Abstract
Severe and moderate acute malnutrition are among the leading causes of mortality among children in low‐ and middle‐income countries. There is strong evidence that growth assessed anthropometrically from conception to 2 years of age marks later risk of ill health. This is central to the concept of the developmental origins of adult disease and is presumed to be related to modification of developmental processes during critical “window(s)” of vulnerability. Interventions to treat acute malnutrition have resulted in dramatic increase in the number of affected children surviving. Ensuring that these children thrive to fulfil their full physical and cognitive potential is a new challenge. Integral to this challenge is the need to be able to measure how earlier insults relate to the ability to survive and thrive to productive adulthood. Despite its obvious value, routine anthropometry does not adequately indicate how earlier adverse exposures affect more refined aspects of growth. Anthropometry is inadequate for predicting how disruption of healthy growth might modulate risk of disease or any subsequent interventions that correct this risk. A clear characterisation of healthy child growth is needed for determining which component best predicts later outcomes. The extent to which postnatal acute malnutrition is a consequence of maternal factors acting preconception or in utero and their relationship to postnatal health and long‐term risk of non‐communicable diseases is not clear. Body‐composition measurement has significant untapped potential allowing us to translate and better understand the relationship between early insults and interventions on early growth in the short‐term and long‐term health outcomes.
Keywords: acute malnutrition, assessment of nutritional status, body composition, chronic disease, DoHaD, growth
Key messages.
The extent to which postnatal acute malnutrition reflects maternal risk factors operating preconception or in utero and their relationship to long‐term disease risk needs to be determined.
Currently used anthropometric indicators do not adequately reflect how early adverse exposures affect short‐term health and are poor at predicting how disruptions and interventions to correct it affects later disease risk.
Body composition represents the convergence of preconceptional, in utero, postnatal and childhood insults, growth, and long‐term disease risks.
Measurement of body composition using valid and reliable techniques will improve understanding of how early life insults interact with.
1. INTRODUCTION
Nutrition is rising to the top of the global policy agenda. Malnutrition is associated with impaired cognitive development, education performance, and adult productivity, undermining the development of nations (Baye, 2017; Black et al., 2013; Global Nutrition Report, 2017). During infancy and early childhood, inappropriate feeding practices, nutrient deficiencies, frequent infections, and intestinal dysfunction are among the many factors resulting in deficits of the three major anthropometric indicators of growth: wasting, underweight, and stunting among children in low‐ and middle‐income countries (LMICs; World Health Organization and United Nations Children's Fund, 2003). Stunting and underweight are associated with increased morbidity and mortality and impaired child development (World Health Organization, 2017).
In 2017, 151 million children under 5 years old were stunted, defined as low height‐for‐age. Fifty‐one million children under 5 years old were wasted, defined as low weight‐for‐length (WLZ) in children under 2 years old and as low weight‐for‐height (WHZ) in older children. The presence of bilateral oedema is used as an additional criterion to either WLZ or WHZ in identifying children with severe wasting which significantly increases the risk of mortality (World Health Organization, 2017). Of the wasted children, 16.4 million were severely wasted (United Nations Children's Fund, World Health Organization, World Bank Group, 2018). This is a conservative estimate of those with severe acute malnutrition (SAM) because the full case definition of SAM includes not just wasting but those with oedematous malnutrition (kwashiorkor), as well as those with low mid‐upper arm circumference (MUAC) (Briend et al., 2016; Frison, Checchi, & Kerac, 2015). Moderate acute malnutrition (MAM) and SAM (defined by low WHZ/WLZ and/or low MUAC) are among the leading causes of mortality in children in LMICs (Collins, 2007; Lenters, Wazny, & Bhutta, 2016; World Health Organization, 2016), predominantly by potentiating the effects of common infectious diseases. At the same time 38 million children under 5 years old were overweight in 2017 (United Nations Children's Fund, World Health Organization, World Bank Group, 2018). Childhood overweight and obesity have increased 10‐fold in 40 years from 1975 to 2016 (NCD Risk Factor Collaboration, 2017).
Emerging evidence indicates undernutrition as a leading cause of death in children age 5 to 14 years old. For example, between 2008 and 2013, acute undernutrition contributed to 34% of deaths in this age group in Ethiopia (Dedefo et al., 2016). Among adults, non‐communicable diseases (NDCs) are emerging as public health problem in LMIC. It is estimated that in 2010 they accounted for half of disability‐adjusted life years (DALYs) lost and for 58% of all deaths in these countries and that 70% of all deaths will be caused by NDCs in these countries (World Health Organization, 2010a, 2010b). The associated economic loss is estimated to be US$ 7 trillion in the period 2011–2025 (World Health Organization, 2014). There is strong evidence that growth from conception to 2 years old reflects the effects of adverse exposures during a critical “window,” which also drive later risk of ill‐health. This complex chain of events is central to the concept of the developmental origins of adult disease (Cameron & Demerath, 2002). Routine postnatal growth monitoring using World Health Organization (WHO) reference growth curves is recommended practice worldwide (World Health Organization, 2017). In the short‐term “growth trajectory” for weight‐for‐age is considered to indicate health and likelihood of attaining full human potential (Ashworth, Shrimpton, & Jamil, 2008). However, height is not normally assessed in routine growth monitoring in LMIC (World Health Organization, 2017).
A big step forward in nutrition over the last two decades has involved expanded programme efforts in the treatment of acute malnutrition. The use of ready‐to‐use therapeutic foods (RUTFs) in the context of community‐based management of severe acute malnutrition (CMAM) has resulted in dramatic increase in the number of affected children being treated and thus surviving. Interventions targeting mothers during gestation such as iron/folic acid, multiple micronutrient supplements (MMN), and MMN‐containing lipid‐based nutritional supplements (LNS) have had positive effect on in utero foetal development (Hambidge & Krebs, 2018) and are likely to translate to better postnatal child survival. Enabling these children to thrive and fulfil their full physical and cognitive potential is the new challenge. An associated challenge is how to measure the full extent of any early nutritional/health/environmental insults and link those with future potential to not just survive but to also thrive. WZHand MUAC are the main indicators used to admit and discharge children from SAM treatment, whereas body mass index (BMI) is used as a screening tool for obesity (World Health Organisation, 2017). However, these anthropometric indicators do not fully reflect the impact of early adverse exposures on health and, much more importantly, are poor at predicting how disruptions and interventions to correct it affects later risk of health and disease. The following questions remain to be addressed.
First, how can healthy child growth be best characterised and defined, what are its components and which of these components best predicts what will happen later? This relates to the question on which anthropometric measures should be used in primary health care to assess nutritional status in light of the double burden of malnutrition (World Health Organisation, 2017). Second, to what extent is SAM/MAM a consequence of maternal risk factors operating before birth either preconception or in utero? Third, to what extent does an episode of SAM/MAM and its treatment modulate the long‐term risk of non‐communicable diseases and which pathways are involved? Fourth, can body‐composition measurement enable more effective translation of a better understanding of the relationships between early insults and interventions on early growth in the short‐term and long‐term health outcomes? Specifically, what is the body composition pre‐SAM and in the stunted‐obese or stunted‐wasted child? A better understanding of body composition at birth, in early life, and during and after the months and years following treatment for SAM and MAM potentially carries the key to predicting longer term risks and improved assessment of the effectiveness of early interventions.
It is desirable that we have the ability to cocurrently identify children at risk of acute malnutrition on the one hand and those at risk of overweight and obesity on the other as per the updated Integrated Management of Childhood Illnesses (IMCI) guideline (World Health Organisation, 2017). Is there single “stone that we may use to kill two birds” at the once? In this review we posit that although routine anthropometry (weight, height, and MUAC) and associated derived indicators such as BMI are useful for programmatic purposes, body composition may be the “Rosetta Stone” (Schoville, 2001) that allows us to translate the relationship between early growth and long‐term health outcomes. (Figure 1; Wootton & Jackson, 1996).
Figure 1.
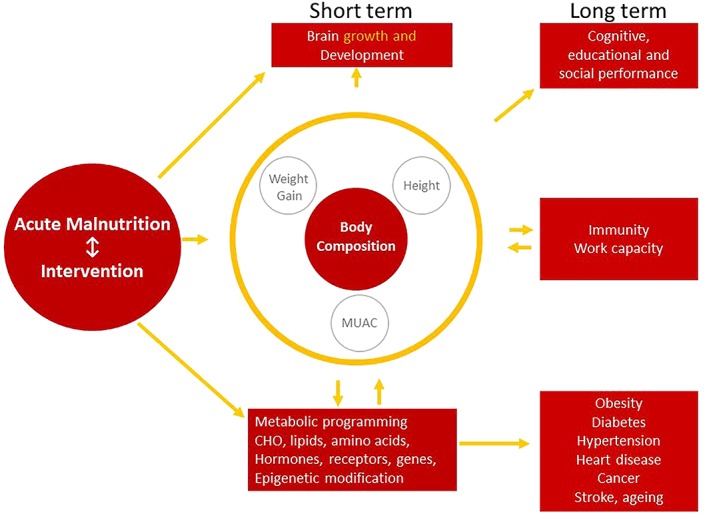
Conceptual framework of pathways underpinning the relationship between early nutritional insults and later outcomes including risk for metabolic dysfunction. Adapted from Jackson, Langley‐Evans, & McCarthy, 1996 and Wootton & Jackson, 1996
1.1. The nature of growth in childhood
Good health during childhood consists of timely development and maturation of physiological, physical, neurocognitive, emotional, and social functions (Cameron, 2008). The achievement of health at all ages is characterised by an ability to cope with environmental challenges, which include physical, chemical, microbiological, behavioural, emotional, and social insults that also determine the timing, magnitude, and duration of growth (Cameron, 2007; Cameron, 2008; Hochberg, 2011). When the sum total of the challenges (allostatic load) exceeds the ability of the individual to cope (allostatic capacity), ill health or pathology supervenes and becomes increasing evident as clinical disease including greater non‐communicable disease (NCD) risk (Wells, 2018). For example, stunting and wasting in early childhood may deplete metabolic capacity thereby increasing the risk of NCDs (Ferraro & Bechere, 2013; Jackson et al., 1996; Wells, 2018; Wootton & Jackson, 1996). Growth is a regulated process by which the organism increases in mass, size, and physical and metabolic complexity. During growth, there are substantial changes in the distribution, architecture and relative masses of body tissues. The pattern of growth and development between conception and adulthood is absolutely dependent on adequate provision of energy and nutrients interacting with genetic, hormonal, and environmental influences (Hochberg, 2011; Jackson, 1990; Jackson & Wootton, 1989; Tapsell et al., 2016).
Thus, normal growth is an ordered process, but its progressive nature means that all later stages are built on the amount and quality of growth in earlier stages. This cumulative process leads to the structural and functional phenotype of the adult, thereby giving a life‐course reflection of healthy lifetime opportunities (Hochberg, 2011; Wootton & Jackson, 1996). The processes of normal growth can be disrupted at any of the four stages (foetal, infancy, childhood, and adolescence) by inadequate nutrition and/or environmental challenge, potentially limiting opportunities for health at all later ages (Gluckman et al., 2005; Prado & Dewey, 2014). High growth and developmental velocity during foetal and early postnatal life (before the age of about 2 years old) implies that restriction of nutrient supply and other insults will have greatest effect when experienced at these stages of life (Hochberg, 2011; Prado & Dewey, 2014; Victora, de Onis, Hallal, Blossner, & Shrimpton, 2010).
Inadequate intake of energy and nutrients during development can result in irreversible alteration of organ and tissue architecture and function (Jackson et al., 1996; Wootton & Jackson, 1996). The consequence of such insults is “programming” of an individual's phenotype, evident as alteration of body structure, composition, and metabolic function resulting in heightened risk for non‐communicable diseases in adulthood (Ross & Beall, 2008).
Experimental studies in animals have demonstrated the existence of “critical periods” (Cameron & Demerath, 2002) in early development during which alteration of nutrient supply may alter structure and function irreversibly. This phenomenon has been labelled “nutritional programming” (Langley‐Evans, 2015; Vaiserman, 2014). A recent review demonstrated how events in the periconceptional period may contribute to developmental programming (Fleming et al., 2018). It offers time‐limited opportunities for intervention, which may reduce the risk of NCDs in later life.
Upon provision of adequate nutritional intake following growth restriction, there is a tendency for growth to be accelerated (Jackson, 1990; Jackson & Wootton, 1989). This is known as “catch‐up” or “compensatory” growth (Adair, 1999; Zhang et al., 2016). There is some evidence that this phenomenon is positively correlated with maternal height (Desmond & Casale, 2017) and the intrauterine‐growth environment (Cho & Suh, 2016; Dunlop, Cedrone, Staples, & Regnault, 2015; Morrison, Duffield, Muhlhausler, Gentili, & McMillen, 2010). Adolescent catch up growth has also been described and is thought to represent previous insults (Prentice et al., 2013). Catch‐up growth may also occur due to improvements in living and psychological conditions, freedom from infections, and via nutrient supplementation (Martorell, Khan, & Schroeder, 1994). During this catch‐up growth, the normal proportionate distribution and relative weight of tissues and organ systems may be disrupted. Consequent alterations in body composition may be mirrored in altered metabolic function and chronic‐disease risk (Cho & Suh, 2016). The tendency for “catch‐up” to occur makes independent effects of prenatal and postnatal nutrient supply on chronic disease risk hard to separate. Postnatal growth occurs in three phases of infancy, childhood, and adolescence, which are easily influenced by environmental factors (Cameron, 2007; Prentice et al., 2013) in ways that may result in individual patterns deviating from generally acceptable patterns.
Removal of the risk of environment hazards by ensuring high standards of hygiene and sanitation is crucial, but alone may not influence growth positively, as demonstrated by the results of one (Arnold, Null, Luby, & Colford, 2018; Null et al., 2018) of the largest randomised studies addressing this theme. Environmental challenges embrace a range of stresses or stressors, notably those associated with infection, neglect or the range of challenges imposed by poverty or social disruption. A good example is the environmental enteric dysfunction (EED) phenomenon that is believed to affect linear growth in yet to be defined ways (Keusch et al., 2013; Owino et al., 2016). Environmental hazards with endocrine disrupting properties and microbial toxins such as mycotoxins, are also thought to be involved in foetal programming and postnatal growth disruption (Gong, Watson, & Routledge, 2016; Owino, Cornelius, & Loechl, 2018; Zheng et al., 2016). The effects of stressors on growth are determined by their timing, character, and the duration of the adverse experiences. Milder insults of relatively short duration are potentially reversible (Lundeen, Behrman, Crookston, & Dearden, 2014; Martorell et al., 1994; Singh, Ashish, & Kumar, 2017), but more severe insults of longer duration or insufficient recovery time can have lasting impact. The specific effect of any particular stressor on the body will be determined by the timing of the insult in relation to the timing of development of particular organs, tissues, or function. Individual organs are most vulnerable to insults during their period of most rapid differentiation and growth, and the effects may be irreversible. Within this complexity of developmental changes, any disruptions to normal growth in either the shorter or longer term are most simply marked by inadequate growth in height, weight, MUAC, or abnormalities in their relative proportions, yet these are relatively indirect measures of specific tissues and processes.
1.2. Interventions to address acute malnutrition
Since the millennium, there have been extensive programmatic efforts to address acute malnutrition in many countries. These programmes often neglect the fact that many acutely malnourished children are concurrently stunted, with wasting increasing the risk of stunting and vice versa—the exact relationship being complex and likely setting specific (Angood, 2014; Briend, Khara, & Dolan, 2015). Standardisation of treatment protocols including development of specific therapeutic products and scale up of the integrated management of acute malnutrition (IMAM) approach has resulted in a significant reduction in SAM case fatality whilst allowing rapid scale up and significant increase in programme and geographical coverage. The high short‐term benefit of the treatment has been confirmed by reduced postdischarge mortality among those who completed the treatment when compared with those who defaulted (Bahwere, Mtimuni, Sadler, Banda, & Collins, 2012). Although, results on relapse rates are contrasting, several studies have shown that relapse rates after an episode of SAM can be low in some settings (Bahwere et al., 2008; Burza et al., 2016; Khanum, Ashworth, & Huttly, 1998; Pecoul, Soutif, Hounkpevi, & Ducos, 1992; Somasse, Dramaix, Bahwere, & Donnen, 2015). There is a strong body of epidemiological evidence that the cooccurrence of undernutrition and infectious diseases increases the risk of death in children suffering from common childhood infectious diseases such as diarrhoea and pneumonia (Jones & Berkley, 2014; Rodriguez, Cervantes, & Ortiz, 2011; World Health Organization, 2010b). Thus, treating acute malnutrition contributes to preventing death from these diseases. In contrast to short‐term benefits, the medium‐ and long‐term benefits of treating acute undernutrition are less well described and less well understood. The main reasons for this are that longer term patient follow‐up is often difficult in the resource‐poor settings where SAM and MAM are common and until recently the main focus of nutrition programmes has been on short term survival. This is however changing, with longer term perspectives becoming increasingly important.
Worldwide reduction in under 5 years old child mortality has made it important for the global community moves beyond the “survive” agenda alone and enables children to also “thrive,” fulfilling their full physical and cognitive potential. For this to happen, systems and approaches to problems must be “transformed,” hence the “Survive, Thrive, and Transform” call of the Global Strategy for Women's, Children's and Adolescent's Health (2016–2030) (World Health Organization, 2015). There is growing interest in how the Developmental Origins of Health and Disease (DOHaD) theory (Barker, 1995; Barker, 2007) applies to acute malnutrition in early childhood. Most focus however has been on in utero exposures to malnutrition, with clear evidence arising that timing of insults and rate of subsequent catch‐up growth are key determinants of long‐term effects. Given the severity of an episode of acute malnutrition, it is plausible that this too would have an independent effect on long‐term risk—but data showing this is limited. In particular, it has not yet been possible to distinguish to what extent SAM/MAM and treatment interventions are on the causal pathway to long‐term NCD‐related risk and to what extent they reflect and are symptoms of earlier in utero and maternal risk factors. For example, one recent study followed up children 7 years old post‐SAM and found both functional and structural impairments including reduced linear growth and a body‐composition profile (waist‐hip ratio) consistent with future NCD risk. Critically, however, this study did not have details of children's birth weights (nor data on whether or not they were preterm at birth) or their detailed clinical/growth histories prior to the episode of SAM (Lelijveld et al., 2016). It did however document the fact that most children were concurrently stunted and that there was some catch‐up of height after the age of 2 years old since the original episode of SAM in some of the survivors.
It is important to understand the limits of the window of plasticity under DOHaD and the factors that most strongly influence long‐term outcomes. In practice, current treatment and prevention programmes focus on short‐term cure. As understanding of long‐term outcomes improves, the details of treatments may change (e.g., composition of therapeutic/supplementary foods and target weight gains in programme). It is also possible that posttreatment interventions may develop as a way of reducing adverse long‐term outcomes (e.g., social support or longer term antibiotic prophylaxis). Lastly, policy makers and funders seeking to address the global epidemic of adult NCDs should be aware that because the major antecedents are in childhood, appropriate investment in child health is a key way to tackle adult problems.
During the treatment of SAM and MAM, the primary objective of treatment is correction of specific nutrient deficits and is measured by correction of deficits in weight. Over the longer term, improvement in wider functional deficits is a preferred objective, which may be better indicated by the correction of height deficits. There is variation in the response of individual children to different aspects of the correction of weight deficits, height deficits, or functional deficits, which may be determined by a range of factors related to prior experience and wider environmental constraints. Enabling correction of height deficits may be achievable in contexts where the wider environmental stressors have been adequately addressed, but this remains to be determined in clinical trials. The outstanding question is whether improving lean mass without excessive deposition of fat mass during treatment of SAM and MAM will lead ultimately to improved functional capability and increased resilience to environmental challenge, thereby mitigating vulnerability to NCD in later life.
1.3. Body composition in relation to growth and long‐term health
Body size, shape, and composition affect health and disease risk (Baumgartner, Heymsfield, & Roche, 1995). At attended delivery, birth weight, length, and head circumference might be measured as major indicators of pregnancy outcome and growth during infancy and childhood, comparing measurements against the recently launched WHO Growth Standards (World Health Organization, 2007). Body composition connotes the components that comprise an individual's body weight such as water, lean, and fat mass (Jackson, 1990; Wells & Fewtrell, 2006). Most commonly, a two‐compartment model is used that assumes that the body is composed of fat and fat‐free mass. Body composition is influenced by many factors including age, sex, race, genes, and dietary and lifestyle behaviours (Baumgartner et al., 1995). Appropriate balance between fat mas and fat‐free mass is necessary for good health but changes with age. Excess body fat is associated with physiological changes that can lead to non‐communicable diseases, such as diabetes, cardiovascular disease (CVD), and some cancers. Abnormal regional distribution of body fat, especially visceral and liver fat accumulation, is linked to increased risk of CVD (Després, 2012; Wells & Fewtrell, 2006). On the other hand, inadequate food intake or increased demand for certain amino acids caused by chronic inflammation can result in muscle wasting and ultimately death (International Atomic Energy Agency, 2009; Owino, Slater, & Loechl, 2017).
Birth height and weight, childhood growth and adiposity, adult attained height, and adult obesity have been linked to increased risk of developing NCD (Li et al., 2015), such as cardiovascular disease (Berenson, Srinivasan, Xu, & Chen, 2016; Ohlsson et al., 2017), nonalcoholic fatty‐liver disease (Ayonrinde et al., 2017; Yan et al., 2017), some cancers (Brown et al., 2018; Hendriks et al., 2018), chronic kidney disease (Eriksson et al., 2018), and dementia (Russ, Kivimäki, Starr, Stamatakis, & Batty, 2014). The double burden of malnutrition where stunting, wasting, and micronutrient deficiencies occur alongside overweight and obesity seems to drive the risk of NCD in ways that are yet to be fully comprehended.
Structural measures of growth such as weight and height do not fully reflect how early adverse exposures affect health and, much more importantly, are poor at predicting how any disruption and any intervention to correct it affects later risk of disease. It is also apparent that body composition seems to be the convergence point between what happens early in life and both short‐ and long‐term metabolic response (Jackson et al., 1996; Wootton & Jackson, 1996). Figure 1 presents the pathways underpinning the relationship between early nutritional insults and later outcomes including risk for metabolic dysfunction.
Intrauterine exposures that influence growth also act through body composition. For example, it has been shown that cord blood adiponectin affects infant size via altered adiposity in African–American children (Schneider, Catalano, Biggio, Gower, & Chandler‐Laney, 2018). Cord‐blood leptin has been linked to smaller birth size and rapid weight gain in the first 6 months of life, whereas cord‐blood adiponectin directly predicted increased central adiposity at 3 years old (Mantzoros et al., 2009). Fat‐free mass and not fat‐mass accretion in infancy is related to linear growth in childhood according to a study from Ethiopia (Admassu et al., 2018). A study from Burkina Faso showed that children treated for MAM using LNS put on more lean mass than fat mass postdischarge (Fabiansen et al., 2017), contrary to the wide‐held concern that weight gain in such interventions is mainly due to increased body fat. Short children in the same study had slower gain of both fat‐free mas and fat mass (Fabiansen et al., 2018). More research is needed to build stronger evidence in this area. Body composition and not body weight has been found to correlate to CVD risk (Segal et al., 1987). Body fat has been shown to be positively correlated with CVD and metabolic syndrome risks in both women and men (Chuang et al., 2012).
The validity of anthropometric measures of body composition has been questioned (Brambilla et al., 2000; Henriksson et al., 2017; Jensen et al., 2015). Yet, over the decades, BMI has been used as the main indicator to estimate unhealthily low (or high) body fat. This is because BMI is a quick, field applicable, and useful screening tool (Buss, 2014) for identifying high risk individuals. However, a major limitation is that BMI represents inadequate or excess weight for height and does not distinguish between fat and fat‐free mass. The relationship between BMI and body fat is also affected by factors such as hydration, maturation, and ethnicity, making BMI a poor indicator of adiposity. It has been demonstrated that people of Asian ethnicities have lower BMI but higher body fat than Caucasians of European descent do (Wang et al., 1994). The accuracy of BMI to predict body fat has also been found to be lower compared with densitometry (Okorodudu et al., 2010).
Infants of similar birth weight, weight, height, or even weight for height can vary substantially in body composition. A study from Ethiopia found that newborn fat mass was determined by sex and birth order and that fat mass was positively associated with birth weight in both boys and girls (Andersen et al., 2011). For example, babies born to optimally healthy mothers in rural India have been characterised as having the thin‐fat phenotype because although small and thin at birth, they have proportionally more body fat and centrally deposited fat than European newborns (Yajnik, 2003). This difference in body composition phenotype appears to be related to birth size and marks a fundamental metabolic difference and greater risk of later non‐communicable diseases during adult life (Kensara et al., 2006). These observations emphasise the importance of more detailed characterisation of body structure and composition to identify high risk of poor health at an early age (Corvalan, Kain, Weisstaub, & Uauy, 2009). Accurate, sensitive, simple, and noninvasive techniques of body composition are needed to fully understand the effect of interventions. Techniques to assess body composition include anthropometry, densitometry (air displacement plethysmography and underwater weighing), dual‐energy X‐ray absorptiometry, isotope dilution, and bioelectrical impedance analysis (International Atomic Energy Agency, 2009; Most, Marlatt, Altazan, & Redman, 2018). Body composition techniques rely on assumptions and each technique has its strengths and weakness in terms of accuracy, precision, and applicability to populations and settings. Available literature suggest that, compared with the gold standard four‐compartment model, isotope dilution using deuterium oxide is more accurate compared with other single techniques in a variety of paediatric populations (Bray, DeLany, Volaufova, Harsha, & Champagne, 2002; Ramírez, Valencia, Moya‐Camarena, Alemán‐Mateo, & Méndez, 2009; Vásquez et al., 2012; Silva et al., 2013; Martinez et al., 2017). The current review is limited to raising the question on why it is important to consider body composition as central indicator of exposure to and recovery from acute malnutrition. Given the rapid technological advancement in this area in recent years, a separate comprehensive review updating on the various body‐composition measurement techniques, their applicability, limitations, and feasibility for use, especially in malnourished children and pregnant and lactating mothers is recommended.
2. CONCLUSION
Acute malnutrition is likely to remain pervasive in LMICs for the foreseeable future, and the intensity of interventions to address it will increase towards achieving global goals. Child growth will continue to be the key benchmark for evaluation of progress. The urgency for more accurate ways to measure healthy growth and to better understand the longer‐term outcomes of insults and interventions cannot be belaboured, we need to look beyond growth to the composition of the body. Better understanding of body composition in the months and years following treatment for SAM and MAM is key to predicting longer term risks of adult disease. Measuring body composition should be based on valid, reliable, and noninvasive approaches. An update of different techniques to measure body composition is urgently needed.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
VO conceived the idea and drafted the manuscript. All authors contributed to discussions and to extensive literature review towards this manuscript during the Consultants Meeting on Growth in Children Recovering from Acute Undernutrition and Risk for Metabolic Dysfunction funded and hosted by the International Atomic Energy Agency in Vienna, Austria, from 5 to 8 December 2016. All authors contributed to writing of the manuscript, reviewed, and approved the final draft before submission.
ACKNOWLEDGMENTS
We are most grateful to Nigel Rollins, Department of Maternal, Newborn, Child and Adolescent Health, World Health Organisation, Switzerland, for significantly contributing to discussions during the consultants meeting and throughout the development of the manuscript. Sincerely thank you to Cornelia Loechl, Carolin Cornelius of the Nutritional and Health‐Related Environmental Studies Section (NAHRES), International Atomic Energy Agency for support in the lead up to and during the consultants meeting. We are grateful to the International Atomic Energy Agency for funding the Consultants Meeting where this paper was conceived.
Owino VO, Murphy‐Alford AJ, Kerac M, et al. Measuring growth and medium‐ and longer‐term outcomes in malnourished children. Matern Child Nutr. 2019;15:e12790 10.1111/mcn.12790
REFERENCES
- Adair, L. S. (1999). Filipino children exhibit catch‐up growth from age 2 to 12 years. Journal of Nutrition, 129, 1140–1148. 10.1093/jn/129.6.1140 [DOI] [PubMed] [Google Scholar]
- Admassu, B. , Ritz, C. , Wells, J. C. K. , Girma, T. , Andersen, G. S. , Belachew, T. , … Kæstel, P. (2018). Accretion of fat‐free mass rather than fat mass in infancy is positively associated with linear growth in childhood. Journal of Nutrition, 148, 607–615. 10.1093/jn/nxy003 [DOI] [PubMed] [Google Scholar]
- Andersen, G. S. , Girma, T. , Wells, J. C. , Kæstel, P. , Michaelsen, K. F. , & Friis, H. (2011). Fat and fat‐free mass at birth: Air displacement plethysmography measurements on 350 Ethiopian newborns. Pediatric Research, 70, 501–506. 10.1203/PDR.0b013e31822d7470 [DOI] [PubMed] [Google Scholar]
- Angood, C . (2014). Review of the links between wasting and stunting (WaSt). Retrieved from http://www.ennonline.net/ourwork/reviews/wastingstunting
- Arnold, B. , Null, C. , Luby, S. P. , & Colford, J. M. Jr. (2018). Implications of WASH benefits trials for water and sanitation. Lancet, 6, e616–e617. [DOI] [PubMed] [Google Scholar]
- Ashworth, A. , Shrimpton, R. , & Jamil, K. (2008). Growth monitoring and promotion: Review of evidence of impact. Maternal & Child Nutrition, 4, 86–117. 10.1111/j.1740-8709.2007.00125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayonrinde, O. T. , Oddy, W. H. , Adams, L. A. , Mori, T. A. , Beilin, L. J. , de Klerk, N. , & Olynyk, J. K. (2017). Infant nutrition and maternal obesity influence the risk of non‐alcoholic fatty liver disease in adolescents. Journal of Hepatology, 67, 568–576. 10.1016/j.jhep.2017.03.029 [DOI] [PubMed] [Google Scholar]
- Bahwere, P. , Mtimuni, A. , Sadler, K. , Banda, T. , & Collins, S. (2012). Long term mortality after community and facility based treatment of severe acute malnutrition: Analysis of data from Bangladesh, Kenya, Malawi and Niger. Journal of Public Health and Epidemiology, 4, 215–225. 10.5897/JPHE11.212 [DOI] [Google Scholar]
- Bahwere, P. , Piwoz, E. , Joshua, M. C. , Sadler, K. , Grobler‐Tanner, C. H. , Guerrero, S. , & Collins, S. (2008). Uptake of HIV testing and outcomes within a Community‐based Therapeutic Care (CTC) programme to treat severe acute malnutrition in Malawi: A descriptive study. BMC Infectious Diseases, 8, 106 10.1186/1471-2334-8-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, D. J. (1995). The fetal and infant origins of disease. European Journal of Clinical Investigation, 25, 457–463. 10.1111/j.1365-2362.1995.tb01730.x [DOI] [PubMed] [Google Scholar]
- Barker, D. J. (2007). The origins of the developmental origins theory. Journal of Internal Medicine, 261, 412–417. 10.1111/j.1365-2796.2007.01809.x [DOI] [PubMed] [Google Scholar]
- Baumgartner, R. N. , Heymsfield, S. B. , & Roche, A. F. (1995). Human body composition and the epidemiology of chronic disease. Obesity Research, 3, 73–95. 10.1002/j.1550-8528.1995.tb00124.x [DOI] [PubMed] [Google Scholar]
- Baye, K. (2017). The sustainable development goals cannot be achieved without improving maternal and child nutrition. Journal of Public Health Policy, 38(1), 137–145. 10.1057/s41271-016-0043-y [DOI] [PubMed] [Google Scholar]
- Berenson, G. S. , Srinivasan, S. R. , Xu, J. H. , & Chen, W. (2016). Adiposity and cardiovascular risk factor variables in childhood are associated with premature death from coronary heart disease in adults: The Bogalusa heart study. American Journal of the Medical Sciences, 352, 448–454. 10.1016/j.amjms.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Alderman, H. , Bhutta, Z. A. , Gillespie, S. , Haddad, L. , Horton, S. , … Webb, P. (2013). Maternal and child nutrition: Building momentum for impact. Lancet, 382, 372–375. [DOI] [PubMed] [Google Scholar]
- Brambilla, P. , Rolland‐Cachera, M. F. , Testolin, C. , Briend, A. , Salvatoni, A. , Testolin, G. , & Chiumello, G. (2000). Lean mass of children in various nutritional states. Comparison between dual‐energy X‐ray absorptiometry and anthropometry. Annals of the New York Academy of Sciences, 904, 433–436. [DOI] [PubMed] [Google Scholar]
- Bray, G. A. , DeLany, J. P. , Volaufova, J. , Harsha, D. W. , & Champagne, C. (2002). Prediction of body fat in 12‐y‐old African American and white children: Evaluation of methods. American Journal of Clinical Nutrition, 76, 980–990. 10.1093/ajcn/76.5.980 [DOI] [PubMed] [Google Scholar]
- Briend, A. , Alvarez, J. L. , Avril, N. , Bahwere, P. , Bailey, J. , Berkley, J. A. , … Whitney, S. (2016). Low mid‐upper arm circumference identifies children with a high risk of death who should be the priority target for treatment. BMC Nutrition, 2, 63 10.1186/s40795-016-0101-7 [DOI] [Google Scholar]
- Briend, A. , Khara, T. , & Dolan, C. (2015). Wasting and stunting—Similarities and differences: Policy and programmatic implications. Food and Nutrition Bulletin, 36(1 Suppl), S15–S23. [DOI] [PubMed] [Google Scholar]
- Brown, K. F. , Rumgay, H. , Dunlop, C. , Ryan, M. , Quartly, F. , Cox, A. , … Parkin, D. M. (2018). The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. British Journal of Cancer, 118, 1130–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burza, S. , Mahajan, R. , Marino, E. , Sunyoto, T. , Shandilya, C. , Tabrez, M. , … Mishra, N. K. (2016). Seasonal effect and long‐term nutritional status following exit from a Community‐Based Management of Severe Acute Malnutrition program in Bihar, India. European Journal of Clinical Nutrition, 70, 437–444. 10.1038/ejcn.2015.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, J. (2014). Limitations of body mass index to assess body fat. Workplace Health & Safety, 62, 264 10.1177/216507991406200608 [DOI] [PubMed] [Google Scholar]
- Cameron, N. (2007). Growth patterns in adverse environments. American Journal of Human Biology, 19, 615–621. 10.1002/ajhb.20661 [DOI] [PubMed] [Google Scholar]
- Cameron, N. (2008). The biology of growth. Nestle Nutrition Workshop Series. Pediatric Program, 61, 1–19. [DOI] [PubMed] [Google Scholar]
- Cameron, N. , & Demerath, E. W. (2002). Critical periods in human growth and their relationship to diseases of aging. American Journal of Physical Anthropology, 35(Suppl), 159–184. [DOI] [PubMed] [Google Scholar]
- Cho, W. K. , & Suh, B. K. (2016). Catch‐up growth and catch‐up fat in children born small for gestational age. Korean Journal of Pediatrics, 59, 1–7. 10.3345/kjp.2016.59.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang, H. H. , Li, W. C. , Sheu, B. F. , Liao, S. C. , Chen, J. Y. , Chang, K. C. , & Tsai, Y. W. (2012). Correlation between body composition and risk factors for cardiovascular disease and metabolic syndrome. BioFactors, 38, 284–291. 10.1002/biof.1027 [DOI] [PubMed] [Google Scholar]
- Collins, S. (2007). Treating severe acute malnutrition seriously. Archives of Diseases in Childhood, 92, 453–461. 10.1136/adc.2006.098327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvalan, C. , Kain, J. , Weisstaub, G. , & Uauy, R. (2009). Impact of growth patterns and early diet on obesity and cardiovascular risk factors in young children from developing countries. Proceedings of the Nutrition Society, 68, 327–337. [DOI] [PubMed] [Google Scholar]
- Dedefo, M. , Zelalem, D. , Eskinder, B. , Assefa, N. , Ashenafi, W. , Baraki, N. , … Haile, A. (2016). Causes of death among children aged 5 to 14 years old from 2008 to 2013 in Kersa Health and Demographic Surveillance System (Kersa HDSS), Ethiopia. PLoS One, 11, e0151929 10.1371/journal.pone.0151929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond, C. , & Casale, D. (2017). Catch‐up growth in stunted children: Definitions and predictors. PLoS One, 12, e0189135 10.1371/journal.pone.0189135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després, J. P. (2012). Body fat distribution and risk of cardiovascular disease. An Update. Circulation, 126, 1301–1313. 10.1161/CIRCULATIONAHA.111.067264 [DOI] [PubMed] [Google Scholar]
- Dunlop, K. , Cedrone, M. , Staples, J. F. , & Regnault, T. R. (2015). Altered fetal skeletal muscle nutrient metabolism following an adverse in utero environment and the modulation of later life insulin sensitivity. Nutrients, 7, 1202–1216. 10.3390/nu7021202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, J. G. , Salonen, M. K. , Kajantie, E. , & Osmond, C. (2018). Prenatal growth and CKD in older adults: Longitudinal findings from the Helsinki Birth Cohort Study, 1924‐1944. American Journal of Kidney Diseases, 71, 20–26. 10.1053/j.ajkd.2017.06.030 [DOI] [PubMed] [Google Scholar]
- Fabiansen, C. , Phelan, K. P. Q. , Cichon, B. , Yaméogo, C. W. , Iuel‐Brockdorff, A. S. , Kurpad, A. , … Friis, H. (2018). Short malnourished children and fat accumulation with food supplementation. Pediatrics, 142(3). pii: e20180679 [DOI] [PubMed] [Google Scholar]
- Fabiansen, C. , Yaméogo, C. W. , Iuel‐Brockdorf, A. S. , Cichon, B. , Rytter, M. J. H. , Kurpad, A. , … Friis, H. (2017). Effectiveness of food supplements in increasing fat‐free tissue accretion in children with moderate acute malnutrition: A randomised 2 × 2 × 3 factorial trial in Burkina Faso. PLoS Medicine, 14(9), e1002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro, A. A. , & Bechere, F. M. T. (2013). Relationship between childhood growth and later outcomes. Nestle Nutrition Institute Workshop Series, 71, 191–197. [DOI] [PubMed] [Google Scholar]
- Fleming, T. P. , Watkins, A. J. , Velazquez, M. A. , Mathers, J. C. , Prentice, A. M. , Stephenson, J. , … Godfrey, K. M. (2018). Origins of lifetime health around the time of conception: Causes and consequences. Lancet, 391, 1842–1852. 10.1016/S0140-6736(18)30312-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frison, S. , Checchi, F. , & Kerac, M. (2015). Omitting edema measurement: How much acute malnutrition are we missing? American Journal of Clinical Nutrition, 102, 1176–1181. [DOI] [PubMed] [Google Scholar]
- Global Nutrition Report (2017). Nourishing the SDGs. Bristol, UK: Development Initiatives. [Google Scholar]
- Gluckman, P. D. , Mark, A. , Hanson, M. A. , Hamish, G. , Spencer, H. G. , & Bateson, P. (2005). Environmental influences during development and their later consequences for health and disease: Implications for the interpretation of empirical studies. Proceedings Biological Sciences, 272, 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Y. , Watson, S. , & Routledge, M. N. (2016). Aflatoxin exposure and associated human health effects. The Official Journal of Food Safety Commission of Japan, 4(1), 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambidge, K. M. , & Krebs, N. F. (2018). Strategies for optimizing maternal nutrition to promote infant development. Reproductive Health, 15(Suppl 1), 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks, S. H. , Schrijnders, D. , van Hateren, K. J. , Groenier, K. H. , Siesling, S. , Maas, A. H. E. M. , … Kleefstra, N. (2018). Association between body mass index and obesity‐related cancer risk in men and women with type 2 diabetes in primary care in the Netherlands: A cohort study (ZODIAC‐56). British Medical Journal Open, e018859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson, H. , Eriksson, B. , Forsum, E. , Flinke, E. , Henriksson, P. , & Löf, M. (2017). Longitudinal assessment of body composition in healthy Swedish children from 1 week until 4 years of age. European Journal of Clinical Nutrition, 71, 1345–1352. 10.1038/ejcn.2017.125 [DOI] [PubMed] [Google Scholar]
- Hochberg, Z. (2011). Evolutionary perspective in child growth. Rambam Maimonides Medical Journal, 2, e0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Atomic Energy Agency (2009). Assessing body composition and total energy expenditure in humans using stable isotope techniques. IAEA Human Health Series No. 3. Vienna: IAEA. https://www-pub.iaea.org/books/IAEABooks/7982/Assessment-of-Body-Composition-and-Total-Energy-Expenditure-in-Humans-Using-Stable-Isotope-Techniques (accessed July 2016).
- Jackson, A. A. (1990). Protein requirements for catch‐up growth. Proceedings of the Nutrition Society, 49, 507–516. [DOI] [PubMed] [Google Scholar]
- Jackson, A. A. , Langley‐Evans, S. C. , & McCarthy, H. D. (1996). Nutritional influence in early life upon obesity and body proportions In Chadwick D. J., & Cardew G. (Eds.), The origins and consequences of obesity. Chichester: John Wiley & Sons. CIBA Foundation Symposium [DOI] [PubMed] [Google Scholar]
- Jackson, A. A. , & Wootton, S. A. (1989). The energy requirements of growth and catch‐up growth In Schurch B., & Scrimshaw N. S. (Eds.), Activity, energy expenditure and energy requirements of infants and children. Proceedings of the International Dietary Energy Consultancy Group Workshop held in Cambridge, USA, November 14 to 17, 1989 United Nations University Press. [Google Scholar]
- Jensen, A. M. , Molgaard, C. , Ejlerskov, K. T. , Chritensen, L. B. , Michaelsen, K. F. , & Briend, A. (2015). Validity of anthropometric measurements to assess body composition, including muscle mass, in 3‐year‐old children from the SKOT cohort. Maternal & Child Nutrition, 11, 398–408. 10.1111/mcn.12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, K. D. J. , & Berkley, J. A. (2014). Severe acute malnutrition and infection. Pediatrics and International Child Health, 34(Suppl 1), 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensara, O. A. , Wooton, S. A. , Phillips, D. I. , Patel, M. , Hoffman, D. J. , Jackson, A. A. , & Elia, M. (2006). Hertfordshire Study Group. Substrate‐energy metabolism and metabolic risk factors for cardiovascular disease in relation to fetal growth and adult body composition. American Journal of Physiology‐Endocrinology and Metabolism, 291, E365–E371. 10.1152/ajpendo.00599.2005 [DOI] [PubMed] [Google Scholar]
- Keusch, G. T. , Rosenberg, I. H. , Denno, D. M. , Duggan, C. , Guerrant, R. L. , Lavery, J. V. , … Brewer, T. (2013). Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low‐ and middle‐income countries. Food and Nutrition Bulletin, 34, 357–364. 10.1177/156482651303400308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanum, S. , Ashworth, A. , & Huttly, S. R. (1998). Growth, morbidity, and mortality of children in Dhaka after treatment for severe malnutrition: a prospective study. American Journal of Clinical Nutrition, 67, 940–945. 10.1093/ajcn/67.5.940 [DOI] [PubMed] [Google Scholar]
- Langley‐Evans, S. C. (2015). Nutrition in early life and the programming of adult disease: A review. Journal of Human Nutrition & Diet, 28(Suppl 1), 1–14. [DOI] [PubMed] [Google Scholar]
- Lelijveld, N. , Seal, A. , Wells, J. C. , Kirkby, J. , Opondo, C. , Chimwezi, E. , … Kerac, M. (2016). Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): A cohort study. The Lancet Glob Health, 4, e654–e662. 10.1016/S2214-109X(16)30133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenters, L. , Wazny, K. , & Bhutta, Z. A. (2016). Management of severe and moderate acute malnutrition in children In Black R. E., Laxminarayan R., Temmerman M., & Walker N. (Eds.), Reproductive, maternal, newborn, and child health: Disease control priorities (3rd ed.). Washington (DC): The International Bank for Reconstruction and Development / The World Bank. [PubMed] [Google Scholar]
- Li, S. , Chen, W. , Sun, D. , Fernandez, C. , Li, J. , Kelly, T. , … Whelton, P. K. (2015). Variability and rapid increase in body mass index during childhood are associated with adult obesity. International Journal of Epidemiology, 44, 1943–1950. 10.1093/ije/dyv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeen, E. A. , Behrman, J. R. , Crookston, B. T. , & Dearden, K. A. (2014). Growth faltering and recovery in children aged 1–8 years in four low‐ and middle‐income countries: Young Lives. Public Health Nutrition, 17, 2131–2137. 10.1017/S1368980013003017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzoros, C. S. , Rifas‐Shiman, S. L. , Williams, C. J. , Fargnoli, J. L. , Kelesidis, T. , & Gillman, M. W. (2009). Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: A prospective cohort study. Pediatrics, 123, 682–689. 10.1542/peds.2008-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, E. E. , Smallwood, C. D. , Quinn, N. L. , Ariagno, K. , Bechard, L. J. , Duggan, C. P. , & Mehta, N. M. (2017). Body composition in children with chronic illness: Accuracy of bedside assessment techniques. Journal of Pediatrics, 190, 56–62. 10.1016/j.jpeds.2017.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell, R. , Khan, L. K. , & Schroeder, D. G. (1994). Reversibility of stunting: Epidemiological findings in children from developing countries. European Journal of Clinical Nutrition, 48(Suppl 1), S45–S57. [PubMed] [Google Scholar]
- Morrison, J. L. , Duffield, J. A. , Muhlhausler, B. S. , Gentili, S. , & McMillen, I. C. (2010). Fetal growth restriction, catch‐up growth and the early origins of insulin resistance and visceral obesity. Pediatric Nephrology, 25, 669–677. 10.1007/s00467-009-1407-3 [DOI] [PubMed] [Google Scholar]
- Most, J. , Marlatt, K. L. , Altazan, A. D. , & Redman, L. M. (2018). Advances in assessing body composition during pregnancy. European Journal of Clinical Nutrition, 72, 645–656. 10.1038/s41430-018-0152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration (2017). Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population‐based measurement studies in 128·9 million children, adolescents, and adults. Lancet, 390, 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Null, C. , Stewart, C. P. , Pickering, A. J. , Dentz, H. N. , Arnold, B. F. , Arnold, C. D. , … Colford, J. M. Jr. (2018). Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: A cluster‐randomised controlled trial. Lancet Global Health, 6, e316–e329. 10.1016/S2214-109X(18)30005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson, C. , Bygdell, M. , Sondén, A. , Jern, C. , Rosengren, A. , & Kindblom, J. M. (2017). BMI increase through puberty and adolescence is associated with risk of adult stroke. Neurology, 89, 363–369. 10.1212/WNL.0000000000004158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okorodudu, D. O. , Jumean, M. F. , Montori, V. M. , Romero‐Corral, A. , Somers, V. K. , Erwin, P. J. , & Lopez‐Jimenez, F. (2010). Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta‐analysis. International Journal of Obesity, 34, 791–799. 10.1038/ijo.2010.5 [DOI] [PubMed] [Google Scholar]
- Owino, V. , Ahmed, T. , Freemark, M. , Kelly, P. , Loy, A. , Manary, M. , & Loechl, C. (2016). Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics, 138. pii: e20160641 [DOI] [PubMed] [Google Scholar]
- Owino, V. O. , Cornelius, C. , & Loechl, C. U. (2018). Elucidating adverse nutritional implications of exposure to endocrine disrupting chemicals and mycotoxins through stable isotope techniques. Nutrients, 10. pii: E401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owino, V. O. , Slater, C. , & Loechl, C. U. (2017). Using stable isotope techniques in nutrition assessments and tracking of global targets post‐2015. Proceedings of the Nutrition Society, 76, 495–503. [DOI] [PubMed] [Google Scholar]
- Pecoul, B. , Soutif, C. , Hounkpevi, M. , & Ducos, M. (1992). Efficacy of a therapeutic feeding centre evaluated during hospitalization and a follow‐up period, Tahoua, Niger, 1987‐1988. Annals of Tropical Paediatrics, 12, 47–54. 10.1080/02724936.1992.11747546 [DOI] [PubMed] [Google Scholar]
- Prado, E. L. , & Dewey, K. H. (2014). Nutrition and brain development in early life. Nutrition Reviews, 72, 267–284. 10.1111/nure.12102 [DOI] [PubMed] [Google Scholar]
- Prentice, A. M. , Ward, K. A. , Goldberg, G. R. , Jarjou, L. M. , Moore, S. E. , Fulford, A. J. , & Prentice, A. (2013). Critical windows for nutritional interventions against stunting. American Journal of Clinical Nutrition, 97, 911–918. 10.3945/ajcn.112.052332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez, E. , Valencia, M. E. , Moya‐Camarena, S. Y. , Alemán‐Mateo, H. , & Méndez, R. O. (2009). Four‐compartment model and validation of deuterium dilution technique to estimate fat‐free mass in Mexican youth. Nutrition, 25, 194–199. 10.1016/j.nut.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Rodriguez, L. , Cervantes, E. , & Ortiz, R. (2011). Malnutrition and gastrointestinal and respiratory infections in children: A public health problem. International Journal of Environmental Public Health, 8, 1174–1205. 10.3390/ijerph8041174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, M. G. , & Beall, M. H. (2008). Adult sequelae of intrauterine growth restriction. Seminars in Perinatology, 32, 213–218. 10.1053/j.semperi.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ, T. C. , Kivimäki, M. , Starr, J. M. , Stamatakis, E. , & Batty, G. D. (2014). Height in relation to dementia death: Individual participant meta‐analysis of 18 UK prospective cohort studies. British Journal of Psychiatry, 205, 348–354. 10.1192/bjp.bp.113.142984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, C. R. , Catalano, P. M. , Biggio, J. R. , Gower, B. A. , & Chandler‐Laney, P. C. (2018). Associations of neonatal adiponectin and leptin with growth and body composition in African American infants. Pediatric Obesity, 13, 485–491. 10.1111/ijpo.12274. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoville, K. (2001). The Rosetta Stone in historical perspective. Journal of the Adventist Theological Society, 12, 1–21. [Google Scholar]
- Segal, K. R. , Dunaif, A. , Gutin, B. , Albu, J. , Nyman, A. , & Pi‐Sunyer, F. X. (1987). Body composition, not body weight, is related to cardiovascular disease risk factors and sex hormone levels in men. Journal of Clinical Investigations, 80, 1050–1055. 10.1172/JCI113159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, D. R. , Ribeiro, A. S. , Pavão, F. H. , Ronque, E. R. , Avelar, A. , Silva, A. M. , & Cyrino, E. S. (2013). Validity of the methods to assess body fat in children and adolescents using multi‐compartment models as the reference method: A systematic review. Revista da Associação Médica Brasileira, 59, 475–486. 10.1016/j.ramb.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Singh, A. U. , Ashish, K. , & Kumar, K. (2017). Birth size, stunting and recovery from stunting in Andhra Pradesh, India: Evidence from the Young Lives Study. Maternal and Child Health Journal, 21, 492–508. 10.1007/s10995-016-2132-8 [DOI] [PubMed] [Google Scholar]
- Somasse, Y. E. , Dramaix, M. , Bahwere, P. , & Donnen, P. (2015). Relapses from acute malnutrition and related factors in a community‐based management programme in Burkina Faso. Maternal & Child Nutrition, 12, 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapsell, L. C. , Neale, E. P. , Satija, A. , & Hu, F. B. (2016). Foods, nutrients, and dietary patterns: Interconnections and implications for dietary guidelines. Advances in Nutrition, 7, 445–454. 10.3945/an.115.011718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Children's Fund, World Health Organization, World Bank Group (2018). Levels and trends in child malnutrition: Key findings of the 2018 Edition of the Joint Child Malnutrition Estimates. <http://data.unicef.org/nutrition>; <http://www.who.int/nutgrowthdb>; <http://data.worldbank.org>.
- Vaiserman, A. M. (2014). Early‐life nutritional programming of longevity. Journal of Developmental Origins of Health and Disease, 5, 325–338. 10.1017/S2040174414000294 [DOI] [PubMed] [Google Scholar]
- Vásquez, F. , Diaz, E. , Lera, L. , Vásquez, L. , Anziani, A. , & Burrows, R. (2012). Methods of body composition and four compartments model in obese school children. Nutrición Hospitalaria, 27, 1079–1085. 10.3305/nh.2012.27.4.5819 [DOI] [PubMed] [Google Scholar]
- Victora, C. G. , de Onis, M. , Hallal, P. C. , Blossner, M. , & Shrimpton, R. (2010). Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics, 125, e473–e480. 10.1542/peds.2009-1519 [DOI] [PubMed] [Google Scholar]
- Wang, J. , Thornton, J. C. , Russell, M. , Burastero, S. , Heymsfield, S. , & Pierson, R. N. (1994). Asians have lower body mass index (BMI) but higher percent body fat than do whites: Comparisons of anthropometric measurements. American Journal of Clinical Nutrition, 60, 23–28. 10.1093/ajcn/60.1.23 [DOI] [PubMed] [Google Scholar]
- Wells, J. C. K. (2018). The capacity‐load model of non‐communicable disease risk: understanding the effects of child malnutrition, ethnicity and the social determinants of health. European Journal of Clinical Nutrition, 72, 688–697. 10.1038/s41430-018-0142-x [DOI] [PubMed] [Google Scholar]
- Wells, J. C. K. , & Fewtrell, M. S. (2006). Measuring body composition. Archives of Diseases in Childhood, 91, 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton, S. A. , & Jackson, A. A. (1996). Influence of under‐nutrition in early life on growth, body composition and metabolic competence In Henry C., & Ulijaszek J. K. (Eds.), Long‐term consequences of early environment: Growth, development and the lifespan developmental perspective (pp. 109–123). Cambridge, England: Cambridge University Press. [Google Scholar]
- World Health Organization (2007). Child growth standards: Methods and development: Length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age. Geneva: WHO; Retrieved from http://www.who.int/childgrowth/publications/technical_report_pub/en/index.html. [Google Scholar]
- World Health Organization (2010a). Global status report on noncommunicable diseases. Retrieved from http://www.who.int/nmh/publications/ncd_report_full_en.pdf
- World Health Organization (2010b). Communicable diseases and severe food shortage: WHO technical note. Geneva: WHO; Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK304206/ [PubMed] [Google Scholar]
- World Health Organization (2014). Global status report on noncommunicable diseases. Retrieved from http://www.who.int/nmh/publications/ncd-status-report-2014/en/
- World Health Organization (2015). Global strategy for women's, children's and adolescents' health, 2016–2030 World Health Assembly, 18–26 May 2015. Retrieved from http://who.int/pmnch/media/events/2015/gs_pager.pdf?ua=1
- World Health Organization (2016). Children: Reducing mortality. Retrieved from http://www.who.int/mediacentre/factsheets/fs178/en/
- World Health Organization . (2017). Guideline. Assessing and managing children at primary health‐care facilities to prevent overweight and obesity in the context of the double burden of malnutrition. Updates for the Integrated Management of Childhood Illness (IMCI). http://apps.who.int/iris/bitstream/handle/10665/259133/9789241550123-eng.pdf;jsessionid=7D36BEB2EAFC6F815F22B205F1EC66B1?sequence=1 [PubMed]
- World Health Organization and United Nations Children's Fund (2003). Global strategy for infant and young child feeding. Retrieved from http://www.who.int/nutrition/publications/infantfeeding/9241562218/en/
- Yajnik, C. S. (2003). Neonatal anthropometry: The thin‐fat Indian baby. The Pune maternal nutrition study. International Journal of Obesity and Related Metabolic Disorders, 27, 173–180. 10.1038/sj.ijo.802219 [DOI] [PubMed] [Google Scholar]
- Yan, Y. , Hou, D. , Zhao, X. , Liu, J. , Cheng, H. , Wang, Y. , & Mi, J. (2017). Childhood adiposity and nonalcoholic fatty liver disease in adulthood. Pediatrics, 139. pii: e20162738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. , Undurraga, E. A. , Zeng, W. , Reyes‐Garcia, V. , Tanner, S. , Leonard, W. R. , … Godoy, R. A. (2016). Catch‐up growth and growth deficits: Nine‐year annual panel child growth for native Amazonians in Bolivia. Annals of Human Biology, 43, 304–315. 10.1080/03014460.2016.1197312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, T. , Zhang, J. , Sommer, K. , Basig, B. A. , Zhang, X. , Braun, J. , … Kelsey, K. (2016). Effects of environmental exposures on fetal and childhood growth trajectories. Annals of Global Health, 82, 41–99. 10.1016/j.aogh.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


