Abstract
This study estimated the prevalence of violations of the International Code of Marketing of Breast‐milk Substitutes (BMS) and subsequent resolutions of the World Health Assembly (Code) at health facilities, points of sale (POS), and on BMS labelling and media in Mexico. We carried out a cross‐sectional survey among 693 mothers with children aged less than 24 months and 48 health providers at public and private health facilities in two states of Mexico. Observational assessment at 20 POS and the health facilities was conducted as well as an analysis of labels on BMS products for sale. Women attending public and private health facilities reported receiving free BMS samples in the previous 6 months (11.1%), and about 80% reported seeing BMS promotion in the mass media. Health providers reported contact with BMS manufacturer representatives in the previous 6 months (15.5%), and only 41.6% of the health providers had knowledge of the Code. BMS promotions were identified at nearly all POS. Analysis of 190 BMS labels showed that 30% included pictures/text idealizing the use of BMS, and all labels incorporated health and nutrition claims. Violations of the Code are prevalent within the health services, POS, and labelling of BMS products. The high percentage of health providers with no knowledge of the Code calls for action at national level to better disseminate and comply with the Code. A transparent, free from commercial influence, and continual monitoring system for Code compliance is needed, including a follow‐up component on sanctions for contraventions of the Code.
Keywords: breastfeeding, breast milk substitutes, International Code of Marketing of Breast‐milk Substitutes, Mexico, violations
Key messages.
Four out of five mothers of infants under 24 months of age recalled seeing BMS publicity in the mass media.
Eleven per cent of them reported receiving free samples of breast milk substitutes at health facilities in two cities of Mexico.
Almost 60% of health providers lack knowledge about the Code.
Nearly all labels of BMS products incorporated health and nutrition claims.
It is urgent to establish actions at national level to disseminate information among health providers about the Code and government strategies currently in place to implement it. Importantly, not only monitoring of violations but also allocation of appropriate sanctions is needed.
1. INTRODUCTION
The contribution of breastfeeding to survival, health, and development in children as well as to the mother's health and human capital development is well known (Victora et al., 2016). Poor breastfeeding practices increase the risk of mortality and morbidity. Suboptimal breastfeeding practices have been linked to more than 800,000 deaths among children worldwide (Black et al., 2013).
Breastfeeding practices are influenced by socio‐economic, cultural, and individual factors, as well as by the presence or absence of public policies that support and promote breastfeeding and regulated marketing strategies of breast milk substitutes (BMS). Among these factors, the promotion of manufactured BMS (including infant formula, follow‐up formula, and growing‐up or toddler milks) results in suboptimal breastfeeding practices. Promotion of BMS negatively affects the choice and ability of mothers to optimally breastfeed their children (Rollins et al., 2016). The International Code of Marketing of Breast‐milk Substitutes was adopted by the World Health Assembly (WHA) in 1981 in response to a rise in child mortality owing to the promotion of BMS (World Health Organization [WHO], 1981). Since then, the WHA has adopted a number of subsequent relevant resolutions to clarify aspects of the Code. Collectively, the International Code and subsequent WHA resolutions are referred to as the Code. The Code outlines the responsibilities of governments, health care systems, and workers, as well as those of the companies that market or manufacture BMS (WHO, 1981).
The Code is a recommendation from the WHA to governments; therefore, its effectiveness in protecting mothers and families as well as health providers from inappropriate and unethical BMS marketing depends on national legislation, monitoring, and enforcement (WHO, 1981). Whereas most countries have some form of Code‐related legal measures in place, in 2018, only 35 countries have legislation incorporating all or most Code provisions (WHO & UNICEF, 2018). A further challenge is the monitoring and enforcement of the Code, which are both necessary to detect and report violations and ensure mechanisms to effectively intervene, to comply with national Code‐related measures. In this regard, with no change to 2018, in 2016, only 32 countries reported to WHO that they have monitoring mechanisms in place, and of those, few are fully functional in practice (WHO & UNICEF, 2016).
Even though some countries monitor compliance with the Code, the results of such monitoring are rarely published in peer‐reviewed journals with broad circulation. Available studies and reports from different countries describe continued and repeated violations to the Code (Access to Nutrition Foundation, 2018; Aguayo, Ross, Kanon, & Ouedraogo, 2003; Champeny et al., 2016; Changing Markets Foundation, 2018; Ching & IBFAN‐ICDC, 2017; Pries et al., 2016; Taylor, 1998).
In Mexico, breastfeeding practices are deteriorating, especially exclusive breastfeeding, particularly among indigenous women (González de Cosío, Escobar‐Zaragoza, González‐Castell, & Rivera‐Dommarco, 2013). A recent study among Mexican women shows that social norms appeared to support breastfeeding but not as internationally recommend. Infant formula, solid food, or local beverages are commonly introduced soon after birth (Swigart et al., 2017). On the other hand, even though Mexico has a National Strategy to protect, promote, and support breastfeeding (Secretaría de Salud, 2014), there is still a low to moderate scaling‐up environment for breastfeeding in Mexico (González de Cosío, Ferré, Mazariegos, Pérez‐Escamilla, & BBF Mexico Committee, 2018). Specifically, for legislation despite Mexican legislation incorporates many Code provisions (WHO & UNICEF, 2018), there is no published information about compliance. The objective of this study was to estimate the prevalence of violations of the Code in Mexico.
2. METHODS
2.1. Study design
The study followed the WHO/UNICEF “Protocol for the Assessment and Monitoring of ‘The Code’ and relevant national measurements” (NetCode protocol; WHO, UNICEF, & NetCode, 2017). This was a cross‐sectional survey among mothers of children aged <24 months and health providers at public and private health facilities in the states of Chihuahua and Puebla. In addition, observational assessment was conducted at points of sale (POS) and health facilities, as well as for labels of all beverages and food products within the scope of the Code, identified in the market of the two aforementioned states.
The study was conducted during January and February 2016. The protocol was approved by the ethics committee of the National Institute of Public Health of Mexico. The Ethical Review Committee of the Pan American Health Organization, regional office of the WHO, considered the study a monitoring exercise and, therefore, not subject to human subjects review. Informed consent was obtained from all study participants.
2.2. Definitions
According to the Code, we considered the following products to be BMS: breast milk substitutes, including any kind of infant formula (for children from 0 to 6 months), follow‐up formula (for children 6–12 months), and growing‐up formula (for 12 months and older); other milk products, foods, and beverages, including bottle‐fed complementary foods for children younger than 24 months, when marketed or otherwise represented to be suitable, with or without modification, for use as a partial or total replacement for breast milk; and feeding bottles and teats (WHO, 1981).
2.3. Participants and sampling procedure
2.3.1. States and municipalities
The sample included 10 urban municipalities, five in Chihuahua and five in Puebla, selected according to their high number of births. Puebla and Chihuahua are located in east‐central and north‐western Mexico, respectively. These two states were selected to be part of the study by the Mexican Secretary of Health, mainly for the following reasons: (a) These two states had different achievements in terms of actions taken to protect, promote, and support breastfeeding; (b) the health authorities of both states were willing to participate in the study; (c) the selected states were representative of two regions of the country, northern and south‐east‐central Mexico; and (d) there was no electoral process that might interfere with development of the study in either state.
A two‐step approach was used for the selection of health facilities, mothers, and health providers, as described below.
2.3.2. Health care facilities
For public health facilities, between four and five health care facilities that provided well‐baby care were randomly selected in each municipality, according to the number of prenatal consults provided in the year prior to the study. Health care units were selected that attended at least 10 mothers of children aged <24 months on at least 2 days in a typical week. For private health facilities, a convenience sample was used; it would have been logistically difficult to obtain a sufficient number of women who attended them. The only two selection criteria for private health facilities were provision of well‐baby care and the consent and authorization of clinic authorities for the study. The goal was to reach 75% and 25% of all public and private health facilities, respectively. Public health facilities were outpatient clinics. For private health facilities, large hospitals were also included in the sample, given the difficulty in identifying a sufficient number of women for the study from small, private, outpatient clinics. However, recruitment from private health facilities was conducted among mothers attending their child's paediatric visits.
2.3.3. Mothers of infants aged <24 months and health providers
At each health care facility, 16 to 17 mothers of infants <24 months of age were identified and invited to participate in the study. Eight to nine mothers with children <6 months and the same number with children between 6 and 23 months were sampled from each selected health facility, giving a total sample size of 693 mothers in the two states. For participants with more than two children <24 months, we applied a computer‐automated sampling method to randomly select only one child per mother.
During the days that we visited each health care facility, one health provider at each facility was interviewed. A total of 48 health providers from the two states were selected, based on their activities with supplying BMS in the health units as well as their availability and willingness to be interviewed.
2.3.4. Points of sale
We selected supermarkets, pharmacies, convenience stores, and corner stores selling products covered under the scope of the Code. Convenience store refers to small‐ to medium‐sized establishments that sell food products, which may be franchises or chains. Corner stores refer to locally owned establishments that are smaller than convenience stores. We visited a total of 20 POS (10 per state) close to the sampled health facilities, with three supermarkets and seven pharmacies, convenience, or corner stores visited in each state (Table 1). Information about these establishments was obtained by asking study participants where baby milk powder or other BMS could be purchased close to the health facility.
Table 1.
Sample characteristics of points of sale (POS), health facilities, mothers, and health professionals included in the study
| Percent | |||
|---|---|---|---|
| Public | Private | Total | |
| Points of sale | ‐ | ‐ | (n = 51) |
| Supermarkets | ‐ | ‐ | 54.9 |
| Convenience storea | ‐ | ‐ | 5.9 |
| Corner storeb | ‐ | ‐ | 7.9 |
| Pharmacies | 31.3 | ||
| Health facilities | (n = 29) | (n = 19) | (n = 48) |
| Primary health centre | 100 | 15.7 | 66.6 |
| Doctors office | ‐ | 10.5 | 4.2 |
| Maternity facility | ‐ | 73.6 | 29.1 |
| (n = 29) | (n = 19) | (n = 48) | |
| Physician | 68.9 | 52.6 | 62.5 |
| Nurse | 27.5 | 15.7 | 22.9 |
| Other (director, administrator, and certified public accounted) | 3.45 | 31.5 | 14.5 |
| Mothers of children <24 months | (n = 525) | (n = 168) | (n = 693) |
| Age of child | |||
| <6 months | 52.0 | 50.0 | 51.5 |
| ≥6 months | 48.0 | 50.0 | 48.4 |
| Age (years; mean ± SD) | 25.5 ± 6.5 | 26.5 ± 6.2 | 25.7 ± 6.4 |
| Number of children <24 months | |||
| 1 | 96.1 | 95.8 | 96.1 |
| >2 | 3.8 | 4.1 | 3.8 |
| Educationc | |||
| None | 0.7 | 1.19 | 0.8 |
| Elementary school | 24.6 | 11.3 | 21.3 |
| Middle school | 45.4 | 27.3 | 41.0 |
| High school or technician | 24.8 | 33.3 | 26.8 |
| Professional or more | 4.3 | 26.7 | 9.8 |
Convenience store refers to small‐median establishments that sell food products and can be franchises or chain.
Corner store refers to local owners and their establishments are smaller than convenience stores.
P value of difference with x 2 test = 0.0001.
The sample size determined was of 693 mothers, the minimum number to detect 10% of the prevalence rate of exposure to BMS promotions within the health system, with confidence intervals at 95% and a measurement error of ±5%, assuming a design effect of 2 to account for the cluster design.
2.4. Data collection
All field workers participated in a 1‐week training workshop, including a pilot study. Questionnaires were administered relative to the current period and up to 6 months prior to the survey, and direct observations were made during visit to the health facility.
2.4.1. Health care facilities
Three different sets of data collection took place in each facility. These included interviews with mothers of children aged <24 months, interviews with health providers, and observation of promotional, informational, or educational materials as well as equipment or materials bearing the logo or name of manufacturers of BMS products under the scope of the Code.
Characteristics of each participating mother and child were collected, including the mother's age and educational attainment and the infant's date of birth and birth order. Structured interviews included items on information and advice received about infant feeding during the first 6 months post‐partum; promotion of BMS in the health facility or the media (including TV, radio, internet, social networks, magazine, billboard, or other means of mass communication), participation in social groups or events sponsored by BMS manufacturers or companies; and whether participants received samples, gifts, or coupons from companies that produced infant formulas and other BMS. For all sections, we investigated the place, person, product, or company involved in BMS advertising.
During the interviews of health providers, we collected information about the number, type, reason, and frequency of contacts they had with BMS company representatives in the previous 6 months. In addition, we asked about their knowledge and training regarding the Code, as well as their awareness of strategies to implement it in Mexico.
Finally, advertising of BMS and other infant products was evaluated in public spaces within the health units. All pamphlets, videos, posters, growth charts, attire or materials used by health professionals, or equipment in the health unit were documented through photographs; samples of these were obtained when possible.
2.4.2. Points of sale
Data collection at each POS included enumeration of products sold that were under the scope of the Code. We recorded a list of the products encountered in the sampled stores. As additional stores were visited, a master list was created by adding new products encountered that had not been found in previous stores. The brand name, subbrand name, descriptive name, and age category of each product were recorded. For each product, the geographical location and type of business were also noted. At all selected retail outlets, any type of promotion related to BMS was recorded and photographed, and promotional materials were also collected. For analysis of BMS labels, we photographed all BMS products available at large supermarkets. We purchased products available at pharmacies, convenience stores, and corner stores, as we were not authorized to photograph products in these stores.
2.4.3. Assessment of materials and product labels
A desk review was performed by one researcher (A. L. T.) to analyse the promotional and educational materials collected from health facilities and POS, as well as the labels on products sold at retail stores. All materials and labels were closely examined according to a predefined checklist of criteria that are based on the Code.
2.5. Data management, processing, and analysis
Data were entered by field workers using Microsoft Access, 2010. Data entry programs were designed using the questionnaires and criteria checklist to assess BMS promotions, educational materials, and labels proposed in the NetCode protocol. Data were sent to the central office of the Instituto Nacional de Salud Pública for quality control and database generation.
After a cleaning process of data out of range or data entry errors, data analysis was carried out. Means and proportions with their respective confidence intervals were calculated, taking into account the effect of the cluster sampling design (health care facilities). Statistically significant differences were tested using the chi‐square (χ 2) test, with a two‐sided P value of 0.05. All analyses were performed using Stata, version 14.1 (StataCorp, College Station, TX, USA).
3. RESULTS
3.1. Sample characteristics
We investigated 29 public and 19 private health facilities in 10 urban municipalities of the states of Chihuahua and Puebla. No differences on maternal characteristics were found between the two states, except for education. Women from Chihuahua had higher education (Data S1). Among the private facilities, 14 were large hospitals. From the same municipalities, we visited 51 POS (35 in Chihuahua and 16 in Puebla—the field team had more opportunities to visit more POS in Chihuahua state). The most common POS visited were supermarket and pharmacies (Table 1).
A total of 693 mothers of children aged <24 months were interviewed (357 women with children <6 months); all were recruited in the waiting rooms of selected health facilities. The average age of mothers was 26 years, with more than 40% of them having completed a middle school education (Table 1). Women attending private health facilities had higher educational levels (P = 0.0001).
We interviewed 48 health professionals (one per health facility); most were physicians, and the average time working in the health facility was 8.7 ± 7.9 years (Table 1).
3.2. Mothers of children aged <24 months
Women attending public and private health facilities reported receiving free samples of BMS in the last 6 months (Table 2), with 76 of 688 women receiving at least one sample (11.1%). A slightly higher percentage of women in private health facilities received samples (14.8% private vs. 10.0% public, P = 0.08). Infant formula was the most common type of BMS sample given to mothers (51 of 76 women receiving free samples—67.1%). More than 80% of interviewed women reported seeing BMS in the mass media, with a higher percentage of women from private health facilities reporting seeing BMS promotions (86.9% private vs. 79.8% public, P = 0.04; Table 2). A total 3.3% of women reported seeing promotion of BMS within the health facilities.
Table 2.
Mothers of children younger than 24 months, at public and private health facilities, who received free BMS sample, recommendations to use BMS, and exposure to publicity in the previous 6 months; health provider contact with BMS manufacturers and Code awarenessa
| Public | Private | Total | |||||
|---|---|---|---|---|---|---|---|
| Percentage | 95% CI | Percentage | 95% CI | Percentage | 95% CI | P valueb | |
| Receiving free samples of BMSc (n = 688) | 10.0 | [6.4, 15.2] | 14.8 | [8.6, 2.4] | 11.1 | [7.8, 15.6] | 0.08 |
| Receiving coupons of BMS (n = 687) | 1.5 | [0.7, 3.1] | 1.7 | [0.4, 6.6] | 1.6 | [0.8, 3.0] | 0.82 |
| Receiving gift of articles or utensils that may promote the use of BMS (n = 688) | 7.6 | [5.3, 10.9] | 11.3 | [6.5, 18.9] | 8.5 | [6.2, 11.7] | 0.29 |
| Seeing promotions/messages of BMS within the health care facility (n = 686) | 3.1 | [1.94, 4.82] | 4.2 | [1.8, 9.2] | 3.3 | [2.2, 4.9] | 0.15 |
| Seeing promotions of BMS in mass media (n = 689) | 79.8 | [74.6, 84.1] | 86.9 | [75.6, 93.4] | 81.5 | [76.9, 85.4] | 0.04 |
| Receiving recommendations about the use of BMS for their children in the last 6 months (n = 689) | 48.4 | [44.6, 52.3] | 40.7 | [32.7, 49.2] | 46.5 | [42.9, 50.3] | 0.14 |
| Receiving recommendations about the use of other beverages and foods for their children (n = 689) | 56.0 | [49.3, 62.5] | 46.4 | [39.0, 54.0] | 53.7 | [48.1, 59.1] | 0.03 |
| Health providers | n = 29 | n = 19 | n = 48 | ||||
| Type of health providers contacted by BMS manufacturer (number of health providers)d | 0.156 | ||||||
| Administrator | 0 | 2 | 2 | ||||
| Director | 0 | 2 | 2 | ||||
| Physician | 1 | 3 | 4 | ||||
| No specified | ‐ | ‐ | 1 | ||||
| Total of number of contacts by BMS manufacturers | n = 1 | n = 10 | n = 11 | N/A | |||
| Via telephone | 0 | 1 | 1 | ||||
| Via e‐mail | 0 | 1 | 1 | ||||
| Via visit at health facility | 1 | 8 | 9 | ||||
| (n = 28) | (n = 13) | (n = 41) | |||||
| Knowledge about the Code | 41.3 | [24.6, 60.3] | 42.1 | [22.0, 65.1] | 41.6 | [28.2, 56.4] | 0.96 |
| Knowledge about national strategies to implement the Code | 55.1 | [36.4, 72.5] | 57.8 | [38.4, 75.2] | 56.2 | [43.5, 71.7] | 0.83 |
Percentage and 95% confidence intervals (CI) or as specified.
χ 2 test. Percentages comparing public versus private health facilities.
Breast milk substitutes (BMS): Comprises breast milk substitutes, including infant formula, follow‐up formula, and growing‐up formula; other milk products, foods, and beverages, including bottle‐fed complementary foods, when marketed or otherwise represented to be suitable, with or without modification, for use as a partial or total replacement of breast milk; and feeding bottles and teats.
Only seven health providers specified BMS manufacturer name, representing ~15.5% of the sample.
Nearly half of respondents (46.5%) reported having received a recommendation for use of BMS in the previous 6 months (Table 2). Nearly 30.5% of respondents received such a recommendation from physicians, followed by a relative or friend (16.5%; data not shown). The BMS manufacturing companies that women recalled the most frequently being recommended included Nestlé (68%) and Mead Johnson (23%; data not shown).
The only difference on the Code violations between the states was those related to receiving free samples of BMS and seeing promotion/messages of BMS within health facilities and in mass media. Mothers in Puebla reported more exposure to free samples and promotions of BMS (Data S1).
3.3. Health providers and facilities
A total nine of 48 health providers reported having contact with BMS manufacturer representatives in the previous 6 months. Of these, only seven specified the name of BMS manufacturer (15.5%; Table 2). The most common type of contact was a representative visit to the health facility, and these were more frequent in private health facilities. Such visits were mainly for the promotion and distribution of coupons and educational materials about BMS products. The manufacturer recalled by health providers as the one that most frequently contacted them was Nestlé.
Only 41.6% of the health providers interviewed (20 of 48) had knowledge of the Code, and 56.2% had some knowledge about national strategies to implement the Code (Table 2). Only 25.0% of health providers reported receiving recent training about the Code (on average 1.0 ± 0.85 years), with no difference between the percentage of providers at public and private health facilities with recent training (data not shown).
Five BMS promotions were found in the health facilities. All of these were at private facilities in the form of BMS company posters and flyers (data not shown). No difference on Code violations were identified by state.
3.4. Points of sale
From the 51 POS visited, 50 of them had publicity of BMS. Promotions, flyers, and other materials were identified within POS with the name of BMS manufacturer. The main publicity observed at the retail establishments visited were price promotions (60%), promotional packages (20%), and free samples (16%) at the retail establishments visited (Figure 1). Promotions and samples of BMS on POS were more common in Puebla, than in Chihuahua, except for low price promotions (Data S1).
Figure 1.
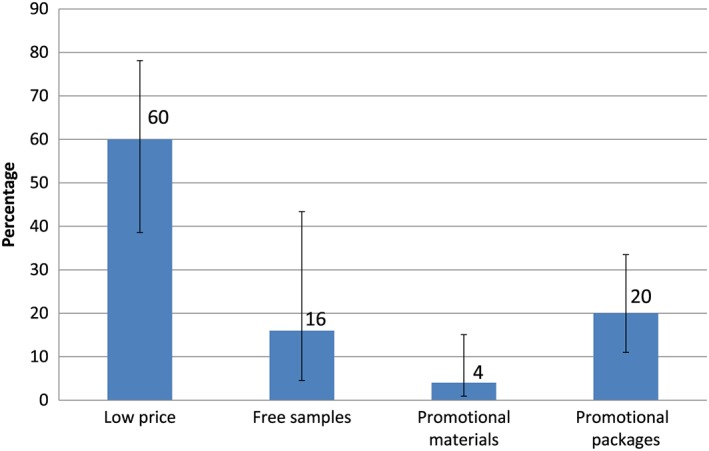
Promotion and samples of breast milk substitutes at points of sale (POS). Percentage and 95% CI; n = 50 POS with promotions
3.5. Analysis of labelling and products
We checked the labelling and inserts of all BMS products against the standards set forth by the Code. Of all BMS products identified at POS (n = 190), 28.9% were infant, 9.5% follow‐on, 10.0% growing‐up formula, and 0.5% baby milks (0.5%), whereas 49.4% were complementary foods and 1.6% supplements and bottled water for children (Data S2).
The Code states on Article 9.1: Labels should be designed to provide the necessary information about the appropriate use of the product, and so as not to discourage breastfeeding, and Article 9.2 states that “Neither the container nor the label should have pictures of infants, nor should they have other pictures or text which may idealize the use of infant formula.” In our study, all (100%) the labels for BMS were in violation with the Code, including nutrition and health claims. Another common violation was text with an invitation to communicate with BMS company (97.8%) and pictures/text that idealized their use (30.6%; Data S2). With respect to infant formula, the most violations were the use of pictures (94.5%) and text (92.3%) that may idealize its use. About 10% of them did not state the superiority of breast milk, as stated in the Code (Figure 2a). As for labels of complementary foods, all of them included health and nutrition claims and nearly all of them (97%) included an invitation to contact the BMS producer. Four per cent of labels of these products did not include the recommended age (Figure 2b).
Figure 2.
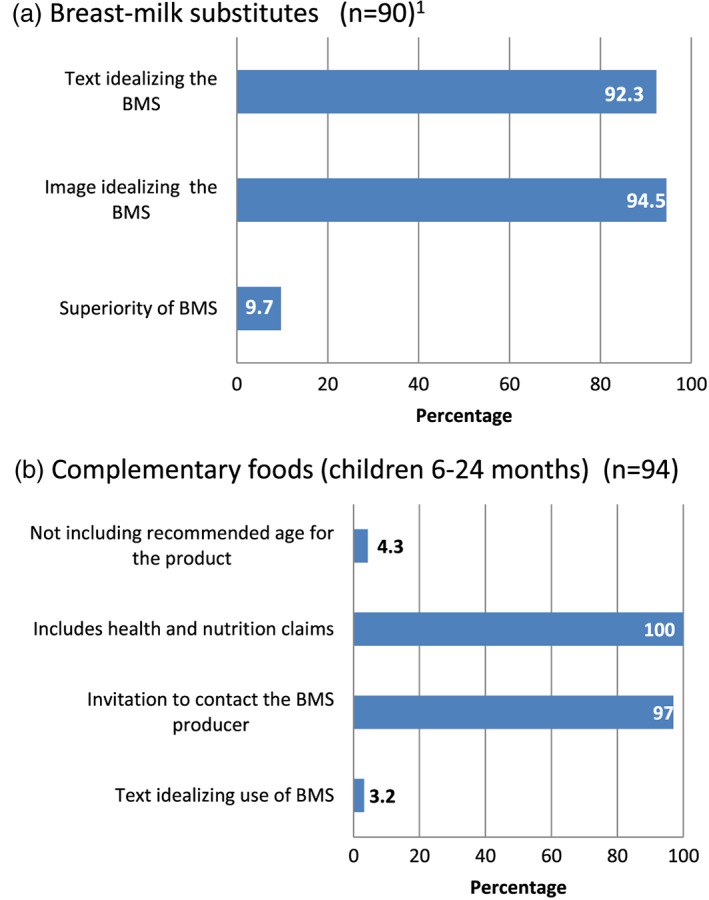
Prevalence of Code violations on the labels of products for children under 24 months of age. (a) Breast milk substitutes (BMS; n = 90): Comprises infant, follow‐on, and growing‐up formulas and baby milks. (b) Complementary foods (children 6–24 months; n = 94)
There were some aspects of labelling that were in compliance with the Code, such as appropriate language, indication of age of introduction, clear descriptions of product composition, storage conditions, and consumption deadlines (Data S2).
4. DISCUSSION
To our knowledge, this is the first study in the world reporting on compliance with the Code using the recently released NetCode protocol. In this study, 11% of mothers of infants under 24 months of age in two states of Mexico reported receiving free samples of infant formula and other BMS products in health facilities. Almost 82% of participants also recalled seeing BMS publicity in mass media and 3% within health facilities. The data show that representatives of BMS manufacturers had contact with health providers. At POS, promotions and free samples on promotional packages were common practices. More than 90% of BMS labels included information or images idealizing the use of infant formula.
Data from national nutrition surveys in Mexico show that rates of exclusive breastfeeding among Mexican women are among the lowest in Latin America (UNICEF, 2016) and are far from the current WHO global target to increase the rate of exclusive breastfeeding in the first 6 months to at least 50% (WHO/UNICEF, 2014).
Among the factors that undermine breastfeeding is the aggressive and unethical BMS marketing and promotion strategies. The negative effects of marketing, promotion, and provision of free samples of infant formula, bottles, and teats to women who are breastfeeding have been previously investigated in other countries (Donnelly, Snowden, Renfrew, & Woolridge, 2000; Frank, Wirtz, Sorenson, & Heeren, 1987; Kaplan & Graff, 2008; Piwoz & Huffman, 2015; Sobela et al., 2011; Victora et al., 2016). We found that BMS promotion and offering mothers free samples of infant formula occur within as well as outside of health facilities in Mexico. Although such promotion of BMS is prohibited by the Code, as well as Mexican regulations (for infant formulas for children under 12 months; Cobo‐Armijo, Charvel, & Hernández‐Ávila, 2017), we found that women reported being exposed to BMS promoted in the mass media frequently.
Producing and marketing BMS is a very profitable business. Between 2014 and 2019, global BMS sales are projected to increase from $45 to $71 billion (Kent, 2015). The increase is mainly owing to growth in infant formula sales in low‐ and middle‐income countries (Kent, 2015). According to EuroMonitor data provided to the WHO, in Mexico, sales of infant formula, follow‐up milks, and growing‐up milks are projected to increase from 1,002 million U.S. dollars in 2014 to 1,145 million U.S. dollars in 2019 (Rollins et al., 2016) corresponding to an increase of 14%. The association between increased BMS sales and breastfeeding practices has seldom been studied. In Mexico, Escobar‐Zaragoza, Hernández‐Ávila, and González de Cosío (2016) reported an inverse relationship between trends in exclusive breastfeeding of children under age 6 months and increased infant formula sales; their analysis included infant formula only and excluded hydrolysed, lactose‐free, soy, antireflux, or any formulas for preterm infants. These authors recognized, however, that this association is not a causal one. However, it can be expected that if exclusive breastfeeding rates decrease, infant formula sales would increase (Escobar‐Zaragoza et al., 2016).
Other authors and organizations have reported violations to the Code in other countries. Among the reported violations are those related to pregnant women and mothers of children aged between 6 and 24 months receiving free BMS samples (Access to Nutrition Foundation, 2018; Aguayo et al., 2003; Changing Markets Foundation, 2018; Ching & IBFAN‐ICDC, 2017; Taylor, 1998), in addition to health facilities receiving donations of BMS that were not used for research or professional purposes and having educational materials with BMS producer logos (Aguayo et al., 2003; Pries et al., 2016). Other studies have identified that promotion of BMS outside of health facilities is a common practice in Cambodia (Pries et al., 2016) and Senegal (Feeley et al., 2016), as is promotion within health facilities (Coriolis, 2014; Pries et al., 2016). BMS promotion at POS has been identified in Senegal, Cambodia, Nepal, and Tanzania (Champeny et al., 2016). Violations of labelling standards of the Code have also been identified in other countries (Ergin et al., 2013; Parrilla‐Rodriguez & Gorrín‐Peralta, 2008). The prevalence and types of violations reported in studies from various countries suggest that systematic Code violation is common in countries worldwide.
Although not explicitly a Code violation, a large number of health providers in Mexico (30.5%) recommend infant formulas, far more than would be expected for the small number of women who for medical reasons should use infant formula. Similar results were reported in Cambodia (Pries et al., 2016) and Senegal (Feeley et al., 2016). As recognized in the Coriolis report Infant Formula Value Chain (Coriolis, 2014), the major global multinationals direct a large part of their sales effort towards health providers rather than retailers. The effect of a health provider's recommendation for the use of BMS on a mother's decisions about feeding practices is well known. Health providers have unparalleled credibility in terms of infant health and feeding recommendations (Champeny et al., 2016; Piwoz & Huffman, 2015). Thus, it is of paramount importance to ensure that health providers have the knowledge to adequately support breastfeeding (or know where to refer mothers who require support) and understand their ethical responsibility not to recommend the use of infant formula when there is no medical reason to do so.
Despite Mexican regulations stating that BMS manufacturers are not allowed to contact health providers, 15.5% of providers reported having contact with BMS manufacturers. Such contact with providers has also been reported in Bangladesh, Poland, South Africa, and Thailand (Coriolis, 2014), as well as in Togo and Burkina Faso (Champeny et al., 2016). In addition, an important and disturbing finding is the lack of knowledge of the Code among health providers in Mexico, similar to health professionals in Togo and Burkina Faso (Champeny et al., 2016). It is urgent to establish actions at a national level to disseminate the Code and develop government strategies to implement it. This would aid in preventing BMS promotion by health care providers.
Our study revealed that all commercial BMS available in Mexico included nutritional and health claims. There are studies showing how health and nutritional claims on BMS labelling can persuade mothers to use these products. For example, mothers with children aged 6–23 months recalled health and nutrition claims made for different BMS products, including impacts on intelligence, growth and development, and claims about vitamin and nutrient content. Women reported that even though they were aware of the benefits of breastfeeding, such information convinced them to try BMS (Pries et al., 2016). Important to mention that both The Code and Codex include a statement prohibiting nutrition and health claims (Food and Agriculture Organization of the United Nations, 1997).
There is evidence showing a potential indirect effect of the proliferation of BMS products in the market on social norms and attitudes about breastfeeding (Piwoz & Huffman, 2015). In our study, we found a great variety of products for children under 24 months old in retail, convenience, and corner stores as well as pharmacies.
Despite the Code clearly prohibiting POS promotion of BMS, this provision has not been enacted in Mexican legislation and regulations. We identified BMS promotion in nearly all retail stores, pharmacies, and convenience and corners stores visited. Efforts are needed to include restrictions on the promotion of BMS at POS in Mexican legislation and regulations.
This study has some limitations, some of them related to the NetCode protocol. First, as indicated in the NetCode protocol, the study was health facility based. Although most women in Mexico attend health facilities for well‐child care, some do not and so are missed in our sampling frame. In addition, it might not be possible to generalize these findings to other states in Mexico because conditions in other cities and rural areas could differ. However, the characteristics of both states selected for the study cover the different spectrum of conditions under which contraventions of the Code might occur. Thus, it is reasonable to believe that similar violations are occurring at similar rates in other major cities of the country. Similarly, the study was done in health facilities at the primary care level. It is important to evaluate Code violations in maternity facilities as well. Another important setting missing in the Code violations assessment in the NetCode protocol is the professional conferences. Finally, our results on the comparison of public versus private health facilities should be considered with caution, given that the sample size calculation was based on one sample proportion.
On the other hand, it is important to highlight some strengths of the NetCode protocol as well. First, the NetCode protocol includes questionnaires designed to evaluate Code violations. Despite that all instruments need to be adapted to each country, the questionnaires are very clear and easy to apply at population level. Even though some settings are missing, as stated before, NetCode protocol includes tools and strategies to evaluate Code violations in primary health facilities, media (mainly television and radio), POS, and products labelling.
Finally, an important challenge to monitoring and enforcing compliance with the Code is the increasing use of Internet platforms, cellular phones, and social media such as Facebook, Twitter, YouTube, and mobile applications to promote and sell BMS products, including online ordering and coupons for free or low‐cost BMS (Abrahams, 2012). As part of this study, we explored the presence of BMS advertisements in social media, the Internet, and television programming. The results will be presented in a separate paper, but briefly, we found a high presence of BMS promotion in all these communication media. Thus, any strategy to establish a monitoring system in Mexico should consider the use of social media as part of the system.
Progress has been made to include some provisions of the Code in the legislation and regulations in Mexico; however, gaps remain that must be addressed. It is important to update Mexican regulations regarding follow‐up and growing‐up formulas; in addition, as stated in the 2016 WHA 69.9 resolution, it is also necessary to include the definition of foods for infants and young children, namely, “all commercially produced food or beverage products that are specifically marketed as suitable for feeding children up to 36 months of age” (WHO, 2016). WHA 69.9 resolution states that products that function as BMS should not be promoted.
In addition, specific implementation actions are needed for effective education, training, and monitoring systems to ensure that health care providers, BMS manufacturers, and distributers (POS) comply with the Code (Cobo‐Armijo et al., 2017). Mexico needs a transparent, free from commercial influence, and continual monitoring system on compliance with the Code enacted by the Government that includes a component to follow‐up on sanctions for violations to the Code.
5. CONCLUSION
Breaches of the Code are prevalent in Mexico within health services, at POS, and in labelling of BMS products sold. The high percentage of health providers with no knowledge of the Code calls for action at national level to better disseminate and comply with the Code, such as continuous training about the Code and the legislation in the country. In addition, an effective, transparent, and free from commercial influence national monitoring system of compliance implemented by the Government of Mexico with Code is also needed, which includes a component to follow‐up on sanctions for contraventions of the Code.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
The authors' responsibilities were as follows: SHC and ALLT had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. SHC, ALLT, TSL, CKL, TGC, PS, and LGS designed the research study. SHC, ALLT, and TSL conducted the research. SHC, ALLT, TSL, CKL, TGC, PS, and LGS proposed and approved modifications to the protocol during the development of the study. SHC and ALLT analysed the data. SHC and ALLT wrote the manuscript and had primary responsibility for the final content. TSL, CKL, PS, TGC, JRD, and LGS gave key insights for data interpretation and for the final manuscript. All authors read and approved the final version of the manuscript.
Supporting information
Data S1. Table S1. Sample characteristics of points of sale (POS), health facilities, mothers, and health professionals included by state included in study.
Table S2. Mothers of children younger than 24 months, at public and private health facilities, who received free Breast Milk Substitutes (BMS) sample, recommendations to use BMS, and exposure to publicity in the previous 6 months; health provider contact with BMS manufacturers and Code awareness.1
Data S2. Examination of labels of products sold in Puebla and Chihuahua, Mexico1.
ACKNOWLEDGEMENTS
We acknowledge support received from the Ministry of Health in facilitating interactions with the Health Authorities of the States (Puebla and Chihuahua), particularly Erika Paola García, Nazarea Herrera Maldonado, Liliana Martínez Peñafiel, and Eduardo Pesqueira from the Centro Nacional de Equidad de Género y Salud Reproductiva. We thank the Health Authorities of Puebla and Chihuahua. We thank Analisa Avila, ELS, from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Hernández‐Cordero S, Lozada‐Tequeanes AL, Shamah‐Levy T, et al. Violations of the International Code of Marketing of Breast‐milk Substitutes in Mexico. Matern Child Nutr. 2019;15:e12682 10.1111/mcn.12682
Footnotes
“The Ethical Review Committee of the Pan American Health Organization/WHO considered the project a monitoring exercise, and therefore, not subject to human subjects review.” There are two reasons for this: first that Pan American Health Organization followed proper protocol in submitting the proposal for ethical approval and second that there are different perspectives from IRB Committees about whether Code monitoring is research in human subjects.
REFERENCES
- Abrahams, S. W. (2012). Milk and social media: Online communities and the International Code of Marketing of Breast‐milk Substitutes. Journal Human Lactation, 28(3), 400–406. [DOI] [PubMed] [Google Scholar]
- Access to Nutrition Foundation . (2018). Access to Nutrition Index. Global Index 2018. Available at: https://www.accesstonutrition.org/sites/gl18.atnindex.org/files/resources/atni_report_global_index_2018.pdf. Accessed on July, 2018
- Aguayo, V. , Ross, J. , Kanon, S. , & Ouedraogo, A. (2003). Monitoring compliance with the International Code of Marketing of Breastmilk Substitutes in west Africa: Multisite cross sectional survey in Togo and Burkina Faso. British Medical Journal, 326, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, R. , Victora, C. G. , Walker, S. , Bhutta, Z. , Christian, P. , de Onis, M. , … The Maternal and Child Nutrition Study Group (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet, 382, 427–451. [DOI] [PubMed] [Google Scholar]
- Champeny, M. , Pereira, C. , Sweet, L. , Khin, M. , Coly, M. , Gueye, Y. , … Huffman, S. L. (2016). Point‐of‐sale promotion of breastmilk substitutes and commercially produced complementary foods in Cambodia, Nepal, Senegal and Tanzania. Maternal & Child Nutrition, 12(2), 126–139. 10.1111/mcn.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changing Markets Foundation . (2018). Busting the myth of science‐based formula. An investigation into Nestlé infant milk products and claims. Milking it. Available at: http://changingmarkets.org/wp-content/uploads/2018/02/BUSTING-THE-MYTH-OF-SCIENCE-BASED-FORMULA.pdf. Accessed on July, 2018
- Ching, C. , & IBFAN‐ICDC . (2017). Overview: Breaking the Rules, Stretching the Rules 2017. World Nutrition, 8(2), 323–330. [Google Scholar]
- Cobo‐Armijo, F. , Charvel, S. , & Hernández‐Ávila, M. (2017). La regulación basada en desempeño: estrategia para incrementar las tasas de lactancia materna. Salud Pública de México, 59(3), 314–320. Available at: http://saludpublica.mx/index.php/spm/rt/printerFriendly/8122/11192. [Accessed: Jul. 2017] [DOI] [PubMed] [Google Scholar]
- Coriolis . (2014). Infant formula value chain. Auckland, New Zealand: Coriolis; Available at: http://www.coriolisresearch.com/pdfs/Coriolis_Infant_Formula_Value_Chain_2014.pdf [Google Scholar]
- Donnelly, A. , Snowden, H. M. , Renfrew, M. J. , & Woolridge, M. W. (2000). Commercial hospital discharge packs for breastfeeding women. The Cochrane Database of Systematic Reviews., 2, CD002075 10.1002/14651858.CD002075 [DOI] [PubMed] [Google Scholar]
- Ergin, A. , Hatipoglu, C. , Bozkurt, A. I. , Erdogan, A. , Güler, S. , Ince, G. , … Yeniay, M. K. (2013). Compliance status of product labels to the International Code on Marketing of Breast Milk Substitutes. Maternal and Child Health Journal, 17(1), 62–67. [DOI] [PubMed] [Google Scholar]
- Escobar‐Zaragoza, L. , Hernández‐Ávila, M. , & González de Cosío, T. (2016). Papel de la comercialización de las fórmulas y leches en México In de Cosío Martínez T. G., & Hernández‐Cordero S. (Eds.), Lactancia Materna en México. Recomendaciones para el diseño e implementación de una política nacional multisectorial de promoción, protección y apoyo de la lactancia materna en México (1st ed.). Mexico, Mx: Academia Nacional de Medicina. [Google Scholar]
- Feeley, A. , Coly, A. , Gueye, N. , Diop, E. , Pries, A. , Champeny, M. , … Huffman, S. L. (2016). Promotion and consumption of commercially produced foods among children: Situation analysis in an urban setting in Senegal. Maternal & Child Nutrition, 12(2), S64–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations . (1997). Guidelines for use of Nutrition and Health Claims CAC/GL 23‐1997. Available at: http://www.fao.org/fao‐who‐codexalimentarius/sh‐proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCAC%2BGL%2B23‐1997%252FCXG_023e.pdf. Consulted on October, 2017
- Frank, D. , Wirtz, S. , Sorenson, J. , & Heeren, T. (1987). Commercial discharge packs and breast‐feeding counseling: Effects on infant‐feeding practices in a randomized trial. Pediatrics, 80(6), 845–854. [PubMed] [Google Scholar]
- González de Cosío, T. , Escobar‐Zaragoza, L. , González‐Castell, L. D. , & Rivera‐Dommarco, J. A. (2013). Prácticas de alimentación infantil y deterioro de la lactancia materna en México. Salud Publica de México, 55(2), S170–S179. [PubMed] [Google Scholar]
- González de Cosío, T. , Ferré, I. , Mazariegos, M. , Pérez‐Escamilla, R. , & on behalf of BBF Mexico Committee . (2018). Scaling up breastfeeding programs in Mexico: Lessons learned from the becoming breastfeeding friendly initiative. Current Developments in Nutrition 2:nzy018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, D. , & Graff, K. M. (2008). Marketing breastfeeding—Reversing corporate influence on infant feeding practices. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 85(4), 486–504. 10.1007/s11524-008-9279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent, G. (2015). Global infant formula: Monitoring and regulating the impacts to protect human health. International Breastfeeding Journal, 10, 6 10.1186/s13006-014-0020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrilla‐Rodriguez, A. M. , & Gorrín‐Peralta, J. J. (2008). Formula labeling violations to the WHO Code: A quantitative and qualitative analysis. Puerto Rico Health Sciences Journal, 27(1), 49–54. [PubMed] [Google Scholar]
- Piwoz, E. , & Huffman, S. (2015). The impact of marketing of breast‐milk substitutes on WHO‐recommended breastfeeding practices. Food and Nutrition Bulletin, 36(4), 373–386. [DOI] [PubMed] [Google Scholar]
- Pries, A. , Huffman, A. L. , Mengkheang, K. , Kroeun, H. , Champeny, M. , Roberts, M. , & Zehner, E. (2016). Pervasive promotion of breastmilk substitutes in Phnom Penh, Cambodia, and high usage by mothers for infant and young child feeding. Maternal & Child Nutrition, 12(2), 38–51. 10.1111/mcn.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins, N. , Bhandari, N. , Hajeebhoy, N. , Horton, S. , Lutter, C. , Martines, J. , … on behalf of The Lancet Breastfeeding Series Group . (2016). Why invest, and what it will take to improve breastfeeding practices? Lancet, 387, 491–504. [DOI] [PubMed] [Google Scholar]
- Secretaría de Salud (2014). Estratega Nacional de Lactancia Materna 2014‐2018. Secretaría de Salud México. Available at: http://cnegsr.salud.gob.mx/contenidos/descargas/SMP/ENLM_2014-2018.pdf. Accessed on July, 2018.
- Sobela, H. , Iellamoa, A. , Rayab, R. , Padillac, A. , Olivéa, J. M. , & Nyunt‐Ua, N. (2011). Is unimpeded marketing for breast milk substitutes responsible for the decline in breastfeeding in the Philippines? An exploratory survey and focus group analysis. Social Science & Medicine, 73, 1445–1448. [DOI] [PubMed] [Google Scholar]
- Swigart, T. M. , Bonvecchio, A. , Theodore, F. L. , Zamudio‐Haas, S. , Villanueva‐Borbolla, M. A. , & Thrasher, J. F. (2017). Breastfeeding practices, beliefs, and social norms in low‐resource communities in Mexico: Insights for how to improve future promotion strategies. PLoS ONE, 12(7), e0180185 10.1371/journal.pone.0180185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, A. (1998). Violations of the international code of marketing of breast milk substitutes: Prevalence in four countries. British Medical Journal, 316(7138), 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF . (2016). The State of the World's Children 2016. A fair chance of every child. United Nations Children's Fund. New York, USA. June 2016. Available at: https://www.unicef.org/publications/files/UNICEF_SOWC_2016.pdf [Accessed September 2018].
- Victora, C. G. , Bahl, R. , Barros, A. , França, G. , Horton, S. , Krasevec, J. , … for The Lancet Breastfeeding Series Group (2016). Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet, 387, 475–490. [DOI] [PubMed] [Google Scholar]
- World Health Organization . (1981). International Code of Marketing of Breastmilk Substitutes. Geneva: WHO. [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2016). Maternal, infant and young child nutrition. Guidance on ending the inappropriate promotion of foods for infants and young children. Report by the Secretariat. Sixty‐Ninth World Health Assembly. A69/7 Add.1 2016. Available at: http://www.who.int/nutrition/topics/guidance-inappropriate-food-promotion-iyc/en/. [Accessed: Jul. 2017]
- World Health Organization & United Nations Children's Fund . (2016). Marketing of breast‐milk substitutes: National implementation of the international code. Status Report 2016. WHO/NMH/NHD/16.1: Geneva.
- World Health Organization , & United Nations Children's Fund . (2018). Marketing of breast‐milk substitutes: National implementation of the international code, status report 2018. Geneva: World Health Organization. Licence: CC BY‐NC‐SA 3.0 IGO [Google Scholar]
- World Health Organization , United Nations Children's Fund , & NetCode . (2017). Protocol for the assessment and monitoring of “The Code” and relevant national measures. Summary. Available at: http://www.who.int/nutrition/netcode/protocol_summary.pdf?ua=1 [Accessed: Jun. 2017].
- World Health Organization/UNICEF . (2014). Global nutrition targets 2025: Breastfeeding policy brief WHO/NMH/NHD/14.7. Geneva: WHO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Table S1. Sample characteristics of points of sale (POS), health facilities, mothers, and health professionals included by state included in study.
Table S2. Mothers of children younger than 24 months, at public and private health facilities, who received free Breast Milk Substitutes (BMS) sample, recommendations to use BMS, and exposure to publicity in the previous 6 months; health provider contact with BMS manufacturers and Code awareness.1
Data S2. Examination of labels of products sold in Puebla and Chihuahua, Mexico1.


