considerations
The study was approved by the University of California, Berkeley Committee on Human Research, the Ethical Review Committee of the Kenya Medical Research Institute, and PATH's Institutional Review Board.
3. RESULTS
3.1. Analysis of nutrition knowledge
Overall nutrition knowledge was significantly higher among community members and CHWs who participated in the intervention compared with comparison groups (Table 1). In addition, community members who participated in the intervention had higher average scores in the breastfeeding practices subscale than the comparison group, whereas CHW participants had higher scores in all subscales (breastfeeding, complementary feeding, and confidence/behaviour).
Table 1.
Nutrition knowledge scores among community members and community health volunteers who participated in the intervention compared to those who did not, using a t test
| Community membersa | Community health volunteersa | |||||
|---|---|---|---|---|---|---|
| Intervention (n = 43) | Comparison (n = 149) | P valueb | Intervention (n = 22) | Comparison (n = 64) | P valueb | |
| Overall nutrition knowledge | 21.9 (3.2) | 20.3 (3.9) | <0.01 | 24.7 (3.3) | 21.2 (3.6) | 0.0001 |
| Subscales | ||||||
| Complementary feeding practices | 9.2 (2.1) | 9.0 (2.1) | 0.25 | 10.3 (1.8) | 9.2 (2.0) | <0.05 |
| Breastfeeding practices | 5.6 (1.3) | 4.6 (1.4) | 0.0000 | 6.4 (0.95) | 5.1 (1.2) | 0.0000 |
| Caregiver confidence and behaviour | 5.2 (1.3) | 4.9 (1.6) | 0.16 | 6.1 (1.4) | 5.0 (1.6) | <0.01 |
Values are in means (SDs); responses characterized as correct or incorrect.
One‐tailed t test.
3.2. Analysis of child dietary outcomes
Analysis of baseline descriptive statistics indicated few differences among caregivers in the intervention and comparison groups (Appendix S2). Intervention participants were significantly younger (P = 0.07) and more food secure (P = 0.05) than the comparison group. There were no significant differences in child characteristics or breastfeeding practices between children in the intervention and comparison groups at baseline.
Following the intervention, children in the intervention group had higher odds of meeting the MMF criteria and consumed a higher number of meals than children in the comparison group (Table 2). Immediately postintervention, children in the intervention group had 2.42 (95% confidence interval [1.09, 5.39]) times higher odds of meeting the MAD criteria than children in the comparison group. The average number of food groups consumed per day and percent of children meeting the MDD and iron consumption indicator criteria did not differ between the groups at any time point.
Table 2.
Comparison of infant and young child feeding (IYCF) practices between the intervention and comparison groups at each time point
| IYCF indicator | Intervention (n baseline = 48)a | Comparison (n baseline = 179)b | Total (n baseline = 227)c | Unadjusted regressiond |
|---|---|---|---|---|
| Mean number of food groups per daye | β (95% CI) | |||
| Baseline | 2.5 (SD = 1.0) | 2.7 (SD = 1.0) | 2.7 (SD = 1.0) | −0.20 [−0.52, 0.11] |
| Immediately post | 3.2 (SD = 1.0) | 3.1 (SD = 1.0) | 3.1 (SD = 1.0) | 0.10 [−0.22, 0.42] |
| 6 months post | 2.8 (SD = 0.8) | 2.8 (SD = 0.8) | 2.8 (SD = 0.8) | <0.01 [−0.26, 0.26] |
| Mean number of meals per day | β (95% CI) | |||
| Baseline | 3.5 (SD = 0.7) | 3.4 (SD = 0.6) | 3.5 (SD = 0.6) | 0.07 [−0.12, 0.27] |
| Immediately post | 3.6 (SD = 0.6) | 3.3 (SD = 0.6) | 3.4 (SD = 0.6) | 0.37 [0.17, 0.57] |
| 6 months post | 3.6 (SD = 0.6) | 3.4 (SD = 0.6) | 3.4 (SD = 0.6) | 0.20 [0.01, 0.40] |
| Minimum dietary diversityf | OR (95% CI) | |||
| Baseline | 5 (10.4%) | 36 (20.1%) | 41 (18.1%) | 0.46 [0.17, 1.25] |
| Immediately post | 19 (39.6%) | 59 (33.9%) | 78 (35.1%) | 1.27 [0.66, 2.47] |
| 6 months post | 6 (13.3%) | 33 (19.5%) | 39 (18.2%) | 0.63 [0.25, 1.62] |
| Minimum meal frequencyg | OR (95% CI) | |||
| Baseline | 36 (75.0%) | 105 (58.7%) | 141 (62.1%) | 2.11 [1.03, 4.33] |
| Immediately post | 33 (68.8%) | 74 (42.5%) | 107 (48.2%) | 2.97 [1.51, 5.87] |
| 6 months post | 30 (66.7%) | 80 (47.3%) | 110 (51.4%) | 2.23 [1.12, 4.43] |
| Minimum acceptable dieth | OR (95% CI) | |||
| Baseline | 3 (6.3%) | 16 (8.9%) | 19 (8.4%) | 0.68 [0.19, 2.43] |
| Immediately post | 12 (25.0%) | 21 (12.1%) | 33 (14.9%) | 2.42 [1.09, 5.39] |
| 6 months post | 2 (4.4%) | 12 (7.1%) | 14 (6.5%) | 0.61 [0.13, 2.82] |
| Consumed iron‐rich foodi | OR (95% CI) | |||
| Baseline | 30 (62.5%) | 134 (74.9%) | 164 (72.3%) | 0.56 [0.29, 1.11] |
| Immediately post | 38 (79.2%) | 127 (73.0%) | 165 (74.3%) | 1.41 [0.65, 3.05] |
| 6 months post | 41 (91.1%) | 151 (89.4%) | 192 (89.7%) | 1.22 [0.39, 3.81] |
Note. CI: confidence interval; OR: odds ratio.
6 months post: Intervention (n = 45).
Immediately post: Comparison (n = 174), 6 months post: Comparison (n = 169).
Immediately post: Total (n = 222), 6 months post: Total (n = 214).
Statistically significant at α < 0.05 bold faced.
Food groups: Grains/roots/tubers, legumes/nuts, dairy, flesh foods, eggs, vitamin A‐rich fruits and vegetables, and other fruits and vegetables (WHO, 2008).
Minimum dietary diversity criteria: Consuming four out of seven designated food groups (grains/roots/tubers, legumes/nuts, dairy, flesh foods, eggs, vitamin A‐rich fruits and vegetables, and other fruits and vegetables) in 24 hr (WHO, 2008).
Minimum meal frequency criteria: Consuming a minimum of three meals in 24 hr if concurrently breastfeeding or four meals per 24 hr if not (WHO, 2008).
Minimum acceptable diet criteria: For children who are currently breastfeeding, both minimum meal frequency and minimum dietary diversity criteria must be met; for children who are not currently breastfeeding, the minimum meal frequency criteria must be met and the child must receive two dairy servings and consume four out of the six non‐dairy food groups described above (WHO, 2008).
Consumption of iron‐rich foods includes flesh foods, iron supplements, iron‐fortified foods such as baby formula, and micronutrient packets with iron (WHO, 2008).
At immediately postintervention, children in the intervention group had a significantly higher mean number of meals per day than children in the comparison group (Table 2). The mean number of meals consumed per day by children in the intervention group increased from 3.5 meals at baseline to 3.6 immediately postintervention, whereas among children in the comparison group, the mean number of meals consumed per day decreased over the same time from 3.4 meals to 3.3 meals. Controlling for differences in HFIAS scores, the increase in the number of meals per day from baseline to immediately postintervention was 0.31 (95% confidence interval [0.03, 0.60], P < 0.05) meals higher for the intervention group (Table 3). None of the other indicators demonstrated a difference in trends between the intervention and comparison groups. The trends in IYCF practices across the three time points are presented in Figure S2.
Table 3.
Difference‐in‐difference multivariate regression analysis of infant and young child feeding (IYCF) indicators between the intervention and comparison groups controlling for household food insecurity scorea
| IYCF indicator | Baseline to immediately post (n = 222)b | Immediately post to 6 months post (n = 212)c | Baseline to 6 months post (n = 214)d |
|---|---|---|---|
| Difference‐in‐difference of means (95% CI)e | |||
| Number of food groups per dayf | 0.34 [−0.11, 0.79] | 0.20 [−0.21, 0.61] | −0.14 [−0.55, 0.27] |
| Number of meals per day | 0.31 [0.03, 0.60] | 0.13 [−0.15, 0.41] | −0.18 [−0.46, 0.01] |
| Difference‐in‐difference of log odds (95% CI)e | |||
| Met minimum dietary diversityg | 1.10 [−0.11, 2.31] | 0.34 [−1.06, 1.74] | −0.86 [−2.12, 0.40] |
| Met minimum meal frequencyh | 0.41 [−0.67, 1.49] | 0.06 [−0.95, 1.07] | −0.31 [−1.31, 0.69] |
| Met minimum acceptable dieti | 1.46 [−0.12, 3.04] | −0.11 [−2.11, 1.89] | −1.43 [−3.18, 0.33] |
| Consumed iron‐rich foodj | 1.12 [−0.04, 2.29] | 0.83 [−0.53, 2.19] | −0.17 [−1.56, 1.22] |
Note. CI: confidence interval.
Household Food Insecurity Access Scale (HFIAS) score ranges from 0 (food secure) to 27 (severely food insecure; Coates et al., 2007); in the study sample, HFIAS scores ranged from 0 to 22.
Baseline to immediately post: Intervention (n = 48), comparison (n = 174).
Immediately post to 6 months post: Intervention (n = 45), comparison (n = 167).
Baseline to 6 months post: Intervention (n = 45), comparison (n = 169).
Statistically significant at α < 0.05 bold faced.
Food groups: Grains/roots/tubers, legumes/nuts, dairy, flesh foods, eggs, vitamin A‐rich fruits and vegetables, and other fruits and vegetables (WHO, 2008).
Minimum dietary diversity criteria: Consuming four out of seven designated food groups (grains/roots/tubers, legumes/nuts, dairy, flesh foods, eggs, vitamin A‐rich fruits and vegetables, and other fruits and vegetables) in 24 hr (WHO, 2008).
Minimum meal frequency criteria: Consuming a minimum of three meals in 24 hr if concurrently breastfeeding or four meals per 24 hr if not (WHO, 2008).
Minimum acceptable diet criteria: For children who are currently breastfeeding, both minimum meal frequency and minimum dietary diversity criteria must be met; for children who are not currently breastfeeding, the minimum meal frequency criteria must be met and the child must receive two dairy servings and consume four out of the six non‐dairy food groups described above (WHO, 2008).
Consumption of iron‐rich foods includes flesh foods, iron supplements, iron‐fortified foods such as baby formula, and micronutrient packets with iron (WHO, 2008).
4. DISCUSSION
Our results suggest that although the Kanyakla Nutrition Program had a 2‐year effect on nutrition knowledge among community members and CHWs, this improved knowledge did not translate into improved IYCF practices.
Trends in diet quality measures, particularly dietary diversity, were similar in the intervention and comparison groups. Children in both groups improved MDD immediately after the intervention and then returned to baseline 6 months after the intervention. This pattern suggests that seasonal trends may play a role in dietary diversity. The maize harvest season coincided with the immediate postintervention time point and may have contributed to the relative increase in dietary diversity among all children. At the first follow‐up time point, the mean number of food groups consumed exceeded three groups, the recommended minimum number for breastfeeding children. Thus, building resilience to seasonal declines in dietary diversity may be particularly important within this population.
A social support intervention alone may be insufficient to surmount barriers to enacting nutrition knowledge. In addition to our quantitative findings regarding increased nutrition knowledge, a qualitative evaluation of the Kanyakla Nutrition Program demonstrated improvements in programme participants' knowledge and confidence regarding nutrition behaviour and feeding young children (DeLorme et al., 2018). The programme also engaged male caregivers, and both male and female participants identified structural barriers to food access (e.g., income and limited access to irrigation) that prevented them from fully enacting their knowledge (DeLorme et al., 2018). Despite the inclusion of the social network component, the findings of this study are similar to studies evaluating maternal education programmes that improved maternal knowledge and attitudes towards appropriate feeding practices but did not significantly improve actual dietary diversity and meal frequency (Agbozo, Colecraft, & Ellahi, 2015; Christian et al., 2016; Gyampoh, Otoo, & Aryeetey, 2014).
Despite the limited effects on IYCF practices, engagement of social networks may have benefits for nutrition knowledge acquisition and retention and may position the Kanyakla Nutrition Program to expand support. The inclusion of community members such as fathers and grandmothers in the social network approach may be advantageous for knowledge attainment and could affect behaviour if engaged in settings with higher levels of food security. Further, the social network component may provide a platform to increase access to nutritious foods to further support households in applying their nutrition knowledge. For example, social networks could be used to support nutrition‐sensitive agricultural and fishing interventions.
A strength of our analytic approach is that the intervention and comparison groups were well matched, providing for an appropriate comparison. In addition, the island's relative isolation, small size, small population, and ethnic homogeneity strengthen the comparison between the intervention and comparison groups. Because of these features, research on a subset of this population can reasonably be applied to the larger community and may have relevance for communities with similar levels of food security.
However, the comparison group does not provide an ideal comparison. The comparison group included both participants who would have joined the intervention given the chance and those who would not, whereas the intervention group only included the former. Our analysis showed that the comparison group had higher levels of household food insecurity and, on average, younger caregivers than the intervention group. Some other possible differences not addressed in the study are that caregivers who are interested in participating may already be knowledgeable about the importance of nutrition, better educated, or perhaps have more time or support allowing them to participate; conversely, they may be driven to participate by the hope of receiving food or other direct benefits. Further, the intensive period of the intervention was relatively short, including six sessions over 12 weeks.
The use of IYCF indicators rather than a direct assessment of dietary intake has benefits and limitations. Proxy indicators are simple to assess and understand, results are directly comparable with other studies, and change over time is easy to monitor (Bhutta et al., 2013; UNICEF, 2011). However, the correlation between dietary indicators and health, nutrition status, and child growth is not universally established (Jones et al., 2014). Indicators may oversimplify complex dietary habits and do not take individual nutritional needs into account. Although 24‐hr dietary recalls do not put a large burden on the participant, they require trained personnel, may not reflect the usual diet especially if some atypical event occurred in the last 24 hr, and have inherent recall bias (Kelada et al., 2001; Prentice et al., 2011). Additionally, by 6 months postintervention, some of the children in the study were older than the age range indicated in WHO guidelines (WHO, 2008).
5. CONCLUSION
Our findings demonstrate the salient challenge that those who improve their knowledge of nutrition may still face substantial barriers to accessing the foods that will improve their nutrition. These barriers may be particularly acute in certain seasons, further amplifying the challenge in smoothing food access across production seasons. Although we find social network groups that engage diverse community members succeeded in augmenting nutrition knowledge, members of these groups were unable to provide each other with the support necessary to change the feeding behaviours for young children in a food insecure setting. Social network interventions to improve nutrition should therefore engage further with opportunities to change not only knowledge and within‐group support but also the food environment, incomes, and diversity of food produced by group members.
CONFLICTS OF INTEREST
The authors contributed to the development of this programme and KJF is a board member at Organic Health Response. The other authors declare no financial conflicts of interest.
CONTRIBUTIONS
KJF, EMM, and LHF designed the study, and KJF, EMM FA, BM, and EB designed the intervention. MM, ERG, and BM analysed the data on IYCF practices. FWA, ERG, and BM analysed the data on nutrition knowledge. MM, ERG, and KJF drafted the manuscript. All authors reviewed and approved the final manuscript.
Supporting information
Data S1. Supporting info item
ACKNOWLEDGMENTS
We gratefully acknowledge the participants in this research study. We thank the staff of Organic Health Response, Ekialo Kiona Center, who designed and carried out this programme and the Mfangano East Community Health Workers who supported the Kanyakla Nutrition Program. We thank Charles Salmen, Matthew Hickey, and MicroClinics International for their work in the conceptualization and formulation of Kanyakla groups on Mfangano Island. We thank the UCSF Global Health Department faculty, staff, and students for their support and feedback.
Fiorella KJ, Gavenus ER, Milner EM, et al. Evaluation of a social network intervention on child feeding practices and caregiver knowledge. Matern Child Nutr. 2019;15:e12782 10.1111/mcn.12782
REFERENCES
- Abila, R. O. (2000). The development of the Lake Victoria fishery: A boon or bane for food security? The Development of the Lake Victoria Fishery: A Boon or … , (June). Retrieved from http://www.cabdirect.org/abstracts/20026791926.html
- Abila, R. O. (2003). Fish trade and food security: Are they reconcilable in Lake Victoria. FAO. Retrieved November 22, 2015, from http://www.fao.org/docrep/006/y4961e/y4961e0d.htm
- Aboud, F. E. , & Singla, D. R. (2012). Challenges to changing health behaviours in developing countries: A critical review. Social Science & Medicine, 75(4), 589–594. 10.1016/j.socscimed.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Agampodi, T. C. , Agampodi, S. B. , Glozier, N. , & Siribaddana, S. (2015). Measurement of social capital in relation to health in low and middle income countries (LMIC): a systematic review. Social Science and Medicine, 128, 95–104. [DOI] [PubMed] [Google Scholar]
- Agbozo, F. , Colecraft, E. , & Ellahi, B. (2015). Impact of type of child growth intervention program on caregivers' child feeding knowledge and practices: A comparative study in Ga West Municipality, Ghana. Food Science & Nutrition 10.1002/fsn3.318, 4, 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubel, J. (2012). The role and influence of grandmothers on child nutrition: Culturally designated advisors and caregivers. Maternal & Child Nutrition, 8(1), 19–35. 10.1111/j.1740-8709.2011.00333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezner‐Kerr, R. , Dakishoni, L. , Shumba, L. , Msachi, R. , & Chirwa, M. (2008). “We grandmothers know plenty”: Breastfeeding, complementary feeding and the multifaceted role of grandmothers in Malawi. Social Science & Medicine, 66(5), 1095–1105. 10.1016/j.socscimed.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Das, J. K. , Rizvi, A. , Gaffey, M. F. , Walker, N. , Horton, S. , … Black, R. E. (2013). Evidence‐based interventions for improvement of maternal and child nutrition: What can be done and at what cost? The Lancet, 382(9890), 452–477. 10.1016/S0140-6736(13)60996-4 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , de Onis, M. , … Uauy, R. (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet, 382(9890), 427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- Bland, J. M. , & Altman, D. G. (1994). One and two sided tests of significance. BMJ, 309(6949), 248 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2540725/. 10.1136/bmj.309.6949.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto, P. R. , Lye, S. J. , Proulx, K. , Yousafzai, A. K. , Matthews, S. G. , Vaivada, T. , … for the Lancet Early Childhood Development Series Steering Committee . (2017). Nurturing care: promoting early childhood development Lancet, 389, 91–102. [DOI] [PubMed] [Google Scholar]
- Campisi, S. C. , Cherian, A. M. , & Bhutta, Z. A. (2017). World Perspecitive on the Epidemiology of Stunting between 1990 and 2015. Hormone Research in Pediatrics, 88(1). [DOI] [PubMed] [Google Scholar]
- Christian, A. K. , Marquis, G. S. , Colecraft, E. K. , Lartey, A. , Sakyi‐Dawson, O. , Ahunu, B. K. , & Butler, L. M. (2016). Caregivers' nutrition knowledge and attitudes are associated with household food diversity and children's animal source food intake across different agro‐ecological zones in Ghana. British Journal of Nutrition, 115(2), 351–360. 10.1017/S0007114515004468 [DOI] [PubMed] [Google Scholar]
- Coates, J. , Swindale, A. , & Bilinsky, P. (2007). Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator guide (v. 3). Washington, DC: Food and Nutrition Technical Assistance Project, Academy for Educational Development. [Google Scholar]
- Cohen, S. M. , & Wills, T. A. (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98(2), 310–357. 10.1037/0033-2909.98.2.310 [DOI] [PubMed] [Google Scholar]
- De Weerdt, J. , & Dercon, S. (2006). Risk‐sharing networks and insurance against illness. Journal of Development Economics, 81(2), 337–356. 10.1016/j.jdeveco.2005.06.009 [DOI] [Google Scholar]
- DeLorme, A. L. , Gavenus, E. R. , Salmen, C. R. , Benard, G. O. , Mattah, B. , Bukusi, E. , & Fiorella, K. J. (2018). Nourishing networks: A social‐ecological analysis of a network intervention for improving household nutrition in Western Kenya. Social Science and Medicine, 197, 95–103. [DOI] [PubMed] [Google Scholar]
- Dewey, K. G. , & Adu‐afarwuah, S. (2008). Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition, 4(Suppl 1), 24–85. 10.1111/j.1740-8709.2007.00124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkingham, J. , & Namazie, C. (2002). Measuring health and poverty: A review of approaches to identifying the poor. Health Systems Resource Centre, 44(0), 70 10.1111/j.1467-7660.2010.01678.x [DOI] [Google Scholar]
- Fiorella, K. J. (2015). Integrating resource access, livelihoods and human health. UC Berkeley.
- Fiorella, K. J. , Camlin, C. S. , Salmen, C. R. , Omondi, R. , Hickey, M. D. , Omollo, D. O. , … Brashares, J. S. (2015). Transactional fish‐for‐sex relationships amid declining fish access in Kenya. World Development, 74(October), 323–332. 10.1016/j.worlddev.2015.05.015 [DOI] [Google Scholar]
- Fiorella, K. J. , Hickey, M. D. , Salmen, C. R. , Nagata, J. M. , Mattah, B. , Magerenge, R. , … Fernald, L. H. (2014). Fishing for food? Analyzing links between fishing livelihoods and food security around Lake Victoria, Kenya. Food Security, 6(6), 851–860. 10.1007/s12571-014-0393-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella, K. J. , Milner, E. M. , Salmen, C. R. , Hickey, M. D. , Omollo, D. O. , Odhiambo, A. , … Brashares, J. S. (2017). Human health alters the sustainability of fishing practices in East Africa. Proceedings of the National Academy of Sciences, 114(16), 4171–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization , & FHI 360 (2016). Minimum dietary diversity for women: A guide for measurement. Rome: FAO; Retrieved from http://www.fao.org/3/a-i5486e.pdf [Google Scholar]
- Food and Agriculture Organization , International Fund for Agricultural Development , UNICEF , World Food Programme , & World Health Organization (2017). The State of Food Security and Nutrition in the World 2017. Building resislience for peace and food security. Rome: FAO. Retrieved from http://www.fao.org/3/a-I7695e.pdf [Google Scholar]
- Gibson, R. (2005). Principles of nutritional assessment (2nd ed.). New York: Oxford University Press. [Google Scholar]
- Graziose, M. M. , Downs, S. M. , O'Brien, Q. , & Fanzo, J. (2018). Systematic review of the design, implementation and effectiveness of mass media and nutrition education interventions for infant and young child feeding. Public Health Nutr, 21, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon, A. , Quinn, V. , Nielsen, J. , & Stone‐Jimenez, M. (2015). Essential nutrition actions and essential hygiene actions reference manual: Health workers and nutrition managers. Washington, DC: CORE Group; Retrieved from https://www.fsnnetwork.org/essential-nutrition-actions-and-essential-hygiene-actions-framework [Google Scholar]
- Gyampoh, S. , Otoo, G. E. , & Aryeetey, R. N. O. (2014). Child feeding knowledge and practices among women participating in growth monitoring and promotion in Accra, Ghana. BMC Pregnancy and Childbirth, 14(1), 180 10.1186/1471-2393-14-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanselman, B. , Ambikapathi, R. , Mduma, E. , Svensen, E. , Caulfield, L. E. , & Patil, C. L. (2018). Associations of land, cattle and food security with infant feeding practices among a rural population living in Manyara, Tanzania. BMC Public Health, 18, 159 10.1186/s12889-018-5074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad, A. , Yakoob, M. Y. , & Bhutta, Z. A. (2011). Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health, 11(Suppl 3), S25 10.1186/1471-2458-11-S3-S25. 10.1186/1471-2458-11-S3-S25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A. D. , Ickes, S. B. , Smith, L. E. , Mbuya, M. N. N. , Chasekwa, B. , Heidkamp, R. A. , … Stoltzfus, R. J. (2014). World Health Organization infant and young child feeding indicators and their associations with child anthropometry: A synthesis of recent findings. Maternal and Child Nutrition, 10(1), 1–17. 10.1111/mcn.12070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschula, S. (2011). Using people to cope with the hunger: Social networks and food transfers amongst HIV/AIDS afflicted households in KwaZulu‐Natal, South Africa. AIDS and Behavior, 15(7), 1490–1502. 10.1007/s10461-011-0006-z [DOI] [PubMed] [Google Scholar]
- Kelada, S. N. , Shelton, E. , Kaufmann, R. B. , Khoury, M. J. , & Genotype, A. D. (2001). Using intake biomarkers to evaluate the extent of dietary misreporting in large sample of adults: The OPEN study. American Journal of Epidemiology, 154(1), 1–13. 10.1093/aje/154.1.1 [DOI] [PubMed] [Google Scholar]
- Kenya Demographic and Health Survey (2014). Kenya Demographic and Health Survey: Key indicators, 1–76. Nairobi: Kenya National Bureau of Statistics; Retrieved from https://dhsprogram.com/pubs/pdf/fr308/fr308.pdf [Google Scholar]
- Liu, L. , Johnson, H. L. , Cousens, S. , Perin, J. , Scott, S. , Lawn, J. E. , … Black, R. E. (2012). Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. The Lancet, 379(9832), 2151–2161. 10.1016/S0140-6736(12)60560-1 [DOI] [PubMed] [Google Scholar]
- Macharia, T. N. , Ochola, S. , Mutua, M. K. , & Kimani‐Murage, E. W. (2018). Association between household food security and infant feeding practices in urban informal settlements in Nairobi, Kenya. Journal of Developmental Origins of Health and Disease, 9(1), 20–29. 10.1017/S2040174417001064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott, B. P. , White, A. , Hadden, L. , Davies, J. C. , & Wallingford, J. C. (2012). World Health Organization (WHO) infant and young child feeding indicators: associations with growth measures in 14 low‐income countries. Maternal & Child Nutrition., 8, 354–370. 10.1111/j.1740-8709.2011.00380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, P. , Ruel, M. T. , & Arimond, M. (2005). Final evaluation of the impact and cost‐effectiveness of World Vision's Integrated Health and Nutrition Program in Central Plateau, Haiti. Washington, DC: FANTA. [Google Scholar]
- Milner, E. M. , Fiorella, K. J. , Mattah, B. J. , Bukusi, E. , & Fernald, L. C. H. (2017). Timing, intensity, and duration of household food insecurity are associated with early childhood development in Kenya. Maternal & Child Nutrition., 14, e12543 10.1111/mcn.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell‐Box, K. M. , & Braun, K. L. (2013). Impact of male‐partner‐focused interventions on breastfeeding initiations, exclusivity, and continuation. Journal of Human Lactation, 29(4), 473–479. 10.1177/0890334413491833 [DOI] [PubMed] [Google Scholar]
- Mukuria, A. G. , Martin, S. L. , Egondi, T. , Bingham, A. , & Thuita, F. M. (2016). Role of social support in improving infant feeding practices in Western Kenya: A quasi‐experimental study. Global Health, Science & Practice, 4(1), 55–72. 10.9745/GHSP-D-15-00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njiru, M. , Kazungu, J. , Ngugi, C. C. , Gichuki, J. , & Muhoozi, L. (2008). An overview of the current status of Lake Victoria fishery: Opportunities, challenges and management strategies. Lakes & Reservoirs: Research & Management, 13, 1–12. 10.1111/j.1440-1770.2007.00358.x [DOI] [Google Scholar]
- Omwoma, S. , Owuor, P. O. , Ongeri, D. M. K. , Umani, M. , Lalah, J. O. , & Schramm, K. W. (2014). Declining commercial fish catches in Lake Victoria's Winam Gulf: The importance of restructuring Kenya's aquaculture programme. Lakes and Reservoirs: Research and Management, 19(3), 206–210. 10.1111/lre.12068 [DOI] [Google Scholar]
- Pasricha, S. R. , Drakesmith, H. , Black, J. , Hipgrave, D. , & Biggs, B. (2013). Control of iron deficiency anemia in low and middle income countries. Blood, 121, 2607–2617. 10.1182/blood-2012-09-453522 [DOI] [PubMed] [Google Scholar]
- Prentice, R. L. , Mossavar‐Rahmani, Y. , Huang, Y. , Van Horn, L. , Beresford, S. A. A. , Caan, B. , & Neuhouser, M. L. (2011). Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. American Journal of Epidemiology, 174(5), 591–603. 10.1093/aje/kwr140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahn, D. E. , & Stifel, D. (2003). Exploring alternative measures of welfare in the absence of expenditure data. Review of Income and Wealth, 49(4), 463–489. 10.1111/j.0034-6586.2003.00100.x [DOI] [Google Scholar]
- Sherbourne, C. D. , & Stewart, A. L. (1991). The MOS social support survey. Social Science & Medicine, 32(6), 705–714. 10.1016/0277-9536(91)90150-B [DOI] [PubMed] [Google Scholar]
- Stata (2017). Stata statistics/data analysis. Texas, USA: StataCorp LP, College Station. [Google Scholar]
- Thuita, F. M. , Martin, S. L. , Ndegwa, K. , Bingham, A. , & Mukuria, A. G. (2015). Engaging fathers and grandmothers to improve maternal and child dietary practices: Planning a community‐based study in western Kenya. African Journal of Food, Agriculture, Nutrition and Development, 15(5), 10386–10405. [Google Scholar]
- Tomlinson, M. , Rahman, A. , Sanders, D. , Maselko, J. , & Rotheram‐Borus, M. J. (2014). Leveraging paraprofessionals and family strengths to improve coverage and penetration of nutrition and early child development services. Annals of the New York Academy of Sciences, 1308, 162–171. https://dx.doi.org/10.1111%2Fnyas.12269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, A. C. , Bangsberg, D. R. , Emenyonu, N. , Senkungu, J. K. , Martin, J. N. , & Weiser, S. D. (2011). The social context of food insecurity among persons living with HIV/AIDS in rural Uganda. Social Science & Medicine, 73(12), 1717–1724. 10.1016/j.socscimed.2011.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF (2011). Infant and young child feeding programming guide. New York, NY: UNICEF; Retrieved from http://files.ennonline.net/attachments/1470/unicef-iycf-programming-guide-may-26-2011.pdf [Google Scholar]
- USAID Infant & Young Child Nutrition Project (2011). Measuring the quality of mother‐child feeding interactions: A study of indicators for responsive feeding. Washington, DC: USAID; Retrieved from http://iycn.wpengine.netdna-cdn.com/files/IYCN_responsive_feeding_breif_083011.pdf [Google Scholar]
- Victora, C. G. , de Onis, M. , Hallal, P. C. , Blossner, M. , & Shrimpton, R. (2010). Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics, 125(3), E473–E480. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2008). Indicators for assessing infant and young child feeding practices: Part 1 definitions. Conclusions of a concensus meeting held 6–8 November 2007 in Washington, DC, USA. Geneva: WHO Press; Retrieved from http://apps.who.int/iris/bitstream/10665/43895/1/9789241596664_eng.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting info item


