Ten novel 3-(2-aryl-2-oxoethyl)-3-hydroxyindolin-2-ones have been synthesized, mostly in excellent yield, and characterized spectroscopically, and nine of these have been dehydrated to the corresponding (E)-3-(2-aryl-2-oxoethylidene)indolin-2-ones, also in excellent yield, and again characterized spectroscopically. The structures of four of the former and five of the latter are reported, and all show different patterns of supramolecular assembly.
Keywords: synthesis, heterocyclic compounds, isatin, 3-hydroxyindolinone, molecular structure, hydrogen bonding, supramolecular assembly, crystal structure
Abstract
An operationally simple and time-efficient approach has been developed for the synthesis of racemic N-substituted 3-(2-aryl-2-oxoethyl)-3-hydroxyindolin-2-ones by a piperidine-catalysed aldol reaction between aryl methyl ketones and N-alkylisatins. These aldol products were used successfully as strategic intermediates for the preparation of N-substituted (E)-3-(2-hetaryl-2-oxoethylidene)indolin-2-ones by a stereoselective dehydration reaction under acidic conditions. The products have all been fully characterized by 1H and 13C NMR spectroscopy, by mass spectrometry and, for a representative selection, by crystal structure analysis. In each of (RS)-1-benzyl-3-hydroxy-3-[2-(4-methoxyphenyl)-2-oxoethyl]indolin-2-one, C24H21NO4, (Ic), and (RS)-1-benzyl-3-{2-[4-(dimethylamino)phenyl]-2-oxoethyl}-3-hydroxyindolin-2-one, C25H24N2O3, (Id), inversion-related pairs of molecules are linked by O—H⋯O hydrogen bonds to form R
2
2(10) rings, which are further linked into chains of rings by a combination of C—H⋯O and C—H⋯π(arene) hydrogen bonds in (Ic) and by C—H⋯π(arene) hydrogen bonds in (Id). The molecules of (RS)-1-benzyl-3-hydroxy-3-[2-oxo-2-(pyridin-4-yl)ethyl]indolin-2-one, C22H18N2O3, (Ie), are linked into a three-dimensional framework structure by a combination of O—H⋯N, C—H⋯O and C—H⋯π(arene) hydrogen bonds. (RS)-3-[2-(Benzo[d][1,3]dioxol-5-yl)-2-oxoethyl]-1-benzyl-3-hydroxyindolin-2-one, C24H19NO5, (If), crystallizes with Z′ = 2 in the space group P
 and the molecules are linked into complex sheets by a combination of O—H⋯O, C—H⋯O and C—H⋯π(arene) hydrogen bonds. In each of (E)-1-benzyl-3-[2-(4-fluorophenyl)-2-oxoethylidene]indolin-2-one, C23H16FNO2, (IIa), and (E)-1-benzyl-3-[2-oxo-2-(thiophen-2-yl)ethylidene]indolin-2-one, C21H15NO2S, (IIg), the molecules are linked into simple chains by a single C—H⋯O hydrogen bond, while those of (E)-1-benzyl-3-[2-oxo-2-(pyridin-4-yl)ethylidene]indolin-2-one, C22H16N2O2, (IIe), are linked by three C—H⋯O hydrogen bonds to form sheets which are further linked into a three-dimensional structure by C—H⋯π(arene) hydrogen bonds. There are no hydrogen bonds in the structures of either (E)-1-benzyl-3-[2-(4-methoxyphenyl)-2-oxoethylidene]indolin-2-one, C24H19NO3, (IIc), or (E)-1-benzyl-5-chloro-3-[2-(4-chlorophenyl)-2-oxoethylidene]indolin-2-one, C23H15Cl2NO2, (IIh), but the molecules of (IIh) are linked into chains of π-stacked dimers by a combination of C—Cl⋯π(arene) and aromatic π–π stacking interactions.
and the molecules are linked into complex sheets by a combination of O—H⋯O, C—H⋯O and C—H⋯π(arene) hydrogen bonds. In each of (E)-1-benzyl-3-[2-(4-fluorophenyl)-2-oxoethylidene]indolin-2-one, C23H16FNO2, (IIa), and (E)-1-benzyl-3-[2-oxo-2-(thiophen-2-yl)ethylidene]indolin-2-one, C21H15NO2S, (IIg), the molecules are linked into simple chains by a single C—H⋯O hydrogen bond, while those of (E)-1-benzyl-3-[2-oxo-2-(pyridin-4-yl)ethylidene]indolin-2-one, C22H16N2O2, (IIe), are linked by three C—H⋯O hydrogen bonds to form sheets which are further linked into a three-dimensional structure by C—H⋯π(arene) hydrogen bonds. There are no hydrogen bonds in the structures of either (E)-1-benzyl-3-[2-(4-methoxyphenyl)-2-oxoethylidene]indolin-2-one, C24H19NO3, (IIc), or (E)-1-benzyl-5-chloro-3-[2-(4-chlorophenyl)-2-oxoethylidene]indolin-2-one, C23H15Cl2NO2, (IIh), but the molecules of (IIh) are linked into chains of π-stacked dimers by a combination of C—Cl⋯π(arene) and aromatic π–π stacking interactions.
Introduction
Almost 60% of drugs based on small organic molecules which are in use for medicinal purposes contain at least one N-heterocyclic ring (Vitaku et al., 2014 ▸). Amongst these, isatin (1H-indole-2,3-dione) and its derivatives have attracted  particular interest because of their broad range of biological and pharmacological activities (Singh & Desta, 2012 ▸; Pakravan et al., 2013 ▸). Isatin derivatives have also been found to be useful synthetic intermediates for the production of both dyestuffs and organic electronic materials (Stalder et al., 2014 ▸; Deng & Zhang, 2014 ▸). These wide-ranging applications have prompted the development of a large range of synthetic approaches to functionalized isatin derivatives (Moradi et al., 2017 ▸; Bogdanov & Mironov, 2018 ▸; Varun et al., 2019 ▸). Amongst these, the addition of nucleophilic units to the prochiral carbonyl group at atom C3 permits the construction of chiral 3-substituted-3-hydroxyindolin-2-ones containing a stereogenic centre at the 3-position (Peddibhotla, 2009 ▸; Mohammadi et al., 2013 ▸). Such species are desirable targets, because many related structural motifs are found in natural products and pharmaceutically active compounds; for example, convolutamydine A is a bioactive alkaloid with significant activity against HL-60 human plomyelocytic leukemia cells (Kamano et al., 1995 ▸), SM-130686 is a novel orally active growth hormone secretagogue (Nagamine et al., 2001 ▸), donaxaridine has shown effective anticancer properties (Kimura et al., 2016 ▸) and maremycins A and B exhibit antibacterial, antifungal and antitumour properties (Duan et al., 2018 ▸).
particular interest because of their broad range of biological and pharmacological activities (Singh & Desta, 2012 ▸; Pakravan et al., 2013 ▸). Isatin derivatives have also been found to be useful synthetic intermediates for the production of both dyestuffs and organic electronic materials (Stalder et al., 2014 ▸; Deng & Zhang, 2014 ▸). These wide-ranging applications have prompted the development of a large range of synthetic approaches to functionalized isatin derivatives (Moradi et al., 2017 ▸; Bogdanov & Mironov, 2018 ▸; Varun et al., 2019 ▸). Amongst these, the addition of nucleophilic units to the prochiral carbonyl group at atom C3 permits the construction of chiral 3-substituted-3-hydroxyindolin-2-ones containing a stereogenic centre at the 3-position (Peddibhotla, 2009 ▸; Mohammadi et al., 2013 ▸). Such species are desirable targets, because many related structural motifs are found in natural products and pharmaceutically active compounds; for example, convolutamydine A is a bioactive alkaloid with significant activity against HL-60 human plomyelocytic leukemia cells (Kamano et al., 1995 ▸), SM-130686 is a novel orally active growth hormone secretagogue (Nagamine et al., 2001 ▸), donaxaridine has shown effective anticancer properties (Kimura et al., 2016 ▸) and maremycins A and B exhibit antibacterial, antifungal and antitumour properties (Duan et al., 2018 ▸).
Several years ago, we reported the synthesis and structures of a range of 3-alkyl-3-hydroxyindolin-2-ones by reaction of isatin itself with a variety of methyl ketones in the presence of piperidine. Although the procedures were straightforward, the yields were consistently rather disappointing, in the range 40–60% (Becerra et al., 2010 ▸). Within the isatin molecule, both simple amide and vinylogous amide fragments can be identified, so that in the conjugate base of isatin the negative charge can be delocalized into both carbonyl groups. Any consequent partial proton transfer from isatin to piperidine is thus likely to be a factor in depressing the overall yields. We therefore reasoned that incorporation of a substituent at the N atom of isatin should prevent any such ionization and thus increase the product yields significantly.
Accordingly, we have now studied the synthesis and structures of a range of 3-(2-aryl-2-oxoethyl)-3-hydroxyindolin-2-ones, (I), and their dehydration to the corresponding chalcones, (II) (see Scheme 1), and we report here the synthesis and spectroscopic characterization of nine compounds of type (I), and eight of type (II), along with the molecular and supramolecular structures of four representative type (I) compounds [(Ic), (Id), (Ie) and (If)] and of five representative type (II) compounds [(IIa), (IIc), (IIe), (IIg) and (IIh)].
Experimental
Synthesis and crystallization
Isatins (A) (see Scheme 1), where X = H or Cl, were converted to the corresponding N-alkyl analogues (B) by reaction with the appropriate alkyl bromide in dimethylformamide solution in the presence of solid caesium carbonate acting as a weak base, giving yields in excess of 90% after a reaction time of 12 h at 298 K. For the synthesis of the 3-hydroxyindolin-2-ones, (I) (see Scheme 1), a mixture of an N-alkylisatin, (B) (1.0 mmol), the appropriate aryl methyl ketone (1.0 mmol) and piperidine (0.2 mmol) in ethanol (10 ml) was stirred at 298 K for 6 h [24 h in the case of compound (Id)], after which time the starting materials were no longer detectable using thin-layer chromatography (TLC). The resulting solid products were collected by filtration, washed with cold ethanol (2 ml) and dried in air to give the products of type (I). Analytical data for compound (Ia): yield 93%, m.p. 437–438 K (literature 437–441 K; Tripathi et al., 2016 ▸); compound (Ib): yield 91%, m.p. 430–431 K (literature 431–433 K; Duan et al., 2013 ▸); compound (Ic): yield 85%, m.p. 430 K (literature 429–431 K; Duan et al., 2013 ▸); compound (Id): yield 36%, m.p. 450 K; compound (Ie): yield 92%, m.p. 479–481 K; compound (If): yield 87%, m.p. 452–453 K; compound (Ig): yield 82%, m.p. 421–422 K (literature 421–423 K; Satish et al., 2015 ▸); compound (Ih): yield 89%, m.p. 423 K; compound (Ii): yield 83%, m.p. 398 K. Colourless crystals of compounds (Ic), (Id), (Ie) and (If) suitable for single-crystal X-ray diffraction analysis were grown by slow evaporation, at ambient temperature and in the presence of air, of solutions in ethanol–dimethylformamide (6:1 v/v).
For the conversion of the 3-hydroxy compounds (I) into the ethylidene products (II), a solution of the appropriate 3-hydroxy compound (I) (0.50 mmol) in glacial acetic acid (2 ml) was stirred at ambient temperature for 10 min, during which time concentrated hydrochloric acid (0.1 ml) was added slowly. The resulting mixtures were then stirred at 333 K for a further 2 h. For each mixture, the pH was then brought to 7.0 by the addition of a concentrated aqueous solution of ammonia, and the resulting solid products were collected by filtration, washed with cold water and dried in air to give the products of type (II). Analytical data for compound (IIa): yield 92%, m.p. 425–426 K; compound (IIb): yield 85%, m.p. 417–418 K; compound (IIc): yield 93%, m.p. 393 K; compound (IIe): yield 97%, m.p. 398 K; compound (IIf): yield 88%, m.p. 388 K; compound (IIg): yield 91%, m.p. 388–389 K; compound (IIh): yield 89%, m.p. 401–403 K; compound (IIi): yield 83%, m.p. 376–378 K. Crystals of compounds (IIa) (orange), and (IIb), (IIc), (IIe) and (IIg) (all red) suitable for single-crystal X-ray diffraction analysis were grown by slow evaporation, at ambient temperature and in the presence of air, of solutions in ethanol–dimethylformamide (initial composition 6:1 v/v).
Spectroscopic data for compounds (Ia), (Ib), (Ic) and (Ig) have been reported recently in the literature (Duan et al., 2013 ▸; Satish et al., 2015 ▸; Tripathi et al., 2016 ▸). Spectroscopic characterization data (1H and 13C NMR, and mass spectra) for the other compounds reported here are provided in the supporting information.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸. Compound (IIa) was handled as a non-merohedral twin with the twin matrix ( 00 0
00 0 0 0.350,0,1) and with refined twin fractions of 0.1953 (14) and 0.8047 (14). Compound (IIe) was also handled as a non-merohedral twin, with the twin matrix (
0 0.350,0,1) and with refined twin fractions of 0.1953 (14) and 0.8047 (14). Compound (IIe) was also handled as a non-merohedral twin, with the twin matrix ( 00 0
00 0 0 0.989,0,1) and refined twin fractions of 0.9654 (6) and 0.0346 (6). In compound (IIg), the thiophene unit is disordered over two sets of atomic sites having unequal occupancies for the minor-disorder component, and the bonded and [1,2]-nonbonded distances were restrained to be the same as the corresponding distances in the major-disorder component, subject to s.u. values of 0.01 and 0.02 Å, respectively; in addition, the anisotropic displacement parameters of pairs of partial-occupancy atoms occupying essentially the same physical space were constrained to be equal. All H atoms, apart from those in the minor-disorder component of compound (IIg), were located in difference maps. H atoms bonded to C atoms were then treated as riding atoms in geometrically idealized positions, with C—H = 0.95 (alkenyl, aryl and heteroaryl), 0.98 (CH3) or 0.99 Å (CH2) and with U
iso(H) = kU
eq(C), where k = 1.5 for the methyl groups, which were permitted to rotate but not to tilt, and 1.2 for all other H atoms bonded to C atoms; the H atoms in the minor-disorder component of compound (IIg) were included on the same basis, giving refined disorder occupancies of 0.9387 (19) and 0.0613 (19). For the H atoms bonded to O atoms, the atomic coordinates were refined with U
iso(H) = 1.5U
eq(O), giving the O—H distances shown in Table 2 ▸.
0 0.989,0,1) and refined twin fractions of 0.9654 (6) and 0.0346 (6). In compound (IIg), the thiophene unit is disordered over two sets of atomic sites having unequal occupancies for the minor-disorder component, and the bonded and [1,2]-nonbonded distances were restrained to be the same as the corresponding distances in the major-disorder component, subject to s.u. values of 0.01 and 0.02 Å, respectively; in addition, the anisotropic displacement parameters of pairs of partial-occupancy atoms occupying essentially the same physical space were constrained to be equal. All H atoms, apart from those in the minor-disorder component of compound (IIg), were located in difference maps. H atoms bonded to C atoms were then treated as riding atoms in geometrically idealized positions, with C—H = 0.95 (alkenyl, aryl and heteroaryl), 0.98 (CH3) or 0.99 Å (CH2) and with U
iso(H) = kU
eq(C), where k = 1.5 for the methyl groups, which were permitted to rotate but not to tilt, and 1.2 for all other H atoms bonded to C atoms; the H atoms in the minor-disorder component of compound (IIg) were included on the same basis, giving refined disorder occupancies of 0.9387 (19) and 0.0613 (19). For the H atoms bonded to O atoms, the atomic coordinates were refined with U
iso(H) = 1.5U
eq(O), giving the O—H distances shown in Table 2 ▸.
Table 1. Experimental details.
Experiments were carried out at 100 K with Mo Kα radiation using a Bruker D8 Venture diffractometer. Absorption was corrected for by multi-scan methods (SADABS; Bruker, 2016 ▸), except for (IIa) and (IIe), where TWINABS (Bruker, 2012) was used.
| (Ic) | (Id) | (Ie) | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | C24H21NO4 | C25H24N2O3 | C22H18N2O3 |
| M r | 387.42 | 400.46 | 358.38 |
| Crystal system, space group | Monoclinic, C2/c | Triclinic, P

|
Triclinic, P

|
| a, b, c (Å) | 18.7572 (14), 13.2095 (10), 16.750 (2) | 9.1028 (8), 10.6434 (9), 11.2539 (10) | 7.8838 (5), 10.1766 (8), 11.8719 (9) |
| α, β, γ (°) | 90, 105.374 (5), 90 | 88.988 (3), 68.422 (3), 81.352 (3) | 87.554 (3), 75.996 (2), 69.428 (2) |
| V (Å3) | 4001.7 (6) | 1001.55 (15) | 864.30 (11) |
| Z | 8 | 2 | 2 |
| μ (mm−1) | 0.09 | 0.09 | 0.09 |
| Crystal size (mm) | 0.14 × 0.13 × 0.10 | 0.23 × 0.19 × 0.16 | 0.25 × 0.16 × 0.06 |
| Data collection | |||
| T min, T max | 0.908, 0.991 | 0.946, 0.986 | 0.949, 0.994 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 18506, 4768, 3469 | 41110, 4590, 4033 | 55488, 4333, 3760 |
| R int | 0.051 | 0.037 | 0.045 |
| (sin θ/λ)max (Å−1) | 0.659 | 0.650 | 0.670 |
| Refinement | |||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.047, 0.110, 1.02 | 0.038, 0.098, 1.05 | 0.038, 0.101, 1.07 |
| No. of reflections | 4768 | 4590 | 4333 |
| No. of parameters | 266 | 276 | 247 |
| No. of restraints | 0 | 0 | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.30, −0.26 | 0.30, −0.22 | 0.41, −0.22 |
| (If) | (IIa) | (IIc) | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | C24H19NO5 | C23H16FNO2 | C24H19NO3 |
| M r | 401.40 | 357.37 | 369.40 |
| Crystal system, space group | Triclinic, P

|
Monoclinic, P21/c | Monoclinic, P21/n |
| a, b, c (Å) | 11.8136 (7), 12.4987 (10), 13.5976 (11) | 7.6021 (6), 20.4880 (13), 10.9319 (7) | 4.9743 (2), 29.1957 (13), 12.4406 (6) |
| α, β, γ (°) | 93.084 (3), 101.883 (2), 95.055 (2) | 90, 96.986 (3), 90 | 90, 100.914 (2), 90 |
| V (Å3) | 1951.7 (3) | 1690.0 (2) | 1774.05 (14) |
| Z | 4 | 4 | 4 |
| μ (mm−1) | 0.10 | 0.10 | 0.09 |
| Crystal size (mm) | 0.25 × 0.16 × 0.06 | 0.14 × 0.14 × 0.10 | 0.45 × 0.06 × 0.04 |
| Data collection | |||
| T min, T max | 0.957, 0.994 | 0.917, 0.990 | 0.948, 0.996 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 125792, 9691, 8134 | 3896, 3896, 2995 | 54500, 4142, 3643 |
| R int | 0.049 | N/A | 0.048 |
| (sin θ/λ)max (Å−1) | 0.667 | 0.652 | 0.653 |
| Refinement | |||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.039, 0.106, 1.08 | 0.058, 0.134, 1.05 | 0.037, 0.095, 1.06 |
| No. of reflections | 9691 | 3896 | 4142 |
| No. of parameters | 547 | 245 | 254 |
| No. of restraints | 0 | 0 | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.38, −0.24 | 0.29, −0.28 | 0.27, −0.23 |
| (IIe) | (IIg) | (IIh) | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | C22H16N2O2 | C21H15NO2S | C23H15Cl2NO2 |
| M r | 340.37 | 345.40 | 408.26 |
| Crystal system, space group | Monoclinic, P21/n | Orthorhombic, P b c a | Triclinic, P

|
| a, b, c (Å) | 7.3457 (6), 18.0675 (16), 13.1813 (13) | 17.5058 (14), 8.8163 (6), 21.2092 (16) | 8.2010 (6), 9.7629 (7), 12.1740 (9) |
| α, β, γ (°) | 90, 105.994 (3), 90 | 90, 90, 90 | 76.755 (3), 87.675 (3), 76.211 (3) |
| V (Å3) | 1681.7 (3) | 3273.4 (4) | 921.34 (12) |
| Z | 4 | 8 | 2 |
| μ (mm−1) | 0.09 | 0.21 | 0.37 |
| Crystal size (mm) | 0.16 × 0.15 × 0.12 | 0.15 × 0.07 × 0.05 | 0.40 × 0.16 × 0.07 |
| Data collection | |||
| T min, T max | 0.878, 0.990 | 0.942, 0.989 | 0.924, 0.974 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 4167, 4167, 3281 | 32005, 4154, 3280 | 51788, 4558, 3867 |
| R int | N/A | 0.065 | 0.053 |
| (sin θ/λ)max (Å−1) | 0.668 | 0.672 | 0.667 |
| Refinement | |||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.044, 0.121, 1.09 | 0.038, 0.092, 1.04 | 0.032, 0.082, 1.11 |
| No. of reflections | 4167 | 4154 | 4558 |
| No. of parameters | 237 | 239 | 253 |
| No. of restraints | 0 | 10 | 0 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.29, −0.19 | 0.29, −0.31 | 0.44, −0.34 |
Table 2. Hydrogen bonds and related short intramolecular contacts (Å, °) for compounds (Ic)–(If), (IIa), (IIe) and (IIg).
Cg1, Cg2 and Cg3 represent the centroids of the C3A/C4–C7/C7A, C11–C16 and C13A/C14–C17/C17A rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A | ||
|---|---|---|---|---|---|---|
| (Ic) | O3—H3⋯O2i | 0.86 (2) | 2.10 (2) | 2.9487 (15) | 171.1 (18) | |
| C6—H6⋯O324ii | 0.95 | 2.41 | 3.297 (2) | 155 | ||
| C31—H31B⋯O2i | 0.99 | 2.48 | 3.312 (2) | 141 | ||
| C4—H4⋯Cg2iii | 0.95 | 2.93 | 3.6101 (18) | 130 | ||
| C14—H14⋯Cg1iv | 0.95 | 2.82 | 3.709 (2) | 156 | ||
| (Id) | O3—H3⋯O2v | 0.868 (18) | 1.918 (18) | 2.7630 (12) | 164.2 (18) | |
| C7—H7⋯O32vi | 0.95 | 2.44 | 3.3343 (16) | 157 | ||
| C1—H1B⋯Cg1vi | 0.99 | 2.96 | 3.8375 (14) | 149 | ||
| (Ie) | O3—H3⋯N321vii | 0.897 (17) | 1.897 (17) | 2.7915 (14) | 174.9 (15) | |
| C4—H4⋯O3v | 0.95 | 2.46 | 3.3842 (14) | 164 | ||
| C7—H7⋯O32viii | 0.95 | 2.51 | 3.3719 (14) | 150 | ||
| C325—H325⋯O2ix | 0.95 | 2.32 | 3.2578 (15) | 171 | ||
| C322—H322⋯Cg1ii | 0.95 | 2.68 | 3.5294 (14) | 149 | ||
| (If) | O13—H13⋯O22 | 0.874 (17) | 1.912 (17) | 2.7794 (12) | 171.1 (17) | |
| O23—H23⋯O12 | 0.874 (17) | 1.912 (17) | 2.7794 (12) | 171.1 (17) | ||
| C131–H13A⋯O22vi | 0.99 | 2.35 | 3.3075 (16) | 161 | ||
| C147—H147⋯O141x | 0.95 | 2.56 | 3.4776 (18) | 163 | ||
| C231—H23A⋯O12v | 0.99 | 2.37 | 3.3107 (15) | 159 | ||
| C242—H24A⋯O23ii | 0.99 | 2.53 | 3.4889 (19) | 163 | ||
| C142—H14A⋯Cg3ii | 0.99 | 2.53 | 3.3289 (15) | 137 | ||
| (IIa) | C15—H15⋯O32xi | 0.95 | 2.49 | 3.278 (3) | 141 | |
| (IIe) | C14—H14⋯O2xii | 0.95 | 2.32 | 3.234 (3) | 161 | |
| C16—H16⋯O2xiii | 0.95 | 2.45 | 3.230 (2) | 140 | ||
| C326—H326⋯O2xiv | 0.95 | 2.58 | 3.494 (2) | 162 | ||
| C6—H6⋯Cg2xiii | 0.95 | 2.64 | 3.566 (2) | 165 | ||
| (IIg) | C5—H5⋯O2xv | 0.95 | 2.59 | 3.5058 (19) | 161 | |
| C323—H323⋯Cg2xvi | 0.95 | 2.93 | 3.744 (3) | 145 |
Symmetry codes: (i) −x +  , −y +
, −y +  , −z + 1; (ii) x, y − 1, z; (iii) x, −y + 1, z +
, −z + 1; (ii) x, y − 1, z; (iii) x, −y + 1, z +  ; (iv) −x +
; (iv) −x +  , −y +
, −y +  , −z + 1; (v) −x + 1, −y + 1, −z + 1; (vi) −x, −y + 1, −z + 2; (vii) x, y + 1, z; (viii) −x + 1, −y + 1, −z; (ix) −x, −y + 1, −z + 1; (x) −x, −y, −z + 1; (xi) −x + 1, y −
, −z + 1; (v) −x + 1, −y + 1, −z + 1; (vi) −x, −y + 1, −z + 2; (vii) x, y + 1, z; (viii) −x + 1, −y + 1, −z; (ix) −x, −y + 1, −z + 1; (x) −x, −y, −z + 1; (xi) −x + 1, y −  , −z +
, −z +  ; (xii) x +
; (xii) x +  , −y +
, −y +  , z −
, z −  ; (xiii) x −
; (xiii) x −  , −y +
, −y +  , z −
, z −  ; (xiv) x +
; (xiv) x +  , −y +
, −y +  , z +
, z +  ; (xv) x −
; (xv) x −  , y, −z +
, y, −z +  ; (xvi) −x + 1, y +
; (xvi) −x + 1, y +  , −z +
, −z +  .
.
Results and discussion
The title compounds were synthesized starting from the readily available isatins (A), (see Scheme 1, where X = H or Cl; Figs. 1–9 ▸ ▸ ▸ ▸ ▸ ▸ ▸ ▸ ▸). The N-alkylation of the starting isatins was explored using both benzyl bromide and 1-hexyl bromide in the presence of caesium carbonate as a weak non-nucleophilic base, giving isolated yields of the N-alkyl intermediates (B) consistently in excess of 90%. Focusing primarily on the N-benzyl intermediate of type (B), the subsequent reactions with aryl methyl ketones in the presence of piperidine did indeed provide generally much higher yields of the products of type (I), usually well above 80%, than had previously been achieved using isatin carrying no substituent at the N atom, which is consistent with the idea of partial proton transfer from the N-unsubstituted isatin to piperidine. The yields in both steps appear to be much the same regardless of whether the substituent at the N atom is benzyl or 1-hexyl, or whether the substituent at C5 is H or Cl. The only exception was found for compound (Id), where the yield was quite low, 36%, even after a much longer reaction time than that required for all the other type (I) products; this may be associated with the strongly electron-donating nature of the dimethylamino group. Acid-catalysed dehydration of nine of products (I) gave the N-substituted (E)-3-(2-aryl-2-oxoethylidene)indolin-2-ones (II), again with yields well above 80%, although, because of the slow formation and poor yields of (Id) in the first step, the dehydration of this intermediate was not pursued.
Figure 1.
The molecular structure of the R enantiomer of compound (Ic), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 2.
The molecular structure of the R enantiomer of compound (Id), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 3.
The molecular structure of the R enantiomer of compound (Ie), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 4.
The R enantiomers of the two independent molecules of compound (If), showing the atom-labelling schemes for (a) molecule 1 and (b) molecule 2. Displacement ellipsoids are drawn at the 30% probability level.
Figure 5.

The molecular structure of compound (IIa), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 6.

The molecular structure of compound (IIc), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 7.

The molecular structure of compound (IIe), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
Figure 8.

The molecular structure of compound (IIg), showing the atom-labelling scheme and the disorder of the thiophene unit. The major-disorder component is drawn using full lines and the minor-disorder component has been drawn using broken lines. Displacement ellipsoids are drawn at the 30% probability level.
Figure 9.

The molecular structure of compound (IIh), showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level.
For all of the products of types (I) and (II) (see Scheme 1), the mass spectra confirm their overall compositions, while the NMR spectra contain all of the expected signals, thus confirming that all the reactions have proceeded as expected and confirming the identity of the products. Each of the aldol products of type (I) contains a stereogenic centre, at atom C3 in (Ic), (Id) and (Ie), and at atoms C13 and C23 in the two independent molecules in (If). In every case, the reference molecule was selected as one having the R configuration at this atom, but the space groups (Table 1 ▸) confirm that, in each case, the compound has crystallized as a racemic mixture; in the absence from the synthesis of any agent capable of inducing enantioselectivity, it can confidently assumed that all of the other products of type (I) are also formed as racemic mixtures. For each of the type (II) products, only a single geometric isomer was isolated, with no chromatographic or spectroscopic evidence for the formation of even traces of any second isomer. As well as confirming the identity and racemic nature of the type (I) products, the crystal structure analyses have established that in each of the products of type (II) examined here the chalcone unit has the E configuration.
In each of the aldol compounds of type (I) (Figs. 1 ▸–4 ▸ ▸ ▸), the orientation of the N-benzyl unit relative to the indolinone nucleus shows some variation, as indicated by the key torsion angles (Table 3 ▸), despite the fact that atoms from the benzyl unit participate in intermolecular hydrogen bonding only in aldol (Ic) (Table 2 ▸). Similar variations in the orientation of the benzyl group are found in the chalcones of type (II) (Table 4 ▸), where this unit participates in intermolecular hydrogen bonding in both (IIa) and (IIe), but not in any of (IIc), (IIg) and (IIh). On the other hand, the conformations of the rest of the molecule relative to the indolinone unit is broadly similar in both type (I) and type (II) compounds, aside from the pyridyl derivative (Ie). In each of 4-methoxy derivatives (Ic) and (IIc), the two exocyclic C—C—O angles at atom C324 (Figs. 1 ▸ and 6 ▸) differ by ca 10°, as typically found (Seip & Seip, 1973 ▸; Ferguson et al., 1996 ▸) for near-planar alkoxyarene units; the deviations of methyl atoms C327 from the planes of the adjacent aryl rings are only 0.112 (3) and 0.183 (2) Å in (Ic) and (IIc), respectively.
Table 3. Selected torsion angles (°) for compounds (Ic)–(If).
| Parameter | (Ic) | (Id) | (Ie) | (If), molecule 1 | (If), molecule 2 |
|---|---|---|---|---|---|
| (x = 1) | (x = 2) | ||||
| Cx2—Nx1—Cx1—Cx11 | 103.75 (16) | 102.09 (12) | 95.90 (12) | 119.11 (13) | 108.60 (13) |
| Nx1—Cx1—Cx11—Cx12 | −28.5 (2) | −40.32 (15) | −76.98 (13) | −54.19 (16) | −27.57 (17) |
| Nx1—Cx2—Cx3—Cx31 | −126.14 (13) | −133.07 (10) | −124.96 (9) | −123.72 (10) | −126.04 (10) |
| Cx2—Cx3—Cx31—Cx32 | 52.57 (17) | 58.79 (13) | 61.83 (12) | 50.92 (13) | 51.93 (13) |
| C3—C31—C32—C321 | −176.52 (13) | 179.12 (10) | |||
| C3—C31—C32—C324 | −179.45 (10) | ||||
| C31—C32—C321—C322 | 175.64 (14) | −176.22 (10) | |||
| C31—C32—C324—C323 | −149.73 (12) | ||||
| Cx3—Cx31—Cx32—Cx45 | −174.30 (10) | −174.80 (10) | |||
| Cx31—Cx32—Cx45—Cx44 | 176.61 (10) | 178.65 (11) |
Table 4. Selected torsion angles (°) for compounds (IIa), (IIc), (IIe), (IIg) and (IIh).
| Parameter | (IIa) | (IIc) | (IIe) | (IIg) | (IIh) |
|---|---|---|---|---|---|
| C2—N1—C1—C11 | 111.4 (2) | 102.92 (13) | 90.35 (19) | 94.94 (16) | 98.83 (15) |
| N1—C1—C11—C12 | −41.8 (3) | −61.57 (14) | −1.1 (2) | −65.13 (18) | −62.18 (16) |
| N1—C2—C3—C31 | −176.39 (19) | −177.72 (10) | 179.61 (15) | 177.30 (12) | −179.37 (12) |
| C2—C3—C31—C32 | 176.1 (2) | 178.62 (11) | −178.71 (16) | 178.91 (13) | 177.52 (13) |
| C3—C31—C32—C321 | −176.0 (2) | 172.77 (11) | 177.57 (14) | ||
| C3—C31—C32—C322 | −175.99 (14) | ||||
| C3—C31—C32—C324 | 179.55 (16) | ||||
| C31—C32—C321—C322 | −178.2 (2) | 169.40 (10) | 175.80 (12) | ||
| C31—C32—C322—S321 | 167.55 (10) | ||||
| C31—C32—C324—C323 | 173.76 (15) |
The molecules of compound (Ic) are linked into a chain of rings by a combination of O—H⋯O, C—H⋯O and C—H⋯π(arene) hydrogen bonds (Table 2 ▸). Inversion-related pairs of molecules are linked by pairs of O—H⋯O hydrogen bonds, forming an  (10) ring (Etter, 1990 ▸; Etter et al., 1990 ▸; Bernstein et al., 1995 ▸) centred at (
(10) ring (Etter, 1990 ▸; Etter et al., 1990 ▸; Bernstein et al., 1995 ▸) centred at ( ,
,  ,
,  ). A second centrosymmetric motif, now centred at (
). A second centrosymmetric motif, now centred at ( ,
,  ,
,  ), is generated by the C—H⋯π(arene) hydrogen bond having atom C14 as the donor and, in combination, these two motifs generate a chain of rings running parallel to the [010] direction, in which the
), is generated by the C—H⋯π(arene) hydrogen bond having atom C14 as the donor and, in combination, these two motifs generate a chain of rings running parallel to the [010] direction, in which the  (10) rings centred at (
(10) rings centred at ( ,
,  + n,
+ n,  ) alternate with the rings generated by C—H⋯π hydrogen bonds, centred at (
) alternate with the rings generated by C—H⋯π hydrogen bonds, centred at ( ,
,  + n,
+ n,  ), where n represents an integer in each case. The chain formation is augmented by a C—H⋯O hydrogen bond having atom C6 as the donor and linking molecules which are related by translation into a C(13) chain motif (Fig. 10 ▸). There are two other short intermolecular contacts in the structure, involving atoms C4 and C31, but these are unlikely to be of real structural significance (Wood et al., 2009 ▸).
), where n represents an integer in each case. The chain formation is augmented by a C—H⋯O hydrogen bond having atom C6 as the donor and linking molecules which are related by translation into a C(13) chain motif (Fig. 10 ▸). There are two other short intermolecular contacts in the structure, involving atoms C4 and C31, but these are unlikely to be of real structural significance (Wood et al., 2009 ▸).
Figure 10.

Part of the crystal structure of compound (Ic), showing the formation of a chain of rings built from O—H⋯O, C—H⋯O and C—H⋯π(arene) hydrogen bonds. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motifs shown have been omitted.
The supramolecular assembly in compound (Id) is very simple, taking the form of a chain of rings running parallel to the [10 ] direction (Fig. 11 ▸). Rings of
] direction (Fig. 11 ▸). Rings of  (10) type, containing O—H⋯O hydrogen bonds (Table 2 ▸) and centred at (
(10) type, containing O—H⋯O hydrogen bonds (Table 2 ▸) and centred at ( + n,
+ n,  ,
,  − n) alternate with rings of
− n) alternate with rings of  (16) type, containing C—H⋯O hydrogen bonds and centred at (n,
(16) type, containing C—H⋯O hydrogen bonds and centred at (n,  ,
,  − n), where n represents an integer in each case. There is a long C—H⋯π(arene) contact within the chain, but there are no direction-specific interactions between adjacent chains.
− n), where n represents an integer in each case. There is a long C—H⋯π(arene) contact within the chain, but there are no direction-specific interactions between adjacent chains.
Figure 11.

Part of the crystal structure of compound (Id), showing the formation of a chain of rings built from O—H⋯O and C—H⋯O hydrogen bonds. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motifs shown have been omitted.
In contrast to the simplicity of the assembly in 4-(dimethylamino)phenyl compound (Id), that in 4-pyridine derivative (Ie) takes the form of the three-dimensional framework structure built from O—H⋯N, C—H⋯π(arene) and multiple C—H⋯O hydrogen bonds (Table 2 ▸), but the formation of the framework structure is readily analysed in terms of three one-dimensional substructures (Ferguson et al., 1998a
▸,b
▸; Gregson et al., 2000 ▸). A combination of O—H⋯N and C—H⋯π(arene) hydrogen bonds links molecules which are related by translation into a chain of rings running parallel to the [010] direction (Fig. 12 ▸). In the second substructure, a combination of the two hydrogen bonds having atoms C4 and C7 as the donors generates a chain of centrosymmetric rings running parallel to the [001] direction, in which  (10) rings centred at (
(10) rings centred at ( ,
,  ,
,  + n) alternate with
+ n) alternate with  (16) rings centred at (
(16) rings centred at ( ,
,  , n), where n represents an integer (Fig. 13 ▸). In the final substructure, the combination of the two hydrogen bonds having atoms C4 and C322 as the donors generates a chain of centrosymmetric rings running parallel to the [100] direction, in which
, n), where n represents an integer (Fig. 13 ▸). In the final substructure, the combination of the two hydrogen bonds having atoms C4 and C322 as the donors generates a chain of centrosymmetric rings running parallel to the [100] direction, in which  (10) rings centred at (
(10) rings centred at ( + n,
+ n,  ,
,  ) alternate with
) alternate with  (16) rings centred at (n,
(16) rings centred at (n,  , n), where n represents an integer (Fig. 14 ▸). The combination of chains along [100], [010] and [001] suffices to generate the three-dimensional framework structure.
, n), where n represents an integer (Fig. 14 ▸). The combination of chains along [100], [010] and [001] suffices to generate the three-dimensional framework structure.
Figure 12.

Part of the crystal structure of compound (Ie), showing the formation of a chain of rings running parallel to the [010] direction and built from O—H⋯N and C—H⋯π(arene) hydrogen bonds. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motifs shown have been omitted.
Figure 13.

Part of the crystal structure of compound (Ie), showing the formation of a chain of rings running parallel to the [001] direction and built from two types of C—H⋯O hydrogen bonds. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motifs shown have been omitted.
Figure 14.

Part of the crystal structure of compound (Ie), showing the formation of a chain of rings running parallel to the [100] direction and built from two types of C—H⋯O hydrogen bonds. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motifs shown have been omitted.
Compound (If) crystallizes with two molecules in the asymmetric unit but, despite this and the resulting number of independent hydrogen bonds (Table 2 ▸), the supramolecular assembly in only two-dimensional and, as with (Ie), this can be analysed in terms of low-dimensional substructures. The two molecules within the selected asymmetric unit (Fig. 15 ▸) are linked by two O—H⋯O hydrogen bonds to form a dimeric unit having approximate, but noncrystallographic, twofold rotation symmetry (Fig. 15 ▸). This finite, or zero-dimensional, substructure can conveniently be regarded as the basic building block in the supramolecular assembly. These dimeric units are linked by the C—H⋯O hydrogen bonds having atoms C131 and C231 as the donors to form a chain of rings running parallel to the [100] direction, in which  (12) rings centred at (n,
(12) rings centred at (n,  ,
,  ) alternate with
) alternate with  (12) rings centred at (
(12) rings centred at ( + n,
+ n,  ,
,  ), where n represents an integer in each case (Fig. 16 ▸). A second one-dimensional substructure arises from the linking of dimeric units which are related by translation by a combination of C—H⋯O and C—H⋯π(arene) hydrogen bonds to form a second chain of rings, this time running parallel to the [010] direction (Fig. 17 ▸). The combination of chains along [100] and [010] gives rise to complex sheets lying parallel to (001), but there are not direction-specific interactions between adjacent sheets.
), where n represents an integer in each case (Fig. 16 ▸). A second one-dimensional substructure arises from the linking of dimeric units which are related by translation by a combination of C—H⋯O and C—H⋯π(arene) hydrogen bonds to form a second chain of rings, this time running parallel to the [010] direction (Fig. 17 ▸). The combination of chains along [100] and [010] gives rise to complex sheets lying parallel to (001), but there are not direction-specific interactions between adjacent sheets.
Figure 15.
Part of the crystal structure of compound (If), showing the linking of the two independent molecules by two independent O—H⋯O hydrogen bonds. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motifs shown have been omitted.
Figure 16.

Part of the crystal structure of compound (If), showing the formation of a chain of rings running parallel to the [100] direction and built from O—H⋯O and C—H⋯O hydrogen bonds. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motifs shown have been omitted.
Figure 17.
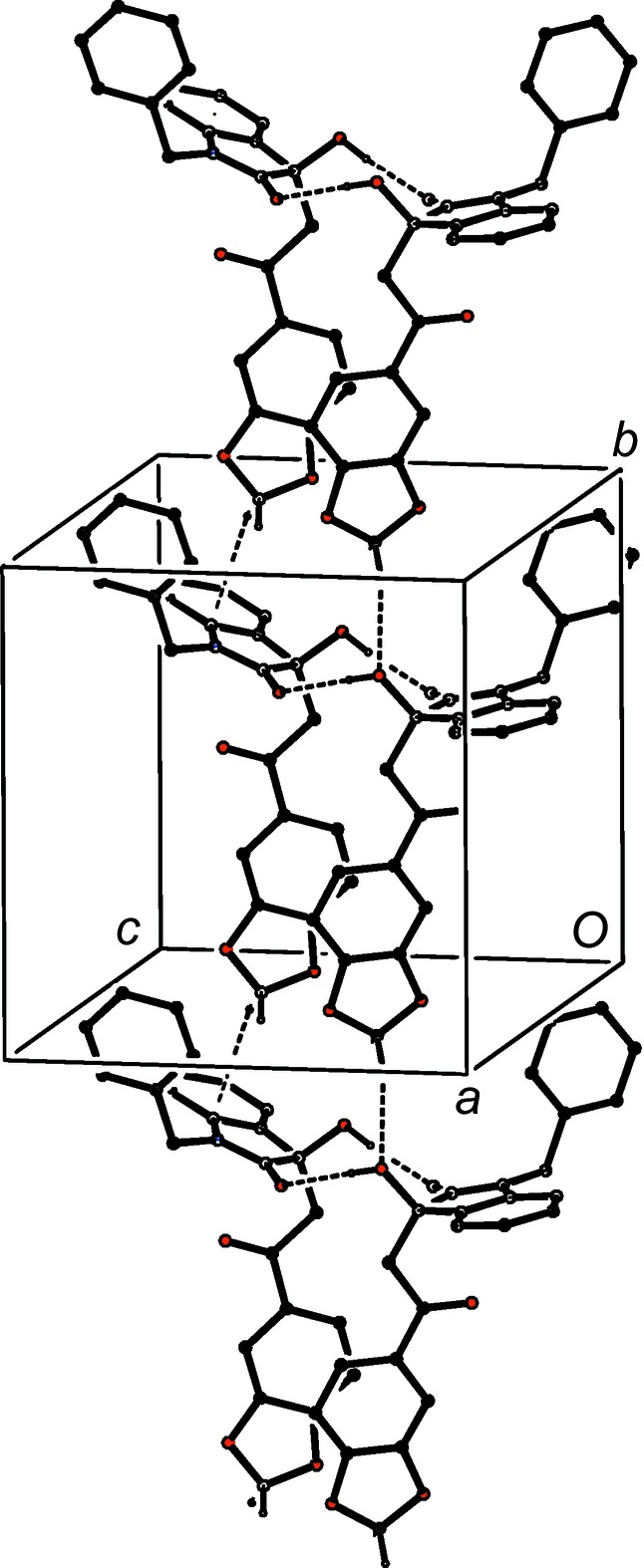
Part of the crystal structure of compound (If), showing the formation of a chain of rings running parallel to the [010] direction and built from O—H⋯O, C—H⋯O and C—H⋯π(arene) hydrogen bonds. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motifs shown have been omitted.
The absence of hydroxy groups in the compounds of type (II) means that the hydrogen bonding in these structures is simpler than that found in compounds of type (I). Thus, for each of compounds (IIc) and (IIh), there are no significant intermolecular hydrogen bonds, while in compound (IIa), a single C—H⋯O hydrogen bond (Table 2 ▸) links molecules which are related by a 21 screw axis into a C(11) chain running parallel to the [010] direction (Fig. 18 ▸). The hydrogen bonding in compound (IIg) is likewise very simple, with a single C—H⋯O hydrogen bond linking molecules which are related by the a-glide plane at z =  to form a C(7) chain running parallel to the [100] direction (Fig. 19 ▸).
to form a C(7) chain running parallel to the [100] direction (Fig. 19 ▸).
Figure 18.

Part of the crystal structure of compound (IIa), showing the formation of a hydrogen-bonded chain running parallel to [010]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motif shown have been omitted.
Figure 19.

Part of the crystal structure of compound (IIg), showing the formation of a hydrogen-bonded chain running parallel to [100]. Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the minor-disorder component and H atoms not involved in the motif shown have been omitted.
A combination of three independent C—H⋯O hydrogen bonds links the molecules of compound (IIe) into a sheet lying parallel to (010) and lying in the domain  < y < 1.0 (Fig. 20 ▸); a second sheet, related to the first by inversion, lies in the domain 0 < y <
< y < 1.0 (Fig. 20 ▸); a second sheet, related to the first by inversion, lies in the domain 0 < y <  , and adjacent sheets are linked by a C—H⋯π(arene) hydrogen bond, so generating a single three-dimensional framework structure.
, and adjacent sheets are linked by a C—H⋯π(arene) hydrogen bond, so generating a single three-dimensional framework structure.
Figure 20.

Part of the crystal structure of compound (IIe), showing the formation of a sheet built from three C—H⋯O hydrogen bonds and lying parallel to (010). Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, H atoms not involved in the motifs shown have been omitted.
Although there are no hydrogen bonds in the structure of compound (IIh), the molecules are nonetheless linked into a chain by the combination of a C—Cl⋯π(arene) interaction and a π–π stacking interaction. In the first of these interactions, atom Cl34 in the molecule at (x, y, z) forms a short contact with the C3A/C4–C7/C7A ring in the molecule at (−x, −y + 1, −z + 1), with geometric parameters Cl⋯Cg = 3.6055 (8) Å and C—Cl⋯Cg = 88.71 (5)°, where Cg represents the centroid of the aryl ring. The Cl⋯Cg distance here may be compared with the average value of 2.6° deduced from a database analysis of such contacts (Imai et al., 2008 ▸), and this interaction generates a cyclic centrosymmetric dimer. In addition, the C3A/C4–C7/C7A ring at (x, y, z) and the C321–C326 ring at (−x + 1, −y + 1, −z + 1) make a dihedral angle of only 7.58 (7)°. The ring-centroid separation is 3.7374 (9) Å and the shortest perpendicular distance from the centroid of one ring to the plane of the other is 3.3592 (6) Å, corresponding to a ring-centroid offset of ca 1.64 Å. The combination of these two interactions thus generates a chain of π-stacked dimers lying parallel to the [100] direction (Fig. 21 ▸).
Figure 21.
Part of the crystal structure of compound (IIh), showing the formation of a chain of π-stacked dimers running parallel to the [100] direction. The Cl⋯(ring centroid) contacts are shown as tapered lines and, for the sake of clarity, H atoms have all been omitted.
The synthetic methodology described here is notable for its operational simplicity, broad substrate scope, good functional group compatibility, and eco-compatibility in terms of energy and waste. We note, in addition, that in each of the hydroxy compounds (Ic), (Id) and (If), paired O—H⋯O hydrogen bonds generate  (10) motifs, which are centrosymmetric in each of (Ic) and (Id), although the ring in (If) exhibits no crystallographic symmetry. By contrast, the structure of pyridyl derivative (Ie) contains no O—H⋯O hydrogen bonds (Table 2 ▸). It is interesting in this context to note that in a series of seven 3-alkyl-3-hydroxyindolin-2-ones, having no substituent on the N atom of the indoline ring, every structure contains a centrosymmetric
(10) motifs, which are centrosymmetric in each of (Ic) and (Id), although the ring in (If) exhibits no crystallographic symmetry. By contrast, the structure of pyridyl derivative (Ie) contains no O—H⋯O hydrogen bonds (Table 2 ▸). It is interesting in this context to note that in a series of seven 3-alkyl-3-hydroxyindolin-2-ones, having no substituent on the N atom of the indoline ring, every structure contains a centrosymmetric  (10) ring embedded within a more complex supramolecular assembly involving N—H⋯O hydrogen bonds and, in some cases, C—H⋯O and C—H⋯π(arene) hydrogen bonds also (Becerra et al., 2010 ▸). Rings of the
(10) ring embedded within a more complex supramolecular assembly involving N—H⋯O hydrogen bonds and, in some cases, C—H⋯O and C—H⋯π(arene) hydrogen bonds also (Becerra et al., 2010 ▸). Rings of the  (10) type also occur in a number of related examples in the Cambridge Structural Database (CSD; Groom et al., 2016 ▸), including examples having CSD refcodes MUMMAY (Chen et al., 2009 ▸), TAWFAZ (Luppi et al., 2005 ▸), TEQVUH (Luppi et al., 2006 ▸) and YIFZIX (Xing et al., 2007 ▸). Finally, we note that the reaction of isatin with cyclohexanone involves both of the α-methylene units of the cyclohexanone, leading to the formation of 3,3′-[(1RS,3SR)-2-oxocyclohexane-1,3-diyl]bis[(3RS,3′SR)-3-hydroxyindolin-2-one] which was crystallized as a dehydrate (Becerra et al., 2013 ▸). The organic components, which exhibit approximate, but noncrystallographic, mirror symmetry are linked by a combination of N—H⋯O and O—H⋯O hydrogen bonds to form sheets containing rings of
(10) type also occur in a number of related examples in the Cambridge Structural Database (CSD; Groom et al., 2016 ▸), including examples having CSD refcodes MUMMAY (Chen et al., 2009 ▸), TAWFAZ (Luppi et al., 2005 ▸), TEQVUH (Luppi et al., 2006 ▸) and YIFZIX (Xing et al., 2007 ▸). Finally, we note that the reaction of isatin with cyclohexanone involves both of the α-methylene units of the cyclohexanone, leading to the formation of 3,3′-[(1RS,3SR)-2-oxocyclohexane-1,3-diyl]bis[(3RS,3′SR)-3-hydroxyindolin-2-one] which was crystallized as a dehydrate (Becerra et al., 2013 ▸). The organic components, which exhibit approximate, but noncrystallographic, mirror symmetry are linked by a combination of N—H⋯O and O—H⋯O hydrogen bonds to form sheets containing rings of  (8),
(8),  (16) and
(16) and  (40) types; these sheets are further linked by water molecules, which themselves form cyclic centrosymmetric
(40) types; these sheets are further linked by water molecules, which themselves form cyclic centrosymmetric  (8) tetramers.
(8) tetramers.
Supplementary Material
Crystal structure: contains datablock(s) global, Ic, Id, Ie, If, IIa, IIc, IIe, IIg, IIh. DOI: 10.1107/S2053229620004143/sk3748sup1.cif
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748Icsup11.cml
Structure factors: contains datablock(s) Ic. DOI: 10.1107/S2053229620004143/sk3748Icsup2.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748Idsup12.cml
Structure factors: contains datablock(s) Id. DOI: 10.1107/S2053229620004143/sk3748Idsup3.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748Iesup13.cml
Structure factors: contains datablock(s) Ie. DOI: 10.1107/S2053229620004143/sk3748Iesup4.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748Ifsup14.cml
Structure factors: contains datablock(s) If. DOI: 10.1107/S2053229620004143/sk3748Ifsup5.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIasup15.cml
Structure factors: contains datablock(s) IIa. DOI: 10.1107/S2053229620004143/sk3748IIasup6.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIcsup16.cml
Structure factors: contains datablock(s) IIc. DOI: 10.1107/S2053229620004143/sk3748IIcsup7.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIesup17.cml
Structure factors: contains datablock(s) IIe. DOI: 10.1107/S2053229620004143/sk3748IIesup8.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIgsup18.cml
Structure factors: contains datablock(s) IIg. DOI: 10.1107/S2053229620004143/sk3748IIgsup9.hkl
Structure factors: contains datablock(s) IIh. DOI: 10.1107/S2053229620004143/sk3748IIhsup10.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIhsup19.cml
Spectroscopic data for compounds (Id)-(If), (Ih), (Ii), (IIa)-(IIc) and (IIe)-(IIi). DOI: 10.1107/S2053229620004143/sk3748sup20.txt
Acknowledgments
The authors thank ‘Centro de Instrumentación Científico-Técnica’ of Universidad de Jaén and its staff for data collection. They also thank Universidad del Valle, Universidad Pedagógica y Tecnológica de Colombia (project SGI-2829), Universidad de Jaén and the Consejería de Innovación, Ciencia y Empresa (Junta de Andalucía, Spain), for financial support. DB also thanks the Asociación Universitaria Iberoamericana de Postgrado for financial support.
References
- Becerra, D., Insuasty, B., Cobo, J. & Glidewell, C. (2010). Acta Cryst. C66, o79–o86. [DOI] [PubMed]
- Becerra, D., Insuasty, B., Cobo, J. & Glidewell, C. (2013). Acta Cryst. C69, 1081–1084. [DOI] [PubMed]
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Bogdanov, A. V. & Mironov, V. (2018). Synthesis, 50, 1601–1609.
- Bruker (2016). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2017). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2018). APEX3. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chen, G., Liu, B., Tang, Y. & Xu, J. (2009). Acta Cryst. E65, o1723. [DOI] [PMC free article] [PubMed]
- Deng, P. & Zhang, Q. (2014). Polym. Chem. 5, 3298–3305.
- Duan, Y., Liu, Y., Huang, T., Zou, Y., Huang, T., Hu, K., Deng, Z. & Lin, S. (2018). Org. Biomol. Chem. 16, 5446–5451. [DOI] [PubMed]
- Duan, Z., Han, J., Qian, P., Zhang, Z., Wang, Y. & Pan, Y. (2013). Org. Biomol. Chem. 11, 6456–6459. [DOI] [PubMed]
- Etter, M. C. (1990). Acc. Chem. Res. 23, 120–126.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Ferguson, G., Glidewell, C., Gregson, R. M. & Meehan, P. R. (1998a). Acta Cryst. B54, 129–138.
- Ferguson, G., Glidewell, C., Gregson, R. M. & Meehan, P. R. (1998b). Acta Cryst. B54, 139–150.
- Ferguson, G., Glidewell, C. & Patterson, I. L. J. (1996). Acta Cryst. C52, 420–423.
- Gregson, R. M., Glidewell, C., Ferguson, G. & Lough, A. J. (2000). Acta Cryst. B56, 39–57. [DOI] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Imai, Y. N., Inoue, Y., Nakanishi, I. & Kitaura, K. (2008). Protein Sci. 17, 1129–1137. [DOI] [PMC free article] [PubMed]
- Kamano, Y., Zhang, H. P., Ichihara, Y., Kizu, H., Komiyama, K. & Pettit, G. R. (1995). Tetrahedron Lett. 36, 2783–2784.
- Kimura, J., Subba Reddy, U. V., Kohari, Y., Seki, C., Mawatari, Y., Uwai, K., Okuyama, Y., Kwon, E., Tokiwa, M., Takeshita, M., Iwasa, T. & Nakano, H. (2016). Eur. J. Org. Chem. 2016, 3748–3756.
- Luppi, G., Cozzi, P. G., Monari, M., Kaptein, B., Broxterman, Q. B. & Tomasini, C. (2005). J. Org. Chem. 70, 7418–7421. [DOI] [PubMed]
- Luppi, G., Monari, M., Corrêa, R. J., Violante, F. de A., Pinto, A. C., Kaptein, B., Broxterman, Q. B., Garden, S. J. & Tomasini, C. (2006). Tetrahedron, 62, 12017–12024.
- Mohammadi, S., Heiran, R., Herrera, R. P. & Marqués-López, E. (2013). ChemCatChem, 5, 2131–2148.
- Moradi, R., Ziarani, G. M. & Lashgari, N. (2017). ARKIVOC, Vol. 2017, Part (i), 148–201; doi: https://doi.org/10.24820/ark.5550190.p009.980.
- Nagamine, J., Nagata, R., Seki, H., Nomura-Akimaru, N., Ueki, Y., Kumagai, K., Taiji, M. & Noguchi, H. (2001). J. Endocrinol. 171, 481–489. [DOI] [PubMed]
- Pakravan, P., Kashanian, S., Khodaei, M. M. & Harding, F. J. (2013). Pharmacol. Rep. 65, 313–335. [DOI] [PubMed]
- Peddibhotla, S. (2009). Curr. Bioact. Compd. 5, 20–38.
- Satish, G., Polu, A., Ramar, T. & Ilangovan, A. (2015). J. Org. Chem. 80, 5167–5175. [DOI] [PubMed]
- Seip, H. M., Seip, R., Christensen, S. B., Brehm, L. & Nimmich, W. (1973). Acta Chem. Scand. 27, 4024–4027.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Singh, G. S. & Desta, Z. Y. (2012). Chem. Rev. 112, 6104–6155. [DOI] [PubMed]
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- Stalder, R., Mei, J., Graham, K. R., Estrada, L. A. & Reynolds, J. R. (2014). Chem. Mater. 26, 664–678.
- Tripathi, R. K. P., Krishnamurthy, S. & Ayyannan, S. R. (2016). ChemMedChem, 11, 119–132. [DOI] [PubMed]
- Varun, Sonam & Kakkar, R. (2019). Med. Chem. Commun. 10, 351–368. [DOI] [PMC free article] [PubMed]
- Vitaku, E., Smith, D. T. & Njardarson, J. T. (2014). J. Med. Chem. 57, 10257–10274. [DOI] [PubMed]
- Wood, P. A., Allen, F. H. & Pidcock, E. (2009). CrystEngComm, 11, 1563–1571.
- Xing, X.-N., Li, L., Zhu, X.-Y. & Chen, J.-R. (2007). Acta Cryst. E63, o2969–o2970.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, Ic, Id, Ie, If, IIa, IIc, IIe, IIg, IIh. DOI: 10.1107/S2053229620004143/sk3748sup1.cif
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748Icsup11.cml
Structure factors: contains datablock(s) Ic. DOI: 10.1107/S2053229620004143/sk3748Icsup2.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748Idsup12.cml
Structure factors: contains datablock(s) Id. DOI: 10.1107/S2053229620004143/sk3748Idsup3.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748Iesup13.cml
Structure factors: contains datablock(s) Ie. DOI: 10.1107/S2053229620004143/sk3748Iesup4.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748Ifsup14.cml
Structure factors: contains datablock(s) If. DOI: 10.1107/S2053229620004143/sk3748Ifsup5.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIasup15.cml
Structure factors: contains datablock(s) IIa. DOI: 10.1107/S2053229620004143/sk3748IIasup6.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIcsup16.cml
Structure factors: contains datablock(s) IIc. DOI: 10.1107/S2053229620004143/sk3748IIcsup7.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIesup17.cml
Structure factors: contains datablock(s) IIe. DOI: 10.1107/S2053229620004143/sk3748IIesup8.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIgsup18.cml
Structure factors: contains datablock(s) IIg. DOI: 10.1107/S2053229620004143/sk3748IIgsup9.hkl
Structure factors: contains datablock(s) IIh. DOI: 10.1107/S2053229620004143/sk3748IIhsup10.hkl
Supporting information file. DOI: 10.1107/S2053229620004143/sk3748IIhsup19.cml
Spectroscopic data for compounds (Id)-(If), (Ih), (Ii), (IIa)-(IIc) and (IIe)-(IIi). DOI: 10.1107/S2053229620004143/sk3748sup20.txt








