Abstract
Studies of iron and its association with the risk of small for gestational age (SGA) show inconsistent results. Consuming iron supplements during pregnancy is controversial because of possible risks. This study assessed the association between iron intake and the risk of having an SGA newborn and whether iron intake is associated with gestational diabetes. A case–control study of 518 pairs of Spanish women who were pregnant and attending five hospitals was conducted. Groups were matched 1:1 for age (±2 years) and hospital. Cases were women with an SGA newborn at delivery. Controls were women with normal‐sized newborns at delivery. Data were gathered on demographic characteristics, socio‐economic status, adverse habits (like smoking), and diet. A 137‐item food frequency questionnaire was completed. Iron intakes were categorized in quintiles (Q1–Q5). Crude odds ratios (ORs) and adjusted ORs (aORs) with 95% confidence intervals (CIs) were estimated by conditional logistic regression. No significant relationship was found between dietary iron intake and SGA. A protective association was found for women receiving iron supplementation >40 mg/day and SGA versus women not taking supplements (aOR = 0.64, 95% CI [0.42, 0.99]). This association was identified in mothers both with (aOR = 0.57, 95% CI [0.40, 0.81]) and without (aOR = 0.64, 95% CI [0.64, 0.97]) anaemia. In women in the control group without anaemia, iron supplementation >40 mg/day was positively associated with gestational diabetes (aOR = 6.32, 95% CI [1.97, 20.23]). Iron supplementation in pregnancy may prevent SGA independently of existing anaemia but may also increase the risk of gestational diabetes.
Keywords: diabetes, diet, iron, maternal nutrition, pregnancy, SGA
Key messages.
The consumption of iron from the diet is unrelated to the risk of SGA.
Iron supplementation >40 mg/day decreases the risk. This intake is associated with a higher frequency of gestational diabetes.
No association between iron supplements and gestational diabetes was found in women with anaemia.
1. INTRODUCTION
Iron is a micronutrient that plays a fundamental role during pregnancy because of its role in the growth, haematopoiesis, and development of the fetus (Marangoni et al., 2016; Peña‐Rosas, De‐Regil, Dowswell & Viteri, 2012; Taylor et al., 2017). Iron deficiency in pregnant women leads to iron‐deficiency anaemia, which affects 14% of the population in industrialized countries and between 35% and 75% of pregnant women in developing countries (United Nations Administrative Committee on Coordination/Standing Committee on Nutrition, 2004; World Health Organization [WHO], 1992).
The evidence supports the effectiveness of routine iron supplementation during pregnancy for improving maternal haematological indices, but the clinical significance for pregnant women and their newborns remains unclear (Brannon, Stover, & Taylor, 2017; Brannon & Taylor, 2017; Cantor, Bougatsos, Dana, Blazina, & McDonagh, 2015; Demuth, Martin, & Weissenborn, 2018; Marangoni et al., 2016; O'Brien & Ru, 2017). Despite this, the WHO recommends universal iron supplements of 30–60 mg/day during pregnancy (WHO, 2016a, 2016b). Supplementation with these micronutrients is not without risks (Calderón Vélez, 2007; Dewey & Oaks, 2017; Marangoni et al., 2016; Scanlon, Yip, Schieve, & Cogswell, 2000; Shastri et al., 2015). Prenatal daily iron supplements are effective in reducing the risk of low birthweight and preventing maternal anaemia and iron deficiency in pregnancy, but women receiving iron are more likely to have higher haemoglobin concentrations. Emerging and preliminary evidence demonstrates a U‐shaped risk in women with both low and high iron status for adverse health outcomes at birth and in infants, including growth, preterm birth, and gestational diabetes mellitus (Calderón Vélez, 2007; Dewey & Oaks, 2017; Marangoni et al., 2016; Scanlon et al., 2000; Shastri et al., 2015).
One of the effects of iron is its relationship with the risk of small for gestational age (SGA; Gómez Roig et al., 2017; Yang et al., 2017). Gómez Roig et al. (2017) studied 46 mothers with SGA newborns and 81 controls, reporting that mothers with a lower dietary iron intake were more frequently in the group with an SGA newborn. Similar results were observed in a study carried out in China, where the highest tertile of iron intake compared with the lowest tertile was negatively associated with SGA (OR = 0.76, 95% CI [0.62, 0.94]); however, this study did not find any association between the consumption of iron supplements and risk of an SGA newborn (Yang et al., 2017).
In Spain, it is recommended by the national public programme to take supplements of iron if a physician considers necessary. In a previous study in northern Spanish women, 20% pregnant women did not take iron supplements and it was associated to an increased risk of low birthweight (Palma et al., 2008). An important limitation of this study was that iron from diet intake was not assessed, and the sample size lacked enough statistical power to detect any association with adverse outcomes due to iron supplements. In this study, we have tried to overcome this drawback by applying a food frequency questionnaire (FFQ) to assess iron intake from diet and to a larger sample. Therefore, the main objectives of this study were to assess the relationship between iron intake (either through diet or through supplements) and SGA and gestational diabetes.
2. METHODS
The study population comprised women from five hospitals in Eastern Andalusia (Spain) serving 1.8 million people: University of Jaen Hospital, Ubeda Hospital, University of Granada Hospitals (two centres), and Poniente Hospital. Case and control groups were enrolled from May 15, 2012, through July 15, 2015. The Ethics Committees of the hospitals authorized this study. Informed consent was sought from every eligible woman.
We estimated the appropriate sample size based on the results of a similar study (Ricci, Chiaffarino, Cipriani, Malvezzi, & Parazzini, 2010). To detect a significant (P < .05, odds ratio [OR] = 0.6) difference between extreme quintiles with a statistical power of 80%, considering possible loss due to unreliable diet answers (10%), final estimated sample size was 1,050 subjects, including 525 cases and 525 controls.
2.1. Cases
The eligibility criteria for cases were delivery of a single, live newborn diagnosed as SGA according to the tables developed for the Spanish population (Delgado‐Beltrán et al., 1996), without congenital malformations during the study period, and residence in the referral area of the hospital. Nineteen women declined to participate. A total of 533 cases were selected: 79 from University of Jaen Hospital, 369 from University of Granada Hospitals, 46 from Ubeda Hospital, and 39 from Poniente Hospital.
2.2. Controls
A matching control based on age at delivery (±2 years) was selected at the same hospital within 1 week of including a case. Eligible women had a normal‐sized newborn with no congenital malformations and resided in the referral area of the hospital. Sixty‐five women in the control group declined to participate. (Figure 1).
Figure 1.
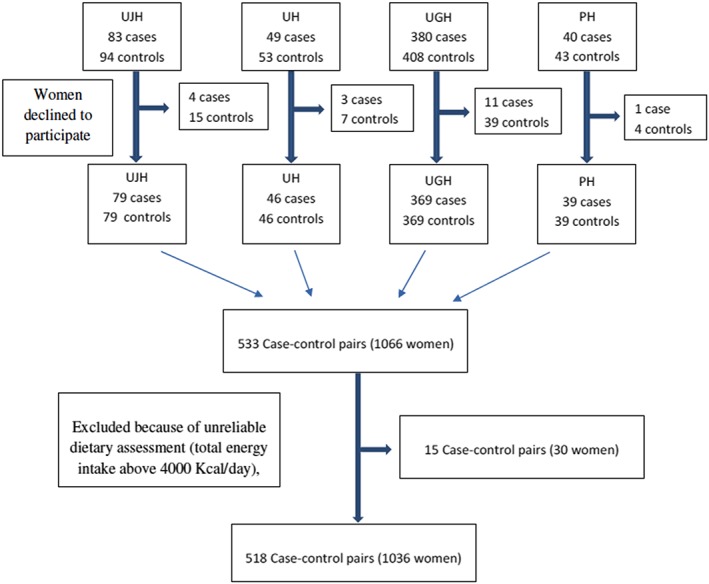
Participants' flowchart
2.3. Data collection
Three sources of data were used: personal interviews (conducted within 2 days after delivery), clinical charts, and prenatal care records. Information was obtained on the following variables: mother's vital statistics (age at pregnancy, race, pre‐pregnancy body mass index [BMI], education level, marital status, income level, and occupation), obstetric history (parity and abortions), previous adverse perinatal outcomes, conditions during pregnancy (infections, pre‐eclampsia, diabetes, and other obstetric conditions), birthweight (weight in grams in the delivery room), prescribed and over‐the‐counter drugs, smoking during pregnancy, and prenatal care (number of visits and date of first visit and weight gain during pregnancy). Social class was coded in five main levels ranging from I (highest) to V (lowest) according to the classification of the Spanish Society of Epidemiology (Álvarez‐Dardet, Alonso, Domingo, & Regidor, 1995), which is similar to that of the Black Report (Townsend & Davidson, 1982). Prenatal care utilization was measured using the Kessner Index (Kessner, Singer, & Kalk, 1993). Alcohol consumption during and before pregnancy was assessed with a structured questionnaire in which the number of drinks and type of drink on weekdays, weekends (including Friday evening), and holidays (including the holiday eve) were recorded.
2.4. Dietary assessment
The baseline questionnaire included a semiquantitative FFQ that was previously validated in Spain (de la Fuente‐Arrillaga, Ruiz, Bes‐Rastrollo, Sampson, & Martinez‐Gonzalez, 2010), which consists of 137 items and open‐ended questions related to information about the use of dietary supplements (Martin‐Moreno et al., 1993). The questionnaire was based on typical portion sizes and had nine options for the frequency of intake in the previous year for each food item (ranging from never or almost never to ≥6 times/day). A dietitian updated the nutrient data bank using the latest available information included in the food composition tables for Spain (Mataix Verdú, 2003; Moreiras, Carbajal, Cabrera, & Cuadrado, 2003). After computing the total energy intake, a total of 15 matched pairs were excluded because of an unreliable dietary assessment (total energy intake >4,000 Kcal/day), leaving 518 pairs for analysis. Eighty women who did not remember the brand of the supplement (43 in SGA newborn group and 38 in control group). The possible cut‐off points of iron contents in supplements were based on the usual concentration of commercial brands: 14, 28, 40, and 80 mg. After a sensitivity analysis, the dose of 40 mg was chosen as it better defines a group at higher risk.
2.5. Statistical analysis
The main outcome is SGA. Gestational diabetes was assessed as a secondary outcome in the control group: This was done so as the group of SGA cases does not represent the general population. Food and nutrient intakes were adjusted for total energy intake using the residuals method, and separate regression models were performed to obtain the residuals (Willet, 1998). Energy‐adjusted food or nutrient intakes were categorized in quintiles. ORs and their 95% confidence intervals (CIs) were estimated by conditional regression logistic models, with adjustment for potential confounders (adjusted OR [aOR]). To determine the variables to be included in the multivariate analysis, the procedure described by Sun, Shook, and Kay (1996) was followed. Intermediate variables were discarded. We ran two stepwise models, one backward and another forward, including variables with a value of P < .2 (Maldonado & Greenland, 1993; Mickey & Greenland, 1989). We constructed a list of predictors of SGA identified in other studies. Using information from stepwise models and the list of predictors, a saturated model was built, and by using a heuristic approach, variables that did not change the coefficient of the bundles by more than 10% were discarded, in order to construct a parsimonious model retaining all important confounders. A priori, we evaluated SGA risk factors in mothers that were related to diet (i.e., tobacco use, pregestational BMI, body weight, and educational level) as potential confounders. The model was adjusted for the following maternal factors: pre‐eclampsia, smoking, BMI, total food energy, previous preterm/low‐birthweight newborn, and anaemia during pregnancy. All P values are two‐tailed. Significance was set at P < .05. Analyses were performed with Stata 14 (College Station, TX).
3. RESULTS
The characteristics of the study population can be seen in Table 1. Smoking habits of the mother, having a previous low‐birthweight newborn and/or SGA newborn, weight gain during pregnancy, BMI, intrauterine growth retardation, and pre‐eclampsia during pregnancy were identified as risk factors for SGA (P < .001).
Table 1.
Description of the study population
| Variable | Cases | Controls | P value |
|---|---|---|---|
| Marital status, n (%) | .036 | ||
| Single | 37 (7.1) | 42 (8.1) | |
| Stable couple | 161 (31.1) | 124 (23.9) | |
| Married | 320 (61.8) | 352 (68.0) | |
| Education level, n (%) | .084 | ||
| Primary | 112 (21.6) | 93 (17.9) | |
| High school, not completed | 42 (8.1) | 28 (5.4) | |
| High school, completed | 185 (35.7) | 190 (36.7) | |
| University | 179 (34.6) | 207 (40.0) | |
| Previous preterm/low‐birthweight newborn, n (%) | 454 (87.6) | 492 (95.0) | <.001 |
| Kessner index (prenatal care), n (%) | .737 | ||
| Adequate | 259 (50.0) | 253 (48.8) | |
| Intermediate | 185 (35.7) | 182 (35.2) | |
| Inadequate | 74 (14.3) | 83 (16.0) | |
| Smoking during pregnancy, n (%) | 149 (28.8) | 80 (15.4) | <.001 |
| Pre‐eclampsia, n (%) | 46 (8.9) | 11 (2.1) | <.001 |
| Intrauterine growth retardation, n (%) | 141 (27.2) | 8 (1.5) | <.001 |
| Weight gain during pregnancy (g/week), mean (SD) | 278 (121) | 310 (114) | <.001 |
| Body mass index, mean (SD) | 23.1 (4.5) | 23.9 (4.1) | <.001 |
| Alcohol intake (g/week), mean (SD) | 4.2 (18.5) | 3.1 (15.2) | .312 |
Dietary intake of iron (milligrams per day) was not associated with the risk of SGA (Table 2). Iron supplementation >40 mg/day showed a protective association with SGA in both the crude analysis (OR = 0.55, 95% CI [0.39, 0.77]) and the adjusted analysis (aOR = 0.64, 95% CI [0.42, 0.99]). Iron supplements were also analysed according to the existence of anaemia; in this situation, the reference was women without anaemia and not taking iron supplements. The number of women with anaemia who did not take iron supplements was very low (n = 23) and this result, although showing a protective association, is not reliable. The results of iron supplements in women without anaemia showed similar results, but significance was only reached with supplements containing iron ˃40 mg/day (aOR = 0.64, 95% CI [0.43, 0.97]). In pregnant women with anaemia, iron supplementation behaved in the same manner (aOR = 0.57, 95% CI [0.40, 0.81]).
Table 2.
Intake of iron in the diet and with supplements during pregnancy and risk of small for gestational age
| Cases n (%) | Controls n (%) | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| Diet iron, quintiles (mg/day) | ||||
| <15.9 | 115 (22.2) | 104 (20.1) | 1 “Reference” | 1 “Reference” |
| 15.9–17.7 | 120 (23.2) | 104 (20.1) | 1.04 [0.71, 1.51] | 1.04 [0.67, 1.59]a |
| 17.8–19.2 | 103 (19.9) | 103 (19.9) | 0.89 [0.62, 1.30] | 0.90 [0.59, 1.37] |
| 19.3–21.3 | 73 (14.5) | 104 (20.1) | 0.65 [0.43, 0.97] | 0.56 [0.35, 0.90] |
| >21.3 | 105 (20.3) | 103 (19.9) | 0.92 [0.63, 1.36] | 0.97 [0.62, 1.52] |
| Iron (supplement) | ||||
| No | 255 (49.2) | 210 (40.5) | 1 “Reference” | 1 “Reference” |
| Yes | 263 (50.8) | 308 (59.5) | 0.68 [0.53, 0.88] | 0.76 [0.55, 1.04]a |
| ≤40 mg/day | 117 (24.6) | 111 (23.1) | 0.82 [0.58, 1.16] | 0.80 [0.54, 1.17] |
| >40 mg/day | 103 (21.6) | 159 (33.1) | 0.55 [0.39, 0.77] | 0.64 [0.42, 0.99] |
| Unknownc | 43 (4.6) | 38 (3.3) | ||
| No anaemia and no iron supplement | 247 (47.7) | 195 (37.6) | 1 “Reference” | 1 “Reference” |
| Anaemia and no iron | 8 (9.7) | 15 (2.9) | 0.37 [0.14, 0.99] | 0.34 [0.12, 0.99]b |
| No anaemia and iron | 158 (30.5) | 171 (33.0) | 0.71 [0.53, 0.96] | 0.73 [0.50, 1.06]b |
| ≤40 mg/day | 87 (16.8) | 87 (16.8) | 0.77 [0.54, 1.10] | 0.73 [0.50, 1.06] |
| >40 mg/day | 71 (13.7) | 84 (16.2) | 0.65 [0.44, 0.95] | 0.64 [0.43, 0.97] |
| Anaemia and iron | 105 (20.3) | 137 (26.5) | 0.59 [0.42, 0.81] | 0.57 [0.40, 0.81]b |
Abbreviations: aOR, adjusted odd ratio; CI, confidence interval; OR, odd ratio.
Adjusted for pre‐eclampsia, smoking, body mass index, iron supplements, total food energy, previous preterm/low‐birthweight newborn, and anaemia during pregnancy.
Adjusted for pre‐eclampsia, smoking, body mass index, total food energy, and previous preterm/low‐birthweight newborn.
Women who did not remember the brand of the supplement.
The relationship between iron intake and gestational diabetes was assessed in the control group (Table 3). First, dietary iron, grouped in intake quintiles, was ascertained. Although no one estimate reached statistical significance, the trend was significant (the higher the intake, the higher the risk) in both crude and adjusted analyses. Iron supplements increased the risk of gestational diabetes when the intake was >40 mg/day (aOR = 3.53, 95% CI [1.37, 9.05]). The results were stratified by anaemia status. No association between iron supplements and gestational diabetes was found in women with anaemia; however, in nonanaemic women, an intake >40 mg/day was associated with a higher risk of gestational diabetes (aOR = 6.32, 95% CI [1.97, 20.23]). The consumption of iron supplements before the 12th week of gestation or before pregnancy (14 women) showed no association with the risk of gestational diabetes (aOR = 0.38, 95% CI [0.04, 3.19]); this association was detected when iron supplements were taken after the first 12 weeks of gestation (aOR = 3.40, 95% CI [1.34, 8.58]).
Table 3.
Intake of iron during pregnancy and risk of gestational diabetes mellitus in the control group
| Total | Gestational diabetes mellitus n (%) | OR (95% CI) | aORa (95% CI) | |
|---|---|---|---|---|
| All control women | ||||
| Dietary iron, quintiles (mg/day) | ||||
| <15.9 | 104 | 4 (3.85) | 1 “Reference” | 1 “Reference” |
| 15.9–17.7 | 104 | 8 (7.69) | 2.08 [0.61, 7.14] | 2.08 [0.61, 7.37] |
| 17.8–19.2 | 103 | 3 (2.91) | 0.75 [0.16, 3.44] | 0.72 [0.15, 3.40] |
| 19.3–21.3 | 104 | 3 (2.88) | 0.74 [0.16, 3.40] | 0.79 [0.17, 3.68] |
| >21.3 | 103 | 10 (9.71) | 2.69 [0.81, 8.87] | 2.71 [0.80, 9.14] |
| P for trend | .036 | .040 | ||
| Iron (supplement) | ||||
| No | 210 | 8 (3.81) | 1 “Reference” | 1 “Reference” |
| Yes: Timing | ||||
| Early (<12 week) | 87 | 1 (1.15) | 0.29 [0.04, 2.38] | 0.38 [0.04, 3.19] |
| Late | 191 | 18 (9.42) | 2.38 [1.01, 5.55] | 3.40 [1.34, 8.58] |
| Yes: dose | ||||
| ≤40 mg/day | 111 | 4 (3.60) | 0.94 [0.28, 3.21] | 1.35 [0.37, 4.89] |
| >40 mg/day | 159 | 15 (9.43) | 2.63 [1.09, 6.37] | 3.53 [1.37, 9.05] |
| Anaemic women | ||||
| Iron (supplement) | ||||
| No | 15 | 1 (1.67) | 1 “Reference” | 1 “Reference” |
| Yes | ||||
| ≤40 mg/day | 24 | 2 (8.33) | 1.27 [0.11, 15.38] | 1 [0.72, 13.70] |
| >40 mg/day | 94 | 7 (7.45) | 1.13 [0.13, 9.87] | 0.98 [0.10, 9.23] |
| Nonanaemic women | ||||
| Iron (supplement) | ||||
| No | 195 | 7 (3.59) | 1 “Reference” | 1 “Reference” |
| Yes | ||||
| ≤40 mg/day | 87 | 2 (2.30) | 0.63 [0.13, 3.11] | 1.08 [0.20, 5.81] |
| >40 mg/day | 65 | 8 (12.31) | 3.77 [1.31, 10.85] | 6.32 [1.97, 20.23] |
Abbreviations: aOR, adjusted odd ratio; CI, confidence interval; OR, odd ratio.
Adjusted for total food energy, body mass index, and education level.
4. DISCUSSION
According to our results, the consumption of iron from the diet is unrelated to the risk of SGA, whereas iron supplementation >40 mg/day decreases the risk. This intake is associated with a higher frequency of gestational diabetes in nonanaemic women in the control group.
In Spain, there is no universal recommendation to take iron supplements during pregnancy; it is an optional criterion of the professional in charge of carrying out pregnancy control individually in each case (Department of Equality, Health and Social Policies of the Junta de Andalucia, 2014).
We used Spanish fetal growth curves to define adequate size for gestational age (Delgado‐Beltrán et al., 1996). The cut‐off points of INTERGROWTH‐21st (2018) for newborn weight are very similar to those taken in the fetal growth curves for Spain (Delgado‐Beltrán et al., 1996). For instance, for boys in INTERGROWTH curves, percentile 10 (P10) for Weeks 37 and 40 are 2.38 and 2.88 kg, respectively, whereas for Spaniards boys are 2.40 and 2.87 kg (very close to the previous figures). The same occurs for girls; P10 in INTERGROWTH‐21 are 2.33 and 2.78 kg for Weeks 37 and 40, very similar to the 2.32 and 2.76 kg in Spain (INTERGROWTH‐21st, 2018). The applied FFQ was validated in a Spanish population previously (Martin‐Moreno et al., 1993; Mataix Verdú, 2003). The control group was selected by density in the same hospitals as the cases to represent the regular dietary intake of the reference pregnant female population from the area serviced by those hospitals and to avoid seasonal differences between cases and controls. A selection bias was unlikely, because the number of women who refused to participate was rather small (4% for the cases of SGA and 12% for the group of newborn adequate for gestational age).
Our study may have several limitations. All questionnaires were recorded after birth but before hospital discharge, with the intention of estimating the average dietary intake during pregnancy. We cannot discard a memory bias in case–control studies, but, if present, we think it will be a nondifferential bias as no relationship between a particular food intake or supplement and SGA beforehand is assumed. Also, interviewers did not know the aims of this study, and we asked to participating women to inform about changes produced in their dietary habits during pregnancy, to ascertain whether the changes were more relevant in cases than in controls, thus putting into evidence a differential misclassification bias. The change of diet during pregnancy and the differences between cases in controls were minimal (1–2% better for cases). Residual confounding cannot be completely ruled out.
We did not observe an association between dietary iron and SGA. However, two other studies reported an inverse relationship between iron intake from the maternal diet and SGA in a Spanish (Gómez Roig et al., 2017) and a Chinese (Yang et al., 2017) population.
According to our results, iron supplements have a protective effect against SGA, even in women without anaemia, when the intake is >40 mg/day. Palma et al. (2008) identified an association between the consumption of iron supplements in women without anaemia and the low‐birthweight newborn, in line with our results. On the contrary, other reports do not support our results. Neither a Chinese study of 7,375 women (Gómez Roig et al., 2017) nor an Indian one of 1,196 women (Shastri et al., 2015) found any association between iron supplementation and SGA.
The association between iron supplementation and gestational diabetes was analysed in the control group because the case group does not represent the general population. Our results agree with other studies in which iron supplements in nonanaemic pregnant women are associated with a higher incidence of gestational diabetes (Dewey & Oaks, 2017; Marangoni et al., 2016). The U.S. Preventive Services Task Force concluded that the evidence is insufficient to recommend for or against routine iron supplementation for nonanaemic pregnant women (Siu & Force USPST, 2015). A case–control study of 107 women with and 214 controls without anaemia, with the aim of prospectively and longitudinally investigating maternal iron status during early to midpregnancy and subsequent risk of gestational diabetes mellitus, suggested that elevated iron stores may be involved in the development of gestational diabetes mellitus from as early as the first trimester (Rawal et al., 2017). We did not detect an association between iron supplements during the first 12 weeks of gestation and the risk of gestational diabetes. In a study carried out on 231 pregnant women with neither diabetes mellitus nor gestational diabetes mellitus, Renz, Hernandez, and Camargo (2018) assessed the effects of iron supplements on HbA1c in anaemic and nonanaemic women. No changes were observed in nonanaemic women taking iron supplements. After a review of the scientific literature on iron supplementation in pregnancy and childhood, Brannon et al. (2017) affirmed that further research is needed to clarify the relationship of high iron status and supplementation in iron‐replete pregnant women and the risk for gestational diabetes. Iron is in food in two forms: haem iron and nonhaem iron. A recent review suggests that the increased risk of gestational diabetes does not result from short exposures to iron supplements during pregnancy but is associated with higher intakes of dietary haem‐containing iron during the preconceptional and early pregnancy period (Khambalia et al., 2016). We assessed preconceptional iron supplements, which was reported by 14 control women, and no case of gestational diabetes was detected.
Contrary to our results, Fu, Li, Zhou, and Liu (2016) concluded that dietary total iron has no relationship with gestational diabetes. On the contrary and agreeing with our results, Zhao et al. (2017) found that an increased dietary intake of iron was positively associated with the risk of gestational diabetes. Qiu et al. (2011) also reported that high levels of dietary haem iron intake during the preconceptional and early pregnancy periods may be associated with an increased risk of gestational diabetes, but the association between gestational diabetes risk and nonhaem dietary iron intake is less clear. Zhang and Rawal (2017) in a systematic review of several randomized control trials and observational studies suggested that dietary iron, particularly haem iron intake during or before pregnancy, is significantly and positively associated with gestational diabetes mellitus even after the adjustment for major dietary factors and other major well‐documented risk factors for gestational diabetes mellitus. Kataria, Wu, Horskjær, Mandrup‐Poulsen, and Ellervik (2018) in a meta‐analysis concluded accumulating evidence that suggests that circulating and dietary iron biomarkers among pregnant women are associated with gestational diabetes mellitus, but the results should be interpreted with caution due to the high heterogeneity of analyses. Randomized trials investigating the benefits of iron reduction in women at high risk for gestational diabetes mellitus are warranted.
The embryonic period (the first 12 weeks of gestation) is characterized by the formation of tissues and organs from the embryonic leaves (organogenesis) and placental growth. It is evident that pregnant women have an increased demand for iron in this period (Kataria et al., 2018; Marangoni et al., 2016). Therefore, the intake of iron supplements would go to the formation of those organs and tissues. After 12 weeks of gestation, the iron needs would decrease because they would no longer be required for the formation of these. This extra iron supply is no longer necessary and may have an influence on the maternal levels. Evidence from experimental studies has demonstrated that higher than normal iron concentrations can lead to pancreatic β cell dysfunction and impaired glucose metabolism (Bowers et al., 2016). In addition, iron is a fundamental element of haemoglobin, and high levels of haemoglobin have been associated with the risk of gestational diabetes (Nastaran, Nourossadat, Abbas, & Hamid, 2012).
5. CONCLUSION
Iron supplementation had a protective role in relation to SGA. No association between iron intake in the maternal diet and SGA was established. Iron supplementation >40 mg/day was associated with a higher frequency of gestational diabetes in nonanaemic women.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
MD‐R and JMM‐G conceived and designed the study. MD‐R and AB‐C analysed the data. CA‐P, NC‐I, IS‐B, and JMM‐G coordinated data collection. The first draft of the paper was written by MD‐R and JMM‐G. All of the authors discussed, made contributions to the article, and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the women who participated in the study and the interviewers.
Martínez‐Galiano JM, Amezcua‐Prieto C, Cano‐Ibañez N, Salcedo‐Bellido I, Bueno‐Cavanillas A, Delgado‐Rodriguez M. Maternal iron intake during pregnancy and the risk of small for gestational age. Matern Child Nutr. 2019;15:e12814 10.1111/mcn.12814
REFERENCES
- Álvarez‐Dardet, C. , Alonso, J. , Domingo, A. , & Regidor, E. (1995). La Medición de la Clase Social en Ciencias de la Salud, Informe de un Grupo de Trabajo de la Sociedad Española de Epidemiología. Madrid, Spain: SG Editores. [Google Scholar]
- Bowers, K. A. , Olsen, S. F. , Bao, W. , Halldorsson, T. I. , Strøm, M. , & Zhang, C. (2016). Plasma concentrations of ferritin in early pregnancy are associated with risk of gestational diabetes mellitus in women in the Danish National Birth Cohort. The Journal of Nutrition, 146(9), 1756–1761. 10.3945/jn.115.227793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon, P. M. , Stover, P. J. , & Taylor, C. L. (2017). Integrating themes, evidence gaps, and research needs identified by workshop on iron screening and supplementation in iron‐replete pregnant women and young children. The American Journal of Clinical Nutrition, 106(Suppl 6), 1703S–1712S. 10.3945/ajcn.117.156083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon, P. M. , & Taylor, C. L. (2017). Iron supplementation during pregnancy and infancy: Uncertainties and implications for research and policy. Nutrients, 9(12), 1327 10.3390/nu9121327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón Vélez, J. (2007). Iron supplementation and oxidative stress in pregnancy: A little‐discussed paradox. Revista Colombiana de Obstetricia y Ginecología, 58(4), 304–308. [Google Scholar]
- Cantor, A. G. , Bougatsos, C. , Dana, T. , Blazina, I. , & McDonagh, M. (2015). Routine iron supplementation and screening for iron deficiency anemia in pregnancy: A systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine, 162(8), 566–576. 10.7326/M14-2932 [DOI] [PubMed] [Google Scholar]
- de la Fuente‐Arrillaga, C. , Ruiz, Z. V. , Bes‐Rastrollo, M. , Sampson, L. , & Martinez‐Gonzalez, M. A. (2010). Reproducibility of an FFQ validated in Spain. Public Health Nutrition, 13, 1364–1372. 10.1017/S1368980009993065 [DOI] [PubMed] [Google Scholar]
- Delgado‐Beltrán, P. , Melchor‐Marcos, J. C. , Rodríguez‐Alarcón, J. , Linares‐Uribe, A. , Fernández‐Llebrez del Rey, L. , Barbazán‐Cortés, M. J. , … Aranguren Dúo, G. (1996). The fetal development curves of newborn infants in the Hospital de Cruces (Vizcaya). I. Weight. Anales de Pediatría, 44(1), 50–54. [PubMed] [Google Scholar]
- Demuth, I. R. , Martin, A. , & Weissenborn, A. (2018). Iron supplementation during pregnancy—A cross‐sectional study undertaken in four German states. BMC Pregnancy and Childbirth, 1s8(1), 491 10.1186/s12884-018-2130-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Equality, Health and Social Policies of the Junta de Andalucia (2014). Pregnancy, childbirth and postpartum integrated healthcare process (3rd ed.). Seville, Spain: Junta de Andalucía. Consejería de Igualdad, Salud y Políticas Sociales. [Google Scholar]
- Dewey, K. G. , & Oaks, B. M. (2017. Dec). U‐shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. The American Journal of Clinical Nutrition, 106(Suppl 6), 1694S–1702S. 10.3945/ajcn.117.156075. Epub 2017 Oct 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, S. , Li, F. , Zhou, J. , & Liu, Z. (2016). The relationship between body iron status, iron intake and gestational diabetes: A systematic review and meta‐analysis. Medicine (Baltimore), 95(2), e2383 10.1097/MD.0000000000002383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez Roig, M. D. , Mazarico, E. , Ferrero, S. , Montejo, R. , Ibáñez, L. , Grima, F. , & Vela, A. (2017). Differences in dietary and lifestyle habits between pregnant women with small fetuses and appropriate‐for‐gestational‐age fetuses. The Journal of Obstetrics and Gynaecology Research, 43(7), 1145–1151. 10.1111/jog.13330 [DOI] [PubMed] [Google Scholar]
- INTERGROWTH‐21st . Standards and Tools. In https://intergrowth21.tghn.org/standards-tools/, consulted on December 26, 2018.
- Kataria, Y. , Wu, Y. , Horskjær, P. H. , Mandrup‐Poulsen, T. , & Ellervik, C. (2018). Iron status and gestational diabetes—A meta‐analysis. Nutrients, 10(5), 621 10.3390/nu10050621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessner, D. M. , Singer, J. , & Kalk, C. E. (1993). Infant death: An analysis by maternal risk and health care. Contrasts in health status. Institute of Medicine, National Academy of Sciences: Washington DC, USA. [Google Scholar]
- Khambalia, A. Z. , Aimone, A. , Nagubandi, P. , Roberts, C. L. , McElduff, A. , Morris, J. M. , … Nassar, N. (2016). High maternal iron status, dietary iron intake and iron supplement use in pregnancy and risk of gestational diabetes mellitus: A prospective study and systematic review. Diabetic Medicine, 33(9), 1211–1221. 10.1111/dme.13056 [DOI] [PubMed] [Google Scholar]
- Maldonado, G. , & Greenland, S. (1993). Simulation study of confounder‐selection strategies. American Journal of Epidemiology, 138, 923–936. 10.1093/oxfordjournals.aje.a116813 [DOI] [PubMed] [Google Scholar]
- Marangoni, F. , Cetin, I. , Verduci, E. , Canzone, G. , Giovannini, M. , Scollo, P. , … Poli, A. (2016). Maternal diet and nutrient requirements in pregnancy and breastfeeding. An Italian consensus document. Nutrients, 8(10), 629 10.3390/nu8100629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Moreno, J. M. , Boyle, P. , Gorgojo, L. , Maisonneuve, P. , Fernandez‐Rodriguez, J. C. , Salvini, S. , & Willett, W. C. (1993). Development and validation of a food frequency questionnaire in Spain. International Journal of Epidemiology, 22, 512–519. 10.1093/ije/22.3.512 [DOI] [PubMed] [Google Scholar]
- Mataix Verdú, J. (2003). Tabla de composición de alimentos españoles. (Spanish food composition tables (4th ed.). Granada, Spain: Universidad de Granada. [Google Scholar]
- Mickey, R. M. , & Greenland, S. (1989). The impact of confounder selection criteria on effect estimation. American Journal of Epidemiology, 129, 125–137. 10.1093/oxfordjournals.aje.a115101 [DOI] [PubMed] [Google Scholar]
- Moreiras, O. , Carbajal, A. , Cabrera, L. , & Cuadrado, C. (2003). Tablas de composición de alimentos. (Spanish food composition tables (7th ed.). Madrid, Spain: Pirámide. [Google Scholar]
- Nastaran, S. A. , Nourossadat, K. , Abbas, H. F. , & Hamid, A. M. (2012). Hemoglobin level during the first trimester of pregnancy in gestational diabetes. Ginekologia Polska, 83(12), 929–933. [PubMed] [Google Scholar]
- O'Brien, K. O. , & Ru, Y. (2017). Iron status of North American pregnant women: An update on longitudinal data and gaps in knowledge from the United States and Canada. The American Journal of Clinical Nutrition, 106(Suppl 6), 1647S–1654S. 10.3945/ajcn.117.155986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma, S. , Perez‐Iglesias, R. , Prieto, D. , Pardo, R. , Llorca, J. , & Delgado‐Rodriguez, M. (2008). Iron but not folic acid supplementation reduces the risk of low birthweight in pregnant women without anaemia: A case‐control study. Journal of Epidemiology and Community Health, 62, 120–124. 10.1136/jech.2006.052985 [DOI] [PubMed] [Google Scholar]
- Peña‐Rosas, J. P. , De‐Regil, L. M. , Dowswell, T. , & Viteri, F. E. (2012). Intermittent oral iron supplementation during pregnancy (Review). The Cochrane Database of Systematic Reviews, 7, CD009997 10.1002/14651858.CD009997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, C. , Zhang, C. , Gelaye, B. , Enquobahrie, D. A. , Frederick, I. O. , & Williams, M. A. (2011). Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care, 34(7), 1564–1569. 10.2337/dc11-0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal, S. , Hinkle, S. N. , Bao, W. , Zhu, Y. , Grewal, J. , Albert, P. S. , … Zhang, C. (2017). A longitudinal study of iron status during pregnancy and the risk of gestational diabetes: Findings from a prospective, multiracial cohort. Diabetologia, 60(2), 249–257. 10.1007/s00125-016-4149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz, P. B. , Hernandez, M. K. , & Camargo, J. L. (2018). Effect of iron supplementation on HbA1c levels in pregnant women with and without anaemia. Clinica Chimica Acta, 478, 57–61. 10.1016/j.cca.2017.12.028 [DOI] [PubMed] [Google Scholar]
- Ricci, E. , Chiaffarino, F. , Cipriani, S. , Malvezzi, M. , & Parazzini, F. (2010). Diet in pregnancy and risk of small for gestational age birth: Results from a retrospective case–control study in Italy. Maternal & Child Nutrition, 6, 297–305. 10.1111/j.1740-8709.2009.00218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon, K. S. , Yip, R. , Schieve, L. A. , & Cogswell, M. E. (2000). High and low hemoglobin levels during pregnancy: Differential risks for preterm birth and small for gestational age. Obstetrics and Gynecology, 96(5 Pt 1), 741–748. [DOI] [PubMed] [Google Scholar]
- Shastri, L. , Mishra, P. E. , Dwarkanath, P. , Thomas, T. , Duggan, C. , Bosch, R. , … Kurpad, A. V. (2015). Association of oral iron supplementation with birth outcomes in non‐anaemic South Indian pregnant women. European Journal of Clinical Nutrition, 69(5), 609–613. 10.1038/ejcn.2014.248 [DOI] [PubMed] [Google Scholar]
- Siu, A. L. , & Force USPST (2015). Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine, 163, 529–536. 10.7326/M15-1707 [DOI] [PubMed] [Google Scholar]
- Sun, G. W. , Shook, T. L. , & Kay, G. L. (1996). Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. Journal of Clinical Epidemiology, 49, 907–916. 10.1016/0895-4356(96)00025-X [DOI] [PubMed] [Google Scholar]
- Taylor, R. M. , Fealy, S. M. , Bisquera, A. , Smith, R. , Collins, C. E. , Evans, T.‐J. , & Hure, A. J. (2017). Effects of nutritional interventions during pregnancy on infant and child cognitive outcomes: A systematic review and meta‐analysis. Nutrients, 9(11), 1265 10.3390/nu9111265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, P. , & Davidson, N. (1982). Inequalities in health, the Black report. Hammonsworth, UK: Penguin. [Google Scholar]
- United Nations Administrative Committee on Coordination/Standing Committee on Nutrition . (2004). Fifth report on the world nutrition situation: Nutrition for improved development outcomes. Geneva, Switzerland: WHO. [Google Scholar]
- World Health Organization . (1992). The prevalence of anaemia in women: A tabulation of available information (2nd ed.). Geneva, Switzerland: WHO. [Google Scholar]
- World Health Organization . (2016a). Guideline: Daily iron and folic acid supplementation during pregnancy. Geneva,Switzerland: WHO. [PubMed] [Google Scholar]
- World Health Organization . (2016b). WHO recommendations on antenatal care for a positive pregnancy experience; Geneva,Switzerland: WHO; [PubMed] [Google Scholar]
- Willet, W. C. (1998). Nutritional epidemiology (2nd ed.). New York, USA: Oxford University Press. [Google Scholar]
- Yang, J. , Cheng, Y. , Pei, L. , Jiang, Y. , Lei, F. , Zeng, L. , … Yan, H. (2017). Maternal iron intake during pregnancy and birth outcomes: A cross‐sectional study in Northwest China. The British Journal of Nutrition, 117(6), 862–871. 10.1017/S0007114517000691 [DOI] [PubMed] [Google Scholar]
- Zhang, C. , & Rawal, S. (2017). Dietary iron intake, iron status, and gestational diabetes. The American Journal of Clinical Nutrition, 106(Suppl 6), 1672S–1680S. 10.3945/ajcn.117.156034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Lian, J. , Tian, J. , Shen, Y. , Ping, Z. , Fang, X. , … Wang, F. (2017). Dietary intake of heme iron and body iron status are associated with the risk of gestational diabetes mellitus: A systematic review and meta‐analysis. Asia Pacific Journal of Clinical Nutrition, 26(6), 1092–1106. 10.6133/apjcn.022017.09 [DOI] [PubMed] [Google Scholar]


