Abstract
Undernutrition is a major public health concern due to its association with the mortality and disease burden of women and children. This study aimed at identifying the extent and determinants of undernutrition among young pregnant women in Ethiopia. A multivariable regression analysis was fitted to identify determinants of undernutrition and anaemia in a sample of 1,393 pregnant women. Risk ratios (RRs) with 95% confidence interval (CI) were estimated. All the analyses were performed using STATA version 14 and adjusted for clustering. The study revealed that 38% of the women were undernourished and 22% were anaemic. Improved maternal education, RR = 0.94, 95% CI [0.89, 0.98]; higher wealth status, RR = 0.72, 95% CI [0.47, 0.95]; higher minimum dietary diversity for women, RR = 0.87, 95% CI [0.77, 0.98]; increased maternal height, RR = 0.96, 95% CI [0.94, 0.98]; and protected water source, RR = 0.93, 95% CI [0.86, 0.96], have decreased the risk of undernutrition while using unimproved toilet, RR = 1.31, 95% CI [1.06, 1.63], and depression, RR = 1.33, 95% CI [1.14, 1.55], increased the risk of anaemia. Animal source food consumption decreased both the risk of undernutrition, RR = 0.85, 95% CI [0.77, 0.94], and anaemia, RR = 0.91, 95% CI [0.85, 0.95]. The burden of undernutrition is still high. Although improved socio‐economic status and dietary practices decreased the risk of undernutrition, poor health and environmental conditions were still significant risk factors. These findings suggest the need to target this set of important determinants to significantly decrease the burden of undernutrition among young pregnant women.
Keywords: adolescent pregnancy, anaemia, ENGINE, Ethiopia, pregnant women, undernutrition
Key messages.
The burden of undernutrition in young pregnancies is still high in Ethiopia.
Improved socio‐economic and educational statuses shown to protect against undernutrition in young pregnancies implying targeting social determinants through educational and economic empowerment of women may result in positive outcomes.
Optimal dietary practices by dietary diversification and consumption of animal source foods have shown to decrease the risk of undernutrition and anaemia.
Pregnancy at adolescence, poor health, and environmental conditions increased the risk of undernutrition.
Prevention of early marriage and pregnancy, improving access to safe water, and improved toilet facilities can also help prevent undernutrition among young pregnant women.
List of abbreviations
- ENGINE
Empowering New Generation in Nutrition and Economic Opportunities
- MDDW
minimum dietary diversity for women
- MUAC
mid‐upper arm circumference
1. INTRODUCTION
Maternal undernutrition remains an important public health condition in low‐ and middle‐income countries (LMICs; Black et al., 2008). Women of reproductive age, especially pregnant women, in developing countries are recognized to be at risk of poor nutritional status, including multiple micronutrient deficiencies, with a high risk of adverse effects on the mother and pregnancy outcomes (Black et al., 2010). Marriage for girls, before or very shortly after puberty, is common in Africa and South Asia, curtailing their growth process. Nearly two thirds of women in sub‐Saharan Africa and in several countries of South Asia have their first child before the age of 20 years (Rah et al., 2008). Evidence shows that in these settings, chronic undernutrition can also delay physical maturation and extend the adolescent growth period beyond 20 years of age (Chulani & Gordon, 2014). The adverse nutritional status in this group is mainly due to adverse environmental conditions, low socio‐economic status, and poor diets (Black et al., 2008; Chhoun et al., 2016; Lindsay, Gibney, & McAuliffe, 2012). Their poor nutritional practices result in nutrient deficiencies and, subsequently, obstetric and nutritional complications (Lindsay et al., 2012).
The spectrum of nutritional problems among pregnant women in LMICs encompasses the entire range of acute and chronic malnutrition and micronutrient deficiencies (Black et al., 2008; Hossain, 2013; Nguyen et al., 2017). These nutritional problems worsen when the pregnancy is during adolescence. An undernourished woman is more likely to give birth to an undernourished child, causing the cycle of undernutrition to be repeated over generations (Leary, 2005; Prentice et al., 2013; United Nations, 1993). Factors at the individual, household, and community level (or a combination thereof) share responsibility for women's vulnerability to undernutrition throughout their lives (Chhoun et al., 2016; Lindsay et al., 2012; Nguyen et al., 2017). Pregnant women in Ethiopia, like in many other LMICs, suffer from a range of nutritional problems. Despite tremendous efforts being put in place to address this challenge, the burden of maternal undernutrition remains high, as 27% remain chronically malnourished (Central Statistical Agency, Ethiopia ICF International Calverton, Maryland, USA, 2012) and 32% of pregnant women are anaemic (Kassa, Muche, Berhe, & Fekadu, 2017). Understanding the wide range and associated impacts of determinants of undernutrition in pregnant women is thus very important in designing targeted interventions. The present study was aimed at identifying and qualifying the impact of sociodemographic, dietary, and health‐related determinants of undernutrition during pregnancy among young and pregnant women in Ethiopia. Determinants of both undernutrition and anaemia were examined to help identify an important set of determinants to be targeted by interventions, focusing on a range of indicators of nutritional status in young and pregnant women.
2. METHODS
2.1. Study setting, design, and sample
Baseline data from the USAID Empowering New Generation in Nutrition and Economic Opportunities (ENGINE) birth cohort study, conducted from January 2014 to March 2016 in the Oromia region of Ethiopia, were used in the present study. Oromia was selected purposively as the largest region in the country targeted by USAID ENGINE. Three districts, namely, Goma, Woliso, and Tiro Afeta, were further selected from the region based on (a) an expected population of more than 3,000 pregnant women (to account for loss to follow up), (b) geographical similarities in agro‐ecology and agricultural production practices, and (c) proximity and accessibility for data collection. Administratively, each district was further subdivided into units called kebeles, which were the study clusters. All recruitment took place at the kebele level.
Trained data collectors, who were nurses by profession, conducted house‐to‐house active surveillance to recruit the study participants with support from the kebele health extension workers. Upon identification, pregnant women who were in their second and third trimester were invited to enrol in the study after receiving informed consent. From the original cohort, a subset of 1,393 pregnant women who were 15–24 years old at the time of pregnancy, and for whom complete data on mid‐upper arm circumference (MUAC) and anaemia were measured, were included in the present study. There were no significant differences among the young pregnancies (15–24) included in the current study and the rest of the cohort in terms of various sample characteristics including socio‐economic, dietary, and morbidity status.
2.2. Data quality control
Enumerators and supervisors were given adequate training on each component of the data collection tool and on taking all the measurements needed in the study using practical application and role playing. A 3‐day pretesting was also conducted in order to ensure correct administration of questions and taking and recording of measurements among the enumerators before commencing actual data collection. An electronic data collection method was applied using Android handheld tablets to minimize errors in data collection and entry. The collected data were cross‐checked by supervisors on a regular basis before sending to a secure online server. Additionally, a data manager regularly checked completeness and consistency of the online data and referred mistakes and incomplete data to the field to be corrected.
2.3. Measurements
Data on household characteristics, socio‐economic and demographic information, antenatal care exposures, dietary data, and household food security status were collected using a structured, pretested, and interviewer‐administered questionnaire using an Android tablet computer at recruitment.
Maternal height was measured using a stadiometer (Weigh and Measure LCC, USA) to the nearest 0.1 cm, without shoes. In the regression analysis, maternal height was analysed as a continuous variable. MUAC of the pregnant women was measured on the left arm midway between the olecranon and acromion using a nonelastic MUAC tape (SECA 201, Germany; Cogill, 2003). A MUAC cut‐off of less than 23 cm was used to identify undernourished pregnant women (Tang et al., 2016). Individual minimum dietary diversity for women (MDDW) was constructed based on data from a 24‐hr qualitative dietary recall interview and according to the standard guideline (Food and Agriculture Organization [FAO] and FHI 360, 2016). The food groups used to construct the score were starchy staples, beans and peas, seeds and nuts, dark green leafy vegetables, dairy, meat and fish, eggs, other vitamin A‐rich fruits and vegetables, other vegetables, and other fruits. Because all of the vegetables reported to be consumed were dark green leafy vegetables, there was no reported consumption under the food group “other vegetables” making MDDW score ranging from 0 to 9 points, each point representing one food group. Women with MDDW score of <5 were considered to have lower micronutrient adequacy. Animal source food (ASF) consumption was constructed by counting consumption of animal source products (flesh foods, egg, and dairy products), and women who consumed any of these food products were considered a consumer. A wealth index was constructed using principal components analysis based on information on housing conditions, ownership of assets, and availability of basic services. Three factors were identified as explaining 63% of the variations in the variables included in the index. Factor loadings having a value of >0.5 were the basis for selecting individual variables for the final factors used in the analysis. The variables included in the final factors were source of drinking water, toilet facility, electricity, type of floor, type of wall, ownership of durable goods, cropland, and livestock. For analytical purposes, the score was then categorized into tertiles of low, medium, and high (Filmer & Pritchett, 2001). Maternal depression was assessed using Patient Health Questionnaire that was validated among pregnant women in the same region our study was conducted (Woldetensay et al., 2018). The possible scores ranged from 0 to 25, and women with mild to severe depression were all classified as having depression (Kroenke, 2012). The HemoCue Hb 201 DM system was used to measure haemoglobin levels and then anaemia values were adjusted for altitude and trimester using standard methods (Cohen & Haas, 1999). Women with haemoglobin level <11 g dl−1 were labelled as anaemic (Daru et al., 2018; WHO, 2011).
2.4. Conceptual model: Blocks and variables
Variables were grouped into three different blocks based on a proposed conceptual framework for promoting maternal nutrition (Figure 1). The first block included sociodemographic and environmental characteristics (maternal age, parity, maternal education, wealth index, toilet type, and source of drinking water). The second included variables on maternal health status (illness during pregnancy and antenatal depression). The third block focused on nutritional and antenatal exposures (dietary diversity score for women, ASF consumption, iron‐folic acid supplement, maternal height, deworming, and antenatal care follow‐up). The outcome variables were maternal MUAC, categorized as <23 and ≥23 cm, and maternal anaemia, categorized as <11 and ≥11 g dl−1.
Figure 1.
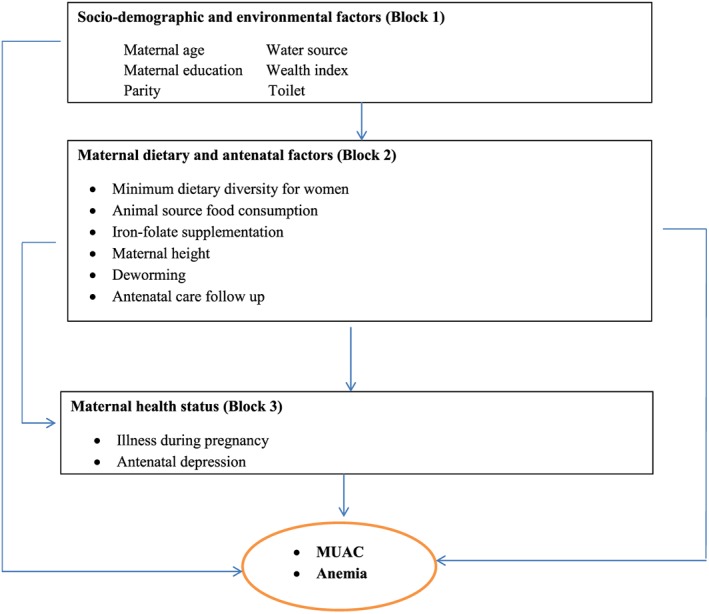
Conceptual framework. MUAC: mid‐upper arm circumference
2.5. Statistical analysis
Exploratory analysis was performed to verify any missing values and outliers before conducting the main analysis. Frequencies, percentages, mean, and standard deviations were used to report descriptive data. The differences in various maternal characteristics across the outcomes measured (MUAC and anaemia) were tested using a chi‐square test. First, a bivariate Poisson regression analysis was performed to estimate risk ratios (RRs) for both of the outcomes. Variables were selected for the next step based on statistical significance at p < 0.25 (Bursac, Gauss, Williams, & Hosmer, 2008) and from a review of relevant literature. Following this, multivariable Poisson regression models were estimated, in which variables from each block were included sequentially using nestreg command on STATA 14 (StataCorp, Texas, USA). This resulted in three regression models (A, B, and C) with a step‐by‐step inclusion of blocks of potential risk factors. The multiple coefficient of determination (R 2) was used to measure the percentage of variance in the outcomes explained by the independent variables for each block. Interaction terms between different covariates were included in the model to test for model improvement. Collinearity among the independent variables in the model was also assessed by measuring their variance inflation factor. All the analyses were performed using robust variance estimators to adjust for subject clustering by study kebele. All tests were two sided, and statistical significance was considered at p < 0.05.
2.6. Ethical issues
Ethical approval was granted from the Institutional Review Board of Jimma University and Tufts University before commencement of the study. Informed consent was obtained from the participants after a detailed explanation of the objectives of the study. During the study, women who had health problems were referred to a nearby health facility to seek proper medical care.
3. RESULTS
The mean (±SD) age of the women was 20 years (±2 years). The study found that 38% of the pregnant women had MUAC of <23 cm, 22% were anaemic, and 12% of them had both conditions. Most of the independent variables were significantly associated to both outcomes as tested using bivariate methods. The descriptive results are presented in Table 1. Consumption of fruits, vegetables, and ASF was found to be much higher among pregnant women with higher MDDW as compared with their counterparts (Figure 2).
Table 1.
Baseline characteristics by nutritional status of pregnant women
| Variables | MUAC < 23 cm n = 1,393 | p value | Anaemia n = 1,393 | p value | |||
|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | ||||
| Maternal age in years | 15–19 | 152 (39) | 237 (61) | 0.208* | 80 (20.6) | 309 (79.4) | 0.479 |
| 20–24 | 379 (38) | 625 (62) | 224 (22.3) | 780 (77.7) | |||
| Educational status | No formal education | 183 (41) | 261 (59) | 0.097** | 115 (26) | 329 (74) | 0.042*** |
| Primary | 309 (38) | 514 (62) | 164 (20) | 659 (80) | |||
| Secondary and above | 39 (31) | 87 (69) | 25 (20) | 101 (80) | |||
| Parity | Primipara | 311 (39) | 485 (61) | 0.399 | 101 (17) | 496 (83) | 0.001*** |
| Multipara | 220 (37) | 377 (63) | 203 (25.5) | 593 (74.5) | |||
| Wealth index | Lowest | 220 (42) | 298 (58) | 0.001*** | 124 (24) | 394 (76) | 0.017*** |
| Middle | 178 (44) | 226 (56) | 98 (24) | 306 (76) | |||
| Highest | 133 (28) | 338 (72) | 82 (17.4) | 389 (82.6) | |||
| Family size | <5 | 448 (38) | 733 (62) | 0.737 | 253 (21.4) | 928 (78.6) | 0.393 |
| ≥5 | 83 (39) | 129 (61) | 51 (24) | 161 (76) | |||
| Toilet type | Improved | 146 (36.5) | 254 (73.5) | 0.705 | 70 (17.5) | 330 (82.5) | 0.043*** |
| Unimproved | 385 (39) | 608 (61) | 234 (23.6) | 754 (76.4) | |||
| Water source | Protected | 341 (36) | 606 (64) | 0.017*** | 202 (21.3) | 745 (78.6) | 0.537 |
| Unprotected | 189 (43) | 254 (57) | 101 (23) | 342 (77) | |||
| MUAC | <23 cm | — | — | 137 (26) | 394 (74) | 0.005*** | |
| ≥23 cm | — | — | 167 (19.3) | 695 (80.7) | |||
| MDDW | <5 | 470 (39) | 745 (61) | 0.138* | 267 (22) | 948 (78) | 0.720 |
| ≥5 | 61 (34) | 117 (66) | 37 (21) | 141 (79) | |||
| Maternal height | Mean (SD) | 156 (5.9) | 158 (5.4) | 0.001*** | 158 (5.7) | 158 (5.6) | 0.753 |
| ASF consumption | Consume | 250 (40.5) | 367 (59.5) | 0.100** | 128 (21) | 489 (79) | 0.385 |
| Not consume | 281 (36) | 495 (64) | 176 (22) | 600 (78) | |||
| Iron‐folic acid supplement | ≤3 months | 369 (38) | 607 (62) | 0.714 | 221 (22.6) | 755 (71.4) | 0.227* |
| >3 months | 162 (39) | 255 (61) | 83 (20) | 334 (80) | |||
| Deworming | Yes | 25 (37) | 43 (63) | 0.814 | 16 (23.5) | 52 (76.5) | 0.727 |
| No | 506 (38) | 819 (62) | 288 (22) | 1,037 (80) | |||
| ANC follow‐up | No | 51 (41.5) | 72 (58.5) | 0.424 | 37 (30) | 86 (70) | 0.020*** |
| At least one | 480 (38) | 790 (62) | 267 (21) | 1,003 (79) | |||
| Any illness | Yes | 103 (43.6) | 133 (56.4) | 0.055** | 50 (21) | 186(79) | 0.975 |
| No | 428 (37) | 729 (63) | 254 (22) | 903(78) | |||
| Depression | Yes | 132 (41) | 189 (59) | 0.207* | 88 (27.4) | 233 (72.6) | 0.006*** |
| No | 399 (37) | 673 (63) | 216 (20) | 856 (80) | |||
Note. ANC: antenatal care; ASF: animal source food; MDDW: minimum dietary diversity for women; MUAC: mid‐upper arm circumference.
Statistical significance at p < 0.25.
Statistical significance at p < 0.10.
Statistical significance at p < 0.05.
Figure 2.
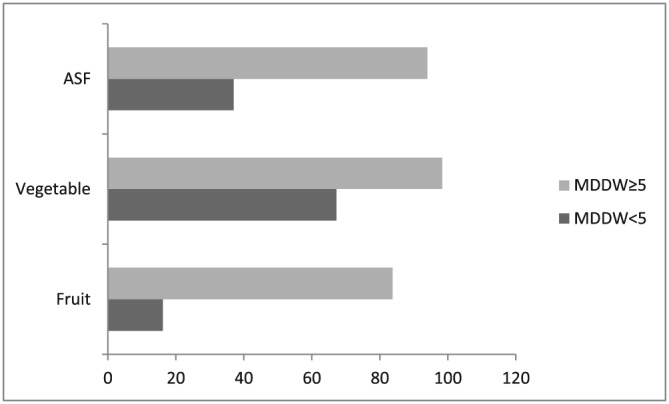
Percentage consumption of fruits, vegetables, and ASF by minimum dietary diversity score for women (MDDW). ASF: animal source food
Tables 2 and 3 show the estimates from the multivariable hierarchical regression analyses after decomposing the variables in the three blocks and three regression models. Model A identified the role of sociodemographic and environmental factors in determining maternal MUAC. Variables in this block explained 15% of the variation in the outcome, with improved maternal educational status, RR = 0.90, 95% CI [0.89, 0.98], and highest wealth tertile, RR = 0.67, 95% CI [0.47, 0.90], significantly protecting against undernutrition. The variables in this block also explained 17% of the variation in maternal anaemia. Younger maternal age (15–19), RR = 1.20, 95% CI [1.07, 1.33], and using an unimproved toilet, RR = 1.41, 95% CI [1.07, 1.86], were significant risk factors for anaemia.
Table 2.
Multivariable hierarchical regression analysis of determinants of undernutrition among young pregnant women in Ethiopia
| Variables |
Model A (Blocks 1) R 2 = 15% RR [95% CI] |
Model B (Blocks 1 & 2) R 2 = 22% RR [95% CI] |
Model C (Blocks 1, 2, & 3) R 2 = 43% RR [95% CI] |
|---|---|---|---|
| Maternal age (15–19) | 1.04 [0.82, 1.31] | 1.03 [0.80, 1.33] | 1.05 [0.78, 1.40] |
| Maternal education | |||
| No formal | Reference | Reference | Reference |
| Primary | 0.98 [0.84, 1.13] | 0.97 [0.84, 1.13] | 0.99 [0.88, 1.12] |
| ≥Secondary | 0.90 [0.89, 0.98]* | 0.90 [0.83, 0.98]* | 0.94 [0.89, 0.98]* |
| Primipara | 0.97 [0.74, 1.27] | 0.98 [0.75, 1.28] | 0.97 [0.70, 1.35] |
| Wealth index | |||
| Lowest | Reference | Reference | Reference |
| Middle | 1.03 [0.83, 1.27] | 1.02 [0.82, 1.27] | 1.03 [0.82, 1.29] |
| Highest | 0.67 [0.47, 0.90]* | 0.67 [0.45, 0.96]* | 0.72 [0.47, 0.95]* |
| Water source (protected) | 0.93 [0.84, 1.02] | 0.92 [0.85, 1.00] | 0.93 [0.86, 0.96]* |
| Any illness (yes) | 1.20 [1.08, 1.33]* | 1.23 [1.09, 1.33]* | |
| Depression (yes) | 1.11 [0.88, 1.39] | 1.17 [0.83, 1.39] | |
| MDDW (≥5) | 0.87 [0.77, 0.98]* | ||
| Maternal height | 0.96 [0.94, 0.98]* | ||
| ASF consume (yes) | 0.82 [0.77, 0.94]* | ||
Note. ASF: animal source food; MDDW: minimum dietary diversity for women; MUAC: mid‐upper arm circumference; RR: risk ratio.
Statistical significance at p < 0.05.
Table 3.
Multivariable hierarchical regression analysis of determinants of maternal anaemia
| Variables |
Model A (Blocks 1) R 2 = 17% RR [95% CI] |
Model B (Blocks 1 & 2) R 2 = 40% RR [95% CI] |
Model C (Blocks 1, 2, & 3) R 2 = 58% RR [95% CI] |
|---|---|---|---|
| Maternal age (15–19) | 1.20 [1.07, 1.33]* | 1.17 [1.08, 1.28]* | 1.14 [1.06, 1.21]* |
| Maternal education | |||
| No formal | Reference | Reference | Reference |
| Primary | 0.86 [0.66, 1.12] | 0.86 [0.66, 1.13] | 0.91 [0.71, 1.16] |
| ≥Secondary | 1.00 [0.70, 1.51] | 1.03 [0.71, 1.52] | 1.15 [0.80, 1.66] |
| Primipara | 0.65 [0.51, 0.81]* | 0.66 [0.52, 0.83]* | 0.65 [0.52, 0.81]* |
| Wealth index | |||
| Lowest | Reference | Reference | Reference |
| Middle | 0.92 [0.74, 1.16] | 0.92 [0.73, 1.16] | 0.98 [0.82, 1.18] |
| Highest | 0.69 [0.39, 1.22] | 0.68 [0.39, 1.19] | 0.79 [0.47, 1.32] |
| Toilet (unimproved) | 1.41 [1.07, 1.86]* | 1.40 [1.06, 1.87] | 1.35 [1.06, 1.63]* |
| Any illness (yes) | 1.00 [0.75, 1.29] | 0.94 [0.77, 1.21] | |
| Depression (yes) | 1.34 [1.09, 1.64]* | 1.36 [1.14, 1.55]* | |
| MUAC (<23 cm) | 1.30 [1.01, 1.57]* | ||
| ASF consume (yes) | 0.91 [0.85, 0.95]* | ||
| Iron‐folic acid supplement (≥3 months) | 0.96 [0.80, 1.15] | ||
| Deworming (yes) | 1.07 [0.66, 1.76] | ||
| ANC follow‐up (≥1) | 0.81 [0.60, 1.08] | ||
Note. ANC: antenatal care; ASF: animal source food; MUAC: mid‐upper arm circumference; RR: risk ratio.
Statistical significance at p < 0.05.
Model B estimates the role of variables from the second block, adjusted for variables from the first block. The variables in this model explained 22% and 40% of the variation in undernutrition and anaemia, respectively. On top of the previous significant determinants, illness during pregnancy, RR = 1.20, 95% CI [1.08, 1.33], and antenatal depression, RR = 1.34, 95% CI [1.09, 1.64], were significant risk factors for undernutrition and maternal anaemia, respectively, after adjusting for sociodemographic and environmental factors.
Model C was constructed by including all variables in the three blocks to estimate the association of dietary practices of the women with their nutritional status, adjusted for the variables from the first two blocks. This model explained 43% and 58% of the variation in undernutrition and maternal anaemia, respectively. Women with higher MDDW had decreased risk of undernutrition, RR = 0.87, 95% CI [0.77, 0.98], and consumption of ASFs significantly decreased the risk of undernutrition, RR = 0.85, 95% CI [0.77, 0.94], and anaemia, RR = 0.91, 95% CI [0.85, 0.95]. It was also shown that a unit increase in maternal height had a protective effect against undernutrition, RR = 0.96, 95% CI [0.94, 0.98].
4. DISCUSSION
This study aimed to identify the burden and determinants of maternal undernutrition among young and pregnant women in Ethiopia. The findings revealed that the level of undernutrition remains high. We also observed that improved maternal educational status, higher wealth status, using a protected water source, and consumption of ASF were protective against the risk of both undernutrition and anaemia whereas higher MDDW and increased maternal stature were protective against undernutrition. On the other hand, young maternal age (15–19 years), illness during pregnancy, unimproved toilet, and depression were risk factors for both of the outcomes in the present study. Improved socio‐economic status has a known association with nutritional status of pregnant women. Its role is linked to its ability to allow women to create a healthy environment for themselves and their family (Ravaoarisoa et al., 2018). Higher educational attainment increases knowledge about healthy antenatal and dietary practices (Ravaoarisoa et al., 2018). Economic disparities, on the other hand, affect the nutritional status of pregnant women, as low economic status limits nutrition and food choices (Chhoun et al., 2016; Mtumwa, Paul, & Vuai, 2016; Nguyen et al., 2017; Ravaoarisoa et al., 2018). As wealth increases, the level of undernutrition among women decreases (Chhoun et al., 2016; Hong & Mishra, 2006). A large survey in Ethiopia found an association between improved economic status and an increased consumption of diversified diets among rural and urban households, as well as a higher consumption of ASF (Workicho et al., 2016). Therefore, empowering women by improving their educational status and income earning can have a positive impact on their nutritional status.
The role of hygiene and sanitation in determining maternal nutritional status has been documented in previous studies (World Health Organization, 2015). The higher risk of malnutrition among pregnant women using unprotected drinking water and an unimproved type of toilet in the present study highlights the importance of hygiene and sanitation during pregnancy. Poor hygiene and sanitation increases the risk of infections including intestinal parasites and diarrheal diseases (Speich, Croll, Fürst, Utzinger, & Keiser, 2016; Strunz et al., 2014; World Health Organization, 2015), causing disturbances in the digestive system that hinder the absorption of essential nutrients, directly resulting in undernutrition. Therefore, improving hygiene and sanitation is one of the most important interventions to combat maternal and child malnutrition in settings where maternal undernutrition is high (Bhutta et al., 2008; World Health Organization, 2015). This study also revealed that poor physical and psychosocial health during pregnancy negatively affected the nutritional status of the young pregnant women. The presence of these conditions during pregnancy increases the risk of undernutrition through affecting dietary intake and absorption of nutrients (Barker, Kirkham, Ng, & Jensen, 2013; Chhoun et al., 2016; Hurley, Caulfield, Sacco, Costigan, & Dipietro, 2005; Nguyen et al., 2017; Teegarden & Bale, 2008). Malaria, tuberculosis, diarrheal diseases, and other infections highly impact nutritional status of pregnant women in impoverished settings (Papathakis & Rollins, 2005; Strunz et al., 2014; Unger, Ashorn, Cates, Dewey, & Rogerson, 2016; World Health Organization, 2015). These disease processes increase energy expenditure and protein catabolism, draining nutritional reserves (Unger et al., 2016). Similarly, depression during pregnancy may also result in maternal undernutrition through engagement in unhealthy behavioural and antenatal practices such as unhealthy eating patterns (Barker et al., 2013; Teegarden & Bale, 2008). In many LMICs, interventions targeted to protect pregnant women from infectious diseases and macronutrient and micronutrient undernutrition remain less integrated. Considering the interaction between these infectious diseases and maternal nutrition through an integrated approach will help substantially address the burden of maternal nutritional problems.
This study has shown that pregnant women consuming a diversified diet and ASFs were at a lesser risk of undernutrition and anaemia. A more diversified diet is associated with adequate levels of calories, fat, protein, and micronutrients, satisfying nutritional requirements and preventing undernutrition among the women (Hoddinott & Yohannes, 2002; National Academy of Science, 1991). In the present study, consumption of fruits, vegetables, and ASF is found to be much higher among pregnant women with higher MDDW than their counterparts. These food groups are known for their rich nutrients, contributing to improving nutritional status in the women. Unfortunately, there are a lot of social, economic, and cultural barriers to consuming ASF in LMICs, especially during pregnancy (Gittelsohn & Vastine, 2003). Despite these barriers, consumption even in relatively small amounts can provide important protein and essential micronutrients that are needed in larger amounts during pregnancy (Darapheak, Takano, Kizuki, Nakamura, & Seino, 2013; Gittelsohn & Vastine, 2003; Speedy, 2003). Programmes working to promote maternal nutrition should consider promoting the consumption of ASFs. This could be done through direct messaging to increase awareness of the importance of ASF and/or through agricultural interventions that increase the availability and accessibility of ASF.
In resource‐poor settings, MUAC is a useful indicator to identify undernutrition when weight and height measurements are not feasible. As with children, its assessment in pregnant women is also simple and can offer an advantage in identifying women at a higher risk of adverse birth outcomes. Despite a wide range of evidence indicating the association between lower MUAC and adverse health and birth outcomes among pregnant women (ref), there has not been an established cut‐off for identifying pregnant women who are undernourished. A recent review suggested a cut‐off of 23 cm could have a higher specificity to identify pregnant women who are at risk of having a low birthweight (Tang et al., 2016), although it is at the expense of its sensitivity. Because the cut‐offs have various degrees of sensitivity and specificity, identifying an optimal MUAC cut‐off is a complex task. It depends on the availability of resources for an intervention, the effectiveness of different interventions, and the degree of expected improvement in the health outcomes. It was suggested that countries and programmes need to conduct a cost–benefit analysis before adopting any specific cut‐off. We used the FAO classification of food groups for generating MDDW (FAO and FHI 360, 2016). We found out that women with higher MDDW had better consumption of fruits, vegetables, and ASF, which are sources of micronutrients implying the link between diversified diet consumption and improved maternal nutritional status. Our findings also substantiated this fact in that those with higher diet diversity and ASF consumption had better MUAC and lower risk of anaemia.
4.1. Implication
Maternal undernutrition, before and during pregnancy, is prevalent in many sub‐Saharan countries. It is a major public health concern due to its association with mortality and overall disease burden for mothers and their children (Black et al., 2008). It is also documented that micronutrient deficiencies during pregnancy result in severe consequences. Anaemia during pregnancy, mainly due to iron deficiency, is one important indicator of maternal malnutrition. Its presence at any level increases the risk of obstetric complications and diminishes productivity (Black et al., 2008; The Manoff Group, 2011). Younger pregnancies are at a greater risk of these adverse health consequences due to competitions for nutrients with their fetus, and the risk of anaemia is much higher as evidenced in the present study. These findings demonstrate the need for improving access to water, hygiene, and sanitation to decrease the level of undernutrition, especially among the very poor and vulnerable rural communities. Although notable efforts have been made in improving access to water, hygiene, and sanitation in Ethiopia (Save the Children/Ethiopia ENGINE Project, 2014), and in decreasing the level of undernutrition (Government of the Federal Democratic Republic of Ethiopia, 2013), the findings indicate that there remains significant work to be done. We can also learn from the findings that the promotion of optimal dietary practices during pregnancy through dietary diversification and consumption of ASF would positively affect the nutritional status of women. Indeed, the revised national nutrition programme in Ethiopia emphasized the promotion of diversified diets as one of the strategies for improving maternal and child undernutrition (Government of the Federal Democratic Republic of Ethiopia, 2013). For this to be effective, it is imperative to work on economic and educational empowerment of women. Recommendations on optimal maternal nutrition behaviours and practices have been promoted in LMICs, although they are not yet as well established as the guidelines for infant and young child nutrition (Huffman et al., 2001). The lesser attention to maternal nutrition may in part reflect a focus on mortality reduction rather than on growth and development. On the other hand, the complexity of the intergenerational aspects of maternal nutrition may also confound the design of interventions. Understanding the important determinants of nutritional status of pregnant women in LMICs, which are for the most part grouped under socio‐economic, health, and dietary exposures, has paramount importance in designing interventions and guidelines for the promotion of optimal maternal nutrition practices. In this regard, we believe that findings from the present study have identified important exposures, which need to be targeted in policies and programmes working towards the improvement of nutritional status of pregnant women in these settings.
This study included a relatively large sample size and explored a range of determinants based on the predefined conceptual framework. The analysis approach we used also helps us understand which set of determinants explain the majority of the variation in both of the outcomes studied so specific recommendations could be made on planning interventions to improve maternal nutritional status. However, the findings of this study should be interpreted with the following limitations in mind. First, we were not able to adjust the anaemia status of the women by presence of inflammation, which could underestimate the burden of the problem. Second, we relied on diet diversity, which is a proxy measure to estimate micronutrient adequacy of the women, due to lack of quantitative data. Maternal height was also measured during pregnancy, which could be affected by the body curvature due to the pregnancy.
5. CONCLUSIONS
Findings from the study indicated that the burden of undernutrition is still high among pregnant women in Ethiopia. We also demonstrated that improved socio‐economic status and dietary practices decrease the risk of undernutrition among pregnant women. On the other hand, young maternal age and poor health and environmental conditions were important risk factors for maternal undernutrition during pregnancy. The findings highlight that a significant proportion of the burden of undernutrition among pregnant women can be decreased by targeting these set of important determinants.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest. The contents of this document are the sole responsibility of the researchers & do not necessarily reflect the views of USAID or the United States Government.
CONTRIBUTIONS
AW conceived and designed the protocol, analysed the data, interpreted the results, and wrote the manuscript. TB, PK, SG, MK, and CL assisted in data analyses, the interpretation of the results, and reviewing the manuscript. All authors have seen and approved the final version of the manuscript.
ACKNOWLEDGMENT
We would like to thank all the data collection team and participants for their time and invaluable work.
Workicho A, Belachew T, Ghosh S, Kershaw M, Lachat C, Kolsteren P. Burden and determinants of undernutrition among young pregnant women in Ethiopia. Matern Child Nutr. 2019;15:e12751 10.1111/mcn.12751
REFERENCES
- Barker, E. D. , Kirkham, N. , Ng, J. , & Jensen, S. K. G. (2013). Prenatal maternal depression symptoms and nutrition, and child cognitive function. British Journal of Psychiatry, 203(06), 417–421. 10.1192/bjp.bp.113.129486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Ahmed, T. , Black, R. E. , Cousens, S. , Dewey, K. , Giugliani, E. , … Sachdev, H. P. S. (2008). What works? Interventions for maternal and child undernutrition and survival. The Lancet, 371(9610), 417–440. 10.1016/S0140-6736(07)61693-6 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Allen, L. H. , Bhutta, Z. A. , Caulfield, L. E. , De Onis, M. , Ezzati, M. , … Group, C. U. S. (2008). Maternal and child undernutrition: Global and regional exposures and health consequences. The Lancet, 371(9608), 243–260. 10.1016/S0140-6736(07)61690-0 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Cousens, S. , Johnson, H. L. , Lawn, J. E. , Rudan, I. , Bassani, D. G. , … Cibulskis, R. (2010). Global, regional, and national causes of child mortality in 2008: A systematic analysis. The Lancet, 375(9730), 1969–1987. 10.1016/S0140-6736(10)60549-1 [DOI] [PubMed] [Google Scholar]
- Bursac, Z. , Gauss, C. H. , Williams, D. K. , & Hosmer, D. W. (2008). Purposeful selection of variables in logistic regression. Source Code for Biology and Medicine, 3(1), 17 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Statistical Agency Addis Ababa, Ethiopia ICF International Calverton, Maryland, USA . (2012). Ethiopia demographic and health survey 2011. Retrieved from https://dhsprogram.com/pubs/pdf/FR255/FR255.pdf
- Chhoun, P. , Khuondyla, P. , Sreymom, O. , Candice, C. , Sovannary, T. , & Siyan, Y. (2016). Social determinants of maternal and child undernutrition in Cambodia: A systematic review. International Journal of Food and Nutritional Science, 3(4), 1–7. 10.15436/2377-0619.16.881 [DOI] [Google Scholar]
- Chulani, V. L. , & Gordon, L. P. (2014). Adolescent growth and development. Primary Care: Clinics in Office Practice, 41(3), 465–487. 10.1016/j.pop.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Cogill, Bruce . (2003). Anthropometric indicators measurement guide. Washington, DC: Food and Nutrition Technical Assistance (FANTA) Project, FHI 360. Retrieved from file:///C:/Users/hp/Zotero/storage/MZUVZR34/anthropometry‐2003‐ENG%20measurment%20guide.pdf [Google Scholar]
- Cohen, J. H. , & Haas, J. D. (1999). Hemoglobin correction factors for estimating the prevalence of iron deficiency anemia in pregnant women residing at high altitudes in Bolivia. Revista Panamericana de Salud Publica, 6(6), 392–399. [DOI] [PubMed] [Google Scholar]
- Darapheak, C. , Takano, T. , Kizuki, M. , Nakamura, K. , & Seino, K. (2013). Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. International Archives of Medicine, 6(1), 29 10.1186/1755-7682-6-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daru, J. , Zamora, J. , Fernández‐Félix, B. M. , Vogel, J. , Oladapo, O. T. , Morisaki, N. , … Khan, K. S. (2018). Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: A multilevel analysis. The Lancet Global Health. 10.1016/S2214-109X(18)30078-0, 6, e548–e554. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization and FHI 360 . (2016). Minimum dietary diversity for women: A guide for measurement. Retrieved from http://www.fao.org/3/a-i5486e.pdf
- Filmer, D. , & Pritchett, L. H. (2001). Estimating wealth effects without expenditure data–or tears: An application to educational enrollments in states of India. Demography, 38(1), 115–132. [DOI] [PubMed] [Google Scholar]
- Gittelsohn, J. , & Vastine, A. E. (2003). Sociocultural and household factors impacting on the selection. Allocation and Consumption of Animal Source Foods: Current Knowledge and Application, 133(11), 4036S–4041S. 10.1093/jn/133.11.4036S [DOI] [PubMed] [Google Scholar]
- Government of the Federal Democratic Republic of Ethiopia . (2013). National nutrition programme. Retrieved from https://www.unicef.org/ethiopia/National_Nutrition_Programme.pdf
- Hoddinott, J. , & Yohannes, Y. (2002). Dietary diversity as a food security indicator. Indicator. [Google Scholar]
- Hong, R. , & Mishra, V. (2006). Effect of wealth inequality on chronic under‐nutrition in Cambodian children. Journal of Health, Population and Nutrition, 89–99. [PubMed] [Google Scholar]
- Hossain, B. (2013). Nutritional status of pregnant women in selected rural and urban area of Bangladesh. Journal of Nutrition & Food Sciences, 03(04). 10.4172/2155-9600.1000219 [DOI] [Google Scholar]
- Huffman, S. L. , Zehner, E. , Harvey, P. , Martin, L. , Piwoz, E. , Ndure, K. , … Quinn, V . (2001). Essential health sector actions to improve maternal nutrition in Africa LINKAGES Project, Academy for Educational Development.
- Hurley, K. M. , Caulfield, L. E. , Sacco, L. M. , Costigan, K. A. , & Dipietro, J. A. (2005). Psychosocial influences in dietary patterns during pregnancy. Journal of the American Dietetic Association, 105(6), 963–966. 10.1016/j.jada.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Kassa, G. M. , Muche, A. A. , Berhe, A. K. , & Fekadu, G. A. (2017). Prevalence and determinants of anemia among pregnant women in Ethiopia: A systematic review and meta‐analysis. BMC Hematology, 17(1), 17 10.1186/s12878-017-0090-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K. (2012). Enhancing the clinical utility of depression screening. Canadian Medical Association Journal, 184(3), 281–282. 10.1503/cmaj.112004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary, S. (2005). Maternal diet in pregnancy and offspring height, sitting height, and leg length. Journal of Epidemiology & Community Health, 59(6), 467–472. 10.1136/jech.2004.029884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, K. L. , Gibney, E. R. , & McAuliffe, F. M. (2012). Maternal nutrition among women from sub‐Saharan Africa, with a focus on Nigeria, and potential implications for pregnancy outcomes among immigrant populations in developed countries: Maternal nutrition among women from sub‐Saharan Africa. Journal of Human Nutrition and Dietetics, 25(6), 534–546. 10.1111/j.1365-277X.2012.01253.x [DOI] [PubMed] [Google Scholar]
- Mtumwa, A. , Paul, E. , & Vuai, S. (2016). Determinants of undernutrition among women of reproductive age in Tanzania mainland. South African Journal of Clinical Nutrition, 29(2), 75–81. 10.1080/16070658.2016.1216509 [DOI] [Google Scholar]
- National Academy of Science (1991). Nutrition during lactation. Washington, D.C.: National Academies Press; 10.17226/1577 [DOI] [Google Scholar]
- Nguyen, P. H. , Sanghvi, T. , Kim, S. S. , Tran, L. M. , Afsana, K. , Mahmud, Z. , … Menon, P. (2017). Factors influencing maternal nutrition practices in a large scale maternal, newborn and child health program in Bangladesh. PLoS One, 12(7), e0179873 10.1371/journal.pone.0179873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathakis, P. , & Rollins, N. (2005). HIV and nutrition: Pregnant and lactating women. THIS PUBLICATION.
- Prentice, A. M. , Ward, K. A. , Goldberg, G. R. , Jarjou, L. M. , Moore, S. E. , Fulford, A. J. , & Prentice, A. (2013). Critical windows for nutritional interventions against stunting. The American Journal of Clinical Nutrition, 97(5), 911–918. 10.3945/ajcn.112.052332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rah, J. H. , Christian, P. , Shamim, A. A. , Arju, U. T. , Labrique, A. B. , & Rashid, M. (2008). Pregnancy and lactation hinder growth and nutritional status of adolescent girls in rural Bangladesh. The Journal of Nutrition, 138(8), 1505–1511. 10.1093/jn/138.8.1505 [DOI] [PubMed] [Google Scholar]
- Ravaoarisoa, L. , Randriamanantsaina, L. , Rakotonirina, J. , Rakotomanga, J. d. D. M. , Donnen, P. , & Dramaix, M. W. (2018). Socioeconomic determinants of malnutrition among mothers in the Amoron'i Mania region of Madagascar: A cross‐sectional study. BMC Nutrition, 4(1). 10.1186/s40795-018-0212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save the Children/Ethiopia ENGINE Project . (2014). Water, hygiene and sanitation (WASH) in rural households in AMHARA, OROMIA, SNNP AND TIGRAY. Retrieved from https://ethiopia.savethechildren.net/sites/ethiopia.savethechildren.net/files/WASH%20Lo%20Res.pdf
- Speedy, A. W. (2003). Global production and consumption of animal source foods. The Journal of Nutrition, 133(11), 4048S–4053S. 10.1093/jn/133.11.4048S [DOI] [PubMed] [Google Scholar]
- Speich, B. , Croll, D. , Fürst, T. , Utzinger, J. , & Keiser, J. (2016). Effect of sanitation and water treatment on intestinal protozoa infection: A systematic review and meta‐analysis. The Lancet Infectious Diseases, 16(1), 87–99. 10.1016/S1473-3099(15)00349-7 [DOI] [PubMed] [Google Scholar]
- Strunz, E. C. , Addiss, D. G. , Stocks, M. E. , Ogden, S. , Utzinger, J. , & Freeman, M. C. (2014). Water, sanitation, hygiene, and soil‐transmitted helminth infection: A systematic review and meta‐analysis. PLoS Medicine, 11(3), e1001620 10.1371/journal.pmed.1001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, A. M. , Chung, M. , Dong, K. , Terrin, N. , Edmonds, A. , Assefa, N. , … Maalouf‐Manasseh, Z. (2016). Determining a global mid‐upper arm circumference cutoff to assess malnutrition in pregnant women. Retrieved from https://www.fantaproject.org/sites/default/files/resources/FANTA-MUAC-cutoffs-pregnant-women-June2016.pdf
- Teegarden, S. L. , & Bale, T. L. (2008). Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiology & Behavior, 93(4–5), 713–723. 10.1016/j.physbeh.2007.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Manoff Group . (2011). Guidance for formative research on maternal nutrition. Retrieved from http://www.manoffgroup.com/GuidanceforFormativeResearchonMaternalNutrition.pdf.pdf
- Unger, H. W. , Ashorn, P. , Cates, J. E. , Dewey, K. G. , & Rogerson, S. J. (2016). Undernutrition and malaria in pregnancy—A dangerous dyad? BMC Medicine, 14(1), 142 10.1186/s12916-016-0695-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations . (1993). Maternal and child nutrition‐SCN news, Number 11. Retrieved from https://www.unscn.org/layout/modules/resources/files/scnnews11.pdf
- WHO . (2011). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Retrieved from file:///C:/users/hp/desktop/4th%20paper%20references/WHO%20haemoglobin%20cutt%20off.Pdf
- Woldetensay, Y. K. , Belachew, T. , Tesfaye, M. , Spielman, K. , Biesalski, H. K. , Kantelhardt, E. J. , & Scherbaum, V. (2018). Validation of the Patient Health Questionnaire (PHQ‐9) as a screening tool for depression in pregnant women: Afaan Oromo version. PLoS One, 13(2), e0191782 10.1371/journal.pone.0191782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workicho, A. , Belachew, T. , Feyissa, G. T. , Wondafrash, B. , Lachat, C. , Verstraeten, R. , & Kolsteren, P. (2016). Household dietary diversity and animal source food consumption in Ethiopia: Evidence from the 2011 Welfare Monitoring Survey. BMC Public Health, 16(1), 1192 10.1186/s12889-016-3861-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2015). Improving nutrition outcomes with better water, sanitation and hygiene: Practical solutions for policies and programmes.


