Abstract
Dietary intake during pregnancy plays a vital role in determining the health of both mother and baby. Maternal undernutrition affects a large proportion of women in low and lower middle income countries (LLMIC) likely influencing high maternal, infant, and child mortality in these countries. Mobile health (mHealth) interventions have been proposed as effective solutions to improve maternal and neonatal health. This paper reviews the literature to evaluate the effectiveness of mHealth interventions on improving dietary/nutrients intake of pregnant women in LLMIC. Eight electronic databases were searched from inception up to April 2018, including the MEDLINE, EMBASE, CINAHL, Cochrane, Web of Science, Scopus, Global Index Medicus, and Maternity and Infant Care. Using Covidence, two reviewers assessed articles for inclusion, assessed study quality and extracted data. Only studies published in English language were included. Data were summarised narratively. In total, 6,778 were identified of which four were included, with three randomised controlled trials and one prepost experimental study. Studies were conducted in India (n = 2), Indonesia (n = 1), and Kenya (n = 1). All articles evaluated the use of nutrient supplements; iron supplements (n = 1), vitamin supplements (composition not mentioned; n = 1), or calcium supplements (n = 1). This review suggests that mHealth interventions can be used to improve intake of micronutrient supplementation and nutritional status of pregnant women in LLMIC. Further studies are needed to address the limited evidence base related to mHealth nutrition interventions targeting dietary intakes of pregnant women in LLMIC.
Keywords: dietary intake, maternal health, mHealth, mobile phone, nutrients intake, pregnancy
Key messages.
Mobile health (mHealth) interventions can be used to improve adherence to taking micronutrient supplements in pregnancy in low and lower middle income countries (LLMICs)
mHealth interventions can be associated to improved weight gain and haemoglobin level during pregnancy. Few studies have been identified on this topic in LLMICs, this means there is limited evidence. Further studies on this topic are recommended to fill this gap
1. INTRODUCTION
Dietary intake during pregnancy plays a vital role in determining both the short‐ and long‐term health of mothers and their infants (Nnam, 2015). Maternal undernutrition affects a large percentage of women in low and lower middle income countries (LLMIC). Poor nutrition is related to high maternal, infant, and child mortality in these countries (Black et al., 2013).
In 2015, trends in maternal mortality reported 239 deaths per 100,000 live births in developing countries (WHO, UNICEF, UNFPA, WB, & UNDP, 2015). The report's findings estimated that 546 maternal deaths per 100,000 live birth occurred in sub‐Saharan Africa under which most low and lower middle income economies fall (WHO et al., 2015). This maternal mortality rate is disproportionate when compared with maternal mortality rates in developed countries of 12 maternal deaths per 100,000 live birth in the same year (WHO et al., 2015).
Nutrition interventions during pregnancy are associated with improved maternal food and nutrient intakes (Dodd et al., 2014) and better nutritional status during both pregnancy and the postnatal period. For example, improved maternal haemoglobin levels have been reported secondary to use of nutritional supplements (Ekstrom et al., 1996; Menendez et al., 1994; Mohamed, 2004) and an adequate diet (Shobeiri, Begum, & Nazari, 2006). Effective nutrition‐sensitive and nutrition‐specific interventions reduce the risk of maternal death (Villar et al., 2003) and low birth weight (da Silva Lopes et al., 2017; Ruel & Alderman, 2013). Low rate of preterm delivery, stillbirth, and neonatal deaths are related to effective nutrition intervention during the antenatal period (Mavalankar, Trivedi, & Gray, 1991; Villar et al., 2003). Growth and development of the infant following birth is influenced by the dietary intake of the mother during pregnancy.
Mobile technologies are being used to scale up cost‐effective evidence‐based interventions to improve maternal outcomes throughout the pregnancy period, during delivery and postnatal period (Schiffman, Darmstadt, Agarwal, & Baqui, 2010). There is an increase in access and uptake of mobile phones in LLMIC (Agarwal et al., 2016; Sanou, 2017). The International Telecommunication Union reported an increase in mobile phone subscribers in developing countries from 1,213 million in 2005 to 6,133 million in 2017 (International Telecommunication Union. [Producer], 2017). The using of mobile technology to improve health is a potentially important way to improve efficiency and reach within health care systems in developing countries (Qiang, Yamamichi, Hausman, Miller, & Altman, 2012). The technology helps these countries to use their limited resources in making the health system more efficient (Qiang et al., 2012). Further, such technology provides new opportunities to permit safe, accessible, coordinated, and effective maternal health care (WHO, 2011).
Several reviews have been published on the effectiveness of mobile health (mHealth) interventions for improving maternal care such as improving health care seeking behaviour and number of antenatal and postnatal visits (Colaci, Chaudhri, & Vasan, 2016; Feroz, Perveen, & Aftab, 2017), improved quality of care (Feroz et al., 2017; Sondaal et al., 2016), skilled attendance at birth (Colaci et al., 2016; Lee et al., 2016), and maternal satisfaction with antenatal care (Colaci et al., 2016). However, no reviews have evaluated the effectiveness of mHealth interventions to improve dietary intake of pregnant women in LLMIC.
2. METHODS
2.1. Search strategy and selection of studies
A detailed protocol was registered with the International Prospective Register for Systematic Reviews number CRD42018098512. We searched eight databases including: MEDLINE, EMBASE, CINAHL, Cochrane, Web of Science, Scopus, Global Index Medicus, Maternity and Infant Care. Various dissertation and thesis were reviewed as well as going through manually the reference list of included articles and relevant systematic reviews.
One author (N.S.) and university librarian developed the search strategy using selected keywords under four groups, relating to mHealth and associated technologies, nutrition and dietary intake, pregnancy, and LLMIC (see Appendix A). Search terms were developed from previous published articles with related review topic. In addition, a hand search was performed using Google search engine. The reference lists of related articles were used to find the eligible articles, and a cited reference search was also performed.
The retrieved records were exported to an EndNote library, and record management was performed using Covidence, which is an online platform (Covidence, 2013). Duplicate records were removed, and articles screened following the inclusion and exclusion criteria by two independent reviewers N. S. and A. A.. Data were extracted from selected published articles and entered on a formulated table by one author and then it was checked by a second reviewer and updated on the table. The discrepancies were resolved by a third independent reviewer (T. B. or M. E. R.).
2.2. Eligibility criteria
The included studies met the following criteria:
2.2.1. Types of study
Study designs included were randomised controlled trials, quasi‐experimental studies, and pre and post studies.
2.2.2. Population
The target population consisted of pregnant women in LLMIC, defined as the countries with a gross national income per capita from $3,955 and below, calculated using the World Bank Atlas method (WB, 2018).
2.2.3. Intervention
Interventions using mHealth technologies with the aim of improving dietary intake (including behavioural changes or fortification/supplementation programs to change food and/or nutrient intake) were included. mHealth is defined as medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices (WHO, 2011). mHealth involves the use and capitalization on a mobile phone's core utility of voice and short message service (SMS) as well as more complex functionalities and applications including general packet radio service, third and fourth generation mobile telecommunications (3G and 4G systems), global positioning system, and Bluetooth technology (WHO, 2011).
2.2.4. Comparator/control
Studies with a comparison group such as control group, placebo, standard therapy, or no treatment were included.
2.3. Outcome
The primary outcome of interest was change in nutrition behaviours (such as dietary intake, nutritional supplements intake, eating habits, and feeding patterns) of pregnant women.
Secondary outcomes of interest include anthropometric outcomes of either women (e.g., pregnancy weight gain) or their offspring (e.g., birth weight, growth pattern); breastfeeding initiation and/or duration; breastfeeding intention; infant dietary intake (other than breastfeeding); offspring health outcome (e.g., presence of foetal alcohol syndrome); offspring birth outcome (e.g., gestational duration, method of delivery, foetal distress); maternal nutrition knowledge (including cooking skills and food safety/hygiene); changes to the food environment for example, food security (access, affordability, adequacy, and appropriateness of the food supply); biomarkers of maternal or offspring food intake (e.g., plasma carotenoids, HbA1c); nutrition‐related health status of mothers or offspring (e.g., improvements in cholesterol, blood pressure, blood glucose).
2.3.1. Data extracted from included studies
Country of origin, study design, study subjects (intervention and control groups information), mode of mHealth delivery such as text messaging, applications, games, videos, mobile calls, voice messages, device used, outcomes of interest (primary and secondary), and results. Data extraction was done by one reviewer and then checked for accuracy by an independent reviewer. Data were synthesised and are described narratively.
2.3.2. Quality assessment
The quality of each included study was assessed by two independent reviewers using quality criteria checklist for primary research developed by The Academy of Nutrition and Dietetics (Academy of Nutrition and Dietetics, 2016). The articles were then ranked either positive/neutral/negative depending on what they scored as per predefined criteria.
3. RESULTS
3.1. Study selection
Figure 1 highlights the process used for the identification of relevant papers from the database searches (n = 6,778 titles). After removing duplicates, 4,948 papers were included for initial screening. Of these, 4,891 papers were excluded during title and abstract screening, leaving 56 papers, which were considered for full text screening. Two of these papers met inclusion criteria. Major reasons for exclusion were not conducted in LLMIC, not the study design population, or outcome of interest (Figure 1). Hand searching of the reference lists from the two included articles and seven relevant systematic reviews on mHealth interventions to improve maternal health broadly was performed. This identified one article, with another identified after performing a cited reference search. From the complete search using all methods, a total of four articles met the inclusion criteria (Figure 1).
Figure 1.
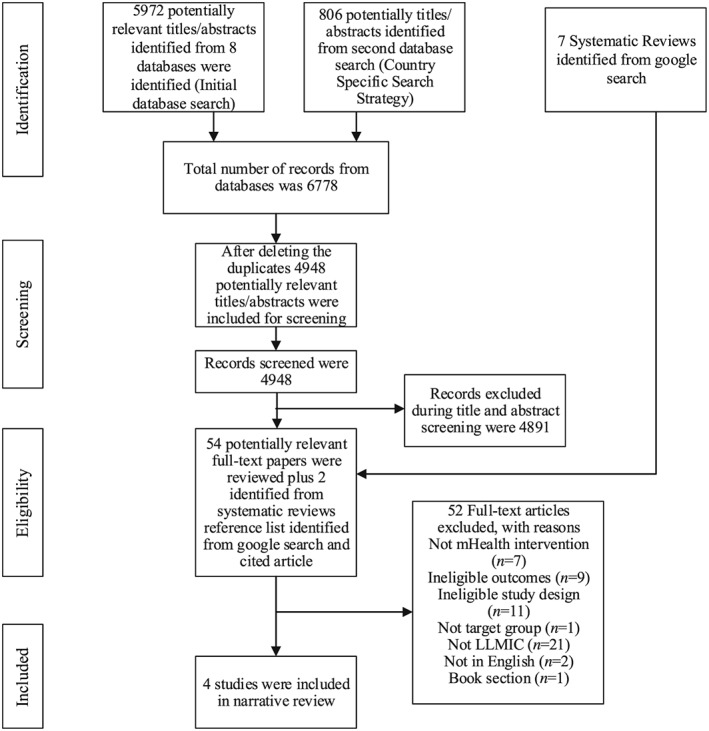
PRISMA flow diagram (Moher, Liberati, Tetzlaff, Altman,, & group, 2009) for database and hand searches of studies on mHealth interventions for improving nutrients intake of pregnant women in low and lower middle income countries
3.2. Characteristics of selected studies
Three of the eligible studies were randomised controlled trials (Bangal, Borawake, Gavhane, & Aher, 2017; Fedha, 2014; Pai et al., 2013), and one was a prepost experimental design (Anitasari & Andrajati, 2017). Two studies were conducted in India (Bangal et al., 2017; Pai et al., 2013), one in Indonesia (Anitasari & Andrajati, 2017), and one in Kenya (Fedha, 2014). Two studies were published in 2017 (Anitasari & Andrajati, 2017; Bangal et al., 2017), one in 2014 (Fedha, 2014), and one was published in 2013 (Pai et al., 2013). The duration of the interventions was 1 month (Anitasari & Andrajati, 2017), 3 months (Pai et al., 2013), 10 months (Fedha, 2014), and 1 year (Bangal et al., 2017).
Sample sizes of the included studies ranged from 74 to 400, with the average age of women ranging from 15 to 45 years and with gestations from 12 to 36 weeks. One study did not specify the gestation stage (Bangal et al., 2017; Table 1).
Table 1.
Characteristics of the included studies
| Author(s), title and year | Objective | Setting | Participant Characteristics | Study Design and Duration | Study groups and Description of Intervention | Limitations |
|---|---|---|---|---|---|---|
| Anitasari & Andrajati, 2017 | To obtain an overview of sociodemographic characteristics and clinical characteristics and to assess the effectiveness of SMS reminders compared to leaflets in complying with taking iron supplements and improving haemoglobin levels in pregnant women, at two public health centres, in Depok City, Indonesia | Two public health centres from March to May 2016, Depok City, Indonesia |
Pregnant women (n = 74) Gestation: 14 to 32 weeks Age (years): <20 (n = 1) 20–30 (n = 53)>35 (n = 20) Education: Elementary (n = 16) Advanced: (n = 58) Occupation: Working (n = 6) not working (n = 68) Parity: Nullipara (n = 21) Primipara (n = 27) Multipara (n = 26) Grande multipara (n = 0) |
Quasi‐experimental study with two intervention groups (pre and post study design) Duration Study: not stated (Recruitment: 3 months) intervention: 1 month |
SMS group (n = 36) received SMS reminders to adhere to iron supplementation once per week for 1 month after administration of supplements. Adherence was measured through eight‐item Morisky Medication Adherence Scale (MMAS‐8) questionnaire and haemoglobin levels (measured by HemoCue). Leaflets group (n = 38) received health education media intervention in form of leaflets once at the time they were given iron supplements Nutrition‐related target of intervention: Iron supplements |
Did not measure the amount of heme food sources consumed each day by the women. Did not account for the impact of side effects (e.g., vomiting) on haemoglobin levels. |
| Bangal et al., 2017 | To improve maternal health and pregnancy outcome by optimum utilisation of antenatal, natal, and postnatal care services, with the use of mobile phone as a medium of communication between health care provider and community in rural area | Rural Medical College, Loni, Ahmednagar. India |
Pregnant women (n = 400) Gestation: not stated Age: Not stated |
Randomised controlled trial Duration Study: 1 year Intervention: Not stated (pregnancy and postnatal period weeks not indicated) |
Intervention group received mobile phone calls, as reminders about next visit and text messages (SMS) on important aspects of antenatal care such as reminder to take supplements at regular intervals in addition to routine antenatal care (n = 200) Control group received routine antenatal care and advice as per hospital protocol (n = 200) nutrition‐related target of intervention: Iron and Calcium supplements |
Person who was calling is not stated |
| Fedha, 2014 | To assess whether the use of mobile technology can increase antenatal service utilisation and hospital deliveries by pregnant women in two health facilities in Njoro Division, Nakuru County in Kenya | Two health facilities in Nakuru, Kenya from April 2012 and July 2012 |
Pregnant women (n = 397) Gestation: 12–36 weeks Age: 20–29 years: (65%) 15–16 years: (1%) >40 years: (1.3%) Marital status: Married: (86.6%) Single: (13.3%) Education: Lower primary: (1.5%) Primary: (52.6%) secondary: (36.8%). Tertiary education: (9.1%) Parity: Primi‐gravidae: (36.3%) Para 1 (27%) Para 2 (18.4%) Para 3 (8.1%) Para 4 (5.0%). > Para (5.4%) |
Randomised controlled trial Duration Study: 10 monthsIntervention: Not stated |
Intervention group received reminders every fortnight relating to next visit to the clinic and advice about their health including iron and vitamin supplements and diet counselling use via mobile phone, however the delivery mode for example, SMS, call was not stated (n = 191) Control group received no mobile phone support, continue with routine services (n = 206) Nutrition‐related target of intervention: Iron and vitamin (not further specified) supplements and diet counselling |
Compliancy was not checked with taking nutrient supplements, researcher relied on information as entered in the client's clinic register, Exclusion criteria were not considered that is, gestation age below 12 weeks to excluded, but they were included in data analysis |
| Pai et al., 2013 | To describe the use of automated voice calls to promote adherence to iron supplements among pregnant women in urban India | Lokmanya Tilak Municipal General Hospital, located near the Dharavi slum in Mumbai, India |
Pregnant women (n = 79) Gestation: 13–28 weeks Mean age: Intervention (24.5 years) and control (24 years) Mean education (years): Intervention (8.3) and control (7.3) Mean prior pregnancies: Intervention (0.8) and control (0.6) |
Randomised controlled trial Duration Study: Not stated Intervention: 3 months |
Intervention group received short audio messages delivered via mobile phone, three times per week for a period of 3 months, encouraging them to take iron supplements in addition to counselling session and a free supply of medication (n = 39) Control group received a counselling session and a free supply of medication (n = 40) Nutrition‐related target of intervention: Iron supplements |
Limited information about and control over women's access to medication. Should have issued medication directly or verify that women received medication. |
Note. SMS: short message service.
3.3. Characteristics of mHealth intervention
The mHealth delivery modes used included SMS (Anitasari & Andrajati, 2017), audio voice messages delivered via mobile phone (Pai et al., 2013), and a combination of mobile phone calls and SMS (Bangal et al., 2017). Participants used their own mobile phones in all included studies (Table 1).
The study conducted in Kenya investigated the impact of a mobile telephone service on the uptake of selected maternal health services by expectant mothers, including supplement use. In addition to routine antenatal clinic (ANC) services, the intervention group were reminded fortnightly of their next visit to the clinic and given advice on pregnancy updates and health advice via mobile phone. The principal investigator was responsible in answering and giving advice to mothers who sought advice via the mobile support program, however further information on the mode of delivery (e.g., SMS, call) via mHealth technology was not provided (Fedha, 2014).
The study by Bangal et al., 2017 aimed to improve maternal health and pregnancy outcomes by using mobile phones as the medium of communication between the health care provider and women. Those in the intervention group received mobile phone calls as a reminder about their next visit and SMS in regard to important aspects of antenatal care at regular intervals (did not mention specific time), over the intervention period of 1 year (Bangal et al., 2017).
A randomised controlled trial from India investigated the use of automated voice calls to promote adherence to taking iron supplements among pregnant women, with compliance to supplement use verified via measurement of blood haemoglobin levels. The intervention group received short pre‐recorded audio messages delivered via mobile phones three times per week for a period of 3 months, encouraging them to take the iron supplements. The duration of each message was 30 s, with the message repeated twice within each call. Each woman was followed‐up 3 months after enrolment. A total of 48 messages were developed and recorded in two languages (Hindi and Marathi). The author and narrator of the messages was a doctor who had practiced in low‐income areas of India (Pai et al., 2013).
The final study was conducted in Indonesia and aimed to assess the effectiveness of SMS reminders to enhance compliance with taking iron supplements and improve haemoglobin levels. The SMS reminders were sent once per week for 1 month following providing the pregnant women with the iron supplements. The details about the content of messages and who narrated were not provided (Anitasari & Andrajati, 2017).
3.4. Types of nutrition outcomes investigated
All articles evaluated the use of nutrient supplements; iron supplements (Anitasari & Andrajati, 2017; Bangal et al., 2017; Fedha, 2014; Pai et al., 2013), vitamin supplements (information on composition not reported; Fedha, 2014), or calcium supplements (Bangal et al., 2017). Anaemia prevalence was studied in one study (Bangal et al., 2017), haemoglobin increase was addressed by two studies (Anitasari & Andrajati, 2017; Pai et al., 2013). One study examined weight gain and rate of low birth weight babies (Bangal et al., 2017). Two studies reported the outcomes for the mothers only (Anitasari & Andrajati, 2017; Pai et al., 2013), and the remaining two studies reported mother and infants outcomes (Bangal et al., 2017; Fedha, 2014). Further details on other reported secondary outcomes are summarised in Table 2.
Table 2.
Primary and secondary outcomes and relevant findings of the included studies
| Author(s), and Year | Primary Outcomes | Secondary Outcomes | Summary of Findings |
|---|---|---|---|
| Anitasari & Andrajati, 2017 | •Intake of iron supplements by pregnant women measured by MMAS‐8 score | •Haemoglobin (Hb) level (g/dL) |
•Low adherence to iron supplementation as measured by MMAS‐8 score (>2): SMS group: 76.3% of women before intervention; 71.1% of women after interventionLeaflet group: 66.7% of women before intervention; 58.3% of women after intervention •High adherence to iron supplementation as measured by MMAS‐8 score (=0): SMS group: 5.3% of women before intervention; 7.9% of women after the intervention Leaflet group: 13.9% of women before intervention; 16.7% of women after intervention •Percentage of women with Hb level ≥11 g/dL: SMS group: 47.22% before intervention; 52.78% after intervention Leaflet group: 55.26% before intervention; 57.69% after intervention •Percent of women with Hb level of 10.9 g/dL–10.0 g/dL: SMS intervention: 36.11% before intervention; 22.22% after intervention Leaflet intervention: 23.68% before intervention; 28.95% after intervention |
| Bangal et al., 2017 | •Percentage of pregnant women who received prophylactic iron and calcium tablets for minimum 3 months |
•Total weight gain during pregnancy (kg) •Birth weight of baby •Need for parenteral and blood transfusion •Attended four antenatal visits |
•Iron and calcium supplementation for more than 3 months: Intervention group 81%; control group 69% (chi square test p < 0.0001) •Satisfactory weight gain (>10 kg): Intervention group 35.00%; control group 25.00% •Anaemia: Intervention group 36%; control group 45% (chi square test p = 0.0119) •Need for blood transfusion: intervention group 0%; control group 3.00% •Need for parenteral iron therapy: Intervention group 2%; control group 5% •The proportion of low birth weight babies: Intervention group 30%; control group 35% •Antenatal visits (≤4): Intervention group 42.5%, control group 76.5% (chi square test p < 0.0001) |
| Fedha, 2014 | •Percentage of pregnant women who received prophylactic iron and/or vitamin supplements •Percentage of pregnant women who received dietary counselling |
•Number of antenatal visits attended •Percentage of intra‐uterine fetal deaths •Percentage of neonatal deaths •Percentage of infants that cried after birth (e.g., immediately, after 5 min, after 10 min) |
•Receive vitamin supplements: Intervention group 39.8%; control group 23.8% (p = 0.001) •Receive iron supplements: Intervention group 91.6%; control group 87.4% (p = 0.170) •Receive diet counselling: intervention group 95%; control group 89.3% (p = 0.027) •Attend less than 4 antenatal visits: intervention group 3.6%; control group 9.7% •Intra uterine fetal death: Intervention group 1%, control group 1.5% (p = 0.715) •Neonatal deaths: Intervention group 1%, control group 3.4% (p = 0.269)the proportion of infants who cried immediately at birth: Intervention group 97.4% and control group 95.6% (p = 0.765). |
| Pai et al., 2013 | •Adherence to taking iron supplements | •Hb level (g/dL) |
•Average Hb levels decreased slightly (by 0.10 g/dL) in the control group but increased (by 0.32 g/dL) in the intervention group. •Taking into account the difference of the differences Hb levels of the intervention group improved by 0.43 g/dL (95% CI = −0.13–0.98) more than the control group (p = 0.13). |
Note. SMS: short message service.
3.5. Effect on mHealth intervention on nutritional‐related outcome
Results from the study conducted in Kenya indicated that a high proportion of the pregnant women in intervention group (91.6%) received iron supplements from ANC compared with control group (87.4%). Author also reported high proportion of pregnant women in intervention group (39.8%) received vitamin supplements compared with control group (23.8%), although the exact type of vitamin supplemented was not reported (Fedha, 2014). However, it is not clear if the participants who received supplements actually used them.
The second study, which was conducted in India, reported that the proportion of women consuming iron and calcium supplements provided during antenatal visits for more than 3 months after enrolment was higher in intervention group compared with control group (81% vs. 69%). Similarly, the proportion of women with anaemia was higher in the control group compared with intervention group (45% vs. 36%). In addition, the proportion of pregnant women needed blood transfusion was significantly higher in control group (3%) than intervention group (0%). In addition, adequate weight gain, which was defined as >10 kg, was observed in 35% of women in the intervention group, compared with 25% of women in control group. A lower proportion of women in the intervention group delivered babies of low birth weight compared with the control group (30% vs. 35%), respectively (Bangal et al., 2017).
The third study, also conducted in India, reported that the mean haemoglobin levels decreased slightly in the control group but increased in the intervention group by 0.32 g/dl (Pai et al., 2013).
The final study, which was conducted in Indonesia, indicated high adherence to taking iron supplements after the SMS intervention from 5.3% before intervention to 7.9% after intervention. The study also reported an increase in the proportion of pregnant women with haemoglobin levels greater or equal to 11 g/dl (47.22% vs. 52.78%) before and after intervention respectively. In addition, low adherence to iron supplementation decreased from 76.3% before the intervention and 71.1% after the intervention (Anitasari & Andrajati, 2017). Table 2 summarises the results of these studies.
3.6. Quality assessment
Two of the studies were evaluated as being of positive quality (Fedha, 2014; Pai et al., 2013), and the remaining two were scored as neutral (Anitasari & Andrajati, 2017; Bangal et al., 2017) using the quality criteria checklist for primary research developed by the Academy of Nutrition and Dietetics (Academy of Nutrition and Dietetics, 2016). Two studies were scored neutral as they were not exceptionally strong as per the validity criteria. The scores for each included study are outlined in Table 3. No study had a negative quality rating.
Table 3.
Quality assessment of mobile health interventions aimed at improving nutrients intake of pregnant women in low and lower middle income countries
| Anitasari & Andrajati, 2017 | Bangal et al., 2017 | Fedha, 2014 | Pai et al., 2013 | |
|---|---|---|---|---|
| Relevance questions | ||||
|
Yes | Yes | Yes | Yes |
|
Yes | Yes | Yes | Yes |
|
Yes | Yes | Yes | Yes |
|
Yes | Yes | Yes | Yes |
| Validity questions | ||||
|
Yes | Yes | Yes | Yes |
|
Yes | No | Yes | Yes |
|
Yes | Unclear | Yes | Yes |
|
No | No | Yes | Yes |
|
No | Unclear | Unclear | Yes |
|
Unclear | Yes | No | Yes |
|
Yes | Yes | Yes | Yes |
|
Yes | Unclear | Yes | Yes |
|
Yes | Unclear | Yes | Yes |
|
Unclear | Yes | Yes | Unclear |
| Quality rating | Neutral | Neutral | + | + |
Rating (+, −, neutral)
If most (six or more) of the answers to the validity questions are “no,” the report should be designated with a minus (−) symbol on the Evidence Worksheet. If the answers to validity criteria Questions 2, 3, 6, and 7 do not indicate that the study is exceptionally strong, the report should be rated as neutral on the Evidence Worksheet. If most of the answers to the above validity questions are “Yes” (including Criteria 2, 3, 6, 7, and at least one additional “yes”), the report should be designated with a plus symbol (+) on the Evidence Worksheet.
4. DISCUSSION
The current review aimed to investigate the effect of mHealth nutrition interventions on maternal and/or infant dietary intake and nutritional status in LLMIC. Findings from the current review suggest that mHealth interventions focusing on micronutrient supplementation are effective in improving nutrient intakes of pregnant women. Overall, few studies were identified, which show the topic is under researched in this setting and therefore supports future research efforts in this area. Despite no studies identified that addresses dietary intake, all of the included studies addressed the use of micronutrient supplements. The four included studies each addressed the use of iron supplements, which is among the micronutrient supplements recommended to be provided to pregnant women in LLMIC (WHO, 2016). Iron supplements are provided often in public hospitals as one component of antenatal care (WHO, 2012) and are provided at no cost to pregnant women during their antenatal visits in some of the LLMIC including Ethiopia (Gebreamlak, Dadi, & Atnafu, 2017) and Tanzania (Kearns, Hurst, Caglia, & Langer, 2014). However, adherence to iron supplementation is low due to reasons including memory lapses, general dislike of “pills,” gastro‐intestinal problems such as constipation (Pai et al., 2013), nausea, vomiting (Hyder, Persson, Chowdhury, & Ekstrom, 2002), and some beliefs that are culturally specific, for example, too many tablets would harm mother and/or her baby (Taye, Abeje, & Mekonen, 2015). The included studies in this review showed improved use of micronutrient supplements (Anitasari & Andrajati, 2017; Bangal et al., 2017;Fedha, 2014; Pai et al., 2013) after pregnant women received education and advice via their mobile phones, which demonstrate the potential for the use of this technology in LLMIC.
Two studies evaluated change in adherence to iron supplementation as the main outcome (Anitasari & Andrajati, 2017; Pai et al., 2013), which is important because pregnant women receive supplements routinely at ANC, which is documented, however checking the adherence of the women to taking these supplements is not collected routinely. Another study, reported improved maternal health care service utilisation as the main outcome and nutritional supplements are one of many maternal health care services addressed (Fedha, 2014). The second study, aimed at improving maternal health and pregnancy outcomes and therefore addressed nutritional supplements, weight gain, haemoglobin levels during delivery, and low birth weight as the indicators of nutrition‐related maternal health and pregnancy outcome (Bangal et al., 2017).
The level of detail reported in the description of the mHealth interventions of the four included studies do not meet some of the criteria of reporting mHealth interventions. In particular, the components of each intervention were not well described in the four studies in the review included. For example, Fedha, 2014 only stated that, the intervention group received prompts and advice about their health and scheduled visits. The actual content of the advice or information provided to participants was not stated in these studies nor was there sufficient information on the mHealth delivery mode used (Fedha, 2014). In 2016, the WHO mHealth technical evidence review group published guidelines for the reporting of interventions delivered via mHealth globally (Agarwal et al., 2016). These guidelines include 16 criteria that they recommend authors adhere to when reporting specific study details. Given these guidelines were published in 2016, it is not appropriate to compare two of the included studies conducted or published prior to this time (Fedha, 2014; Pai et al., 2013). However, use of the guidelines in reporting mHealth interventions when reporting future studies would ensure sufficient detail is included to assist in implementation and evaluation in other settings, including LLMIC. For example, user feedback and cost analysis are two key criteria that impact on scalability of mHealth interventions, however only two studies (Bangal et al., 2017; Pai et al., 2013) and one study (Pai et al., 2013) included in this review reported on these outcomes, respectively.
While there are some systematic reviews on the effect of mHealth interventions on maternal health in LLMICs, none has addressed nutrition outcomes specifically. The main outcomes reported from previous reviews are related to antenatal and postnatal care attendance, facility‐based birthing, attendance of skilled personnel at the birth, and vaccination rates (Colaci et al., 2016; Feroz et al., 2017; Lee et al., 2016; Watterson, Walsh, & Madeka, 2015). A scoping review by Oyeyemi & Wynn, 2015 reports how use of cell phones and radio could help in reducing delays in getting appropriate medical advice and assistance to pregnant women in LLMICs (Oyeyemi & Wynn, 2015). The current review is, to our knowledge, the first that has investigated the effect of mHealth interventions specifically on improving nutrient intakes of pregnant women in LLMIC. Only four studies met the inclusion criteria for the current review, with these mHealth interventions found to be effective in improving the use of micronutrient supplements by pregnant women in these settings. The limited number of studies is likely to be due to the fact that these technologies have only recently become readily available and utilised in these countries. Use of mHealth technologies can help address the language barrier and allow increased reach and access to health information and support among isolated communities (Aranda‐Jan, Mohutsiwa‐Dibe, & Loukanova, 2014). Given the importance of optimising nutrient intake during pregnancy, further high quality randomised controlled trials are needed to evaluate the effectiveness of mobile phones on improving nutrient intakes of pregnant women in LLMIC.
The quality of studies included was either neutral or positive. However, there are specific methodological improvements that should be made by researchers seeking to conduct similar research. For the two studies scored as being neutral quality (Anitasari & Andrajati, 2017; Bangal et al., 2017), most questions were rated as “no” or “neutral” for the validity questions. It is not easy to blind subjects and or research assistants on this type of intervention, especially if mode of delivery is SMS as it is easy to tell who received messages and who did not. However, it is possible to blind those who analyse and interpret the findings. Three of the included studies (Anitasari & Andrajati, 2017; Bangal et al., 2017; Fedha, 2014) did not mention the use of blinding in addressing bias at any stage of study implementation, and the use of blinding to minimise bias is one of the quality assessment criteria and therefore an important consideration for mHealth researchers.
A limitation of this systematic review is that it has included studies conducted in LLMIC and therefore there might be studies conducted in other settings that address the effectiveness of mHealth intervention towards improving dietary intakes of pregnant women. A further limitation is the language of the studies included, which resulted in the exclusion of all studies published in language other than English; we might have missed the articles published in other languages but the relevant intervention, study design, participant, and outcome.
The strengths of the review include the systematic and comprehensive search strategy using eight databases. Hand search also was performed, which allows the identification of articles that could not be obtained using the selected databases. Another strength is the use of two independent reviewers to screen articles and assess the quality of the included studies. In addition, the third reviewer solves conflicts independently, all these improve fairness.
5. CONCLUSION
The current systematic review indicates that mHealth interventions can be used to improve adherence to taking nutrient supplements in pregnancy, resulting in improvements of some indicators of nutritional status of pregnant women and birth outcomes in LLMIC. Many women use mobile phones in these settings and therefore, it is worthwhile to research the effectiveness of using these technologies to optimise not only micronutrient intake but also nutrition counselling, nutrition education, and the overall dietary intake.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
NS was involved in designing the review, developing search strategy, performing search, screening the articles (title and abstract; full text), data extraction, drafting the manuscript, and critical revision of the manuscript.
MR was involved in designing the review, resolving screening conflicts, resolving quality assessment conflicts, critical revision of the manuscript, and supervision.
TB was involved in designing the review, resolving screening conflicts, resolving quality assessment conflicts, critical revision of the manuscript, and supervision.
CC was involved in designing the review, resolving screening conflicts, resolving quality assessment conflicts, critical revision of the manuscript, and supervision.
AA screening of the articles (title and abstract; full text), critical revision of the manuscript.
ACKNOWLEDGMENTS
Authors wish to thank the faculty librarian, Debbie Booth for her help with development of search strategy, Clare Cummins, for her contribution in quality assessment of the articles and reviewing data extraction tables. Naomi Saronga undertook this research as a partial fulfilment of research degree (PhD in Nutrition and Dietetics) with the University of Newcastle, Australia, and is supported by an International Postgraduate Award Scholarship. Clare E. Collins is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship and a Gladys M Brawn Senior Research Fellowship from Faculty of Health and Medicine, the University of Newcastle, Australia. Tracy Burrows is supported by a Gladys M Brawn Senior Research Fellowship from the Faculty of Health and Medicine, the University of Newcastle, Australia.
APPENDIX 1: SEARCH STRATEGY A.
User‐Computer Interface or multimedia or cell phones or computers or handheld or Mobile Applications or mobile health or mhealth or m‐health or ehealth or e‐health or digital health or smartphone or smartphones or phone or phones or cellphone or cellphones or telephone or mobile application or mobile app* or mobile technolog* or health technolog* or health application or health applications or iPad or sms or mms or text messag* or USSD or pda or laptop* or palmtop* or palm‐top* or Personal Digital Assistant* or computer* or interactive voice response or multimedia or mobilephone or iphone or ipod or podcast* or android* or palm pilot* or wireless device* or wireless technolog* or telemonitor or elearning or e‐learning or electronic mail or email or e‐mail or internet or web based or website or social media or social network or chat or online AND
Dietary intake* or Nutrient* Intake* or Food intake* or Feeding Behavio* or Energy Intake or Eating or Diet or Food or Dietary Pattern* or Dietary habit* or Eating pattern* or Eating Habit* or Eating Behavio* or Feeding pattern* or feeding habit* or Nutrition* behavio* or Nutrition* habit or dietary evaluation or Nutrition or Nutrient* or Supplement* or Nutrition* supplement* or iron or folic acid or folate or vitamin* or mineral* or vitamin supplement* or mineral supplement* AND
Pregnancy or pregnan* or expectant mother* or prenatal care or pregnant women or maternal or reproductive health or family planning or newborn* or antenatal or obstetric* or postnatal or postpartum or prenatal or perinatal or infant* or interpartum or maternal health services or delivery or antenatal care or maternal care AND
Low income or lower middle income or developing countr* or resource poor or Afghanistan or Guinea or Rwanda or Benin or Guinea Bissau or Senegal or Burkina Faso or Haiti or Sierra Leone or Burundi or Korea Dem People* Rep or Somalia or Central African Republic or Liberia or South Sudan or Chad or Madagascar or Tanzania or Comoros or Malawi or Togo or Congo Dem Rep or Mali or Uganda or Eritrea or Mozambique or Zimbabwe or Ethiopia or Nepal or Gambia or Niger or Angola or Indonesia or Philippines or Armenia or Jordan or Sao Tome) and Principe) or Bangladesh or Kenya or Solomon Islands or Bhutan or Kiribati or Sri Lanka or Bolivia or Kosovo or Sudan or Cabo Verde or Kyrgyz Republic or Swaziland or Cambodia or Lao PDR or Syrian Arab Republic or Cameroon or Lesotho or Tajikistan or Congo Rep or Mauritania or Timor Leste or Cote dIvoire or Micronesia Fed Sts or Tunisia or Djibouti or Moldova or Ukraine or Egypt Arab Rep or Mongolia or Uzbekistan or El Salvador or Morocco or Vanuatu or Georgia or Myanmar or Vietnam or Ghana or Nicaragua or West Bank) and Gaza) or Guatemala or Nigeria or Yemen Rep or Honduras or Pakistan or Zambia or India or Papua New Guinea
Saronga NJ, Burrows T, Collins CE, Ashman AM, Rollo ME. mHealth interventions targeting pregnancy intakes in low and lower‐middle income countries: Systematic review. Matern Child Nutr. 2019;15:e12777 10.1111/mcn.12777
REFERENCES
- Academy of Nutrition and Dietetics . (2016). Evidence analysis manual: Steps in the academy evidence analysis process. Retrieved from Chicago, USA: http://www.andeal.org
- Agarwal, S. , LeFevre, A. E. , Lee, J. , L'Engle, K. , Mehl, G. , Sinha, C. , & Labrique, A. (2016). Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ, 352, i1174 10.1136/bmj.i1174 [DOI] [PubMed] [Google Scholar]
- Anitasari, D. , & Andrajati, R. (2017). Effectiveness of short message service reminders and leaflets in complying with iron supplementation in pregnant women in Depok City, Indonesia. Asian Journal of Pharmaceutical and Clinical Research, 10(17), 42 10.22159/ajpcr.2017.v10s5.23092 [DOI] [Google Scholar]
- Aranda‐Jan, C. , Mohutsiwa‐Dibe, N. , & Loukanova, S. (2014). Systematic review on what works, what does not work and why of implementation of mobile health (mHealth) projects in Africa. BMC Public Health, 14(1). 10.1186/1471-2458-14-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangal, V. , Borawake, S. , Gavhane, S. , & Aher, K. (2017). Use of mobile phone for improvement in maternal health: A randomized control trial. International Journal of Reproduction Contraception Obstetrics and Gynecology, 6(12), 5458–5463. 10.18203/2320-1770.ijrcog20175260 [DOI] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , de Onis, M. , … Maternal and Child Nutrition Study Group (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet, 382(9890), 427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- Colaci, D. , Chaudhri, S. , & Vasan, A. (2016). mHealth interventions in low‐income countries to address maternal health: A systematic review. Annals of Global Health, 82(5), 922–935. 10.1016/j.aogh.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Covidence . (2013). Systematic Review Software. Retrieved 2018 https://www.covidence.org/home
- Dodd, J. M. , Cramp, C. , Sui, Z. , Yelland, L. N. , Deussen, A. R. , Grivell, R. M. , … Limit Randomised Trial Group (2014). The effects of antenatal dietary and lifestyle advice for women who are overweight or obese on maternal diet and physical activity: The LIMIT randomised trial. BMC Medicine, 12, 161 10.1186/s12916-014-0161-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom, E. C. , Kavishe, F. P. , Habicht, J. P. , Frongillo, E. A. Jr. , Rasmussen, K. M. , & Hemed, L. (1996). Adherence to iron supplementation during pregnancy in Tanzania: Determinants and hematologic consequences. American Journal of Clinical Nutrition, 64(3), 368–374. 10.1093/ajcn/64.3.368 [DOI] [PubMed] [Google Scholar]
- Fedha, T. (2014). Impact of mobile telephone on maternal health service care: A case of Njoro division. Open Journal of Preventive Medicine, 04, 365–376. 10.4236/ojpm.2014.45044 [DOI] [Google Scholar]
- Feroz, A. , Perveen, S. , & Aftab, W. (2017). Role of mHealth applications for improving antenatal and postnatal care in low and middle income countries: A systematic review. BMC Health Services Research, 17(1), 704 10.1186/s12913-017-2664-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreamlak, B. , Dadi, A. F. , & Atnafu, A. (2017). High adherence to Iron/folic acid supplementation during pregnancy time among antenatal and postnatal care attendant mothers in governmental health centers in Akaki Kality sub city, Addis Ababa, Ethiopia: Hierarchical negative binomial poisson regression. PLoS ONE, 12(1), e0169415 10.1371/journal.pone.0169415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder, S. M. , Persson, L. A. , Chowdhury, A. M. , & Ekstrom, E. C. (2002). Do side‐effects reduce compliance to iron supplementation? A study of daily‐ and weekly‐dose regimens in pregnancy. Journal of Health, Population, and Nutrition, 20(2), 175–179. [PubMed] [Google Scholar]
- International Telecommunication Union. (Producer) . (2017, June 27, 2018). Statistics, mobile phone subscribers 2017. Retrieved from http://www.itu.int/en/ITU-D/Statistics/Pages/stat/default.aspx
- Kearns, A. , Hurst, T. , Caglia, J. , & Langer, A. (2014). Focused antenatal care in Tanzania. Retrieved from https://cdn2.sph.harvard.edu/wp-content/uploads/sites/32/2014/09/HSPH-Tanzania5.pdf
- Lee, S. H. , Nurmatov, U. B. , Nwaru, B. I. , Mukherjee, M. , Grant, L. , & Pagliari, C. (2016). Effectiveness of mHealth interventions for maternal, newborn and child health in low‐ and middle‐income countries: Systematic review and meta‐analysis. Journal of Global Health, 6(1), 010401 10.7189/jogh.06.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavalankar, D. V. , Trivedi, C. R. , & Gray, R. H. (1991). Levels and risk factors for perinatal mortality in Ahmedabad, India. Bulletin of the World Health Organization, 69(4), 435–442. [PMC free article] [PubMed] [Google Scholar]
- Menendez, C. , Todd, J. , Alonso, P. L. , Francis, N. , Lulat, S. , Ceesay, S. , … Greenwood, B. M. (1994). The effects of iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Transactions of the Royal Society of Tropical Medicine & Hygiene, 88(5), 590–593. 10.1016/0035-9203(94)90176-7 [DOI] [PubMed] [Google Scholar]
- Mohamed, K. (2004). Iron supplementation in pregnancy. The Cochrane Library, 4. [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & Group, P (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnam, N. M. (2015). Improving maternal nutrition for better pregnancy outcomes. Proceedings of the Nutrition Society, 74(04), 454–459. 10.1017/S0029665115002396 [DOI] [PubMed] [Google Scholar]
- Oyeyemi, S. O. , & Wynn, R. (2015). The use of cell phones and radio communication systems to reduce delays in getting help for pregnant women in low‐ and middle‐income countries: A scoping review. Global Health Action, 8, 28887 10.3402/gha.v8.28887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai, N. , Supe, P. , Kore, S. , Nandanwar, Y. , Hegde, A. , Cutrell, E. , & Thies, W. (2013). Using automated voice calls to improve adherence to Iron supplements during pregnancy: A pilot study Proceedings of the Sixth International Conference on Information and Communication Technologies and Development: Full Papers, 1, 153–163.
- Qiang, C. , Yamamichi, M. , Hausman, V. , Miller, R. , & Altman, D. (2012). Mobile applications for the health sector. Retrieved from Washington DC, USA: http://documents.worldbank.org/curated/en/751411468157784302/pdf/726040WP0Box370th0report00Apr020120.pdf
- Ruel, M. T. , & Alderman, H. (2013). Nutrition‐sensitive interventions and programmes: How can they help to accelerate progress in improving maternal and child nutrition? The Lancet, 382(9891), 536–551. 10.1016/S0140-6736(13)60843-0 [DOI] [PubMed] [Google Scholar]
- Sanou, B. (2017). ICT facts and figures 2017. Retrieved from Geneva, Switzerland: https://www.itu.int/en/ITU-D/Statistics/Documents/facts/ICTFactsFigures2017.pdf
- Schiffman, J. , Darmstadt, G. L. , Agarwal, S. , & Baqui, A. H. (2010). Community‐based intervention packages for improving perinatal health in developing countries: A review of the evidence. Seminars in Perinatology, 34(6), 462–476. 10.1053/j.semperi.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Shobeiri, F. , Begum, K. , & Nazari, M. (2006). A prospective study of maternal hemoglobin status of Indian women during pregnancy and pregnancy outcome. Nutrition Research, 26(5), 209–213. 10.1016/j.nutres.2006.05.008 [DOI] [Google Scholar]
- da Silva Lopes, K. , Ota, E. , Shakya, P. , Dagvadorj, A. , Balogun, O. O. , Pena‐Rosas, J. P. , … Mori, R. (2017). Effects of nutrition interventions during pregnancy on low birth weight: An overview of systematic reviews. BMJ Global Health, 2(3), e000389 10.1136/bmjgh-2017-000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondaal, S. F. , Browne, J. L. , Amoakoh‐Coleman, M. , Borgstein, A. , Miltenburg, A. S. , Verwijs, M. , & Klipstein‐Grobusch, K. (2016). Assessing the effect of mHealth interventions in improving maternal and neonatal care in low‐ and middle‐income countries: A systematic review. PLoS ONE, 11(5), e0154664 10.1371/journal.pone.0154664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taye, B. , Abeje, G. , & Mekonen, A. (2015). Factors associated with compliance of prenatal iron folate supplementation among women in Mecha district, Western Amhara: A cross‐sectional study. The Pan African Medical Journal, 20, 43 10.11604/pamj.2015.20.43.4894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar, J. , Merialdi, M. , Gulmezoglu, A. M. , Abalos, E. , Carroli, G. , Kulier, R. , & de Onis, M. (2003). Nutritional interventions during pregnancy for the prevention or treatment of maternal morbidity and preterm delivery: An overview of randomized controlled trials. Journal of Nutrition, 133(5 Suppl 2), 1606S–1625S. 10.1093/jn/133.5.1606S [DOI] [PubMed] [Google Scholar]
- Watterson, J. L. , Walsh, J. , & Madeka, I. (2015). Using mHealth to improve usage of antenatal care, postnatal care, and immunization: A systematic review of the literature. BioMed Research International, 2015, 1–9. 10.1155/2015/153402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WB . (2018). World Bank country and lending groups. Country Classification Retrieved from https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- WHO . (2011). New horizons for health through mobile technologies. Retrieved from Geneva, Switzerland: http://www.who.int/goe/publications/goe_mhealth_web.pdf
- WHO . (2012). Guideline: Daily iron and folic acid supplementation in pregnant women. Retrieved from Geneva, Switzerland: http://apps.who.int/iris/bitstream/handle/10665/77770/978924?sequence=1 [PubMed]
- WHO . (2016). WHO recommendations on antenatal care for a positive pregnancy experience. Retrieved from Switzerland: http://apps.who.int/iris/bitstream/handle/10665/250796/9789241549912-eng.pdf;jsessionid=8E9F4F5943560EC0D6EC77EA6DE8FED4?sequence=1 [PubMed]
- WHO, UNICEF, UNFPA, WB, & UNDP . (2015). Trends in Maternal Mortality: 1990 to 2015. Retrieved from


