Key Points
Question
Do people with multiple sclerosis have an increased risk of macrovascular disease and mortality?
Findings
In this population-based matched cohort study of 84 823 people with or without multiple sclerosis, those with multiple sclerosis were associated with an increased risk of macrovascular disease, even after controlling for sociodemographic variables and traditional vascular risk factors. People with multiple sclerosis were also associated with a 3.5-fold increased risk of all-cause mortality and a 1.5-fold increased risk of cardiovascular disease mortality; treatment with statins was associated with lower mortality rates among people with multiple sclerosis.
Meaning
These findings show that multiple sclerosis is associated with an increased risk of cardiovascular and cerebrovascular disease, which is not completely accounted for by traditional vascular risk factors.
Abstract
Importance
People with multiple sclerosis (MS) are associated with an increased risk of cardiovascular disease and mortality; however, evidence from population-based studies is sparse.
Objective
To assess whether the risk of macrovascular events and mortality differs among people with MS compared with a matched population without MS in England.
Design, Setting, and Participants
A population-based retrospective matched cohort study was conducted in general practices registered with the Clinical Practice Research Datalink in England between January 1, 1987, and September 30, 2018, with a mean (SD) follow-up of 11.3 (6.5) years. A total of 12 251 patients with MS were matched with up to 6 people without MS (n = 72 572) by age, sex, and general practice. People with 3 or more diagnoses of MS recorded during the study period were included. The first MS diagnosis was considered as index date.
Exposures
Multiple sclerosis status. Analyses were also stratified by sex.
Main Outcomes and Measures
Main outcomes were acute coronary syndrome, cerebrovascular disease, any macrovascular disease (including peripheral arterial disease), and mortality (all-cause mortality and cardiovascular disease–specific mortality). Cox proportional hazards regression and Fine and Gray proportional subhazard regression models were used to assess differences in rates.
Results
A total of 12 251 people with MS (66.9% women; mean [SD] age, 44.9 [13.3] years) were matched with 72 572 people without MS (69.8% women; mean [SD] age, 44.9 [13.3] years). As compared with people without MS, people with MS were associated with a 28% increased hazard of acute coronary syndrome (hazard ratio [HR], 1.28; 95% CI, 1.09-1.51), 59% increased hazard of cerebrovascular disease (HR, 1.59; 95% CI, 1.32-1.92), 32% increased hazard of any macrovascular disease (HR, 1.32; 95% CI, 1.15-1.52), 3.5-fold increased hazard of all-cause mortality (HR, 3.46; 95% CI, 3.28-3.65), and 1.5-fold increased hazard in cardiovascular disease mortality (HR, 1.47; 95% CI, 1.27-1.71). Differences in macrovascular events were more pronounced among women than men. Mortality risk was also higher for women than men. Treatment with lipid-lowering medications (mainly statins) was associated with lower mortality rates among people with MS.
Conclusions and Relevance
This study suggests that MS is associated with an increased risk of cardiovascular and cerebrovascular disease that is not completely accounted for by traditional vascular risk factors. Given the adverse effects of these comorbidities on outcomes in patients with MS, further investigation is needed.
This cohort study assesses whether the risk of macrovascular events and mortality differs among people with multiple sclerosis compared with a matched population without multiple sclerosis in England.
Introduction
The understanding of the importance of vascular risk factors and their management in multiple sclerosis (MS) has improved over time.1,2,3,4,5,6 Compared with the general population, people with MS were associated with a higher prevalence of hypertension and hyperlipidemia,1 being overweight or obese, having ever smoked, and having lower levels of physical activity.7
People with MS may also be at increased risk of macrovascular events, such as cerebrovascular disease and acute myocardial infarction, even after controlling for traditional vascular risk factors.1,8 However, most studies assessing the association between vascular risk factors and MS were not population based,1,8 and in many cases, they were conducted in specific settings with limited generalizability to the general population.9,10,11 Some studies did not control for health behaviors, while, for others, the study period terminated before the modern era of MS and cardiac treatments.12 In addition, some limited evidence suggests that women with MS may have a higher risk of vascular disease than men with MS.13
Mortality among people with MS remains higher than in the general population.7,14 Nevertheless, little is known about whether MS also confers an increased risk of vascular disease–related mortality and whether people with MS might benefit from tighter control of vascular risk factors.
Therefore, we used a large data set representative of the English population to evaluate the association between vascular risk factors and the risk of macrovascular disease and mortality among people with MS compared with the general population.
Methods
Study Design
We conducted a population-based retrospective matched cohort study that included people with MS registered with general practices in England, diagnosed between January 1, 1987, and September 30, 2018. The Independent Scientific Advisory Committee of the UK Clinical Practice Research Datalink (CPRD) granted ethics approval (protocol number: 18_279R). Informed consent was not required for use of this anonymized data set.
Data Source
We used data from the CPRD, one of the largest databases of electronic medical records globally.15 The CPRD holds anonymized routinely collected longitudinal primary care records, covering approximately 7% of the UK population; it is representative in terms of age, sex, and race/ethnicity.16,17 Within the CPRD we focused on the English data set, as linkages to Hospital Episode Statistics and Office for National Statistics mortality data are available.15
Study Population
Similar to a previously adopted algorithm to identify people with MS in the CPRD,18 we identified possible cases of MS based on diagnostic and management primary care codes, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes, and on prescription of disease-modifying therapies used exclusively to treat MS. To improve case finding, management primary care codes were also considered (eTable 1 in the Supplement). When linkage to secondary care data was available, Hospital Episode Statistics data were also considered to confirm the MS diagnosis (ICD-10 code G35). Based on prior work in the CPRD requiring 2 or more MS events and associated symptom and treatment codes19 as well as findings from other studies validating the use of administrative health care data to identify patients with MS,20 to reduce the risk of misclassification, we defined patients with MS as those with 3 or more MS events recorded in their available clinical history. Date of the first MS diagnosis was considered the index date.
Additional inclusion criteria for patients with MS were: (1) diagnosis after January 1, 1987, when magnetic resonance imaging was available to support the diagnosis; (2) continuous registration with the CPRD practice for 1 year or more before the first MS event to ensure that information regarding key covariates was available at onset; (3) defined sex (male or female); (4) valid date of birth; (5) age 18 years or older at cohort entry; (6) MS events recorded before the date of death; and (7) validity of patients’ clinical records in terms of continuous follow-up and data recording defined by the CPRD definition of “up to standard” (eFigure in the Supplement). Up to standard is deemed as the date at which the practice is considered to have high-quality data, based on continuity in data and death recording. Individuals were considered eligible if the clinical information recorded in the year before the index date and the follow-up were considered up to standard.
People with MS were randomly matched with up to 6 people without MS by age, sex, and general practice. Controls had up-to-standard clinical data recorded during the study period and did not have MS or any other demyelinating disease event recorded (eg, optic neuritis, transverse myelitis, acute disseminated encephalomyelitis, and central nervous system demyelination not elsewhere classifiable), which minimized the possibility of including controls who might develop MS in the future. People with MS were matched with multiple controls to reduce variance.21 We assigned the controls the index date of their matched patient with MS. People were followed until their death or the end of the study period (September 30, 2018). People who survived to the end of the study period were censored at the date of last data collection for the CPRD practice.
Study Variables
Consistent with previous CPRD research,22,23,24,25,26,27 we defined study variables using comprehensive primary care code lists and ICD-10 codes (eTable 2 in the Supplement). Prescribing data were extracted using British National Formulary codes. Study outcomes included the following incident vascular events occurring after the index date: acute coronary syndrome, cerebrovascular disease, and macrovascular disease (either acute coronary syndrome, cerebrovascular disease, or peripheral arterial disease), as well as mortality, including all-cause mortality and cardiovascular disease–specific mortality.
Study covariates included individuals’ sociodemographic characteristics (age [continuous], sex, race/ethnicity [white or nonwhite], and index of multiple deprivation [quintiles]28); vascular risk factors, including smoking status (current smoker, former smoker, or nonsmoker), diagnoses of type 2 diabetes and clinical depression, treatment with lipid-lowering, oral antidiabetic, antiplatelet, anticoagulant, and antihypertensive therapies in the index year; and year of MS diagnosis. Treatment with antihypertensive medications was used as a proxy for hypertension, consistent with previous studies.29 We also included the number of primary care visits in the year before the index year to account for differences in health care use between the MS and matched cohorts (surveillance bias).
Statistical Analysis
Differences in study variables between people with MS and controls in the index year were assessed using χ2 tests, t tests, and Kruskal-Wallis tests, as appropriate. We used multivariable Cox proportional hazards regression models to model differences in the hazard of each outcome of interest. For the analyses of incident vascular events, people with a history of the relevant outcome at baseline were excluded from the analysis. For analyses of cardiovascular-specific mortality we used competing-risk regressions based on the Fine and Gray proportional subhazard model where noncardiovascular disease mortality was the competing event and cumulative incidence functions were computed for subgroups of interest. The proportional hazards assumption was met as assessed using plots of log (−log survival time) against log survival time and Schoenfeld residuals against survival time. We also used linear regression of Schoenfeld residuals on time to test for independence between residuals and time. To better understand the role of each covariate in the regression models and avoid overfitting, models were first adjusted for presence of MS, age, and sex. Successively, models were adjusted for other sociodemographic characteristics (eg, ethnicity and deprivation), then for vascular risk factors and comorbidities, then for vascular treatments. Finally, models were adjusted for primary care visits and year of diagnosis. To compare fit of different models we used Akaike Information Criterion. We repeated these analyses after stratifying by sex to assess effect modification.
Complementary Analyses
We conducted a sensitivity analysis in which we included only patients with incident MS (and their matched controls) diagnosed from 2002 onward, the year of full implementation of the 2001 McDonald et al30 criteria for the diagnosis of MS in UK clinical practice. As lipid-lowering treatment at baseline was associated with all-cause and cardiovascular mortality but with opposite trajectories, we investigated a possible interaction between treatment with lipid-lowering medication and MS status by adding an interaction term between the 2 variables and repeated the regression analyses for all outcomes. Because the use of different vascular treatments and the occurrence of comorbidities during the study period might modify the risk of all-cause mortality and macrovascular events, we conducted an additional sensitivity analysis in which treatments and comorbidities were included as time-varying variables in the Cox proportional hazards regression models.
Results were presented as hazard ratios (HR) and subhazard ratios (SHR) and 95% CIs, as appropriate. We used the Nelson-Aalen cumulative hazard curves to plot the estimated risk of MS status on incident macrovascular disease and mortality. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. We used Stata, version 15 MP (StataCorp LLC) to conduct statistical analyses.
Results
We identified 12 251 people with MS diagnosed between January 1, 1987, and September 30, 2018, and 72 572 matched controls. On average, each patient with MS was matched with a mean (SD) of 5.9 (0.3) controls. Mean (SD) follow-up time was 10.3 (6.3) years for people with MS and 11.5 (6.5) years for controls (Table). Among the patients with MS, 66.9% were women, and the mean (SD) age was 44.9 (13.3) years; among the controls, 69.8% were women, and the mean (SD) age was 44.9 (13.3) years. More patients with MS smoked than controls (37.9% vs 29.4%), while the proportion of individuals using cardiovascular medications in the 2 cohorts was broadly similar (antihypertensive medication, 5.6% vs 5.7%; lipid-lowering medication, 2.7% vs 2.9%; antiplatelet medication, 2.7% vs 2.0%; anticoagulant medication, 0.5% vs 0.5%). When restricting the study period to 2002-2018, a total of 7957 people with MS were included and matched with 47 175 controls. Characteristics of this subcohort were similar to those of the main cohort (Table).
Table. Characteristics of the Study Population at Baselinea.
| Study period | Patients with MS | Controls | P value | |||
|---|---|---|---|---|---|---|
| 1987-2018 | 2002-2018 | 1987-2018 | 2002-2018 | 1987-2018 | 2002-2018 | |
| No. | 12 251 | 7957 | 72 572 | 47 175 | ||
| Follow-up time, mean (SD), y | 10.3 (6.3) | 8.3 (4.6) | 11.5 (6.5) | 9.1 (4.6) | <.001 | <.001 |
| Female sex, % | 69.9 | 70.3 | 69.8 | 70.1 | .75 | .95 |
| Age, mean (SD), y | 44.9 (13.3) | 44.8 (13.6) | 44.9 (13.3) | 44.9 (13.6) | .73 | .75 |
| White ethnicity, % | 93.9 | 93.6 | 91.2 | 91.0 | <.001 | <.001 |
| Smoking status, % | ||||||
| Nonsmoker | 47.1 | 46.4 | 58.1 | 60.1 | <.001 | <.001 |
| Ex-smoker | 15.0 | 16.9 | 12.5 | 13.1 | ||
| Current | 37.9 | 36.7 | 29.4 | 26.8 | ||
| Diabetes, % | 7.2 | 6.8 | 5.0 | 4.6 | <.001 | <.001 |
| Depression, % | 20.9 | 23.0 | 8.7 | 8.8 | <.001 | <.001 |
| Medication, % | ||||||
| Antidiabetic | 1.2 | 1.4 | 1.2 | 1.4 | .97 | .79 |
| Antihypertensive | 5.6 | 5.6 | 5.7 | 6.0 | .97 | .13 |
| Lipid lowering | 2.7 | 3.7 | 2.9 | 3.9 | .35 | .44 |
| Antiplatelet | 2.7 | 3.1 | 2.0 | 2.3 | <.001 | <.001 |
| Anticoagulant | 0.5 | 0.5 | 0.5 | 0.5 | .43 | >.99 |
| No. of primary care visits in previous year, mean (SD) | 7.8 (11.2) | 8.8 (12.5) | 2.9 (5.9) | 3.2 (6.5) | <.001 | <.001 |
| Index of multiple deprivation, % | ||||||
| 1st Quintile (least deprived) | 14.4 | 14.6 | 14.4 | 14.6 | >.99 | >.99 |
| 2nd Quintile | 18.5 | 19.4 | 18.5 | 19.3 | ||
| 3rd Quintile | 17.8 | 18.2 | 17.8 | 18.2 | ||
| 4th Quintile | 19.2 | 19.0 | 19.2 | 19.0 | ||
| 5th Quintile (most deprived) | 20.4 | 20.0 | 20.4 | 20.1 | ||
| Missing data | 9.7 | 8.9 | 9.7 | 8.9 | ||
Abbreviation: MS, multiple sclerosis.
Study baseline was defined as the year of MS diagnosis (index year). For matched controls, a matched index year was assigned. All patients with MS and matched controls from 1987 to 2018 were included in the main analysis, while a sensitivity analysis restricted the sample to only diagnoses from 2002 to 2018. Univariate baseline differences in study variables between patients with MS and controls were assessed using χ2 tests, t tests, and Kruskal-Wallis tests, as appropriate.
Incident Macrovascular Disease
During the study period, the incidence among patients with MS per 100 000 person-years of acute coronary syndrome was 204.5 (95% CI, 179.6-233.0), of cerebrovascular disease was 159.6 (95% CI, 138.1-184.4), and of composite macrovascular events was 291.8 (95% CI, 261.2-326.0). The incidence of acute coronary syndrome was 116.8 (95% CI, 109.3-124.8) among controls, the incidence of cerebrovascular disease was 81.4 (95% CI, 75.3-87.9) among controls, and the incidence of composite macrovascular events was 159.1 (95% CI, 150.2-168.4) among controls (Figure 1; eTable 3 in the Supplement). Compared with controls, on multivariable analysis, people with MS had an increased hazard of acute coronary syndrome (HR, 1.28; 95% CI, 1.09-1.51), cerebrovascular disease (HR, 1.59; 95% CI, 1.32-1.92), and any macrovascular disease (HR, 1.32; 95% CI, 1.15-1.52). In stratified analyses, differences between cohorts were observed in women but not in men. Compared with women without MS, those with MS had an increased hazard of acute coronary syndrome (HR, 1.42; 95% CI, 1.16-1.75), cerebrovascular disease (HR, 1.78; 95% CI, 1.41-1.23), and any macrovascular disease (HR, 1.49; 95% 1.26-1.77).
Figure 1. Cumulative Hazards of Macrovascular Events for Patients With Multiple Sclerosis (MS) and Matched Controls.
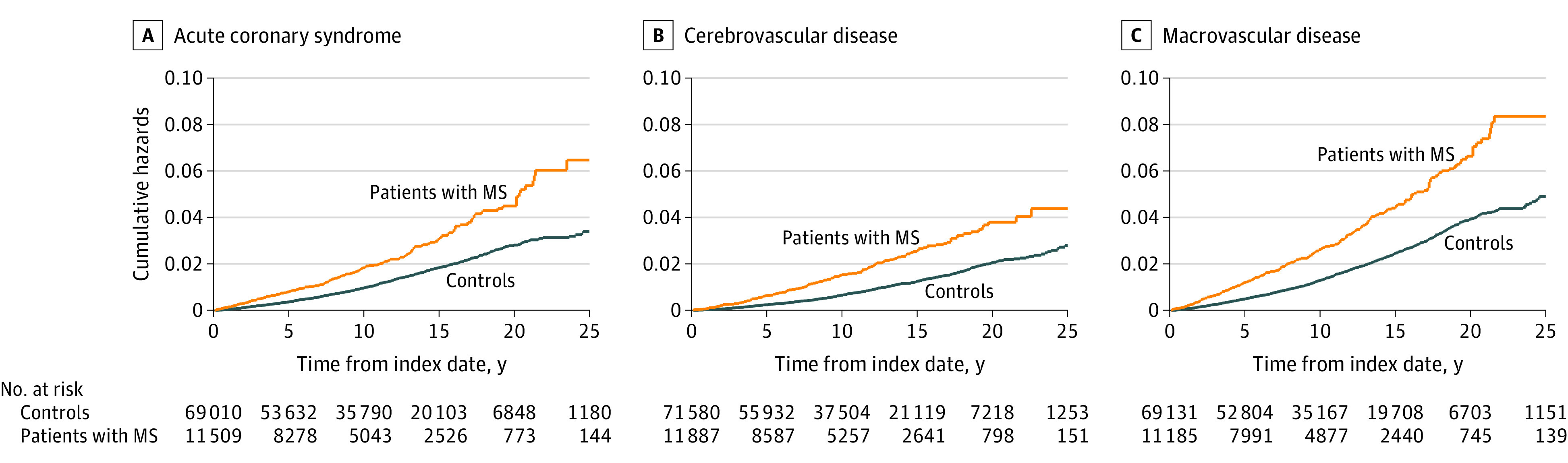
For the definition of any macrovascular disease, the following conditions were included: acute coronary syndrome, cerebrovascular disease, and peripheral arterial disease.
In the sensitivity analysis limiting the cohort to incident cases of MS diagnosed in 2002-2018, general findings were consistent. However, differences in rates of macrovascular disease were also observed in men. Compared with men without MS, men with MS had an increased hazard of acute coronary syndrome (HR, 1.81; 95% CI, 1.22-2.69) and any macrovascular disease (HR, 1.67; 95% CI, 1.15-2.43).
All-Cause Mortality and Cardiovascular Disease Mortality
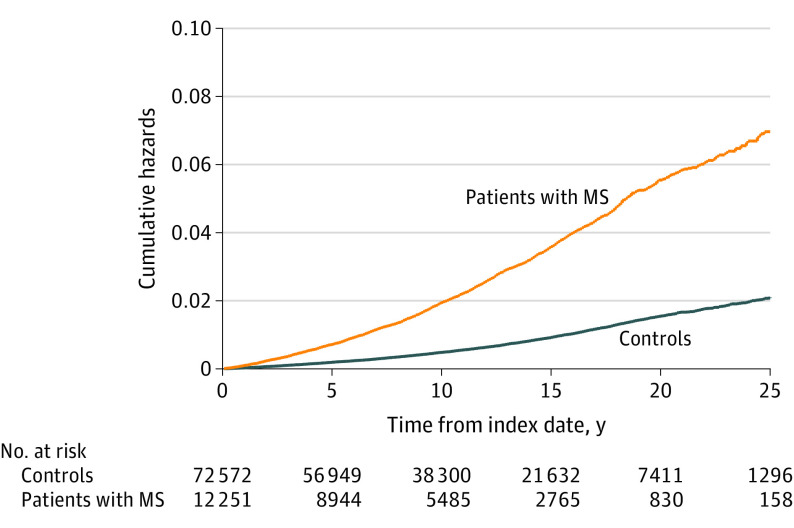
During the study period, the observed mortality rate per 100 000 person-years was 2223.3 events (95% CI, 2140.5-2309.2) for people with MS and 619.5 events (95% CI, 602.5-637.0) for controls (eTable 3 in the Supplement). In adjusted analyses, compared with controls, people with MS had an increased risk of all-cause mortality (HR, 3.46; 95% CI, 3.28-3.65) and cardiovascular disease mortality (HR, 1.47; 95% CI, 1.27-1.71). When stratifying by sex, compared with women without MS, women with MS had a 3.5-fold increase in all-cause mortality (HR, 3.52; 95% CI, 3.28-3.77) and a 1.3-fold increase in cardiovascular disease mortality (SHR, 1.30; 95% CI, 1.04-1.62). As compared with men without MS, those with MS had a 2.7-fold increased risk of all-cause mortality (HR, 2.74; 95% CI, 2.35-3.18) and a 1.5-fold increased risk of cardiovascular disease mortality (SHR, 1.54; 95% CI, 1.06-2.23). Sensitivity analysis restricting the study period to 2002-2018 onward confirmed findings from the main analysis (Figure 2 and Figure 3).
Figure 2. Cumulative Hazards of All-Cause Mortality for People With Multiple Sclerosis (MS) and Matched Controls in England.
Figure 3. Association Between Multiple Sclerosis (MS) and Risk of Macrovascular Disease and Mortality Between January 1987 and September 2018 in England.
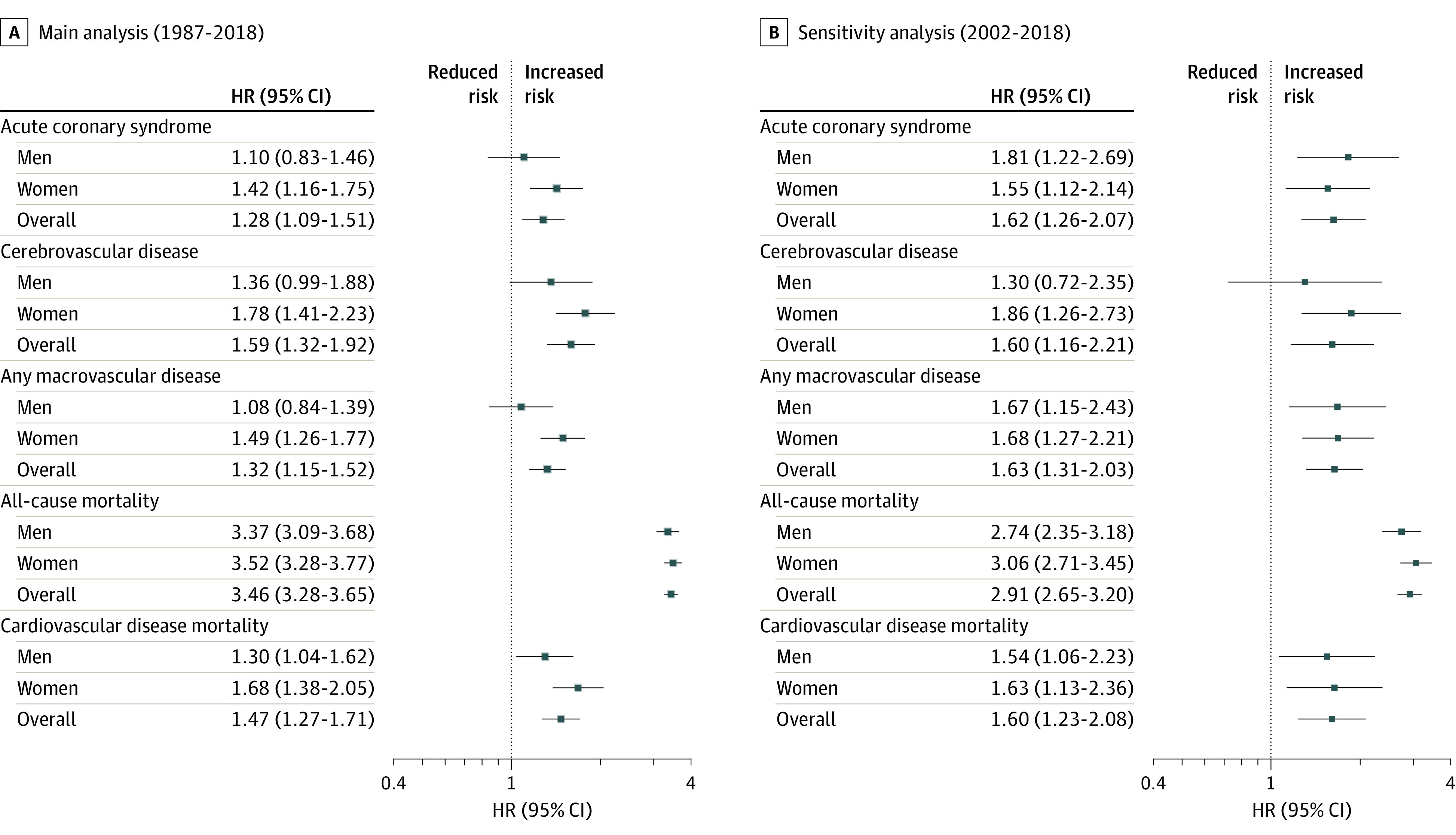
Analyses of mortality outcomes included all individuals with a diagnosis of MS after January 1, 1987, who met case definition criteria for MS and matched controls (see Methods). For analyses of macrovascular diseases, people with a history of the condition at baseline were excluded from the analysis. Date of MS diagnosis was considered as the index date, and a matched index date was assigned to matched controls. Multivariable Cox proportional hazards regression models were used to model the association between MS status and incident rates of acute coronary syndrome, cerebrovascular disease, any macrovascular disease (including acute coronary syndrome, cerebrovascular disease, and peripheral arterial disease), and all-cause mortality. To model differences in rates of cardiovascular disease mortality, competing-risk regressions based on the Fine and Gray proportional subhazard model was used that considered noncardiovascular disease mortality as a competing event, and cumulative incidence functions were computed for subgroups of interest. Regression analyses were adjusted for the following baseline covariates: age; sex; race/ethnicity; index of multideprivation; smoking status; diagnosis of diabetes or depression; use of lipid-lowering, oral antidiabetic, antihypertensive, antiplatelet, and anticoagulation medications; and number of primary care visits in the year before the diagnosis of MS and for the year of diagnosis of MS. For the sensitivity analysis, only patients with MS (and matched controls) who received a diagnosis during the period from 2002 to 2018 were included. HR indicates hazard ratio.
Complementary Analyses
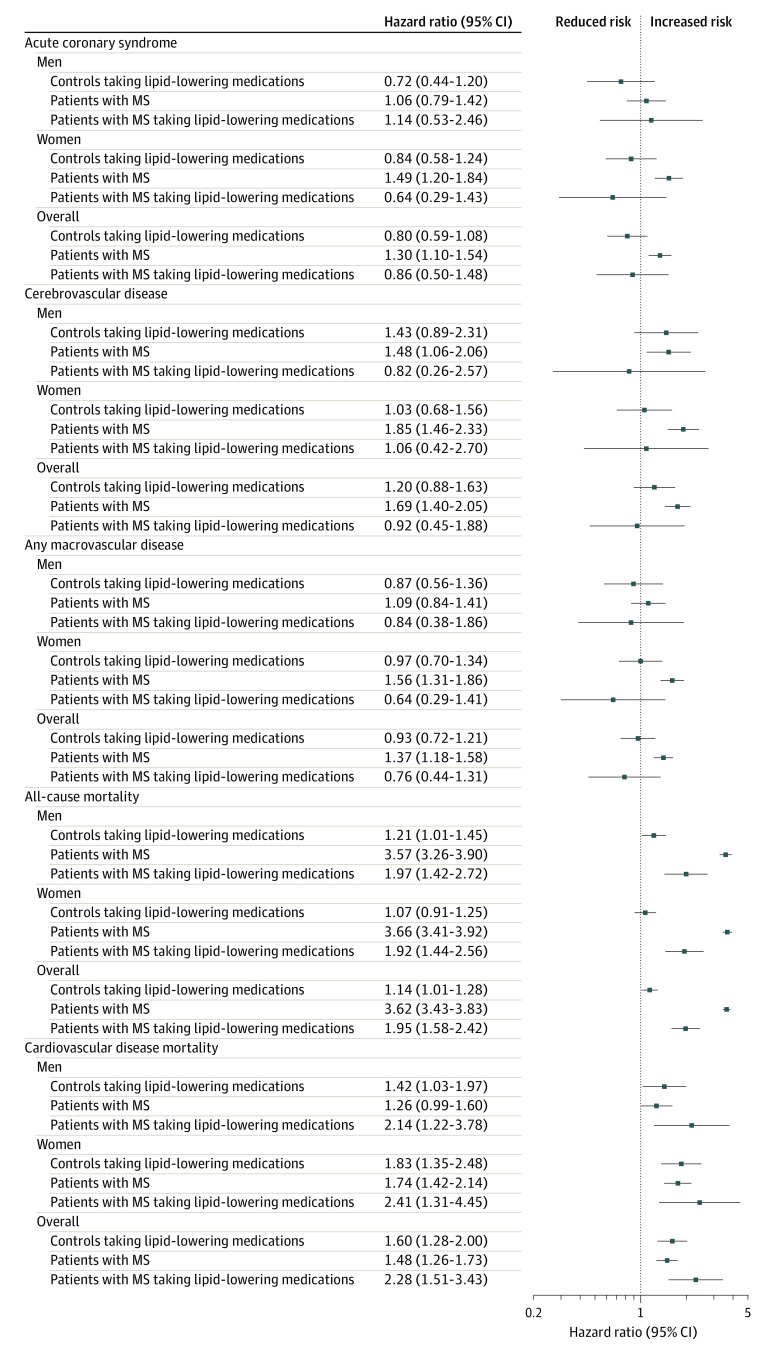
Almost 3% of the study population was taking lipid-lowering medications during the index year (2.7% for people with MS, statins in 94.6%; and 2.9% for controls, statins in 94.3%). In adjusted analyses for the people with MS taking lipid-lowering medications, mortality rates were relatively lower, while cardiovascular disease mortality rates were relatively higher than for those not taking them. Compared with controls not taking lipid-lowering medications, those with MS not taking lipid-lowering medications had 3.6-fold increased mortality rates (HR, 3.62; 95% CI, 3.43-3.83), while those with MS taking lipid-lowering medications had 2-fold increased mortality rates (HR, 1.95; 95% CI, 1.58-2.42). No sex-related differences were observed.
Compared with controls not taking lipid-lowering medications, patients with MS not taking lipid-lowering medications had 1.5-fold increased cardiovascular disease mortality rates (SHR, 1.48; 95% CI, 1.26-1.73) and those with MS taking lipid-lowering medications had 2.3-fold increased mortality rates (SHR, 2.28; 95% CI, 1.51-3.43). When stratified by sex, findings were broadly similar (Figure 4).
Figure 4. Association Between Multiple Sclerosis (MS), Treatment With Lipid-Lowering Medications, and Risk of Macrovascular Disease and Mortality Between January 1987 and September 2018 in England.
Findings from sensitivity analyses using time-varying Cox proportional hazards regression models confirmed those from main analysis (eTable 4 in the Supplement). In the sensitivity analysis, men with MS also had an increased risk of vascular disease (acute coronary syndrome: HR, 1.33; 95% CI, 1.02-1.76; cerebrovascular disease: 1.63; 1.20-2.24; and any macrovascular disease: 1.33; 1.04-1.71; eTable 4 in the Supplement). However, differences again remained less pronounced than for women with MS.
Discussion
We conducted a population-based matched cohort study of 84 823 people with or without MS. Even after controlling for sociodemographic factors and traditional vascular risk factors, people with MS still had an increased risk of macrovascular disease including acute coronary syndrome, cerebrovascular disease, and any macrovascular disease. Compared with the general population, those with MS were associated with a 3.5-fold increased risk of all-cause mortality and a 1.5-fold increased risk of cardiovascular disease mortality. Although there was no difference in incidence rates of macrovascular disease among men in the main analysis, differences were present among women.
Our findings regarding the increased risk of acute coronary syndrome (including unstable angina and acute myocardial infarction) and stroke are consistent with those of prior studies that used retrospective matched cohort designs, but did not account for smoking status. In a Swedish study, the risk of acute myocardial infarction was 85% higher and the risk of stroke was 71% higher in the population with MS, after adjustment for age, sex, birth country, and comorbidities.11 In a Danish study, the risk of acute myocardial infarction and stroke was also elevated in people with MS after adjusting for age, sex, diagnosis year, and comorbidity.12 In a Canadian study, the risk of acute myocardial infarction was 63% higher in the population with MS after adjustment for age, sex, socioeconomic status, diabetes, hypertension, hyperlipidemia, and chronic obstructive pulmonary disease as a proxy for smoking status.8
Women with MS had a greater risk of macrovascular disease than men, consistent with previous findings from Swedish and Canadian studies.11,13 Although the risk of macrovascular disease was not increased in men in the main analyses, it was increased in the sensitivity analyses during the time period when MS was diagnosed via magnetic resonance imaging (2002-2018), and that accounted for changes in treatment and comorbidities over time, suggesting that these temporal changes were important confounders. Prior studies have shown sex-specific differences in the burden of comorbidity in MS. At the time of diagnosis, men have a disproportionately higher prevalence of diabetes, depression, anxiety, and epilepsy compared with women with MS.13 The reasons for sex differences are uncertain but may reflect differences in unmeasured risk factors such as diet, other comorbidities, or effectiveness of risk factor management.
The increased risk of vascular disease even after accounting for the traditional risk factors that account for most of the risk of these conditions is not unique to MS. Systemic inflammation is a recognized risk factor for atherosclerosis,31 and other immune-mediated and inflammatory diseases confer an increased risk of cardiovascular disease, including psoriasis, rheumatoid arthritis, and severe atopic eczema.32,33,34,35,36,37,38,39 Further investigation is warranted to evaluate the mechanisms underlying our findings.
The increased all-cause mortality observed in the cohort with MS is consistent with prior studies,14,32 although few of these studies have accounted for health behaviors such as smoking. Comorbidity, including ischemic heart disease and stroke, confers an increased risk of death in MS, highlighting the importance of preventing and managing these conditions to improve survival. Cardiovascular-specific mortality rates have been the subject of less attention, although cardiovascular disease is the second or third leading cause of death in MS after MS itself.34,40
Treatment with lipid-lowering medications (approximately 95% of which were statins) seemed to have a protective association with all-cause mortality in people with MS. The role of statins in neuroprotection in MS has been investigated during the last 2 decades, with numerous mechanisms postulated, such as direct vasculoprotection and enhanced perfusion41,42,43 and reduction in free radical damage either by improved blood flow and reducing hypoxia-mediated reactive oxygen species production or through direct inhibition of cytotoxic pathways44,45; also, statins may exert a neuroprotective effect by preventing glutamate-mediated excitotoxic effects.46 One direct association seen was the 43% reduction in brain atrophy in a cohort of people with secondary progressive MS randomized to receive high-dose simvastatin compared with controls.47
Strengths and Limitations
This study had several strengths, including the population-based design, large sample size, and the use of more than 30 years of follow-up data from primary and secondary care settings. To our knowledge, this was the first study to explore at population level a possible protective role of lipid-lowering medications on all-cause mortality in people with MS.
Several limitations also merit discussion. First, we included data from January 1987 to September 2018, a period during which changes in the standard of care for MS and vascular disease occurred. However, we controlled our analyses for the year of MS diagnosis and we conducted a sensitivity analysis restricting our analyses to only patients with MS and matched controls with index year after the full implementation of the 2001 McDonald et al30 criteria in England. In addition, when using routinely collected data, miscoding, misclassification, and misdiagnosis may occur. However, the CPRD is a reliable, widely used data source and is subject to regular quality checks.15 Furthermore, we restricted MS diagnoses to people with 3 or more MS events recorded during the study period to improve case finding. We could not account for cardiovascular risk factors such as physical inactivity and obesity. Although they might be considered as proxies of MS disability, the associated risk of macrovascular disease for these risk factors is low and not enough to attenuate our findings of increased risk.48 Failure to account for these factors might have a greater association with mortality, although these associations are also modest.49,50
Conclusions
This study suggests that MS is associated with an increased risk of cardiovascular and cerebrovascular disease that is not completely accounted for by traditional vascular risk factors. Given the adverse effects of these comorbidities on outcomes in patients with MS, further investigation is needed.
eTable 1. Codes for the Definition and Detection of Multiple Sclerosis and Demyelinating Disease in Primary Care Settings
eTable 2. Codes for the Definition and Detection of Selected Study Outcomes in Primary and Secondary Care Settings
eTable 3. Estimated Incident Rates of Study Outcomes Stratified by MS Status and Sex
eTable 4. Results from Time-Varying Cox Regression Analyses Modeling the Association Between Multiple Sclerosis and Risk of Macrovascular Disease and Mortality Between January 1987 and September 2018 in England
eFigure. Study Diagram
References
- 1.Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult Scler. 2015;21(3):318-331. doi: 10.1177/1352458514564485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552. doi: 10.1212/WNL.0b013e31828154f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munger KL, Bentzen J, Laursen B, et al. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler. 2013;19(10):1323-1329. doi: 10.1177/1352458513483889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ascherio A. Environmental factors in multiple sclerosis. Expert Rev Neurother. 2013;13(12)(suppl):3-9. doi: 10.1586/14737175.2013.865866 [DOI] [PubMed] [Google Scholar]
- 5.Sundström P, Nyström L, Hallmans G. Smoke exposure increases the risk for multiple sclerosis. Eur J Neurol. 2008;15(6):579-583. doi: 10.1111/j.1468-1331.2008.02122.x [DOI] [PubMed] [Google Scholar]
- 6.D’hooghe MB, Haentjens P, Nagels G, De Keyser J. Alcohol, coffee, fish, smoking and disease progression in multiple sclerosis. Eur J Neurol. 2012;19(4):616-624. doi: 10.1111/j.1468-1331.2011.03596.x [DOI] [PubMed] [Google Scholar]
- 7.Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol. 2017;13(6):375-382. doi: 10.1038/nrneurol.2017.33 [DOI] [PubMed] [Google Scholar]
- 8.Marrie RA, Garland A, Schaffer SA, et al. Traditional risk factors may not explain increased incidence of myocardial infarction in MS. Neurology. 2019;92(14):e1624-e1633. doi: 10.1212/WNL.0000000000007251 [DOI] [PubMed] [Google Scholar]
- 9.Allen NB, Lichtman JH, Cohen HW, Fang J, Brass LM, Alderman MH. Vascular disease among hospitalized multiple sclerosis patients. Neuroepidemiology. 2008;30(4):234-238. doi: 10.1159/000128103 [DOI] [PubMed] [Google Scholar]
- 10.Mincu RI, Magda SL, Mihaila S, et al. Impaired cardiac function in patients with multiple sclerosis by comparison with normal subjects. Sci Rep. 2018;8(1):3300. doi: 10.1038/s41598-018-21599-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadidi E, Mohammadi M, Moradi T. High risk of cardiovascular diseases after diagnosis of multiple sclerosis. Mult Scler. 2013;19(10):1336-1340. doi: 10.1177/1352458513475833 [DOI] [PubMed] [Google Scholar]
- 12.Christiansen CF, Christensen S, Farkas DK, Miret M, Sørensen HT, Pedersen L. Risk of arterial cardiovascular diseases in patients with multiple sclerosis: a population-based cohort study. Neuroepidemiology. 2010;35(4):267-274. doi: 10.1159/000320245 [DOI] [PubMed] [Google Scholar]
- 13.Marrie RA, Patten SB, Tremlett H, et al. ; CIHR Team in the Epidemiology and Impact of Comorbidity on Multiple Sclerosis . Sex differences in comorbidity at diagnosis of multiple sclerosis: a population-based study. Neurology. 2016;86(14):1279-1286. doi: 10.1212/WNL.0000000000002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kingwell E, van der Kop M, Zhao Y, et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia, Canada. J Neurol Neurosurg Psychiatry. 2012;83(1):61-66. doi: 10.1136/jnnp-2011-300616 [DOI] [PubMed] [Google Scholar]
- 15.Clinical Practice Research Datalink. Clinical Practice Research Datalink. Accessed January 2, 2019. https://www.cprd.com
- 16.Chaudhry Z, Mannan F, Gibson-White A, et al. Outputs and growth of primary care databases in the United Kingdom: bibliometric analysis. J Innov Health Inform. 2017;24(3):942. doi: 10.14236/jhi.v24i3.942 [DOI] [PubMed] [Google Scholar]
- 17.Mathur R, Bhaskaran K, Chaturvedi N, et al. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health (Oxf). 2014;36(4):684-692. doi: 10.1093/pubmed/fdt116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Disanto G, Zecca C, MacLachlan S, et al. Prodromal symptoms of multiple sclerosis in primary care. Ann Neurol. 2018;83(6):1162-1173. doi: 10.1002/ana.25247 [DOI] [PubMed] [Google Scholar]
- 19.Persson R, Lee S, Yood MU, et al. Multi-database study of multiple sclerosis: identification, validation and description of MS patients in two countries. J Neurol. 2019;266(5):1095-1106. doi: 10.1007/s00415-019-09238-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culpepper WJ, Marrie RA, Langer-Gould A, et al. ; United States Multiple Sclerosis Prevalence Workgroup (MSPWG) . Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology. 2019;92(10):e1016-e1028. doi: 10.1212/WNL.0000000000007043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1-21. doi: 10.1214/09-STS313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang KC, Lee JT, Vamos EP, et al. Impact of the National Health Service Health Check on cardiovascular disease risk: a difference-in-differences matching analysis. CMAJ. 2016;188(10):E228-E238. doi: 10.1503/cmaj.151201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang KC, Soljak M, Lee JT, et al. Coverage of a national cardiovascular risk assessment and management programme (NHS Health Check): retrospective database study. Prev Med. 2015;78:1-8. doi: 10.1016/j.ypmed.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 24.Vamos EP, Harris M, Millett C, et al. Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ. 2012;345:e5567. doi: 10.1136/bmj.e5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vamos EP, Pape UJ, Curcin V, et al. Effectiveness of the influenza vaccine in preventing admission to hospital and death in people with type 2 diabetes. CMAJ. 2016;188(14):E342-E351. doi: 10.1503/cmaj.151059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang KC, Vamos EP, Palladino R, Majeed A, Lee JT, Millett C. Impact of the NHS Health Check on inequalities in cardiovascular disease risk: a difference-in-differences matching analysis. J Epidemiol Community Health. 2019;73(1):11-18. doi: 10.1136/jech-2018-210961 [DOI] [PubMed] [Google Scholar]
- 27.Palladino R, Vamos EP, Chang KC, Khunti K, Majeed A, Millett C. Evaluation of the diabetes screening component of a national cardiovascular risk assessment programme in England: a retrospective cohort study. Sci Rep. 2020;10(1):1231. doi: 10.1038/s41598-020-58033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GOV.UK English indices of deprivation 2015. Accessed September 5, 2018. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015
- 29.Hippisley-Cox J, Coupland C. Development and validation of QDiabetes-2018 risk prediction algorithm to estimate future risk of type 2 diabetes: cohort study. BMJ. 2017;359:j5019. doi: 10.1136/bmj.j5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50(1):121-127. doi: 10.1002/ana.1032 [DOI] [PubMed] [Google Scholar]
- 31.Toth PP. Subclinical atherosclerosis: what it is, what it means and what we can do about it. Int J Clin Pract. 2008;62(8):1246-1254. doi: 10.1111/j.1742-1241.2008.01804.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krökki O, Bloigu R, Ansakorpi H, Reunanen M, Remes AM. Neurological comorbidity and survival in multiple sclerosis. Mult Scler Relat Disord. 2014;3(1):72-77. doi: 10.1016/j.msard.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 33.Salter A, Tyry T, Wang G, Fox RJ, Cutter G, Marrie RA. Examining the joint effect of disability, health behaviors, and comorbidity on mortality in MS. Neurol Clin Pract. 2016;6(5):397-408. doi: 10.1212/CPJ.0000000000000269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247. doi: 10.1212/WNL.0000000000001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverwood RJ, Forbes HJ, Abuabara K, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ. 2018;361:k1786. doi: 10.1136/bmj.k1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735-1741. doi: 10.1001/jama.296.14.1735 [DOI] [PubMed] [Google Scholar]
- 37.del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44(12):2737-2745. doi: [DOI] [PubMed] [Google Scholar]
- 38.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52(3):722-732. doi: 10.1002/art.20878 [DOI] [PubMed] [Google Scholar]
- 39.Roifman I, Beck PL, Anderson TJ, Eisenberg MJ, Genest J. Chronic inflammatory diseases and cardiovascular risk: a systematic review. Can J Cardiol. 2011;27(2):174-182. doi: 10.1016/j.cjca.2010.12.040 [DOI] [PubMed] [Google Scholar]
- 40.Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain. 2004;127(Pt 4):844-850. doi: 10.1093/brain/awh104 [DOI] [PubMed] [Google Scholar]
- 41.Giannopoulos S, Katsanos AH, Tsivgoulis G, Marshall RS. Statins and cerebral hemodynamics. J Cereb Blood Flow Metab. 2012;32(11):1973-1976. doi: 10.1038/jcbfm.2012.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endres M, Laufs U, Huang Z, et al. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95(15):8880-8885. doi: 10.1073/pnas.95.15.8880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu G, Fitzgerald ME, Wen Z, et al. Atorvastatin therapy is associated with greater and faster cerebral hemodynamic response. Brain Imaging Behav. 2008;2(2):94. doi: 10.1007/s11682-007-9019-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Most PJ, Dolga AM, Nijholt IM, Luiten PG, Eisel UL. Statins: mechanisms of neuroprotection. Prog Neurobiol. 2009;88(1):64-75. doi: 10.1016/j.pneurobio.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 45.Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997;100(11):2671-2679. doi: 10.1172/JCI119812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmeer C, Kretz A, Isenmann S. Statin-mediated protective effects in the central nervous system: general mechanisms and putative role of stress proteins. Restor Neurol Neurosci. 2006;24(2):79-95. [PubMed] [Google Scholar]
- 47.Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383(9936):2213-2221. doi: 10.1016/S0140-6736(13)62242-4 [DOI] [PubMed] [Google Scholar]
- 48.Yusuf S, Hawken S, Ounpuu S, et al. ; INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937-952. doi: 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 49.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71-82. doi: 10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lear SA, Hu W, Rangarajan S, et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390(10113):2643-2654. doi: 10.1016/S0140-6736(17)31634-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Codes for the Definition and Detection of Multiple Sclerosis and Demyelinating Disease in Primary Care Settings
eTable 2. Codes for the Definition and Detection of Selected Study Outcomes in Primary and Secondary Care Settings
eTable 3. Estimated Incident Rates of Study Outcomes Stratified by MS Status and Sex
eTable 4. Results from Time-Varying Cox Regression Analyses Modeling the Association Between Multiple Sclerosis and Risk of Macrovascular Disease and Mortality Between January 1987 and September 2018 in England
eFigure. Study Diagram