This cohort study examines the association between cumulative exposure to residential segregation during 25 years of young adulthood and cognitive performance in midlife among black individuals in the United States.
Key Points
Question
Is cumulative exposure to residential segregation in young adulthood associated with midlife cognitive performance among black individuals in the US?
Findings
This cohort study of 1568 black participants in the Coronary Artery Risk Development in Young Adults study found that relative to living in low-segregation neighborhoods, black participants who were exposed to highly segregated neighborhoods in young adulthood exhibited worse performance in processing speed.
Meaning
The findings suggest that cumulative exposure to residential segregation is associated with poor cognitive performance among black individuals as early as midlife, which may explain black-white disparities in dementia risk at older age.
Abstract
Importance
Neighborhood-level residential segregation is implicated as a determinant for poor health outcomes in black individuals, but it is unclear whether this association extends to cognitive aging, especially in midlife.
Objective
To examine the association between cumulative exposure to residential segregation during 25 years of young adulthood among black individuals and cognitive performance in midlife.
Design, Setting, and Participants
The ongoing prospective cohort Coronary Artery Risk Development in Young Adults (CARDIA) Study recruited 5115 black and white participants aged 18 to 30 years from 4 field centers at the University of Alabama, Birmingham; University of Minnesota, Minneapolis; Northwestern University, Chicago, Illinois; and Kaiser Permanente, Oakland, California. Data were acquired from February 1985 to May 2011. Among the surviving CARDIA cohort, 3671 (71.8%) attended examination year 25 of the study in 2010, when cognition was measured, and 3008 (81.9%) of those completed the cognitive assessments. To account for time-varying confounding and differential censoring, marginal structural models using inverse probability weighting were applied. Data were analyzed from April 16 to July 20, 2019.
Main Outcomes and Measures
Racial residential segregation was measured using the Getis-Ord Gi* statistic, and the mean cumulative exposure to segregation was calculated across 6 follow-up visits from baseline to year 25 of the study, then categorized into high, medium, and low segregation. Cognitive function was measured at year 25 of the study, using the Digit Symbol Substitution Test (DSST), Stroop color test (reverse coded), and Rey Auditory Verbal Learning Test. To facilitate comparison of estimates, z scores were calculated for all cognitive tests.
Results
A total of 1568 black participants with available cognition data were included in the analysis. At baseline, participants had a mean (SD) age of 25 (4) years and consisted of 936 women (59.7%). Greater cumulative exposure to segregated neighborhoods was associated with a worse DSST z score (for high segregation, β = −0.37 [95% CI, −0.61 to −0.13]; for medium segregation, β = −0.25 [95% CI, −0.51 to 0.0002]) relative to exposure to low segregation.
Conclusions and Relevance
In this cohort study, exposure to residential segregation throughout young adulthood was associated with worse processing speed among black participants as early as in midlife. This association may potentially explain black-white disparities in dementia risk at older age.
Introduction
Several studies have reported disparities in cognitive performance,1,2,3 risk of dementia,1,4,5,6 and markers of brain aging7,8,9 between non-Hispanic black and white older adults in the United States, largely attributed to structural racism.10 In particular, neighborhood-level racial residential segregation continues to persist despite the passage of the Fair Housing Act of 196811 and has been associated with many adverse health outcomes.10,12,13 Furthermore, residential segregation has been associated with worse built environment,13 psychosocial factors,14 educational quality,15 health behaviors,16 environmental exposures,17 and cardiometabolic disease,18,19,20 all of which may contribute to cognitive and brain aging.21,22,23,24,25,26,27 The association between racial residential segregation and cardiometabolic health factors, such as obesity and elevated blood pressure, is a particularly salient and clinically translatable pathway, because cardiometabolic health is a major risk factor for cognitive decline and brain aging.28 Educational quality is another important pathway owing to its association with cognitive reserve and dementia risk.27,29 However, literature examining the association of racial residential segregation with cognitive function is sparse.
Growing evidence suggests that maintaining cognitive function is a lifelong process and that several of the most important risk factors may begin earlier in the life course.30,31,32 Therefore, we examined the association between cumulative exposure to residential segregation during 25 years of young adulthood and cognitive performance in middle-aged black adults who are members of the Coronary Artery Risk Development in Young Adults (CARDIA) study.
Methods
Source Population and Analytic Sample
The CARDIA study is an ongoing, multicenter, prospective cohort study that focuses on the development and determinants of subclinical and clinical cardiovascular disease. Recruitment methods have previously been described.33 Briefly, starting in 1985, 5115 black and white participants aged 18 to 30 years were recruited from 4 field centers: the University of Alabama at Birmingham; University of Minnesota, Minneapolis; Northwestern University, Chicago, Illinois; and Kaiser Permanente, Oakland, California. Within each center, recruitment was balanced by sex, age, and educational level. The institutional review board at each field site and supporting sites approved the study. At each visit, participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
In 2010, cognitive testing was added to the CARDIA cohort 25-year follow-up examination. Race was self-reported, and race categories (white and black) were defined by CARDIA investigators. Given that racial residential segregation is distinctively experienced by black individuals in the United States, we restricted our analytical sample to participants who self-identified as black (n = 2636). We then excluded persons who were missing the cognitive battery (n = 1068). Our final analytical sample included 1568 black participants who had available cognitive data. A comparison of black participants who were included vs excluded from the final analytical sample is presented in eTable 1 in the Supplement.
Exposure of Interest: Racial Residential Segregation From 1985 to 2010
Neighborhood-level residential segregation is represented by the Getis and Ord local Gi* statistic, as previously described.19,34 Briefly, the Gi* statistic is a widely accepted measure of relative racial composition of one’s neighborhood compared with the larger metropolitan area. The Gi* statistic was calculated for black racial composition. The calculation of the Gi* statistic has been previously described in detail in a supplemental methods section by Kershaw et al.19 Briefly, CARDIA participants’ geocoded addresses were linked to tract-level census data at each of the available CARDIA visits (1985-1986, 1992-1993, 1995-1996, 2000-2001, 2005-2006, and 2010-2011). The proportion of black residents in one’s census tract was then compared with the mean proportion of black residents in the surrounding metropolitan area or county, with a spatial weight included to account for racial composition of each tract compared with neighboring tracts. The Gi* statistic produces a z score representing the number of SDs that the racial composition of one’s census tract is from the greater surrounding metropolitan area.
We created a measure of cumulative exposure to racial residential segregation, the exposure of interest, by calculating the mean Gi* statistic for each person across the follow-up period. We then categorized exposure to racial residential segregation as high (Gi* > 1.96), medium (Gi* range, 0-1.96), and low (Gi* < 0), based on critical z score values at the 5% significance level (95% CI).20 The higher the Gi* statistic, the greater the representation of black residents in the census tract compared with the larger metropolitan area. A description of the mean Gi* statistic at each time point is presented in eTable 2 in the Supplement.
Primary Outcome of interest: Cognitive Performance in 2010
Cognitive testing in the CARDIA study has been previously described.31 In brief, cognitive performance was measured using 3 different tests representing distinct domains of cognition. The Digit Symbol Substitution Test (DSST) is a subtest of the Wechsler Adult Intelligence Scale and measures performance on a speed test (range, 0-133 points).35 The interference score on the Stroop color test (executive skills) measures the additional amount of processing needed to respond to one stimulus while suppressing another. The test was scored by seconds needed to spell out color words printed in a different color plus number of errors.36 Stroop scores were reverse coded such that greater scores indicated better performance. The Rey Auditory Verbal Learning Test (RAVLT) measures verbal memory and assesses the ability to memorize and retrieve words (range, 0-15 points).37 All 3 cognitive measures were treated as continuous. Furthermore, given that the cognitive tests have different value ranges, each test was z scored based on the sample mean and SD at the time of cognitive testing to facilitate comparison of estimates. Higher z scores indicate better performance.
Covariates
The covariates chosen for this analysis were based on prior literature regarding factors that act as both possible confounders and mediators of the segregation-cognition association.14,15,18,20,27,28,29 Covariate data were measured at each examination, except sex (male or female) and field center, which were collected at baseline. Data on age, marital status (married vs not), years of education, physical activity (in standardized units based on duration and intensity), income (based on income categories, ranging from ≤$5000 to ≥$100 000), and mean alcohol consumption (in milliliters per day) were self-reported in response to validated standardized questionnaires administered by trained research associates. Smoking status (current smoking vs not) questionnaires were self-administered. Body mass index was calculated using measured weight in kilograms divided by height in meters squared. Blood pressure was measured 3 times at 1-minute intervals, and systolic blood pressure was calculated as the mean of the last 2 measures. Fasting glucose level was measured using the hexokinase UV method by American BioScience Laboratories, Van Nuys, California, and subsequently, samples were analyzed using the hexokinase-glucose-6-phosphate dehydrogenase method by Linco Research, St Louis, Missouri, as previously described.38 Depressive symptoms were measured using the Center for Epidemiologic Studies–Depressive Scale (20-item version).
Statistical Analysis
Data were analyzed from April 16 to July 20, 2019. First, we summarized baseline characteristics of our sample stratified by cumulative racial residential segregation status (low, medium, or high) using frequencies and column percentages for categorical variables, means and SDs for normally distributed variables, and medians and interquartile range for nonnormally distributed variables. Next, we examined the association of cumulative exposure of racial residential segregation (1985-2010) with cognition in 2010. Given the longitudinal nature of the study spanning 25 years and the repeated measures of racial residential segregation and covariates, we posited that several factors may influence one’s exposure to racial residential segregation but can also be influenced by exposure to segregated neighborhoods.18 Therefore, these factors may act as both confounders (ie, a third variable that determines both the exposure and outcome) and mediators (ie, a third variable that is determined by the exposure and subsequently determines the outcome, which means it is on the proposed pathway) of the association of interest. This data structure, illustrated by the directed acyclic graph in the Figure, reflects time-varying confounding, which cannot be accounted for with traditional regression methods. The Figure depicts all time-varying confounders of interest, including income, marital status, years of education, and health status, which is represented by physical activity, smoking status, body mass index, systolic blood pressure, depressive symptoms, fasting glucose level, and alcohol consumption. To address this methodological concern, we used marginal structural models, which require the construction of inverse probability weights to account for the time-varying confounding structure.39,40 In addition, using the same method, we computed inverse probability of censoring weights due to loss to follow-up (but not death) to address selective attrition (a summary of participants lost to follow-up at each time point is presented in eTable 3 in the Supplement). For each participant, we then multiplied their weight for the exposure (segregation) and loss to follow-up to compute a final weight. A detailed description of the construction of the inverse probability weights is presented in the eMethods in the Supplement. To estimate the association of cumulative exposure to racial residential segregation with cognition, we used generalized estimating equations with an identity link, exchangeable working correlation matrix, and the cumulative weights computed above.41 In these models, we computed robust standard errors to account for the correlation between participants living in the same census tract. In all models, we additionally adjusted for the following time-invariant covariates: center, age, sex, and baseline years of education. Estimates and 95% CIs were presented, and statistically significant results were indicated by 95% CIs that do not contain the null. All analyses were conducted in SAS, version 9.4 (SAS Institute, Inc).
Figure. Directed Acyclic Graph for Time-Varying Confounding of Racial Residential Segregation and Cognitive Function.
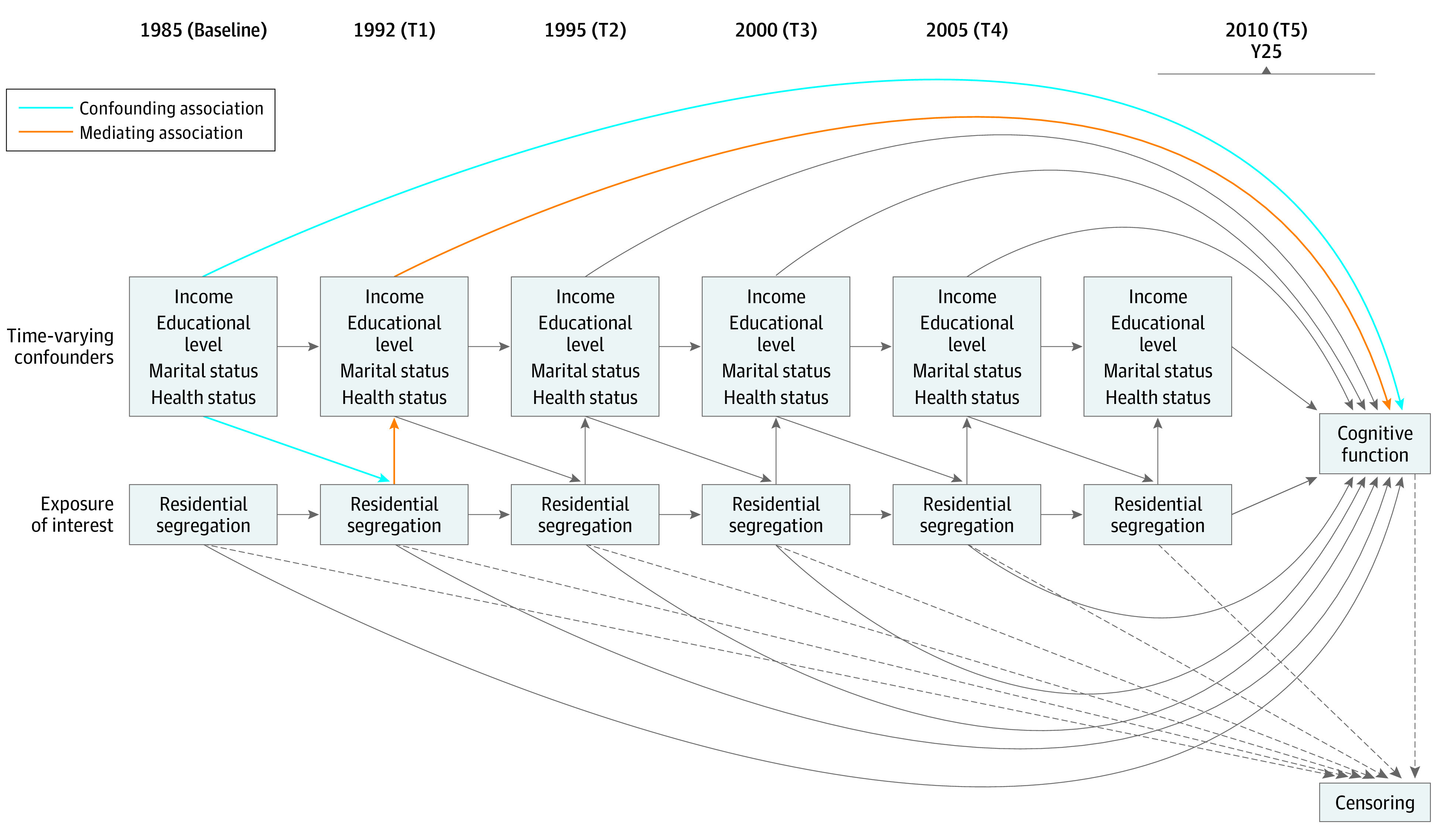
Participants are from the Coronary Artery Risk Development in Young Adults study. Unmeasured confounders and lines from time-varying confounders to censoring at study times (T) are not diagrammed here for clarity. Time-varying confounders are lagged by T − 1. Health status variables include physical activity, smoking status, body mass index, systolic blood pressure, fasting glucose level, depressive symptoms, and alcohol consumption. Dotted lines indicate censoring at each time.
Missing Data
All regression models to compute the weights were calculated using 10 multiply imputed data sets to account for missing covariate data across the time points, assuming missingness at random and a multivariate normal distribution. A summary of missingness of covariates at each time point is presented in eTable 4 in the Supplement.
Results
Table 1 outlines the baseline sample characteristics, stratified by baseline segregation category. Most participants at baseline were living in neighborhoods characterized by high racial residential segregation. Mean (SD) baseline age across segregation categories was 25 (4) years; 936 were women (59.7%) and 632 were men (40.3%). Compared with living in medium- or low-segregation neighborhoods, those living in high-segregation neighborhoods had slightly fewer years of education (mean [SD], 13 [2] vs 14 [2] and 14 [2] years, respectively) and lower median income ($21 000 [IQR, $14 000-$43 000] vs $30 000 [IQR, $21 000-$43 000] and $30 000 [IQR, $21 000-$43 000], respectively), were more likely to be married (268 [20.8%] vs 45 [23.9%] and 16 [17.0%], respectively) and current smokers (396 [30.8%] vs 44 [23.4%] and 21 [22.3%], respectively), and exhibited lower median total physical activity (290 [IQR, 144-500] vs 351 [IQR, 172-579] and 354 [IQR, 187-580] exercise units, respectively). Systolic blood pressure, body mass index, number of depressive symptoms, and alcohol consumption were similar across segregation categories. Compared with black participants who were included in this analysis, those who were excluded had lower median household income ($20 500 [IQR, $14 000-$42 500] vs $30 000 [IQR, $14 000-$42 500]), were less likely to be married (174 of 1068 [16.3%] vs 329 of 1568 [21.0%]), and were more likely to be current smokers (423 of 1068 [39.6%] vs 461 of 1568 [29.4%]) (eTable 1 in the Supplement).
Table 1. Sample Characteristics at Baseline Stratified by Cumulative Racial Residential Segregation Categorya.
| Characteristic | Racial residential segregation category | ||
|---|---|---|---|
| High (n = 1286) | Medium (n = 188) | Low (n = 94) | |
| Sociodemographic | |||
| Age, mean (SD), y | 24 (4) | 25 (4) | 25 (3) |
| Educational level, mean (SD), y | 13 (2) | 14 (2) | 14 (2) |
| Income, median (IQR), $1000 | 21 (14-43) | 30 (21-43) | 30 (21-43) |
| Married, No. (%) | 268 (20.8) | 45 (23.9) | 16 (17.0) |
| Women, No. (%) | 781 (60.7) | 105 (55.9) | 50 (53.2) |
| Clinical risk factors | |||
| Systolic blood pressure, mean (SD), mm Hg | 111 (11) | 111 (11) | 111 (9) |
| Body mass index, mean (SD)b | 25 (6) | 26 (6) | 25 (5) |
| Fasting glucose level, mean (SD), mg/dL | 81 (11) | 82 (9) | 80 (7) |
| CES-D score, median (IQR)c | 10 (6-17) | 11 (6-16) | 9 (6-15) |
| Health behaviors | |||
| Current smoker, No. (%) | 396 (30.8) | 44 (23.4) | 21 (22.3) |
| Total physical activity, median (IQR), exercise units | 290 (144-500) | 351 (172-579) | 354 (187-580) |
| Mean alcohol consumption, median (IQR), mL | 2 (0-11) | 2 (0-11) | 2 (0-10) |
Abbreviations: CES-D, Center for Epidemiologic Studies–Depressive Scale; IQR, interquartile range.
SI conversion factor: To convert glucose level to millimoles per liter, multiply by 0.0555.
Includes 1568 participants at baseline (1985). Data are from the 1985-2010 Coronary Artery Risk Development in Young Adults study.
Calculated as weight in kilograms divided by height in meters squared.
Scores range from 0 to 60, with higher scores indicating greater number of depressive symptoms.
Table 2 displays results from marginal structural models examining the association between cumulative exposure to racial residential segregation and cognitive performance, accounting for time-varying confounding and censoring at each visit. Generally, greater cumulative exposure to racial residential segregation throughout young adulthood was associated with worse DSST performance (z score units) in middle-aged participants in a dose-response fashion (high segregation, β = −0.37 [95% CI, −0.61 to −0.13]; medium segregation, β = −0.25 [95% CI, −0.51 to 0.0002]) relative to low segregation. In comparison, the coefficient for baseline age was −0.07 (95% CI, −0.08 to −0.06), such that 1 extra year of age was associated with a 0.07-point decrease in DSST z score. Associations with Stroop and RAVLT performance did not reach statistical significance (Table 2).
Table 2. Association Between Cumulative Exposure to Racial Residential Segregation Throughout Young Adulthood and Midlife Cognitive Functiona.
| Racial residential segregation | Cognitive measure, β (95% CI) | ||
|---|---|---|---|
| DSST | Stroop color test | RAVLT | |
| High | −0.37 (−0.61 to −0.13) | −0.16 (−0.46 to 0.13) | −0.13 (−0.37 to 0.11) |
| Medium | −0.25 (−0.51 to 0.0002) | −0.07 (−0.38 to 0.24) | −0.07 (−0.33 to 0.18) |
| Low | 1 [Reference] | 1 [Reference] | 1 [Reference] |
Abbreviations: DSST, Digit Symbol Substitution Test; RAVLT, Rey Auditory Verbal Learning Test.
Includes 1568 participants at baseline (1985). Midlife cognition was measured in 2010 in the 1985-2010 Coronary Artery Risk Development in Young Adults study. Estimates are from marginal structural models. Cognitive scores are calculated as z scores to facilitate comparison across estimates, and Stroop scores were additionally reverse coded. Marginal structural models were adjusted for baseline age, visit, examination center, sex, and baseline years of education. Estimates are summarized across results from 10 multiply imputed data sets.
Discussion
In this study, we found that greater cumulative exposure to segregated neighborhoods during 25 years of young adulthood was associated with worse processing speed (DSST score) in midlife among black participants in the CARDIA study. The estimate for the association of high racial residential segregation with DSST was more than 5-fold that of the association of age with DSST. By using marginal structural models, we accounted for time-varying confounding that otherwise would have resulted in biased estimates of the association of racial residential segregation on cognitive function. These findings support the notion that long-term exposure to racial residential segregation in young adulthood is associated with cognitive aging as early as midlife.
Our study contributes to the sparse literature examining residential segregation and cognitive performance, especially in the context of young adulthood among black individuals living in the United States. Previous studies have shown that neighborhood-level black composition and measures of black-white segregation were not significantly associated with cognition,42,43 which is in contrast to our study. For example, black-white residential segregation measured using the isolation index was not associated with cognitive function or decline among participants of the Health and Retirement Study.42 The difference in findings may be attributable to different measures of segregation, modeling techniques, and age distribution between samples.
Racial residential segregation has been called a fundamental cause of black-white health disparities because it influences the distribution of resources and opportunities that can protect health and/or increase risk of disease.13 Consistent with this theory, there are many pathways through which segregation could influence cognitive performance. For example, residential segregation is believed to result in concentrated poverty and limit opportunities for socioeconomic mobility,13 and several studies6,44,45,46 have shown that lower neighborhood-level socioeconomic status is related to worse cognitive performance, although some associations were more evident with baseline cognitive status as opposed to cognitive change over time.44,47 In addition, educational quality operationalized by state- and classroom-level features, which are strongly influenced by segregation, has also been shown to be associated with cognitive performance and to explain black-white disparities in late life.48
Segregated neighborhoods are often economically disinvested,13 which can shape the landscape of the built environment as well, and a worse neighborhood built environment has been associated with worse cognition in older adults.44,49 Segregated neighborhoods, concentrated poverty, and income inequality often go hand in hand,13,50 and poverty and income inequality have been associated with worse cognitive function.31,45,51 Finally, individuals living in segregated neighborhoods may be more likely to be exposed to environmental pollutants,52,53 and exposure to environmental pollutants is known to influence brain and cognitive aging.17,54 Taken together, future work is warranted to replicate our findings and explore neighborhood-level or contextual mediators, all of which can inform intervention on the policy or community level.
In addition to the aforementioned contextual pathways of interest, several individual-level factors may serve as possible downstream mediators and potential targets for clinical intervention.27 Residential segregation has been associated with psychosocial factors,14 health behaviors,16 and cardiometabolic disease,18,19,20 and subsequently, these factors have been associated with cognitive decline in older populations.21,22,23,24,25,26 Because many of these factors represent vascular disease or are known to influence vascular disease risk, these data are consistent with our findings with the measure of processing speed (DSST), which is associated with cerebrovascular injury.55 We posit that the association of racial residential segregation with cognitive performance is mediated by complex downstream pathways influencing both contextual risk factors and individual social, psychological, and health-related conditions. Future studies should examine the extent to which these factors mediate this association to identify the most effective targets for intervention. To enhance translation to the clinic, future work should also consider how intervention on these proximal risk factors in late life may mitigate the risk of earlier life exposure to racial residential segregation on cognitive health.
In this study, we used marginal structural models to account for time-varying confounding and differential censoring across the study period. Assessing the validity of estimates from this model requires a thorough evaluation of several assumptions. First, although the exchangeability assumption cannot be tested pragmatically, we included the most important determinants of segregation and cognitive performance based on previous literature. Second, the consistency assumption is more difficult to fulfill in the context of social exposures.56 We defined segregation using the Gi* statistic and subsequent categorization, which captures the clustering dimension of racial residential segregation. However, measures of other dimensions of segregation may not yield the same associations observed herein. The possible violation of this assumption is further concerning, given other evidence showing that racial residential segregation operationalized by measures other than the Gi* statistic is not associated with cognition.42,43 Furthermore, segregation status is not as well-defined an intervention as other more explicitly defined exposures, such as medication use. Considering the manipulability criterion also aids in assessment of this assumption57; in other words, interventions to treat segregation may occur in many different ways, including policy-level interventions, natural phenomena, and changes in economic or social factors. Residential segregation as we have defined it in this study likely does not meet this criterion; however, future work can aim to examine other definitions of segregation as well as examine downstream, well-defined exposures. Finally, we examined the positivity assumption graphically and did not observe any obvious violations.
Strengths and Limitations
This study has several strengths. First, we leveraged a unique data set with repeated measures of health and social factors across early adulthood in black participants, and this allowed us to examine our associations of interest accounting for time-varying confounding and differential censoring. Second, studies examining racial residential segregation in the context of cognitive function are limited, and thus our findings contribute to an important yet sparse literature.
Several limitations to this work are worth noting. First, although we attempted to account for time-varying confounding using inverse probability of treatment weights and marginal structural models, we caution about the causality of the findings. Future studies are warranted to replicate our findings in other cohorts. Second, although inverse probability weighting is a powerful method for accounting for time-varying confounding and potential bias due to attrition, it is limited by the specification of the model, and the inverse probability weight estimator can be unstable if cell sizes are small. Third, cognition was examined at only 1 point, but next steps should include examining cognitive trajectories and dementia incidence. We examined different domains of cognitive function, but both validity and reliability can be strengthened by having data on a more thorough neuropsychological battery.58 Fourth, although there are several hypothesized pathways through which racial residential segregation may influence cognitive function, we were not able to identify specific mechanisms in this study. Future studies using analytical approaches, such as structural equation modeling, are needed to better elucidate these pathways. Fifth, although we used census tracts to approximate neighborhood level segregation, previous research in multiple population-based epidemiological studies have shown high correlations (range, 0.85-0.96) between neighborhood indicators measured at the block group and census tract levels.59 In addition, because the Gi* statistic is a spatial autocorrelation measure, it reflects the composition of the tract in which the participant lives and neighboring tracts, thus accounting for some uncertainty involved in using census tracts as proxies for neighborhoods. In addition, our measure of segregation is the result of people who may have changed their neighborhood of residence as well as changes in the racial composition of the neighborhoods over time.19 Separating one from the other is beyond the scope of this analysis, and as such our analysis focused on the overall measure of racial composition.
Conclusions
Taken together, these data suggest that exposure to racial residential segregation during young adulthood is associated with worse cognitive function as early as midlife. Interventions targeting mediating pathways of this association may help attenuate the risk of cognitive impairment in black residents of segregated neighborhoods. More importantly, our findings suggest that policies that address segregation and the uneven distribution of resources, such as the Health in All Policies,60 may be beneficial for reducing inequities in cognitive performance.
eTable 1. Comparison of Baseline Covariates Between Blacks Included and Excluded From the Analysis, CARDIA Study
eTable 2. Summary Statistics for Gi* Statistic at Each Point, CARDIA Study (n = 1568)
eTable 3. Number (Percentage) of Participants Censored (Lost to Follow-Up) and Active at Each Point, CARDIA Study (n = 1568)
eTable 4. Frequency (Percentage) of Missing Values in Covariates at Each Point, CARDIA Study (n = 1568)
eMethods. Inverse Probability Weights
eReferences.
References
- 1.Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151-159. doi: 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahodne LB, Manly JJ, Azar M, Brickman AM, Glymour MM. Racial disparities in cognitive performance in mid- and late adulthood: analyses of two cohort studies. J Am Geriatr Soc. 2016;64(5):959-964. doi: 10.1111/jgs.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Díaz-Venegas C, Downer B, Langa KM, Wong R. Racial and ethnic differences in cognitive function among older adults in the USA. Int J Geriatr Psychiatry. 2016;31(9):1004-1012. doi: 10.1002/gps.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015-2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15(1):17-24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216-224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaffe K, Falvey C, Harris TB, et al. ; Health ABC Study . Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. doi: 10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264-273. doi: 10.1001/jamaneurol.2018.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zahodne LB, Manly JJ, Narkhede A, et al. Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and whites. Curr Alzheimer Res. 2015;12(7):632-639. doi: 10.2174/1567205012666150530203214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol. 2008;65(8):1053-1061. doi: 10.1001/archneur.65.8.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463. doi: 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- 11.Massey DS. The Legacy of the 1968 Fair Housing Act. Sociol Forum (Randolph N J). 2015;30(suppl 1):571-588. doi: 10.1111/socf.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White K, Borrell LN. Racial/ethnic residential segregation: framing the context of health risk and health disparities. Health Place. 2011;17(2):438-448. doi: 10.1016/j.healthplace.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404-416. doi: 10.1016/S0033-3549(04)50068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bécares L, Nazroo J, Jackson J. Ethnic density and depressive symptoms among African Americans: threshold and differential effects across social and demographic subgroups. Am J Public Health. 2014;104(12):2334-2341. doi: 10.2105/AJPH.2014.302047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan JR, Stowell J, Oakley DO Choosing segregation: racial imbalance in American public schools, 1990-2000. Published March 29, 2002. Accessed January 23, 2019. https://eric.ed.gov/?id=ED471516
- 16.Borrell LN, Kiefe CI, Diez-Roux AV, Williams DR, Gordon-Larsen P. Racial discrimination, racial/ethnic segregation, and health behaviors in the CARDIA study. Ethn Health. 2013;18(3):227-243. doi: 10.1080/13557858.2012.713092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112(17):1645-1653. doi: 10.1289/ehp.7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pool LR, Carnethon MR, Goff DC Jr, Gordon-Larsen P, Robinson WR, Kershaw KN. Longitudinal associations of neighborhood-level racial residential segregation with obesity among blacks. Epidemiology. 2018;29(2):207-214. doi: 10.1097/EDE.0000000000000792 [DOI] [PubMed] [Google Scholar]
- 19.Kershaw KN, Robinson WR, Gordon-Larsen P, et al. Association of changes in neighborhood-level racial residential segregation with changes in blood pressure among black adults: the CARDIA study. JAMA Intern Med. 2017;177(7):996-1002. doi: 10.1001/jamainternmed.2017.1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the Multi-ethnic Study of Atherosclerosis. Circulation. 2015;131(2):141-148. doi: 10.1161/CIRCULATIONAHA.114.011345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters R, Booth A, Rockwood K, Peters J, D’Este C, Anstey KJ. Combining modifiable risk factors and risk of dementia: a systematic review and meta-analysis. BMJ Open. 2019;9(1):e022846. doi: 10.1136/bmjopen-2018-022846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahodne LB, Sol K, Kraal Z. Psychosocial pathways to racial/ethnic inequalities in late-life memory trajectories. J Gerontol B Psychol Sci Soc Sci. 2019;74(3):409-418. doi: 10.1093/geronb/gbx113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahodne LB, Manly JJ, Smith J, Seeman T, Lachman ME. Socioeconomic, health, and psychosocial mediators of racial disparities in cognition in early, middle, and late adulthood. Psychol Aging. 2017;32(2):118-130. doi: 10.1037/pag0000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeki Al Hazzouri A, Vittinghoff E, Byers A, et al. Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci. 2014;69(5):595-601. doi: 10.1093/gerona/glt139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461-468. doi: 10.1212/WNL.0b013e318227b227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabia S, Nabi H, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Health behaviors from early to late midlife as predictors of cognitive function: the Whitehall II study. Am J Epidemiol. 2009;170(4):428-437. doi: 10.1093/aje/kwp161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223-254. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- 28.Gardener H, Wright CB, Rundek T, Sacco RL. Brain health and shared risk factors for dementia and stroke. Nat Rev Neurol. 2015;11(11):651-657. doi: 10.1038/nrneurol.2015.195 [DOI] [PubMed] [Google Scholar]
- 29.Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, et al. ; Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup . Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2018;S1552-5260(18)33491-5. doi: 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pais J. Intergenerational neighborhood attainment and the legacy of racial residential segregation: a causal mediation analysis. Demography. 2017;54(4):1221-1250. doi: 10.1007/s13524-017-0597-8 [DOI] [PubMed] [Google Scholar]
- 31.Zeki Al Hazzouri A, Elfassy T, Sidney S, Jacobs D, Pérez Stable EJ, Yaffe K. Sustained economic hardship and cognitive function: the Coronary Artery Risk Development in Young Adults Study. Am J Prev Med. 2017;52(1):1-9. doi: 10.1016/j.amepre.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaffe K, Vittinghoff E, Pletcher MJ, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129(15):1560-1567. doi: 10.1161/CIRCULATIONAHA.113.004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105-1116. doi: 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- 34.Getis A, Ord K. The analysis of spatial association by use of distance statistics. Geogr Anal. 1992;24(3):189-206. doi: 10.1111/j.1538-4632.1992.tb00261.x [DOI] [Google Scholar]
- 35.Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III). Psychological Corporation; 1997. [Google Scholar]
- 36.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109(2):163-203. doi: 10.1037/0033-2909.109.2.163 [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. J Clin Psychol. 1984;40(3):785-787. doi: [DOI] [PubMed] [Google Scholar]
- 38.Bancks MP, Carnethon MR, Jacobs DR Jr, et al. Fasting glucose variability in young adulthood and cognitive function in middle age: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care. 2018;41(12):2579-2585. doi: 10.2337/dc18-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656-664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550-560. doi: 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 41.Hernán MA, Robins JM. Causal Inference: What If. Chapman & Hall/CRC; 2020. [Google Scholar]
- 42.Kovalchik SA, Slaughter ME, Miles J, Friedman EM, Shih RA. Neighbourhood racial/ethnic composition and segregation and trajectories of cognitive decline among US older adults. J Epidemiol Community Health. 2015;69(10):978-984. doi: 10.1136/jech-2015-205600 [DOI] [PubMed] [Google Scholar]
- 43.Aneshensel CS, Ko MJ, Chodosh J, Wight RG. The urban neighborhood and cognitive functioning in late middle age. J Health Soc Behav. 2011;52(2):163-179. doi: 10.1177/0022146510393974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Besser LM, McDonald NC, Song Y, Kukull WA, Rodriguez DA. Neighborhood environment and cognition in older adults: a systematic review. Am J Prev Med. 2017;53(2):241-251. doi: 10.1016/j.amepre.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D, Griffin BA, Kabeto M, Escarce J, Langa KM, Shih RA. Lagged associations of metropolitan statistical area- and state-level income inequality with cognitive function: the Health and Retirement Study. PLoS One. 2016;11(6):e0157327. doi: 10.1371/journal.pone.0157327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeki Al Hazzouri A, Haan MN, Osypuk T, Abdou C, Hinton L, Aiello AE. Neighborhood socioeconomic context and cognitive decline among older Mexican Americans: results from the Sacramento Area Latino Study on Aging. Am J Epidemiol. 2011;174(4):423-431. doi: 10.1093/aje/kwr095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosso AL, Flatt JD, Carlson MC, et al. Neighborhood socioeconomic status and cognitive function in late life. Am J Epidemiol. 2016;183(12):1088-1097. doi: 10.1093/aje/kwv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sisco S, Gross AL, Shih RA, et al. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):557-567. doi: 10.1093/geronb/gbt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Besser LM, Rodriguez DA, McDonald N, et al. Neighborhood built environment and cognition in non-demented older adults: the Multi-ethnic Study of Atherosclerosis. Soc Sci Med. 2018;200:27-35. doi: 10.1016/j.socscimed.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawachi I, Kennedy BP. Health and social cohesion: why care about income inequality? BMJ. 1997;314(7086):1037-1040. doi: 10.1136/bmj.314.7086.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grasset L, Glymour MM, Elfassy T, et al. Relation between 20-year income volatility and brain health in midlife: the CARDIA study. Neurology. 2019;93(20):e1890-e1899. doi: 10.1212/WNL.0000000000008463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cushing L, Morello-Frosch R, Wander M, Pastor M. The haves, the have-nots, and the health of everyone: the relationship between social inequality and environmental quality. Annu Rev Public Health. 2015;36:193-209. doi: 10.1146/annurev-publhealth-031914-122646 [DOI] [PubMed] [Google Scholar]
- 53.Morello-Frosch R, Jesdale BM. Separate and unequal: residential segregation and estimated cancer risks associated with ambient air toxics in US metropolitan areas. Environ Health Perspect. 2006;114(3):386-393. doi: 10.1289/ehp.8500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Power MC, Adar SD, Yanosky JD, Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology. 2016;56:235-253. doi: 10.1016/j.neuro.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rehkopf DH, Glymour MM, Osypuk TL. The consistency assumption for causal inference in social epidemiology: when a rose is not a rose. Curr Epidemiol Rep. 2016;3(1):63-71. doi: 10.1007/s40471-016-0069-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaufman JS, Cooper RS. Seeking causal explanations in social epidemiology. Am J Epidemiol. 1999;150(2):113-120. doi: 10.1093/oxfordjournals.aje.a009969 [DOI] [PubMed] [Google Scholar]
- 58.Weuve J, Proust-Lima C, Power MC, et al. ; MELODEM Initiative . Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement. 2015;11(9):1098-1109. doi: 10.1016/j.jalz.2015.06.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diez-Roux AV, Kiefe CI, Jacobs DR Jr, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies [published correction appears in Ann Epidemiol. 2001;30(4):924]. Ann Epidemiol. 2001;11(6):395-405. doi: 10.1016/s1047-2797(01)00221-6 [DOI] [PubMed] [Google Scholar]
- 60.de Leeuw E. Engagement of sectors other than health in integrated health governance, policy, and action. Annu Rev Public Health. 2017;38:329-349. doi: 10.1146/annurev-publhealth-031816-044309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Comparison of Baseline Covariates Between Blacks Included and Excluded From the Analysis, CARDIA Study
eTable 2. Summary Statistics for Gi* Statistic at Each Point, CARDIA Study (n = 1568)
eTable 3. Number (Percentage) of Participants Censored (Lost to Follow-Up) and Active at Each Point, CARDIA Study (n = 1568)
eTable 4. Frequency (Percentage) of Missing Values in Covariates at Each Point, CARDIA Study (n = 1568)
eMethods. Inverse Probability Weights
eReferences.


