Abstract
Introduction:
In patients with stage IA lung adenocarcinoma (ADC), sublobar resection and tumor spread through air spaces (STAS) are associated with high rates of locoregional recurrence, half of which occur within the regional lymph nodes (LNs). Our objective was to investigate the association between occult LN metastasis (ONM) and STAS and to assess their prognostic value in patients with clinical stage IA lung ADC.
Methods:
The association between STAS and ONM was analyzed in patients who underwent lobectomy and LN dissection for clinical stage IA lung ADC (n=809). Multivariable logistic regression analysis was conducted to identify predictors of ONM. Site-specific recurrence by surgical procedure was investigated in patients with pathologic N0 disease (n=1055) using a competing-risks approach.
Results:
ONM was identified in 129 patients (16%)—one-third of ONMs were located only in intrapulmonary nodes. STAS was more common in patients with ONM (67% vs. 39%; P<0.001) and in patients with multiple ONMs (86%−89% vs. 60%−67%). STAS was a significant predictor of ONM on multivariable analysis, independent of tumor size, maximum standardized uptake value, and lymphovascular invasion. In STAS-positive (high ONM risk) patients, the risk of recurrence in the treated lobe and regional lymph nodes increased as the extent of resection decreased (recurrence risk: lobectomy < segmentectomy < wedge resection). In STAS-negative patients, the risk of locoregional recurrence did not differ by procedure type.
Conclusion:
Presence of STAS predicts ONM in patients with clinical stage IA lung ADC and can help stratify risk of recurrence by extent and type of resection.
Keywords: Lung adenocarcinoma, non-small cell lung cancer, lymph node metastasis, sublobar resection, pathological staging
Introduction
Lobectomy with hilar and mediastinal lymph node (LN) dissection is the standard of care for the management of patients with early-stage non-small cell lung cancer (NSCLC).1 This follows the results of the Lung Cancer Study Group randomized trial from 1995,2 which showed that sublobar resection was associated with a higher risk of locoregional recurrence than lobectomy for patients with T1N0M0 NSCLC.
An analysis of patients with stage I NSCLC from the Surveillance, Epidemiology, and End Results database (1998–2009) showed that both the incidence of early-stage NSCLC and the use of sublobar resection (segmentectomy and wedge resection) have been increasing3 despite ongoing concerns about the high risk of recurrence associated with these procedures.2,4,5 Lung adenocarcinoma (ADC) is the most common histologic type of NSCLC, and 25% of cases of lung ADC are diagnosed at stage IA.6 In patients with small, peripheral lung ADC, which is often treated with sublobar resection, accurate staging to confirm node-negative (N0) status is key. (18) F-fluorodeoxyglucose–positron emission tomography (PET) is routinely used in lung cancer workup on the basis of its higher sensitivity for the primary tumor, mediastinal LN metastasis, and distant metastasis, compared with conventional staging.1,7–9 However, even in patients with clinical N0 lung ADC identified on both computed tomography (CT) and PET, occult LN metastasis (ONM) still occurs at a high rate (both N1 and N2, 15%−21%; only N2, 9%−14%).10–13
Lung ADC is associated with a higher risk of ONM than other histologic types of NSCLC.11,14 We previously established that increasing percentage of micropapillary (MIP) subtype is associated with a higher risk of mediastinal ONM in patients with early-stage lung ADC without PET-positive mediastinal LNs.15 We also found that the presence of MIP subtype (≥5% of the tumor) was associated with a higher risk of locoregional recurrence in patients with small lung ADCs undergoing sublobar resection.16 On the basis of this observation, we investigated the lung parenchyma surrounding the tumor and identified a previously unrecognized pattern of invasion: tumor spread through air spaces (STAS), which is defined as tumor cells existing within air spaces in the lung parenchyma beyond the tumor edge. We were the first to report that STAS is significantly associated with a higher risk of locoregional recurrence following sublobar resection for lung ADC.17 In addition, we reported that in patients with stage IA lung ADC who underwent sublobar resection, a resection margin equal to more than the tumor diameter does not protect against locoregional recurrence, unlike in patients who underwent lobectomy.5 The prognostic importance of STAS has been validated in cohorts from multiple institutional databases18–23 and for other lung cancer histologic subtypes.24–27
Given that approximately half of locoregional recurrences occur within regional LNs, we hypothesized that STAS might be associated with the risk of ONM in patients with clinical N0 lung ADC. In the present study, we investigated the incidence, number, location, and size of ONMs in patients with clinical stage (c-Stage) IA lung ADC (N0 on CT and PET) and evaluated the association between ONM and STAS. Additionally, we hypothesized that the incidence of locoregional recurrence would be higher in STAS-positive patients undergoing sublobar resection than in those undergoing lobectomy.
Methods
Study Cohort and Data Collection
This retrospective study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK). The MSK Thoracic Surgery Service’s prospectively maintained lung cancer database was reviewed to identify consecutive patients who had been surgically treated for c-Stage IA lung ADC between January 1, 2000, and December 31, 2014. Exclusion criteria are shown in Figure 1. CT and (18) F-fluorodeoxyglucose–PET analyses performed within 3 months before surgery were reviewed for 1822 patients. Patients with tumor diameter >3 cm or LN short-axis diameter >1 cm on CT scan or patients with suspected hilar or mediastinal LN metastasis on PET scan (maximum standardized uptake value [SUVmax] ≥2.5)28–30 were excluded from the analysis. Patient demographic information was obtained from the MSK Thoracic Surgery Service’s prospectively maintained lung cancer database. Data on clinicopathological variables were obtained by reviewing patient medical records specifically for the purposes of this study, to determine clinical characteristics and follow-up status. Staging was based on the eighth edition of the American Joint Committee on Cancer Staging Manual.31
Figure 1.
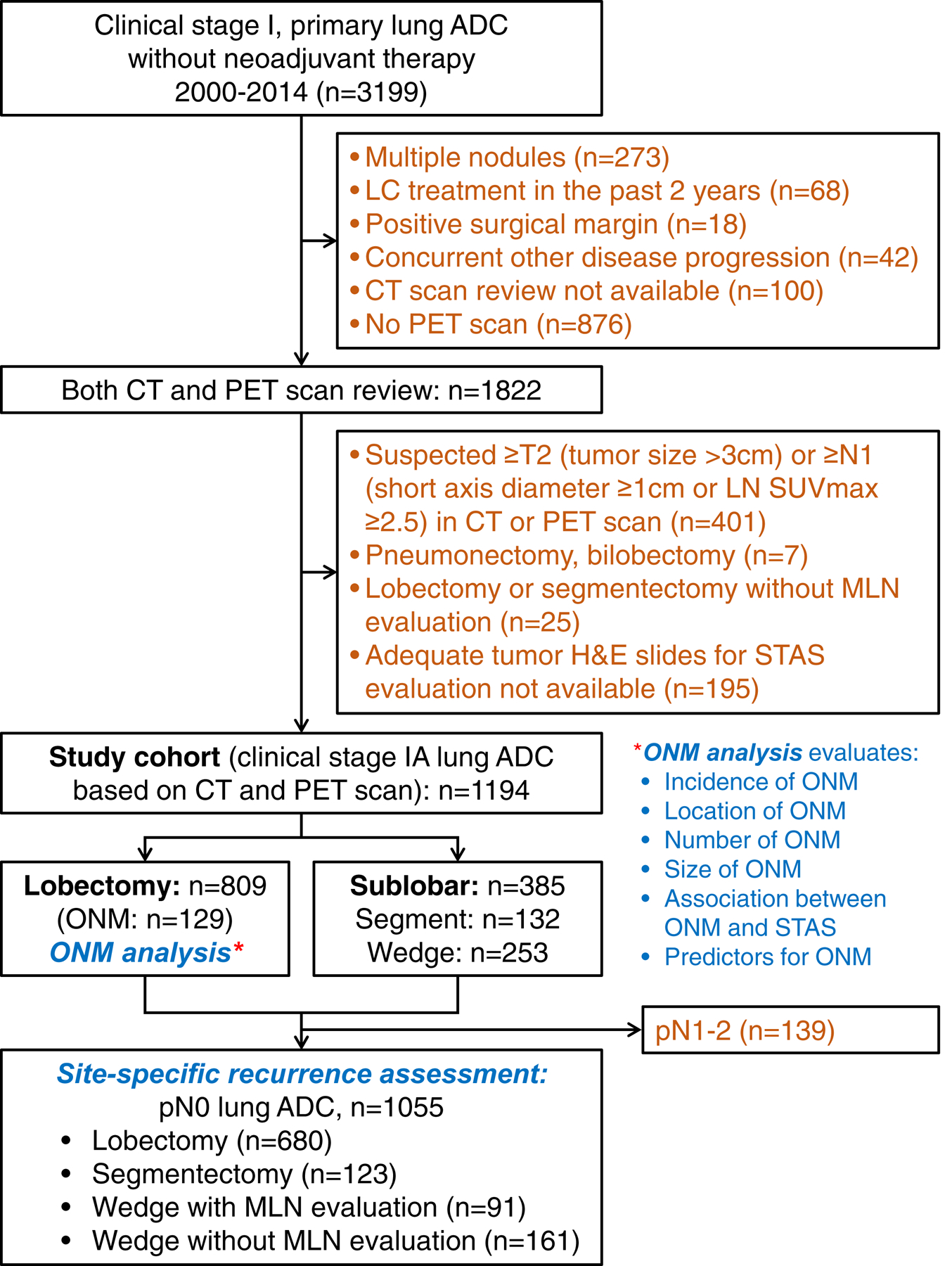
CONSORT diagram. ADC, adenocarcinoma; CT, computed tomography; LC, lung cancer; MLN, mediastinal lymph node; ONM, occult lymph node metastasis; PET, positron emission tomography; STAS, spread through air spaces; SUVmax, maximum standardized uptake value.
Patient follow-up status was updated as of July 2017. All recurrences were confirmed by clinical, radiologic, and pathologic assessment and were classified as local, regional LN, regional lung, or distant.32 Local recurrence was defined as recurrence in the staple line or the lung parenchyma within the treated lobe. Regional LN recurrence was defined as recurrence within the ipsilateral hilar or mediastinal LNs. Regional lung recurrence was defined as recurrence within the ipsilateral lobes other than the resected lobe.32 In cases where a new tumor developed in the lung or pleura and a biopsy specimen was available, the histologic profile was reviewed to determine whether the new tumor was a metachronous primary tumor, a recurrence, or a metastasis; this was completed in accordance with the method developed by our group.33 In total, 1194 patients with c-Stage IA lung ADC met the inclusion criteria.
Histologic Evaluation
All available hematoxylin and eosin–stained tumor and LN slides were reviewed by two pathologists (S.L. and W.D.T.), who were blinded to patient clinical outcomes, using an Olympus BX51 microscope (Olympus, Tokyo, Japan) with a standard 22-mm diameter eyepiece. Any discrepancies among the pathologists during assignment of predominant subtypes were later resolved via consensus using a multihead microscope.
Presence of tumor STAS was defined as tumor cells in clusters, solid nests, or aggregates of single cells within air spaces beyond the edge of the main tumor.17 Artifacts were excluded on the basis of previously described criteria.17 The percentage of each histologic pattern was recorded in 5% increments. Tumors were classified, in accordance with the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification and the 2015 World Health Organization classification, as adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma, which was subdivided into lepidic-predominant (LEP), acinar-predominant (ACI), papillary-predominant (PAP), MIP-predominant, solid-predominant (SOL), colloid-predominant (COL), and invasive mucinous (IMA) adenocarcinoma.34,35 Tumors were grouped by architectural grade as low (AIS, MIA, or LEP), intermediate (PAP or ACI), or high (MIP, SOL, COL, or IMA).36 Visceral pleural invasion (VPI), lymphovascular invasion (LVI), and necrosis were also investigated. In cases with LN metastasis, the largest diameter of metastatic area was measured using a ruler.
Evaluation of ONM
ONM was evaluated in 809 patients who underwent lobectomy with mediastinal LN evaluation. Pathologic reports were reviewed and the presence and location of ONM were evaluated for all cases. The location of ONM was classified, on the basis of nodal station,31 as either intrapulmonary (only #12-#14), hilar (#10, #11, with or without intrapulmonary LNs), and mediastinal (#2-#9, with or without N1 [#10–14] nodes). The number of metastatic LN stations was recorded. The size of the ONM was defined as the largest diameter among the metastatic areas.
Site-Specific Risk of Recurrence by Type of Resection
To investigate whether recurrence pattern is associated with risk of ONM, site-specific CIR was evaluated in patients with pathologic N0 disease (n=1055) who underwent lobectomy, segmentectomy, and wedge resection with pathologic mediastinal LN evaluation by either dissection or sampling and in patients who underwent wedge resection without mediastinal LN evaluation. Recurrence was classified as local (treated lobe, including the resection line), regional LN (ipsilateral hilar or mediastinal LN), regional lung (other ipsilateral lobe), or distant recurrence.32
Statistical Analysis
Patient clinicopathologic characteristics were summarized and compared using Fischer’s exact test and the χ2 test (for categorical variables), the Wilcoxon rank-sum test (to compare continuous variables between two groups), or the Kruskal-Wallis test (to compare continuous variables among more than two groups). Logistic regression analysis was used to identify risk factors for ONM. Multivariable models were constructed starting with variables with P<0.1 in the univariable analyses. To assess the marker of interest (STAS), the multivariable model building procedure was conducted in three phases: (1) identify a set of preoperative factors associated with ONM; (2) include STAS in the model from part 1, assuming STAS can be detected intraoperatively using frozen section analysis37; and (3) include postoperative factors in the model to investigate whether STAS can predict ONM independently of other pathologic factors, such as LVI. Recurrence patterns were summarized using a CIR approach and were compared between surgery types using Gray’s test for competing-risks events. Two statistical comparisons were conducted: (1) between three procedures (lobectomy, segmentectomy, and wedge resection) with mediastinal LN evaluation and (2) between four procedures (including wedge resection without LN evaluation). All P values were two-sided with a 5% alpha level. Statistical tests were conducted using Stata 13.1 (StataCorp, College Station, TX) and R 3.1.1 (R Development Core, Vienna, Austria).
Results
Patient Clinicopathologic Characteristics and ONM Status
The clinicopathologic characteristics of patients who underwent lobectomy are reported in Table 1. Of the 809 patients who underwent lobectomy, 129 (16%) had ONM identified. Of the 129 patients with ONM, 31% had only intrapulmonary ONM, 19% had hilar ONM, and 50% had mediastinal ONM. Fifty-seven percent had ONM in 1 station, 29% had 2 stations, and 15% had ≥3 stations. The median size of ONM was 3 mm (25th-75th percentile, 2–6 mm).
Table 1.
Patient clinicopathologic characteristics in the lobectomy cohort and their association with occult lymph node metastasis and spread through air spaces status
| Variable | Overall cohort (N=809) | ONM (−) (N=680) | ONM (+) (N=129) | P | STAS (−) (N=459) | STAS (+) (N=350) | P |
|---|---|---|---|---|---|---|---|
| Age, years | 68 (61–75) | 68 (61–75) | 68 (63–73) | 0.5 | 68 (61–75) | 68 (61–74) | 0.7 |
| Sex | |||||||
| Female | 530 (66) | 434 (64) | 96 (74) | 0.020 | 305 (66) | 225 (64) | 0.6 |
| Male | 279 (34) | 246 (36) | 33 (26) | 154 (34) | 125 (36) | ||
| Smoking | |||||||
| Never | 167 (21) | 144 (21) | 23 (18) | 0.5 | 111 (24) | 56 (16) | 0.003 |
| Former | 548 (68) | 460 (68) | 88 (68) | 305 (66) | 243 (69) | ||
| Current | 94 (12) | 76 (11) | 18 (14) | 43 (9) | 51 (15) | ||
| Pack-year index | 25 (4–50) | 25 (4–50) | 30 (9–50) | 0. 5 | 23 (1–45) | 30 (11–50) | 0.003 |
| CT tumor size, cm | 2.0 (1.5–2.5) | 1.9 (1.5–2.4) | 2.2 (1.7–2.7) | <0.001 | 1.9 (1.5–2.4) | 2.0 (1.5–2.5) | 0.15 |
| Tumor SUVmax | 2.7 (1.5–5.2) | 2.4 (1.3–4.4) | 4.7 (3.0–7.9) | <0.001 | 2.1 (1.1–3.9) | 3.8 (2.0–6.9) | <0.001 |
| ONM | |||||||
| Present | 129 (16) | 42 (9) | 87 (25) | <0.001 | |||
| Location | |||||||
| Intrapulmonarya | 40 (5) | 14 (3) | 26 (7) | <0.001 | |||
| Hilarb | 24 (3) | 9 (2) | 15 (4) | ||||
| Mediastinalc | 65 (8) | 19 (4) | 46 (13) | ||||
| Number of metastatic stations | |||||||
| 1 | 73 (9) | 28 (6) | 45 (13) | <0.001 | |||
| 2 | 37 (5) | 10 (2) | 27 (8) | ||||
| ≥3 | 19 (2) | 4 (1) | 15 (4) | ||||
| Size of metastatic area, mmd | 3 (2–6) | 4 (2–5) | 3 (1–6) | 0. 3 | |||
| pN (8th ed.) | |||||||
| N1a (single N1) | 55 (7) | 22 (5) | 33 (9) | <0.001 | |||
| N1b (multiple N1) | 9 (1) | 1 (0) | 8 (2) | ||||
| N2a (single N2) | 51 (6) | 17 (4) | 34 (10) | ||||
| N2b (multiple N2) | 14 (2) | 2 (0) | 12 (3) | ||||
| STAS present | 350 (43) | 263 (39) | 87 (67) | <0.001 | |||
| Pathologic tumor size, cm | 1.8 (1.4–2.4) | 1.7 (1.3–2.2) | 2.0 (1.6–2.5) | <0.001 | 1.7 (1.2–2.2) | 1.9 (1.5–2.5) | <0.001 |
| Invasive tumor size, cm | 1.5 (1.0–2.0) | 1.4 (1.0–2.0) | 2.0 (1.5–2.5) | <0.001 | 1.2 (0.8–1.8) | 1.8 (1.3–2.3) | <0.001 |
| LVI | 377 (47) | 269 (40) | 108 (84) | <0.001 | 155 (34) | 222 (63) | <0.001 |
| VPI | 140 (17) | 96 (14) | 44 (34) | <0.001 | 53 (12) | 87 (25) | <0.001 |
| Necrosis (n=792) | 96 (12) | 80 (12) | 16 (13) | 0.7 | 38 (8) | 58 (17) | 0.003 |
| Predominant subtype | |||||||
| AIS | 1 (0) | 1 (0) | 0 (0) | <0.001 | 1 (0) | 0 (0) | <0.001 |
| MIA | 47 (6) | 47 (7) | 0 (0) | 47 (10) | 0 (0) | ||
| Lepidic | 59 (7) | 55 (8) | 4 (3) | 53 (12) | 6 (2) | ||
| Acinar | 369 (46) | 308 (45) | 61 (47) | 205 (45) | 164 (47) | ||
| Papillary | 123 (15) | 110 (16) | 13 (10) | 69 (15) | 54 (15) | ||
| Micropapillary | 56 (7) | 36 (5) | 20 (16) | 11 (2) | 45 (13) | ||
| Solid | 130 (16) | 99 (15) | 31 (24) | 54 (12) | 76 (22) | ||
| IMA | 22 (3) | 22 (3) | 0 (0) | 17 (4) | 5 (1) | ||
| Colloid | 2 (0) | 2 (0) | 0 (0) | 2 (0) | 0 (0) | ||
| Histologic grade | |||||||
| Low | 107 (13) | 103 (15) | 4 (3) | <0.001 | 101 (22) | 6 (2) | <0.001 |
| Intermediate | 493 (61) | 419 (62) | 74 (57) | 274 (60) | 219 (63) | ||
| High | 209 (26) | 158 (23) | 51 (40) | 84 (18) | 125 (36) | ||
| Mutation status (n=683) | |||||||
| Wild-type | 337 (49) | 288 (49) | 49 (52) | 0.7 | 184 (48) | 153 (51) | 0.3 |
| EGFR | 150 (22) | 133 (23) | 17 (18) | 93 (24) | 57 (19) | ||
| KRAS | 196 (29) | 168 (29) | 28 (30) | 107 (28) | 89 (30) |
Data are no. (%) or median (25–75 percentile). AIS, adenocarcinoma in situ; CT, computed tomography; IMA, invasive mucinous adenocarcinoma; LVI, lymphovascular invasion; MIA, minimally invasive adenocarcinoma; ONM, occult lymph node metastasis; STAS, spread through air spaces; SUVmax, maximum standardized uptake value; VPI, visceral pleural invasion.
Only intrapulmonary lymph node metastasis (#12-#14), without hilar and mediastinal metastasis.
Hilar lymph node metastasis (#10, #11) without mediastinal metastasis, with or without intrapulmonary metastasis.
Mediastinal lymph node metastasis (#2-#9), with or without hilar/intrapulmonary metastasis.
Largest diameter of the metastatic area.
The presence of ONM was significantly associated with female sex, larger tumor CT size, higher tumor SUVmax, presence of STAS, larger pathologic total and invasive tumor size, presence of LVI, presence of VPI, and a lower proportion of low-grade and a higher proportion of high-grade histologic subtypes (Table 1). The location and incidence of ONM, by resected lobes, are shown in supplemental data, Table S1.
STAS and ONM
Of the 809 patients who underwent lobectomy, 350 (43%) had STAS identified. The presence of STAS was significantly associated with current smoker status, higher pack-year index, higher tumor SUVmax, higher number of ONMs, larger pathologic total and invasive tumor size, presence of LVI, presence of VPI, presence of necrosis, and a lower proportion of low-grade and a higher proportion of high-grade histologic subtypes (Table 1).
Figure 2 demonstrates the relationship between ONM status and incidence of STAS. The incidence of STAS was higher in patients with ONM than in those without ONM (67% vs. 39%; P<0.001) and higher in patients with multiple ONMs (N1b or N2b) than in those with a single ONM (N1a or N2a) (86%−89% vs. 60%−67%). The incidence of STAS increased with increasing number of ONMs (0=39%, 1=62%, 2=73%, 3=79%; P<0.001).
Figure 2.
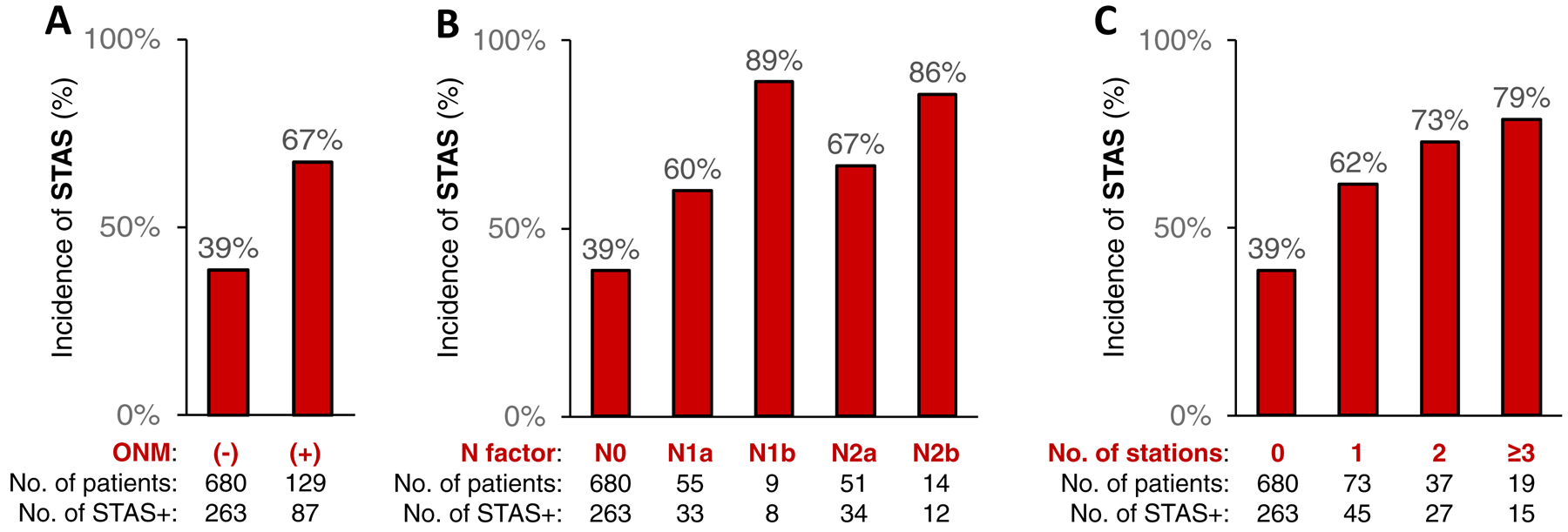
Percentage of STAS-positive patients by ONM status – A) incidence of STAS by ONM, B) incidence of STAS in N1 and N2 lymph nodes, and C) incidence of STAS and number of positive lymph node stations. ONM, occult lymph node metastasis; STAS, spread through air spaces.
Predictors of ONM
In univariable logistic analysis, female sex, larger CT tumor size, higher tumor SUVmax, presence of STAS, larger pathologic total and invasive tumor size, presence of LVI, presence of VPI, and higher-grade histologic subtypes were significantly associated with ONM (Table 2).
Table 2.
Univariable and multivariable logistic regression analysis for predicting occult lymph node metastasis
| Multivariable models | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Preoperative factors | Preoperative + STAS | Both pre- and postoperative | |||||||||
| Factor | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Preoperative factors | ||||||||||||
| Age (per 1-year increase) | 1.00 | 0.98–1.02 | 0.7 | |||||||||
| Male (vs female) | 0.61 | 0.40–0.93 | 0.021 | 0.60 | 0.39–0.93 | 0.023 | 0.57 | 0.37–0.90 | 0.015 | 0.50 | 0.31–0.78 | 0.003 |
| Smoking (vs never) | ||||||||||||
| Former | 1.20 | 0.73–1.97 | 0.5 | |||||||||
| Current | 1.48 | 0.75–2.92 | 0.3 | |||||||||
| Pack-year index (per 1 index increase) | 1.00 | 0.99–1.01 | 1.0 | |||||||||
| CT tumor size (per 1-cm increase) | 2.04 | 1.46–2.86 | <0.001 | 1.55 | 1.08–2.21 | 0.016 | 1.59 | 1.10–2.29 | 0.013 | |||
| Tumor SUVmax (per 1 value increase) | 1.16 | 1.11–1.22 | <0.001 | 1.15 | 1.09–1.21 | <0.001 | 1.12 | 1.06–1.18 | <0.001 | 1.06 | 1.00–1.12 | 0.037 |
| STAS | ||||||||||||
| Present (vs absent) | 3.28 | 2.20–4.90 | <0.001 | 2.82 | 1.87–4.28 | <0.001 | 1.89 | 1.23–2.93 | 0.004 | |||
| Postoperative factors | ||||||||||||
| Pathologic tumor size (per 1-cm increase) | 1.82 | 1.43–2.31 | <0.001 | |||||||||
| Invasive tumor size (per 1-cm increase) | 2.29 | 1.80–2.91 | <0.001 | 1.42 | 1.06–1.90 | 0.019 | ||||||
| LVI (vs absent) | 7.86 | 4.80–12.85 | <0.001 | 5.21 | 3.08–8.83 | <0.001 | ||||||
| VPI (vs absent) | 3.15 | 2.06–4.81 | <0.001 | |||||||||
| Necrosis (vs absent) | 1.13 | 0.63–2.00 | 0.7 | |||||||||
| Histologic grade (vs low) | ||||||||||||
| Intermediate | 4.55 | 1.63–12.73 | 0.004 | |||||||||
| High | 8.31 | 2.92–23.69 | <0.001 | |||||||||
| Mutation (vs wild-type) | ||||||||||||
| EGFR | 0.75 | 0.42–1.35 | 0.3 | |||||||||
| KRAS | 0.98 | 0.59–1.62 | 0.9 | |||||||||
CI, confidence interval; CT, computed tomography; LVI, lymphovascular invasion; OR, odds ratio; STAS, spread through air spaces; SUVmax, maximum standardized uptake value; VPI, visceral pleural invasion.
In the first multivariable model, which included only preoperative factors, female sex, larger CT tumor size, and higher SUVmax were independent risk factors for ONM. In the second multivariable model, which included STAS in addition to preoperative factors, STAS was a significant risk factor for ONM (odds ratio, 2.82 [95% confidence interval, 1.87–4.28]; P<0.001), independent of sex, CT tumor size, and SUVmax. In the third multivariable model, which included both preoperative and postoperative pathologic factors, STAS remained a significant risk factor for ONM (odds ratio, 1.89 [95% confidence interval, 1.23–2.93]; P=0.004), independent of sex, SUVmax, invasive tumor size, and LVI.
ONM Risk-Based Recurrence Pattern Assessment: Site-Specific 5-Year CIR by Procedure
Table 3 shows site-specific 5-year CIR by type of resection. The top panel shows the results from the overall cohort of patients. Patients were divided into two groups on the basis of risk of ONM as determined by STAS status: patients with STAS were considered high ONM risk (middle panel), and patients without STAS were considered low ONM risk (bottom panel).
Table 3.
Site-specific 5-year cumulative incidence of recurrence between procedures in patients with pN0 lung adenocarcinoma: Occult lymph node metastasis risk-based recurrence pattern assessment
| MLN evaluation (+)a | MLN evaluation (−)c | |||||
|---|---|---|---|---|---|---|
| Cohort, site | Lobectomy | Segmentectomy | Wedge | Pb | Wedge | Pd |
| Overall | N=680 | N=123 | N=91 | N=161 | ||
| Local (treated lobe) | 0 (N/A) | 3 (1–9) | 5 (2–14) | <0.001 | 14 (9–21) | <0.001 |
| Regional LN (ipsilateral hilar/mediastinal) | 1 (1–3) | 4 (1–10) | 8 (3–19) | 0.011 | 14 (9–21) | <0.001 |
| Regional lung (ipsilateral another lobe) | 3 (2–5) | 2 (0–7) | 1 (0–9) | 0.7 | 7 (3–13) | 0.053 |
| Distant | 9 (7–12) | 7 (4–14) | 12 (6–23) | 0.5 | 12 (8–20) | 0.3 |
| High ONM risk (STAS positive) | N=263 | N=37 | N=34 | N=75 | ||
| Local (treated lobe) | 0 (N/A) | 3 (0–20) | 8 (2–32) | <0.001 | 23 (14–36) | <0.001 |
| Regional LN (ipsilateral hilar/mediastinal) | 2 (1–6) | 9 (3–26) | 21 (9–50) | <0.001 | 22 (14–35) | <0.001 |
| Regional lung (ipsilateral another lobe) | 5 (3–10) | 6 (1–22) | 3 (0–24) | 0.9 | 11 (5–23) | 0.4 |
| Distant | 14 (10–19) | 11 (4–29) | 22 (10–50) | 0.7 | 21 (13–35) | 0.5 |
| Low ONM risk (STAS negative) | N=417 | N=86 | N=57 | N=86 | ||
| Local (treated lobe) | 0 (N/A) | 3 (1–12) | 4 (1–17) | 0.002 | 7 (3–15) | <0.001 |
| Regional LN (ipsilateral hilar/mediastinal) | 1 (0–3) | 2 (0–12) | 0 (N/A) | 0.8 | 6 (3–15) | <0.001 |
| Regional lung (ipsilateral another lobe) | 1 (0–3) | 0 (N/A) | 0 (N/A) | 0.5 | 3 (1–10) | 0.3 |
| Distant | 6 (4–9) | 6 (2–15) | 7 (3–19) | 0.7 | 5 (2–13) | 0.9 |
Data are % (95% confidence interval). LN, lymph node; MLN, mediastinal lymph node; N/A, not applicable; ONM, occult lymph node metastasis; STAS, spread through air spaces.
Pathologic evaluation of at least one MLN by either dissection or sampling.
Comparison between three procedures with MLN evaluation.
No pathologic evaluation of MLNs.
Comparison between four procedures, including wedge resection without MLN evaluation.
In the overall cohort, the incidence of local (treated lobe) and regional LN recurrence were lowest in patients who underwent lobectomy; the incidence increased as the extent of resection decreased (recurrence risk: lobectomy < segmentectomy < wedge resection) and was highest in patients who underwent wedge resection without mediastinal LN evaluation. There was no statistically significant difference in the incidence of distant recurrence across the four procedures.
Differences in local and regional LN recurrence across procedures were more evident in the high ONM risk cohort (patients with STAS) than in the overall cohort. Of note, the incidence of regional LN recurrence was significantly higher after sublobar resection than after lobectomy; the risk was highest after wedge resection, regardless of mediastinal LN evaluation.
In the low ONM risk cohort (patients without STAS), risk of local recurrence was higher after sublobar resection than after lobectomy but was similar after segmentectomy and wedge resection with mediastinal LN evaluation. The risk of regional LN recurrence did not differ by extent of resection, with the exception of a higher risk in patients treated with wedge resection without mediastinal LN evaluation.
Table 4 shows patient clinicopathologic characteristics and a comparison of the four surgical procedures. Greater extent of resection (lobectomy > segmentectomy > wedge) was associated with younger age, lower pack-year index, larger CT tumor size, higher tumor SUVmax, larger pathologic total and invasive tumor size, and a lower proportion of low-grade subtypes.
Table 4.
Patient clinicopathologic characteristics and comparison between surgical procedures in patients who underwent lobectomy, segmentectomy, and wedge resection for pN0 lung adenocarcinoma
| MLN Evaluation (+)a | MLN Evaluation (−)b | ||||
|---|---|---|---|---|---|
| Characteristic | Lobectomy (N=680) | Segmentectomy (N=123) | Wedge (N=91) | Wedge (N=161) | P |
| Age, years | 68 (61–75) | 68 (61–75) | 71 (65–76) | 70 (63–77) | 0.004 |
| Sex | |||||
| Female | 434 (64) | 85 (69) | 63 (69) | 101 (63) | 0.5 |
| Male | 246 (36) | 38 (31) | 28 (31) | 60 (37) | |
| Smoking | |||||
| Never | 144 (21) | 25 (20) | 9 (10) | 25 (16) | 0.14 |
| Former | 460 (68) | 81 (66) | 70 (77) | 116 (72) | |
| Current | 76 (11) | 17 (14) | 12 (13) | 20 (12) | |
| Pack-year index | 25 (4–50) | 25 (5–60) | 31 (15–56) | 36 (10–56) | 0.045 |
| CT tumor size, cm | 1.9 (1.5–2.4) | 1.7 (1.3–2.2) | 1.5 (1.2–1.9) | 1.5 (1.1–1.8) | <0.001 |
| Tumor SUVmax | 2.4 (1.3–4.4) | 2.4 (1.2–4.1) | 2.1 (1.2–2.9) | 1.9 (0.0–3.6) | 0.001 |
| T factor | |||||
| Tis | 1 (0) | 0 (0) | 0 (0) | 2 (1) | <0.001 |
| T1a | 129 (19) | 34 (28) | 22 (24) | 52 (32) | |
| T1a(mi) | 47 (7) | 17 (14) | 16 (18) | 26 (16) | |
| T1b | 304 (45) | 52 (42) | 34 (37) | 49 (30) | |
| T1c | 90 (13) | 6 (5) | 0 (0) | 2 (1) | |
| T2a | 97 (14) | 14 (11) | 19 (21) | 29 (18) | |
| T2b | 4 (1) | 0 (0) | 0 (0) | 0 (0) | |
| T3 | 8 (1) | 0 (0) | 0 (0) | 1 (1) | |
| Pathologic tumor size, cm | 1.7 (1.3–2.2) | 1.5 (1.1–2.0) | 1.3 (1.0–1.8) | 1.2 (1.0–1.6) | <0.001 |
| Invasive tumor size, cm | 1.4 (1.0–2.2) | 1.1 (0.7–1.5) | 1.0 (0.5–1.5) | 1.0 (0.6–1.4) | <0.001 |
| LVI | 269 (40) | 41 (33) | 26 (29) | 59 (37) | 0.2 |
| VPI | 96 (14) | 11 (9) | 19 (21) | 29 (18) | 0.051 |
| Necrosis (n=1041) | 80 (12) | 9 (7) | 5 (6) | 10 (6) | 0.053 |
| STAS | 263 (39) | 37 (30) | 34 (37) | 75 (47) | 0.043 |
| Predominant subtype | |||||
| AIS | 1 (0) | 0 (0) | 0 (0) | 2 (1) | 0.022 |
| MIA | 47 (7) | 17 (14) | 16 (18) | 26 (16) | |
| Lepidic | 55 (8) | 12 (10) | 12 (13) | 14 (9) | |
| Acinar | 308 (45) | 51 (41) | 31 (34) | 63 (39) | |
| Papillary | 110 (16) | 14 (11) | 10 (11) | 22 (14) | |
| Micropapillary | 36 (5) | 5 (4) | 6 (7) | 7 (4) | |
| Solid | 99 (15) | 17 (14) | 11 (12) | 23 (14) | |
| IMA | 22 (3) | 7 (6) | 5 (5) | 4 (2) | |
| Colloid | 2 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Histologic grade | |||||
| Low | 103 (15) | 30 (24) | 28 (31) | 42 (26) | 0.001 |
| Intermediate | 419 (62) | 64 (52) | 41 (45) | 85 (53) | |
| High | 158 (23) | 29 (24) | 22 (24) | 34 (21) | |
| Mutation status (n=890) | |||||
| Wild-type | 288 (49) | 49 (51) | 36 (49) | 61 (47) | 0.11 |
| EGFR | 133 (23) | 17 (18) | 7 (10) | 26 (20) | |
| KRAS | 168 (29) | 31 (32) | 30 (41) | 44 (34) | |
Data are no. (%) or median (25–75 percentile). AIS, adenocarcinoma in situ; CT, computed tomography; IMA, invasive mucinous adenocarcinoma; LVI, lymphovascular invasion; MIA, minimally invasive adenocarcinoma; MLN, mediastinal lymph node; ONM, occult lymph node metastasis; STAS, spread through air spaces; SUVmax, maximum standardized uptake value; VPI, visceral pleural invasion.
Pathologic evaluation of at least one MLN by either dissection or sampling.
No pathologic evaluation of MLNs.
Discussion
The novelty of the present study is reflected in its evaluation of detailed characteristics of ONM, including location, number, and size, using a large cohort of patients with c-Stage IA lung ADC. Of significance, (1) one-third of ONMs were located in intrapulmonary LNs without hilar and mediastinal LN metastasis, and half of ONMs were 3 mm or smaller, suggesting potential difficulty in detecting ONMs by hilar and mediastinal sampling during sublobar resection; (2) STAS was associated with a high risk of ONM, especially multiple ONMs, and was a significant predictor of ONM on multivariable analysis, independent of tumor size, SUVmax, and LVI; and (3) in patients with STAS, risk of recurrence within treated lobes and regional LNs was higher after sublobar resection than after lobectomy, suggesting that lobectomy may be the most appropriate procedure for patients with STAS.
Previous studies have reported radiologic or pathologic predictors of ONM, such as larger tumor size, higher SUVmax, presence of LVI, and MIP histologic subtype.11,15,38 Although pathologic findings are strongly associated with ONM, it would be difficult to use these factors for pre- or intraoperative decisions regarding resection type. We have reported that detection of MIP histologic subtype on frozen section analysis39 had low sensitivity (37%) despite high specificity (94%). However, we also assessed the potential utility of frozen section analysis for detecting STAS intraoperatively and found that frozen section analysis for STAS had better sensitivity and similar specificity, compared with frozen section for MIP subtype, with substantial interpathologist agreement.37 Our three-phase multivariable models for predicting ONM demonstrated that (1) SUVmax and tumor size were independent predictors of ONM; (2) STAS was an independent predictor of ONM, suggesting that intraoperative detection of STAS (assuming frozen section analysis is feasible) will be useful for predicting ONM, in addition to preoperative radiological findings; and (3) STAS remained a significant predictor of ONM, independent of LVI and invasive tumor size, when preoperative and postoperative factors were included in the analysis. Together, these findings suggest that STAS is a clinically useful and significant factor for predicting ONM in patients with c-Stage IA lung ADC.
To avoid locoregional recurrence following sublobar resection, various options have been proposed, such as selecting patients by imaging studies,40 achieving adequate surgical margins,41 performing segmentectomy rather than wedge resection,42 and including adequate LN evaluation.43 In the present study, we evaluated site-specific risk of recurrence by risk of ONM (based on STAS status). The results of this analysis showed that (1) wedge resection without mediastinal LN evaluation was associated with a significantly higher risk of locoregional recurrence, regardless of the risk of ONM, supporting the importance of adequate mediastinal LN evaluation in all patients undergoing sublobar resection; (2) in patients with a high risk of ONM (based on positive STAS status), decreased extent of resection was associated with an increased risk of recurrence within the treated lung and regional LNs; and (3) in patients with a low risk of ONM (based on negative STAS status), the risk of regional LN recurrence was similar between sublobar resection with mediastinal LN evaluation and lobectomy and the risk of any type of recurrence was similar between segmentectomy and wedge resection (with mediastinal LN evaluation). These findings suggest that assessing the risk of ONM by STAS status—in addition to performing mediastinal LN evaluation to detect ONM—may help to identify appropriate candidates for sublobar resection. A multi-institutional, prospective study determining the accuracy and predictive value of detecting STAS on frozen section is a first step, as confirmation of presence of STAS can help determine the type of resection for small-sized lung ADC. The information derived from such a study (location, extent, and histological subtypes of STAS cells) can help determine the nature of the prospective, therapeutic study investigating the appropriate extent of resection for high-risk, small-sized lung ADC. The presence of STAS in other NSCLC histologic subtypes has been reported. The utility of STAS in predicting ONM in NSCLC other than lung ADC is an ongoing area of investigation.
One of the limitations of the present study was the potential selection bias between surgical procedures. Another limitation was the relatively small number of patients who underwent sublobar resection. These limitations might have affected our results. Nevertheless, the association between STAS and ONM demonstrated in our study is provocative and needs to be investigated in a prospective study. Following the results of the National Lung Screening Trial, which showed that screening with low-dose CT reduces mortality attributable to lung cancer, the detection of early-stage lung cancer has been expected to increase.44 In addition, the age of patients with lung cancer in the United States has been increasing, with associated higher risks of postoperative morbidity and noncancer-specific mortality.45,46 The above factors underscore the importance of investigating the risk factors for ONM.
In conclusion, we have demonstrated that, in patients with c-Stage IA lung ADC, ONMs are small and frequently located in intrapulmonary LNs, suggesting a potential difficulty in detecting ONM by LN sampling during sublobar resection. STAS is a significant predictor of ONM, independent of SUVmax, tumor size, and LVI. In patients who are eligible for both lobar and sublobar resection, intraoperative identification of STAS can help to determine the most appropriate type of resection to perform.
Supplementary Material
Acknowledgements
We thank Krishna E. Tobon and David B. Sewell of the MSK Thoracic Surgery Service for their editorial assistance.
Funding: The authors’ laboratory work is supported by grants from the National Institutes of Health (R01 CA236615, R01CA235667, and P30 CA008748), the U.S. Department of Defense (CA170630, BC132124, CA180889, and LC160212), the Joanne and John DallePezze Foundation, the Derfner Foundation, Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Abbreviations:
- ACI
acinar predominant
- ADC
adenocarcinoma
- AIS
adenocarcinoma in situ
- CIR
competing risks analysis
- COL
colloid predominant
- CT
computed tomography
- N0
node-negative
- LEP
lepidic predominant
- LN
lymph node
- LVI
lymphovascular invasion
- MIA
minimally invasive adenocarcinoma
- MIP
micropapillary predominant
- MUC
mucinous predominant
- NSCLC
non-small cell lung cancer
- ONM
occult lymph node metastasis
- PAP
papillary predominant
- PET
positron emission tomography
- SOL
solid predominant
- STAS
spread through air spaces
- SUVmax
maximum standard uptake value
- VPI
visceral pleural invasion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.National Comprehensive Cancer Centers: NCCN clinical practice guidelines in oncology (NCCN Guidelines): Non-small cell lung cancer v4. 2018. Available at: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed June 25, 2018.
- 2.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622; discussion 622–613. [DOI] [PubMed] [Google Scholar]
- 3.Varlotto JM, Medford-Davis LN, Recht A, et al. Identification of stage I non-small cell lung cancer patients at high risk for local recurrence following sublobar resection. Chest. 2013;143:1365–1377. [DOI] [PubMed] [Google Scholar]
- 4.Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg. 2011;92:1819–1823; discussion 1824–1815. [DOI] [PubMed] [Google Scholar]
- 5.Bains S, Eguchi T, Warth A, et al. Procedure-specific risk prediction for recurrence in patients undergoing lobectomy or sublobar resection for small (</=2 cm) lung adenocarcinoma: an international cohort analysis. J Thorac Oncol. 2019;14:72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:990–1003. [DOI] [PubMed] [Google Scholar]
- 7.Reed CE, Harpole DH, Posther KE, et al. Results of the American College of Surgeons Oncology Group Z0050 trial: the utility of positron emission tomography in staging potentially operable non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126:1943–1951. [DOI] [PubMed] [Google Scholar]
- 8.van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359:1388–1393. [DOI] [PubMed] [Google Scholar]
- 9.Nitadori J, Bograd AJ, Morales EA, et al. Preoperative consolidation-to-tumor ratio and SUVmax stratify the risk of recurrence in patients undergoing limited resection for lung adenocarcinoma </=2 cm. Ann Surg Oncol. 2013;20:4282–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirmani BH, Rintoul RC, Win T, et al. Stage migration: results of lymph node dissection in the era of modern imaging and invasive staging for lung cancer. Eur J Cardiothorac Surg. 2013;43:104–109; discussion 109–110. [DOI] [PubMed] [Google Scholar]
- 11.Bille A, Woo KM, Ahmad U, et al. Incidence of occult pN2 disease following resection and mediastinal lymph node dissection in clinical stage I lung cancer patients. Eur J Cardiothorac Surg. 2017;51:674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veeramachaneni NK, Battafarano RJ, Meyers BF, et al. Risk factors for occult nodal metastasis in clinical T1N0 lung cancer: a negative impact on survival. Eur J Cardiothorac Surg. 2008;33:466–469. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Caro A, Garcia S, Reguart N, et al. Incidence of occult mediastinal node involvement in cN0 non-small-cell lung cancer patients after negative uptake of positron emission tomography/computer tomography scan. Eur J Cardiothorac Surg. 2010;37:1168–1174. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Nagai K, Yoshida J, et al. Clinical predictors of N2 disease in the setting of a negative computed tomographic scan in patients with lung cancer. J Thorac Cardiovasc Surg. 1999;117:593–598. [DOI] [PubMed] [Google Scholar]
- 15.Yeh YC, Nitadori J, Kadota K, et al. Micropapillary histology is associated with occult lymph node metastasis (pN2) in patients with clinically N2-negative (cN0/N1) lung adenocarcinoma. J Thorac Oncol. 2013;8:S671–S672. [Google Scholar]
- 16.Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol. 2015;10:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warth A, Muley T, Kossakowski CA, et al. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol. 2015;39:793–801. [DOI] [PubMed] [Google Scholar]
- 19.Masai K, Sakurai H, Sukeda A, et al. Prognostic impact of margin distance and tumor spread through air spaces in limited resection for primary lung cancer. J Thorac Oncol. 2017;12:1788–1797. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto J, Nakajima T, Suzuki H, et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2016;152:64–72 e61. [DOI] [PubMed] [Google Scholar]
- 21.Dai C, Xie H, Su H, et al. Tumor spread through air spaces affects the recurrence and overall survival in patients with lung adenocarcinoma >2 to 3 cm. J Thorac Oncol. 2017;12:1052–1060. [DOI] [PubMed] [Google Scholar]
- 22.Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg. 2016;23:567–572. [DOI] [PubMed] [Google Scholar]
- 23.Onozato ML, Kovach AE, Yeap BY, et al. Tumor islands in resected early-stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am J Surg Pathol. 2013;37:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu S, Tan KS, Kadota K, et al. Spread through air spaces (STAS) is an independent predictor of recurrence and lung cancer-specific death in squamous cell carcinoma. J Thorac Oncol. 2017;12:223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadota K, Kushida Y, Katsuki N, et al. Tumor spread through air spaces is an independent predictor of recurrence-free survival in patients with resected lung squamous cell carcinoma. Am J Surg Pathol. 2017;41:1077–1086. [DOI] [PubMed] [Google Scholar]
- 26.Aly RG, Eguchi T, Kadota K, et al. Spread through air spaces (STAS) correlates with prognosis in lung neuroendocrine tumors (LNET). Mod Pathol. 2018. (suppl; abstr);31:724. [Google Scholar]
- 27.Toyokawa G, Yamada Y, Tagawa T, et al. High frequency of spread through air spaces in resected small cell lung cancer. Anticancer Res. 2018;38:1821–1825. [DOI] [PubMed] [Google Scholar]
- 28.Broderick SR, Patterson GA. Performance of integrated positron emission tomography/computed tomography for mediastinal nodal staging in non-small cell lung carcinoma. Thorac Surg Clin. 2013;23:193–198. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Ren S, Zhang Y, et al. Risk factors for predicting the occult nodal metastasis in T1–2N0M0 NSCLC patients staged by PET/CT: potential value in the clinic. Lung Cancer. 2013;81:213–217. [DOI] [PubMed] [Google Scholar]
- 30.Kanzaki R, Higashiyama M, Fujiwara A, et al. Occult mediastinal lymph node metastasis in NSCLC patients diagnosed as clinical N0–1 by preoperative integrated FDG-PET/CT and CT: risk factors, pattern, and histopathological study. Lung Cancer. 2011;71:333–337. [DOI] [PubMed] [Google Scholar]
- 31.AJCC Cancer Staging Manual. Springer International Publishing; 2017. [Google Scholar]
- 32.Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012;142:1620–1635. [DOI] [PubMed] [Google Scholar]
- 33.Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol. 2009;33:1752–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Travis WD, Brambilla E, Burke AP, et al. WHO classification of tumours of the lung, pleura, thymus and heart, 4th edn., International Agency for Research on Cancer (IARC): Lyon, 2015. Lyon, France: International Agency for Research on Cancer (IARC); 2015. [Google Scholar]
- 36.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eguchi T, Kameda K, Lu SH, et al. Lobectomy is associated with better outcomes than sublobar resection in spread through air spaces (STAS)-positive T1 lung adenocarcinoma: a propensity score-matched analysis. J Thorac Oncol. 2019;14:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon Y, Kim KS, Lee KY, et al. Clinicopathologic factors associated with occult lymph node metastasis in patients with clinically diagnosed N0 lung adenocarcinoma. Ann Thorac Surg. 2016;101:1928–1935. [DOI] [PubMed] [Google Scholar]
- 39.Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of </= 3 cm: accuracy and interobserver agreement. Histopathology. 2015;66:922–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg. 2005;129:991–996. [DOI] [PubMed] [Google Scholar]
- 41.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–932; discussion 932–923. [DOI] [PubMed] [Google Scholar]
- 42.Landreneau JP, Schuchert MJ, Weyant R, et al. Anatomic segmentectomy and brachytherapy mesh implantation for clinical stage I non-small cell lung cancer (NSCLC). Surgery. 2014;155:340–346. [DOI] [PubMed] [Google Scholar]
- 43.Stiles BM, Kamel MK, Nasar A, et al. The importance of lymph node dissection accompanying wedge resection for clinical stage IA lung cancer. Eur J Cardiothorac Surg. 2017;51:511–517. [DOI] [PubMed] [Google Scholar]
- 44.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. [DOI] [PubMed] [Google Scholar]
- 46.Eguchi T, Bains S, Lee MC, et al. Impact of increasing age on cause-specific mortality and morbidity in patients with stage i non-small-cell lung cancer: a competing risks analysis. J Clin Oncol. 2017;35:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


