Key Points
Question
What is the comparative effectiveness of a short-course of antibiotic treatment for pyelonephritis in children vs a prolonged-course of antibiotics?
Findings
In this comparative effectiveness research study of 791 children (aged 6 months to 18 years) with pyelonephritis, no difference was found in the odds of treatment failure for children prescribed a short-course of antibiotics (median 8 days) vs a prolonged-course of antibiotics (median 11 days).
Meaning
Results of this study suggest that short-courses of antibiotic therapy may be as effective as prolonged-courses for the treatment of pyelonephritis in children.
Abstract
Importance
National guidelines recommend treating children with pyelonephritis for 7 to 14 days of antibiotic therapy, yet data are lacking to suggest a more precise treatment duration.
Objective
To compare the clinical outcomes of children receiving a short-course vs a prolonged-course of antibiotic treatment for pyelonephritis.
Design, Setting, and Participants
Retrospective observational study using inverse probability of treatment weighted propensity score analysis of data from 5 hospitals in Maryland between July 1, 2016, and October 1, 2018. Participants were children aged 6 months to 18 years with a urine culture growing Escherichia coli, Klebsiella species, or Proteus mirabilis with laboratory and clinical criteria for pyelonephritis.
Exposures
Treatment of pyelonephritis with a short-course (6 to 9 days) vs a prolonged-course (10 or more days) of antibiotics.
Main Outcomes and Measures
Composite outcome of treatment failure within 30 days of completing antibiotic therapy: (a) unanticipated emergency department or outpatient visits related to urinary tract infection symptoms, (b) hospital readmission related to UTI symptoms, (c) prolongation of the planned, initial antibiotic treatment course, or (d) death. A subsequent urinary tract infection caused by a drug-resistant bacteria within 30 days was a secondary outcome.
Results
Of 791 children who met study eligibility criteria (mean [SD] age 9.2 [6.3] years; 672 [85.0%]) were girls, 297 patients (37.5%) were prescribed a short-course and 494 patients (62.5%) were prescribed a prolonged-course of antibiotics. The median duration of short-course therapy was 8 days (interquartile range, 7-8 days), and the median duration of prolonged-course therapy was 11 days (interquartile range, 11-12 days). Baseline characteristics were similar between the groups in the inverse probability of treatment weighted cohort. There were 79 children (10.1%) who experienced treatment failure. The odds of treatment failure were similar for patients prescribed a short-course vs a prolonged-course of antibiotics (11.2% vs 9.4%; odds ratio, 1.22; 95% CI, 0.75-1.98). There was no significant difference in the odds of a drug-resistant uropathogen for patients with a subsequent urinary tract infection within 30 days when prescribed a short-courses vs prolonged-course of antibiotics (40% vs 64%; odds ratio, 0.36; 95% CI, 0.09-1.43).
Conclusions and Relevance
The study findings suggest that short-course antibiotic therapy may be as effective as prolonged-courses for children with pyelonephritis, and may mitigate the risk of future drug-resistant urinary tract infections. Additional studies are needed to confirm these findings.
This comparative effectiveness research study assesses the clinical outcomes of children receiving a short-course (<10 days) vs a prolonged-course (10 or more days) of antibiotics for the treatment of pyelonephritis.
Introduction
Pyelonephritis, an infection of the upper urinary tract, can lead to both short-term and long-term morbidity, including sepsis, acute kidney injury, renal scarring, and chronic hypertension.1,2,3,4 To alleviate symptoms during the acute phase of the infection and reduce the potential for long-term consequences, pediatric guidelines recommend treating pyelonephritis with a total of 7 to 14 days of antibiotic therapy.5,6
A growing body of evidence supports the noninferiority of short durations of therapy (in the range of 5-7 days) compared with prolonged durations for producing clinical cure in adults with pyelonephritis.7,8,9,10 However, limited data explore this question in children.4,5 Moreover, there is a need for more robust evidence in children with urological abnomalities.5,6 The benefits of a short course of antibiotic therapy for pyelonephritis include improved patient convenience, a decreased likelihood of adverse drug events, a reduced risk of the emergence of antibiotic resistant organisms, and reduced abdominal discomfort.11
Since 2016, The Johns Hopkins Hospital Pediatric Antibiotic Treatment Guidelines, accessible to clinicians at all 5 of the hospitals contributing data to the current study, recommend treating children with pyelonephritis with 7 days of antibiotic therapy (eFigure 1 in the Supplement). These guidelines were developed by a multidisciplinary team including primary care clinicians, emergency medicine clinicians, hospitalists, urologists, nephrologists, intensivists, and infectious diseases clinicians based on a review of the available evidence in adult and pediatric populations and expert opinion where gaps in data were evident. However, despite the availability of local treatment guidelines, variability remains in prescribed treatment durations for children with pyelonephritis, providing an opportunity to investigate the association between varying durations of antibiotic therapy and patient outcomes. Our objective was to compare the clinical outcomes of children receiving a short-course (<10 days) vs a prolonged-course (≥10 days) of antibiotics for the treatment of pyelonephritis.
Methods
Study Setting and Sample
This was a retrospective multicenter study of children aged 6 months to 18 years who presented for medical care between July 1, 2016, and October 1, 2018, to 1 of 5 hospitals in The Johns Hopkins Health System, which includes The Johns Hopkins Hospital, Bayview Medical Center, Howard County General Hospital, Sibley Memorial Hospital, and Suburban Hospital, and their associated outpatient clinics. The investigators were provided with a list of children with urinary cultures growing Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, or Proteus mirabilis from the clinical microbiology laboratory for the 5 hospitals. All data were collected by manual medical record review and entered into a REDCap (Vanderbilt) database by 2 research assistants (both physicians) closely supervised by 2 of us (M.T.F. and P.D.T.). Clinical treatment failure for all children was adjudicated by 2 of us (M.T.F. and P.D.T.). Abstractors were not blinded to the study hypothesis. Children meeting microbiological criteria6 (in addition to ≥5 white blood cells per high powered field on urinalysis) with fever (temperature >38.0 °C) and at least 1 of the following clinical signs or symptoms were included (a) rigors, (b) hypotension (age appropriate ranges for normal blood pressure used), (c) flank pain/costovertebral angle tenderness, (d) emesis, (e) persistent tachycardia (age appropriate ranges of normal heart rate used with additional requirement of a fluid bolus within the first 24 hours of presentation), or (f) irritability without another identified source. Patients had to receive at least 6 calendar days of an in vitro active antibiotic for pyelonephritis. Patients were excluded if they met the aforementioned criteria but received an antibiotic not expected to reach appropriate levels in the kidney parenchyma (ie, nitrofurantoin or fosfomycin) or had a renal abscess.
Additional data, including demographic characteristics, preexisting medical conditions, severity of illness, microbiologic status, treatment, and clinical outcomes, were obtained by manual medical record review. The Maryland Chesapeake Regional Information System for our Patients network was used to evaluate microbiological, clinical, or treatment data at outside emergency departments or inpatient facilities within the state of Maryland or District of Columbia after hospital discharge.12 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for comparative effectiveness research.13 This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board, with a waiver of informed consent as the study was considered minimal risk to participants.
Exposure and Outcome
The primary exposure was treatment of pyelonephritis with a short-course of antibiotics (6-9 days). Unexposed patients received a prolonged-course of antibiotics (≥10 days). The decision to categorize 6 to 9 days as short-course therapy was made after visual inspection of durations of therapy prescribed in the cohort (Figure 1). The duration of therapy included all inpatient and outpatient antibiotic days for pyelonephritis. The primary outcome of treatment failure was indicated by a composite outcome including 1 or more of the following events within 30 days of completing antibiotic treatment: (a) unanticipated emergency department or outpatient visit related to urinary tract infection (UTI) symptoms, (b) hospital readmission related to UTI symptoms, (c) prolongation of the planned initial antibiotic treatment course, or (d) death.
Figure 1. Histogram of Total Inpatient and Outpatient Days of Antibiotic Therapy for 791 Children With Pyelonephritis.
Isolation of an incident antibiotic resistant organism from the urine, defined as an antibiotic minimum inhibitory concentration increase of more than 2-fold comparing the initial and any subsequent urine cultures within 30 days, was a secondary outcome.
Statistical Analysis
Baseline categorical data were compared using Pearson χ2 test or Fisher exact test, as appropriate. Continuous data were compared using the Wilcoxon rank sum test. Because of an expected uneven distribution of baseline variables in exposed and unexposed patients, propensity scores estimating the probability of being prescribed short-course therapy were generated for each patient. Multivariable logistic regression was conducted with receipt of short-course antibiotics as the dependent variable. Covariates of interest were determined a priori based on clinical knowledge and review of the relevant literature. The covariates used to generate propensity scores included age (categorized as 6 months to 3 years, >3 years to 12 years, and 13 years to 18 years); female sex; immunocompromised system; preexisting urologic abnormalities; the specific pathogen recovered in the urine culture; bloodstream infection with the same uropathogen; hospitalization; and intensive care unit admission specifically for pyelonephritis (as a proxy for severity of illness).
Inverse probability of treatment weighting (IPTW) was performed to balance differences in baseline characteristics between the 2 groups.14 Patients who received a short-course of antibiotic therapy were weighted by the inverse of the propensity score and those receiving a prolonged-course of antibiotic therapy were weighted by the inverse of 1 minus the propensity score. A new pseudopopulation (ie, the IPTW cohort) was created in which each patient was assigned an increased or decreased weight. For example, a patient with a high propensity score (ie, a patient with a high probability of receiving short-course therapy) who received a short course of therapy was given a decreased weight, and a patient with a high probability (ie, high propensity score) of receiving short-course therapy who received a prolonged course of therapy was given an increased weight, often changing the number of patients with any specific baseline characteristic between the full cohort and IPTW cohort.14 Stabilized weights were generated to prevent the inclusion of extreme weights. Baseline characteristics were considered balanced if standardized mean difference values were less than 10% (Figure 2). In the final analysis, odds ratios (ORs) and 95% CIs for the primary composite outcome were estimated using logistic regression, adjusting for any variables with standardized mean differences greater than 10% after IPTW.
Figure 2. Standardized Mean Differences in Baseline Characteristics of Children With Pyelonephritis in Unweighted and Weighted Cohorts.
ICU indicates intensive care unit.
Exploratory Subgroup Analysis
In addition to the primary analysis comparing the association of short-course vs prolonged-course antibiotic therapy with treatment failure for children with pyelonephritis, 3 subgroup analyses exploring this study question were performed using the IPTW cohort. These subgroups included the following: (a) outcomes based on age group (6 months to 3 years, 4 to 13 years, and 14 to 18 years), (b) outcomes based on the presence or absence of underlying urological abnormalities, and (c) outcomes based on antibiotic class prescribed after antibiotic susceptibility data were known (first-generation cephalosporins, third-generation cephalosporins, trimethoprim-sulfamethoxazole [TMP-SMX], and fluoroquinolones). A 2-sided P < .05 was considered statistically significant for all tests. Statistical analysis was completed using Stata, version 15.0 (StataCorp LP).
Results
Overview of Eligible Population
There were 1782 patients aged 6 months to 18 years with E coli, Klebsiella spp, or P mirabilis recovered from their urine cultures. In total, 630 patients were excluded for failure to meet all microbiological and clinical criteria for pyelonephritis. An additional 361 patients were excluded for incomplete documentation of antibiotic treatment. Of 791 children who met study eligibility criteria (mean [SD] age 9.2 [6.3] years; 672 [85.0%]) were girls, and 297 patients (37.5%) were prescribed a short-course and 494 patients (62.5%) were prescribed a prolonged-course of antibiotics for pyelonephritis.
In the full cohort, the short-course and prolonged-course groups were generally similar with respect to baseline characteristics but there were a few notable differences (Table 1). Children under 3 years of age were less likely to receive short-course therapy than prolonged-course therapy (18.5% [55 of 297] vs 35.8% [177 of 494]; P < .001), whereas adolescents were more likely to receive short-course therapy than prolonged-course therapy (40.7% [121 of 297] vs 27.1% [134 of 494]; P = .30). These differences did not persist in the IPTW cohort (Table 1). The propensity score distribution in the short-course and prolonged-course groups of the IPTW cohort are available in eFigure 2 and eFigure 3 in the Supplement.
Table 1. Baseline Characteristics of Children With Pyelonephritis by Duration of Antibiotic Therapy, Before and After Propensity Score Weighting.
| Characteristic | Full cohort (n = 791) | Inverse probability of treatment weighted cohort (n = 787)a | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | P value | Standardized mean differences | No. (%) | P value | Standardized mean differences | |||
| Short-course 6-9 d (n = 297) | Prolonged-course ≥10 d (n = 494) | Short-course 6-9 d (n = 296a) | Prolonged-course ≥10 d (n = 491a) | |||||
| Age category | ||||||||
| 6 mo-3 y | 55 (18.5) | 177 (35.8) | <.001 | −0.396 | 86 (29.2) | 142 (29.0) | .95 | −0.002 |
| 4-13 y | 121 (40.7) | 183 (37.0) | .30 | 0.076 | 114 (38.7) | 189 (38.4) | .94 | 0.009 |
| 14-18 y | 121 (40.7) | 134 (27.1) | <.001 | 0.290 | 95 (32.1) | 160 (32.6) | .88 | −0.008 |
| Female sex | 250 (84.2) | 422 (85.4) | .63 | −0.035 | 250 (84.5) | 415 (84.6) | .96 | 0.001 |
| Immunocompromiseb | 4 (1.3) | 17 (3.4) | .08 | −0.137 | 7 (2.4) | 11 (2.3) | .93 | −0.018 |
| Underlying urologic abnormalitiesc | 78 (26.3) | 104 (21.1) | .09 | 0.123 | 69 (23.5) | 114 (23.2) | .93 | 0.005 |
| Pregnant | 5 (1.7) | 14 (2.8) | .31 | −0.077 | 6 (2.2) | 11 (2.3) | .93 | −0.014 |
| Uropathogen | ||||||||
| Escherichia coli | 255 (85.9) | 425 (86.0) | .95 | −0.005 | 257 (87.1) | 424 (86.4) | .80 | 0.027 |
| Klebsiella species | 33 (11.1) | 54 (10.9) | .94 | 0.006 | 29 (9.9) | 52 (10.6) | .75 | −0.028 |
| Proteus mirabilis | 9 (3.0) | 15 (3.0) | >.99 | −0.000 | 9 (3.0) | 15 (3.0) | .96 | −0.003 |
| Bacteremia with same uropathogen | 1 (0.3) | 8 (1.6) | .10 | −0.130 | 3 (0.6) | 3 (0.9) | .69 | −0.020 |
| Hospitalized | 63 (21.2) | 109 (22.1) | .78 | −0.021 | 64 (21.5) | 104 (21.1) | .90 | −0.006 |
| Intensive care unit | 2 (0.7) | 4 (0.8) | .83 | −0.016 | 3 (0.9) | 4 (0.8) | .94 | 0.008 |
| Treatment regimend | ||||||||
| First-generation cephalosporin | 118 (39.7) | 89 (18.0) | <.001 | 0.493 | 127 (42.8) | 85 (17.4) | <.001 | 0.577 |
| Third-generation cephalosporin | 42 (14.1) | 215 (43.5) | <.001 | −0.685 | 46 (15.4) | 206 (42.0) | <.001 | −0.619 |
| Trimethoprim-sulfamethoxazole | 65 (21.9) | 105 (21.3) | .83 | 0.015 | 60 (20.1) | 116 (23.6) | .27 | −0.085 |
| Fluoroquinolone | 30 (10.1) | 29 (5.9) | .03 | 0.156 | 23 (7.9) | 31 (6.4) | .41 | 0.055 |
All numbers of children in the inverse probability of treatment weighted cohort are rounded to the nearest whole number; some categories might not add up to 100%.
Includes receipt of a solid organ transplant, chemotherapy in the previous 6 months, hematopoietic stem cell transplant in the previous 12 months, or actively receiving biologic agents or high-dose steroids for autoimmune conditions.
Neurogenic bladder, high-grade vesicoureteral reflux, nephrolithiasis, ureteral stent, urinary catheter (including a Foley catheter) not removed during treatment course, suprapubic catheter, or recent urologic surgery.
Includes agents prescribed after antibiotic susceptibility data were available; not all agents listed.
In the IPTW cohort, the median duration of antibiotic therapy in the short-course group was 8 days (interquartile range, 7-8 days) vs 11 days (interquartile range, 11-12 days) in the prolonged-course group. Females represented 84.5% (665 of 787) of the IPTW cohort and E coli was the most frequent uropathogen recovered and identified in 86.5% (681 of 787) of urine cultures (Table 1).
The most common antibiotics prescribed within the first 24 hours included ceftriaxone (28.1%), cephalexin (20.1%), and TMP-SMX (19.6%). Antibiotics prescribed after urine culture and antibiotic susceptibility data became available are described in Table 1. Ninety-four percent (740 of 787) of patients were transitioned to oral antibiotics at any point during their treatment course (median time to transition was 2 days [interquartile range, 2-3 days] after therapy initiation).
Overall Treatment Failure
In the IPTW cohort, there were 79 patients (10.1%) who experienced the composite outcome of treatment failure. There were 10 patients (1.3%) who were readmitted to the hospital for UTI symptoms, 53 patients (6.8%) who experienced an unanticipated emergency department or outpatient visit because of UTI symptoms; 65 patients (8.3%) who were prescribed additional antibiotics for lingering UTI symptoms; and 1 patient (0.1%) who died during the treatment course. These categories were not mutually exclusive. There were 33 patients (11.2%) who experienced treatment failure after being prescribed a short course of antibiotic therapy vs 46 patients (9.4%) who experienced treatment failure after being prescribed a prolonged course of antibiotic therapy (P = .42). The odds of achieving the primary outcome for the short-course group vs the prolonged-course group within 30 days of discontinuing antibiotic therapy were 1.22 (95% CI, 0.75-1.98).
Exploratory Subgroup Analyses
No differences in outcomes were observed for children who received short-course vs prolonged-course antibiotic therapy for pyelonephritis within the aged 6 months to 3 years category, the aged 4 to 13 years category, or the aged 14 to 18 years category (Table 2). There was, however, a significantly greater proportion of children who experienced overall treatment failure in the aged 4 to 13 years group (14.5%) compared with children aged 6 months to 3 years (7.4%) and children aged 14 to 18 years (7.8%), regardless of the treatment duration prescribed (OR, 1.71; 95% CI, 1.05-2.79; P < .01) (Table 2).
Table 2. Duration of Therapy for the Treatment of Pyelonephritis in 787 Children, by Age Group and Presence of Urologic Abnormalitiesa,b.
| Category | Treatment failure, No. (%) | Odds ratio (95% CI) | P value | ||
|---|---|---|---|---|---|
| Overall | Short-course therapy | Prolonged-course therapy | |||
| Age category | |||||
| 6 mo-3 y (n = 228) | 17 (7.4) | 3/86 (3.5) | 14/142 (9.9) | 0.52 (0.14-1.90) | .33 |
| 4-13 y (n = 303) | 44 (14.5) | 20/114 (17.5) | 24/189 (12.7) | 1.40 (0.72-2.72) | .32 |
| 14-18 y (n = 255) | 20 (7.8) | 12/95 (12.6) | 8/160 (5.0) | 1.71 (0.66-4.44) | .27 |
| Urologic abnormalityc | |||||
| Present (n = 183) | 35 (19.1) | 16/69 (23.2) | 19/114 (16.6) | 1.49 (0.69-3.24) | .31 |
| Absent (n = 604) | 45 (7.4) | 18/227 (7.9) | 27/377 (7.2) | 1.07 (0.56-2.04) | .83 |
All results are based on the inverse-probability of treatment weighted propensity score cohort and all numbers of children are rounded to the nearest whole number.
Comparing treatment failure across the 3 age categories P < .001.
Comparing treatment failure between the group with urologic abnormalities and the group without urologic abnormalities P < .001.
Similarly, there were no differences in the outcomes of children receiving short-course vs prolonged-course therapy in either the 23.2% (183 of 787) of children with underlying urologic abnormalities (OR, 1.49; 95% CI, 0.69-3.24) or the 76.7% (604 of 787) of children without urologic abnormalities (OR, 1.07; 95% CI, 0.56-2.04) (Table 2). However, 19.1% (35 of 183) vs 7.4% (45 of 604) of children with and without urologic abnormalities experienced treatment failure, respectively (OR, 2.59; 95% CI, 1.42-4.70; P = .03).
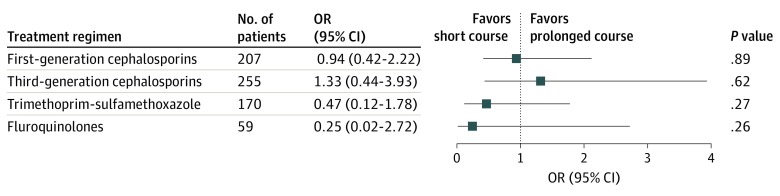
An exploratory subgroup analysis was also performed in the IPTW cohort by antibiotic class used for culture-directed therapy (ie, the antibiotic prescribed after antibiotic susceptibility data became known) including first-generation cephalosporins, third-generation cephalosporins, TMP-SMX, or fluoroquinolones (Figure 3). No significant differences were observed in clinical outcomes when comparing short-course and prolonged-course therapy with stratification by antibiotic class.
Figure 3. Odds of Treatment Failure for Children Prescribed a Short-Course of Antibiotics vs a Prolonged-Course of Antibiotics, by Antibiotic Class.
OR indicates odds ratio.
Subsequent Antibiotic Resistance
For the 37 children in the IPTW cohort who experienced a UTI recurrence who had culture data available within 30 days, 6 of 15 children (40%) in the short-course group and 14 of 22 children (64%) in the prolonged-course group were infected with an incident antibiotic resistant organism (OR, 0.36; 95% CI, 0.09-1.43).
Discussion
Our findings suggest that children receiving a short course (median 8 days) vs a prolonged course (median 11 days) of antibiotic therapy for the treatment of pyelonephritis experience similar clinical outcomes. Although results did not achieve statistical significance, children who received a short course of antibiotic therapy may be less likely to have subsequent infections within the following 30 days with a more resistant strain of the same uropathogen (40% vs 64%). This finding adds to the growing body of evidence supporting shorter durations of therapy than traditionally prescribed for the treatment of common bacterial infections.15,16
To our knowledge, this is the first comparative effectiveness research study evaluating the optimal duration of therapy for pyelonephritis in children. Our data provide evidence that may help guide treatment recommendations for a common pediatric infection, particularly when combined with the high-quality randomized clinical trial data supporting shorter (5-7 days) durations of therapy for adults with pyelonephritis.7,8,9,10 Furthermore, our study included populations frequently excluded from traditional comparative effectiveness UTI studies, including those with immunocompromised systems, structural urologic abnormalities, and previous UTIs, increasing the generalizability of our findings to the pediatric population at highest risk for the development of pyelonephritis.
We found that children aged 4 to 13 years had a significantly higher odds of treatment failure (regardless of the duration of therapy prescribed) than children aged less than 4 years or greater than 14 years. We hypothesize that this finding was likely due to differences in risk factors in the aged 4 to 13 years group compared with younger and older children. A possible explanation for the development of UTIs in healthy children less than aged 4 years is evolving elimination and hygiene practices during infancy and toilet training periods1,17,18,19; whereas children older than 14 years are more likely to experience UTIs related to sexual activity.16,17 Children between these ages, however, are less likely to develop UTIs unless there are anatomical abnormalities of the urinary tract, neurological abnormalities, or functional bowel control conditions.1 The presence of underlying urologic abnormalities was independently associated with treatment failure, regardless of the duration of therapy prescribed.
Additionally, we explored whether treatment durations in children may need to differ based on the antibiotic agent selected. Evidence from the adult population indicates that treatment with fluoroquinolones or TMP-SMX is associated with improved clinical outcomes compared with oral β-lactam regimens,10,20,21 and if β-lactams are selected for treatment, prolonged treatment durations in the range of 10-14 days are recommended in contrast with a course of 5 to 7 days with the former.7,8,9,10,22 It is postulated that higher failure rates with oral β-lactams are owing to a greater likelihood of persistent vaginal bacterial colonization after antibiotic treatment has been completed.10,20 We did not find differences in outcomes based on antibiotic class in the full cohort. However, our findings do not preclude the possibility that differences may have been observed had our study population been larger.
Limitations
Our study has several limitations. This was an observational study. We attempted to mitigate the known confounding by indication of why some children would be more likely to receive short-course therapy through the generation of propensity scores and IPTW; however residual confounding cannot be ruled out. Second, we were limited in our ability to gather detailed outcomes data after hospital discharge. Although we had access to all inpatient and outpatient facilities within The Johns Hopkins Health System and access to all emergency department visits and hospitalizations in the state of Maryland and District of Columbia, we were unable to capture relevant postdischarge data from urgent care centers or primary care practices that were not part of The Johns Hopkins Health System. We do not believe, however, that missing data disproportionately affected one study group more than the other. Third, although we determined a definition for pyelonephritis that was applied evenly for children in the short-course and prolonged-course groups, this definition may pose challenges for nonverbal children (of all ages). There is the possibility that misclassification occurred in which some children diagnosed with pyelonephritis may have had an unrelated infectious diagnosis. Finally, we were unable to assess adherence with antibiotic therapy in the outpatient setting.
Conclusions
Our findings suggest that short courses of antibiotic therapy may be as effective as prolonged courses for children with pyelonephritis. Given the high incidence of UTIs in children and the increasing concern for antibiotic resistance and other negative sequelae associated with antibiotic overuse, a shorter duration of therapy for pyelonephritis could have implications for public health.
eFigure 1. The Johns Hopkins Hospital Pediatric Antibiotic Treatment Guidelines
eFigure 2. Propensity Score Distribution for Children Receiving a Short-Course of Therapy for Pyelonephritis
eFigure 3. Propensity Score Distribution for Children Receiving a Prolonged-Course of Therapy for Pyelonephritis
References
- 1.Raszka WV Jr, Khan O. Pyelonephritis. Pediatr Rev. 2005;26(10):364-370. doi: 10.1542/pir.26-10-364 [DOI] [PubMed] [Google Scholar]
- 2.Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics. 2010;126(6):1084-1091. doi: 10.1542/peds.2010-0685 [DOI] [PubMed] [Google Scholar]
- 3.Morello W, La Scola C, Alberici I, Montini G. Acute pyelonephritis in children. Pediatr Nephrol. 2016;31(8):1253-1265. doi: 10.1007/s00467-015-3168-5 [DOI] [PubMed] [Google Scholar]
- 4.Strohmeier Y, Hodson EM, Willis NS, Webster AC, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database Syst Rev. 2014;(7):CD003772. doi: 10.1002/14651858.CD003772.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts KB; Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management . Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330 [DOI] [PubMed] [Google Scholar]
- 6.Subcommittee on Urinary Tract Infection . Reaffirmation of AAP clinical practice guideline: The diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6):e20163026. doi: 10.1542/peds.2016-3026 [DOI] [PubMed] [Google Scholar]
- 7.Dinh A, Davido B, Etienne M, et al. Is 5 days of oral fluoroquinolone enough for acute uncomplicated pyelonephritis? The DTP randomized trial. Eur J Clin Microbiol Infect Dis. 2017;36(8):1443-1448. doi: 10.1007/s10096-017-2951-6 [DOI] [PubMed] [Google Scholar]
- 8.Eliakim-Raz N, Yahav D, Paul M, Leibovici L. Duration of antibiotic treatment for acute pyelonephritis and septic urinary tract infection–7 days or less versus longer treatment: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2013;68(10):2183-2191. doi: 10.1093/jac/dkt177 [DOI] [PubMed] [Google Scholar]
- 9.Klausner HA, Brown P, Peterson J, et al. A trial of levofloxacin 750 mg once daily for 5 days versus ciprofloxacin 400 mg and/or 500 mg twice daily for 10 days in the treatment of acute pyelonephritis. Curr Med Res Opin. 2007;23(11):2637-2645. doi: 10.1185/030079907X233340 [DOI] [PubMed] [Google Scholar]
- 10.Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. doi: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 11.Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308-1315. doi: 10.1001/jamainternmed.2017.1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesapeake Regional Information System for our Patients (CRISP). Accessed March 24, 2019. https://www.crisphealth.org/
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 14.Amoah J, Stuart EA, Cosgrove SE, et al. Comparing propensity score methods versus traditional regression analysis for the evaluation of observational data: a case study evaluating the treatment of gram-negative bloodstream infections. Clin Infect Dis. Published online February 18, 2020. doi: 10.1093/cid/ciaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spellberg B, Rice LB. Duration of antibiotic therapy: Shorter is better. Ann Intern Med. 2019;171(3):210-211. doi: 10.7326/M19-1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanretty AM, Gallagher JC. Shortened courses of antibiotics for bacterial infections: A systematic review of randomized controlled trials. Pharmacotherapy. 2018;38(6):674-687. doi: 10.1002/phar.2118 [DOI] [PubMed] [Google Scholar]
- 17.Azzarone G, Liewehr S, O’Connor K. Cystitis. Pediatr Rev. 2007;28(12):474-476. doi: 10.1542/pir.28-12-474 [DOI] [PubMed] [Google Scholar]
- 18.Chang SL, Shortliffe LD. Pediatric urinary tract infections. Pediatr Clin North Am. 2006;53(3):379-400, vi. doi: 10.1016/j.pcl.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 19.Kavitha J, Aravind MA, Jayachandran G, Priya S. Risk factors for urinary tract infection in pediatric patients. Int J Contemp Pediatr. 2017;5(1):184-189. doi: 10.18203/2349-3291.ijcp20175583 [DOI] [Google Scholar]
- 20.Hooton TM, Scholes D, Gupta K, Stapleton AE, Roberts PL, Stamm WE. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA. 2005;293(8):949-955. doi: 10.1001/jama.293.8.949 [DOI] [PubMed] [Google Scholar]
- 21.Cronberg S, Banke S, Bergman B, et al. Fewer bacterial relapses after oral treatment with norfloxacin than with ceftibuten in acute pyelonephritis initially treated with intravenous cefuroxime. Scand J Infect Dis. 2001;33(5):339-343. doi: 10.1080/003655401750173922 [DOI] [PubMed] [Google Scholar]
- 22.Fox MT, Melia MT, Same RG, Conley AT, Tamma PD. A seven-day course of TMP-SMX may be as effective as a seven-day course of ciprofloxacin for the treatment of pyelonephritis. Am J Med. 2017;130(7):842-845. doi: 10.1016/j.amjmed.2017.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. The Johns Hopkins Hospital Pediatric Antibiotic Treatment Guidelines
eFigure 2. Propensity Score Distribution for Children Receiving a Short-Course of Therapy for Pyelonephritis
eFigure 3. Propensity Score Distribution for Children Receiving a Prolonged-Course of Therapy for Pyelonephritis