Abstract
Background:
Stents are essential tools in the management of pulmonary arterial (PA) stenosis in patients with congenital heart disease. Although stents can usually be reexpanded as children grow, resistant in-stent or peri-stent obstruction can complicate the management of PA stents. Angioplasty with ultra-high-pressure (UHP) balloons may facilitate successful treatment of stent-associated PA stenoses that are resistant to high-pressure dilation.
Methods and Results:
We reviewed patients who underwent UHP angioplasty of in-stent or peri-stent PA stenoses that were resistant to high-pressure redilation. A resistant stenosis was defined as a residual balloon waist during high-pressure redilation of the stent, along with a pressure gradient and/or angiographic stenosis. Thirty-four lesions in 29 patients, including 8 with multiple concentric, overlapping, or adjacent stents, were included. The median age at UHP angioplasty was 9 years, and a median of 4 years had elapsed since unsuccessful high-pressure angioplasty. Thirty-one of the 34 (91% [81% to 100%]) UHP angioplasty procedures were successful in relieving the resistant stenosis. Balloon:waist diameter ratios were conservative (median 1.26), reflecting the ability of UHP balloons to “fracture” nearly all obstructions. After UHP dilation, lesion diameter increased by a median of 3.1 mm (36%), significantly more than after previous high-pressure dilation (1.3 mm, 19%; P<0.001). In 5 lesions, UHP angioplasty fractured the stent, allowing further vessel expansion. There were no vascular or other complications.
Conclusions:
UHP angioplasty was safe and effective for treatment of stent-related resistant PA stenosis in this series; the ability to fracture maximally expanded stents may extend the utility of stents in the pediatric population
Since their first use in patients with congenital heart disease nearly 20 years ago, balloon-expandable intravascular stents have become indispensable tools in the management of essentially all forms of large vessel obstruction.1–10 Although stents have a number of advantages and are useful in a variety of circumstances, there are also potential limitations and drawbacks to stenting. One of the most important potential disadvantages to the use of stents is in infants and very young children with congenital cardiovascular disease, that the child may outgrow the known maximum diameter of the stent. In general, endovascular stents can be expanded after their initial deployment,4,5 but a combination of factors may contribute to difficulty enlarging a stent beyond a certain point, including but not limited to (1) the diameter limitation intrinsic to all stents once they are fully expanded, (2) stress hardening that occurs on crimping and expansion, which may alter the effective strain modulus of the stent sufficiently to resist further expansion, (3) underlying resistant vascular stenosis, and (4) the vascular and neointimal reaction that occurs in response to dilation and stent placement. Until recently, there have been few options for the management of obstruction across previously placed stents that are fully expanded or highly resistant to redilation.
Since 2004, we have selectively employed angioplasty balloons that are layered with woven ultrahigh molecular weight polyethylene (UHMWPE) and have rated burst pressures as high as 27 atm, for ultra-high-pressure (UHP) angioplasty of resistant pulmonary arterial (PA) stenoses, reexpansion of vascular stents, dilation of calcified right ventricular outflow tract conduits, and other applications. In this study, we reviewed our early experience with UHP angioplasty of stenoses within or adjacent to previously placed stents in the PA circulation that had already been proven resistant to high-pressure dilation.
METHODS
Patients and Resistant Stenoses
The computerized database of the Cardiovascular Program at Children’s Hospital was queried to identify patients who under-went redilation of one or more previously placed PA stents with a Conquest or Atlas UHP balloon (Bard Peripheral Vascular, Inc, Tempe, Ariz) inflated to high pressure and who had previously undergone redilation (ie, dilation after the initial deployment) of the same stent with another type of balloon. Patients may have undergone initial stent placement at Children’s Hospital or elsewhere, but had to have undergone at least one attempted redilation of the stenosis with a high-pressure balloon at Children’s Hospital as the most recent intervention on the stenosis in question.
This cohort was reviewed to determine whether there was a resistant stenosis within or immediately adjacent to the previously placed and redilated stent. A resistant stenosis (referred to hereafter as “lesion”) was considered one that was not success- fully reexpanded by a previous attempt at redilation and was specifically defined by the presence of a residual waist in the stent or PA immediately adjacent to the stent on the balloon used for the previously attempted reexpansion, along with either a pressure gradient or obvious angiographic stenosis relative to the proximal and distal PA branches. The reported assessment of the operator regarding the success of the dilation was also taken into consideration. The resistant waist on a high-pressure balloon may have been seen during a previous catheterization at Children’s Hospital Boston, or during the same procedure as the UHP dilation, but a waist on the balloon used to implant the stent initially was not sufficient for inclusion. We included lesions in which the residual waist on a high-pressure balloon was due to a fully expanded and shortened stent being too small for the vessel as well as lesions with multiple concentric, overlapping, or immediately adjacent stents.
Cardiac Catheterization and Stent Dilation
Catheterization reports and angiograms were reviewed from the catheterizations during which the stent was initially placed, during which the lesion was unsuccessfully redilated with a non-UHP balloon, and at which the UHP balloon was used, to determine the clinical details of stent placement and redilation. The location of the lesion, type, size, and number of stents, number and details of previous and current stent expansions, and adverse events were recorded. Types and sizes of high-pressure balloons were recorded, but inflation pressures were not always available. Stent appearance was described, preexisting stent fractures and neointimal proliferation within the stent were noted, diameters of the stent at multiple points of the lesion, and of the adjacent PA were measured, along with the diameter of the waist in the UHP balloon. The balloon waist was measured on the first UHP balloon, when the balloon proximal and distal to the lesion was fully or nearly fully expanded. The balloon:waist ratio was calculated as the ratio of the diameter of the first UHP balloon inflated to high pressure, to the diameter of the waist on the balloon and was not necessarily the same as the balloon:lesion diameter ratio in cases with in-stent neointimal proliferation or peri-stent stenosis. Hemodynamic data were recorded before and after high-pressure and UHP dilations. For lesions within the stent, the lesion diameter was considered the minimum diameter of the lumen within the stent. Lesions spanning the edge of the stent were considered in-stent lesions.
Since 2004, Conquest (5 to 12 mm diameter) and Atlas (12 to 26 mm diameter) balloons have been used in our laboratory at the discretion of the interventional cardiologist. These balloons were developed and approved for treatment of stenotic hemodialysis fistulas and contain a cross-matrix woven layer of UHMWPE. They have rated burst pressures ranging from 18 to 27 atm, but can be inflated to substantially higher pressures without rupturing.11 The type of balloon was determined on the basis of size (the only diameter at which there is overlap between the 2 balloons is 12 mm). Balloon size was determined at the operator’s discretion, typically, in part, on the basis of information from previous unsuccessful attempts to expand the stent with other balloons of known diameter, and from measured waist, lesion, and/or adjacent PA diameters. In general, balloon sizing was more conservative (lower balloon:waist diameter ratio) than for standard or high-pressure PA angioplasty procedures. The balloon was inflated with a Max30 Inflation Device (Bard Peripheral Vascular, Inc, Tempe, Ariz), which has a gauge that reads up to 30 atm but has a higher inflation capacity, at the discretion of the operator, usually until the waist was eliminated, the balloon ruptured, the pressure exceeded 30 atm, or there was another clinical indication for deflation. Balloon pressures >30 atm were reported as 30 atm. Angiography was performed before and after dilations.
If multiple UHP balloon sizes were used, the balloon that first eliminated the waist was used for analysis in cases of successful UHP balloon dilation, whereas the final attempted UHP balloon was used for analysis in cases of unsuccessful UHP angioplasty.
Data Analysis
The primary outcomes were (1) a categorical assessment of successful or unsuccessful UHP angioplasty, and (2) relative change in narrowest lesion diameter between UHP and previous non-UHP dilations. A successful UHP angioplasty was defined as one in which the resistant waist within or adjacent to the stent was eliminated, or in which a completely expanded and shortened stent was fractured by UHP angioplasty, or in which there was a small residual waist in the balloon but with a diameter that equaled or exceeded that of unobstructed proximal and distal PA segments. The unit of analysis was the lesion. For cases in which there was >1 lesion in a given patient, the lesions were considered to be independent. Each lesion served as its own control, by virtue of the fact that all vessels studied were resistant to a previous attempt at reexpansion with one or more high-pressure balloons. Comparison of increases in lesion diameter between UHP and previous high-pressure angioplasty procedures was performed by paired t test analysis. The proportion of patients who underwent successful UHP angioplasty is reported, with 95% CIs. Data are presented as mean±standard deviation or median (range). The study was conducted according to a protocol approved by the Committee for Clinical Investigations at Children’s Hospital. All authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Patients and Resistant Stenoses
Thirty-four lesions in 29 patients satisfied our inclusion criteria, and were treated with UHP balloon angioplasty after previous unsuccessful attempts at redilation. Patient demographics and lesion-related data are summarized in the Table. The median age at the time of the most recent high-pressure angioplasty procedure was 9 years (2 to 28 years), and at the time of UHP dilation was 14 years (2 to 40 years); a median of 4 years (0 to 12 years) had elapsed between the 2 procedures. Three examples are depicted in Figures 1 through 3. The primary catheterizing physician was the same for the UHP and previous high-pressure angioplasty procedures in all but 3 lesions.
Table.
Patient Demographics and Lesion Data
| Variable | No. Patients | Percent of Total |
|---|---|---|
| Patients | 29 | 100 |
| Primary diagnosis | ||
| Tetralogy of Fallot | 19 | 66 |
| With pulmonary atresia | 17 | 59 |
| With pulmonary stenosis | 2 | 7 |
| Truncus arteriosus | 4 | 14 |
| Transposition/malposition complexes | 3 | 10 |
| Peripheral pulmonary stenosis | 3 | 10 |
| Age, yrs |
||
| 1–5 | 2 | 7 |
| 5–18 | 24 | 83 |
| >18 | 3 | 10 |
| Lesions | 34 | 100 |
| No. stents |
||
| 1 | 26 | 76 |
| 2 | 7 | 21 |
| 3 | 1 | 3 |
| Vessel location |
||
| Main left pulmonary artery | 14 | 41 |
| Main right pulmonary artery | 13 | 38 |
| Left lower pulmonary artery | 4 | 12 |
| Right intermediate pulmonary artery | 2 | 6 |
| Right upper pulmonary artery | 1 | 3 |
| Waist location |
||
| In stent | 26* | 77 |
| Adjacent to stent | 10 | 29 |
| Stent type |
||
| Palmaz iliac (P128, P188, P308) | 18 | 41† |
| Palmaz Genesis | 16 | 36 |
| Palmaz renal (P104, P154, P204) | 9 | 20 |
| Palmaz coronary | 1 | 2 |
Two patients had separate waists within and adjacent to the stent.
Percentage of total stents (n=44).
Figure 1.
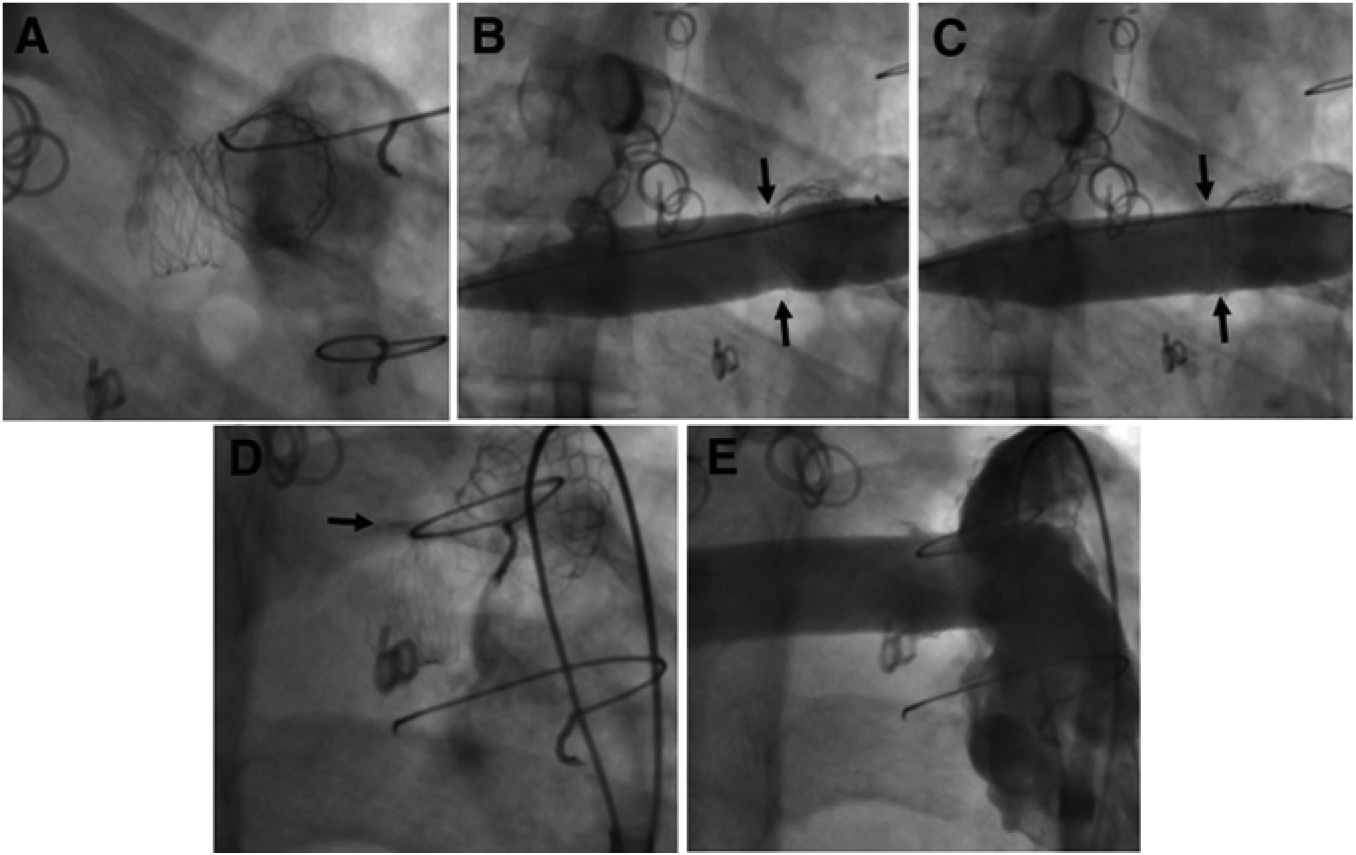
A, in this patient with tetralogy of Fallot, there is a resistant stenosis associated with a previously placed Palmaz P188 stent in the proximal right PA. This stent could not be further expanded with a 15-mm high-pressure balloon. B, A 14-mm diameter Atlas is inflated, and initially reveals a waist (arrows) resulting from maximal expansion of the stent. C, At 20-atm inflation pressure, the stent is fractured longitudinally and the waist is eliminated (arrows). D, The fractured edges of the stent can be appreciated superiorly (arrow). E, After UHP dilation, the caliber of the right PA is uniform.
Figure 3.
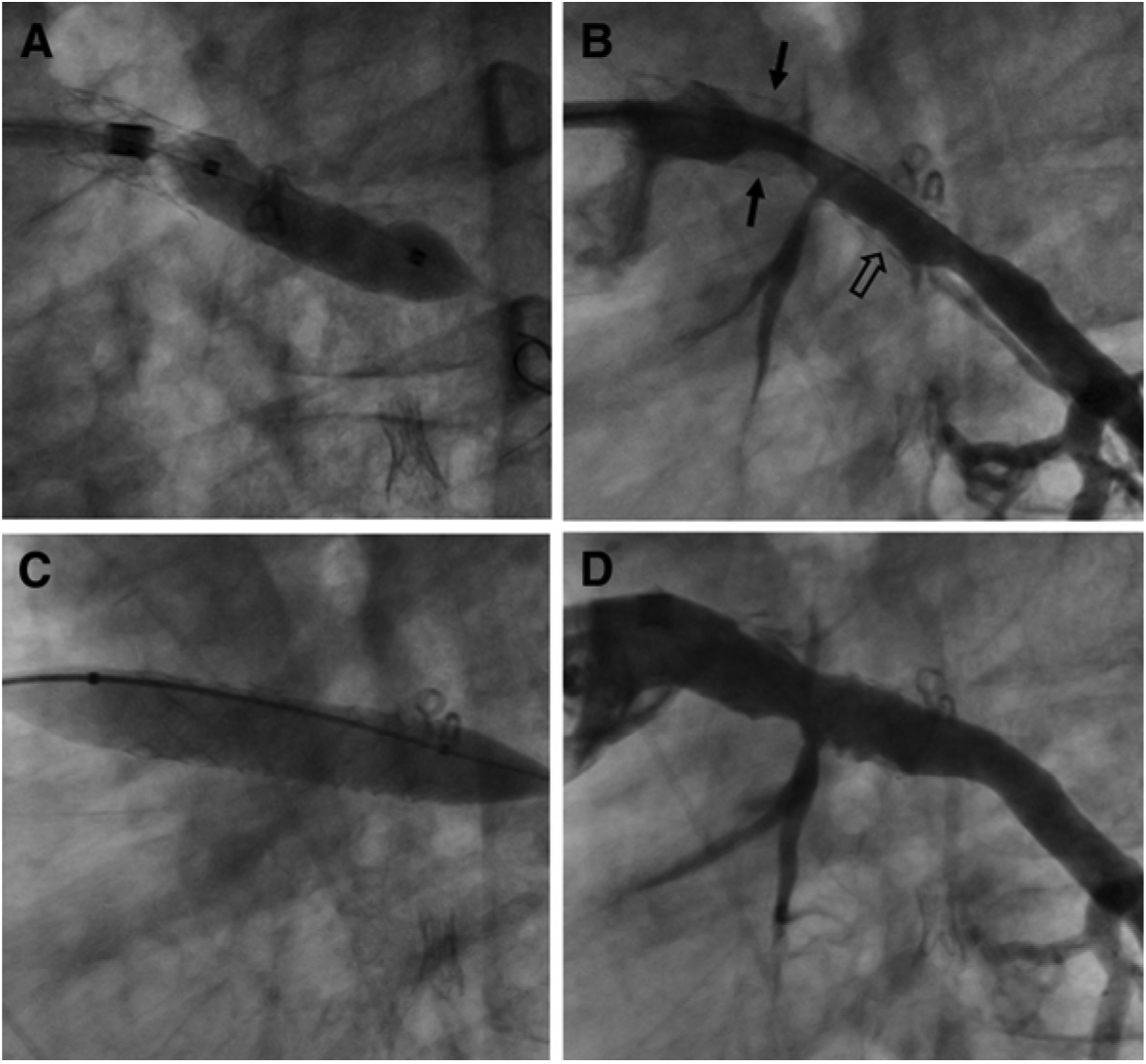
A, A 15-mm balloon expanded to high pressure was unable to relieve the waist in this lesion with 2 concentric stents, which is caused by complete expansion and shortening of a Palmaz P154 stent, across which a Palmaz P188 stent has been placed. B, The waist and the fully expanded outer stent can be appreciated in this fluoroscopic image (arrows). C and D, A 12-mm Atlas balloon inflated to 28 atm was successful at relieving the resistant waist in the stents and fracturing the fully expanded and shortened Palmaz P154 stent (arrows).
In 8 of the treated lesions (24%), there were multiple stents; these were adjacent without overlap in 2 lesions, partially overlapping in 4 lesions, and concentric in 2 (ie, the second stent completely covered the first stent at both ends; Figure 3). In 17 lesions (50%), the distance between the stent and angiographic contrast column was >1 mm at the level of the stenosis, consistent with neointimal proliferation (Figure 2). Most resistant waists (77%) were within rather than adjacent to the stent, and in 2 patients there were separate waists both within and adjacent to the stent (Table). In 26 patients, additional PA branches were dilated during the UHP catheterization.
Figure 2.
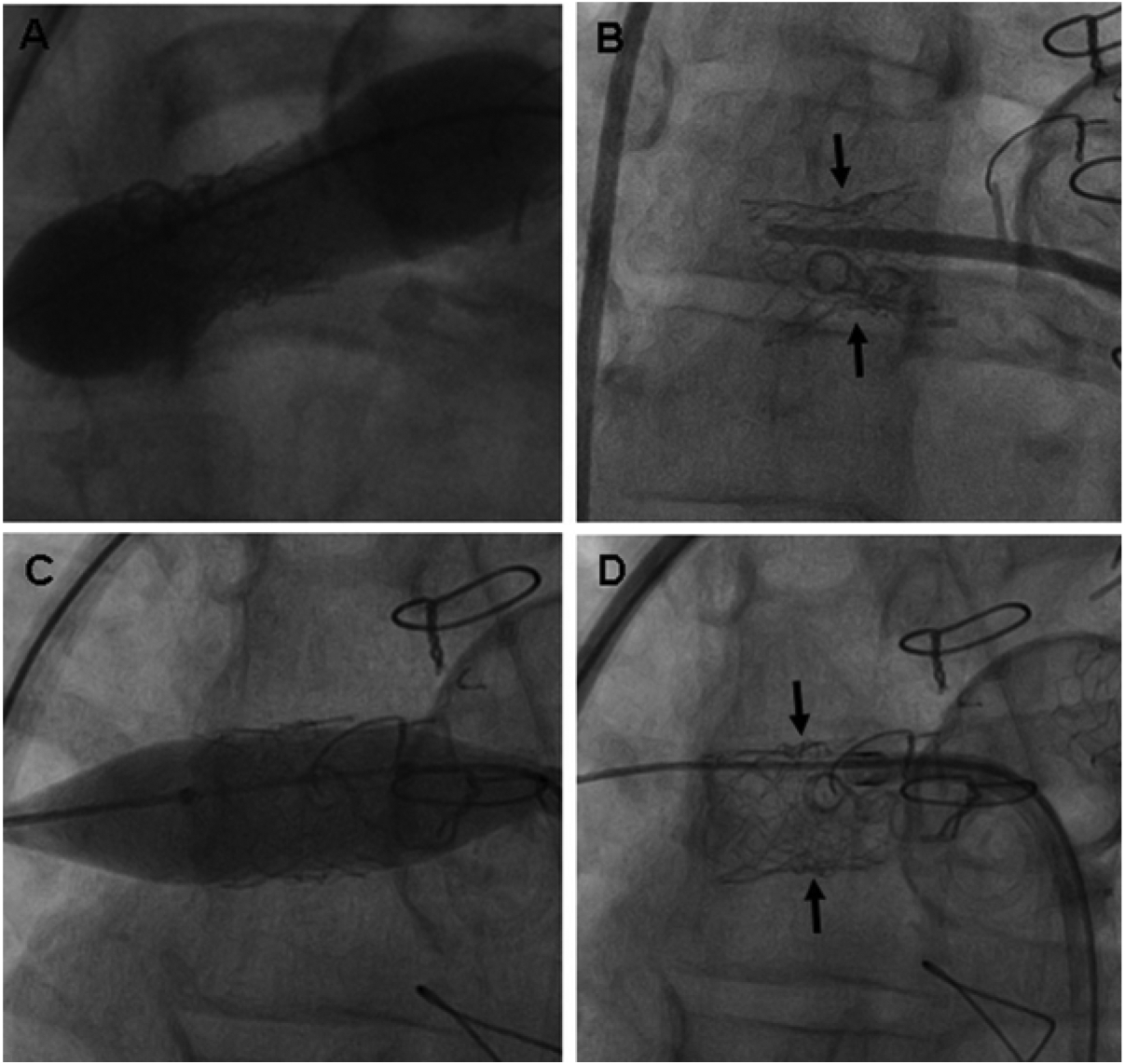
A, In this patient with tetralogy of Fallot and multiple branch PA stenoses, the left lower PA stent (Palmaz P154) was resistant to high-pressure redilation using a 7-mm balloon. At the subsequent catheterization, there was stenosis and neo- intimal proliferation within the left lower PA stent (open arrow), and substantial neointima within the distal portion of the proximal left PA stent (arrows). The left lower PA distal to the stent and the segment of PA between the 2 stents are also obstructed. C, A 10-mm Conquest balloon inflated to 28 atm successfully expanded the left lower PA stent and the adjacent PA stenoses. D, After UHP angioplasty, the left lower PA stent and adjacent PA segments are substantially enlarged.
Cardiac Catheterization and Stent Radiation
Prior High-Pressure Redilation
The most recent previous high-pressure balloon dilation was performed during the same catheterization as the UHP balloon dilation in 4 lesions (12%) and at a previous catheterization in the other 30. A variety of high-pressure balloons were used for the most recent high-pressure dilation, although the exact inflation pressure was often not recorded.
By definition, all of the previous high-pressure dilation procedures were unsuccessful, with resistant waists and angiographic/hemodynamic evidence of obstruction. In many cases, however, the redilation did achieve some enlargement of the stent. During the most recent previous dilation, the median increase in lesion diameter was 1.3 mm (0 to 3 mm), which represented a median change of 19% (0% to 91%). Balloon rupture at inflation pressures above the rate burst pressure occurred in 7 of the 34 (21%) high-pressure balloon dilation procedures.
UHP Redilation
At the time of UHP redilation, a Conquest balloon was used in 10 lesions, with a median initial diameter of 9 mm (6 to 10 mm), and an Atlas balloon was used in 24 lesions, with a median initial diameter of 14 mm (12 to 18 mm). The median inflation pressure for Conquest balloons was 24 atm (22 to 30 atm), and for Atlas balloons was 18 atm (14 to 30 atm). In 19 lesions, two or more sizes of UHP balloon were used. The median balloon:waist ratio was 1.26 (range: 1.12 to 1.62, interquartile range: 1.26 to 1.30).
Thirty-one of the 34 (91% [95% CIs 81% to 100%]) UHP balloon angioplasty procedures were successful in relieving the resistant in-stent or peri-stent PA stenosis (Figures 1 through 3). In 27 dilations (79%), the residual waist was eliminated. After UHP dilation, the minimum lesion diameter increased by a median of 3.1 mm (1 to 8.7 mm), or 36% (7% to 220%). By paired analysis, the increase in minimum lesion diameter after UHP dilation was significantly greater than after high-pressure dilation, as was the relative increase (ratio of increase to starting diameter) (both P<0.001).
After UHP balloon dilation, there was a residual waist in 7 stents. In all but one of these, an Atlas balloon was used. In these 7 cases, the median change in lesion diameter was 20%, with an increase in absolute diameter of 2.0 mm. Of the 7 UHP balloon dilations with residual waists, 4 were considered successful based on the elimination of a pressure gradient and angiographic stenosis, with near resolu- tion of the waist. In one patient, who had undergone previous partial surgical resection of a stent at the site of the lesion, UHP angioplasty was unsuccessful because of puncture of multiple Atlas balloons by the sharp end of a cut strut. In the other 2 failed UHP dilations, the balloons were inflated to relatively modest pressures (14 and 18 atm), and the decision was made by the operator not to proceed; no anatomic or technical reasons for failure were identified.
In 5 lesions (11%), the stent fractured as a result of UHP angioplasty (Figures 1 and 3). Of these, 4 were longitudinal breaks of fully shortened Palmaz (Cordis Endovascu- lar, Warren, NJ) iliac or renal stents, whereas 1 Palmaz Genesis (Cordis Endovascular, Miami, Fla) stent incurred multiple circumferential fractures without embolization of the fragments. Aside from the case in which multiple UHP balloons were punctured, there were no balloon ruptures. There were no vascular ruptures or significant tears associated with UHP angioplasty.
DISCUSSION
In our experience, UHP angioplasty balloons fabricated with woven UHMWPE have proven to be consistently effective for treatment of resistant obstruction within or adjacent to previously implanted PA stents, with successful dilation in 91% of lesions proven to be resistant with other balloons, and no dilation-related complications. The obstructions included in this series took many forms, including completely expanded and shortened stents that were too small for the vessel, obstructions treated with multiple concentric or overlapping stents, obstructions immediately distal or proximal to the stent, and obstructions in patients of various ages with a range of underlying cardiovascular anomalies. In 5 of 34 lesions, UHP angioplasty was effectively able to enlarge a completely expanded and shortened stent by breaking it longitudinally. Because limited expansion capacity is one of the factors that often figure into decisions about PA stent placement in infants and small children, the ability to break fully expanded and shortened stents with UHP balloons should alter the risk:benefit analysis when considering whether stenting is appropriate in very young patients.
Resistant Stenosis Within or Adjacent to Previously Place PA Stents
Lesions that cannot be treated effectively with a standard or high-pressure angioplasty balloon are sometimes termed “resistant” stenoses.12–14 Resistant stenosis across or adjacent to previously implanted PA stents may be due to vascular or stent-related factors. In simple terms, vascular factors may include a resistant stenosis of the vessel itself that was not actually relieved at the time of stent placement, fibrosis at the site of surgical anastomosis or augmentation, mechanical effects of PA remodeling after previous angioplasty/stenting injury, or resistance imparted by in-stent or peri-stent neointimal proliferation. Stent-related factors contributing to a resistant stenosis may include full expansion/shortening of the stent and stress hardening due to crimping, expansion, and reexpansion.
Effective balloon angioplasty for PA stenosis requires disruption (ie, tearing) of the intima and/or media.15 The force necessary to achieve this disruption varies from lesion to lesion, and likely depends on a variety of factors. The force applied to a stenosis is a function of the surface tension generated at the site of stenosis, or the “waist,” which depends on the size and compliance of the dilating balloon, as well as the pressure to which the balloon is inflated. If adequate angioplasty is not achieved before stenting at the time of stent placement, that is, if the resistant lesion is stretched without tearing the intima and/or media, the presence of a stent within the underlying resistant stenosis may create an even more resistant lesion.
Most lesions in this series were located within previously placed stents, but a subset were immediately adjacent to the stent. Lesions adjacent to a stent were included in the study because they pose some of the same problems as in-stent stenosis and are often the lesion for which the stent was placed. Most stents used for PA stenting are closed-cell designs that necessarily shorten as they are expanded. Because the original length of the stent and location of stent placement relative to the stenosis are variable, the distance between the waist and the edge of the stent may be on the order of only 1 to 2 mm. As a result of stent shortening, even when a stent is well centered over an underlying PA stenosis, the relationship between the stent and the underlying stenosis may shift as the stent is reexpanded and shortened. On basis of the review of serial angiograms in these patients, it is our impression that, in some cases, the location of a resistant PA stenosis immediately adjacent to a stent is the result of the original stent shortening off of the underlying resistant stenosis with reexpansion.
As we and others have previously reported, cutting balloons, which score the intima and create planes for intimal/medial disruption, are often highly effective for the treatment of resistant PA stenoses.12,16,17 However, cutting balloons are not appropriate for treating resistant in-stent stenosis unless the stenosis is due only to neointimal tissue, and are only available in diameters up to 8 mm at this time, so they cannot be used for larger lesions.
UHP Angioplasty Balloons
The UHP balloons used in this series were developed for the treatment of resistant stenoses related to dialysis fistulas, for which they have been shown to be effective.11 UHMWPE is a very long-chain form of polyethylene with a high tensile strain modulus and toughness. The woven UHMWPE that coats the Conquest and Atlas balloons is applied with the fibers aligned orthogonally in the long-axis and true circumferential directions. Accordingly, at ultrahigh inflation pressures, even when there is a waist in the balloon, the UHMWPE fibers do not elongate and the phenomenon of eccentric overexpansion (ie, “dog-boning”) does not occur. With proper selection of balloon size, this allows all of the developed surface tension to be applied at the point of resistance (waist), which makes for a balloon that is not only very strong but also mechanically efficient at high pressures. The noncompliant behavior of UHMWPE-coated balloons also minimizes the potential adverse effects of eccentric overexpansion of more compliant balloons, namely, transmission of excess wall stress to vascular segments adjacent to the lesion, which may increase the risk of vascular injury.
Safety
There were no PA ruptures or other adverse events associated with UHP angioplasty in this series. As long as technique and balloon size selection are appropriate, there is no reason that UHP angioplasty should pose any greater risk than conventional or high-pressure angioplasty. Given the very high stresses that can be generated with noncompliant UHP balloons, we believe that conservative balloon sizing is critical to minimize the risk of PA rupture. In this series, the balloon:waist diameter ratios were generally in the 1.2 to 1.3 range, which is smaller than typically applied with standard or high-pressure PA angioplasty.13,14
In 5 cases, UHP angioplasty resulted in axial (ie, longitudinal) fracture of the stent. In theory, the free edges that are created when the stent fractures have the potential to puncture the PA. This complication did not occur in our experience, and mechanistically seems unlikely, as long as the balloon is not markedly oversized and the orientation of the stent edges is not altered substantially relative to the vessel wall.
Because of the woven arrangement of UHMWPE fibers, in which there are small spaces within the weave, these balloons may be punctured by sharp calcium spicules or cut stent edges, as occurred in one patient in this series and several others in whom we have used these balloons for different indications. Even in such cases, however, the defects in the balloon were very small, as the UHMWPE fibers appeared not to tear, and it was possible to deflate and resheathe the balloon adequately. We have not experienced any cases of explosive balloon rupture in this series or with other applications of these balloons.
Operators should be aware that the balloons used in this series, in particular the Atlas, are relatively stiff and have a long nose that may limit their applicability in some circumstances. In addition, it is important to pay close attention to the nose during balloon inflation, particularly if the balloon is inflated across a curved/angled vessel, as straightening of the balloon at high pressure may result in substantial excursion of the nose, potentially posing a risk of distal vascular injury.
Other uses for UHP Balloons in Patients with Congenital Heart Disease
In our experience, UHP balloon angioplasty has also proven effective for the treatment of resistant PA stenoses unrelated to previously placed stents, or for dilating calcified right ventricular outflow tract conduits (data not presented). We have also found UHP balloons useful for dilating PA branches that have been jailed by a previously or concurrently placed stent, through a cell of the jailing stent, often fracturing the struts of the cell that is dilated and allowing adequate expansion of the jailed branch.
Limitations
This study is limited by its retrospective, nonrandomized design. Also, there is no standard definition of or absolute means of determining a “resistant stenosis,” and we made this judgment based on a retrospective review of angio- grams and reports, taking into account the assessment of the operator if clearly stated. Accordingly, it is possible that lesions were misclassified. It is possible that vascular remodeling in the interim between high-pressure and UHP dilation may have altered the compliant characteristics of the lesion, such that the comparison between temporally separated high-pressure and UHP procedures may be confounded.
CONCLUSIONS
UHP angioplasty using UHMWPE-coated balloons seems to be effective and safe for the treatment of in-stent and peri-stent PA stenoses that are refractory to redilation with conventional and high-pressure balloons. Further study will be needed to determine the appropriate use and safety of UHP balloons in the treatment of PA and other vascular stenoses in patients with congenital heart disease.
Clinical Perspective.
In a series of 29 patients with congenital heart disease and a total of 34 pulmonary arterial stenoses within or adjacent to previously placed stents that were resistant to previous attempts at high-pressure dilation, ultra-high-pressure balloon angioplasty was effective at relieving the obstruction 91% of the time. Although this is a small series, our results support the use of ultra-high-pressure balloons in this application. The ability of ultra-high-pressure balloons to fracture completely expanded and shortened stents may overcome one of the major limitations to the use of stents in small children and effectively extend the utility of stents in the pediatric population. Given the high inflation pressures and noncompliant behavior of ultra-high-pressure balloons, we believe that it is important to use conservative balloon:waist ratios to minimize potential trauma at and adjacent to the lesion being treated.
Sources of funding:
This study was supported in part by the Children’s Hospital Department of Cardiology Annie Gorman Fund.
Footnotes
Disclosures: None
REFERENCES
- 1.O’Laughlin MP, Perry SB, Lock JE, Mullins CE. Use of endovascular stents in congenital heart disease. Circulation. 1991;83:1923–1939. [DOI] [PubMed] [Google Scholar]
- 2.O’Laughlin MP, Slack MC, Grifka RG, Perry SB, Lock JE, Mullins CE. Implantation and intermediate-term follow-up of stents in congenital heart disease. Circulation. 1993;88:605– 614. [DOI] [PubMed] [Google Scholar]
- 3.Fogelman R, Nykanen D, Smallhorn J, McCrindle BW, Freedom RM, Benson LN. Endovascular stents in the pulmonary circulation: clinical impact on management and medium-term follow-up. Circulation. 1995;92:881– 885. [DOI] [PubMed] [Google Scholar]
- 4.McMahon CJ, El-Said HG, Grifka RG, Fraley JK, Nihill MR, Mullins CE. Redilation of endovascular stents in congenital heart disease: factors implicated in the development of restenosis and neointimal proliferation. J Am Coll Cardiol. 2001;38:521–526. [DOI] [PubMed] [Google Scholar]
- 5.Duke C, Rosenthal E, Qureshi SA. The efficacy and safety of stent redilatation in congenital heart disease. Heart. 2003;89:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon CJ, El Said HG, Vincent JA, Grifka RG, Nihill MR, Ing FF, Fraley JK, Mullins CE. Refinements in the implantation of pulmonary arterial stents: impact on morbidity and mortality of the procedure over the last two decades. Cardiol Young. 2002;12:445– 452. [DOI] [PubMed] [Google Scholar]
- 7.Forbes TJ, Rodriguez-Cruz E, Amin Z, Benson LN, Fagan TE, Hel- lenbrand WE, Latson LA, Moore P, Mullins CE, Vincent JA. The Genesis stent: a new low-profile stent for use in infants, children, and adults with congenital heart disease. Catheter Cardiovasc Interv. 2003;59:406 – 414. [DOI] [PubMed] [Google Scholar]
- 8.Peng LF, McElhinney DB, Nugent AW, Powell AJ, Marshall AC, Bacha EA, Lock JE. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a fifteen-year experience. Circulation. 2006;113:2598 –2605. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AM, McElhinney DB, Lock JE, Landzberg MJ, Lang P, Marshall AC. Acute and intermediate outcomes, and evaluation of injury to the aortic wall, as based on 15 years experience of implanting stents to treat aortic coarctation. Cardiol Young. 2007;17:1–12. [DOI] [PubMed] [Google Scholar]
- 10.Tzifa A, Marshall AC, McElhinney DB, Lock JE, Geggel RL. Endo- vascular treatment for superior vena cava occlusion or obstruction in a pediatric and young adult population: a 22-year experience. J Am Coll Cardiol. 2007;49:1003–1009. [DOI] [PubMed] [Google Scholar]
- 11.Trerotola SO, Stavropoulos SW, Shlansky-Goldberg R, Tuite CM, Kobrin S, Rudnick MR. Hemodialysis-related venous stenosis: treatment with ultrahigh-pressure angioplasty balloons. Radiology. 2004;231:259 –262. [DOI] [PubMed] [Google Scholar]
- 12.Bergersen LJ, Perry SB, Lock JE. Effect of cutting balloon angioplasty on resistant pulmonary artery stenosis. Am J Cardiol. 2003;91:185–189. [DOI] [PubMed] [Google Scholar]
- 13.Gentles TL, Lock JE, Perry SB. High pressure balloon angioplasty for branch pulmonary artery stenosis: early experience. J Am Coll Cardiol. 1993;22:867– 872. [DOI] [PubMed] [Google Scholar]
- 14.Bergersen L, Gauvreau K, Lock JE, Jenkins KJ. Recent results of pulmonary arterial angioplasty: the differences between proximal and distal lesions. Cardiol Young. 2005;15:597– 604. [DOI] [PubMed] [Google Scholar]
- 15.Edwards BS, Lucas RV Jr, Lock JE, Edwards JE. Morphologic changes in the pulmonary arteries after percutaneous balloon angioplasty for pulmonary arterial stenosis. Circulation. 1985;71:195–201. [DOI] [PubMed] [Google Scholar]
- 16.Bergersen L, Jenkins KJ, Gauvreau K, Lock JE. Follow-up results of cutting balloon angioplasty used to relieve stenoses in small pulmonary arteries. Cardiol Young. 2005;15:605– 610. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama H, Veldtman GR, Norgard G, Lee KJ, Chaturvedi R, Benson LN. Bladed balloon angioplasty for peripheral pulmonary artery stenosis. Catheter Cardiovasc Interv. 2004;62:71–77. [DOI] [PubMed] [Google Scholar]


